MFAP5 Activates ITGA5 to Drive Tooth Germ Mineralization Through the MAPK/ERK Pathway: Insights from Single-Cell Transcriptomics
Abstract
1. Introduction
2. Results
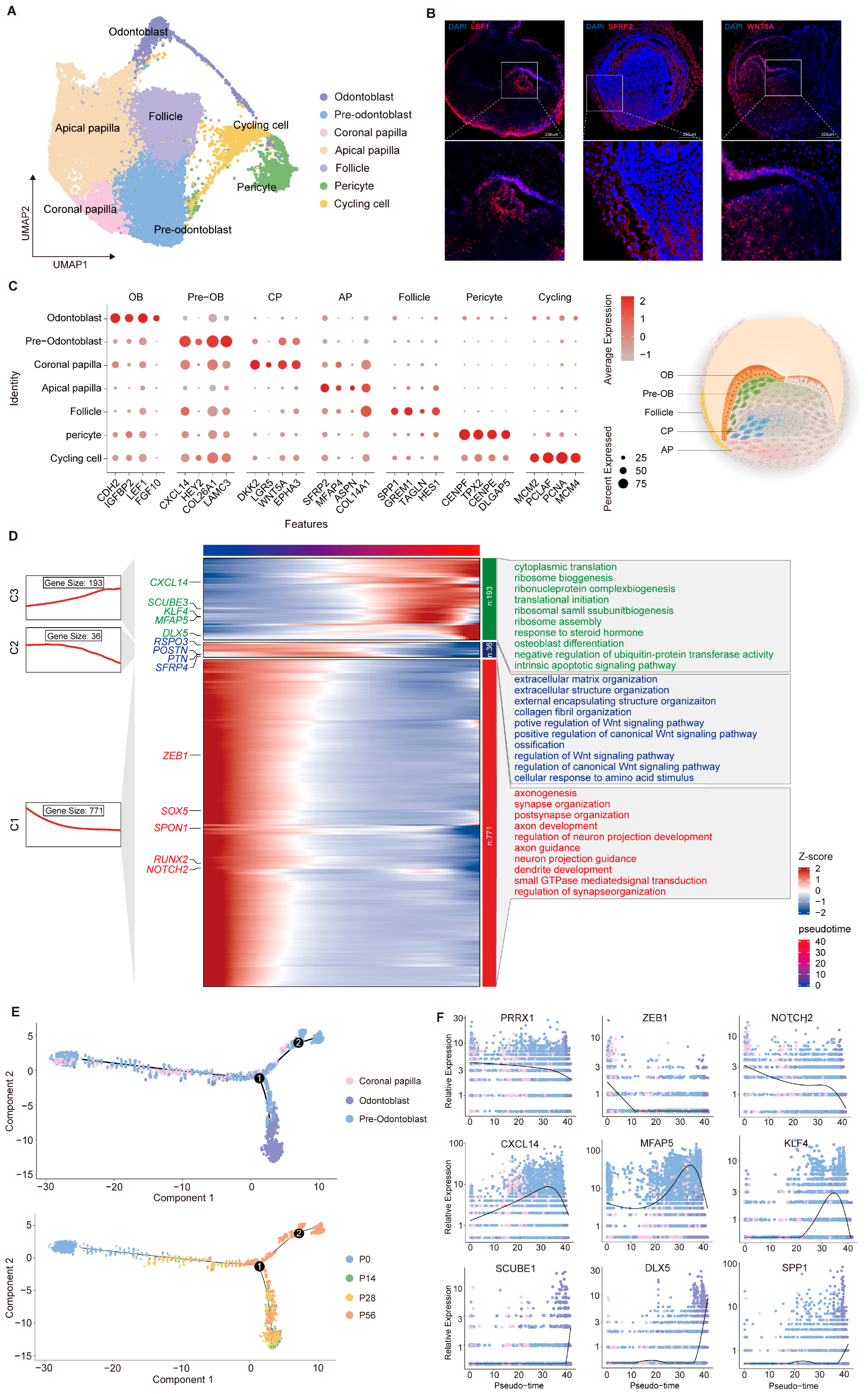
2.1. Cellular Landscape of the Developing Tooth Germ
2.2. Transcriptomic Diversity and Dynamic Changes in Dental Epithelial Cells
2.3. Transcriptomic Diversity and Dynamic Changes in Dental Mesenchymal Cells
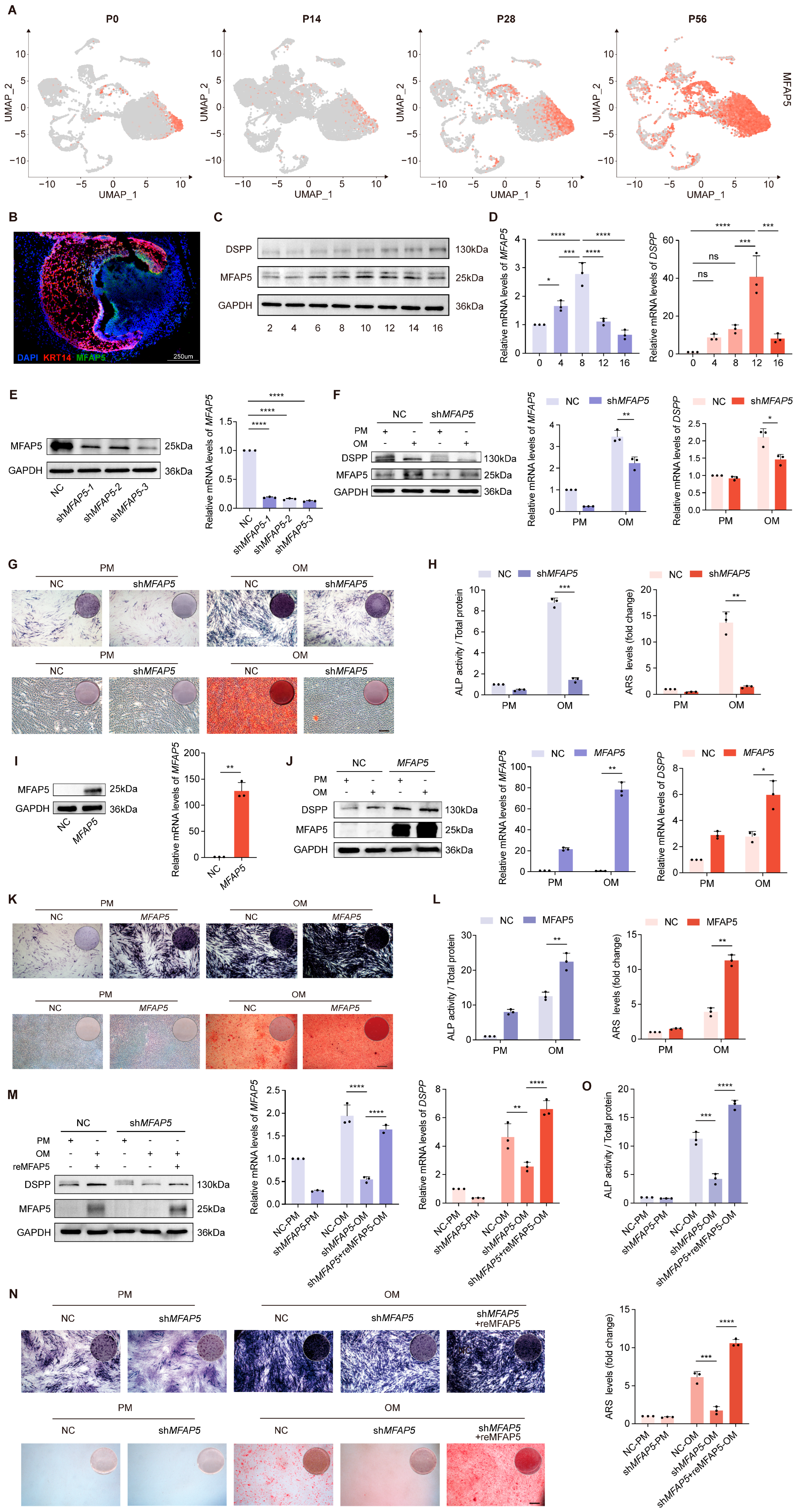
2.4. MFAP5 in the Mineralization Maturation of Dentin Cells
2.5. MFAP5 Regulates MAPK Signaling via ITGA5 Dependency
3. Discussion
4. Materials and Methods
4.1. Experimental Animal Ethics and Sample Collection
4.2. Tissue Dissociation and Preparation of Single-Cell Suspensions
4.3. 10× Genomics cDNA Library Preparation
4.4. Single-Cell RNA Sequencing Analysis
4.5. Lentiviral Transduction
4.6. Protein Extraction and Western Blotting
4.7. RNA Extraction and Quantitative Real-Time Reverse Transcription PCR (qRT-PCR)
4.8. Immunofluorescence
4.9. Alkaline Phosphatase (ALP) and Alizarin Red S (ARS) Staining and Quantification
4.10. RNA Sequencing and Analysis
4.11. Co-Immunoprecipitation (Co-IP)
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lacruz, R.S.; Habelitz, S.; Wright, J.T.; Paine, M.L. Dental Enamel Formation and Implications for Oral Health and Disease. Physiol. Rev. 2017, 97, 939–993. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gong, X.; Xu, X.; Wang, X.; Sun, Y. Tooth Number Abnormality: From Bench to Bedside. Int. J. Oral Sci. 2023, 15, 5. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Chai, Y. Regulatory Mechanisms of Jaw Bone and Tooth Development. Curr. Top. Dev. Biol. 2019, 133, 91–118. [Google Scholar] [CrossRef] [PubMed]
- Balic, A.; Thesleff, I. Tissue Interactions Regulating Tooth Development and Renewal. Curr. Top. Dev. Biol. 2015, 115, 157–186. [Google Scholar] [CrossRef]
- Juuri, E.; Balic, A. The Biology Underlying Abnormalities of Tooth Number in Humans. J. Dent. Res. 2017, 96, 1248–1256. [Google Scholar] [CrossRef]
- Li, Z.; Yu, M.; Tian, W. An Inductive Signalling Network Regulates Mammalian Tooth Morphogenesis with Implications for Tooth Regeneration. Cell Prolif. 2013, 46, 501–508. [Google Scholar] [CrossRef]
- Yu, T.; Klein, O.D. Molecular and Cellular Mechanisms of Tooth Development, Homeostasis and Repair. Dev. Camb. Engl. 2020, 147, dev184754. [Google Scholar] [CrossRef]
- Li, J.; Parada, C.; Chai, Y. Cellular and Molecular Mechanisms of Tooth Root Development. Dev. Camb. Engl. 2017, 144, 374–384. [Google Scholar] [CrossRef]
- Jussila, M.; Thesleff, I. Signaling Networks Regulating Tooth Organogenesis and Regeneration, and the Specification of Dental Mesenchymal and Epithelial Cell Lineages. Cold Spring Harb. Perspect. Biol. 2012, 4, a008425. [Google Scholar] [CrossRef]
- Yang, S.; Huang, F.; Zhang, F.; Sheng, X.; Fan, W.; Dissanayaka, W.L. Emerging Roles of YAP/TAZ in Tooth and Surrounding: From Development to Regeneration. Stem Cell Rev. Rep. 2023, 19, 1659–1675. [Google Scholar] [CrossRef]
- Calamari, Z.T.; Hu, J.K.-H.; Klein, O.D. Tissue Mechanical Forces and Evolutionary Developmental Changes Act Through Space and Time to Shape Tooth Morphology and Function. BioEssays 2018, 40, e1800140. [Google Scholar] [CrossRef]
- Huang, P.; Jiang, R.X.; Wang, F.; Qiao, W.W.; Ji, Y.T.; Meng, L.Y.; Bian, Z. PIEZO1 Promotes Odontoblast-Mediated Reactionary Dentinogenesis via SEMA3A. J. Dent. Res. 2024, 103, 889–898. [Google Scholar] [CrossRef]
