Therapy-Induced Senescence (TIS) and SASP: The p53-Mediated Interplay in Cancer Progression and Treatment
Abstract
1. Introduction
2. Understanding Cellular Senescence
2.1. Triggers and Master Regulatory Pathways of Cellular Senescence
2.1.1. Key Regulators of the p53 Pathway: An Elaborate Balance
2.1.2. Cellular Checkpoints Governing p53 Activation and Stabilization
DNA Damage Response (DDR) Checkpoint
Oncogenic Stress Checkpoint
Nucleolar Stress Checkpoint
Metabolic Stress Checkpoint
2.2. Characteristics of Senescent Cells: The Distinct Pathological Identity
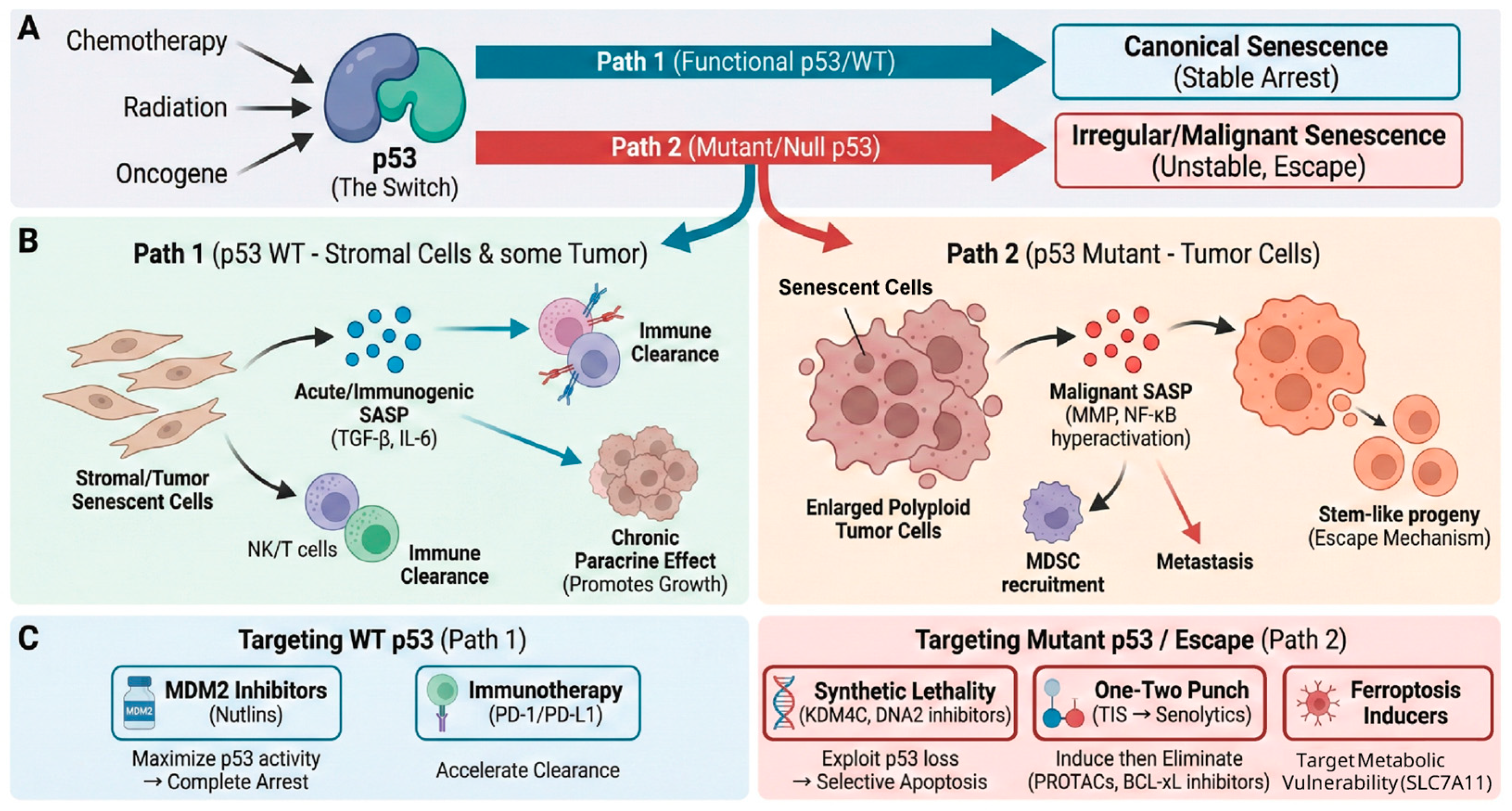
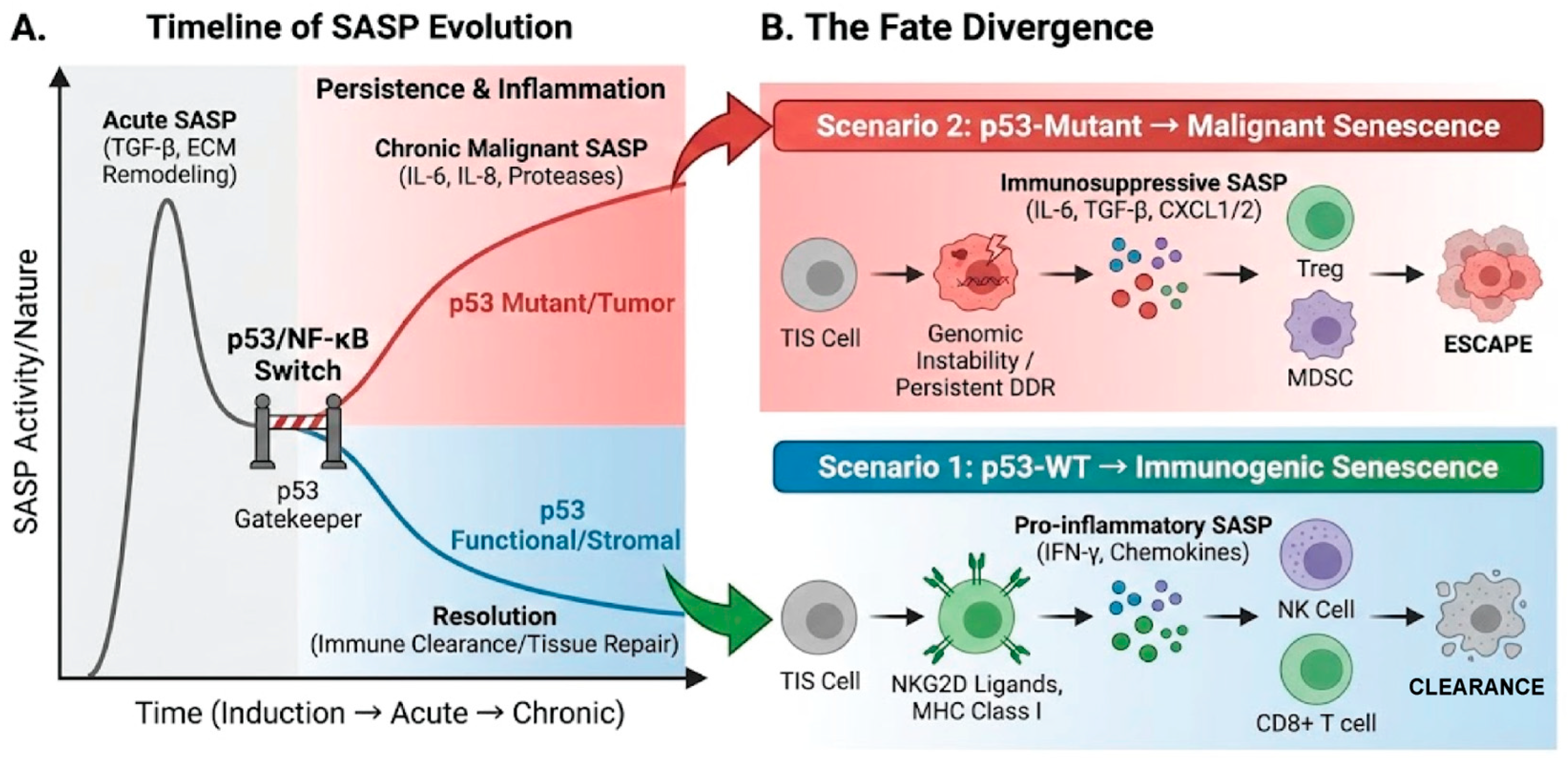
2.3. The Duality of Cellular Senescence
3. Interaction Between Cellular Senescence and the Hallmarks of Cancer
3.1. Cellular Senescence: The Primary Barrier Against Carcinogenesis
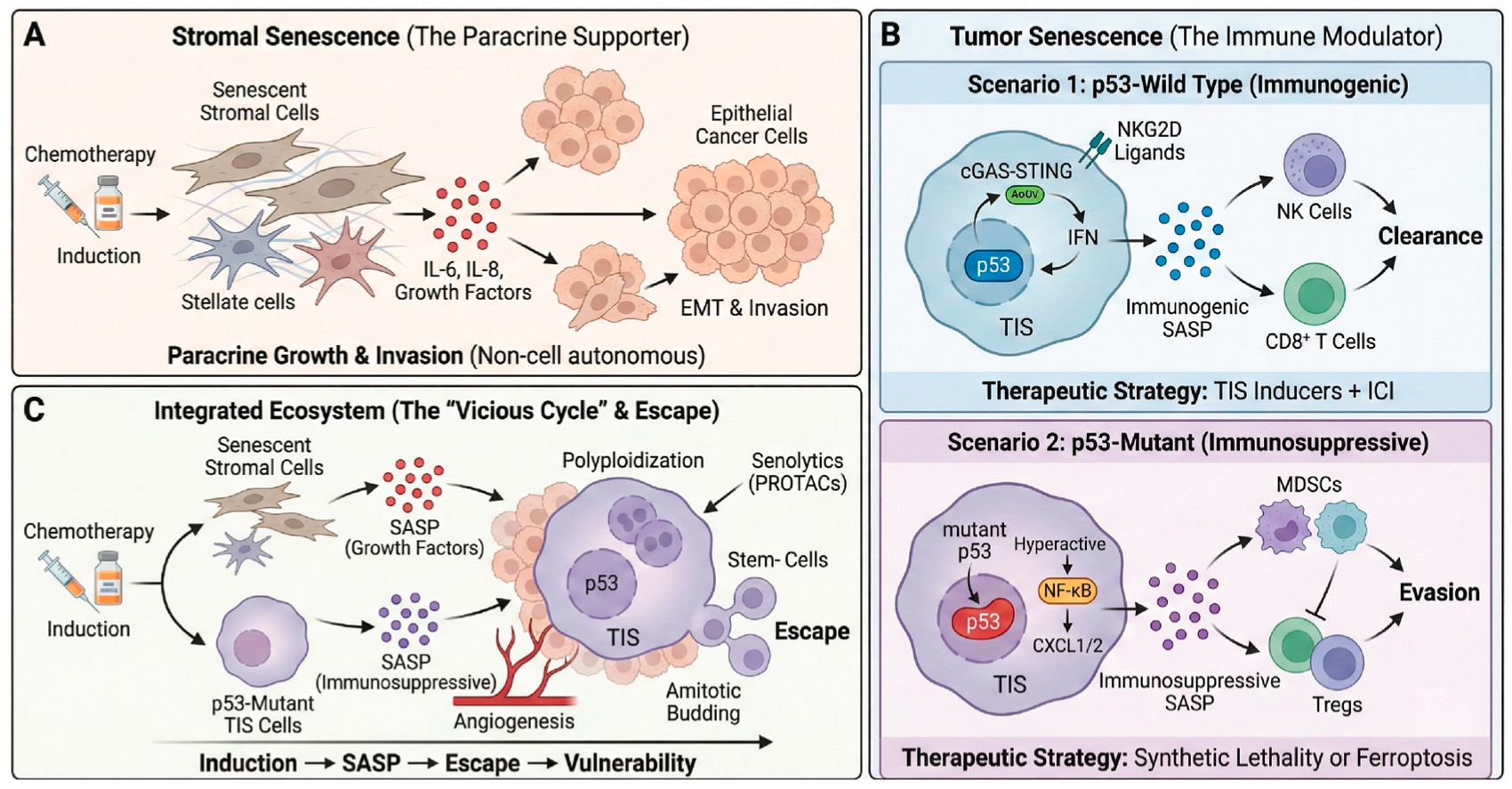
3.2. TIS and SASP: Key Drivers of Malignancy
3.2.1. Invasion and Metastasis
3.2.2. Inducing Angiogenesis
3.2.3. Sustaining Proliferative Signaling
3.2.4. Evading Immune Destruction
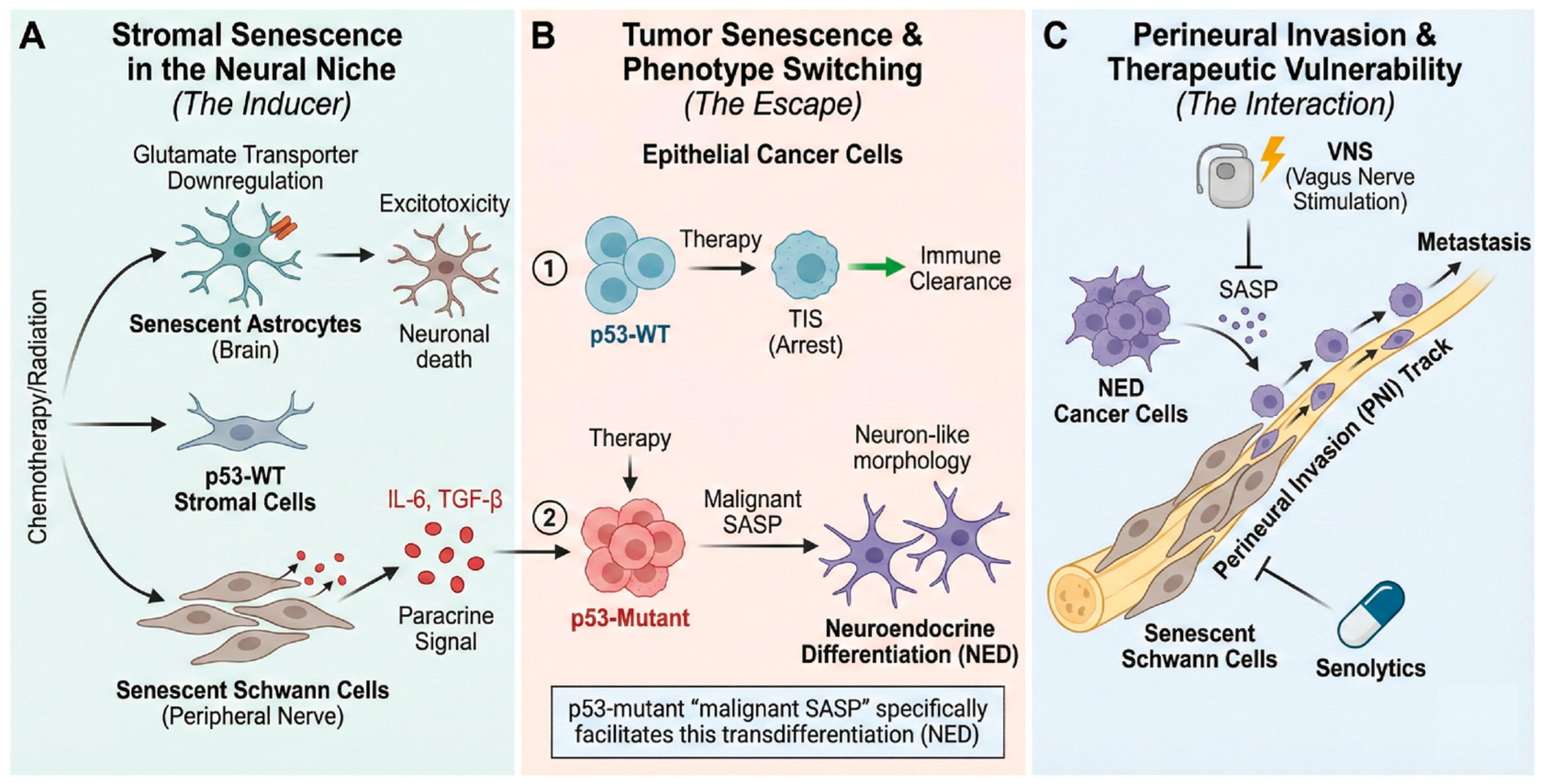
3.3. SASP and Tumor Innervation: An Interaction
3.3.1. Brain TME and Neuro-Immune Modulation
3.3.2. Direct Effects of SASP on Nerve Cells and Cancer Cells
3.3.3. SASP, Nerve-Related Cells, and Cancer Metastasis
3.4. p53 Mutation and Cellular Senescence: Evasion, Incompleteness, and Malignancy
3.4.1. Senescence Evasion (Bypass)
3.4.2. Incomplete Senescence and Escape
3.4.3. Malignant SASP and GOFs
3.5. Metabolic Rewiring and Ferroptosis Resistance
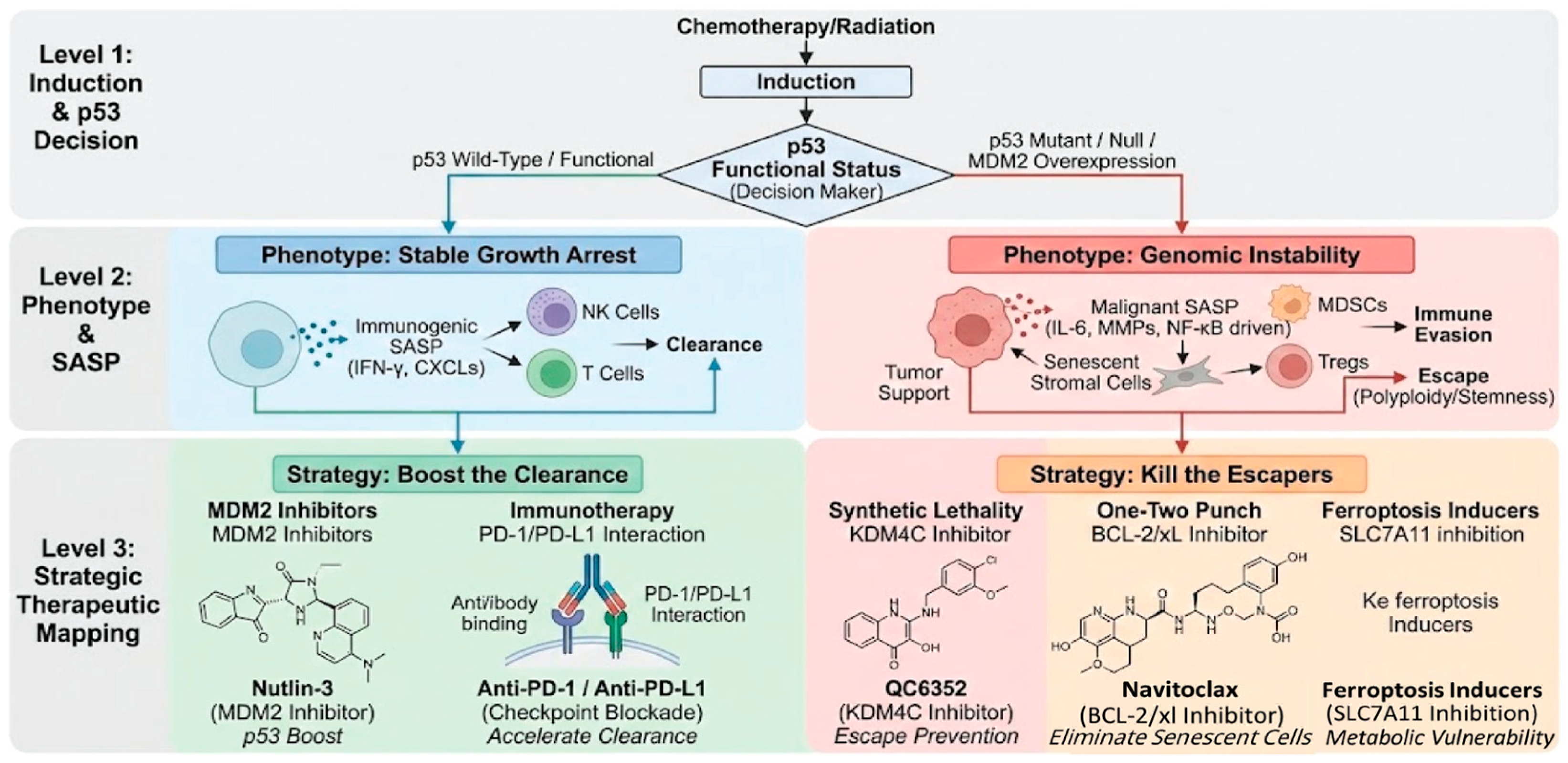
4. Novel Anticancer Therapeutic Strategies Targeting Cellular Senescence
4.1. Strategy 1: Reactivating the p53 Barrier—p53 Activators
4.1.1. MDM2/MDM4 Inhibitors
4.1.2. Mutant p53 Reactivators
4.2. Strategy 2: Targeted Elimination—Senolytics
4.3. Strategy 3: Modulating the Secretome—Senomorphics
4.4. Strategy 4: Harnessing the Immune System—Immunotherapeutic Approaches
4.5. Strategy 5: Targeting p53 Mutant Weaknesses—Synthetic Lethality
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPK | AMP-activated Protein Kinase 1 |
| ATM | Ataxia Telangiectasia Mutated 2 |
| ATR | Ataxia Telangiectasia and Rad3-related |
| CAR-T | Chimeric Antigen Receptor T-cell |
| CTL | Cytotoxic T Lymphocyte |
| DDR | DNA Damage Response |
| DLBCL | Diffuse Large B-cell Lymphoma |
| DSB | DNA Double-Strand Break |
| EMT | Epithelial–Mesenchymal Transition |
| GDF15 | Growth Differentiation Factor 15 |
| GOF | Gain-of-Function |
| HCC | Hepatocellular Carcinoma |
| HNSCC | Head and Neck Squamous Cell Carcinoma |
| ICD | Immunogenic Cell Death |
| ICI | Immune Checkpoint Inhibitor |
| MDSC | Myeloid-Derived Suppressor Cell |
| MMP | Matrix Metalloproteinase |
| NED | Neuroendocrine Differentiation |
| NK | Natural Killer |
| OCCC | Ovarian Clear Cell Carcinoma |
| OIS | Oncogene-Induced Senescence |
| PNI | Perineural Invasion |
| PROTAC | Proteolysis Targeting Chimera |
| ROS | Reactive Oxygen Species |
| SA-β-gal | Senescence-Associated β-galactosidase |
| SAHF | Senescence-Associated Heterochromatin Foci |
| SASP | Senescence-Associated Secretory Phenotype |
| TASc | Tumor-Associated Schwann Cell |
| TIS | Therapy-Induced Senescence |
| TME | Tumor Microenvironment |
| TNBC | Triple-Negative Breast Cancer |
| Treg | Regulatory T cell |
| uPAR | Urokinase Plasminogen Activator Receptor |
| VNS | Vagus Nerve Stimulation |
References
- Khan, S.U.; Fatima, K.; Aisha, S.; Malik, F. Unveiling the mechanisms and challenges of cancer drug resistance. Cell Commun. Signal. 2024, 22, 109. [Google Scholar] [CrossRef] [PubMed]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Fitsiou, E.; Soto-Gamez, A.; Demaria, M. Biological functions of therapy-induced senescence in cancer. In Seminars in Cancer Biology; Academic Press: New York, NY, USA, 2022; pp. 5–13. [Google Scholar]
- Winkler, F.; Venkatesh, H.S.; Amit, M.; Batchelor, T.; Demir, I.E.; Deneen, B.; Gutmann, D.H.; Hervey-Jumper, S.; Kuner, T.; Mabbott, D. Cancer neuroscience: State of the field, emerging directions. Cell 2023, 186, 1689–1707. [Google Scholar] [CrossRef]
- Al Mamun, A.; Sufian, M.A.; Uddin, M.S.; Sumsuzzman, D.M.; Jeandet, P.; Islam, M.S.; Zhang, H.-J.; Kong, A.-N.; Sarwar, M.S. Exploring the role of senescence inducers and senotherapeutics as targets for anticancer natural products. Eur. J. Pharmacol. 2022, 928, 174991. [Google Scholar] [CrossRef] [PubMed]
