Research on Cold Resistance of Kandelia obovata Transplanted to Zhoushan Area at the mRNA Level
Abstract
1. Introduction
2. Results
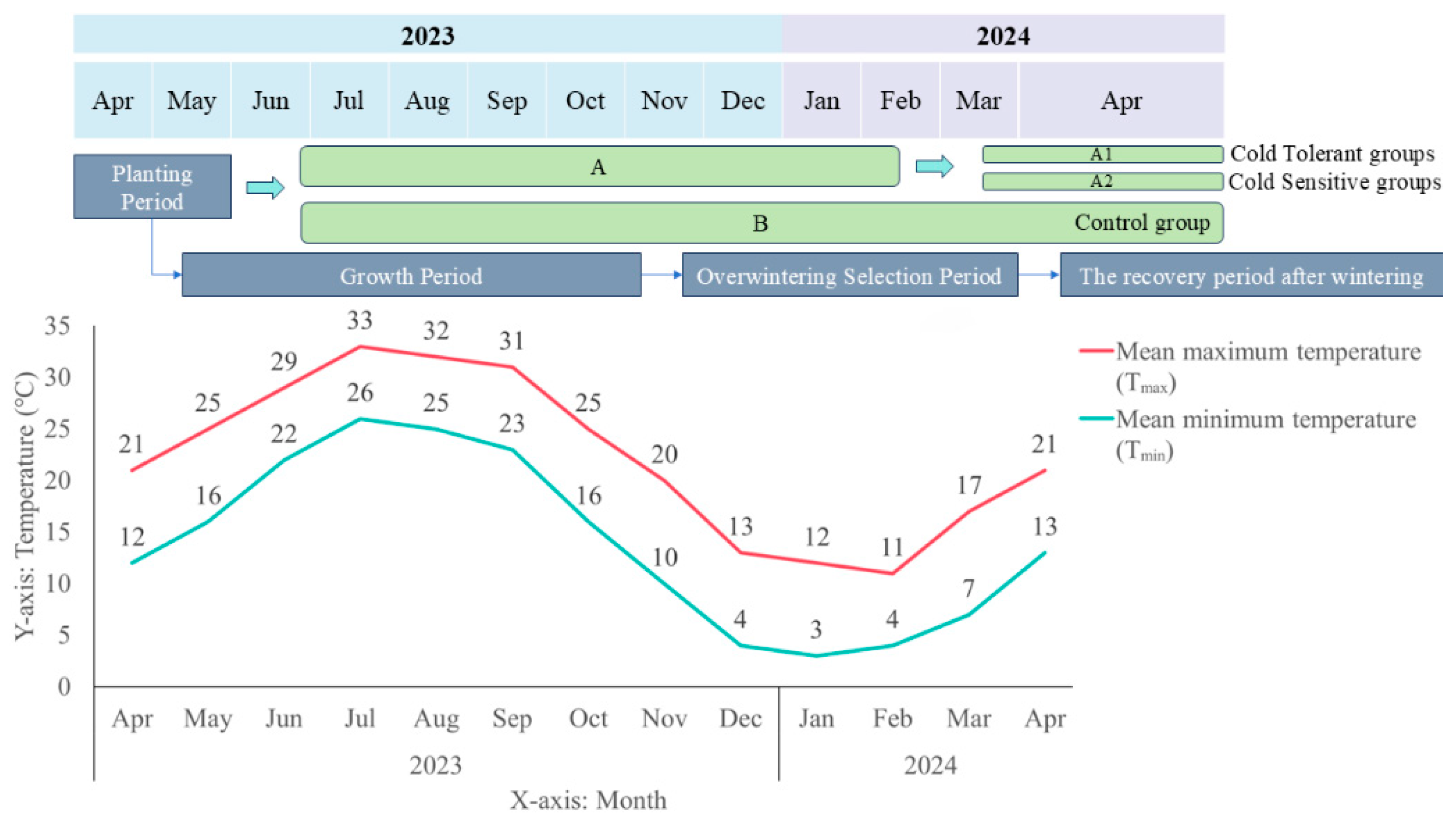
2.1. Comparison of the Survival Rate of Kandelia obovata After Overwintering
2.2. Physiological Differences Between K. obovata Populations Under Low-Temperature Stress After Overwintering
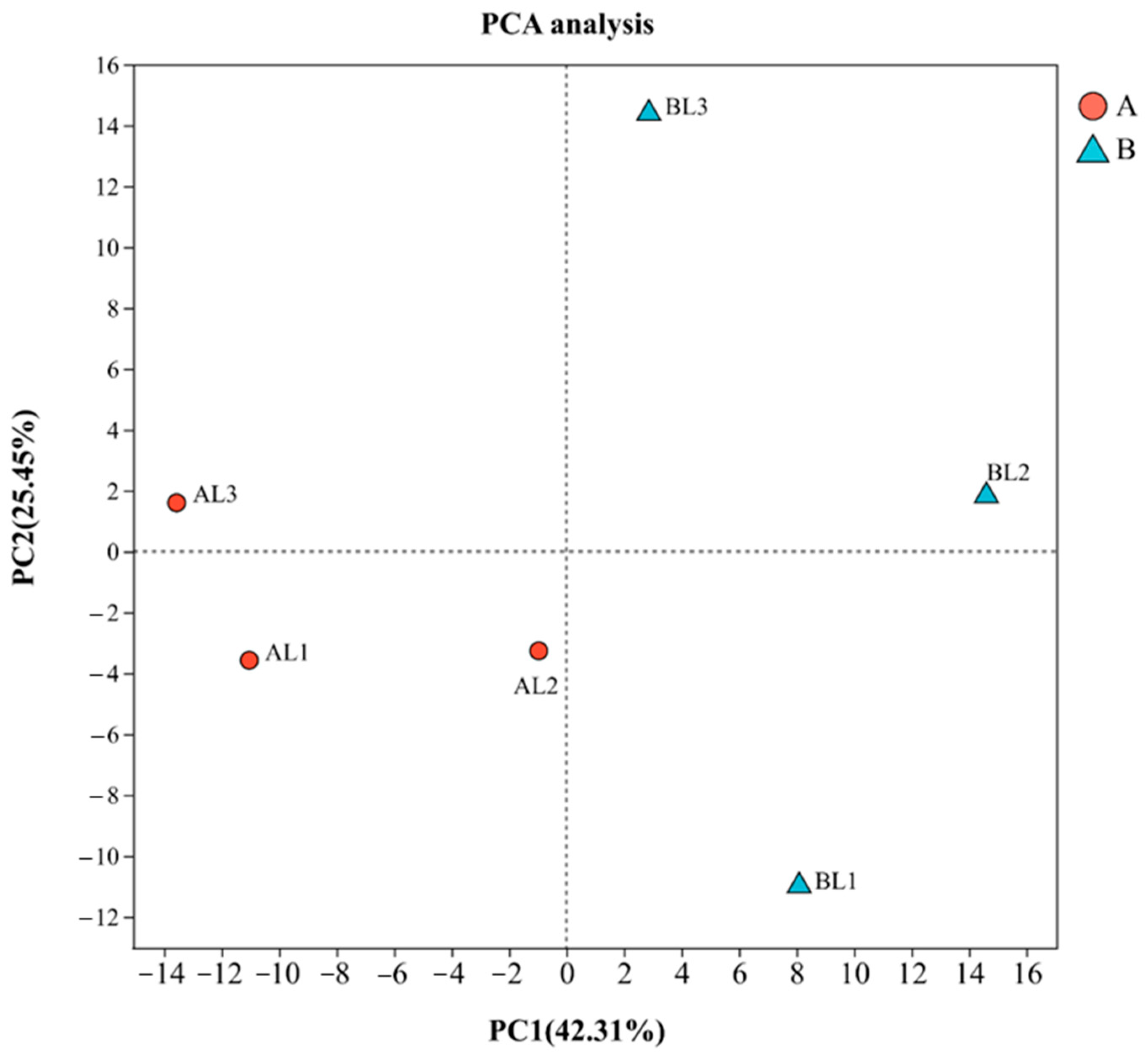
2.3. Quality Control Analysis of Raw Sequencing Data
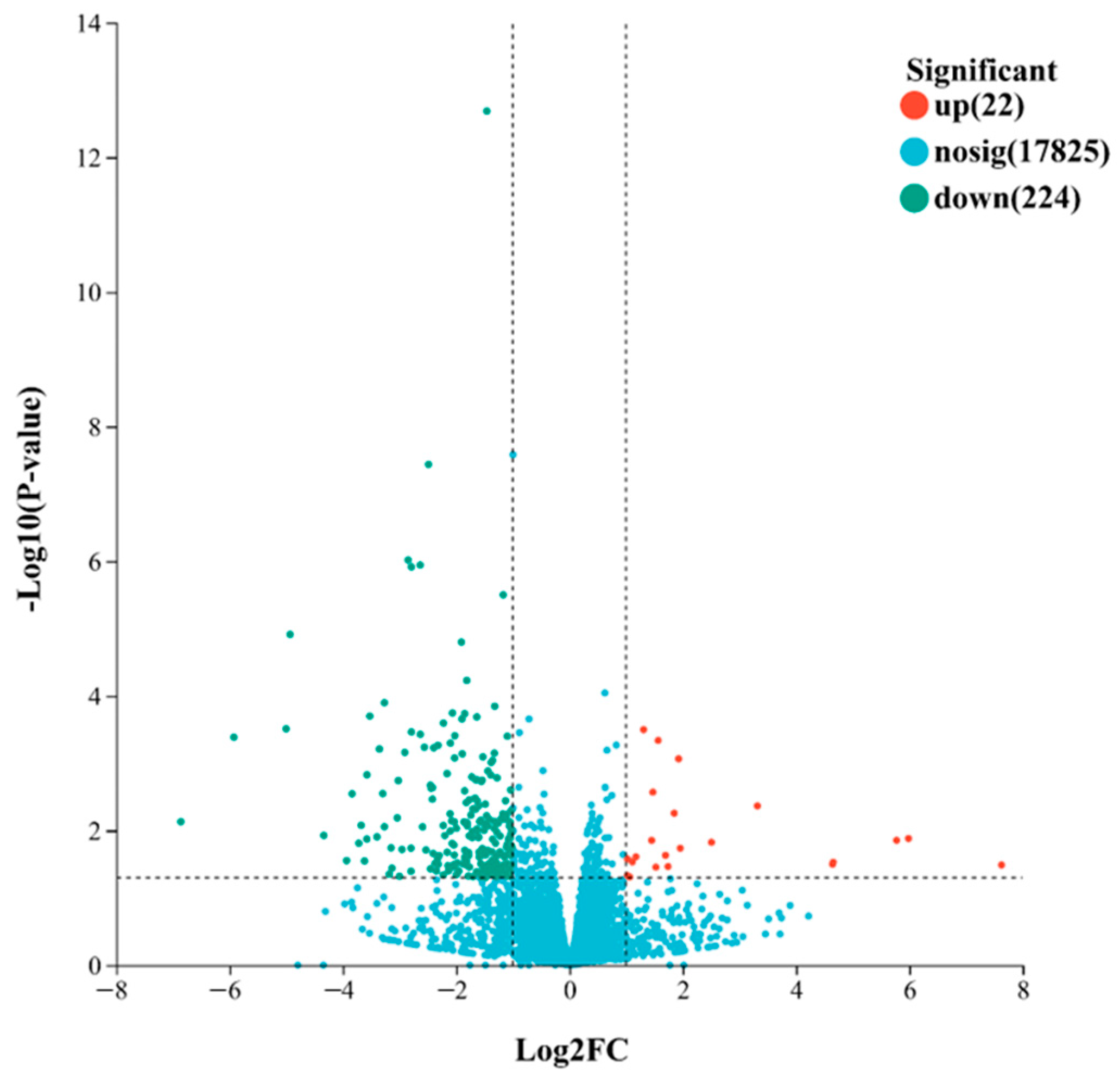
2.4. Differential Gene Expression Analysis
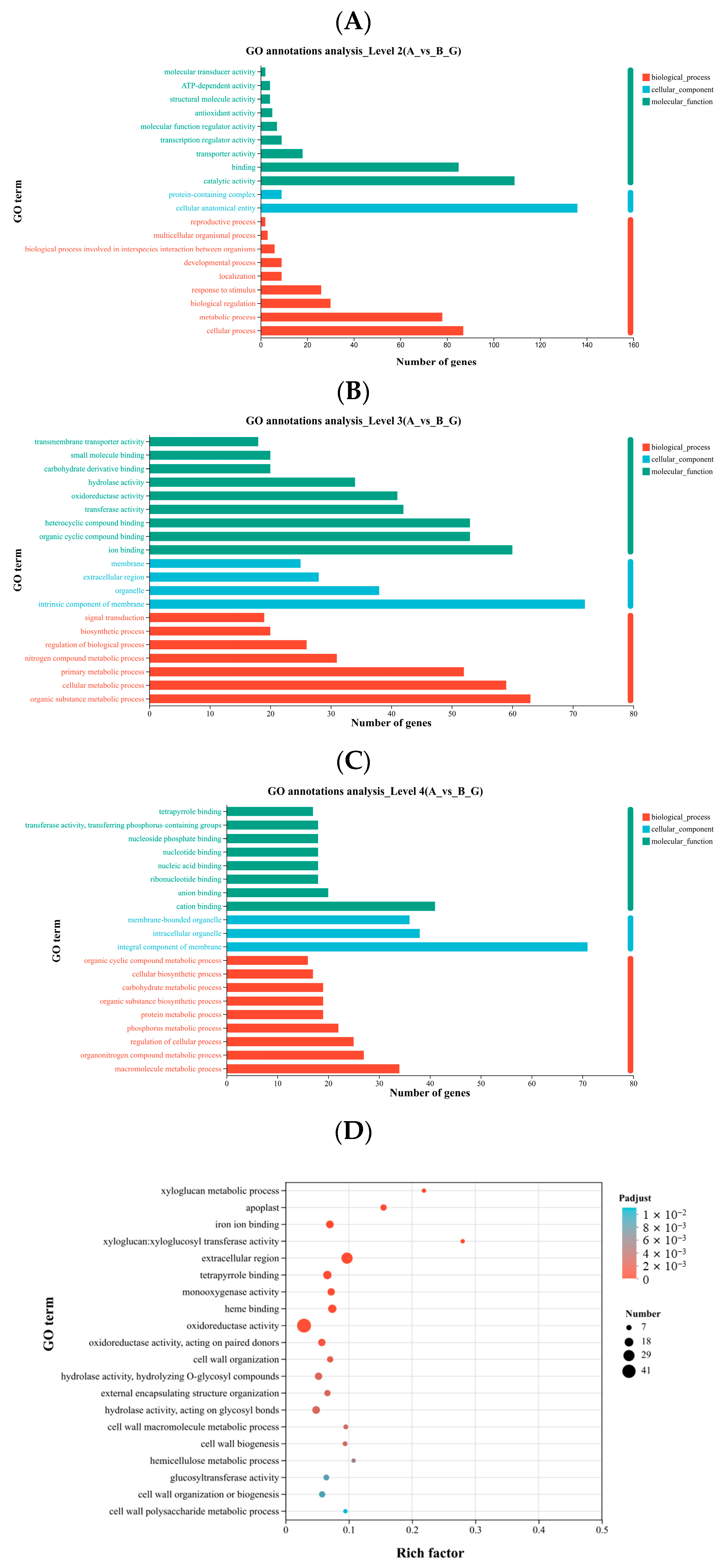
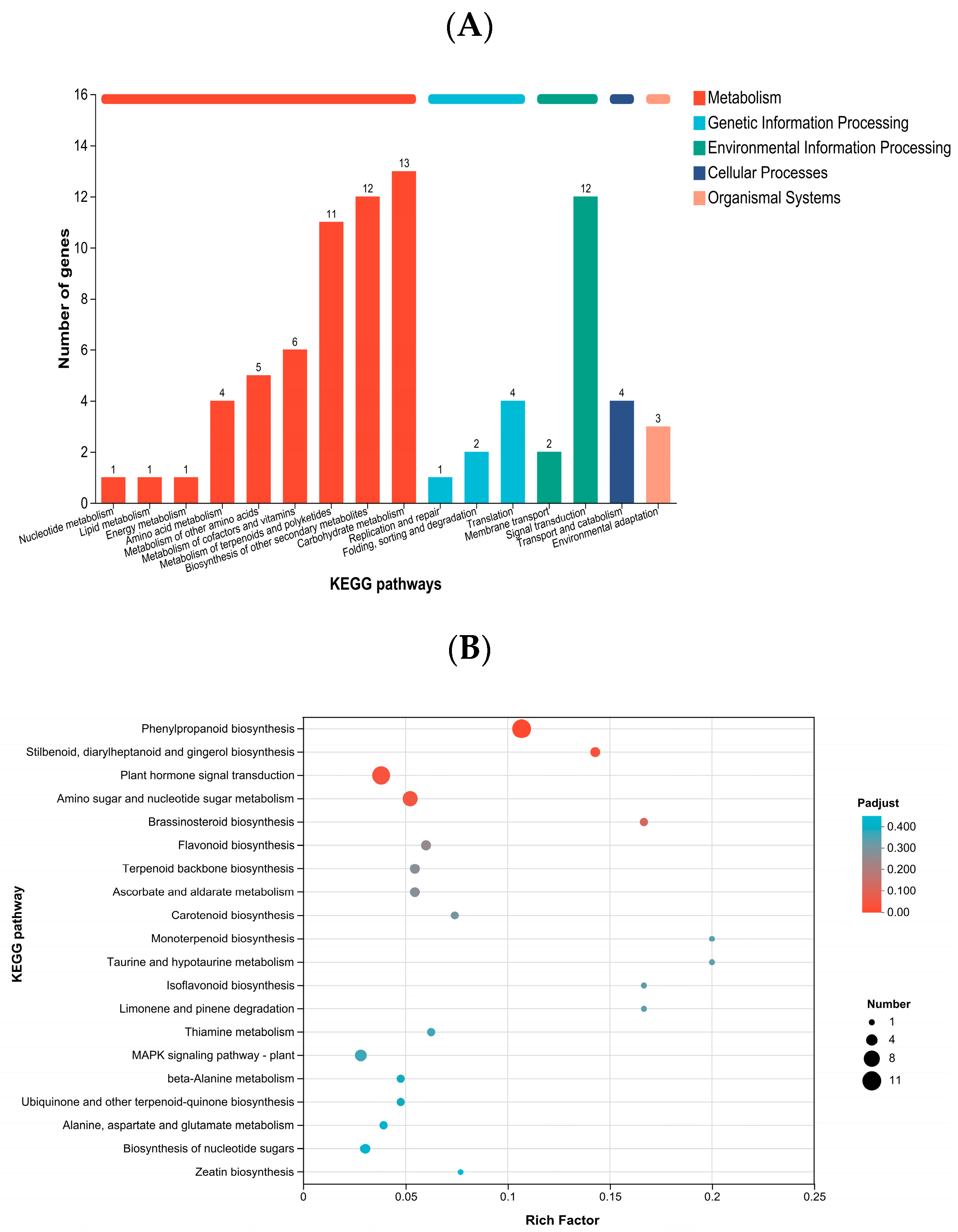
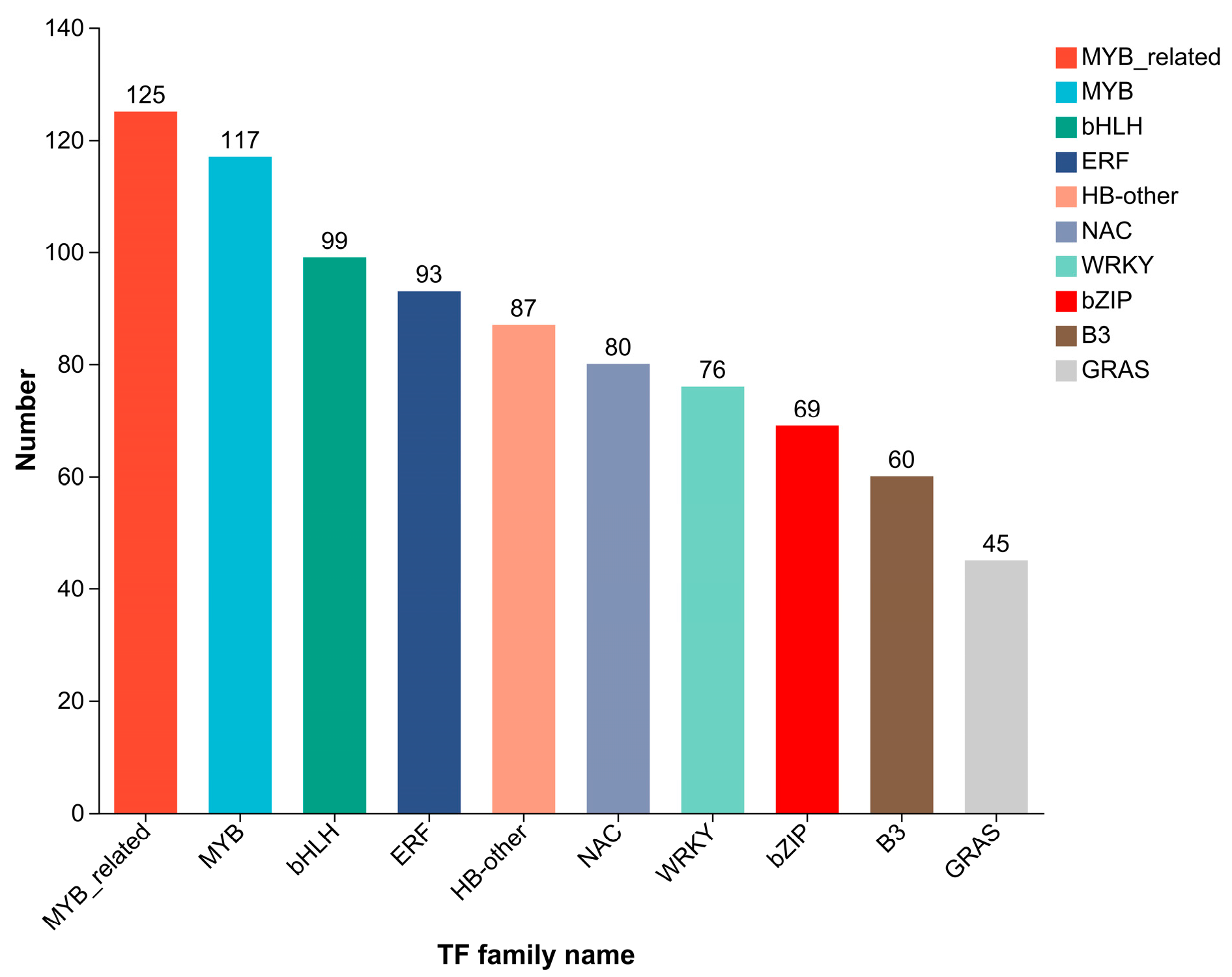
2.5. GO and KEGG Annotation and Enrichment Analysis of Differentially Expressed Genes
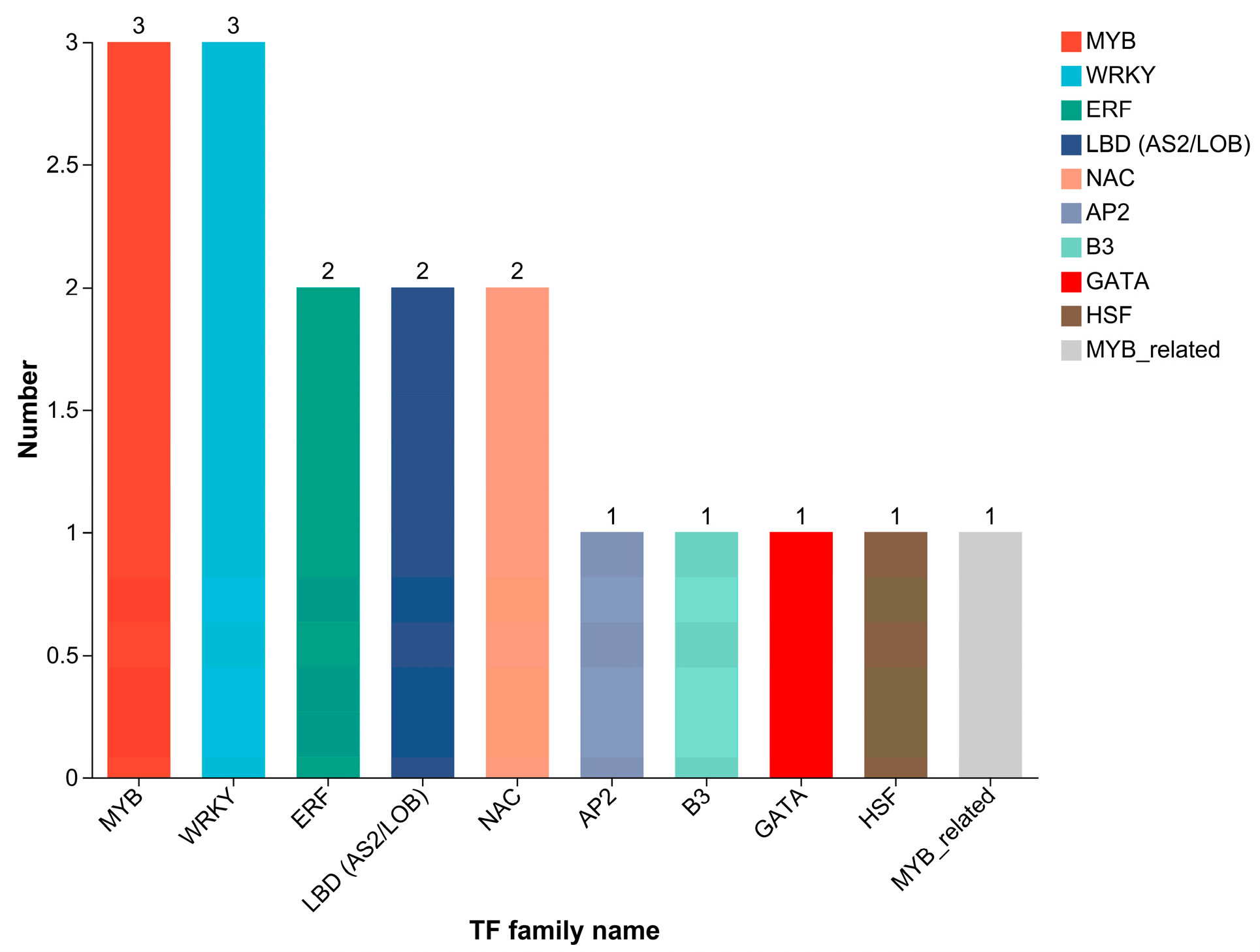
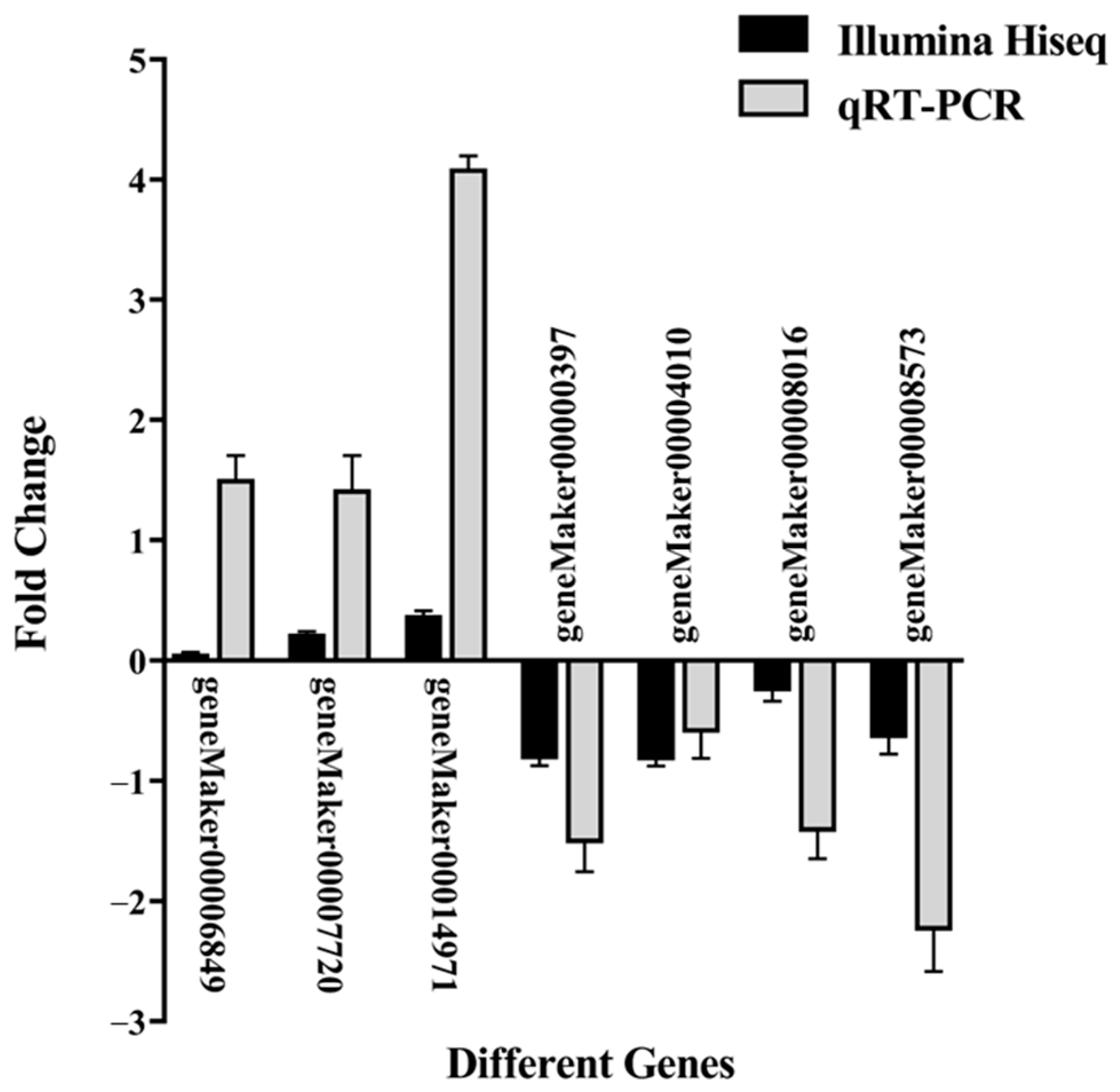
2.6. Transcription Factor Prediction and qRT-PCR Validation
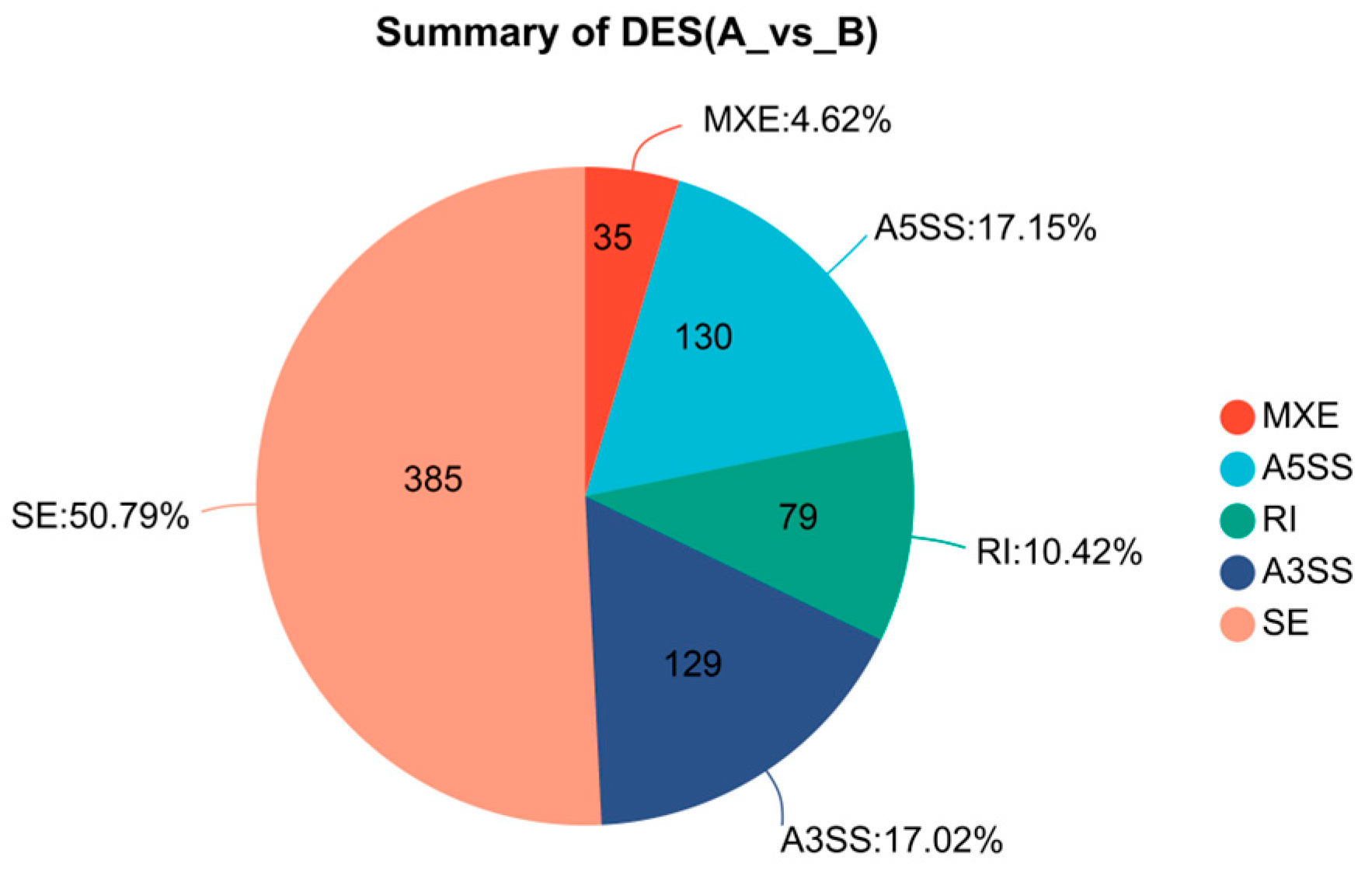
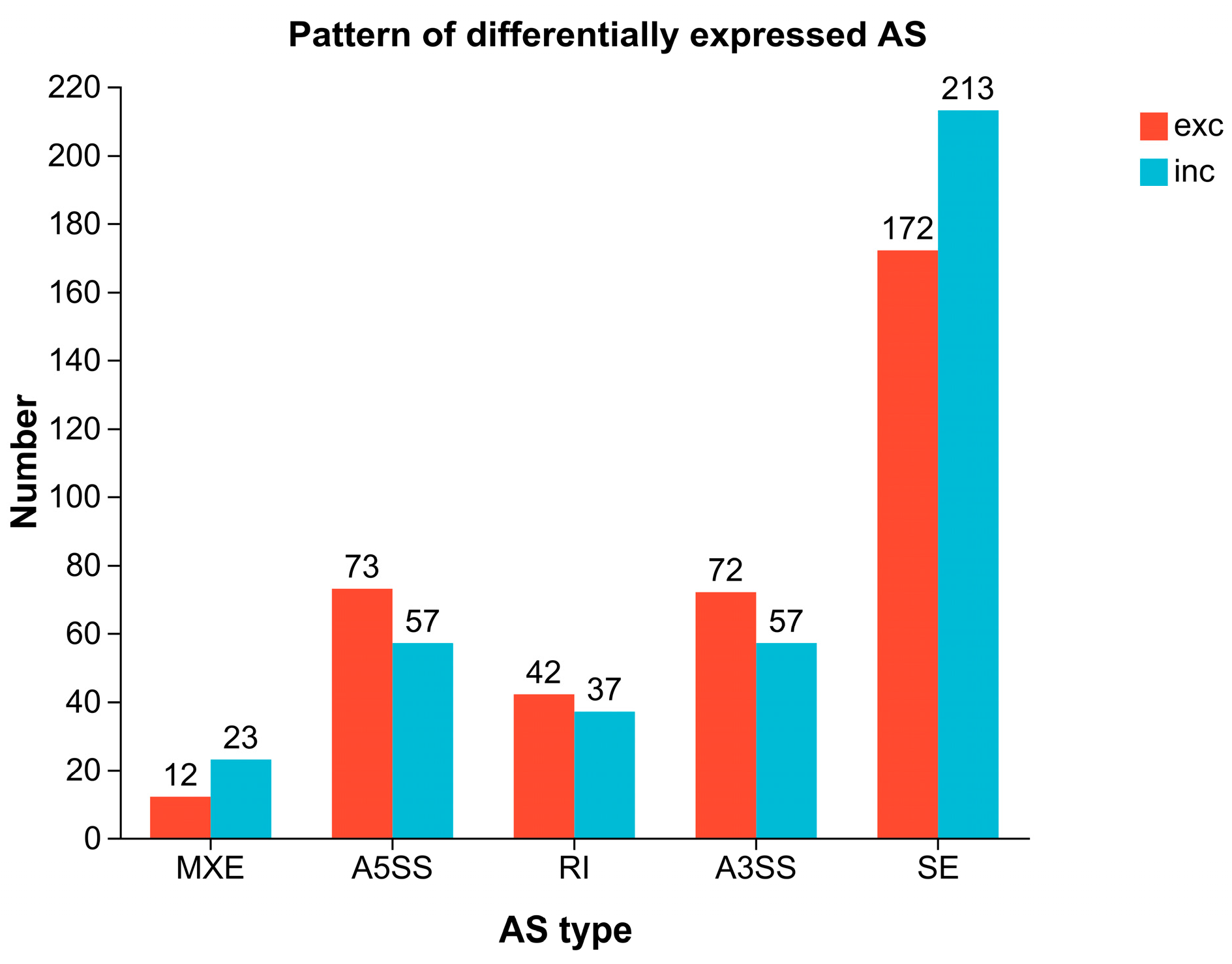
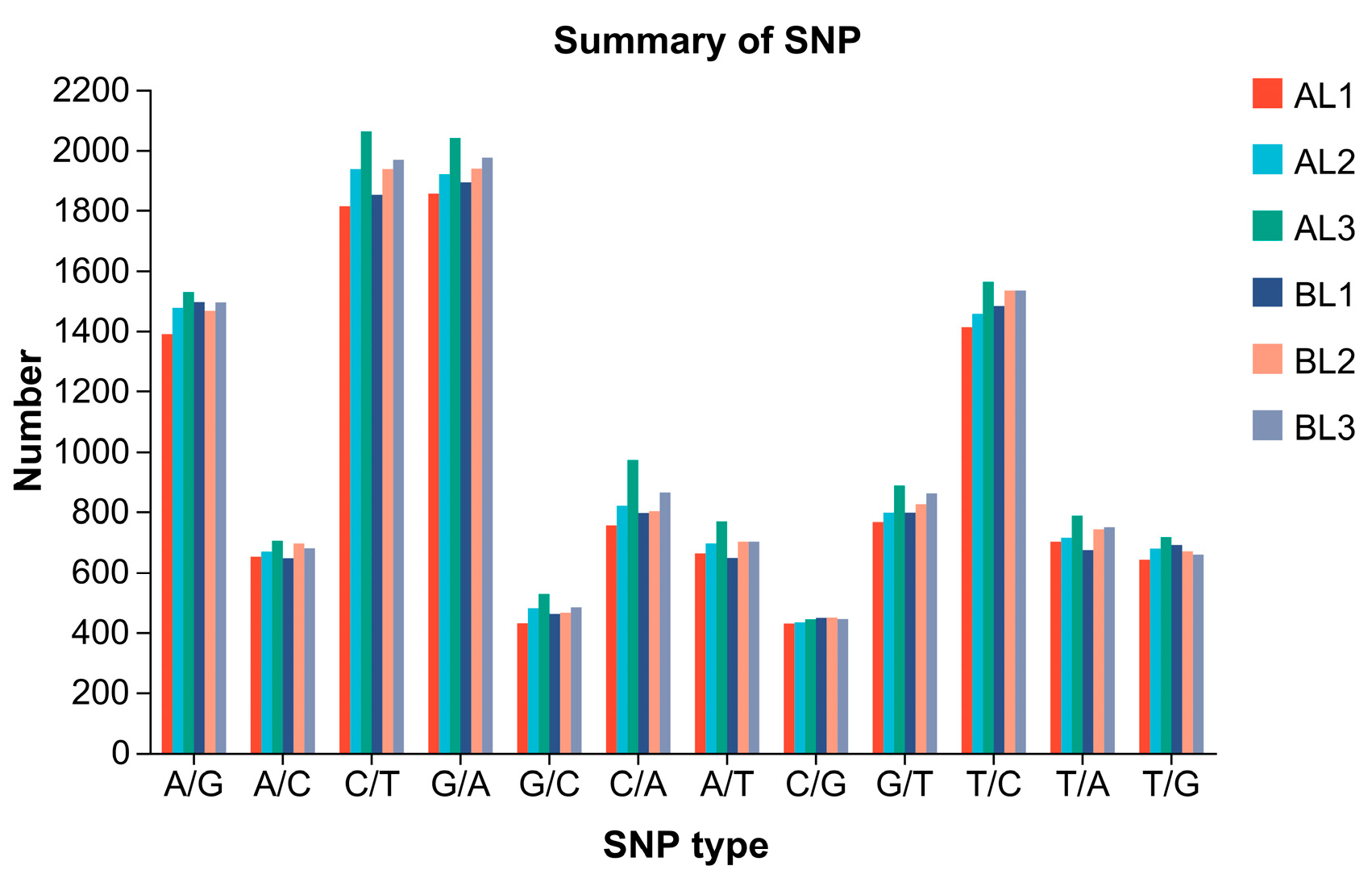
2.7. Alternative Splicing and SNP Analysis
3. Discussion
3.1. Physiological Differences Between Groups
3.2. Differential Expression Between Groups
3.3. Differential Transcription Factor Expression Between Groups
3.4. Genetic Variation Between Groups
4. Materials and Methods
4.1. Experimental Materials
4.2. Experimental Methods
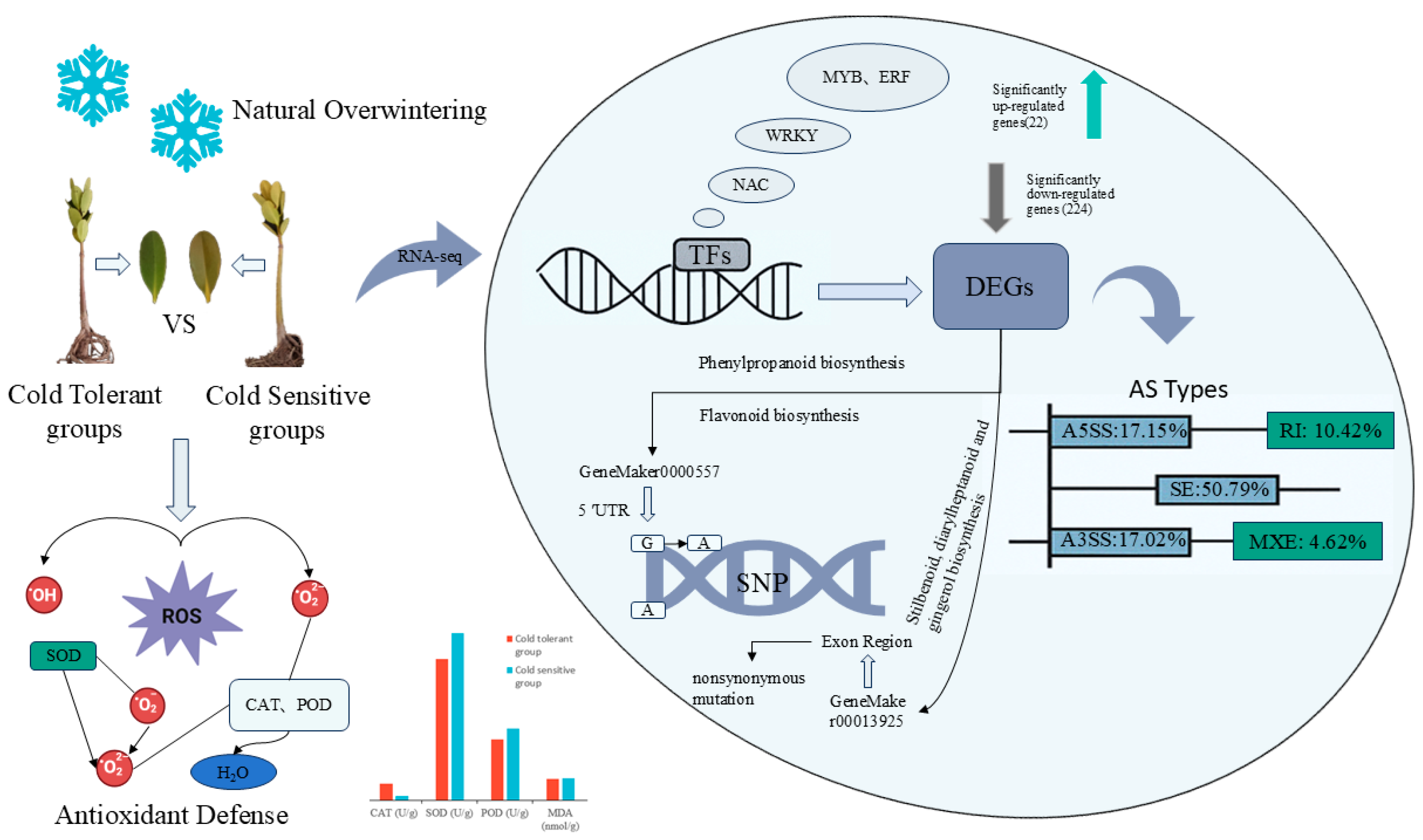
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, X.; Dai, Z.; Yuan, R.; Guo, Z.; Xi, H.; He, Z.; Wei, M. Effects of salinity on assembly characteristics and function of microbial communities in the phyllosphere and rhizosphere of salt-tolerant Avicennia marina mangrove species. Microbiol. Spectr. 2023, 11, e0300022. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, J.; Qin, G.; Zhang, J.; Zhou, J.; Wu, J.; Zhang, L.; Thapa, P.; Sanders, C.J.; Santos, I.R.; et al. Coastal blue carbon in China as a nature-based solution toward carbon neutrality. Innovation 2023, 4, 100481. [Google Scholar] [CrossRef] [PubMed]
