Synthesis and Characterization of Bioactive Oligoitaconates with Amino Acid Functional Groups for Tissue Engineering
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
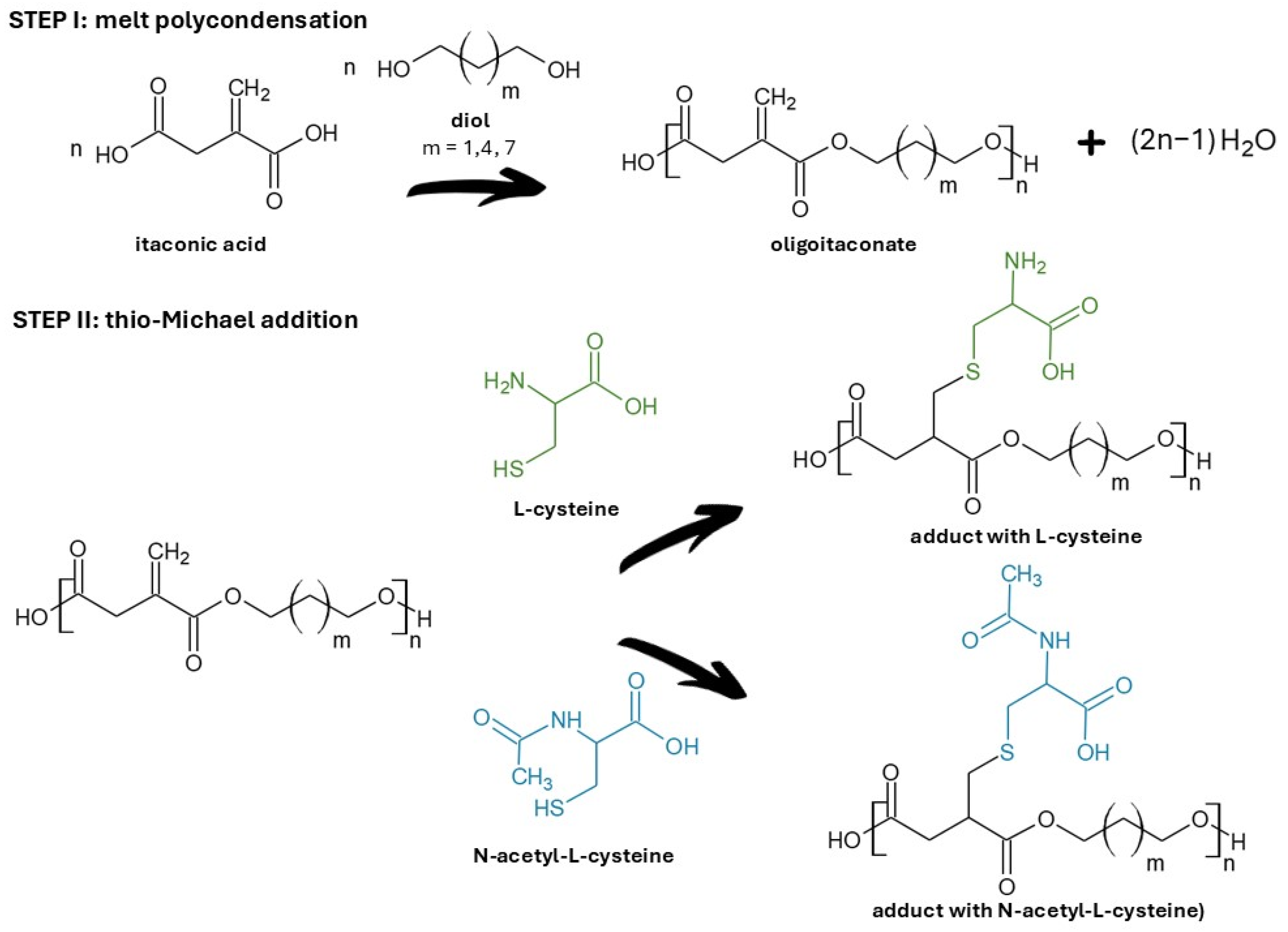
4.2. Oligoester Synthesis
4.3. Thio-Michael Addition
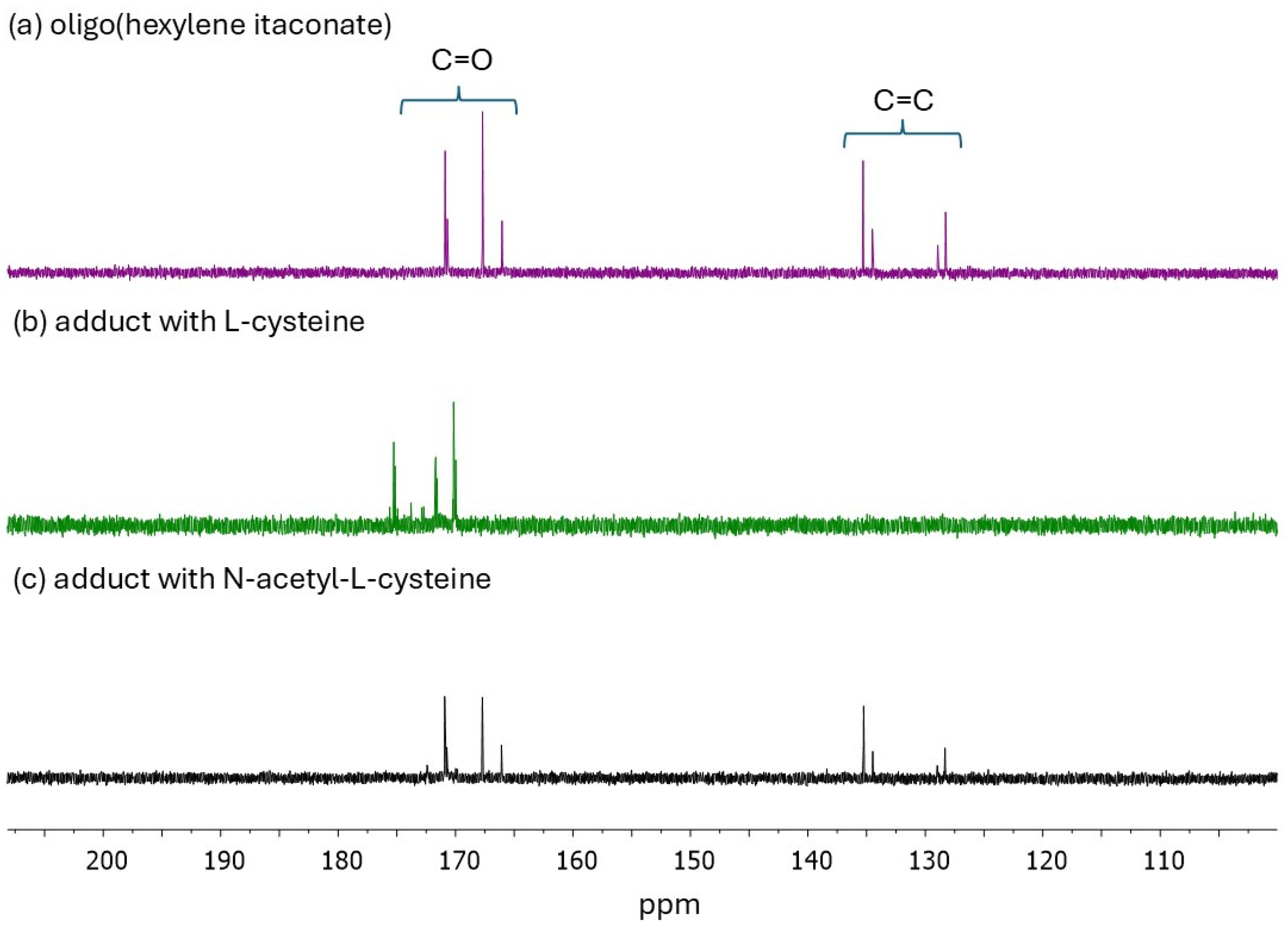
4.4. Nuclear Magnetic Resonance (NMR)
- —summarized value of the integral signals from double bond protons of oligoitaconate,
- —the integral value of the signal from mesaconic acid,
- —summarized value of the integral signals from the double bond protons of the unreacted itaconic acid,
- —summarized value of the integral signals from the Ordelts reaction,
- —the integral value of the signal from the itaconic acid radical polymerization.
- —number of moles of t-BuOH used for NMR analysis (mol),
- —summarized value of the integrals from double bond protons,
- —the value of the integral of the signal from the CH3- group of t-BuOH,
- —mass of oligomer sample (g).
- —double bond content in 100 g of oligoester before modification (molC=C/100 g),
- —double bond content in 100 g of oligoester after modification (molC=C/100 g).