- D’adda Di Fagagna, F. Living on a break: Cellular senescence as a DNA-damage response. Nat. Rev. Cancer 2008, 8, 512–522. [Google Scholar] [CrossRef]
- Levine, A.J. p53: 800 million years of evolution and 40 years of discovery. Nat. Rev. Cancer 2020, 20, 471–480. [Google Scholar] [CrossRef]
- Wang, H.; Guo, M.; Wei, H.; Chen, Y. Targeting p53 pathways: Mechanisms, structures and advances in therapy. Signal Transduct. Target. Ther. 2023, 8, 92. [Google Scholar] [CrossRef]
- Serrano, M.; Blasco, M.A. Cancer and ageing: Convergent and divergent mechanisms. Nat. Rev. Mol. Cell Biol. 2007, 8, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Piano, A.; Titorenko, V.I. The intricate interplay between mechanisms underlying aging and cancer. Aging Dis. 2014, 6, 56. [Google Scholar] [CrossRef]
- Liu, B.; Peng, Z.; Zhang, H.; Zhang, N.; Liu, Z.; Xia, Z.; Huang, S.; Luo, P.; Cheng, Q. Regulation of cellular senescence in tumor progression and therapeutic targeting: Mechanisms and pathways. Mol. Cancer 2025, 24, 106. [Google Scholar] [CrossRef]
- Haupt, Y.; Maya, R.; Kazaz, A.; Oren, M. Mdm2 promotes the rapid degradation of p53. Nature 1997, 387, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Kubbutat, M.H.; Jones, S.N.; Vousden, K.H. Regulation of p53 stability by Mdm2. Nature 1997, 387, 299–303. [Google Scholar] [CrossRef]
- Wang, W.; Albadari, N.; Du, Y.; Fowler, J.F.; Sang, H.T.; Xian, W.; McKeon, F.; Li, W.; Zhou, J.; Zhang, R. MDM2 inhibitors for cancer therapy: The past, present, and future. Pharmacol. Rev. 2024, 76, 414–453. [Google Scholar] [CrossRef]
- Li, M.; Brooks, C.L.; Wu-Baer, F.; Chen, D.; Baer, R.; Gu, W. Mono-versus polyubiquitination: Differential control of p53 fate by Mdm2. Science 2003, 302, 1972–1975. [Google Scholar] [CrossRef]
- Grier, J.D.; Xiong, S.; Elizondo-Fraire, A.C.; Parant, J.M.; Lozano, G. Tissue-specific differences of p53 inhibition by Mdm2 and Mdm4. Mol. Cell. Biol. 2006, 26, 192–198. [Google Scholar] [CrossRef]
- Xiong, S.; Zhang, Y.; Zhou, X.; Pant, V.; Mirani, A.; Gencel-Augusto, J.; Chau, G.; You, M.J.; Lozano, G. Dependence on Mdm2 for Mdm4 inhibition of p53 activity. Cancer Lett. 2025, 621, 217622. [Google Scholar] [CrossRef]
- Luo, J.; Nikolaev, A.Y.; Imai, S.-I.; Chen, D.; Su, F.; Shiloh, A.; Guarente, L.; Gu, W. Negative control of p53 by Sir2α promotes cell survival under stress. Cell 2001, 107, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Ong, A.L.; Ramasamy, T.S. Role of Sirtuin1-p53 regulatory axis in aging, cancer and cellular reprogramming. Ageing Res. Rev. 2018, 43, 64–80. [Google Scholar] [CrossRef] [PubMed]
- Pomerantz, J.; Schreiber-Agus, N.; Liégeois, N.J.; Silverman, A.; Alland, L.; Chin, L.; Potes, J.; Chen, K.; Orlow, I.; Lee, H.-W. The Ink4a tumor suppressor gene product, p19Arf, interacts with MDM2 and neutralizes MDM2’s inhibition of p53. Cell 1998, 92, 713–723. [Google Scholar] [CrossRef]
- Sherr, C.J. The INK4a/ARF network in tumour suppression. Nat. Rev. Mol. Cell Biol. 2001, 2, 731–737. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Pratico, D.; Bahijri, S.; Tuomilehto, J.; Uversky, V.N.; Ren, J. Hallmarks of cellular senescence: Biology, mechanisms, regulations. Exp. Mol. Med. 2025, 57, 1482–1491. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.-L.; Duncavage, E.J.; Mathew, R.; Den Besten, W.; Pei, D.; Naeve, D.; Yamamoto, T.; Cheng, C.; Sherr, C.J.; Roussel, M.F. Arf induces p53-dependent and-independent antiproliferative genes. Cancer Res. 2003, 63, 1046–1053. [Google Scholar]
- Gu, W.; Roeder, R.G. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell 1997, 90, 595–606. [Google Scholar] [CrossRef]
- Wang, K.; Gong, Z.; Chen, Y.; Zhang, M.; Wang, S.; Yao, S.; Liu, Z.; Huang, Z.; Fei, B. KDM4C-mediated senescence defense is a targetable vulnerability in gastric cancer harboring TP53 mutations. Clin. Epigenetics 2023, 15, 163. [Google Scholar] [CrossRef]
- Soriani, A.; Zingoni, A.; Cerboni, C.; Iannitto, M.L.; Ricciardi, M.R.; Di Gialleonardo, V.; Cippitelli, M.; Fionda, C.; Petrucci, M.T.; Guarini, A. ATM-ATR–dependent up-regulation of DNAM-1 and NKG2D ligands on multiple myeloma cells by therapeutic agents results in enhanced NK-cell susceptibility and is associated with a senescent phenotype. Blood J. Am. Soc. Hematol. 2009, 113, 3503–3511. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.; Lin, A.W.; McCurrach, M.E.; Beach, D.; Lowe, S.W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 1997, 88, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Kuilman, T.; Michaloglou, C.; Mooi, W.J.; Peeper, D.S. The essence of senescence. Genes Dev. 2010, 24, 2463–2479. [Google Scholar] [CrossRef]
