Integrative Landscape of Dry AMD Pathogenesis, Models, and Emerging Therapeutic Strategies
Abstract
1. Introduction
1.1. Genetics Contributions to AMD
| Gene | Effect in AMD | Human Relevance/Phenotype | Best Use | Limitation |
|---|---|---|---|---|
| CFH | Key regulator of complement system. Decrease in CFH level and variants increases risk of advanced AMD and drusen formation [29] | Early macular drusen [30] | To evaluate genetic risk and guide advanced AMD treatment strategies [31] | Genetic testing not advised for routine patient counseling [32] |
| ARMS2 | ARMS2 is strongly associated with AMD development, and plays important role in extracellular matrix and mitochondrial function in retinal cells [33] | HTRA1 mutations are associated with both GA and choroidal neovascularization (CNV) [34] | Used in genetic risk assessment models for AMD prognosis. | Genetic tests often use a limited number of genes and are biased towards European ancestry [35] |
| HTRA1 | HTRA1 is strongly associated with extracellular matrix (ECM) remodeling in AMD [36] | HTRA1 contributes to AMD, affecting drusen formation and compromising BrM [36] | Studying drusen pathogenesis and BrM function | Unable to fully replicate late-stage AMD, like GA |
| C3 | Polymorphisms are associated with an increased risk of AMD, especially advanced forms. The rs2230199 (R102G) variant is considered a likely causal variant in Caucasians [37] | Involved in the complement system; specific variants can increase AMD risk | Included in predictive models for AMD prevalence and incidence [31] | The clinical importance of biomarker correlations is still unclear, requiring further study [38] |
