Marine- and Plant-Based Nanoemulsion Platforms Enhance the Anticancer Activity of Curcumin In Vitro
Abstract
1. Introduction
2. Results and Discussion
2.1. Lipid Classes
2.2. Fatty Acid Composition of Oils and Lecithins
2.3. Solubility Test of Curcumin in Oils
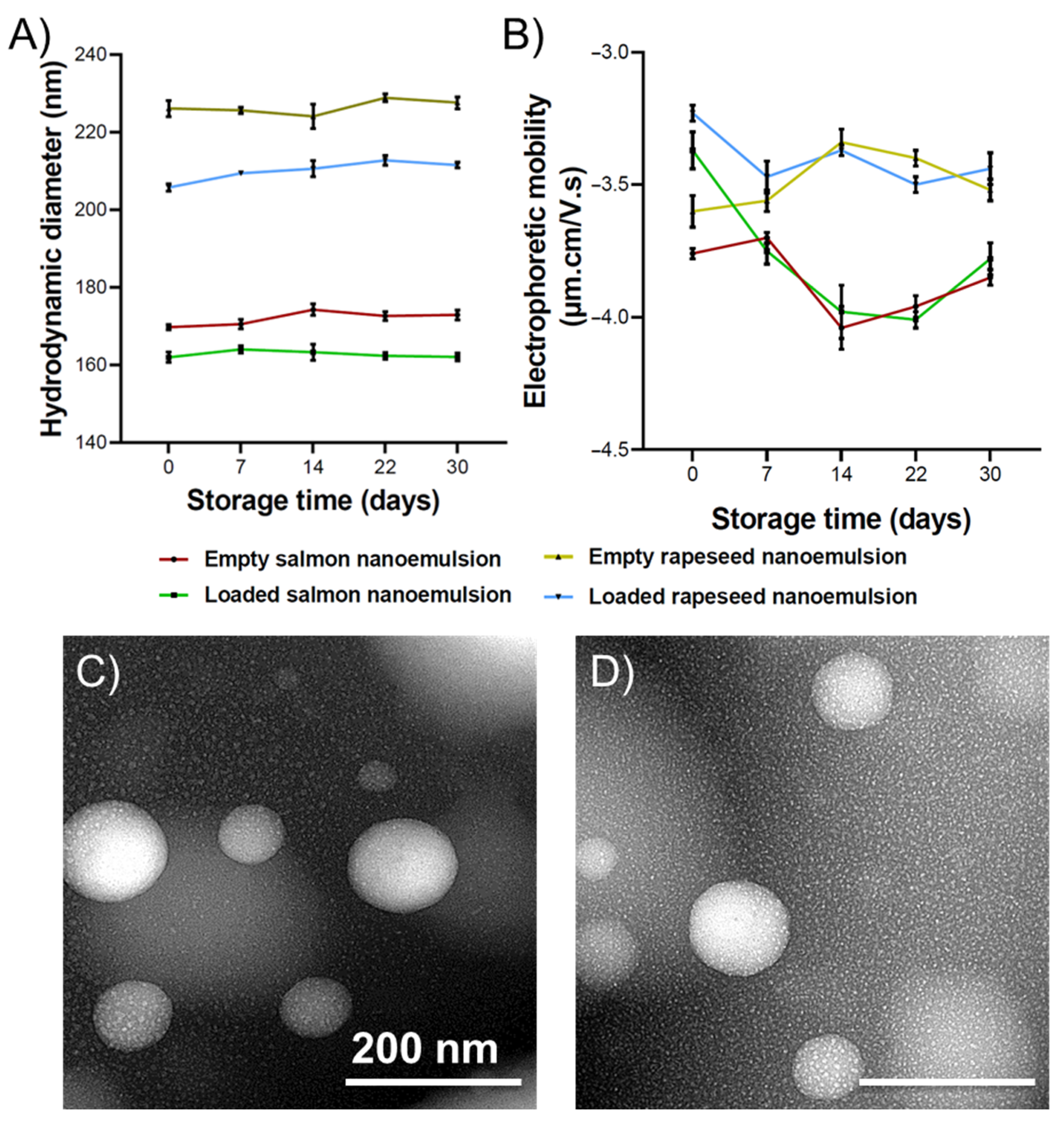