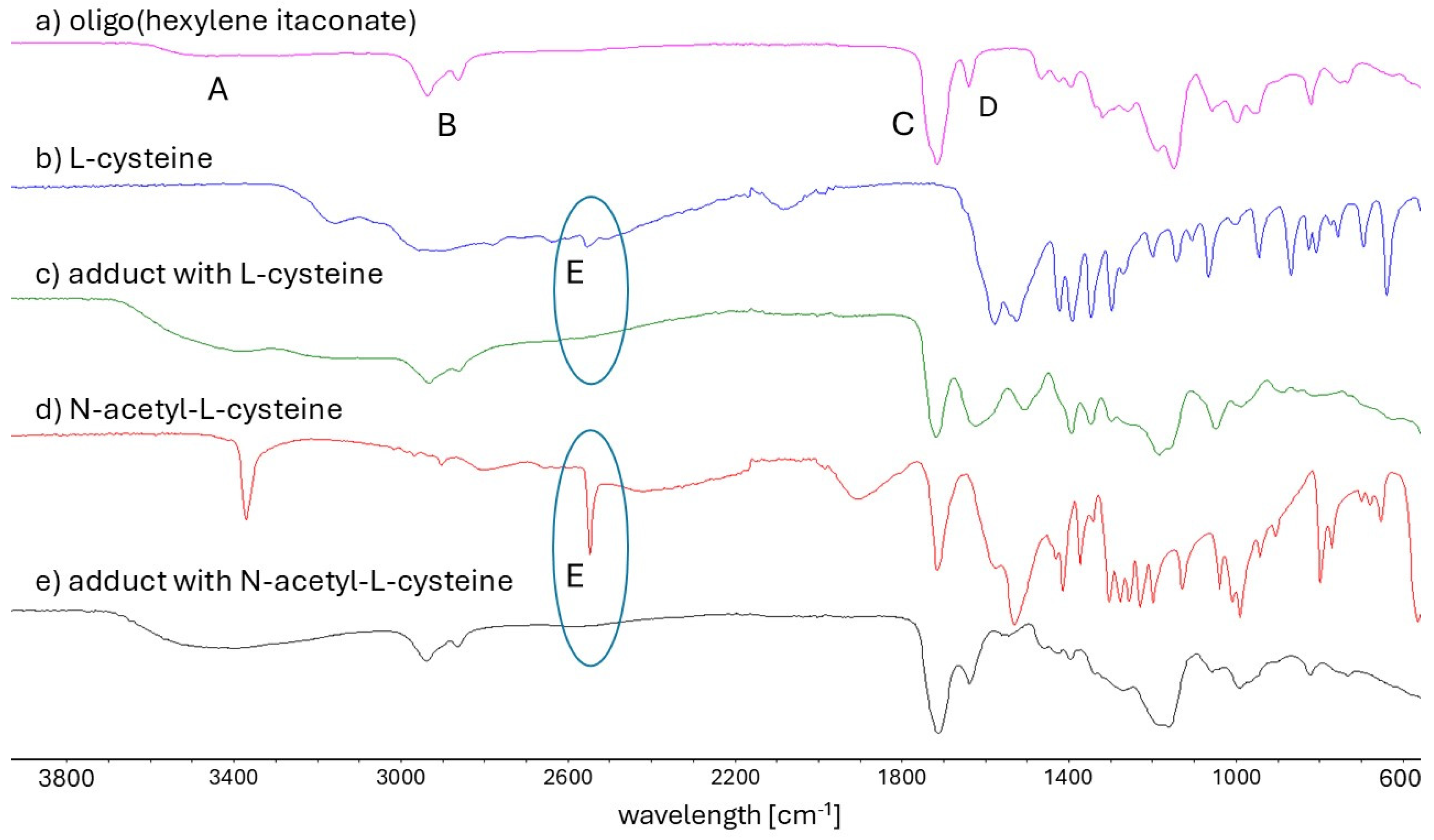
4.5. Fourier Transform Infrared Spectroscopy (FT-IR)
4.6. Gel Permeation Chromatography/Size Exclusion Chromatography (GPC/SEC)
4.7. Acid Number (AN)
- —the volume of 1 M NaOH solution used for sample titration (cm3),
- —the volume of 1 M NaOH solution used for blank titration (cm3),
- —the concentration of the titration solution (1 M),
- —mass of oligomer sample (g).
4.8. Ester Number (EN)
- —the volume of 1 M HCl solution used for sample titration (cm3),
- —the volume of 1 M HCl solution used for blank titration (cm3),
- —the concentration of the titration solution (1 M),
- —mass of oligomer sample (g),
- —acid number (mgKOH/g).
4.9. Esterification Degree (ED)
- —acid number (mgKOH/g).
- —ester number (mgKOH/g).