- Greenblatt, M.B.; Kim, J.-M.; Oh, H.; Park, K.H.; Choo, M.-K.; Sano, Y.; Tye, C.E.; Skobe, Z.; Davis, R.J.; Park, J.M.; et al. P38α MAPK Is Required for Tooth Morphogenesis and Enamel Secretion. J. Biol. Chem. 2015, 290, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.; Groeger, S.; Meyle, J.; Ruf, S. MAPK and β-Catenin Signaling: Implication and Interplay in Orthodontic Tooth Movement. Front. Biosci. Landmark Ed. 2022, 27, 54. [Google Scholar] [CrossRef]
- Yan, H.; Zhou, J.; Wu, T. ECM Stiffness-Mediated Regulation of Glucose Metabolism in MSCs Osteogenic Differentiation: Mechanisms, Intersections, and Implications. Oral Sci. Homeost. Med. 2025, 1, 9610025. [Google Scholar] [CrossRef]
- Cruz Walma, D.A.; Yamada, K.M. Extracellular Matrix in Human Craniofacial Development. J. Dent. Res. 2022, 101, 495–504. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular Matrix Structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef] [PubMed]
- Mecham, R.P.; Gibson, M.A. The Microfibril-Associated Glycoproteins (MAGPs) and the Microfibrillar Niche. Matrix Biol. 2015, 47, 13–33. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, H.; Meng, L.; Yu, S.; Liu, Z.; Xue, J. Microfibril-Associated Glycoprotein-2 Promoted Fracture Healing via Integrin Avβ3/PTK2/AKT Signaling. Lab. Investig. 2023, 103, 100121. [Google Scholar] [CrossRef]
- Li, H.; Zhou, W.; Sun, S.; Zhang, T.; Zhang, T.; Huang, H.; Wang, M. Microfibrillar-Associated Protein 5 Regulates Osteogenic Differentiation by Modulating the Wnt/β-Catenin and AMPK Signaling Pathways. Mol. Med. Camb. Mass 2021, 27, 153. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-J.; Lin, C.-Y.; Stitziel, N.O. Genetics of the Extracellular Matrix in Aortic Aneurysmal Diseases. Matrix Biol. 2018, 71–72, 128–143. [Google Scholar] [CrossRef]
- Barbier, M.; Gross, M.-S.; Aubart, M.; Hanna, N.; Kessler, K.; Guo, D.-C.; Tosolini, L.; Ho-Tin-Noe, B.; Regalado, E.; Varret, M.; et al. MFAP5 Loss-of-Function Mutations Underscore the Involvement of Matrix Alteration in the Pathogenesis of Familial Thoracic Aortic Aneurysms and Dissections. Am. J. Hum. Genet. 2014, 95, 736–743. [Google Scholar] [CrossRef]
- Chen, Z.; Yan, X.; Li, K.; Ling, Y.; Kang, H. Stromal Fibroblast-Derived MFAP5 Promotes the Invasion and Migration of Breast Cancer Cells via Notch1/Slug Signaling. Clin. Transl. Oncol. 2020, 22, 522–531. [Google Scholar] [CrossRef]
- Turecamo, S.E.; Walji, T.A.; Broekelmann, T.J.; Williams, J.W.; Ivanov, S.; Wee, N.K.; Procknow, J.D.; McManus, M.R.; Randolph, G.J.; Scheller, E.L.; et al. Contribution of Metabolic Disease to Bone Fragility in MAGP1-Deficient Mice. Matrix Biol. J. Int. Soc. Matrix Biol. 2018, 67, 1–14. [Google Scholar] [CrossRef]
- Craft, C.S.; Zou, W.; Watkins, M.; Grimston, S.; Brodt, M.D.; Broekelmann, T.J.; Weinbaum, J.S.; Teitelbaum, S.L.; Pierce, R.A.; Civitelli, R.; et al. Microfibril-Associated Glycoprotein-1, an Extracellular Matrix Regulator of Bone Remodeling. J. Biol. Chem. 2010, 285, 23858–23867. [Google Scholar] [CrossRef] [PubMed]
- Walji, T.A.; Turecamo, S.E.; Sanchez, A.C.; Anthony, B.A.; Abou-Ezzi, G.; Scheller, E.L.; Link, D.C.; Mecham, R.P.; Craft, C.S. Marrow Adipose Tissue Expansion Coincides with Insulin Resistance in MAGP1-Deficient Mice. Front. Endocrinol. 2016, 7, 87. [Google Scholar] [CrossRef]
- Burns, J.S.; Rasmussen, P.L.; Larsen, K.H.; Schrøder, H.D.; Kassem, M. Parameters in Three-Dimensional Osteospheroids of Telomerized Human Mesenchymal (Stromal) Stem Cells Grown on Osteoconductive Scaffolds That Predict in Vivo Bone-Forming Potential. Tissue Eng. Part A 2010, 16, 2331–2342. [Google Scholar] [CrossRef]