| TIMP3 | A susceptibility locus for AMD, with roles in extracellular matrix degradation | Mutations are linked to Sorsby’s fundus dystrophy, a rare form of macular degeneration | Identifying rare coding variants to pinpoint causal genes within known genetic loci [30] | Systematically identifying associations with rare variants requires extremely large sample sizes and specific study designs [30] |
| APOE | Associated with AMD and plays a role in high-density lipoprotein metabolism. | Involved in lipid transport and metabolism; the ε2 allele was the first genetic risk factor identified for AMD [39] | Included in models to assess the joint effects of genetic, ocular, and environmental variables for AMD [31] | Not all of the genetic contribution to AMD is explained by known loci, suggesting other genes with smaller effects exist [40] |
| LIPC | Influences susceptibility to AMD, associated with high-density lipoprotein cholesterol levels. Predicted higher LIPC expression in AMD cases is expected to result in lower blood HDL levels [29] | Encodes hepatic lipase (HL), which regulates HDL concentration [29] | Part of the broader genetic analysis to understand AMD etiology [29] | Single expression quantitative trait locus (eQTL) analysis has limitations, as the causative signal remains elusive for many variants [29] |
| CETP | Associated with AMD and high-density lipoprotein cholesterol levels. Lower predicted CETP expression is significantly associated with AMD in some tissues, aligning with findings that increased HDL is linked to AMD risk [29] | Involved in cholesterol ester transfer, with CETP deficiency leading to high HDL levels [29] | Used in genetic studies to understand lipid metabolism pathways in AMD [29] | The exact mechanisms of how gene expression regulation relates to AMD progression need further elucidation [29] |
