EBV Early Lytic Antigens, EBNA2 and PDL-1, in Progressive Multiple Sclerosis Brain: A Coordinated Contribution to Viral Immune Evasion
Abstract
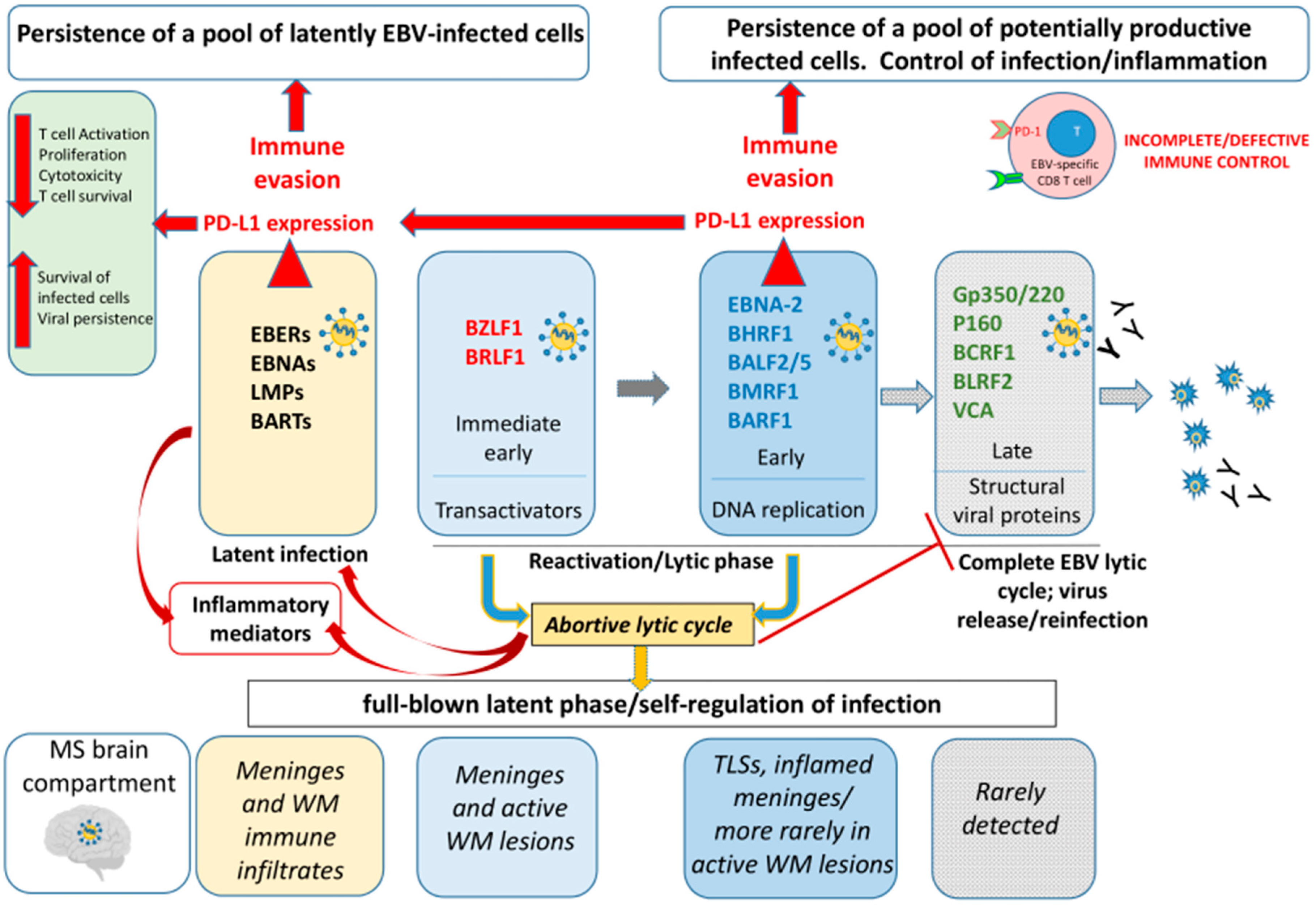
1. Introduction
2. Results
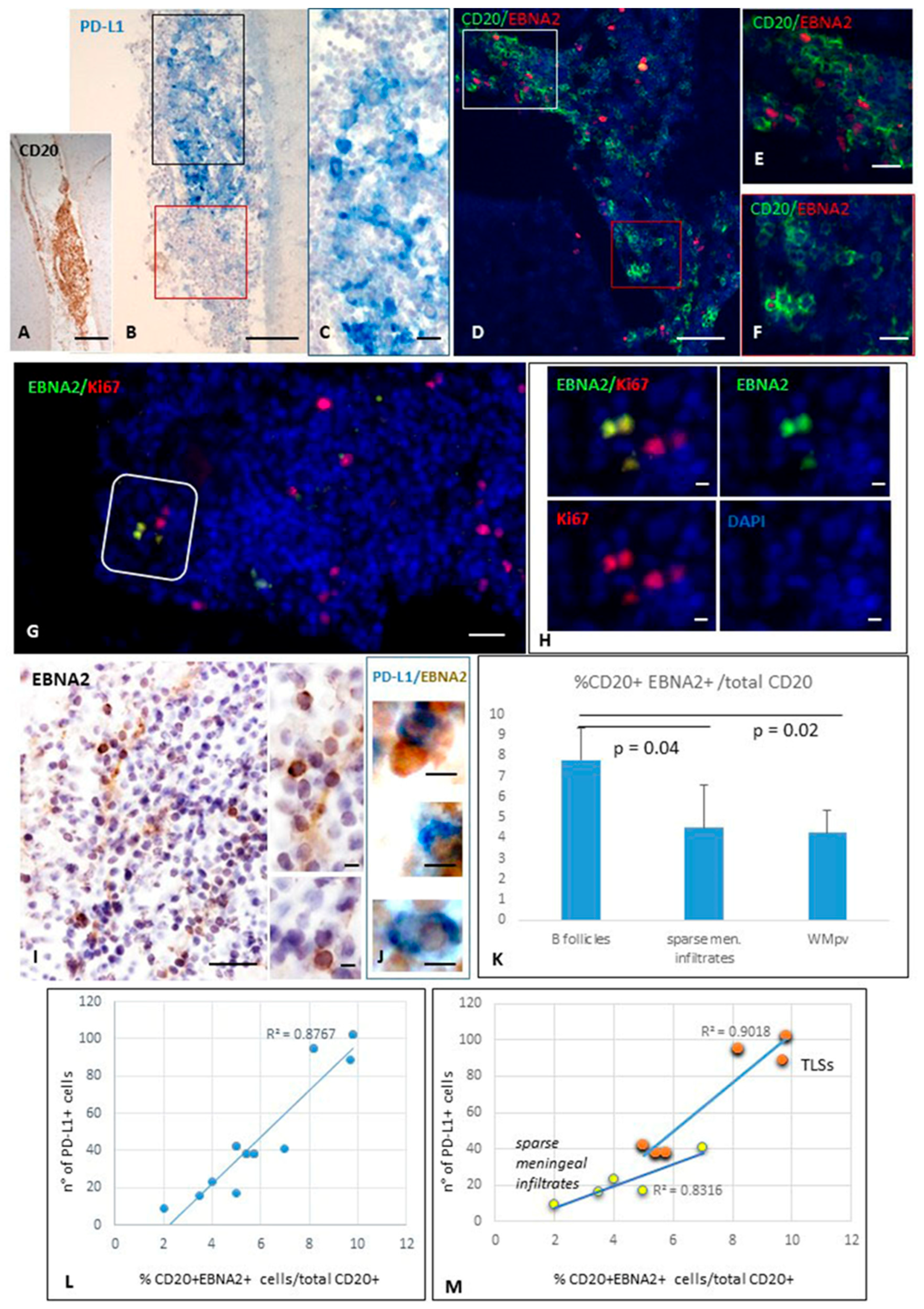
2.1. EBNA2-Positive B Cells in MS Inflammatory Infiltrates Co-Express PD-L1, and Their Number Positively Correlates with Intracerebral PD-L1 Expression
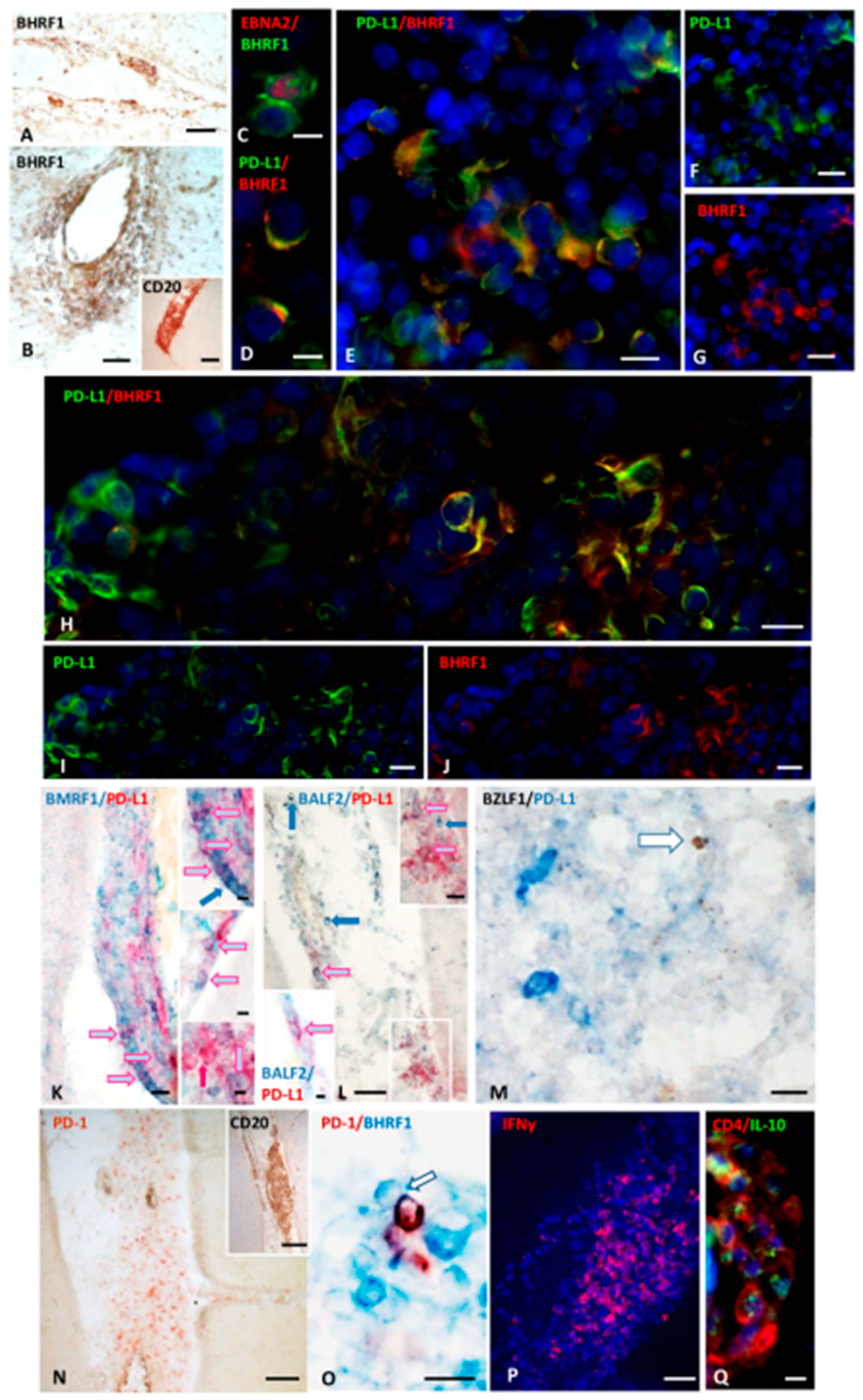
2.1.1. Presence and Distribution of Cells Expressing the EBV E Antigen BHRF1 in MS Brain
2.1.2. Cells Expressing EBNA2 and Early Lytic Antigens BHRF1, BALF2, and BMRF1 Co-Express PD-L1
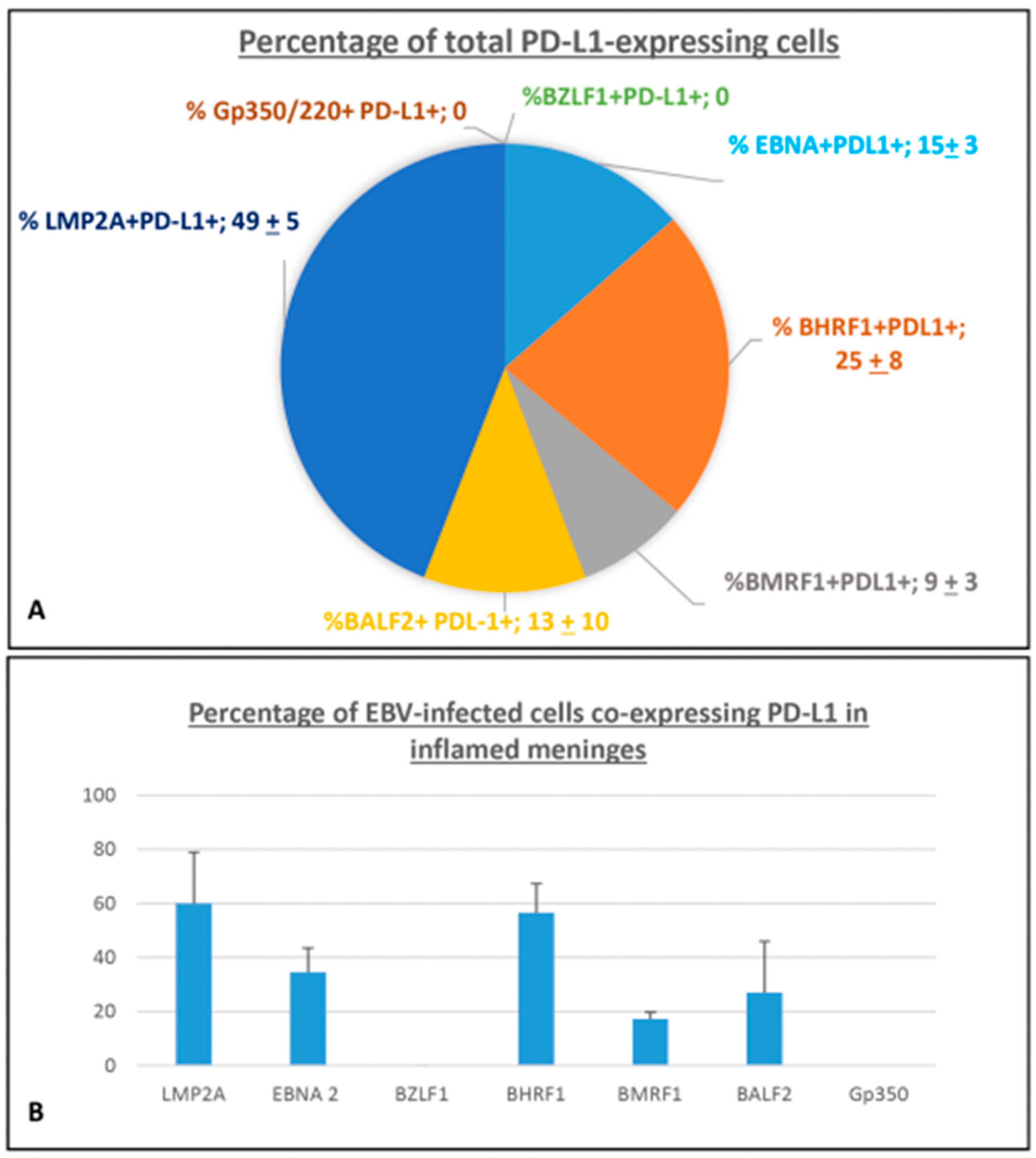
2.1.3. Quantification of EBV-Infected Cells Co-Expressing PD-L1
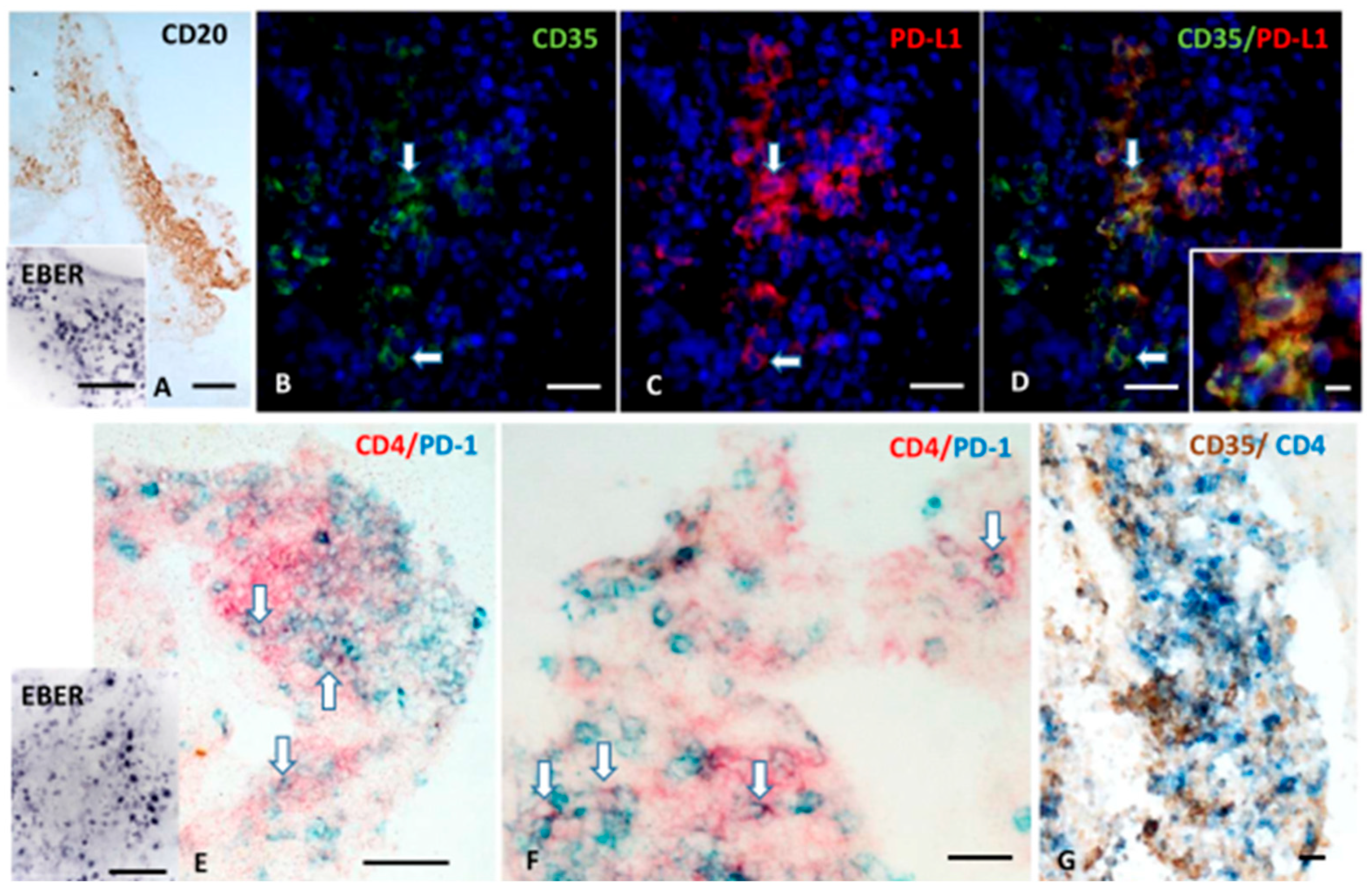
2.1.4. FDCs Express PD-L1, and Intrafollicular CD4+ T Cells Express PD-1 Within EBV-Storing Meningeal TLSs
3. Discussion
4. Materials and Methods
4.1. Tissue Samples
4.2. Neuropathological Assessment
4.3. Immunohistochemistry
4.4. Cell Counts and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BALF2 | BamHI A Rightward Reading Frame 2 |
| BHRF1 | BamHI H Rightward Reading Frame 1 |
| BMRF1 | BamHI M Rightward Reading Frame 1 |
| BZLF1 | BamHI Z Leftward Reading Frame 1 |
| CNS | Central Nervous System |
| CSF | Cerebrospinal Fluid |
| DMTs | Disease-Modifying Therapies |
| E | Early |
| EBNA | Epstein–Barr Nuclear Antigen |
| EBV | Epstein–Barr Virus |
| FDCs | Follicular Dendritic Cells |
| gp350/220 | Glycoprotein 350/220 |
| HLA | Human Leukocyte Antigen |
| IE | Immediate Early |
| IFNγ | Interferon Gamma |
| IL-10 | Interleukin 10 |
| IRF3 | Interferon Regulatory Factor 3 |
| L | Late |
| LMP | Latent Membrane Protein |
| MS | Multiple Sclerosis |
| NK cells | Natural Killer Cells |
| PD-1 | Programmed Cell Death Protein 1 |
| PD-L1 | Programmed Death-Ligand 1 |
| RRMS | Relapsing-Remitting Multiple Sclerosis |
| SPMS | Secondary Progressive Multiple Sclerosis |
| Tfh cells | Follicular Helper T cells |
| TLR3 | Toll-like Receptor 3 |
| TLSs | Tertiary Lymphoid Structures |
| Trm cells | Tissue-Resident Memory T cells |
| WM | White Matter |
References
- Serafini, B.; Rosicarelli, B.; Magliozzi, R.; Stigliano, E.; Aloisi, F. Detection of ectopic B cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 2004, 14, 164–174. [Google Scholar] [CrossRef]
- Magliozzi, R.; Howell, O.W.; Vora, A.; Serafini, B.; Nicholas, R.; Puopolo, M.; Reynolds, R.; Aloisi, F. Meningeal B cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 2007, 130, 1089–1104. [Google Scholar] [CrossRef] [PubMed]
- Machado-Santos, J.; Saji, E.; Tröscher, A.R.; Paunovic, M.; Liblau, R.; Gabriely, G.; Bien, C.G.; Bauer, J.; Lassmann, H. The compartmentalized inflammatory response in the multiple sclerosis brain is composed of tissue-resident CD8+ T lymphocytes and B cells. Brain 2018, 141, 2066–2082. [Google Scholar] [CrossRef] [PubMed]
- Serafini, B.; Benincasa, L.; Rosicarelli, B.; Aloisi, F. EBV infected cells in the multiple sclerosis brain express PD-L1: How the virus and its niche may escape immune surveillance. J. Neuroimmunol. 2024, 389, 578314. [Google Scholar] [CrossRef]
- Magliozzi, R.; Howell, O.W.; Calabrese, M.; Reynolds, R. Meningeal inflammation as a driver of cortical grey matter pathology and clinical progression in multiple sclerosis. Nat. Rev. Neurol. 2023, 19, 461–476. [Google Scholar] [CrossRef] [PubMed]
- Bery, A.I.; Shepherd, H.M.; Li, W.; Krupnick, A.S.; Gelman, A.E.; Kreisel, D. Role of tertiary lymphoid organs in the regulation of immune responses in the periphery. Cell. Mol. Life Sci. 2022, 79, 359. [Google Scholar] [CrossRef] [PubMed]
- Howell, O.W.; Reeves, C.A.; Nicholas, R.; Carassiti, D.; Radotra, B.; Gentleman, S.M.; Serafini, B.; Aloisi, F.; Roncaroli, F.; Magliozzi, R.; et al. Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain 2011, 134, 2755–2771. [Google Scholar] [CrossRef] [PubMed]
- Bjornevik, K.; Cortese, M.; Healy, B.C.; Kuhle, J.; Mina, M.J.; Leng, Y.; Elledge, S.J.; Niebuhr, D.W.; Scher, A.I.; Munger, K.L.; et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 2022, 375, 296–301. [Google Scholar] [CrossRef]
- Bjornevik, K.; Münz, C.; Cohen, J.I.; Ascherio, A. Epstein-Barr virus as a leading cause of multiple sclerosis: Mechanisms and implications. Nat. Rev. Neurol. 2023, 19, 160–171. [Google Scholar] [CrossRef]