- Zhu, S.; Ye, L.; Bennett, S.; Xu, H.; He, D.; Xu, J. Molecular Structure and Function of Microfibrillar-Associated Proteins in Skeletal and Metabolic Disorders and Cancers. J. Cell. Physiol. 2021, 236, 41–48. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, Q.; Chen, L.; Gao, S.; Meng, L.; Liu, Y.; Wang, Y.; Li, T.; Xue, J. MAGP2 Promotes Osteogenic Differentiation during Fracture Healing through Its Crosstalk with the β-Catenin Pathway. J. Cell. Physiol. 2024, 239, e31183. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ding, Y.; Wang, J.; Lyu, F.; Tang, Q.; Song, J.; Luo, Z.; Wan, Q.; Lan, X.; Xu, Z.; et al. Single-Cell RNA Sequencing in Oral Science: Current Awareness and Perspectives. Cell Prolif. 2022, 55, e13287. [Google Scholar] [CrossRef] [PubMed]
- Hemeryck, L.; Hermans, F.; Chappell, J.; Kobayashi, H.; Lambrechts, D.; Lambrichts, I.; Bronckaers, A.; Vankelecom, H. Organoids from Human Tooth Showing Epithelial Stemness Phenotype and Differentiation Potential. Cell. Mol. Life Sci. CMLS 2022, 79, 153. [Google Scholar] [CrossRef]
- Jing, J.; Feng, J.; Yuan, Y.; Guo, T.; Lei, J.; Pei, F.; Ho, T.-V.; Chai, Y. Spatiotemporal Single-Cell Regulatory Atlas Reveals Neural Crest Lineage Diversification and Cellular Function during Tooth Morphogenesis. Nat. Commun. 2022, 13, 4803. [Google Scholar] [CrossRef]
- Wang, F.; Xiao, J.; Cong, W.; Li, A.; Song, T.; Wei, F.; Xu, J.; Zhang, C.; Fan, Z.; Wang, S. Morphology and Chronology of Diphyodont Dentition in Miniature Pigs, Sus Scrofa. Oral Dis. 2014, 20, 367–379. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Fang, D.; Shi, S. The Miniature Pig: A Useful Large Animal Model for Dental and Orofacial Research. Oral Dis. 2007, 13, 530–537. [Google Scholar] [CrossRef]
- Wu, X.; Hu, J.; Li, G.; Li, Y.; Li, Y.; Zhang, J.; Wang, F.; Li, A.; Hu, L.; Fan, Z.; et al. Biomechanical Stress Regulates Mammalian Tooth Replacement via the Integrin Β1-RUNX2-Wnt Pathway. EMBO J. 2020, 39, e102374. [Google Scholar] [CrossRef]
- Yu, W.; Sun, Z.; Sweat, Y.; Sweat, M.; Venugopalan, S.R.; Eliason, S.; Cao, H.; Paine, M.L.; Amendt, B.A. Pitx2-Sox2-Lef1 Interactions Specify Progenitor Oral/Dental Epithelial Cell Signaling Centers. Development 2020, 147, dev186023. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Duan, Y.; Wang, K.; Fu, H.; Liao, Y.; Wang, T.; Zhang, Z.; Kang, F.; Zhang, B.; Zhang, H.; et al. Dental Niche Cells Directly Contribute to Tooth Reconstitution and Morphogenesis. Cell Rep. 2022, 41, 111737. [Google Scholar] [CrossRef] [PubMed]
- Krivanek, J.; Soldatov, R.A.; Kastriti, M.E.; Chontorotzea, T.; Herdina, A.N.; Petersen, J.; Szarowska, B.; Landova, M.; Matejova, V.K.; Holla, L.I.; et al. Dental Cell Type Atlas Reveals Stem and Differentiated Cell Types in Mouse and Human Teeth. Nat. Commun. 2020, 11, 4816. [Google Scholar] [CrossRef]
- Andersson, K.; Dahllöf, G.; Lindahl, K.; Kindmark, A.; Grigelioniene, G.; Åström, E.; Malmgren, B. Mutations in COL1A1 and COL1A2 and Dental Aberrations in Children and Adolescents with Osteogenesis Imperfecta—A Retrospective Cohort Study. PLoS ONE 2017, 12, e0176466. [Google Scholar] [CrossRef] [PubMed]
- Tucker, A.S.; Headon, D.J.; Schneider, P.; Ferguson, B.M.; Overbeek, P.; Tschopp, J.; Sharpe, P.T. Edar/Eda Interactions Regulate Enamel Knot Formation in Tooth Morphogenesis. Dev. Camb. Engl. 2000, 127, 4691–4700. [Google Scholar] [CrossRef]
