Dynamic Changes in Oxidative Stress Biomarkers in a Child with Idiopathic Nephrotic Syndrome: A Longitudinal Case Study
Abstract
1. Introduction
2. Results
2.1. Case Presentation
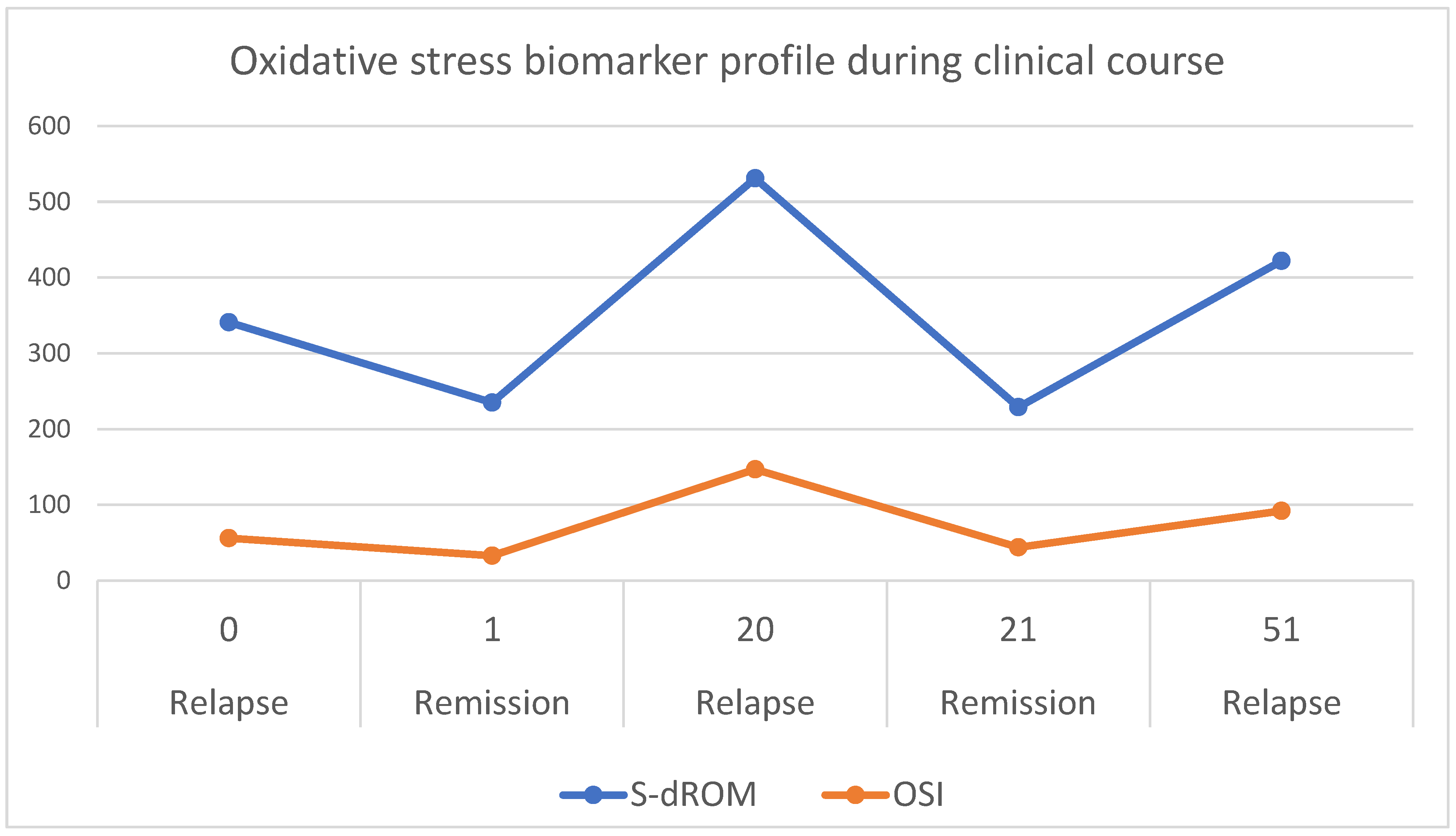
2.2. Biomarker Dynamics Across Clinical Timepoints
3. Discussion
3.1. Limitations and Generalizability
3.2. Confounding by Treatment Effects and Lack of Controls
- (1)
- The temporal pattern shows d-ROM peaks during clinically active relapse phases (Week 0: 341 U.CARR; Week 20: 531 U.CARR) with decreases during remission (Week 1: 235 U.CARR; Week 21: 229 U.CARR), suggesting correlation with disease activity independent of treatment timing.