- Kitao, M.; Utsugi, H.; Kuramoto, S.; Tabuchi, R.; Fujimoto, K.; Lihpai, S. Light-dependent photosynthetic characteristics indicated by chlorophyll fluorescence in five mangrove species native to Pohnpei Island, Micronesia. Physiol. Plant. 2003, 117, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.-J.; Chen, J.; Ghoto, K.; Hu, W.-J.; Gao, G.-F.; Luo, M.-R.; Li, Z.; Simon, M.; Zhu, X.-Y.; Zheng, H.-L. Proteomic analysis on mangrove plant Avicennia marina leaves reveals nitric oxide enhances the salt tolerance by up-regulating photosynthetic and energy metabolic protein expression. Tree Physiol. 2018, 38, 1605–1622. [Google Scholar] [CrossRef]
- Liu, H.; An, X.; Liu, X.; Yang, S.; Liu, Y.; Wei, X.; Li, X.; Chen, Q.; Wang, J. Molecular mechanism of salinity and waterlogging tolerance in mangrove Kandelia obovata. Front. Plant Sci. 2024, 15, 1354249. [Google Scholar] [CrossRef]
- Du, Z.; You, S.; Zhao, X.; Xiong, L.; Li, J. Genome-wide identification of WRKY genes and their responses to chilling stress in Kandelia obovata. Front. Genet. 2022, 13, 875316. [Google Scholar] [CrossRef]
- Zhou, Y.-Y.; Wang, Y.-S.; Sun, C.-C. Molecular Cloning and Expression Analysis of the Typical class III chitinase genes from three mangrove species under heavy metal stress. Plants 2023, 12, 1681. [Google Scholar] [CrossRef]
- Britto Martins de Oliveira, J.; Corrêa Junior, D.; Parente, C.E.T.; Frases, S. Fungi in Mangrove: Ecological Importance, Climate Change Impacts, and the Role in Environmental Remediation. Microorganisms 2025, 13, 878. [Google Scholar] [CrossRef]
- Mossa, M.; De Padova, D. Interaction between waves and vegetation. Sci. Rep. 2025, 15, 6157. [Google Scholar] [CrossRef]
- Zhao, J.; Li, C.; Wang, T.; Li, C.; Shen, J.; Liu, Y.; Wu, P. Distribution pattern of mangrove fish communities in China. Biology 2022, 11, 1696. [Google Scholar] [CrossRef]
- Chen, L.; Wang, W.; Li, Q.Q.; Zhang, Y.; Yang, S.; Osland, M.J.; Huang, J.; Peng, C. Mangrove species’ responses to winter air temperature extremes in China. Ecosphere 2017, 8, e01865. [Google Scholar] [CrossRef]