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef]
- Rodier, F.; Campisi, J. Four faces of cellular senescence. J. Cell Biol. 2011, 192, 547–556. [Google Scholar] [CrossRef]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G. Cellular senescence: Defining a path forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef]
- Suryadevara, V.; Hudgins, A.D.; Rajesh, A.; Pappalardo, A.; Karpova, A.; Dey, A.K.; Hertzel, A.; Agudelo, A.; Rocha, A.; Soygur, B. SenNet recommendations for detecting senescent cells in different tissues. Nat. Rev. Mol. Cell Biol. 2024, 25, 1001–1023. [Google Scholar] [CrossRef]
- Narita, M.; Nuñez, S.; Heard, E.; Narita, M.; Lin, A.W.; Hearn, S.A.; Spector, D.L.; Hannon, G.J.; Lowe, S.W. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 2003, 113, 703–716. [Google Scholar] [CrossRef]
- Wang, B.; Han, J.; Elisseeff, J.H.; Demaria, M. The senescence-associated secretory phenotype and its physiological and pathological implications. Nat. Rev. Mol. Cell Biol. 2024, 25, 958–978. [Google Scholar] [CrossRef] [PubMed]
- Krizhanovsky, V.; Yon, M.; Dickins, R.A.; Hearn, S.; Simon, J.; Miething, C.; Yee, H.; Zender, L.; Lowe, S.W. Senescence of activated stellate cells limits liver fibrosis. Cell 2008, 134, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Marin, I.; Boix, O.; Garcia-Garijo, A.; Sirois, I.; Caballe, A.; Zarzuela, E.; Ruano, I.; Attolini, C.S.-O.; Prats, N.; López-Domínguez, J.A. Cellular senescence is immunogenic and promotes antitumor immunity. Cancer Discov. 2023, 13, 410–431. [Google Scholar] [CrossRef]
- Baker, D.J.; Wijshake, T.; Tchkonia, T.; LeBrasseur, N.K.; Childs, B.G.; Van De Sluis, B.; Kirkland, J.L.; Van Deursen, J.M. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011, 479, 232–236. [Google Scholar] [CrossRef]
- Chaib, S.; Tchkonia, T.; Kirkland, J.L. Cellular senescence and senolytics: The path to the clinic. Nat. Med. 2022, 28, 1556–1568. [Google Scholar] [CrossRef] [PubMed]
- Harley, C.B.; Futcher, A.B.; Greider, C.W. Telomeres shorten during ageing of human fibroblasts. Nature 1990, 345, 458–460. [Google Scholar] [CrossRef]
- Hoare, M.; Ito, Y.; Kang, T.-W.; Weekes, M.P.; Matheson, N.J.; Patten, D.A.; Shetty, S.; Parry, A.J.; Menon, S.; Salama, R. NOTCH1 mediates a switch between two distinct secretomes during senescence. Nat. Cell Biol. 2016, 18, 979–992. [Google Scholar] [CrossRef]
- Pribluda, A.; Elyada, E.; Wiener, Z.; Hamza, H.; Goldstein, R.E.; Biton, M.; Burstain, I.; Morgenstern, Y.; Brachya, G.; Billauer, H. A senescence-inflammatory switch from cancer-inhibitory to cancer-promoting mechanism. Cancer Cell 2013, 24, 242–256. [Google Scholar] [CrossRef] [PubMed]
- Cohn, R.L.; Gasek, N.S.; Kuchel, G.A.; Xu, M. The heterogeneity of cellular senescence: Insights at the single-cell level. Trends Cell Biol. 2023, 33, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Theodorakis, N.; Feretzakis, G.; Tzelves, L.; Paxinou, E.; Hitas, C.; Vamvakou, G.; Verykios, V.S.; Nikolaou, M. Integrating machine learning with multi-omics technologies in geroscience: Towards personalized medicine. J. Pers. Med. 2024, 14, 931. [Google Scholar] [CrossRef]
- Sanborn, M.A.; Wang, X.; Gao, S.; Dai, Y.; Rehman, J. Unveiling the cell-type-specific landscape of cellular senescence through single-cell transcriptomics using SenePy. Nat. Commun. 2025, 16, 1884. [Google Scholar] [CrossRef]
- Coppé, J.-P.; Patil, C.K.; Rodier, F.; Sun, Y.; Muñoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.-Y.; Campisi, J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008, 6, e301. [Google Scholar] [CrossRef]
- Loo, T.M.; Miyata, K.; Tanaka, Y.; Takahashi, A. Cellular senescence and senescence-associated secretory phenotype via the cGAS-STING signaling pathway in cancer. Cancer Sci. 2020, 111, 304–311. [Google Scholar] [CrossRef]
- Li, T.; Chen, Z.J. The cGAS–cGAMP–STING pathway connects DNA damage to inflammation, senescence, and cancer. J. Exp. Med. 2018, 215, 1287–1299. [Google Scholar] [CrossRef]
- Paul, P.; Kumar, A.; Parida, A.S.; De, A.K.; Bhadke, G.; Khatua, S.; Tiwari, B. p53-mediated regulation of LINE1 retrotransposon-derived R-loops. J. Biol. Chem. 2025, 301, 108200. [Google Scholar] [CrossRef] [PubMed]