2.4. Physicochemical and Morphological Properties of Nanoemulsions
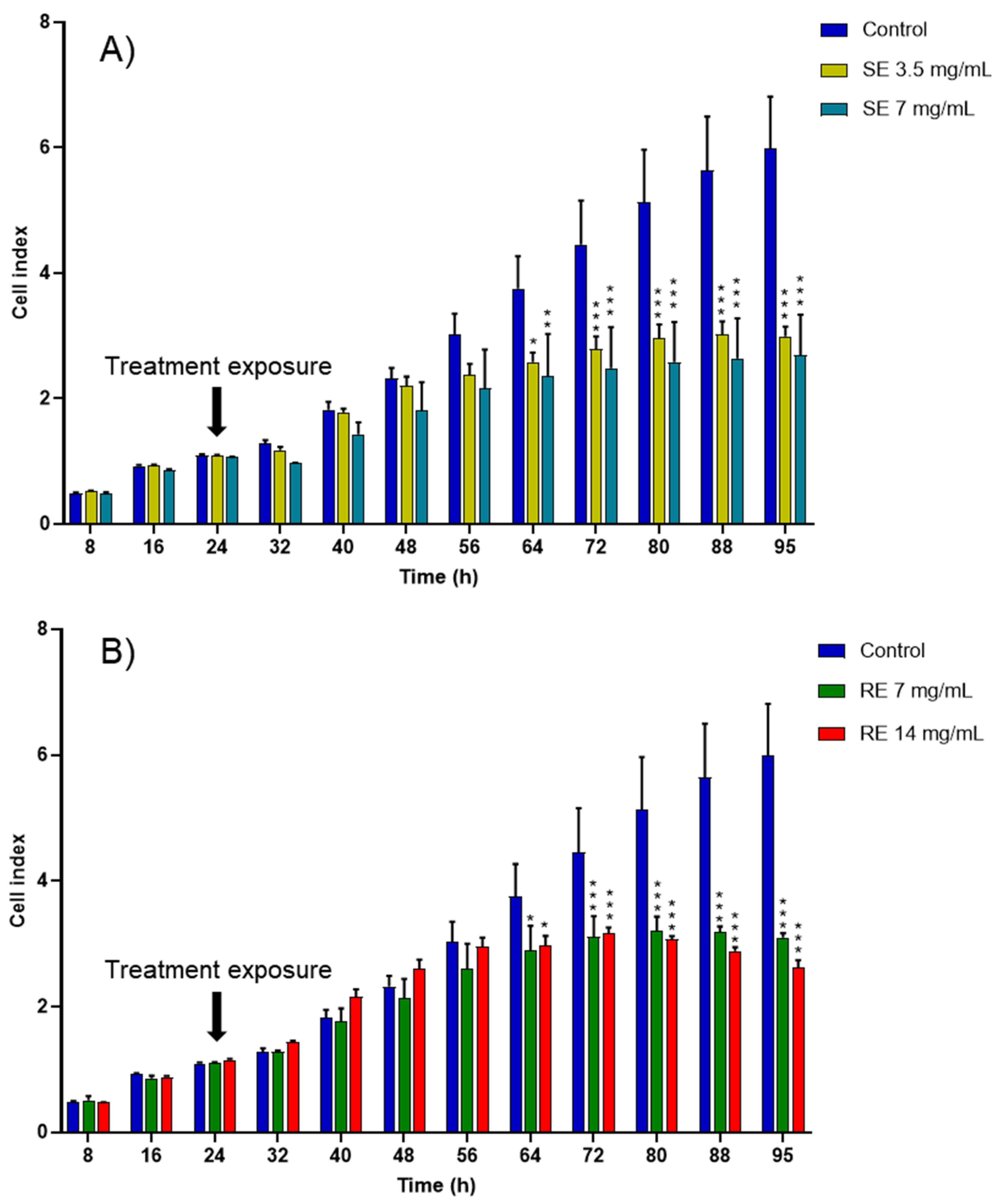
2.5. Cytotoxicity Analysis
3. Materials and Methods
3.1. Lipid Classes
- -
- Neutral lipids: cholesterol, tripalmitin, 1.2-dipalmitoyl-snglycerol, and 1-monostearoyl-rac-glycerol.