4.10. Differential Scanning Calorimetry (DSC)
4.11. Thermogravimetry (TG)
4.12. Solubility
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A1 | Adduct with L-cysteine |
| A2 | Adduct with N-acetyl-L-cysteine |
| AD | Addition degree |
| AN | Acid number |
| Cys | L-cysteine |
| DSC | Differential Scanning Calorimetry |
| DMF | Dimethylformamide |
| DMSO | Dimethyl sulfoxide |
| ED | Esterification degree |
| EN | Ester number |
| FT-IR | Fourier Transform Infrared Spectroscopy |
| GPC | Gas Permeation Chromatography |
| HDO | 1,6-hexanediol |
| IA | Itaconic acid |
| MW | Molecular weight |
| NAC | N-acetyl-L-cysteine |
| NDO | 1,9-nonanediol |
| NIPS | non-solvent-induced phase separation |
| NMR | Nuclear magnetic resonance |
| PDO | 1,3-propanediol |
| OHI | oligo(hexylene itaconate) |
| PNI | oligo(nonylene itaconate) |
| OPI | oligo(propylene itaconate) |
| SEC | Size Exclusion Chromatography |
| t-BuOH | tert-butanol |
| Td | Decomposition temperature |
| TG | Thermogravimetry |
| THF | Tetrahydrofuran |
| Tg | Glass transition temperature |
| Đ | polydispersity |
| xDB | double bond content in 100 g of polymer |
References
- Gnanasekaran, R.; Dhandapani, B.; Gopinath, K.P.; Iyyappan, J. Synthesis of Itaconic Acid from Agricultural Waste Using Novel Aspergillus niveus. Prep. Biochem. Biotechnol. 2018, 48, 605–609. [Google Scholar] [CrossRef]
- Okabe, M.; Lies, D.; Kanamasa, S.; Park, E.Y. Biotechnological Production of Itaconic Acid and Its Biosynthesis in Aspergillus terreus. Appl. Microbiol. Biotechnol. 2009, 84, 597–606. [Google Scholar] [CrossRef]
- Steiger, M.G.; Blumhoff, M.L.; Mattanovich, D.; Sauer, M. Biochemistry of Microbial Itaconic Acid Production. Front. Microbiol. 2013, 4, 23. [Google Scholar] [CrossRef]
- Wierckx, N.; Agrimi, G.; Lübeck, P.S.; Steiger, M.G.; Mira, N.P.; Punt, P.J. Metabolic Specialization in Itaconic Acid Production: A Tale of Two Fungi. Curr. Opin. Biotechnol. 2020, 62, 153–159. [Google Scholar] [CrossRef]
- Strelko, C.L.; Lu, W.; Dufort, F.J.; Seyfried, T.N.; Chiles, T.C.; Rabinowitz, J.D.; Roberts, M.F. Itaconic Acid Is a Mammalian Metabolite Induced during Macrophage Activation. J. Am. Chem. Soc. 2011, 133, 16386–16389. [Google Scholar] [CrossRef]
- Li, P.; Ma, S.; Dai, J.; Liu, X.; Jiang, Y.; Wang, S.; Wei, J.; Chen, J.; Zhu, J. Itaconic Acid as a Green Alternative to Acrylic Acid for Producing a Soybean Oil-Based Thermoset: Synthesis and Properties. ACS Sustain. Chem. Eng. 2017, 5, 1228–1236. [Google Scholar] [CrossRef]
- Willke, T.; Vorlop, K.D. Biotechnological Production of Itaconic Acid. Appl. Microbiol. Biotechnol. 2001, 56, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Robert, T.; Friebel, S. Itaconic Acid—A Versatile Building Block for Renewable Polyesters with Enhanced Functionality. Green Chem. 2016, 18, 2922–2934. [Google Scholar] [CrossRef]
- Saini, N.; Singh, S.; Manickam, S.; Cruz-Martins, N.; Kumar, V.; Verma, R.; Kumar, D. Itaconic Acid and Its Applications for Textile, Pharma and Agro-Industrial Purposes. Sustainability 2022, 14, 13777. [Google Scholar] [CrossRef]
- Perković, I.; Beus, M.; Schols, D.; Persoons, L.; Zorc, B. Itaconic Acid Hybrids as Potential Anticancer Agents. Mol. Divers. 2022, 26, 1–14. [Google Scholar] [CrossRef]
- Ernst, P.; Zlati, F.; Kever, L.; Wirtz, A.; Goldbaum, R.; Pietruszka, J.; Wynands, B.; Frunzke, J.; Wierckx, N. Selective Production of the Itaconic Acid-Derived Compounds 2-Hydroxyparaconic and Itatartaric Acid. Metab. Eng. Commun. 2024, 19, e00252. [Google Scholar] [CrossRef]