| C2/CFB | AMD susceptibility loci involved in the complement system. Variants are significantly related to progression to advanced AMD [31] | C2 and CFB are components of the complement pathway. | Included in predictive models for AMD prevalence and incidence [31] | Genetic risk predictions in multifactorial diseases like AMD have limitations [41] |
| CFI | Susceptibility locus for AMD, part of the complement system. Predicted lower expression of CFI in AMD cases compared to controls [29] | Regulates the alternative complement pathway [29] | Contributes to understanding the genetic basis of AMD [29] | Gene expression in diseased tissue may differ significantly from healthy tissue, which current transcriptome-wide association study (TWAS) models do not fully capture [29] |
| RLPB1 | Predicted to have lower gene expression in AMD cases in retinal tissue. One of six genes potentially exclusive to retina affects [29] | Encodes cellular retinaldehyde-binding protein 1; mutations cause diseases such as retinitis punctata albescens and rod-cone dystrophy [29] | Understanding retina-specific effects in AMD etiology [29] | Changes in retinal gene expression can only partly explain genome-wide association study (GWAS) association signals. Data on RPE or choroid tissue gene expression is not yet available to draw further conclusions [29] |
1.2. Environmental Risk Factors and Pathogenic Mechanisms
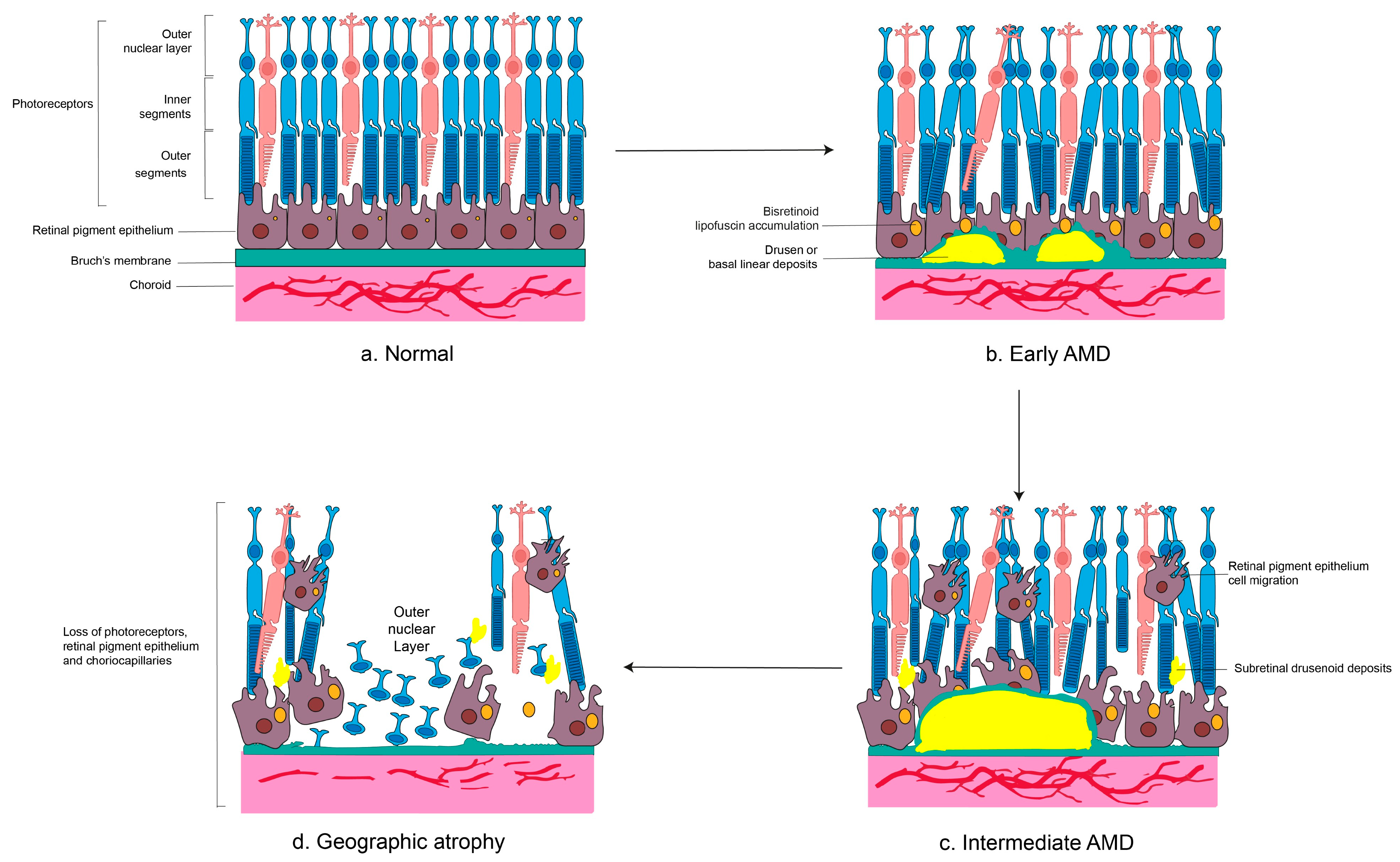
1.3. Pathological Hallmarks of Dry AMD
2. Events and Cellular Pathways in Dry AMD
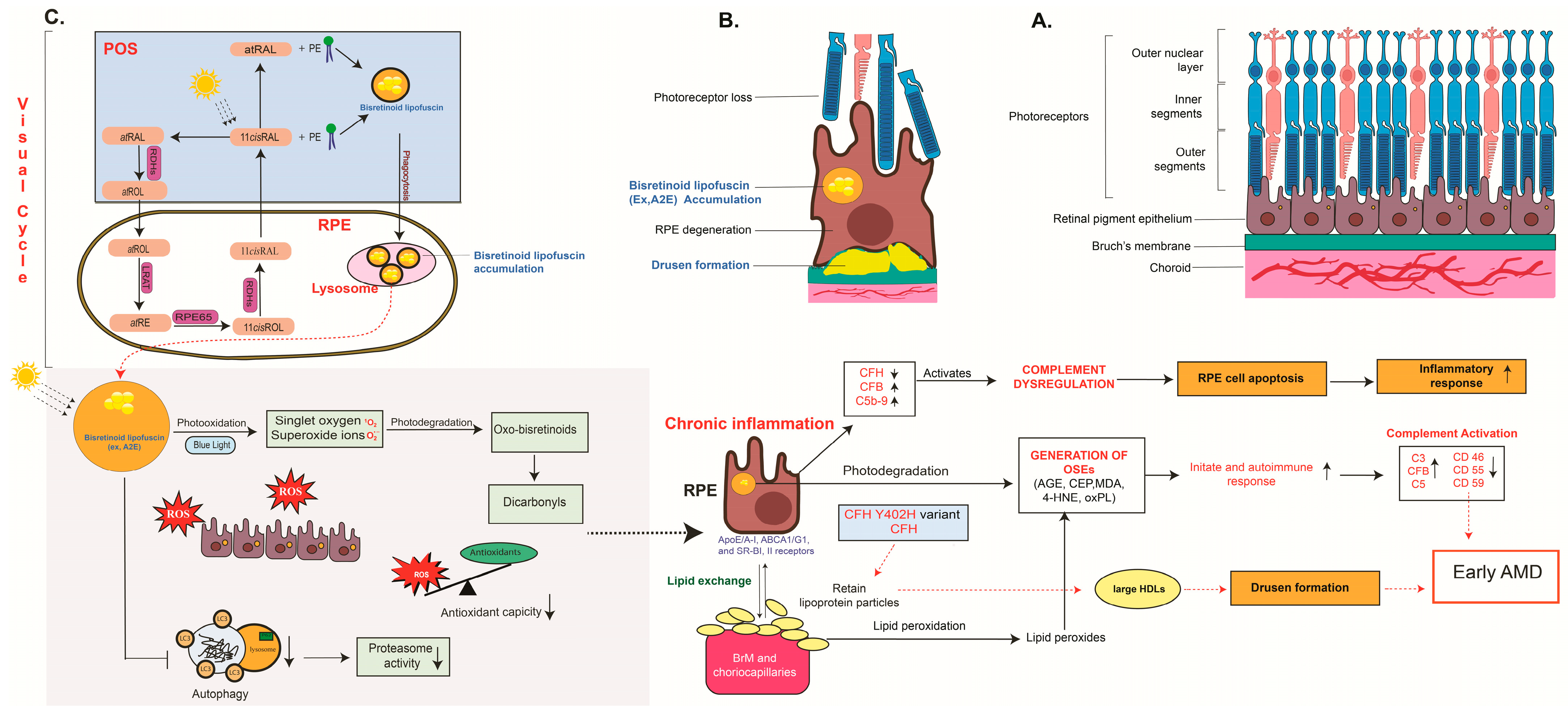
2.1. Oxidative Stress
2.2. Lipid Polymorphism and Lipid Dysregulation
2.3. Inflammation and Immune Activation