- Kassai, Y.; Munne, P.; Hotta, Y.; Penttilä, E.; Kavanagh, K.; Ohbayashi, N.; Takada, S.; Thesleff, I.; Jernvall, J.; Itoh, N. Regulation of Mammalian Tooth Cusp Patterning by Ectodin. Science 2005, 309, 2067–2070. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Fang, Y.; Qian, Y.; Liu, Y.; Yang, X.; Huang, H.; Huang, H.; Li, Y.; Zhang, X.; Zhang, Z.; et al. Wnt Production in Dental Epithelium Is Crucial for Tooth Differentiation. J. Dent. Res. 2019, 98, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Lav, R.; Li, J.; Tucker, A.S.; Green, J.B.A. Molar Bud-to-Cap Transition Is Proliferation Independent. J. Dent. Res. 2019, 98, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Lesot, H.; Kieffer-Combeau, S.; Fausser, J.L.; Meyer, J.M.; Perrin-Schmitt, F.; Peterková, R.; Peterka, M.; Ruch, J.V. Cell-Cell and Cell-Matrix Interactions during Initial Enamel Organ Histomorphogenesis in the Mouse. Connect. Tissue Res. 2002, 43, 191–200. [Google Scholar] [CrossRef]
- Sasaki, T.; Ito, Y.; Xu, X.; Han, J.; Bringas, P.; Maeda, T.; Slavkin, H.C.; Grosschedl, R.; Chai, Y. LEF1 Is a Critical Epithelial Survival Factor during Tooth Morphogenesis. Dev. Biol. 2005, 278, 130–143. [Google Scholar] [CrossRef]
- Kratochwil, K.; Galceran, J.; Tontsch, S.; Roth, W.; Grosschedl, R. FGF4, a Direct Target of LEF1 and Wnt Signaling, Can Rescue the Arrest of Tooth Organogenesis in Lef1−/− Mice. Genes Dev. 2002, 16, 3173–3185. [Google Scholar] [CrossRef]
- Zeng, X.; He, P. Spatio-Temporal Regulation of IGFs in Enamel Development: Molecular Mechanisms From Ameloblast Polarity to Mineralization Homeostasis. Stem Cells Int. 2025, 2025, 9665706. [Google Scholar] [CrossRef]
- Kawasaki, K.; Kawasaki, M.; Watanabe, M.; Idrus, E.; Nagai, T.; Oommen, S.; Maeda, T.; Hagiwara, N.; Que, J.; Sharpe, P.T.; et al. Expression of Sox Genes in Tooth Development. Int. J. Dev. Biol. 2015, 59, 471–478. [Google Scholar] [CrossRef]
- Chiba, Y.; Tian, T.; Yoshizaki, K.; Wang, X.; Yamada, A.; Fukumoto, S. Keratin 15 Regulates Cell Proliferation in Outer Enamel Epithelium. J. Dent. Res. 2025. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Donato, R.; Sorci, G.; Giambanco, I. S100A6 Protein: Functional Roles. Cell. Mol. Life Sci. CMLS 2017, 74, 2749–2760. [Google Scholar] [CrossRef]
- Otake, S.; Saito, K.; Chiba, Y.; Yamada, A.; Fukumoto, S. S100a6 Knockdown Promotes the Differentiation of Dental Epithelial Cells toward the Epidermal Lineage Instead of the Odontogenic Lineage. FASEB J. 2024, 38, e23608. [Google Scholar] [CrossRef]
- Miao, X.; Niibe, K.; Zhang, M.; Liu, Z.; Nattasit, P.; Ohori-Morita, Y.; Nakamura, T.; Jiang, X.; Egusa, H. Stage-Specific Role of Amelx Activation in Stepwise Ameloblast Induction from Mouse Induced Pluripotent Stem Cells. Int. J. Mol. Sci. 2021, 22, 7195. [Google Scholar] [CrossRef]
- He, P.; Zhang, Y.; Kim, S.O.; Radlanski, R.J.; Butcher, K.; Schneider, R.A.; DenBesten, P.K. Ameloblast Differentiation in the Human Developing Tooth: Effects of Extracellular Matrices. Matrix Biol. 2010, 29, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Zhang, Y.; Zheng, H.; Zhao, K.; Yang, Y.; Lai, B.; Deng, X.; Wei, Y. Spatiotemporal Molecular Architecture of Lineage Allocation and Cellular Organization in Tooth Morphogenesis. Adv. Sci. 2024, 11, e2403627. [Google Scholar] [CrossRef]
- Hermans, F.; Hemeryck, L.; Bueds, C.; Torres Pereiro, M.; Hasevoets, S.; Kobayashi, H.; Lambrechts, D.; Lambrichts, I.; Bronckaers, A.; Vankelecom, H. Organoids from Mouse Molar and Incisor as New Tools to Study Tooth-Specific Biology and Development. Stem Cell Rep. 2023, 18, 1166–1181. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhang, H.; Camhi, H.; Seymen, F.; Koruyucu, M.; Kasimoglu, Y.; Kim, J.-W.; Kim-Berman, H.; Yuson, N.M.R.; Benke, P.J.; et al. Analyses of Oligodontia Phenotypes and Genetic Etiologies. Int. J. Oral Sci. 2021, 13, 32, Erratum in Int. J. Oral Sci. 2021, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Mustonen, T.; Tümmers, M.; Mikami, T.; Itoh, N.; Zhang, N.; Gridley, T.; Thesleff, I. Lunatic Fringe, FGF, and BMP Regulate the Notch Pathway during Epithelial Morphogenesis of Teeth. Dev. Biol. 2002, 248, 281–293. [Google Scholar] [CrossRef]