- Damania, B.; Kenney, S.C.; Raab-Traub, N. Epstein-Barr virus: Biology and clinical disease. Cell 2022, 185, 3652–3670. [Google Scholar] [CrossRef] [PubMed]
- Veroni, C.; Aloisi, F. The CD8 T cell-Epstein-Barr virus-B cell trialogue: A central issue in multiple sclerosis pathogenesis. Front. Immunol. 2021, 12, 665718. [Google Scholar] [CrossRef] [PubMed]
- Lyu, L.; Li, Q.; Wang, C. EBV Latency Programs: Molecular and Epigenetic Regulation and Its Role in Disease Pathogenesis. J. Med. Virol. 2025, 97, e70501. [Google Scholar] [CrossRef] [PubMed]
- Sausen, D.G.; Poirier, M.C.; Spiers, L.M.; Smith, E.N. Mechanisms of T cell evasion by Epstein-Barr virus and implications for tumor survival. Front. Immunol. 2023, 14, 1289313. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhang, Y.; Luo, B. The lytic phase of Epstein-Barr virus plays an important role in tumorigenesis. Virus Genes 2023, 59, 1–12. [Google Scholar] [CrossRef]
- Cao, Y.; Xie, L.; Shi, F.; Tang, M.; Li, Y.; Hu, J.; Zhao, L.; Zhao, L.; Yu, X.; Luo, X.; et al. Targeting the signaling in Epstein–Barr virus-associated diseases: Mechanism, regulation, and clinical study. Sig. Transduct. Target. Ther. 2021, 6, 15. [Google Scholar] [CrossRef]
- Long, X.; Yang, J.; Zhang, X.; Yang, Z.; Li, Y.; Wang, F.; Li, X.; Kuang, E. BRLF1 suppresses RNA Pol III-mediated RIG-I inflammasome activation in the early EBV lytic lifecycle. EMBO Rep. 2021, 22, e50714. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadou, E.; Stroopinsky, D.; Alimperti, S.; Jiao, A.L.; Pyzer, A.R.; Cippitelli, C.; Pepe, G.; Severa, M.; Rosenblatt, J.; Etna, M.P.; et al. Epstein-Barr virus-encoded EBNA2 alters immune checkpoint PD-L1 expression by downregulating miR-34a in B-cell lymphomas. Leukemia 2019, 33, 132–147. [Google Scholar] [CrossRef]
- Leopizzi, M.; Mundo, L.; Messina, E.; Campolo, F.; Lazzi, S.; Angeloni, A.; Marchese, C.; Leoncini, L.; Giordano, C.; Slack, F.; et al. Epstein-Barr virus–encoded EBNA2 downregulates ICOSL by inducing miR-24 in B-cell lymphoma. Blood 2024, 143, 429–443. [Google Scholar] [CrossRef]
- Su, C.; Lu, F.; Soldan, S.S.; Lamontagne, R.J.; Tang, H.Y.; Napoletani, G.; Farrell, P.J.; Tempera, I.; Kossenkov, A.V.; Lieberman, P.M. EBNA2 driven enhancer switching at the CIITA-DEXI locus suppresses HLA class II gene expression during EBV infection of B-lymphocytes. PLoS Pathog. 2021, 17, e1009834. [Google Scholar] [CrossRef]
- Yanagi, Y.; Okuno, Y.; Narita, Y.; Masud, H.M.A.; Watanabe, T.; Sato, Y.; Kanda, T.; Kimura, H.; Murata, T. RNAseq analysis identifies involvement of EBNA2 in PD-L1 induction during Epstein-Barr virus infection of primary B cells. Virology 2021, 557, 44–54. [Google Scholar] [CrossRef]
- Hahn, A.M.; Huye, L.E.; Ning, S.; Webster-Cyriaque, J.; Pagano, J.S. Interferon regulatory factor 7 is negatively regulated by the Epstein-Barr virus immediate-early gene, BZLF-1. J. Virol. 2005, 79, 10040–10052. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Long, X.; Huang, L.; Yang, M.; Yuan, Y.; Wang, Y.; Delecluse, H.-J.; Kuang, E. Epstein-Barr virus BZLF1-mediated downregulation of proinflammatory factors is essential for optimal lytic viral replication. J. Virol. 2015, 90, 887–903. [Google Scholar] [CrossRef] [PubMed]
- Michaud, F.; Coulombe, F.; Gaudreault, É.; Paquet-Bouchard, C.; Rola-Pleszczynski, M.; Gosselin, J. Epstein-Barr virus interferes with the amplification of IFNα secretion by activating suppressor of cytokine signaling 3 in primary human monocytes. PLoS ONE 2010, 5, e11908. [Google Scholar] [CrossRef] [PubMed]
- Buschle, A.; Hammerschmidt, W. Epigenetic lifestyle of Epstein-Barr virus. Semin. Immunopathol. 2020, 42, 131–142. [Google Scholar] [CrossRef]
- Adamson, A.L.; Darr, D.; Holley-Guthrie, E.; Johnson, R.A.; Mauser, A.; Swenson, J. Epstein-Barr virus immediate-early proteins BZLF1 and BRLF1 activate the ATF2 transcription factor by increasing the levels of phosphorylated p38 and c-Jun N-terminal kinases. J. Virol. 2000, 74, 1224–1233. [Google Scholar] [CrossRef]
- Hislop, A.D.; Ressing, M.E.; van Leeuwen, D.; Pudney, V.A.; Horst, D.; Koppers-Lalic, D.; Croft, N.P.; Neefjes, J.J.; Rickinson, A.B.; Wiertz, E.J. A CD8+ T cell immune evasion protein specific to Epstein–Barr virus and its close relatives in Old World primates. J. Exp. Med. 2007, 204, 1863–1873. [Google Scholar] [CrossRef]