2.4. Mitochondrial Dysfunction
2.5. Autophagy and Drusen Biogenesis, and RPE–Choriocapillaris Interdependence
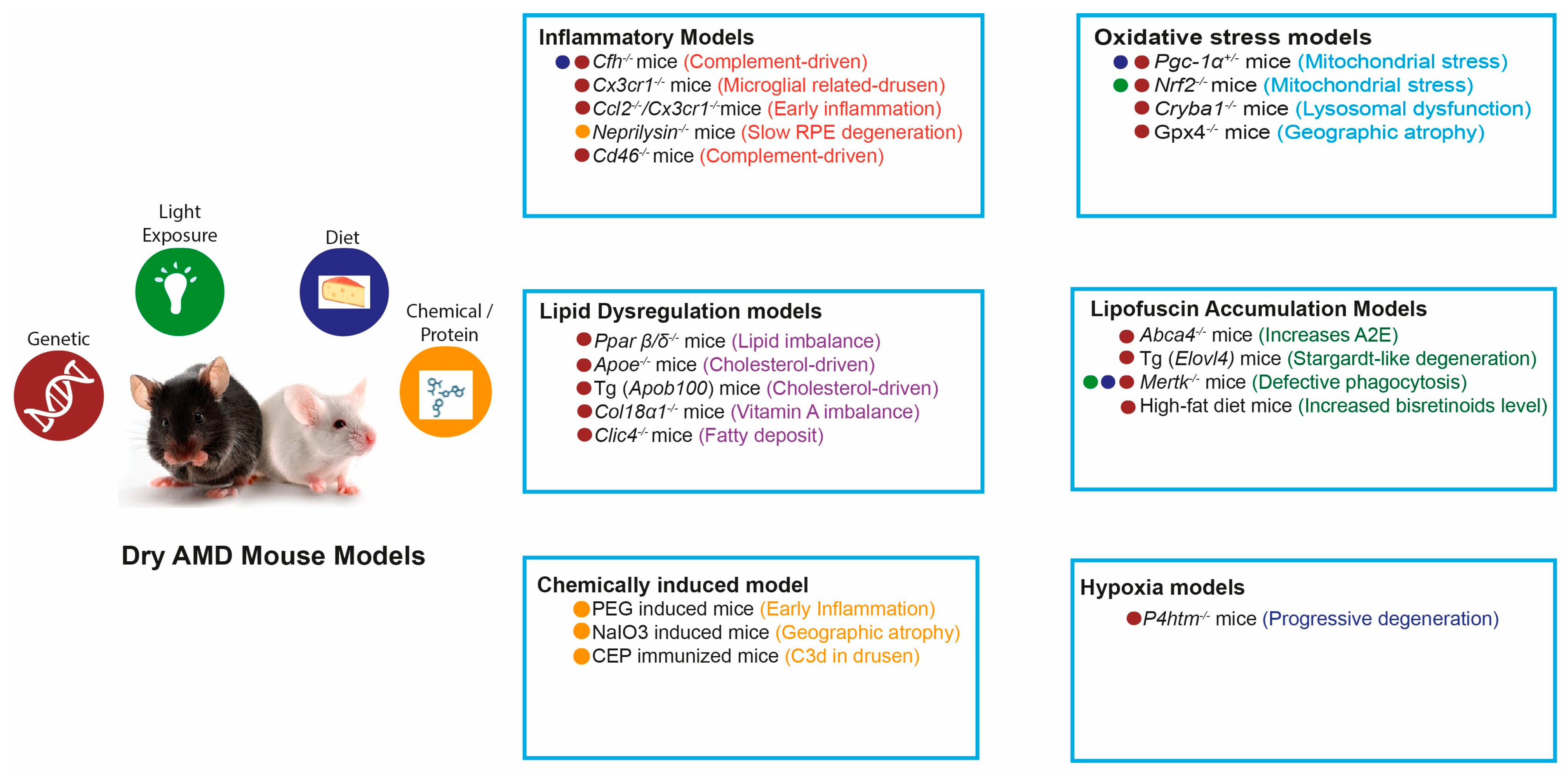
3. Mechanistic Mouse Models of Dry AMD
3.1. Oxidative Stress-Driven Models
3.2. Lipid Dysregulation-Driven Drusen-like Deposit Models
3.3. Inflammation and Immune Dysregulation-Driven Models
3.4. RPE Lipofuscin Accumulation Models
3.4.1. Genetic Models Involving Visual Cycle Enzymes
3.4.2. Lipid Metabolism and Photoreceptor Vulnerability
3.4.3. Impaired Phagocytosis and Retinal Debris Accumulation
3.4.4. Environmental and Metabolic Risk Factor Models
3.5. Immunological and Stress-Induced Mouse Models
3.5.1. Complement and Immune Activation Models
3.5.2. Ferroptosis-Associated Models
3.5.3. Chemically Induced Models
3.6. Emerging Alternatives to Traditional Mouse Models
3.6.1. iPSC-Derived RPE Organoids
3.6.2. Nonhuman Primate Models
3.6.3. Ex Vivo Human RPE Models
3.6.4. Other Emerging and Alternative Models
| Model System | Key Advantage | Major Limitation |
|---|---|---|
| Mouse models | High degree of genetic conservation with humans. Well-established tools for genetic manipulation (CRISPR, KO/KI, transgenics) enabling mechanistic studies of complement dysregulation, lipid metabolism, and oxidative stress. Rapid aging, short reproductive cycles, and relatively low maintenance costs enable large-scale and longitudinal studies. Wide availability of standardized strains and experimental reagents [156]. | Lack a macula and fovea, limiting direct modeling of macular-specific degeneration and drusen formation. AMD is multifactorial; most mouse models reproduce single pathogenic pathways rather than full disease complexity. Limited genetic diversity reduces generalizability to heterogeneous human populations. Strain-specific variability can influence phenotype and treatment responses [156]. |