- (2)
- Elevated OSI during subclinical relapse (Week 51: OSI 92, d-ROM 422) occurred before clinician-detected clinical signs, suggesting that oxidative stress reflects underlying disease processes rather than solely treatment effects.
- (3)
- Our published cohort study (n = 20) demonstrated significantly higher d-ROM values at first disease presentation/relapse (before GC initiation in most patients) compared to remission (p = 0.0458) [20], supporting disease-driven oxidative burden.
- (1)
- PAT values showed patterns suggesting GC-induced modulation of antioxidant defenses. GCs have complex, dose- and duration-dependent effects on oxidative stress: while chronic high-dose exposure can induce oxidative damage through increased mitochondrial ROS production and depletion of antioxidant enzymes [35,36], therapeutic doses may enhance antioxidant gene expression and reduce inflammatory ROS generation [37].
3.3. Lack of Pediatric Reference Ranges
- (1)
- Age-dependent oxidative metabolism: Children exhibit different metabolic rates, growth-related oxidative demands, and antioxidant enzyme maturation compared to adults, potentially affecting baseline d-ROM and PAT values.
- (2)
- Developmental variation: Reference ranges likely vary across pediatric age groups (neonates, infants, children, adolescents), yet age-stratified data are lacking.
- (3)
- Disease-specific considerations: Pediatric INS involves unique pathophysiological features (minimal change disease predominance, higher steroid responsiveness) that may influence oxidative stress profiles differently than adult nephrotic syndrome.
- (4)
- Therapeutic implications: Clinical decision thresholds based on adult values may not accurately identify children at risk for relapse or steroid resistance.
4. Materials and Methods
4.1. Study Design and Ethical Considerations
4.2. Clinical Setting and Patient Timeline
- Initial presentation: mild relapse
- Remission (early response to therapy)
- Relapse
- Remission
- Relapse
4.3. Sample Collection
4.4. Oxidative Stress Biomarkers
4.4.1. d-ROMs Test (Derivatives of Reactive Oxygen Metabolites)
4.4.2. PAT (Plasma Antioxidant Test)
4.4.3. Oxidative Stress Index (OSI)
4.5. Oxidative Status Categorization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ROS | reactive oxygen species |
| MDA | malondialdehyde |
| SOD | superoxide dismutase |
| GSH | glutathione |
| TAC | total antioxidant capacity |
| TAS | total antioxidant status |
| GC | glucocorticoid |
| d-ROMs test | derivatives of Reactive Oxygen Metabolites |
| PAT | Plasma Antioxidant Test |
| OSI | Oxidative Stress Index |
References
- da Silva Filha, R.; Burini, K.; Pires, L.G.; Brant Pinheiro, S.V.; Simões E Silva, A.C. Idiopathic Nephrotic Syndrome in Pediatrics: An Up-to-date. Curr. Pediatr. Rev. 2022, 18, 251–264. [Google Scholar] [CrossRef]
- Rodriguez, J.M.; Monsalves-Alvarez, M.; Henriquez, S.; Llanos, M.N.; Troncoso, R. Glucocorticoid resistance in chronic diseases. Steroids 2016, 115, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Sahali, D.; Sendeyo, K.; Mangier, M.; Audard, V.; Zhang, S.Y.; Lang, P.; Ollero, M.; Pawlak, A. Immunopathogenesis of idiopathic nephrotic syndrome with relapse. Semin. Immunopathol. 2014, 36, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Hackl, A.; Zed, S.E.D.A.; Diefenhardt, P.; Binz-Lotter, J.; Ehren, R.; Weber, L.T. The role of the immune system in idiopathic nephrotic syndrome. Mol. Cell. Pediatr. 2021, 8, 18. [Google Scholar] [CrossRef]
- Ponticelli, C.; Glassock, R.J.; Coppo, R. Minimal change disease. In Treatment of Primary Glomerulonephritis, 3rd online ed.; Ponticelli, C., Glassock, R.J., Eds.; Oxford Clinical Nephrology Series; Oxford University Press: Oxford, UK, 2019; Available online: https://academic.oup.com/book/25000/chapter-abstract/188998931?redirectedFrom=fulltext (accessed on 6 June 2025).