- Yang, J.; Wu, A.; Li, J.; Wei, H.; Qin, J.; Tian, H.; Fan, D.; Wu, W.; Chen, S.; Tong, X.; et al. Structured and unstructured intraspecific propagule trait variation across environmental gradients in a widespread mangrove. Ecol. Evol. 2024, 14, e10835. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Li, G.; Zhang, S.; Yang, Y.; Li, Y.; Dong, Z.; Guo, Y.; Wang, Z. Global warming exacerbates the risk of habitat loss for regional mangrove species. Sci. Rep. 2025, 15, 19710. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lu, X.; Yang, S.; Liu, Y.; Wang, W.; Wei, X.; Ji, H.; Zhang, B.; Xin, W.; Wen, J.; et al. Role of exogenous abscisic acid in freezing tolerance of mangrove Kandelia obovata under natural frost condition at near 32° N. BMC Plant Biol. 2022, 22, 593. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, W.; Qiu, J.; Huang, L.; Huang, X.; Chen, S. Comparison of physiological characteristics of Kandelia obovata at different ages in winter in the northernmost mangrove transplanted area of China. Acta Ecol. Sin. 2013, 33, 132–138. [Google Scholar] [CrossRef]
- Inoue, T.; Akaji, Y.; Baba, S.; Noguchi, K. Temperature dependence of O2 respiration in mangrove leaves and roots: Implications for seedling dispersal phenology. New Phytol. 2023, 237, 100–112. [Google Scholar] [CrossRef]
- Zhang, J.; Ouyang, S.; Cai, X.; Yang, S.; Chen, Q.; Yang, J.; Song, Z.; Zhang, W.; Wang, Y.; Zhu, Y.; et al. Phenotypic adaptation and genomic variation of Kandelia obovata associated with its northern introduction along southeastern coast of China. Front. Plant Sci. 2025, 16, 1512620. [Google Scholar] [CrossRef]
- He, Z.; Sun, H.; Peng, Y.; Hu, Z.; Cao, Y.; Lee, S.Y. Colonization by native species enhances the carbon storage capacity of exotic mangrove monocultures. Carbon Balance Manag. 2020, 15, 28. [Google Scholar] [CrossRef]
- Lu, W.-X.; Zhang, B.-H.; Zhang, Y.-Y.; Yang, S.-C. Differentiation of cold tolerance in an artificial population of a mangrove species, Kandelia obovata, is associated with geographic origins. Front. Plant Sci. 2022, 12, 695746. [Google Scholar] [CrossRef]