- Zhao, T.; Zhong, Q.; Sun, Z.; Yu, X.; Sun, T.; An, Z. Decoding SFRP2 Progenitors in Sustaining Tooth Growth at Single-Cell Resolution. Stem Cell Res. Ther. 2025, 16, 58. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kim, S.; Shin, Y.; Lee, H.-S.; Jeon, M.; Kim, S.-O.; Cho, S.-W.; Ruparel, N.B.; Song, J.S. Comparative Gene Expression Analysis of the Coronal Pulp and Apical Pulp Complex in Human Immature Teeth. J. Endod. 2016, 42, 752–759. [Google Scholar] [CrossRef]
- Fjeld, K.; Kettunen, P.; Furmanek, T.; Kvinnsland, I.H.; Luukko, K. Dynamic Expression of Wnt Signaling-Related Dickkopf1, -2, and -3 mRNAs in the Developing Mouse Tooth. Dev. Dyn. 2005, 233, 161–166. [Google Scholar] [CrossRef]
- Sui, B.-D.; Zheng, C.-X.; Zhao, W.-M.; Xuan, K.; Li, B.; Jin, Y. Mesenchymal Condensation in Tooth Development and Regeneration: A Focus on Translational Aspects of Organogenesis. Physiol. Rev. 2023, 103, 1899–1964. [Google Scholar] [CrossRef]
- Peng, L.; Dong, G.; Xu, P.; Ren, L.B.; Wang, C.L.; Aragon, M.; Zhou, X.D.; Ye, L. Expression of Wnt5a in Tooth Germs and the Related Signal Transduction Analysis. Arch. Oral Biol. 2010, 55, 108–114. [Google Scholar] [CrossRef]
- Qiu, X.; Hill, A.; Packer, J.; Lin, D.; Ma, Y.-A.; Trapnell, C. Single-Cell mRNA Quantification and Differential Analysis with Census. Nat. Methods 2017, 14, 309–315. [Google Scholar] [CrossRef]
- Xu, X.; Gong, X.; Zhang, L.; Zhang, H.; Sun, Y. PRX1-Positive Mesenchymal Stem Cells Drive Molar Morphogenesis. Int. J. Oral Sci. 2024, 16, 15. [Google Scholar] [CrossRef]
- Tao, H.; Lin, H.; Sun, Z.; Pei, F.; Zhang, J.; Chen, S.; Liu, H.; Chen, Z. Klf4 Promotes Dentinogenesis and Odontoblastic Differentiation via Modulation of TGF-β Signaling Pathway and Interaction With Histone Acetylation. J. Bone Miner. Res. 2019, 34, 1502–1516. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Xu, L.; Liu, H.; Sun, Q.; Chen, Z.; Yuan, G.; Chen, Z. KLF4 Promotes the Odontoblastic Differentiation of Human Dental Pulp Cells. J. Endod. 2011, 37, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Acampora, D.; Merlo, G.R.; Paleari, L.; Zerega, B.; Postiglione, M.P.; Mantero, S.; Bober, E.; Barbieri, O.; Simeone, A.; Levi, G. Craniofacial, Vestibular and Bone Defects in Mice Lacking the Distal-Less-Related Gene Dlx5. Development 1999, 126, 3795–3809. [Google Scholar] [CrossRef]
- Zeng, L.; Zhao, N.; Han, D.; Liu, H.; Liu, Y.; Wang, Y.; Feng, H. DLX3 Mutation Negatively Regulates Odontogenic Differentiation of Human Dental Pulp Cells. Arch. Oral Biol. 2017, 77, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Fan, J.; Cao, Y.; Gao, R.; Fan, Z. Distal-Less Homeobox 5 Promotes the Osteo-/Dentinogenic Differentiation Potential of Stem Cells from Apical Papilla by Activating Histone Demethylase KDM4B through a Positive Feedback Mechanism. Exp. Cell Res. 2019, 374, 221–230. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the Extracellular Matrix in Development and Disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Deford, P.; Brown, K.; Richards, R.L.; King, A.; Newburn, K.; Westover, K.; Albig, A.R. MAGP2 Controls Notch via Interactions with RGD Binding Integrins: Identification of a Novel ECM-Integrin-Notch Signaling Axis. Exp. Cell Res. 2016, 341, 84–91. [Google Scholar] [CrossRef]