- De Cecco, M.; Ito, T.; Petrashen, A.P.; Elias, A.E.; Skvir, N.J.; Criscione, S.W.; Caligiana, A.; Brocculi, G.; Adney, E.M.; Boeke, J.D. L1 drives IFN in senescent cells and promotes age-associated inflammation. Nature 2019, 566, 73–78. [Google Scholar] [CrossRef]
- Mathavarajah, S.; Dellaire, G. LINE-1: An emerging initiator of cGAS-STING signalling and inflammation that is dysregulated in disease. Biochem. Cell Biol. 2023, 102, 38–46. [Google Scholar] [CrossRef]
- Lujambio, A.; Akkari, L.; Simon, J.; Grace, D.; Tschaharganeh, D.F.; Bolden, J.E.; Zhao, Z.; Thapar, V.; Joyce, J.A.; Krizhanovsky, V. Non-cell-autonomous tumor suppression by p53. Cell 2013, 153, 449–460. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, L.; Ding, L.; Zhang, C.; Li, Y.; Wang, B.; Shi, J.; Zhang, J. NFATc1 facilitates hepatocellular carcinoma progression by regulating the senescence-associated secretory phenotype. Sci. Rep. 2025, 15, 24824. [Google Scholar] [CrossRef]
- Gilbert, L.A.; Hemann, M.T. DNA damage-mediated induction of a chemoresistant niche. Cell 2010, 143, 355–366. [Google Scholar] [CrossRef]
- Ling, Y.-W.; Duan, J.-L.; Jiang, Z.-J.; Yang, Z.; Liu, J.-J.; Song, P.; Fang, Z.-Q.; Yue, Z.-S.; He, F.; Dou, K.-F. Diabetes reshapes pancreatic cancer-associated endothelial niche by accelerating senescence. Nat. Commun. 2025, 16, 8654. [Google Scholar] [CrossRef] [PubMed]
- Krtolica, A.; Parrinello, S.; Lockett, S.; Desprez, P.-Y.; Campisi, J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: A link between cancer and aging. Proc. Natl. Acad. Sci. USA 2001, 98, 12072–12077. [Google Scholar] [CrossRef] [PubMed]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef]
- Ruhland, M.K.; Loza, A.J.; Capietto, A.-H.; Luo, X.; Knolhoff, B.L.; Flanagan, K.C.; Belt, B.A.; Alspach, E.; Leahy, K.; Luo, J. Stromal senescence establishes an immunosuppressive microenvironment that drives tumorigenesis. Nat. Commun. 2016, 7, 11762. [Google Scholar] [CrossRef]
- Wiklund, F.E.; Bennet, A.M.; Magnusson, P.K.; Eriksson, U.K.; Lindmark, F.; Wu, L.; Yaghoutyfam, N.; Marquis, C.P.; Stattin, P.; Pedersen, N.L. Macrophage inhibitory cytokine-1 (MIC-1/GDF15): A new marker of all-cause mortality. Aging Cell 2010, 9, 1057–1064. [Google Scholar] [CrossRef]
- Pence, B.D.; Yarbro, J.R.; Emmons, R.S. Growth differentiation factor-15 is associated with age-related monocyte dysfunction. Aging Med. 2021, 4, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.; Lehoczki, A.; Haskó, G.; Lohoff, F.W.; Ungvari, Z.; Pacher, P. Global and tissue-specific transcriptomic dysregulation in human aging: Pathways and predictive biomarkers. GeroScience 2025, 47, 5917–5936. [Google Scholar] [CrossRef]
- Roth, P.; Junker, M.; Tritschler, I.; Mittelbronn, M.; Dombrowski, Y.; Breit, S.N.; Tabatabai, G.; Wick, W.; Weller, M.; Wischhusen, J. GDF-15 contributes to proliferation and immune escape of malignant gliomas. Clin. Cancer Res. 2010, 16, 3851–3859. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Z.; Fan, N.; Deng, P.; Huang, Q.; Bruns, C.; Zhao, Y. GDF15 connecting ageing and cancer: Mechanistic insights and therapeutic opportunities. Ageing Cancer Res. Treat. 2025, 2, 202509. [Google Scholar] [CrossRef]
- Brem, S. Vagus nerve stimulation: Novel concept for the treatment of glioblastoma and solid cancers by cytokine (interleukin-6) reduction, attenuating the SASP, enhancing tumor immunity. Brain Behav. Immun.-Health 2024, 42, 100859. [Google Scholar] [CrossRef]
- Eom, Y.; Lee, S.H. Xylene Impairs Neuronal Development by Dysregulating Calcium Homeostasis and Neuronal Activity in Developing Hippocampal Neurons. Biomol. Ther. 2025, 33, 830. [Google Scholar] [CrossRef]
- Carreno, G.; Guiho, R.; Martinez-Barbera, J.P. Cell senescence in neuropathology: A focus on neurodegeneration and tumours. Neuropathol. Appl. Neurobiol. 2021, 47, 359–378. [Google Scholar] [CrossRef]
- Huang, L.; Qi, G.; Chen, G.; Duan, J.; Dai, C.; Lu, Y.; Zhou, Q. Tumor-associated Schwann cells as new therapeutic target in non-neurological cancers. Cancer Lett. 2025, 624, 217748. [Google Scholar] [CrossRef]
- Yin, Y.; Zhou, Y.; Zhou, J.; Zhao, L.; Hu, H.; Xiao, M.; Niu, B.; Peng, J.; Dai, Y.; Tang, Y. Cisplatin causes erectile dysfunction by decreasing endothelial and smooth muscle content and inducing cavernosal nerve senescence in rats. Front. Endocrinol. 2023, 14, 1096723. [Google Scholar] [CrossRef] [PubMed]