| In Vitro Models (iPSC-RPE, 2D/3D Organoids) | Human-derived RPE and retinal cells capture patient-specific genotypes, including AMD risk variants (CFH, ARMS2). Highly controlled environments allow precise manipulation of oxidative stress, complement activity, and lipid metabolism. Suitable for high-throughput drug screening and mechanistic assays. Reduced animal use offers ethical advantages. 3D retinal organoids partially recapitulate retinal layering and early photoreceptor development [157,158,159]. | Unable to fully replicate in vivo retinal microenvironment, including vasculature, immune components, and choroidal support. Limited capacity to model aging, a central driver of AMD pathogenesis. Organoid generation is time-consuming, variable, and costly. Conventional 2D cultures may exhibit altered physiology or incomplete RPE maturation. Systemic interactions contributing to AMD cannot be modeled [157,159]. |
| Nonhuman Primates (NHPs) | Possess a true macula and fovea, closely resembling human retinal anatomy. Retinal physiology, photoreceptor architecture, and choroidal vasculature are highly comparable to humans. Can develop age-related drusen and RPE alterations. Essential for late-stage preclinical validation of pharmacokinetics, delivery strategies, safety, and efficacy. Provide the closest translational bridge between rodent studies and human clinical trials [159,160,161]. | Limited availability and very high housing and experimental costs. Long lifespan prolongs aging and disease-induction studies. Substantial ethical constraints due to cognitive capacity and similarity to humans. Genetic manipulation is more difficult and less established than in mice. Species- and protocol-dependent variability may affect reproducibility [159,162,163]. |