- -
- Phospholipids: sphingomyelin, lyso-phosphatidylcholine, L-a-phosphatidylinositol, L-a-phosphatidyl-L-serine, 3 sn-phosphatidyleth-anolamine, and L-a-phosphatidylcholine.
3.2. Fatty Acids Composition
3.3. Curcumin Solubility in Rapeseed and Salmon Oils
3.4. Preparation of Differents Nanoemulsions Containing Curcumin
3.5. The Size and Electrophoretic Mobility of Nanoemulsions
3.6. The Stability of Nanoemulsion
3.7. Transmission Electron Microscopy
3.8. In Vitro Evaluation of the Anticancer Activity
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rehman, M.; Tahir, N.; Sohail, M.F.; Qadri, M.U.; Duarte, S.O.D.; Brandão, P.; Esteves, T.; Javed, I.; Fonte, P. Lipid-Based Nanoformulations for Drug Delivery: An Ongoing Perspective. Pharmaceutics 2024, 16, 1376. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Kather, F.S.; Boddu, S.H.S.; Shah, J.; Nair, A.B. Innovations in Nanoemulsion Technology: Enhancing Drug Delivery for Oral, Parenteral, and Ophthalmic Applications. Pharmaceutics 2024, 16, 1333. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Noh, Y.; McClements, D.J.; Choi, S.J. Impact of Hydrophilic Substances on Ostwald Ripening in Emulsions Stabilized by Varied Hydrophilic Group Surfactants. Npj Sci. Food 2024, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Tanuku, S.; Velisila, D.; Thatraju, D.; Vadaga, A.K. Nanoemulsion Formulation Strategies for Enhanced Drug Delivery: Review Article. J. Pharma Insights Res. 2024, 2, 125–138. [Google Scholar] [CrossRef]
- Izadiyan, Z.; Webster, T.; Kia, P.; Kalantari, K.; Misran, M.; Rasouli, E.; Maghareh Esfahan, Z.; Shameli, K. Nanoemulsions Based Therapeutic Strategies: Enhancing Targeted Drug Delivery against Breast Cancer Cells. Int. J. Nanomed. 2025, 20, 6133–6162. [Google Scholar] [CrossRef]
- Mehmood, T.; Ahmad, A.; Ahmed, A.; Ahmed, Z. Optimization of Olive Oil Based O/W Nanoemulsions Prepared through Ultrasonic Homogenization: A Response Surface Methodology Approach. Food Chem. 2017, 229, 790–796. [Google Scholar] [CrossRef]
- Patil, J.; Pawde, D.M.; Bhattacharya, S.; Srivastava, S. Phospholipid Complex Formulation Technology for Improved Drug Delivery in Oncological Settings: A Comprehensive Review. AAPS PharmSciTech 2024, 25, 91. [Google Scholar] [CrossRef]
- Hennebelle, M.; Villeneuve, P.; Durand, E.; Lecomte, J.; Van Duynhoven, J.; Meynier, A.; Yesiltas, B.; Jacobsen, C.; Berton-Carabin, C. Lipid Oxidation in Emulsions: New Insights from the Past Two Decades. Prog. Lipid Res. 2024, 94, 101275. [Google Scholar] [CrossRef]
- Omidvari, E.; Samandari, M.; Ghanbariamin, D.; Mollocana Lara, E.C.; Quint, J.; Saeedinejad, F.; Bouizi, Y.; Bouguet-Bonnet, S.; Elkhoury, K.; Sanchez-Gonzalez, L.; et al. Nanoliposome Functionalized Colloidal GelMA Inks for 3D Printing of Scaffolds with Multiscale Porosity. Biofabrication 2024, 17, 015039. [Google Scholar] [CrossRef]
- Linder, M.; Matouba, E.; Fanni, J.; Parmentier, M. Enrichment of Salmon Oil with N-3 PUFA by Lipolysis, Filtration and Enzymatic Re-Esterification. Eur. J. Lipid Sci. Technol. 2002, 104, 455–462. [Google Scholar] [CrossRef]