- Ruoslahti, E. RGD and Other Recognition Sequences for Integrins. Annu. Rev. Cell Dev. Biol. 1996, 12, 697–715. [Google Scholar] [CrossRef]
- Balic, A. Concise Review: Cellular and Molecular Mechanisms Regulation of Tooth Initiation. Stem Cells 2019, 37, 26–32. [Google Scholar] [CrossRef]
- Ye, Q.; Bhojwani, A.; Hu, J.K. Understanding the Development of Oral Epithelial Organs through Single Cell Transcriptomic Analysis. Dev. Camb. Engl. 2022, 149, dev200539. [Google Scholar] [CrossRef]
- Hermans, F.; Bueds, C.; Hemeryck, L.; Lambrichts, I.; Bronckaers, A.; Vankelecom, H. Establishment of Inclusive Single-Cell Transcriptome Atlases from Mouse and Human Tooth as Powerful Resource for Dental Research. Front. Cell Dev. Biol. 2022, 10, 1021459. [Google Scholar] [CrossRef]
- Chiba, Y.; Saito, K.; Martin, D.; Boger, E.T.; Rhodes, C.; Yoshizaki, K.; Nakamura, T.; Yamada, A.; Morell, R.J.; Yamada, Y.; et al. Single-Cell RNA-Sequencing From Mouse Incisor Reveals Dental Epithelial Cell-Type Specific Genes. Front. Cell Dev. Biol. 2020, 8, 841. [Google Scholar] [CrossRef]
- Zheng, Y.; Lu, T.; Zhang, L.; Gan, Z.; Li, A.; He, C.; He, F.; He, S.; Zhang, J.; Xiong, F. Single-Cell RNA-Seq Analysis of Rat Molars Reveals Cell Identity and Driver Genes Associated with Dental Mesenchymal Cell Differentiation. BMC Biol. 2024, 22, 198. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yu, Y.; Zhou, Y.; Zhao, J.; Zhang, W.; Zou, D.; Song, W.; Wang, S. A Single-Cell Interactome of Human Tooth Germ from Growing Third Molar Elucidates Signaling Networks Regulating Dental Development. Cell Biosci. 2021, 11, 178. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Y.; Chen, S.; Chen, X.; Zhang, Y.; Chen, H.; Liao, Y.; Zhang, J.; Wu, D.; Chu, H.; et al. Single Cell Atlas of Developing Mouse Dental Germs Reveals Populations of CD24+ and Plac8+ Odontogenic Cells. Sci. Bull. 2022, 67, 1154–1169. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Alves, T.; Miao, M.Z.; Wu, Y.C.; Li, G.; Lou, J.; Hasturk, H.; Van Dyke, T.E.; Kantarci, A.; Wu, D. Single-Cell Transcriptomic Analysis of Dental Pulp and Periodontal Ligament Stem Cells. J. Dent. Res. 2024, 103, 71–80. [Google Scholar] [CrossRef]
- Ren, H.; Wen, Q.; Zhao, Q.; Wang, N.; Zhao, Y. Atlas of Human Dental Pulp Cells at Multiple Spatial and Temporal Levels Based on Single-Cell Sequencing Analysis. Front. Physiol. 2022, 13, 993478, Erratum in Front. Physiol. 2023, 14, 1331650. [Google Scholar] [CrossRef]
- Easter, Q.T.; Fernandes Matuck, B.; Beldorati Stark, G.; Worth, C.L.; Predeus, A.V.; Fremin, B.; Huynh, K.; Ranganathan, V.; Ren, Z.; Pereira, D.; et al. Single-Cell and Spatially Resolved Interactomics of Tooth-Associated Keratinocytes in Periodontitis. Nat. Commun. 2024, 15, 5016. [Google Scholar] [CrossRef]
- Liu, J.; He, J.; Zhang, Z.; Liu, L.; Cao, Y.; Zhang, X.; Cai, X.; Luo, X.; Lei, X.; Zhang, N.; et al. Single-Cell Transcriptomics Identifies PDGFRA+ Progenitors Orchestrating Angiogenesis and Periodontal Tissue Regeneration. Int. J. Oral Sci. 2025, 17, 56. [Google Scholar] [CrossRef]
- Alghadeer, A.; Hanson-Drury, S.; Patni, A.P.; Ehnes, D.D.; Zhao, Y.T.; Li, Z.; Phal, A.; Vincent, T.; Lim, Y.C.; O’Day, D.; et al. Single-Cell Census of Human Tooth Development Enables Generation of Human Enamel. Dev. Cell 2023, 58, 2163–2180.e9. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wu, D.; Zhou, H.; Shen, Z.; Gu, X.; Wei, W.; Wu, C.; Chen, S.; Xia, J.; Zhang, C.; et al. Comparative Transcriptomic Analysis of Human Maxillary and Mandibular Tooth Germs Reveals Discrepancies in Gene Expression Patterns. Oral Sci. Homeost. Med. 2025, 1, 9610032. [Google Scholar] [CrossRef]