- Blaine, J.; Dylewski, J. Regulation of the Actin Cytoskeleton in Podocytes. Cells 2020, 9, 1700. [Google Scholar] [CrossRef]
- Rangel, P.X.M.; Priyadarshini, A.; Tian, X. New Insights into the Immunity and Podocyte in Glomerular Health and Disease: From Pathogenesis to Therapy in Proteinuric Kidney Disease. Integr. Med. Nephrol. Androl. 2021, 8, 5. [Google Scholar] [CrossRef]
- Su, H.; Wan, C.; Song, A.; Qiu, Y.; Xiong, W.; Zhang, C. Oxidative Stress and Renal Fibrosis: Mechanisms and Therapies. Adv. Exp. Med. Biol. 2019, 1165, 585–604. [Google Scholar] [CrossRef] [PubMed]
- Solin, M.L.; Ahola, H.; Haltia, A.; Ursini, F.; Montine, T.; Roveri, A.; Kerjaschki, D.; Holthöfer, H. Lipid peroxidation in human proteinuric disease. Kidney Int. 2001, 59, 481–487. [Google Scholar] [CrossRef]
- Parmar, G.S.; Mistry, K.N.; Gang, S. Correlation of Serum Albumin and Creatinine with Oxidative Stress Markers In Patients Having Nephrotic Syndrome. Int. J. Pharm. Pharm. Sci. 2021, 13, 20–24. [Google Scholar] [CrossRef]
- Cornelli, U.; Terranova, R.; Luca, S.; Cornelli, M.; Alberti, A. Bioavailability and antioxidant activity of some food supplements in men and women using the D-Roms test as a marker of oxidative stress. J. Nutr. 2001, 131, 3208–3211. [Google Scholar] [CrossRef]
- Vassalle, C.; Boni, C.; Di Cecco, P.; Ndreu, R.; Zucchelli, G.C. Automation and validation of a fast method for the assessment of in vivo oxidative stress levels. Clin. Chem. Lab. Med. 2006, 44, 1372–1375. [Google Scholar] [CrossRef] [PubMed]
- Fukui, T.; Yamauchi, K.; Maruyama, M.; Yasuda, T.; Kohno, M.; Abe, Y. Significance of measuring oxidative stress in lifestyle-related diseases from the viewpoint of correlation between d-ROMs and BAP in Japanese subjects. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2011, 34, 1041–1045. [Google Scholar] [CrossRef]
- Benedetti, S.; Primiterra, M.; Finco, A.; Gorni, D.; Catalani, S.; Battistelli, S.; Cornelli, U. Determination of Plasma Antioxidant Power in Capillary Blood through the Innovative system PAT (Plasma Antioxidant Test). Free Radic. Antioxid. 2018, 8, 149–152. [Google Scholar]
- Osredkar, J.; Pucko, S.; Lukić, M.; Fabjan, T.; Alič, E.B.; Kumer, K.; Rodriguez, M.M.; Jereb, M. The Predictive Value of Oxidative Stress Index in Patients with Confirmed SARS-COV-2 Infection. Acta Chim. Slov. 2022, 69, 564–570. [Google Scholar] [CrossRef]
- Paulis, G.; Paulis, A.; De Giorgio, G.; Quattrocchi, S. Measurement of Oxidative Stress Index (OSI) in Penile Corpora Cavernosa and Peripheral Blood of Peyronie’s Disease Patients: A Report of 49 Cases. Metabolites 2024, 14, 55. [Google Scholar] [CrossRef]
- Masaki, N.; Sato, A.; Horii, S.; Kimura, T.; Toya, T.; Yasuda, R.; Namba, T.; Yada, H.; Kawamura, A.; Adachi, T. Usefulness of the d-ROMs test for prediction of cardiovascular events. Int. J. Cardiol. 2016, 222, 226–232. [Google Scholar] [CrossRef]