- Suárez, E.R.; Mugford, P.F.; Rolle, A.J.; Burton, I.W.; Walter, J.A.; Kralovec, J.A. 13C-NMR Regioisomeric Analysis of EPA and DHA in Fish Oil Derived Triacylglycerol Concentrates. J. Am. Oil Chem. Soc. 2010, 87, 1425–1433. [Google Scholar] [CrossRef]
- Lauritzen, L. The Essentiality of Long Chain N-3 Fatty Acids in Relation to Development and Function of the Brain and Retina. Prog. Lipid Res. 2001, 40, 1–94. [Google Scholar] [CrossRef] [PubMed]
- Belhaj, N.; Arab-Tehrany, E.; Linder, M. Oxidative Kinetics of Salmon Oil in Bulk and in Nanoemulsion Stabilized by Marine Lecithin. Process Biochem. 2010, 45, 187–195. [Google Scholar] [CrossRef]
- Li, J.; Elkhoury, K.; Barbieux, C.; Linder, M.; Grandemange, S.; Tamayol, A.; Francius, G.; Arab-Tehrany, E. Effects of Bioactive Marine-Derived Liposomes on Two Human Breast Cancer Cell Lines. Mar. Drugs 2020, 18, 211. [Google Scholar] [CrossRef]
- Coonrod, D.; Brick, M.A.; Byrne, P.F.; DeBonte, L.; Chen, Z. Inheritance of Long Chain Fatty Acid Content in Rapeseed (Brassica napus L.). Euphytica 2008, 164, 583–592. [Google Scholar] [CrossRef]
- Bianchi, A.; Velot, É.; Kempf, H.; Elkhoury, K.; Sanchez-Gonzalez, L.; Linder, M.; Kahn, C.; Arab-Tehrany, E. Nanoliposomes from Agro-Resources as Promising Delivery Systems for Chondrocytes. Int. J. Mol. Sci. 2020, 21, 3436. [Google Scholar] [CrossRef]
- Velot, É.; Elkhoury, K.; Kahn, C.; Kempf, H.; Linder, M.; Arab-Tehrany, E.; Bianchi, A. Efficient TGF-Β1 Delivery to Articular Chondrocytes In Vitro Using Agro-Based Liposomes. Int. J. Mol. Sci. 2022, 23, 2864. [Google Scholar] [CrossRef]
- Rapti, E.; Adamantidi, T.; Efthymiopoulos, P.; Kyzas, G.Z.; Tsoupras, A. Potential Applications of the Anti-Inflammatory, Antithrombotic and Antioxidant Health-Promoting Properties of Curcumin: A Critical Review. Nutraceuticals 2024, 4, 562–595. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Mohammed, D.M.; Alkafaas, S.S.; Ghosh, S.; Negm, S.H.; Salem, H.M.; Fahmy, M.A.; Mosa, W.F.A.; Ibrahim, E.H.; et al. Curcumin, an Active Component of Turmeric: Biological Activities, Nutritional Aspects, Immunological, Bioavailability, and Human Health Benefits—A Comprehensive Review. Front. Immunol. 2025, 16, 1603018. [Google Scholar] [CrossRef]
- Gülseren, İ.; Guri, A.; Corredig, M. Effect of Interfacial Composition on Uptake of Curcumin–Piperine Mixtures in Oil in Water Emulsions by Caco-2 Cells. Food Funct. 2014, 5, 1218. [Google Scholar] [CrossRef]
- Metzler, M.; Pfeiffer, E.; Schulz, S.I.; Dempe, J.S. Curcumin Uptake and Metabolism. BioFactors 2013, 39, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Kather, F.; Morsy, M.; Boddu, S.; Attimarad, M.; Shah, J.; Shinu, P.; Nair, A. Advances in Nanocarrier Systems for Overcoming Formulation Challenges of Curcumin: Current Insights. Nanomaterials 2024, 14, 672. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.; Li, Y.; McClements, D.J.; Xiao, H. Nanoemulsion- and Emulsion-Based Delivery Systems for Curcumin: Encapsulation and Release Properties. Food Chem. 2012, 132, 799–807. [Google Scholar] [CrossRef]