- van de Weijer, M.L.; Luteijn, R.D.; Wiertz, E.J.H.J. Viral immune evasion: Lessons in MHC class I antigen presentation. Semin. Immunol. 2015, 27, 125–137. [Google Scholar] [CrossRef]
- Hoebe, E.K.; Le Large, T.Y.S.; Tarbouriech, N.; Oosterhoff, D.; De Gruijl, T.D.; Middeldorp, J.M.; Greijer, A.E. Epstein-Barr virus-encoded BARF1 protein is a decoy receptor for macrophage colony stimulating factor and interferes with macrophage differentiation and activation. Viral Immunol. 2012, 25, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Fitzsimmons, L.; Cartlidge, R.; Chang, C.; Sejic, N.; Galbraith, L.C.A.; Suraweera, C.D.; Croom-Carter, D.; Dewson, G.; Tierney, R.J.; Bell, A.I.; et al. EBV BCL-2 homologue BHRF1 drives chemoresistance and lymphomagenesis by inhibiting multiple cellular pro-apoptotic proteins. Cell Death Differ. 2020, 27, 1554–1568. [Google Scholar] [CrossRef] [PubMed]
- Vilmen, G.; Glon, D.; Siracusano, G.; Lussignol, M.; Shao, Z.; Hernandez, E.; Perdiz, D.; Quignon, F.; Mouna, L.; Poüs, C.; et al. BHRF1, a BCL2 viral homolog, disturbs mitochondrial dynamics and stimulates mitophagy to dampen type I IFN induction. Autophagy 2021, 17, 1296–1315. [Google Scholar] [CrossRef]
- Salamun, S.G.; Sitz, J.; De La Cruz-Herrera, C.F.; Yockteng-Melgar, J.; Marcon, E.; Greenblatt, J.; Fradet-Turcotte, A.; Frappier, L. The Epstein-Barr virus BMRF1 protein activates transcription and inhibits the DNA damage response by binding NuRD. J. Virol. 2019, 93, e01070-19. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Deng, Y.; Guo, Y.; Xu, Z.; Li, Y.; Ou, X.; Xie, L.; Lu, M.; Zhong, J.; Li, B.; et al. Epstein–Barr virus early protein BFRF1 suppresses IFN-β activity by inhibiting the activation of IRF3. Front. Immunol. 2020, 11, 513383. [Google Scholar] [CrossRef]
- Angelini, D.F.; Serafini, B.; Piras, E.; Severa, M.; Coccia, E.M.; Rosicarelli, B.; Ruggieri, S.; Gasperini, C.; Buttari, F.; Centonze, D.; et al. Increased CD8+ T cell response to Epstein-Barr virus lytic antigens in the active phase of multiple sclerosis. PLoS Pathog. 2013, 9, e1003220. [Google Scholar] [CrossRef]
- Chao, T.Y.; Cheng, Y.Y.; Wang, Z.Y.; Fang, T.F.; Chang, Y.R.; Fuh, C.S.; Su, M.T.; Su, Y.W.; Hsu, P.H.; Su, Y.C.; et al. Subcellular distribution of BALF2 and the role of Rab1 in the formation of Epstein-Barr virus cytoplasmic assembly compartment and virion release. Microbiol. Spectr. 2023, 11, e0436922. [Google Scholar] [CrossRef] [PubMed]
- Bussey, K.A.; Murthy, S.; Reimer, E.; Chan, B.; Hatesuer, B.; Schughart, K.; Glaunsinger, B.; Adler, H.; Brinkmann, M.M. Endosomal Toll-Like Receptors 7 and 9 Cooperate in Detection of Murine Gammaherpesvirus 68 Infection. J. Virol. 2019, 93, e01173-18. [Google Scholar] [CrossRef] [PubMed]
- van Gent, M.; Griffin, B.D.; Berkhoff, E.G.; van Leeuwen, D.; Boer, I.G.J.; Buisson, M.; Hartgers, F.C.; Burmeister, W.P.; Wiertz, E.J.; Ressing, M.E. EBV lytic-phase protein BGLF5 contributes to TLR9 downregulation during productive infection. J. Immunol. 2011, 186, 1694–1702. [Google Scholar] [CrossRef] [PubMed]
- van Gent, M.; Gram, A.M.; Boer, I.G.J.; Geerdink, R.J.; Lindenbergh, M.F.S.; Jan Lebbink, R.; Wiertz, E.J.; Ressing, M.E. Silencing the shutoff protein of Epstein–Barr virus in productively infected B cells points to (innate) targets for immune evasion. J. Gen. Virol. 2015, 96, 858–865. [Google Scholar] [CrossRef]
- Rowe, M.; Glaunsinger, B.; van Leeuwen, D.; Zuo, J.; Sweetman, D.; Ganem, D.; Middeldorp, J.; Wiertz, E.J.H.J.; Ressing, M.E. Host shutoff during productive Epstein–Barr virus infection is mediated by BGLF5 and may contribute to immune evasion. Proc. Natl. Acad. Sci. USA 2007, 104, 3366–3371. [Google Scholar] [CrossRef]
- Todorović, N.; Ambrosio, M.R.; Amedei, A. Immune modulation by Epstein-Barr virus lytic cycle: Relevance and implication in oncogenesis. Pathogens 2024, 13, 876. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Ling, C.; Johannsen, E.; Nagaraja, T.; Swaminathan, S. Negative autoregulation of Epstein-Barr virus (EBV) replicative gene expression by EBV SM protein. J. Virol. 2009, 83, 8041–8050. [Google Scholar] [CrossRef] [PubMed]
- Serafini, B.; Rosicarelli, B.; Veroni, C.; Mazzola, G.A.; Aloisi, F. Epstein-Barr virus in the cerebrospinal fluid and blood of patients with multiple sclerosis. J. Virol. 2019, 93, e00980-19. [Google Scholar] [CrossRef]