- Liu, W.; Zheng, C.; Chen, J.; Qiu, J.; Huang, Z.; Wang, Q.; Ye, Y. Cold acclimation improves photosynthesis by regulating the ascorbate–glutathione cycle in chloroplasts of Kandelia obovata. J. For. Res. 2019, 30, 755–765. [Google Scholar] [CrossRef]
- Peng, Y.-L.; Wang, Y.-S.; Fei, J.; Sun, C.-C.; Cheng, H. Ecophysiological differences between three mangrove seedlings (Kandelia obovata, Aegiceras corniculatum, and Avicennia marina) exposed to chilling stress. Ecotoxicology 2015, 24, 1722–1732. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Z.; Kong, X.; Khan, A.; Ullah, N.; Zhang, X. Plant coping with cold stress: Molecular and physiological adaptive mechanisms with future perspectives. Cells 2025, 14, 110. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Mur, L.A.; Wang, Q.; Hou, X.; Zhao, C.; Chen, Z.; Wu, J.; Guo, Q. ROS scavenging and ion homeostasis is required for the adaptation of halophyte Karelinia caspia to high salinity. Front. Plant Sci. 2022, 13, 979956. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Zhang, H.; Liu, Y.; Wang, X.; Dong, D.; Yuan, X.; Li, X.; Zhang, X.; Li, X.; Zhang, N.; et al. CsSNAT positively regulates salt tolerance and growth of cucumber by promoting melatonin biosynthesis. Environ. Exp. Bot. 2020, 175, 104036. [Google Scholar] [CrossRef]
- Guan, Y.; Hu, W.; Xu, Y.; Sarengaowa; Ji, Y.; Yang, X.; Feng, K. Proteomic analysis validates previous findings on wounding-responsive plant hormone signaling and primary metabolism contributing to the biosynthesis of secondary metabolites based on metabolomic analysis in harvested broccoli (Brassica oleracea L. var. italica). Food Res. Int. 2021, 145, 110388. [Google Scholar] [CrossRef]
- Fei, J.; Wang, Y.-S.; Cheng, H.; Su, Y.-B.; Zhong, Y.-J.; Zheng, L. The Kandelia obovata transcription factor KoWRKY40 enhances cold tolerance in transgenic Arabidopsis. BMC Plant Biol. 2022, 22, 274. [Google Scholar] [CrossRef]