- Neslihan Gursoy, R.; Benita, S. Self-Emulsifying Drug Delivery Systems (SEDDS) for Improved Oral Delivery of Lipophilic Drugs. Biomed. Pharmacother. 2004, 58, 173–182. [Google Scholar] [CrossRef]
- Hasan, M.; Belhaj, N.; Benachour, H.; Barberi-Heyob, M.; Kahn, C.J.F.; Jabbari, E.; Linder, M.; Arab-Tehrany, E. Liposome Encapsulation of Curcumin: Physico-Chemical Characterizations and Effects on MCF7 Cancer Cell Proliferation. Int. J. Pharm. 2014, 461, 519–528. [Google Scholar] [CrossRef]
- Benedet, J.A.; Umeda, H.; Shibamoto, T. Antioxidant Activity of Flavonoids Isolated from Young Green Barley Leaves toward Biological Lipid Samples. J. Agric. Food Chem. 2007, 55, 5499–5504. [Google Scholar] [CrossRef]
- Kabri, T.; Arab-Tehrany, E.; Belhaj, N.; Linder, M. Physico-Chemical Characterization of Nano-Emulsions in Cosmetic Matrix Enriched on Omega-3. J. Nanobiotechnol. 2011, 9, 41. [Google Scholar] [CrossRef]
- Small, D.M. Lateral Chain Packing in Lipids and Membranes. J. Lipid Res. 1984, 25, 1490–1500. [Google Scholar] [CrossRef]
- Mazzarino, L.; Travelet, C.; Ortega-Murillo, S.; Otsuka, I.; Pignot-Paintrand, I.; Lemos-Senna, E.; Borsali, R. Elaboration of Chitosan-Coated Nanoparticles Loaded with Curcumin for Mucoadhesive Applications. J. Colloid Interface Sci. 2012, 370, 58–66. [Google Scholar] [CrossRef]
- Nirmala, R.; Park, H.-M.; Navamathavan, R.; Kang, H.-S.; El-Newehy, M.H.; Kim, H.Y. Lecithin Blended Polyamide-6 High Aspect Ratio Nanofiber Scaffolds via Electrospinning for Human Osteoblast Cell Culture. Mater. Sci. Eng. C 2011, 31, 486–493. [Google Scholar] [CrossRef]
- Moussa, Z.; Chebl, M.; Patra, D. Interaction of Curcumin with 1,2-Dioctadecanoyl-Sn-Glycero-3-Phosphocholine Liposomes: Intercalation of Rhamnolipids Enhances Membrane Fluidity, Permeability and Stability of Drug Molecule. Colloids Surf. B Biointerfaces 2017, 149, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Karewicz, A.; Bielska, D.; Gzyl-Malcher, B.; Kepczynski, M.; Lach, R.; Nowakowska, M. Interaction of Curcumin with Lipid Monolayers and Liposomal Bilayers. Colloids Surf. B Biointerfaces 2011, 88, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Civelek, N.; Bilge, D. Investigating the Molecular Effects of Curcumin by Using Model Membranes. Food Biophys. 2022, 17, 232–247. [Google Scholar] [CrossRef]
- Zweers, M.L.T.; Grijpma, D.W.; Engbers, G.H.M.; Feijen, J. The Preparation of Monodisperse Biodegradable Polyester Nanoparticles with a Controlled Size. J. Biomed. Mater. Res. B Appl. Biomater. 2003, 66B, 559–566. [Google Scholar] [CrossRef]
- Yen, F.-L.; Wu, T.-H.; Lin, L.-T.; Cham, T.-M.; Lin, C.-C. Nanoparticles Formulation of Cuscuta Chinensis Prevents Acetaminophen-Induced Hepatotoxicity in Rats. Food Chem. Toxicol. 2008, 46, 1771–1777. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, Y.; Wang, Y.-W.; Huang, M.-T.; Ho, C.-T.; Huang, Q. Enhancing Anti-Inflammation Activity of Curcumin through O/W Nanoemulsions. Food Chem. 2008, 108, 419–424. [Google Scholar] [CrossRef]