- Raynard, C.; Ma, X.; Huna, A.; Tessier, N.; Massemin, A.; Zhu, K.; Flaman, J.m.; Moulin, F.; Goehrig, D.; Medard, J.j. NF-κB-dependent secretome of senescent cells can trigger neuroendocrine transdifferentiation of breast cancer cells. Aging Cell 2022, 21, e13632. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Ngoc, D.T.M.; Choi, J.-H.; Lee, C.-H. Unveiling the neural environment in cancer: Exploring the role of neural circuit players and potential therapeutic strategies. Cells 2023, 12, 1996. [Google Scholar] [CrossRef]
- Lee, C.; Cho, J.; Lee, K. Tumour regression via integrative regulation of neurological, inflammatory, and hypoxic tumour microenvironment. Biomol. Ther. 2020, 28, 119–130. [Google Scholar] [CrossRef]
- Bhat, S.; Adiga, D.; Shukla, V.; Guruprasad, K.P.; Kabekkodu, S.P.; Satyamoorthy, K. Metastatic suppression by DOC2B is mediated by inhibition of epithelial-mesenchymal transition and induction of senescence. Cell Biol. Toxicol. 2022, 38, 237–258. [Google Scholar] [CrossRef]
- Chen, J. The cell-cycle arrest and apoptotic functions of p53 in tumor initiation and progression. Cold Spring Harb. Perspect. Med. 2016, 6, a026104. [Google Scholar] [CrossRef]
- Cho, J. Understanding tumor dormancy: From experimental models to mechanisms and therapeutic strategies. Biomol. Ther. 2025, 33, 770. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, K.; Guo, Y.; Guo, X.; Hou, K.; Hou, J.; Luo, Y.; Liu, J.; Jia, S. Gain-of-Function p53N236S Mutation Drives the Bypassing of HRasV12-Induced Cellular Senescence via PGC–1α. Int. J. Mol. Sci. 2023, 24, 3790. [Google Scholar] [CrossRef]
- Collado, M.; Serrano, M. Senescence in tumours: Evidence from mice and humans. Nat. Rev. Cancer 2010, 10, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Quaas, M.; Steiner, L.; Engeland, K. The p53-p21-DREAM-CDE/CHR pathway regulates G2/M cell cycle genes. Nucleic Acids Res. 2016, 44, 164–174. [Google Scholar] [CrossRef]
- Joruiz, S.M.; Von Muhlinen, N.; Horikawa, I.; Gilbert, M.R.; Harris, C.C. Distinct functions of wild-type and R273H mutant Δ133p53α differentially regulate glioblastoma aggressiveness and therapy-induced senescence. Cell Death Dis. 2024, 15, 454. [Google Scholar] [CrossRef]
- Chen, C.; Chen, J.; Zhang, Y.; Zhang, Q.; Shi, H. Senescence-associated secretory phenotype in lung cancer: Remodeling the tumor microenvironment for metastasis and immune suppression. Front. Oncol. 2025, 15, 1605085. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.K.; Haynes, J.; Collignon, E.; Brown, K.R.; Wang, Y.; Nixon, A.M.; Bruce, J.P.; Wintersinger, J.A.; Mer, A.S.; Lo, E.B. Colorectal cancer cells enter a diapause-like DTP state to survive chemotherapy. Cell 2021, 184, 226–242. e221. [Google Scholar] [CrossRef] [PubMed]
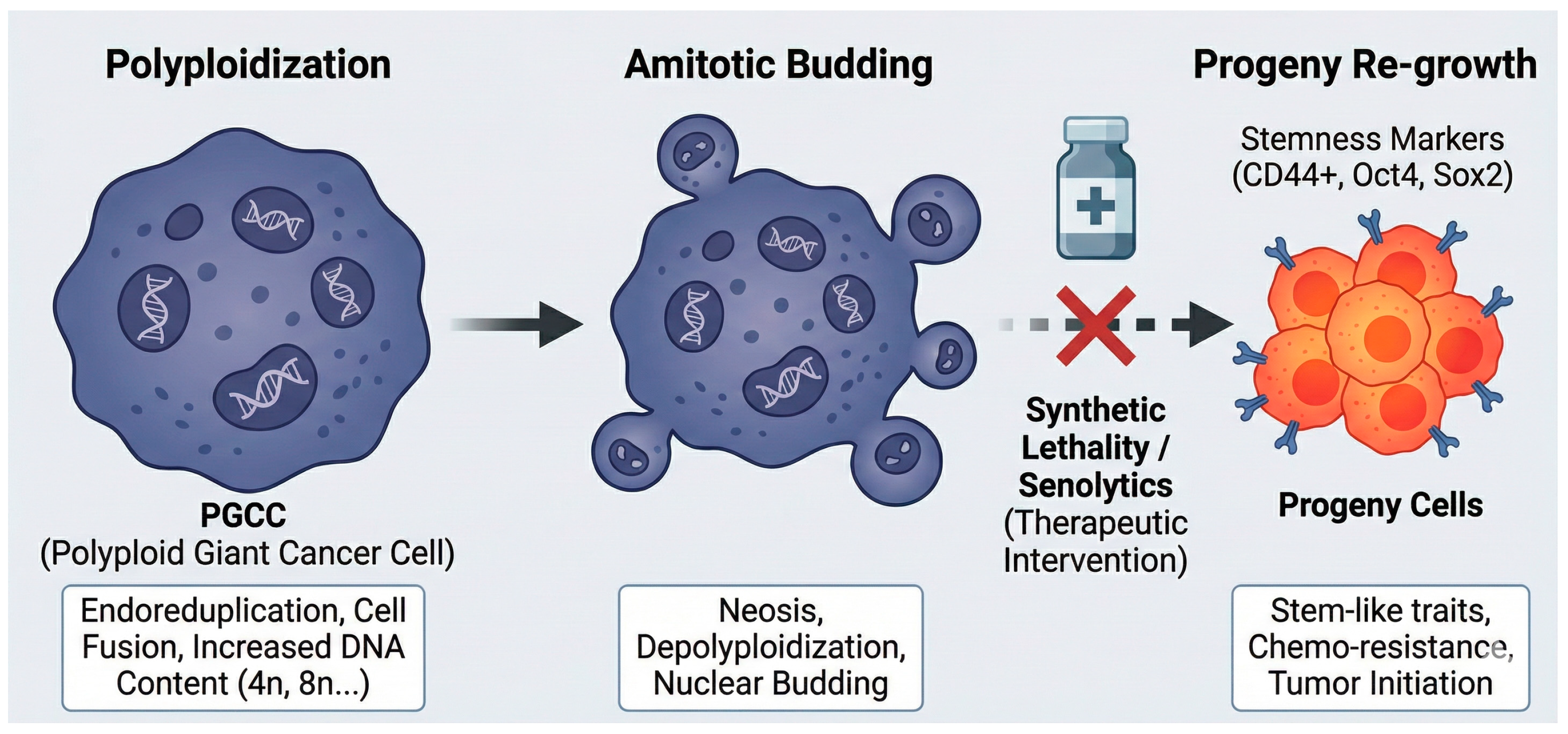
- Zhou, X.; Zhou, M.; Zheng, M.; Tian, S.; Yang, X.; Ning, Y.; Li, Y.; Zhang, S. Polyploid giant cancer cells and cancer progression. Front. Cell Dev. Biol. 2022, 10, 1017588. [Google Scholar] [CrossRef]
- Sikora, E.; Czarnecka-Herok, J.; Bojko, A.; Sunderland, P. Therapy-induced polyploidization and senescence: Coincidence or interconnection? In Seminars in Cancer Biology; Academic Press: New York, NY, USA, 2022; pp. 83–95. [Google Scholar]
- Saleh, T.; Tyutyunyk-Massey, L.; Murray, G.F.; Alotaibi, M.R.; Kawale, A.S.; Elsayed, Z.; Henderson, S.C.; Yakovlev, V.; Elmore, L.W.; Toor, A. Tumor cell escape from therapy-induced senescence. Biochem. Pharmacol. 2019, 162, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-H.; Petty, C.A.; Dixon-McDougall, T.; Lopez, M.V.; Tyshkovskiy, A.; Maybury-Lewis, S.; Tian, X.; Ibrahim, N.; Chen, Z.; Griffin, P.T. Chemically induced reprogramming to reverse cellular aging. Aging 2023, 15, 5966. [Google Scholar] [CrossRef]
- Browder, K.C.; Reddy, P.; Yamamoto, M.; Haghani, A.; Guillen, I.G.; Sahu, S.; Wang, C.; Luque, Y.; Prieto, J.; Shi, L. In vivo partial reprogramming alters age-associated molecular changes during physiological aging in mice. Nat. Aging 2022, 2, 243–253. [Google Scholar] [CrossRef]
- Haraoka, Y.; Akieda, Y.; Nagai, Y.; Mogi, C.; Ishitani, T. Zebrafish imaging reveals TP53 mutation switching oncogene-induced senescence from suppressor to driver in primary tumorigenesis. Nat. Commun. 2022, 13, 1417. [Google Scholar] [CrossRef]