- Sethy, B.; Hsieh, C.-F.; Lin, T.-J.; Hu, P.-Y.; Chen, Y.-L.; Lin, C.-Y.; Tseng, S.-N.; Horng, J.-T.; Hsieh, P.-W. Design, Synthesis, and Biological Evaluation of Itaconic Acid Derivatives as Potential Anti-Influenza Agents. J. Med. Chem. 2019, 62, 2390–2403. [Google Scholar] [CrossRef]
- Zhu, X.; Guo, Y.; Liu, Z.; Yang, J.; Tang, H.; Wang, Y. Itaconic Acid Exerts Anti-Inflammatory and Antibacterial Effects via Promoting Pentose Phosphate Pathway to Produce ROS. Sci. Rep. 2021, 11, 18173. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Alfaro, A.C.; Young, T.; Green, S.; Zarate, E.; Merien, F. Itaconic Acid Inhibits Growth of a Pathogenic Marine Vibrio Strain: A Metabolomics Approach. Sci. Rep. 2019, 9, 5937. [Google Scholar] [CrossRef]
- Luan, H.H.; Medzhitov, R. Food Fight: Role of Itaconate and Other Metabolites in Antimicrobial Defense. Cell Metab. 2016, 24, 379–387. [Google Scholar] [CrossRef]
- Veerabagu, U.; Jaikumar, G.; Fushen, L. A Facile Synthesis of Itaconic Acid Based Biodegradable Co-Polyesters: An In-Vitro Anticancer Evaluation and Controlled Drug Delivery System. J. Polym. Environ. 2019, 27, 2756–2768. [Google Scholar] [CrossRef]
- Marcelo, G.; Ferreira, I.C.; Viveiros, R.; Casimiro, T. Development of Itaconic Acid-Based Molecular Imprinted Polymers Using Supercritical Fluid Technology for pH-Triggered Drug Delivery. Int. J. Pharm. 2018, 542, 125–131. [Google Scholar] [CrossRef]
- Vuong, M.D.L.; Haouas, M.; Ural, M.S.; Desmaële, D.; Martineau-Corcos, C.; Gref, R. Degradation of Polymer-Drug Conjugate Nanoparticles Based on Lactic and Itaconic Acid. Int. J. Mol. Sci. 2022, 23, 14461. [Google Scholar] [CrossRef] [PubMed]
- Ranjha, N.; Mudassir, J.; Akhtar, N. Methyl Methacrylate-Co-Itaconic Acid (MMA-Co-IA) Hydrogels for Controlled Drug Delivery. J. Sol-Gel Sci. Technol. 2008, 47, 23–30. [Google Scholar] [CrossRef]
- Razzaq, R.; Ranjha, N.; Rashid, Z.; Nasir, B. Preparation and Evaluation of Novel pH-Sensitive Poly(Butyl Acrylate-co-itaconic Acid) Hydrogel Microspheres for Controlled Drug Delivery. Adv. Polym. Technol. 2016, 37, 247–255. [Google Scholar] [CrossRef]
- Shi, X.; Zhou, H.; Wei, J.; Mo, W.; Li, Q.; Lv, X. The Signaling Pathways and Therapeutic Potential of Itaconate to Alleviate Inflammation and Oxidative Stress in Inflammatory Diseases. Redox Biol. 2022, 58, 102553. [Google Scholar] [CrossRef] [PubMed]
- Cordes, T.; Michelucci, A.; Hiller, K. Itaconic Acid: The Surprising Role of an Industrial Compound as a Mammalian Antimicrobial Metabolite. Annu. Rev. Nutr. 2015, 35, 451–473. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, R.; Zahedi, P.; Goodarzi, V. Application of Poly(Glycerol Itaconic Acid) (PGIt) and Poly(ɛ-Caprolactone) Diol (PCL-Diol) as Macro Crosslinkers Containing Cloisite Na+ to Application in Tissue Engineering. J. Polym. Environ. 2024, 32, 3392–3406. [Google Scholar] [CrossRef]
- Miętus, M.; Kolankowski, K.; Gołofit, T.; Denis, P.; Bandzerewicz, A.; Spychalski, M.; Mąkosa-Szczygieł, M.; Pilarek, M.; Wierzchowski, K.; Gadomska-Gajadhur, A. From Poly(Glycerol Itaconate) Gels to Novel Nonwoven Materials for Biomedical Applications. Gels 2023, 9, 788. [Google Scholar] [CrossRef]
- Potorac, S.; Popa, M.; Verestiuc, L.; Le Cerf, D. New Semi-IPN Scaffolds Based on HEMA and Collagen Modified with Itaconic Anhydride. Mater. Lett. 2012, 67, 95–98. [Google Scholar] [CrossRef]