- Hitomi, Y.; Masaki, N.; Ishinoda, Y.; Ido, Y.; Iwashita, M.; Yumita, Y.; Kagami, K.; Yasuda, R.; Ikegami, Y.; Toya, T.; et al. Effectiveness of the d-ROMs oxidative stress test to predict long-term cardiovascular mortality. Int. J. Cardiol. 2022, 354, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Vassalle, C.; Vigna, L.; Bianchi, S.; Maffei, S.; Novembrino, C.; De Giuseppe, R.; de Liso, F.; Vannucci, A.; Tirelli, S.; Maiavacca, R.; et al. A Biomarker of Oxidative Stress as a Nontraditional Risk Factor in Obese Subjects. Biomark. Med. 2013, 7, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Kopač, M.; Jerin, A.; Bohinc, E.; Osredkar, J. Correlation of Oxidative Stress Biomarkers with Activity of Pediatric Idiopathic Nephrotic Syndrome. Biomedicines 2025, 13, 1984. [Google Scholar] [CrossRef]
- Mulat, S.Y.; Mihajloviic, M.; Antonic, T.; Miloševski-Lomic, G.; Peco-Antic, A.; Jovanovic, D.; Paripovic, D.; Stefanovic, A. Pediatric nephrotic syndrome: The interplay of oxidative stress and inflammation. J. Med. Biochem. 2024, 43, 424–435. [Google Scholar] [CrossRef]
- Kinra, S.; Rath, B.; Kabi, B.C. Indirect quantification of lipid peroxidation in steroid responsive nephrotic syndrome. Arch. Dis. Child. 2000, 82, 76–78. [Google Scholar] [CrossRef]
- Begenik, H.; Soyoral, Y.U.; Erkoc, R.; Emre, H.; Taskın, A.; Tasdemir, M.; Aslan, M. Serum malondialdehyde levels, myeloperoxidase and catalase activities in patients with nephrotic syndrome. Redox Rep. 2013, 18, 107–112. [Google Scholar] [CrossRef]
- Reddy, P.; Sindgikar, S.P.; Shenoy, R.D.; Shenoy, V. Oxidative stress in childhood steroid sensitive nephrotic syndrome and its correlation with DNA damage. Int. J. Contemp. Pediatr. 2016, 3, 768–772. [Google Scholar] [CrossRef]
- Mishra, O.P.; Schaefer, F. Oxidative stress in children with nephrotic syndrome. Pediatr. Nephrol. 2012, 27, 157. [Google Scholar] [CrossRef]
- Ilatovskaya, D.V.; Behr, A.; Staruschenko, A.; Hall, G.; Palygin, O. Mechanistic Insights into Redox Damage of the Podocyte in Hypertension. Hypertension 2025, 82, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Farrokhi, F.R.; Rezaee, R.; Sahebkar, A. Oxidative stress induces renal failure: A review of possible molecular pathways. J. Cell. Biochem. 2018, 119, 2990–2998. [Google Scholar] [CrossRef]
- Wan, C.; Su, H.; Zhang, C. Role of NADPH Oxidase in Metabolic Disease-Related Renal Injury: An Update. Oxidative Med. Cell. Longev. 2016, 2016, 7813072. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Zhang, A.; Huang, S. Serum levels of malondialdehyde, vitamin C and E in idiopathic nephrotic syndrome: A meta-analysis. Ren. Fail. 2014, 36, 994–999. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, M.A.; Mendoza-Núñez, V.M. Oxidative Stress Indexes for Diagnosis of Health or Disease in Humans. Oxidative Med. Cell. Longev. 2019, 2019, 4128152. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Duan, N.; Kravitz, R.L.; Schmid, C.H. Single-patient (n-of-1) trials: A pragmatic clinical decision methodology for population research. J. Clin. Epidemiol. 2013, 66, S21–S28. [Google Scholar] [CrossRef] [PubMed]