- Mahat, D.B.; Kumra, H.; Castro, S.A.; Metcalf, E.; Nguyen, K.; Morisue, R.; Ho, W.W.; Chen, I.; Sullivan, B.; Yim, L.H. Mutant p53 exploits enhancers to elevate immunosuppressive chemokine expression and impair immune checkpoint inhibitors in pancreatic cancer. Immunity 2025, 58, 1688–1705.e9. [Google Scholar] [CrossRef]
- Liu, K.; Garan, L.A.W.; Lin, F.-T.; Lin, W.-C. Mutant p53 variants differentially impact replication initiation and activate cGAS-STING to affect immune checkpoint inhibition. Commun. Biol. 2025, 8, 1522. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shi, M.; Liu, B.; Zhang, X.; Lin, W.; Yang, Y.; Huang, Z.; Yang, D.; Chu, T.; Zheng, D. Low-dose statins restore innate immune response in breast cancer cells via suppression of mutant p53. Front. Pharmacol. 2025, 16, 1492305. [Google Scholar] [CrossRef]
- Dixon, S.J.; Stockwell, B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014, 10, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Su, L.-J.; Zhang, J.-H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.-Y. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxidative Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef]
- Jung, K.H.; Kim, S.E.; Go, H.G.; Lee, Y.J.; Park, M.S.; Ko, S.; Han, B.S.; Yoon, Y.-C.; Cho, Y.J.; Lee, P. Synergistic renoprotective effect of melatonin and zileuton by inhibition of ferroptosis via the AKT/mTOR/NRF2 signaling in kidney injury and fibrosis. Biomol. Ther. 2023, 31, 599. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, W. p53 in ferroptosis regulation: The new weapon for the old guardian. Cell Death Differ. 2022, 29, 895–910. [Google Scholar] [CrossRef]
- Toledo, F.; Wahl, G.M. MDM2 and MDM4: P53 regulators as targets in anticancer therapy. Int. J. Biochem. Cell Biol. 2007, 39, 1476–1482. [Google Scholar] [CrossRef] [PubMed]
- Vassilev, L.T.; Vu, B.T.; Graves, B.; Carvajal, D.; Podlaski, F.; Filipovic, Z.; Kong, N.; Kammlott, U.; Lukacs, C.; Klein, C. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 2004, 303, 844–848. [Google Scholar] [CrossRef]
- Shangary, S.; Wang, S. Small-molecule inhibitors of the MDM2-p53 protein-protein interaction to reactivate p53 function: A novel approach for cancer therapy. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 223–241. [Google Scholar] [CrossRef]
- Saleh, M.N.; Patel, M.R.; Bauer, T.M.; Goel, S.; Falchook, G.S.; Shapiro, G.I.; Chung, K.Y.; Infante, J.R.; Conry, R.M.; Rabinowits, G. Phase 1 trial of ALRN-6924, a dual inhibitor of MDMX and MDM2, in patients with solid tumors and lymphomas bearing wild-type TP53. Clin. Cancer Res. 2021, 27, 5236–5247. [Google Scholar] [CrossRef]
- Michaeli, O.; Luz, I.; Vatarescu, M.; Manko, T.; Weizman, N.; Korotinsky, Y.; Tsitrina, A.; Braiman, A.; Arazi, L.; Cooks, T. APR-246 as a radiosensitization strategy for mutant p53 cancers treated with alpha-particles-based radiotherapy. Cell Death Dis. 2024, 15, 426. [Google Scholar] [CrossRef]
- Yosef, R.; Pilpel, N.; Tokarsky-Amiel, R.; Biran, A.; Ovadya, Y.; Cohen, S.; Vadai, E.; Dassa, L.; Shahar, E.; Condiotti, R. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat. Commun. 2016, 7, 11190. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Nakamura, K.; Nakagawa-Saito, Y.; Takenouchi, S.; Suzuki, S.; Togashi, K.; Sugai, A.; Mitobe, Y.; Seino, M.; Ohta, T. Senolytic Elimination of Senescent Ovarian Clear Cell Carcinoma Cells Induced by CEP-1347 with the BH3 Mimetic Navitoclax. Anticancer Res. 2025, 45, 4841–4851. [Google Scholar] [CrossRef]
- Osman, A.A.; Monroe, M.M.; Ortega Alves, M.V.; Patel, A.A.; Katsonis, P.; Fitzgerald, A.L.; Neskey, D.M.; Frederick, M.J.; Woo, S.H.; Caulin, C. Wee-1 kinase inhibition overcomes cisplatin resistance associated with high-risk TP53 mutations in head and neck cancer through mitotic arrest followed by senescence. Mol. Cancer Ther. 2015, 14, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.Y.; Liu, Y.; Lu, Y.Q.; Tang, Z.M.; Zhu, Z.X.; Zhu, M.Y.; Chen, H.Y.; Hui, H.; Xu, J.Y.; Li, H. Chidamide Accelerates the Death of Senescence-Like Diffuse Large B-Cell Lymphoma Cells with TP53 Mutation Induced by Doxorubicin. FASEB J. 2025, 39, e71167. [Google Scholar] [CrossRef]
- Schreiber, A.R.; Smoots, S.G.; Jackson, M.M.; Bagby, S.M.; Dus, E.D.; Dominguez, A.T.; Binns, C.A.; Pitts, T.M.; Diamond, J.R. Potentiating doxorubicin activity through BCL-2 inhibition in p53 wild-type and mutated triple-negative breast cancer. Front. Oncol. 2025, 15, 1549282. [Google Scholar] [CrossRef]