- Chen, L.W.; Wang, S.S.; Hung, C.H.; Hung, Y.H.; Lin, C.L.; Chang, P.J. The Epstein-Barr Virus Lytic Protein BMLF1 Induces Upregulation of GRP78 Expression through ATF6 Activation. Int. J. Mol. Sci. 2021, 22, 4024. [Google Scholar] [CrossRef]
- McKenzie, J.; Lopez-Giraldez, F.; Delecluse, H.J.; Walsh, A.; El-Guindy, A. The Epstein-Barr virus immunoevasins BCRF1 and BPLF1 are expressed by a mechanism independent of the canonical late pre-initiation complex. PLoS Pathog. 2016, 12, e1006008. [Google Scholar] [CrossRef] [PubMed]
- Pudney, V.A.; Leese, A.M.; Rickinson, A.B.; Hislop, A.D. CD8+ immunodominance among Epstein-Barr virus lytic cycle antigens directly reflects the efficiency of antigen presentation in lytically infected cells. J. Exp. Med. 2005, 201, 349–360. [Google Scholar] [CrossRef]
- Morales-Sánchez, A.; Fuentes-Panana, E.M. The Immunomodulatory Capacity of an Epstein-Barr Virus Abortive Lytic Cycle: Potential Contribution to Viral Tumorigenesis. Cancers 2018, 10, 98. [Google Scholar] [CrossRef]
- Hudson, K.; Cross, N.; Jordan-Mahy, N.; Leyland, R. The extrinsic and intrinsic roles of PD-L1 and its receptor PD-1: Implications for immunotherapy treatment. Front. Immunol. 2020, 11, 568931. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, G.; Romano, M.; Nova-Lamperti, E.; Werner Sunderland, M.; Nerviani, A.; Scotta, C.; Bombardieri, M.; Quezada, S.A.; Sacks, S.H.; Noelle, R.J.; et al. PD-L1 signaling on human memory CD4+ T cells induces a regulatory phenotype. PLoS Biol. 2021, 19, e3001199. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.W.; Wang, H.; Zhang, W.W.; Wang, J.H.; Liu, W.J.; Xia, Z.J.; Huang, H.Q.; Jiang, W.Q.; Zhang, Y.J.; Wang, L. PD-L1 is upregulated by EBV-driven LMP1 through NF-κB pathway and correlates with poor prognosis in natural killer/T-cell lymphoma. J. Hematol. Oncol. 2016, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Yanagi, Y.; Hara, Y.; Mabuchi, S.; Watanabe, T.; Sato, Y.; Kimura, H.; Murata, T. PD-L1 upregulation by lytic induction of Epstein-Barr Virus. Virology 2022, 568, 31–40. [Google Scholar] [CrossRef]
- Shi, J.; Hou, S.; Fang, Q.; Liu, X.; Liu, X.; Qi, H. PD-1 Controls Follicular T Helper Cell Positioning and Function. Immunity 2018, 49, 264–274.e4. [Google Scholar] [CrossRef] [PubMed]
- Wahbeh, F.; Sabatino, J.J. The association between multiple sclerosis and Epstein-Barr virus: Epidemiology and immunology. Neurol. Neuroimmunol. Neuroinflamm. 2025, 12, e200460. [Google Scholar] [CrossRef] [PubMed]
- Sollid, L.M. Epstein-Barr virus as a driver of multiple sclerosis. Sci. Immunol. 2022, 7, eabo7799. [Google Scholar] [CrossRef] [PubMed]
- Sattarnezhad, N.; Kockum, I.; Thomas, O.G.; Liu, Y.; Ho, P.P.; Barrett, A.K.; Comanescu, A.I.; Wijeratne, T.U.; Utz, P.J.; Alfredsson, L.; et al. Antibody reactivity against EBNA1 and GlialCAM differentiates multiple sclerosis patients from healthy controls. Proc. Natl. Acad. Sci. USA 2025, 122, e2424986122. [Google Scholar] [CrossRef] [PubMed]
- Münz, C. Altered EBV specific immune control in multiple sclerosis. J. Neuroimmunol. 2024, 390, 578343. [Google Scholar] [CrossRef]
- Hassani, A.; Corboy, J.R.; Al-Salam, S.; Khan, G. Epstein-Barr virus is present in the brain of most cases of multiple sclerosis and may engage more than just B cells. PLoS ONE 2018, 13, e0192109. [Google Scholar] [CrossRef] [PubMed]
- Veroni, C.; Serafini, B.; Rosicarelli, B.; Fagnani, C.; Aloisi, F. Transcriptional profile and Epstein-Barr virus infection status of laser-cut immune infiltrates from the brain of patients with progressive multiple sclerosis. J. Neuroinflamm. 2018, 15, 18. [Google Scholar] [CrossRef]
- Serafini, B.; Rosicarelli, B.; Franciotta, D.; Magliozzi, R.; Reynolds, R.; Cinque, P.; Andreoni, L.; Trivedi, P.; Salvetti, M.; Faggioni, A.; et al. Dysregulated Epstein-Barr virus infection in the multiple sclerosis brain. J. Exp. Med. 2007, 204, 2899–2912. [Google Scholar] [CrossRef] [PubMed]
- Serafini, B.; Severa, M.; Columba-Cabezas, S.; Rosicarelli, B.; Veroni, C.; Chiappetta, G.; Magliozzi, R.; Reynolds, R.; Coccia, E.M.; Aloisi, F. Epstein-Barr virus latent infection and BAFF expression in B cells in the multiple sclerosis brain: Implications for viral persistence and intrathecal B-cell activation. J. Neuropathol. Exp. Neurol. 2010, 69, 677–693. [Google Scholar] [CrossRef]