- Mogollón, I.; Ahtiainen, L. Live Tissue Imaging Sheds Light on Cell Level Events During Ectodermal Organ Development. Front. Physiol. 2020, 11, 818. [Google Scholar] [CrossRef] [PubMed]
- Jheon, A.H.; Seidel, K.; Biehs, B.; Klein, O.D. From Molecules to Mastication: The Development and Evolution of Teeth. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 165–182. [Google Scholar] [CrossRef]
- Gibson, M.A.; Hughes, J.L.; Fanning, J.C.; Cleary, E.G. The Major Antigen of Elastin-Associated Microfibrils Is a 31-kDa Glycoprotein. J. Biol. Chem. 1986, 261, 11429–11436. [Google Scholar] [CrossRef]
- Craft, C.S.; Broekelmann, T.J.; Mecham, R.P. Microfibril-Associated Glycoproteins MAGP-1 and MAGP-2 in Disease. Matrix Biol. 2018, 71–72, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Leonardo, T.R.; Romana-Souza, B.; Shi, J.; Keiser, S.; Yuan, H.; Altakriti, M.; Ranzer, M.J.; Ferri-Borgogno, S.; Mok, S.C.; et al. Microfibril-Associated Protein 5 and the Regulation of Skin Scar Formation. Sci. Rep. 2023, 13, 8728. [Google Scholar] [CrossRef]
- Liu, F.; Millar, S.E. Wnt/Beta-Catenin Signaling in Oral Tissue Development and Disease. J. Dent. Res. 2010, 89, 318–330. [Google Scholar] [CrossRef]
- Wang, J.; Martin, J.F. Hippo Pathway: An Emerging Regulator of Craniofacial and Dental Development. J. Dent. Res. 2017, 96, 1229–1237. [Google Scholar] [CrossRef]
- Chen, Y.; Petho, A.; Ganapathy, A.; George, A. DPP an Extracellular Matrix Molecule Induces Wnt5a Mediated Signaling to Promote the Differentiation of Adult Stem Cells into Odontogenic Lineage. Sci. Rep. 2024, 14, 26187. [Google Scholar] [CrossRef]
- Wu, H.; Teng, P.-N.; Jayaraman, T.; Onishi, S.; Li, J.; Bannon, L.; Huang, H.; Close, J.; Sfeir, C. Dentin Matrix Protein 1 (DMP1) Signals via Cell Surface Integrin. J. Biol. Chem. 2011, 286, 29462–29469. [Google Scholar] [CrossRef] [PubMed]
- Bellahcène, A.; Castronovo, V.; Ogbureke, K.U.E.; Fisher, L.W.; Fedarko, N.S. Small Integrin-Binding Ligand N-Linked Glycoproteins (SIBLINGs): Multifunctional Proteins in Cancer. Nat. Rev. Cancer 2008, 8, 212–226. [Google Scholar] [CrossRef]
- Liu, M.M.; Li, W.T.; Xia, X.M.; Wang, F.; MacDougall, M.; Chen, S. Dentine Sialophosphoprotein Signal in Dentineogenesis and Dentine Regeneration. Eur. Cells Mater. 2021, 42, 43–62. [Google Scholar] [CrossRef]
- Staines, K.A.; MacRae, V.E.; Farquharson, C. The Importance of the SIBLING Family of Proteins on Skeletal Mineralisation and Bone Remodelling. J. Endocrinol. 2012, 214, 241–255, Erratum in J. Endocrinol. 2013, 219, X1. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Yang, Y.; Xu, H.; Jin, A.; Huang, X.; Gao, X.; Sun, S.; Liu, Y.; Liu, J.; Lu, T.; et al. The Odontoblastic Differentiation of Dental Mesenchymal Stem Cells: Molecular Regulation Mechanism and Related Genetic Syndromes. Front. Cell Dev. Biol. 2023, 11, 1174579. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.-M.; Kong, Y.-Y.; Li, Y.-Y.; Zhang, W. MagT1 Regulated the Odontogenic Differentiation of BMMSCs Induced byTGC-CM via ERK Signaling Pathway. Stem Cell Res. Ther. 2019, 10, 48. [Google Scholar] [CrossRef]
- Kong, Y.; Hu, X.; Zhong, Y.; Xu, K.; Wu, B.; Zheng, J. Magnesium-Enriched Microenvironment Promotes Odontogenic Differentiation in Human Dental Pulp Stem Cells by Activating ERK/BMP2/Smads Signaling. Stem Cell Res. Ther. 2019, 10, 378. [Google Scholar] [CrossRef]
- Kumar, S.; Boehm, J.; Lee, J.C. P38 MAP Kinases: Key Signalling Molecules as Therapeutic Targets for Inflammatory Diseases. Nat. Rev. Drug Discov. 2003, 2, 717–726. [Google Scholar] [CrossRef]