- Zhang, B.; Gao, Y.; Zhang, L.; Zhou, Y. The plant cell wall: Biosynthesis, construction, and functions. J. Integr. Plant Biol. 2021, 63, 251–272. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, T.; Cai, X.; Ouyang, S.; Yang, J.; Song, Z.; Zhang, W.; Wang, Y.; Zhu, Y.; Nan, P. Comparative transcriptomic and metabolomic analysis of two related Kandelia obovata populations in response to cold wave. Phenomics 2025, 1–17. [Google Scholar] [CrossRef]
- Du, Z.; You, S.; Yang, D.; Tao, Y.; Zhu, Y.; Sun, W.; Chen, Z.; Li, J. Comprehensive analysis of the NAC transcription factor gene family in Kandelia obovata reveals potential members related to chilling tolerance. Front. Plant Sci. 2022, 13, 1048822. [Google Scholar] [CrossRef]
- Pang, X.; Xue, M.; Ren, M.; Nan, D.; Wu, Y.; Guo, H. Ammopiptanthus mongolicus stress-responsive NAC gene enhances the tolerance of transgenic Arabidopsis thaliana to drought and cold stresses. Genet. Mol. Biol. 2019, 42, 624–634. [Google Scholar] [CrossRef]
- Li, J.; Chen, S.; Yin, Y.; Shan, Q.; Zheng, C.; Chen, Y. Genome-Wide Analysis of bHLH family genes and identification of members associated with cold/drought-induced photoinhibition in Kandelia obovata. Int. J. Mol. Sci. 2023, 24, 15942. [Google Scholar] [CrossRef] [PubMed]
- An, J.-P.; Wang, X.-F.; Zhang, X.-W.; Xu, H.-F.; Bi, S.-Q.; You, C.-X.; Hao, Y.-J. An apple MYB transcription factor regulates cold tolerance and anthocyanin accumulation and undergoes MIEL1-mediated degradation. Plant Biotechnol. J. 2020, 18, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Illgen, S.; Zintl, S.; Zuther, E.; Hincha, D.K.; Schmülling, T. Characterisation of the ERF102 to ERF105 genes of Arabidopsis thaliana and their role in the response to cold stress. Plant Mol. Biol. 2020, 103, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Khatab, A.A.; Hu, L.; Zhao, L.; Yang, J.; Wang, L.; Xie, G. Genome-wide association mapping identifies new candidate genes for cold stress and chilling acclimation at seedling stage in rice (Oryza sativa L.). Int. J. Mol. Sci. 2022, 23, 13208. [Google Scholar] [CrossRef]