- Serafini, B.; Muzio, L.; Rosicarelli, B.; Aloisi, F. Radioactive in situ hybridization for Epstein-Barr virus-encoded small RNA supports presence of Epstein-Barr virus in the multiple sclerosis brain. Brain 2013, 136, e233. [Google Scholar] [CrossRef]
- Tzartos, J.S.; Khan, G.; Vossenkamper, A.; Cruz-Sadaba, M.; Lonardi, S.; Sefia, E.; Meager, A.; Elia, A.; Middeldorp, J.M.; Clemens, M.; et al. Association of innate immune activation with latent Epstein-Barr virus in active MS lesions. Neurology 2012, 78, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.A.; Or-Geva, N.; Aftab, B.T.; Khanna, R.; Croze, E.; Steinman, L.; Han, M.H. Molecular signature of Epstein-Barr virus infection in MS brain lesions. Neurol. Neuroimmunol. Neuroinflamm. 2018, 5, e466. [Google Scholar] [CrossRef] [PubMed]
- Orr, N.; Steinman, L. Epstein-Barr virus and the immune microenvironment in multiple sclerosis. Proc. Natl. Acad. Sci. USA 2025, 122, e2425670122. [Google Scholar] [CrossRef]
- Jaquiéry, E.; Jilek, S.; Schluep, M.; Meylan, P.; Lysandropoulos, A.; Pantaleo, G.; Du Pasquier, R.A. Intrathecal immune responses to EBV in early MS. Eur. J. Immunol. 2010, 40, 878–887. [Google Scholar] [CrossRef] [PubMed]
- van Nierop, G.P.; van Luijn, M.M.; Michels, S.S.; Melief, M.J.; Janssen, M.; Langerak, A.W.; Ouwendijk, W.J.D.; Hintzen, R.Q.; Verjans, G.M.G.M. Phenotypic and functional characterization of T cells in white matter lesions of multiple sclerosis patients. Acta Neuropathol. 2017, 134, 383–401. [Google Scholar] [CrossRef] [PubMed]
- Veroni, C.; Marnetto, F.; Granieri, L.; Bertolotto, A.; Serafini, B.; Rosicarelli, B.; Magliozzi, R.; Reynolds, R.; Aloisi, F. Immune and Epstein-Barr virus gene expression in cerebrospinal fluid and peripheral blood mononuclear cells from patients with relapsing-remitting multiple sclerosis. J. Neuroimmunol. 2015, 283, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Gouzouasis, V.; Anagnostouli, M.; Kalli, M.; Evangelopoulos, M.E.; Kilidireas, C. Epstein-Barr virus reactivation is associated with altered immune cell profiles in peripheral blood and cerebrospinal fluid of treatment-naïve multiple sclerosis patients. J. Neuroimmunol. 2025, 391, 578758. [Google Scholar] [CrossRef] [PubMed]
- Soldan, S.S.; Su, C.; Monaco, M.C.; Yoon, L.; Kannan, T.; Zankharia, U.; Patel, R.J.; Dheekollu, J.; Vladimirova, O.; Dowling, J.W.; et al. Multiple sclerosis patient-derived spontaneous B cells have distinct EBV and host gene expression profiles in active disease. Nat. Microbiol. 2024, 9, 1540–1554. [Google Scholar] [CrossRef] [PubMed]
- Latham, L.B.; Lee, M.J.; Lincoln, J.A.; Ji, N.; Forsthuber, T.G.; Lindsey, J.W. Antivirus Immune Activity in Multiple Sclerosis Correlates with MRI Activity. Acta Neurol. Scand. 2016, 133, 17–24. [Google Scholar] [CrossRef]
- Serafini, B.; Rosicarelli, B.; Veroni, C.; Aloisi, F. Tissue-resident memory T cells in the multiple sclerosis brain and their relationship to Epstein-Barr virus infected B cells. J. Neuroimmunol. 2023, 376, 578036. [Google Scholar] [CrossRef]
- Läderach, F.; Piteros, I.; Fennell, É.; Bremer, E.; Last, M.; Schmid, S.; Rieble, L.; Campbell, C.; Ludwig-Portugall, I.; Bornemann, L.; et al. EBV induces CNS homing of B cells attracting inflammatory T cells. Nature 2025, 646, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Magliozzi, R.; Bellini, B.; Mensi, A.; Pezzini, F.; Ghezzi, L.; Cross, A.; Mechelli, R.; Bellucci, G.; Salvetti, M.; Muraro, P.; et al. Unravelling the immune-pathological profile of proliferative B cells infected by Epstein-Barr virus in multiple sclerosis leptomeninges. Mult. Scler. J. 2025, 31, 97. [Google Scholar]
- Yap, L.F.; Wong, A.K.C.; Paterson, I.C.; Young, L.S. Functional Implications of Epstein-Barr Virus Lytic Genes in Carcinogenesis. Cancers 2022, 14, 5780. [Google Scholar] [CrossRef]
- Hickish, T.; Robertson, D.; Clarke, P.; Hill, M.; di Stefano, F.; Clarke, C.; Cunningham, D. Ultrastructural localization of BHRF1: An Epstein-Barr virus gene product which has homology with bcl-2. Cancer Res. 1994, 54, 2808–2811. [Google Scholar] [PubMed]
- Fang, W.; Zhang, J.; Hong, S.; Zhan, J.; Chen, N.; Qin, T.; Tang, Y.; Zhang, Y.; Kang, S.; Zhou, T.; et al. EBV-driven LMP1 and IFN-γ up-regulate PD-L1 in nasopharyngeal carcinoma: Implications for oncotargeted therapy. Oncotarget 2014, 5, 12189–12202. [Google Scholar] [CrossRef] [PubMed]