- Mehta, K.; Pantazis, P.; McQueen, T.; Aggarwal, B.B. Antiproliferative Effect of Curcumin (Diferuloylmethane) against Human Breast Tumor Cell Lines: Anticancer. Drugs 1997, 8, 470–481. [Google Scholar] [CrossRef]
- Kuo, M.-L.; Huang, T.-S.; Lin, J.-K. Curcumin, an Antioxidant and Anti-Tumor Promoter, Induces Apoptosis in Human Leukemia Cells. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 1996, 1317, 95–100. [Google Scholar] [CrossRef]
- Er, E.; Oliver, L.; Cartron, P.-F.; Juin, P.; Manon, S.; Vallette, F.M. Mitochondria as the Target of the Pro-Apoptotic Protein Bax. Biochim. Biophys. Acta BBA-Bioenerg. 2006, 1757, 1301–1311. [Google Scholar] [CrossRef]
- Murphy, K.M.; Ranganathan, V.; Farnsworth, M.L.; Kavallaris, M.; Lock, R.B. Bcl-2 Inhibits Bax Translocation from Cytosol to Mitochondria during Drug-Induced Apoptosis of Human Tumor Cells. Cell Death Differ. 2000, 7, 102–111. [Google Scholar] [CrossRef]
- Ahmad, I.; Ahmad, S.; Samad, M.A.; Adam, A.M.; Zughaibi, T.A.; Alhosin, M.; Shakil, S.; Khan, M.S.; Alsaieedi, A.A.; Kumer, A.; et al. Synergistic Inhibition of Colon Cancer Cell Proliferation via P53, Bax, and Bcl-2 Modulation by Curcumin and Plumbagin Combination. ACS Omega 2025, 10, 19045–19060. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wanming, D.; Zhang, D.; Liu, Q.; Kang, J. Water-Soluble Antioxidants Improve the Antioxidant and Anticancer Activity of Low Concentrations of Curcumin in Human Leukemia Cells. Pharmazie 2005, 60, 57–61. [Google Scholar] [PubMed]
- Sandur, S.K.; Ichikawa, H.; Pandey, M.K.; Kunnumakkara, A.B.; Sung, B.; Sethi, G.; Aggarwal, B.B. Role of Pro-Oxidants and Antioxidants in the Anti-Inflammatory and Apoptotic Effects of Curcumin (Diferuloylmethane). Free Radic. Biol. Med. 2007, 43, 568–580. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Ahmad, S.; Ahmad, A.; Zughaibi, T.A.; Alhosin, M.; Tabrez, S. Curcumin, Its Derivatives, and Their Nanoformulations: Revolutionizing Cancer Treatment. Cell Biochem. Funct. 2024, 42, e3911. [Google Scholar] [CrossRef]
- Kunwar, A.; Barik, A.; Mishra, B.; Rathinasamy, K.; Pandey, R.; Priyadarsini, K.I. Quantitative Cellular Uptake, Localization and Cytotoxicity of Curcumin in Normal and Tumor Cells. Biochim. Biophys. Acta BBA-Gen. Subj. 2008, 1780, 673–679. [Google Scholar] [CrossRef]
- Colquhoun, A.; Schumacher, R.I. γ-Linolenic Acid and Eicosapentaenoic Acid Induce Modifications in Mitochondrial Metabolism, Reactive Oxygen Species Generation, Lipid Peroxidation and Apoptosis in Walker 256 Rat Carcinosarcoma Cells. Biochim. Biophys. Acta BBA-Mol. Cell Biol. Lipids 2001, 1533, 207–219. [Google Scholar] [CrossRef]
- Ding, W.-Q.; Vaught, J.L.; Yamauchi, H.; Lind, S.E. Differential Sensitivity of Cancer Cells to Docosahexaenoic Acid-Induced Cytotoxicity: The Potential Importance of down-Regulation of Superoxide Dismutase 1 Expression. Mol. Cancer Ther. 2004, 3, 1109–1117. [Google Scholar] [CrossRef]