- Lobo, M.A.; Moeyaert, M.; Cunha, A.B.; Babik, I. Single-case design, analysis, and quality assessment for intervention research. J. Neurol. Phys. Ther. 2017, 41, 187–197. [Google Scholar] [CrossRef]
- Feng, Y.L.; Tang, X.L. Effect of glucocorticoid-induced oxidative stress on the expression of Cbfa1. Chem. Biol. Interact. 2014, 207, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Patani, A.; Balakrishnan, M.; Pathak, S.; Jindal, D.; Pant, K.; Pant, P.; Rathore, R.S.; Misra, N.; Parmar, H.S.; Chawla, P.A. Harnessing the power of nutritional antioxidants against adrenal hormone imbalance-associated oxidative stress. Front. Endocrinol. 2023, 14, 1271521. [Google Scholar] [CrossRef] [PubMed]
- Herrera, E.A.; Verkerk, M.M.; Derks, J.B.; Giussani, D.A. Antioxidant treatment alters peripheral vascular dysfunction induced by postnatal glucocorticoid programming in guinea pigs. PLoS ONE 2010, 5, E9250. [Google Scholar] [CrossRef]
| Time Point—Weeks After Start of Study Period (Disease Activity) | d-ROM (U. CARR) | PAT (U. CARR) | OSI | Oxidative Situation | Interpretation |
|---|---|---|---|---|---|
| Start of study period (relapse) | 341 | 2046 | 56 | III | Moderate oxidative activity, limited defense |
| 1 (remission) | 235 | 2271 | 33 | IV | Reduced oxidative load, good antioxidant reserve |
| 20 (relapse) | 531 | 2437 | 147 | II | High oxidative stress, borderline defense |
| 21 (remission) | 229 | 2117 | 44 | IV | Low ROS with improved antioxidant status |
| 51 (relapse) | 422 | 2081 | 92 | II–III | Slightly elevated oxidative burden, marginal defense |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Osredkar, J.; Kopač, M. Dynamic Changes in Oxidative Stress Biomarkers in a Child with Idiopathic Nephrotic Syndrome: A Longitudinal Case Study. Int. J. Mol. Sci. 2026, 27, 216. https://doi.org/10.3390/ijms27010216
Osredkar J, Kopač M. Dynamic Changes in Oxidative Stress Biomarkers in a Child with Idiopathic Nephrotic Syndrome: A Longitudinal Case Study. International Journal of Molecular Sciences. 2026; 27(1):216. https://doi.org/10.3390/ijms27010216
Chicago/Turabian StyleOsredkar, Joško, and Matjaž Kopač. 2026. "Dynamic Changes in Oxidative Stress Biomarkers in a Child with Idiopathic Nephrotic Syndrome: A Longitudinal Case Study" International Journal of Molecular Sciences 27, no. 1: 216. https://doi.org/10.3390/ijms27010216
APA StyleOsredkar, J., & Kopač, M. (2026). Dynamic Changes in Oxidative Stress Biomarkers in a Child with Idiopathic Nephrotic Syndrome: A Longitudinal Case Study. International Journal of Molecular Sciences, 27(1), 216. https://doi.org/10.3390/ijms27010216