- Pulvirenti, N.; Righetti, C.; Clemente, F.; Geginat, J. Regulatory T-cells in multiple sclerosis produce IL-10 in the central 1 nervous system but are activated by Epstein-Barr Virus. bioRxiv 2024. bioRxiv: 2024.07.30.605745. [Google Scholar] [CrossRef]
- Gilardini Montani, M.S.; Santarelli, R.; Falcinelli, L.; Gonnella, R.; Granato, M.; Di Renzo, L.; Cuomo, L.; Vitillo, M.; Faggioni, A.; Cirone, M. EBV up-regulates PD-L1 on the surface of primary monocytes by increasing ROS and activating TLR signaling and STAT3. J. Leukoc. Biol. 2018, 104, 821–832. [Google Scholar] [CrossRef]
- O’Malley, G.; Treacy, O.; Lynch, K.; Naicker, S.D.; Leonard, N.A.; Lohan, P.; Dunne, P.D.; Ritter, T.; Egan, L.J.; Ryan, A.E. Stromal Cell PD-L1 Inhibits CD8+ T-cell Antitumor Immune Responses and Promotes Colon Cancer. Cancer Immunol. Res. 2018, 6, 1426–1441. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Chen, M.S.; Lee, T.Y.; Huang, T.S.; Cho, D.Y.; Chen, J.Y. Epstein-Barr Virus BRLF1 Induces PD-L1 Expression in Nasopharyngeal Carcinoma Cells. Viral Immunol. 2024, 37, 115–123. [Google Scholar] [CrossRef]
- Moyano, A.; Ferressini, N.; De Matteo, E.; Preciado, M.V.; Chabay, P. PD-L1 is upregulated in CD163+ tonsillar macrophages from children undergoing EBV primary infection. Front. Immunol. 2022, 13, 940910. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, A.; Abe, H.; Kunita, A.; Saito, R.; Kanda, T.; Yamashita, H.; Seto, Y.; Ishikawa, S.; Fukayama, M. Viral loads correlate with upregulation of PD-L1 and worse patient prognosis in Epstein–Barr Virus-associated gastric carcinoma. PLoS ONE 2019, 14, e0211358. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Mezzadra, R.; Schumacher, T.N. Regulation and function of the PD-L1 checkpoint. Immunity 2018, 48, 434–452. [Google Scholar] [CrossRef] [PubMed]
- Schönrich, G.; Raftery, M.J. The PD-1/PD-L1 axis and virus infections: A delicate balance. Front. Cell. Infect. Microbiol. 2019, 9, 207. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, P.B.; Livbjerg, A.H.; Hansen, H.J.; Petersen, T.; Höllsberg, P. Epstein-Barr Virus peptide presented by HLA-E is predominantly recognized by CD8(bright) cells in multiple sclerosis patients. PLoS ONE 2012, 7, e46120. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.D.M.; Alves, C.E.D.C.; Pontes, G.S. Epstein-Barr virus: The mastermind of immune chaos. Front. Immunol. 2024, 15, 1297994. [Google Scholar] [CrossRef] [PubMed]
| EBV Latency III Gene/Protein | Function | Role in Immune Evasion | Detection in MS Brain | References |
|---|---|---|---|---|
| EBNA2 | Transactivator of viral and cellular genes; it regulates the viral transcription of latency genes and host cell genes; mediates B cell immortalization. | Inhibits the transcription of microRNA-34a (miR-34), increasing the expression of PD-L1; induces miR-24 to reduce ICOSL expression in tumors; impairs MHC-II transcription; creates an anti-inflammatory setting inducing IL-18 receptor on B cells | Yes | [17,18,19,20] |
| EBV immediate early lytic cycle Gene/Protein | ||||
| BZLF1/transcription factor Z | Starter of the EBV lytic cycle, induces the synthesis of E lytic viral proteins; encodes the transcription factor Z, one of the promoters of early (E) lytic genes that encode the DNA viral replication proteins; mitogen activity. | Interferes with the secretion of IFN-α mediated by the JAK/STAT signaling pathway; inhibits the activation of Interferon Regulatory Factor (IRF7); inhibits the release of inflammatory factors TNF-α and IFN-γ; prevents NF-κB activation. | Yes | [21,22,23,24] |
| BRLF1/RTA protein | Viral transactivator, permits an ordered cascade of viral gene expression | Suppresses inflammasome activation (specifically RIG-1) and antiviral responses in infected cells; by interacting with subunits of RNA polymerase III suppresses the transcription of viral and cellular RNAs that can be detected by the host’s immune system. Induces BARF1 production | n.r. | [16,25] |
| EBV early lytic cycle Gene/Protein | ||||
| BNLF2a | Immunoevasin | It inhibits the transporter associated with antigen processing, blocking antigen presentation to T cells and preventing immune recognition of infected cells. | n.r. | [26,27] |