- Hong, M.Y. Fish Oil Increases Mitochondrial Phospholipid Unsaturation, Upregulating Reactive Oxygen Species and Apoptosis in Rat Colonocytes. Carcinogenesis 2002, 23, 1919–1926. [Google Scholar] [CrossRef]
- Rose, D. Effects of Dietary Fatty Acids on Breast and Prostate Cancers: Evidence from in Vitro Experiments and Animal Studies. Am. J. Clin. Nutr. 1997, 66, 1513S–1522S. [Google Scholar] [CrossRef]
- Gerber, M. Background Review Paper on Total Fat, Fatty Acid Intake and Cancers. Ann. Nutr. Metab. 2009, 55, 140–161. [Google Scholar] [CrossRef]
- Terry, P.D.; Terry, J.B.; Rohan, T.E. Long-Chain (n-3) Fatty Acid Intake and Risk of Cancers of the Breast and the Prostate: Recent Epidemiological Studies, Biological Mechanisms, and Directions for Future Research. J. Nutr. 2004, 134, 3412S–3420S. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Kumlin, M.; Ingelman-Sundberg, M.; Wolk, A. Dietary Long-Chain N−3 Fatty Acids for the Prevention of Cancer: A Review of Potential Mechanisms. Am. J. Clin. Nutr. 2004, 79, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Nagendraprabhu, P.; Sudhandiran, G. Astaxanthin Inhibits Tumor Invasion by Decreasing Extracellular Matrix Production and Induces Apoptosis in Experimental Rat Colon Carcinogenesis by Modulating the Expressions of ERK-2, NFkB and COX-2. Investig. New Drugs 2011, 29, 207–224. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhang, J.; Wang, M.; Liu, W.; Gu, X.; Lv, C. Astaxanthin Induces Mitochondria-Mediated Apoptosis in Rat Hepatocellular Carcinoma CBRH-7919 Cells. Biol. Pharm. Bull. 2011, 34, 839–844. [Google Scholar] [CrossRef]
- Tripathi, D.N.; Jena, G.B. Astaxanthin Intervention Ameliorates Cyclophosphamide-Induced Oxidative Stress, DNA Damage and Early Hepatocarcinogenesis in Rat: Role of Nrf2, P53, P38 and Phase-II Enzymes. Mutat. Res. Toxicol. Environ. Mutagen. 2010, 696, 69–80. [Google Scholar] [CrossRef]
- Anuchapreeda, S.; Fukumori, Y.; Okonogi, S.; Ichikawa, H. Preparation of Lipid Nanoemulsions Incorporating Curcumin for Cancer Therapy. J. Nanotechnol. 2012, 2012, 270383. [Google Scholar] [CrossRef]
- Schultze, E.; Coradini, K.; Dos Santos Chaves, P.; Da Silva, L.P.; Buss, J.; Guterres, S.S.; Collares, T.; Beck, R.C.R.; Pohlmann, A.R.; Seixas, F.K. Drug-Loaded Nanoemulsion as Positive Control Is an Alternative to DMSO Solutions for in Vitro Evaluation of Curcumin Delivery to MCF-7 Cells. Pharmacol. Rep. 2017, 69, 1408–1412. [Google Scholar] [CrossRef]
- Ravindran, J.; Prasad, S.; Aggarwal, B.B. Curcumin and Cancer Cells: How Many Ways Can Curry Kill Tumor Cells Selectively? AAPS J. 2009, 11, 495–510. [Google Scholar] [CrossRef]
- Morrison, W.R.; Smith, L.M. Preparation of Fatty Acid Methyl Esters and Dimethylacetals from Lipids with Boron Fluoride–Methanol. J. Lipid Res. 1964, 5, 600–608. [Google Scholar] [CrossRef]
- Khan, N.; Kurnik-Łucka, M.; Kudrycka, M.; Gil, K.; Latacz, G. Optimization of Impedance-Based Real-Time Assay in xCELLigence RTCA SP16 Device for the Analysis of Fully Differentiated Caco-2 Cells. Appl. Sci. 2025, 15, 8298. [Google Scholar] [CrossRef]