- Kolankowski, K.; Miętus, M.; Ruśkowski, P.; Gadomska-Gajadhur, A. Optimisation of Glycerol and Itaconic Anhydride Polycondensation. Molecules 2022, 27, 4627. [Google Scholar] [CrossRef]
- Arunprasert, K.; Pornpitchanarong, C.; Rojanarata, T.; Ngawhirunpat, T.; Opanasopit, P.; Patrojanasophon, P. Mussel-Inspired Poly(Hydroxyethyl Acrylate-Co-Itaconic Acid)-Catechol/Hyaluronic Acid Drug-in-Adhesive Patches for Transdermal Delivery of Ketoprofen. Int. J. Pharm. 2022, 629, 122362. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, T.; Newland, B.; Liu, W.; Wang, W.; Wang, W. Catechol Functionalized Hyperbranched Polymers as Biomedical Materials. Prog. Polym. Sci. 2018, 78, 47–55. [Google Scholar] [CrossRef]
- Zou, Y.; Yan, F.; Tong, R.; Mo, M.; Li, Z. Progress in the Design Principle of Biomedical Tissue Adhesive Hydrogel with Naturally Derived Substance. J. Polym. Sci. 2024, 62, 4341–4359. [Google Scholar] [CrossRef]
- Ding, Y.; Yu, L.; Mao, Z. Adhesion Mechanisms and Design Strategies for Bioadhesives. Colloid Interface Sci. Commun. 2024, 63, 100809. [Google Scholar] [CrossRef]
- Raos, G.; Zappone, B. Polymer Adhesion: Seeking New Solutions for an Old Problem. Macromolecules 2021, 54, 10617–10644. [Google Scholar] [CrossRef]
- Bayramgil, P.N. Grafting of Hydrophilic Monomers Onto Cellulosic Polymers for Medical Applications. In Biopolym. Grafting; Thakur, V.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 81–114. [Google Scholar]
- Chen, Q.; Zhang, D.; Zhang, W.; Zhang, H.; Zou, J.; Chen, M.; Li, J.; Yuan, Y.; Liu, R. Dual Mechanism β-Amino Acid Polymers Promoting Cell Adhesion. Nat. Commun. 2021, 12, 562. [Google Scholar] [CrossRef]
- Klimek, K.; Ginalska, G. Proteins and Peptides as Important Modifiers of the Polymer Scaffolds for Tissue Engineering Applications—A Review. Polymers 2020, 12, 844. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.J.; Stupp, S.I. Poly(Amino Acid) Bioadhesives for Tissue Repair. J. Biomater. Sci. Polym. Ed. 2000, 11, 1023–1038. [Google Scholar] [CrossRef]
- Zhu, S.; Dou, W.; Zeng, X.; Chen, X.; Gao, Y.; Liu, H.; Li, S. Recent Advances in the Degradability and Applications of Tissue Adhesives Based on Biodegradable Polymers. Int. J. Mol. Sci. 2024, 25, 5249. [Google Scholar] [CrossRef]
- Chrószcz-Porębska, M.; Gadomska-Gajadhur, A. Cysteine Conjugation: An Approach to Obtain Polymers with Enhanced Muco- and Tissue Adhesion. Int. J. Mol. Sci. 2024, 25, 12177. [Google Scholar] [CrossRef]
- Otasevic, V.; Korac, B. Amino Acids: Metabolism. In Encyclopedia of Food and Health; Caballeo, B., Fingals, P.M., Toldrá, F., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 149–155. [Google Scholar]
- Goddard, J.M.; Hotchkiss, J.H. Polymer Surface Modification for the Attachment of Bioactive Compounds. Prog. Polym. Sci. 2007, 32, 698–725. [Google Scholar] [CrossRef]
- Guarnei, A.; Cutifani, V.; Cespugli, M.; Pellis, A.; Vassallo, R.; Asaro, F.; Ebert, C.; Gardossi, L. Functionalization of Enzymatically Synthesized Rigid Poly(itaconate)s via Post-Polymerization Aza-Michael Addition of Primary Amines. Adv. Synth. Catal. 2019, 361, 2559–2573. [Google Scholar] [CrossRef]
- Farmer, T.J.; Macuarie, J.W.; Comerford, W.; Pellis, A.; Clark, J.H. Insights Into Post-Polymerisation Modification of Bio-Based Unsaturated Itaconate and Fumarate Polyesters via aza-Michael Addition: Understanding the Effects of C=C Isomerization. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 1935–1945. [Google Scholar] [CrossRef] [PubMed]