- Sonoyama, W.; Liu, Y.; Fang, D.; Yamaza, T.; Seo, B.-M.; Zhang, C.; Liu, H.; Gronthos, S.; Wang, C.-Y.; Wang, S.; et al. Mesenchymal Stem Cell-Mediated Functional Tooth Regeneration in Swine. PLoS ONE 2006, 1, e79. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Ma, Z.-F.; Huo, N.; Tang, L.; Han, C.; Duan, Y.-Z.; Jin, Y. Porcine Tooth Germ Cell Conditioned Medium Can Induce Odontogenic Differentiation of Human Dental Pulp Stem Cells. J. Tissue Eng. Regen. Med. 2011, 5, 354–362. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, F.; Fan, Z.; Wu, T.; He, J.; Wang, J.; Zhang, C.; Wang, S. Whole-Tooth Regeneration by Allogeneic Cell Reassociation in Pig Jawbone. Tissue Eng. Part A 2019, 25, 1202–1212. [Google Scholar] [CrossRef]
- Li, Y.; Lu, D.; Xu, F.; Yang, J.; Li, D.; Yang, C.; Chen, X.; Wang, X.; Qing, J.; Zhang, H.; et al. EGR1 Promotes Craniofacial Bone Regeneration via Activation of ALPL+PDGFD+ Periosteal Stem Cells. Adv. Sci. 2025, 12, e10243. [Google Scholar] [CrossRef]
- Zhou, Z.; Cui, D.; Sun, M.-H.; Huang, J.-L.; Deng, Z.; Han, B.-M.; Sun, X.-W.; Xia, S.-J.; Sun, F.; Shi, F. CAFs-Derived MFAP5 Promotes Bladder Cancer Malignant Behavior through NOTCH2/HEY1 Signaling. FASEB J. 2020, 34, 7970–7988. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Tanaka, T.; Tokuda, T.; Kashiwagi, T.; Kaida, K.; Hieda, A.; Umezaki, Y.; Hashimoto, Y.; Imai, K.; Matsumoto, N.; et al. Local Controlled Release of Polyphenol Conjugated with Gelatin Facilitates Bone Formation. Int. J. Mol. Sci. 2015, 16, 14143–14157. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Lv, L.; Li, W.; Zhang, X.; Jiang, Y.; Ge, W.; Zhou, Y. Protein Deubiquitinase USP7 Is Required for Osteogenic Differentiation of Human Adipose-Derived Stem Cells. Stem Cell Res. Ther. 2017, 8, 186. [Google Scholar] [CrossRef]
- Yu, X.; Wan, Q.; Ye, X.; Cheng, Y.; Pathak, J.L.; Li, Z. Cellular Hypoxia Promotes Osteogenic Differentiation of Mesenchymal Stem Cells and Bone Defect Healing via STAT3 Signaling. Cell. Mol. Biol. Lett. 2019, 24, 64. [Google Scholar] [CrossRef] [PubMed]



| shRNA | Target Sequence |
|---|---|
| shMFAP5-1 | GCATCGGCCGGTTAAACAATG |
| shMFAP5-2 | GCTGCTGTTTCTTGCTGCATT |
| shMFAP5-3 | GGAGATCTGCTCTCGTCTTGT |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| GAPDH | AAGGTCGGAGTCAACGGATTTG | TCCTGGAAGATGGTGATGGGAT |
| MFAP5 [106] | GGGTCAATAGTCAACGAGGAGA | CTGTAGCGGGATCATTCACCA |
| DSPP | ATATTGAGGGCTGGAATGGGGA | TTTGTGGCTCCAGCATTGTCA |
| ITGA5 | GGCTTCAACTTAGACGCGGAG | TGGCTGGTATTAGCCTTGGGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Wang, X.; Gu, L.; Zhang, P.; Zhou, Y. MFAP5 Activates ITGA5 to Drive Tooth Germ Mineralization Through the MAPK/ERK Pathway: Insights from Single-Cell Transcriptomics. Int. J. Mol. Sci. 2026, 27, 394. https://doi.org/10.3390/ijms27010394
Wang X, Gu L, Zhang P, Zhou Y. MFAP5 Activates ITGA5 to Drive Tooth Germ Mineralization Through the MAPK/ERK Pathway: Insights from Single-Cell Transcriptomics. International Journal of Molecular Sciences. 2026; 27(1):394. https://doi.org/10.3390/ijms27010394
Chicago/Turabian StyleWang, Xu, Lanxin Gu, Ping Zhang, and Yongsheng Zhou. 2026. "MFAP5 Activates ITGA5 to Drive Tooth Germ Mineralization Through the MAPK/ERK Pathway: Insights from Single-Cell Transcriptomics" International Journal of Molecular Sciences 27, no. 1: 394. https://doi.org/10.3390/ijms27010394
APA StyleWang, X., Gu, L., Zhang, P., & Zhou, Y. (2026). MFAP5 Activates ITGA5 to Drive Tooth Germ Mineralization Through the MAPK/ERK Pathway: Insights from Single-Cell Transcriptomics. International Journal of Molecular Sciences, 27(1), 394. https://doi.org/10.3390/ijms27010394