- Rahman, A.; Yadav, N.S.; Byeon, B.; Ilnytskyy, Y.; Kovalchuk, I. Genomic and epigenomic changes in the progeny of cold-stressed Arabidopsis thaliana plants. Int. J. Mol. Sci. 2024, 25, 2795. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhong, Y.; Ye, C.; Liang, P.; Pan, X.; Zhang, Y.Y.; Zhang, Y.; Shen, Y. Multi-omics analyses on Kandelia obovata reveal its response to transplanting and genetic differentiation among populations. BMC Plant Biol. 2021, 21, 341. [Google Scholar] [CrossRef]
- Wang, S.-M.; Wang, Y.-S.; Cheng, H. Comparative transcriptomics and metabolomics analyses of Avicennia marina and Kandelia obovata under chilling stress during seedling stage. Int. J. Mol. Sci. 2023, 24, 16989. [Google Scholar] [CrossRef]
- Hong, L.; Su, W.; Zhang, Y.; Ye, C.; Shen, Y.; Li, Q.Q. Transcriptome profiling during mangrove viviparity in response to abscisic acid. Sci. Rep. 2018, 8, 770. [Google Scholar] [CrossRef]

| Group | Number Planted (Plants) | Number Survived (Plants) | Survival Rate (%) | Number Dead (Plants) | Mortality Rate (%) |
|---|---|---|---|---|---|
| A1 (cold-tolerant) | 1500 | 1339 | 89.3 | 161 | 10.7 |
| A2 (cold-sensitive) | 1500 | 86 | 5.72 | 1414 | 94.28 |
| B (control) | 3000 | 1351 | 45.03 | 1649 | 54.97 |
| Detection Type | Cold-Tolerant Group | Cold-Sensitive Group |
|---|---|---|
| Chlorophyll a (mg/g) | 0.28 ± 0.05 a | 0.32 ± 0.02 a |
| Chlorophyll b (mg/g) | 0.68 ± 0.09 a | 0.70 ± 0.03 a |
| Total chlorophyll content (mg/g) | 0.96 ± 0.06 a | 1.01 ± 0.01 a |
| CAT (U/g) | 14.72 ± 0.19 a | 4.01 ± 0.89 b |
| SOD (U/g) | 1213.76 ± 66.24 a | 1435.10 ± 23.89 b |
| POD (U/g) | 52.63 ± 6.11 a | 61.89 ± 0.97 a |
| MDA (nmol/g) | 18.40 ± 0.83 a | 19.37 ± 0.81 a |
| Sample | Clean Reads | Clean Bases | Error Rate (%) | Q20 (%) | Q30 (%) | GC Content (%) |
|---|---|---|---|---|---|---|
| AL1 | 42,780,366 | 6,437,354,464 | 0.0118 | 98.90 | 96.45 | 45.93 |
| AL2 | 46,537,646 | 6,992,527,814 | 0.0117 | 98.96 | 96.63 | 45.71 |
| AL3 | 49,719,668 | 7,469,910,026 | 0.0116 | 99.00 | 96.77 | 45.84 |
| BL1 | 44,857,924 | 6,742,092,163 | 0.0116 | 98.98 | 96.73 | 45.45 |