- Novais, E.J.; Tran, V.A.; Johnston, S.N.; Darris, K.R.; Roupas, A.J.; Sessions, G.A.; Shapiro, I.M.; Diekman, B.O.; Risbud, M.V. Long-term treatment with senolytic drugs Dasatinib and Quercetin ameliorates age-dependent intervertebral disc degeneration in mice. Nat. Commun. 2021, 12, 5213. [Google Scholar] [CrossRef]
- Patterson, C.M.; Balachander, S.B.; Grant, I.; Pop-Damkov, P.; Kelly, B.; McCoull, W.; Parker, J.; Giannis, M.; Hill, K.J.; Gibbons, F.D. Design and optimisation of dendrimer-conjugated Bcl-2/xL inhibitor, AZD0466, with improved therapeutic index for cancer therapy. Commun. Biol. 2021, 4, 112. [Google Scholar] [CrossRef]
- Kang, D.; Lim, Y.; Ahn, D.; Lee, J.; Park, C.-J. Peptide inhibitors targeting FOXO4-p53 interactions and inducing senescent cancer cell-specific apoptosis. J. Med. Chem. 2025, 68, 15683–15694. [Google Scholar] [CrossRef] [PubMed]
- Peris, I.; Romero-Murillo, S.; Martinez-Balsalobre, E.; Farrington, C.C.; Arriazu, E.; Marcotegui, N.; Jimenez-Munoz, M.; Alburquerque-Prieto, C.; Torres-Lopez, A.; Fresquet, V.; et al. Activation of the PP2A-B56alpha heterocomplex synergizes with venetoclax therapies in AML through BCL2 and MCL1 modulation. Blood 2023, 141, 1047–1059. [Google Scholar] [CrossRef]
- Ma, Z.; Zhou, J. NDA submission of vepdegestrant (ARV-471) to US FDA: The beginning of a new era of PROTAC degraders. J. Med. Chem. 2025, 68, 14129–14136. [Google Scholar]
- He, Y.; Zhang, X.; Chang, J.; Kim, H.-N.; Zhang, P.; Wang, Y.; Khan, S.; Liu, X.; Zhang, X.; Lv, D. Using proteolysis-targeting chimera technology to reduce navitoclax platelet toxicity and improve its senolytic activity. Nat. Commun. 2020, 11, 1996. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, W.; Lv, D.; Zhang, X.; Zhang, X.; Ortiz, Y.T.; Budamagunta, V.; Campisi, J.; Zheng, G.; Zhou, D. Inhibition of USP7 activity selectively eliminates senescent cells in part via restoration of p53 activity. Aging Cell 2020, 19, e13117. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Wang, Z.; Li, H.; Wang, D.; Wu, Z.; Bai, F.; Wang, Q.; Luo, W.; Zhang, G.; Xiong, Y. USP7 inhibition promotes early osseointegration in senile osteoporotic mice. J. Dent. Res. 2025, 104, 86–96. [Google Scholar] [CrossRef]
- Korenev, G.; Yakukhnov, S.; Druk, A.; Golovina, A.; Chasov, V.; Mirgayazova, R.; Ivanov, R.; Bulatov, E. USP7 inhibitors in cancer immunotherapy: Current status and perspective. Cancers 2022, 14, 5539. [Google Scholar] [CrossRef]
- Yang, Y.; Jn-Simon, N.; He, Y.; Sun, C.; Zhang, P.; Hu, W.; Tian, T.; Zeng, H.; Basha, S.; Huerta, A.S. A BCL-xL/BCL-2 PROTAC effectively clears senescent cells in the liver and reduces MASH-driven hepatocellular carcinoma in mice. Nat. Aging 2025, 5, 386–400. [Google Scholar] [CrossRef]
- González-Gualda, E.; Pàez-Ribes, M.; Lozano-Torres, B.; Macias, D.; Wilson, J.R., III; González-López, C.; Ou, H.L.; Mirón-Barroso, S.; Zhang, Z.; Lérida-Viso, A. Galacto-conjugation of Navitoclax as an efficient strategy to increase senolytic specificity and reduce platelet toxicity. Aging Cell 2020, 19, e13142. [Google Scholar] [CrossRef]
- Laberge, R.-M.; Sun, Y.; Orjalo, A.V.; Patil, C.K.; Freund, A.; Zhou, L.; Curran, S.C.; Davalos, A.R.; Wilson-Edell, K.A.; Liu, S. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat. Cell Biol. 2015, 17, 1049–1061, Erratum in: Nat. Cell Biol. 2021, 23, 564–565. https://doi.org/10.1038/s41556-021-00655-4. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, M.; Tchkonia, T.; Ding, H.; Ogrodnik, M.; Lubbers, E.R.; Pirtskhalava, T.; White, T.A.; Johnson, K.O.; Stout, M.B.; Mezera, V. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc. Natl. Acad. Sci. USA 2015, 112, E6301–E6310. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.; Scuoppo, C.; Wang, X.; Fang, X.; Balgley, B.; Bolden, J.E.; Premsrirut, P.; Luo, W.; Chicas, A.; Lee, C.S. Control of the senescence-associated secretory phenotype by NF-κB promotes senescence and enhances chemosensitivity. Genes. Dev. 2011, 25, 2125–2136. [Google Scholar] [CrossRef]
- Wang, T.; Liu, W.; Shen, Q.; Tao, R.; Li, C.; Shen, Q.; Lin, Y.; Huang, Y.; Yang, L.; Xie, G. Combination of PARP inhibitor and CDK4/6 inhibitor modulates cGAS/STING-dependent therapy-induced senescence and provides “one-two punch” opportunity with anti-PD-L1 therapy in colorectal cancer. Cancer Sci. 2023, 114, 4184–4201. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Chen, Y.; Yang, X.; Wang, Y.; He, J.; Wang, T.; Fan, Q.; Deng, L.; Tu, J.; Tan, H. Mitotic SENP3 activation couples with cGAS signaling in tumor cells to stimulate anti-tumor immunity. Cell Death Dis. 2022, 13, 640. [Google Scholar] [CrossRef]