| Fatty Acids | Salmon Oil | Rapeseed Oil | Salmon Lecithin | Rapeseed Lecithin | ||||
|---|---|---|---|---|---|---|---|---|
| % | SD | % | SD | % | SD | % | SD | |
| C14 | 3.87 | 0.03 | - | - | 1.60 | 0.01 | - | - |
| C16 | 12.67 | 0.13 | 4.54 | 0.00 | 16.14 | 0.09 | 7.65 | 0.02 |
| C18 | 3.21 | 0.02 | 1.45 | 0.00 | 4.68 | 0.02 | 1.46 | 0.01 |
| C21 | 0.30 | 0.30 | - | - | 1.93 | 0.02 | - | - |
| C22 | - | - | - | - | 0.78 | 0.29 | 0.29 | 0.29 |
| C23 | - | - | - | - | 1.18 | 0.07 | - | - |
| SFA | 20.05 | - | 5.99 | - | 26.31 | - | 9.4 | - |
| C16:1 | 4.29 | 0.02 | 0.25 | 0.00 | 1.54 | 0.06 | 0.29 | 0.00 |
| C17:1 | 0.39 | 0.01 | - | - | 1.20 | 0.01 | - | - |
| C18:1n9 | 36.36 | 0.23 | 63.02 | 0.06 | 19.96 | 0.30 | 53.75 | 0.23 |
| C20:1n11 | 5.17 | 0.12 | 1.21 | 0.01 | 0.42 | 0.10 | 0.68 | 0.01 |
| MUFA | 46.21 | - | 64.48 | - | 23.12 | - | 54.72 | - |
| C18:2n6 | 11.46 | 0.18 | 18.81 | 0.07 | 5.81 | 0.07 | 27.95 | 0.06 |
| C18:3n3 | 4.24 | 0.08 | 9.22 | 0.03 | 2.70 | 0.02 | 6.54 | 0.09 |
| C20:2n6 | 1.52 | 0.02 | 0.25 | 0.02 | 0.29 | 0.03 | 0.17 | 0.02 |
| C20:3n6 | 0.27 | 0.00 | - | - | 0.30 | 0.02 | - | - |
| C20:3n3 | 0.40 | 0.00 | - | - | 0.31 | 0.04 | - | - |
| C20:4n6 | 0.50 | 0.72 | - | - | 2.32 | 0.10 | - | - |
| C20:5n3(EPA) | 4.94 | 0.02 | - | - | 9.40 | 0.06 | - | - |
| C22:4n6 | 0.52 | 0.01 | - | - | 1.68 | 0.03 | - | - |
| C22:5n3 | 2.46 | 0.05 | - | - | 3.22 | 0.06 | - | - |
| C22:6n3(DHA) | 6.43 | 0.04 | - | - | 23.41 | 0.29 | - | - |
| PUFA | 32.74 | - | 28.28 | - | 49.13 | - | 34.66 | - |
| n-3/n-6 | 1.28 | - | 0.51 | - | 3.75 | - | 0.23 | - |
| DHA/EPA | 1.30 | - | - | - | 2.49 | - | - | - |
| Cur | SE | RE | Cur-SE | Cur-RE | |
|---|---|---|---|---|---|
| IC50 | 12.1 ± 0.98 µM | 5.66 ± 0.51 mg/mL | 9.37 ± 0.55 mg/mL | 3.96 ± 0.32 mg/mL SE 2.83 ± 0.23 µM Cur | 4.37 ± 0.12 mg/mL RE 1.56 ± 0.04 µM Cur |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Hasan, M.; Elkhoury, K.; Kahn, C.J.F.; Linder, M.; Arab-Tehrany, E. Marine- and Plant-Based Nanoemulsion Platforms Enhance the Anticancer Activity of Curcumin In Vitro. Int. J. Mol. Sci. 2026, 27, 29. https://doi.org/10.3390/ijms27010029
Hasan M, Elkhoury K, Kahn CJF, Linder M, Arab-Tehrany E. Marine- and Plant-Based Nanoemulsion Platforms Enhance the Anticancer Activity of Curcumin In Vitro. International Journal of Molecular Sciences. 2026; 27(1):29. https://doi.org/10.3390/ijms27010029
Chicago/Turabian StyleHasan, Mahmoud, Kamil Elkhoury, Cyril J. F. Kahn, Michel Linder, and Elmira Arab-Tehrany. 2026. "Marine- and Plant-Based Nanoemulsion Platforms Enhance the Anticancer Activity of Curcumin In Vitro" International Journal of Molecular Sciences 27, no. 1: 29. https://doi.org/10.3390/ijms27010029
APA StyleHasan, M., Elkhoury, K., Kahn, C. J. F., Linder, M., & Arab-Tehrany, E. (2026). Marine- and Plant-Based Nanoemulsion Platforms Enhance the Anticancer Activity of Curcumin In Vitro. International Journal of Molecular Sciences, 27(1), 29. https://doi.org/10.3390/ijms27010029