| BL2 | 50,516,336 | 7,585,021,404 | 0.0115 | 99.02 | 96.86 | 45.60 |
| BL3 | 45,178,010 | 6,789,571,579 | 0.0116 | 98.99 | 96.74 | 45.64 |
| Sample | Clean Reads | Total Mapped (%) | Multiple Mapped (%) | Uniquely Mapped (%) |
|---|---|---|---|---|
| AL1 | 42,780,366 | 97.95 | 3.39 | 94.56 |
| AL2 | 46,537,646 | 97.89 | 3.67 | 94.22 |
| AL3 | 49,719,668 | 97.88 | 3.73 | 94.15 |
| BL1 | 44,857,924 | 97.79 | 3.96 | 93.83 |
| BL2 | 50,516,336 | 97.93 | 3.39 | 94.54 |
| BL3 | 45,178,010 | 98.12 | 3.16 | 94.97 |
| KEGG Pathway | Pathway ID | Number of DEGs | p-Value | Padjust | First Category | Second Category |
|---|---|---|---|---|---|---|
| Phenylpropanoid biosynthesis | map00940 | 11 | 5.98 × 10−8 | 3.23 × 10−6 | Metabolism | Biosynthesis of other secondary metabolites |
| Amino sugar and nucleotide sugar metabolism | map00520 | 7 | 1.50 × 10−3 | 0.0404 | Metabolism | Carbohydrate metabolism |
| Plant hormone signal transduction | map04075 | 10 | 1.76 × 10−3 | 0.0317 | Environmental Information Processing | Signal transduction |
| Stilbenoid, diarylheptanoid and gingerol biosynthesis | map00945 | 3 | 2.21 × 10−3 | 0.0299 | Metabolism | Biosynthesis of other secondary metabolites |
| Brassinosteroid biosynthesis | map00905 | 2 | 9.62 × 10−3 | 0.104 | Metabolism | Metabolism of terpenoids and polyketides |
| Type | AL1 | AL2 | AL3 | BL1 | BL2 | BL3 | |
|---|---|---|---|---|---|---|---|
| Transition (Ti) | A/G | 1388 | 1474 | 1529 | 1493 | 1464 | 1492 |
| C/T | 1814 | 1936 | 2060 | 1852 | 1936 | 1967 | |
| G/A | 1856 | 1919 | 2038 | 1892 | 1937 | 1974 | |
| T/C | 1411 | 1454 | 1563 | 1480 | 1534 | 1534 | |
| Total | 6469 | 6783 | 7189 | 6717 | 6871 | 6967 | |
| Transversion (Tv) | A/C | 650 | 667 | 703 | 645 | 694 | 678 |
| G/C | 429 | 479 | 525 | 460 | 464 | 482 | |
| C/A | 754 | 818 | 971 | 794 | 800 | 862 | |
| A/T | 661 | 694 | 766 | 646 | 700 | 700 | |
| C/G | 428 | 432 | 442 | 447 | 448 | 443 | |
| G/T | 764 | 795 | 885 | 795 | 823 | 859 | |
| T/A | 700 | 713 | 785 | 672 | 741 | 748 | |