- Ruscetti, M.; Leibold, J.; Bott, M.J.; Fennell, M.; Kulick, A.; Salgado, N.R.; Chen, C.-C.; Ho, Y.-J.; Sanchez-Rivera, F.J.; Feucht, J. NK cell–mediated cytotoxicity contributes to tumor control by a cytostatic drug combination. Science 2018, 362, 1416–1422. [Google Scholar] [CrossRef]
- Amor, C.; Feucht, J.; Leibold, J.; Ho, Y.-J.; Zhu, C.; Alonso-Curbelo, D.; Mansilla-Soto, J.; Boyer, J.A.; Li, X.; Giavridis, T. Senolytic CAR T cells reverse senescence-associated pathologies. Nature 2020, 583, 127–132, Erratum in: Nature 2024, 627, E9. https://doi.org/10.1038/s41586-024-07197-3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Srihari, S.; Singla, J.; Wong, L.; Ragan, M.A. Inferring synthetic lethal interactions from mutual exclusivity of genetic events in cancer. Biol. Direct 2015, 10, 57. [Google Scholar] [CrossRef]
- Wang, T.; Tang, T.; Jiang, Y.; He, T.; Qi, L.; Chang, H.; Qiao, Y.; Sun, M.; Shan, C.; Zhu, X. PRIM2 promotes cell cycle and tumor progression in p53-mutant lung cancer. Cancers 2022, 14, 3370. [Google Scholar] [CrossRef]
- Folly-Kossi, H.; Graves, J.D.; Garan, L.A.W.; Lin, F.-T.; Lin, W.-C. DNA2 nuclease inhibition confers synthetic lethality in cancers with mutant p53 and synergizes with PARP inhibitors. Cancer Res. Commun. 2023, 3, 2096–2112. [Google Scholar] [CrossRef]
- DeNicola, G.M.; Karreth, F.A.; Humpton, T.J.; Gopinathan, A.; Wei, C.; Frese, K.; Mangal, D.; Yu, K.H.; Yeo, C.J.; Calhoun, E.S. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 2011, 475, 106–109. [Google Scholar] [CrossRef]
- Cummings, S.R.; Lui, L.-Y.; Zaira, A.; Mau, T.; Fielding, R.A.; Atkinson, E.J.; Patel, S.; LeBrasseur, N. Biomarkers of cellular senescence and major health outcomes in older adults. Geroscience 2025, 47, 3407–3415, Erratum in: Geroscience 2025, 47, 6117. https://doi.org/10.1007/s11357-025-01619-4. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- St. Sauver, J.L.; Weston, S.A.; Atkinson, E.J.; Mc Gree, M.E.; Mielke, M.M.; White, T.A.; Heeren, A.A.; Olson, J.E.; Rocca, W.A.; Palmer, A.K. Biomarkers of cellular senescence and risk of death in humans. Aging Cell 2023, 22, e14006. [Google Scholar] [CrossRef] [PubMed]
- Farr, J.N.; Monroe, D.G.; Atkinson, E.J.; Froemming, M.N.; Ruan, M.; LeBrasseur, N.K.; Khosla, S. Characterization of human senescent cell biomarkers for clinical trials. Aging Cell 2025, 24, e14489. [Google Scholar] [CrossRef]
- Fukumoto, T.; Shimosawa, T.; Yakabe, M.; Yoshida, S.; Yoshida, Y. Recent advances in biomarkers for senescence: Bridging basic research to clinic. Geriatr. Gerontol. Int. 2025, 25, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.A.; Atkinson, E.J.; Aversa, Z.; White, T.A.; Heeren, A.A.; Achenbach, S.J.; Mielke, M.M.; Cummings, S.R.; Pahor, M.; Leeuwenburgh, C. Associations between biomarkers of cellular senescence and physical function in humans: Observations from the lifestyle interventions for elders (LIFE) study. Geroscience 2022, 44, 2757–2770. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Lee, C.H.; Minh Nguyen, T.; Lee, Y.; Choi, S.G.; Nguyen, P.N.; Park, J.H.; Park, M.K. Therapy-Induced Senescence (TIS) and SASP: The p53-Mediated Interplay in Cancer Progression and Treatment. Int. J. Mol. Sci. 2026, 27, 357. https://doi.org/10.3390/ijms27010357
Lee CH, Minh Nguyen T, Lee Y, Choi SG, Nguyen PN, Park JH, Park MK. Therapy-Induced Senescence (TIS) and SASP: The p53-Mediated Interplay in Cancer Progression and Treatment. International Journal of Molecular Sciences. 2026; 27(1):357. https://doi.org/10.3390/ijms27010357
Chicago/Turabian StyleLee, Chang Hoon, Tuan Minh Nguyen, Yongook Lee, Seoung Gyu Choi, Phuong Ngan Nguyen, Jung Ho Park, and Mi Kyung Park. 2026. "Therapy-Induced Senescence (TIS) and SASP: The p53-Mediated Interplay in Cancer Progression and Treatment" International Journal of Molecular Sciences 27, no. 1: 357. https://doi.org/10.3390/ijms27010357
APA StyleLee, C. H., Minh Nguyen, T., Lee, Y., Choi, S. G., Nguyen, P. N., Park, J. H., & Park, M. K. (2026). Therapy-Induced Senescence (TIS) and SASP: The p53-Mediated Interplay in Cancer Progression and Treatment. International Journal of Molecular Sciences, 27(1), 357. https://doi.org/10.3390/ijms27010357