- Moore, O.B.; Hanson, P.A.; Comerford, J.W.; Pellis, A.; Farme, T.J. Improving the Post-polymerization Modification of Bio-Based Itaconate Unsaturated Polyesters: Catalyzing Aza-Michael Additions with Reusable Iodine on Acidic Alumina. Front. Chem. 2019, 7, 501. [Google Scholar] [CrossRef] [PubMed]
- Chanda, S.; Ramakrishnan, S. Poly(alkylene itaconate)s—An Interesting Class of Polyesters with Periodically Located Exo-Chain Double Bonds Susceptible to Michael Addition. Polym. Chem. 2015, 6, 2108–2114. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, S.; Lu, W.; Wang, Y.; Zhang, P.; Yao, Q. Electrospun Fibers and Their Application in Drug Controlled Release, Biological Dressings, Tissue Repair, and Enzyme Immobilization. RSC Adv. 2019, 9, 25712–25729. [Google Scholar] [CrossRef]
- Wang, X.; Pellerin, C.; Bazuin, C.G. Enhancing the Electrospinnability of Low Molecular Weight Polymers Using Small Effective Cross-Linkers. Macromolecules 2016, 49, 891–899. [Google Scholar] [CrossRef]
- Saquing, C.D.; Tang, C.; Monian, B.; Bonino, C.A.; Manasco, J.L.; Alsberg, E.; Khan, S.A. Alginate–Polyethylene Oxide Blend Nanofibers and the Role of the Carrier Polymer in Electrospinning. Ind. Eng. Chem. Res. 2013, 52, 8692–8704. [Google Scholar] [CrossRef]
- Syed, M.H.; Khan, M.M.R.; Zahari, M.A.K.M.; Beg, M.D.H.; Abdullah, N. Current Issues and Potential Solutions for the Electrospinning of Major Polysaccharides and Proteins: A Review. Int. J. Biol. Macromol. 2023, 253, 126735. [Google Scholar] [CrossRef]
- Perez-Puyana, V.M.; Romero, A.; Guerrero, A.; Moroni, L.; Wieringa, P.A. Enabling Low Molecular Weight Electrospinning through Binary Solutions of Polymer Blends. Next Mater. 2025, 6, 100306. [Google Scholar] [CrossRef]
- Zhang, M.; June, S.M.; Long, T.E. Principles of Step-Growth Polymerization (Polycondensation and Polyaddition). In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Moller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 7–47. [Google Scholar]
- Jackson, P.A.; Widen, J.C.; Harki, D.A.; Brummond, K.M. Covalent Modifiers: A Chemical Perspective on the Reactivity of α,β-Unsaturated Carbonyls with Thiols via Hetero-Michael Addition Reactions. J. Med. Chem. 2017, 60, 839–885. [Google Scholar] [CrossRef]
- Xie, R.; Weisen, A.R.; Lee, Y.; Aplan, M.A.; Fenton, A.M.; Masucci, A.E.; Kempe, F.; Sommer, M.; Pester, C.W.; Colby, R.H.; et al. Glass Transition Temperature from the Chemical Structure of Conjugated Polymers. Nat. Commun. 2020, 11, 893. [Google Scholar] [CrossRef]
- Brady, J.; Dürig, T.; Lee, P.I.; Li, J.-X. Polymer Properties and Characterization. In Developing Solid Oral Dosage Forms; Qiu, Y., Chen, Y., Zhang, G.G.Z., Yu, L., Mantri, R.V., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 181–223. [Google Scholar]
- Chen, W.; Karde, V.; Cheng, T.N.H.; Ramli, S.S.; Heng, J.Y.Y. Surface Hydrophobicity: Effect of Alkyl Chain Length and Network Homogeneity. Front. Chem. Sci. Eng. 2021, 15, 90–98. [Google Scholar] [CrossRef]
- Puka-Sundvall, M.; Eriksson, P.; Nilsson, M.; Sandberg, M.; Lehmann, A. Neurotoxicity of Cysteine: Interaction with Glutamate. Brain Res. 1995, 705, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Clemente Plaza, N.; Reig García-Galbis, M.; Martínez-Espinosa, R.M. Effects of the Usage of L-Cysteine (l-Cys) on Human Health. Mol. J. Synth. Chem. Nat. Prod. Chem. 2018, 23, 575. [Google Scholar] [CrossRef] [PubMed]