| T/G | 640 | 677 | 715 | 689 | 668 | 657 | |
| Total | 5026 | 5275 | 5793 | 5148 | 5338 | 5429 | |
| Ti/Tv | 1.29 | 1.29 | 1.24 | 1.30 | 1.29 | 1.28 | |
| Total | 11,495 | 12,058 | 12,982 | 11,865 | 12,209 | 12,396 | |
| Gene ID | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|
| MYB-related-14971 | GCAGGTTCGGAGCAGCAATTATC | ACATCTGATTTAGTGCGTTCGTTGG |
| MYB-7720 | TTCAGACCTTCTACCGACAACCATC | GAGTGGCTGCTGAGTTTGTTTGG |
| LBD (AS2/LOB)-6849 | GCTGTGGTGGATGGCGAGAG | TGTTGGCATCACGGACTCCTTG |
| LBD (AS2/LOB)-8016 | TTTCCTCCTTGAACCCTCACATCTC | CCCAGGTCACACTAGCACACTATTC |
| GATA-397 | CGACCACATTGACGACCTCCTC | ACTCGGACTCAGCAGACCAAATG |
| ERF-4010 | CCATCGGGACCACCAACTAAAGG | AGGCGACCACGTATCCAATGC |
| ERF-8573 | TGGATCGAAGACATGGCAAGAGG | GTTGAGATGGAGCGGAGTAGATGG |
| KoACT2 (internal reference) | ACCGAGGCTCCTCTTAATCC | AGCTGGCACATTGAAGGTCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Li, H.; Sun, Z.; Li, W.; Yin, X.; Xu, X.; Zhang, X.; Yan, X.; Wang, X.; Li, Y.; Ma, A. Research on Cold Resistance of Kandelia obovata Transplanted to Zhoushan Area at the mRNA Level. Int. J. Mol. Sci. 2026, 27, 429. https://doi.org/10.3390/ijms27010429
Li H, Sun Z, Li W, Yin X, Xu X, Zhang X, Yan X, Wang X, Li Y, Ma A. Research on Cold Resistance of Kandelia obovata Transplanted to Zhoushan Area at the mRNA Level. International Journal of Molecular Sciences. 2026; 27(1):429. https://doi.org/10.3390/ijms27010429
Chicago/Turabian StyleLi, Haozhe, Zhibin Sun, Weiye Li, Xiaolong Yin, Xian Xu, Xiaolin Zhang, Xiaojun Yan, Xinan Wang, Yuanyuan Li, and Aijun Ma. 2026. "Research on Cold Resistance of Kandelia obovata Transplanted to Zhoushan Area at the mRNA Level" International Journal of Molecular Sciences 27, no. 1: 429. https://doi.org/10.3390/ijms27010429
APA StyleLi, H., Sun, Z., Li, W., Yin, X., Xu, X., Zhang, X., Yan, X., Wang, X., Li, Y., & Ma, A. (2026). Research on Cold Resistance of Kandelia obovata Transplanted to Zhoushan Area at the mRNA Level. International Journal of Molecular Sciences, 27(1), 429. https://doi.org/10.3390/ijms27010429