| BARF1 | Oncogenic, mitogen and immortalizing activity in human epithelial cells. | By blocking CSF-1’s normal function, BARF1 inhibits the development and activation of mononuclear cells, reduces IFN-α release, and hinders NK cell activation and cytotoxicity; up-regulates anti-apoptotic Bcl-2. | Intrathecal Ab synthesis | [28] |
| BHRF1/vBcl-2 protein | Inhibition of apoptosis; cell survival | It gives rise to viral miRNAs which suppress interferon production and target immune-related genes like CXCL-11 and RIG-I, a key viral sensor; blocking the nuclear translocation of Interferon Regulatory Factor 3, protects BZLF1-sensitized cells from NK cell killing; inhibits apoptosis. | Yes | [29,30] |
| BMRF1/DNA polymerase processivity factor (PPF) | Viral genome replication and activation of EBV genes. It functions as viral EBV DNA polymerase accessory protein; plays a role in transcriptional activation of some EBV genes for late lytic protein synthesis. | Inactivating the host’s DNA damage response pathway, suppresses the signaling cascade at double-strand DNA breaks, thus inhibiting immune surveillance | Yes | [31] |
| BFRF1 | One of the two essential proteins of the core nuclear egress complex (NEC, with BALF2), essential for the anchoring of the viral capsid, recruitment of factors to reorganize the inner nuclear membrane to allow the viral capsid to exit the nucleus into the cytoplasm. | Suppresses the host’s type I interferon (IFN-I) response, a crucial part of innate antiviral immunity, by blocking the activation pathway of the IRF3 transcription factor. | Yes | [32,33] |
| BALF2/p138 | Component of the NEC. Single stranded DNA binding protein; present in the tegument layer of mature virions. Possible role in both DNA replication and virion assembly. | Prevents host programmed cell death, contributing to maintaining the viral infection within host cells. | Yes | [34] |
| BGLF5 | Viral effector of global mRNA degradation resulting in a severe restriction of cellular gene expression. Genome instability. | EBV-induced host shutoff of host protein synthesis in productively infected cells, resulting in reduced surface display of HLA molecules for T cell recognition. Decreases both RNA and protein expression of TLR9. | n.r. | [35,36,37,38,39] |
| BMLF1/EB2-SM-Mta | Upregulates expression of GRP78, a central regulator of the unfolded protein response, to maintain host’s cell ER homeostasis and ensure a fully productive replication. | It downregulates BHRF1. | Presence of BMLF1-specific CD8+T cells | [40,41,42] |
| EBV Late phase Gene/Protein | ||||
| BCRF1 | IL-10 homolog/immunomodulatory protein | Suppression of IFN γ production | n.r. | [43] |
| Case Code | Multiple Sclerosis | Sex/Age at Death (years) | Disease Duration (years) | Cause of Death | Post-Mortem Delay (hours) | Immunotherapy | Tissue Processing | Number of Brain Tissue Blocks Analysed |
|---|---|---|---|---|---|---|---|---|
| MS92 | SPMS | F/37 | 17 | MS | 26 | Age 21: ACTH | PFA fixed and frozen | 1 |
| Snap frozen | 1 | |||||||
| MS180 | SPMS | F/44 | 18 | MS | 9 | Not reported | PFA fixed and frozen | 1 |
| Snap frozen | 1 | |||||||
| MS234 | PRMS | F/39 | 15 | Pneumonia | 15 | Not reported | PFA fixed and frozen | 1 |
| Snap frozen | 1 | |||||||
| MS342 | SPMS | F/35 | 5 | MS | 9 | Not reported | PFA fixed and frozen | 2 |
| MS352 | SPMS | M/43 | 19 | MS | 26 | Age 32: methylprednisolone Age 33: Campath-1H | PFA fixed and frozen | 1 |
| MS408 | SPMS | M/39 | 10 | Pneumonia, sepsis | 21 | Steroids, Mitoxantron, Avonex | PFA fixed and frozen | 1 |
| MS121 | PRMS | F/49 | 14 | MS | 24 | Age 46: methylprednisolone | Snap frozen | 1 |
| MS330 | SPMS | F/59 | 39 | Pneumonia | 21 | Not reported | PFA fixed and frozen | 1 |
| Formalin fixed-paraffin embedded | 1 | |||||||
| Case Code | Non Neurological Control | Sex/Age at Death (years) | Disease Duration (years) | Cause of Death | Post-Mortem Delay (hours) | Immunotherapy | Tissue Processing | Number of Tissue Blocks Analysed |
| C14 | Signs of ischaemia | M/64 | Myocardial infarction | 18 | PFA fixed and frozen | 1 | ||
| C16 | None | M/92 | Cardiac failure/old age | 13 | PFA fixed and frozen | 1 | ||
| C32 | Age-related changes | M/88 | Prostate cancer | 22 | PFA fixed and frozen | 1 | ||
| C41 | WM and perivascular oedema; mild inflammation | M/54 | Lung cancer | 20 | PFA fixed and frozen | 1 |