- Dang, A.; Hui, C.M.; Ferebee, R.; Kubiak, J.; Li, T.; Matyjaszewski, K.; Bockstaller, M.R. Thermal Properties of Particle Brush Materials: Effect of Polymer Graft Architecture on the Glass Transition Temperature in Polymer-Grafted Colloidal Systems. Macromol. Symp. 2013, 331–332, 9–16. [Google Scholar] [CrossRef]
- Fang, M.; Wang, K.; Lu, H.; Yang, Y.; Nutt, S. Single-Layer Graphene Nanosheets with Controlled Grafting of Polymer Chains. J. Mater. Chem. 2010, 20, 1982–1992. [Google Scholar] [CrossRef]
- Baljon, A.R.C.; Van Weert, M.H.M.; DeGraaff, R.B.; Khare, R. Glass Transition Behavior of Polymer Films of Nanoscopic Dimensions. Macromolecules 2005, 38, 2391–2399. [Google Scholar] [CrossRef]
- Pokorný, V.; Štejfa, V.; Havlín, J.; Fulem, M.; Růžička, K. Heat Capacities of L-Cysteine, L-Serine, L-Threonine, L-Lysine, and L-Methionine. Molecules 2023, 28, 451. [Google Scholar] [CrossRef]
- Mahumane, G.D.; Kumar, P.; Pillay, V.; Choonara, Y.E. Repositioning N-Acetylcysteine (NAC): NAC-Loaded Electrospun Drug Delivery Scaffolding for Potential Neural Tissue Engineering Application. Pharmaceutics 2020, 12, 934. [Google Scholar] [CrossRef]



| Sample | AN (mgKOH/g) | EN (mgKOH/g) | ED (%) | MW | ||||
|---|---|---|---|---|---|---|---|---|
| avg | SD | avg | SD | avg | SD | MW (g/mol) | Đ | |
| oligo(propylene itaconate) OPI | 193 | 12 | 691 | 42 | 88 a,b | 3 | 562 | 1.50 |
| oligo(hexylene itaconate) OHI | 129 a | 21 | 509 | 39 | 85 a,c | 3 | 690 | 1.51 |
| oligo(nonylene itaconate) ONI | 122 a | 11 | 448 | 20 | 88 b,c | 2 | 960 | 1.84 |
| Sample | Td (°C) | Tg (°C) | |||
|---|---|---|---|---|---|
| Td5% | Td30% | Td50% | Td85% | ||
| OPI | 182 | 263 | 360 | 420 | −38 |
| OHI | 170 | 283 | 381 | 422 | −53 |
| ONI | 214 | 331 | 396 | 427 | −50 |
| OPIA1 | 98 | 204 | 310 | 433 | −21 |
| OHIA1 | 125 | 205 | 305 | 401 | −22 |
| ONIA1 | 70 | 246 | 351 | 415 | −55 |
| OPIA2 | 73 | 244 | 331 | 418 | −25 |
| OHIA2 | 76 | 261 | 357 | 418 | −31 |
| ONIA2 | 134 | 285 | 371 | 418 | −44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Chrószcz-Porębska, M.; Waśkiewicz, S.; Gołofit, T.; Gadomska-Gajadhur, A. Synthesis and Characterization of Bioactive Oligoitaconates with Amino Acid Functional Groups for Tissue Engineering. Int. J. Mol. Sci. 2026, 27, 324. https://doi.org/10.3390/ijms27010324
Chrószcz-Porębska M, Waśkiewicz S, Gołofit T, Gadomska-Gajadhur A. Synthesis and Characterization of Bioactive Oligoitaconates with Amino Acid Functional Groups for Tissue Engineering. International Journal of Molecular Sciences. 2026; 27(1):324. https://doi.org/10.3390/ijms27010324
Chicago/Turabian StyleChrószcz-Porębska, Marta, Sylwia Waśkiewicz, Tomasz Gołofit, and Agnieszka Gadomska-Gajadhur. 2026. "Synthesis and Characterization of Bioactive Oligoitaconates with Amino Acid Functional Groups for Tissue Engineering" International Journal of Molecular Sciences 27, no. 1: 324. https://doi.org/10.3390/ijms27010324
APA StyleChrószcz-Porębska, M., Waśkiewicz, S., Gołofit, T., & Gadomska-Gajadhur, A. (2026). Synthesis and Characterization of Bioactive Oligoitaconates with Amino Acid Functional Groups for Tissue Engineering. International Journal of Molecular Sciences, 27(1), 324. https://doi.org/10.3390/ijms27010324