| Antigen | Specificity | Source | Host & Clonality | Dilution | Tissue Processing Post-Fixation | Antigen Retrieval |
| CD20 | B cells | ScyTeK Laboratories, The Hague, The Netherlands | Mouse monoclonal IgG2a, k (clone L26) | Ready to use | FFPE/FF/SF Acetone | Citrate buffer (for FFPE/FF) |
| CD4 | T cells | Quartett, Berlin, Germany | Rabbit monoclonal IgG (clone QR032) | 1:250 | FF/SF Acetone | Citrate buffer (for IL-10 co-staining) Tris-EDTA-citrate buffer pH 7.8 (for PD-1, and CD35 co-staining) |
| CD8 | T cells | Thermo Fisher Scientific Rockford, Illinois, USA | Rabbit polyclonal IgG | 1:800 | FF/SF Acetone | Citrate buffer (for FF) |
| CD35 | Follicular dendritic cells/stromal cells | NeoBiotechnologies, Union City, CA, USA | Mouse monoclonal IgG2a, k (Clone CD35 CR1/6378) | 1:300 | FF Acetone | Tris-EDTA-citrate buffer (for CD4 co-staining) |
| PD-1 | ScyTeK Laboratories | Mouse monoclonal IgG1, k (clone NAT105) | 1:100 (for FFPE) 1:200 (for FF/SF) | FFPE/FF Acetone | Citrate buffer (for BHRF1 co-staining) | |
| PD-L1 | Quartett | Rabbit monoclonal IgG (clone QR001) | 1:120 | FF Acetone | Tris-EDTA-citrate buffer (for CD35, EBNA2, BALF-2, BZLF-1, BMRF-1 and gp250/350 co-staining) | |
| PD-L1 | Abcam, Cambridge, UK | Mouse monoclonal IgG2a, k (clone ABM4E54) | 1:100 | FF Acetone | Citrate buffer (for BHRF1 co-staining) | |
| IFN γ | Abcam | Rabbit polyclonal IgG | 1:100 | SF Acetone | Citrate buffer | |
| IL-10 | Thermo Fisher Scientific | Rat monoclonal IgG, k (clone JES3-9D7) | 1:150 | FF Acetone | Citrate buffer (for CD4 co-staining) | |
| Ki67 | Proliferating cells | Spring Biosience Abcam, Cambridge, UK | Rabbit monoclonal IgG (clone SP6) | 1:150 | FF Acetone | Tris-EDTA-citrate buffer (for EBNA2 co-staining) |
| EBNA-2 | EBV latency III antigen | Celltech, Torino, Italy | Mouse monoclonal IgG1 (clone PE2) | 1:10 | FFPE/FF/SF Acetone | Citrate buffer Tris-EDTA-citrate buffer (for PD-L1 and KI67 co-staining) |
| EBNA-2 | EBV latency III antigen | Merck KGaA, Darmstadt, Germania | Rat monoclonal IgG2a, k (clone R3) | 1:100 | FF Acetone | Citrate buffer (for BHRF1 co-staining) |
| BZLF-1 | EBV immediate-early lytic antigen | Novus Biologicals, Colorado, USA | Mouse monoclonal IgG1 (clone BZ.1) | 1:50 (FFPE) 1:100 (FF) | FFPE/FF Acetone | Citrate buffer Tris-EDTA-citrate buffer (for PD-L1 co-staining) |
| BHRF1 | EBV early lytic antigen | Cusabio, Houston, USA | Rabbit polyclonal IgG | 1:200 | FFPE/FF/SF Acetone | Citrate buffer (for PD-1, PD-L1 and EBNA-2 co-staining) |
| BALF-2 | EBV early lytic antigen | Kind gift of Prof. J.M. Middeldorp | Mouse monoclonal (Clone OT13N2) | 1:350 | FFPE/FF Acetone | Citrate buffer Tris-EDTA-citrate buff |
| BMRF-1 | EBV early lytic antigen | Kind gift of Prof. J.M. Middeldorp | Mouse monoclonal (clone OT14E2) | 1:470 | FFPE/FF Acetone | Citrate buffer Tris-EDTA-citrate buffer (for PD-L1 co-staining) |
| gp250/350 | EBV late lytic antigen | Thermo Fisher Scientific | Mouse monoclonal, IgG1 (clone C61H) | 1:10 | FFPE/FF Acetone | Citrate buffer |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Benincasa, L.; Rosicarelli, B.; Meloni, C.; Serafini, B. EBV Early Lytic Antigens, EBNA2 and PDL-1, in Progressive Multiple Sclerosis Brain: A Coordinated Contribution to Viral Immune Evasion. Int. J. Mol. Sci. 2026, 27, 437. https://doi.org/10.3390/ijms27010437
Benincasa L, Rosicarelli B, Meloni C, Serafini B. EBV Early Lytic Antigens, EBNA2 and PDL-1, in Progressive Multiple Sclerosis Brain: A Coordinated Contribution to Viral Immune Evasion. International Journal of Molecular Sciences. 2026; 27(1):437. https://doi.org/10.3390/ijms27010437
Chicago/Turabian StyleBenincasa, Lucia, Barbara Rosicarelli, Chiara Meloni, and Barbara Serafini. 2026. "EBV Early Lytic Antigens, EBNA2 and PDL-1, in Progressive Multiple Sclerosis Brain: A Coordinated Contribution to Viral Immune Evasion" International Journal of Molecular Sciences 27, no. 1: 437. https://doi.org/10.3390/ijms27010437
APA StyleBenincasa, L., Rosicarelli, B., Meloni, C., & Serafini, B. (2026). EBV Early Lytic Antigens, EBNA2 and PDL-1, in Progressive Multiple Sclerosis Brain: A Coordinated Contribution to Viral Immune Evasion. International Journal of Molecular Sciences, 27(1), 437. https://doi.org/10.3390/ijms27010437