4. Dry AMD Therapeutics
4.1. Current Approaches
4.1.1. AREDS/AREDS2 Supplements
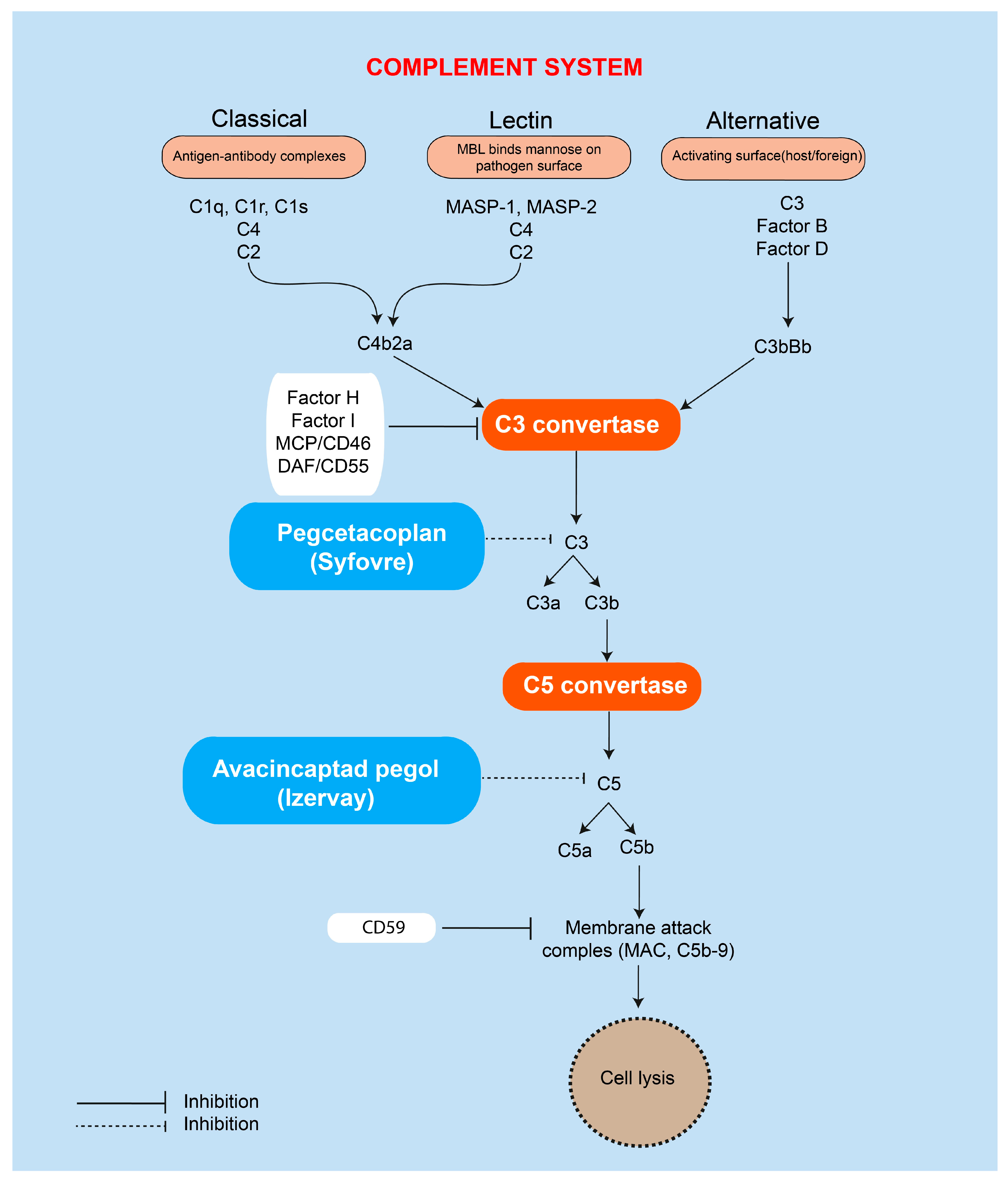
4.1.2. Pegcetacoplan (Syfovre)
4.1.3. Avacincaptad Pegol (Izervay)
4.2. Emerging and Investigational Therapies
4.2.1. Complement Pathway Inhibitors
4.2.2. Inflammation Modulators and Steroids
4.2.3. Visual Cycle Modulators
4.2.4. Neuroprotective and Mitochondrial Protective Agents
4.2.5. Photobiomodulation (PBM)
4.2.6. Gene Therapy and Long-Acting Vector-Based Approaches
4.2.7. Other Experimental Candidates
4.3. Lessons from Clinical Failures
5. Conclusions
6. Future Direction
6.1. Retinal Organoids in Drug Screening
6.2. Emerging Therapies and Clinical Innovation
6.3. Longitudinal Multimodal Imaging
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- la Cour, M.; Kiilgaard, J.F.; Nissen, M.H. Age-related macular degeneration: Epidemiology and optimal treatment. Drugs Aging 2002, 19, 101–133. [Google Scholar] [CrossRef]
- Duker, J.S.; Waheed, N.K.; Goldman, D.R. 6.1—Dry Age-Related Macular Degeneration. In Handbook of Retinal OCT: Optical Coherence Tomography, 2nd ed.; Duker, J.S., Waheed, N.K., Goldman, D.R., Eds.; Elsevier: Philadelphia, PA, USA, 2022; pp. 42–45. [Google Scholar]
- Zając-Pytrus, H.M.; Pilecka, A.; Turno-Kręcicka, A.; Adamiec-Mroczek, J.; Misiuk-Hojło, M. The Dry Form of Age-Related Macular Degeneration (AMD): The Current Concepts of Pathogenesis and Prospects for Treatment. Adv. Clin. Exp. Med. 2015, 24, 1099–1104. [Google Scholar] [CrossRef]
- Nashine, S. Potential Therapeutic Candidates for Age-Related Macular Degeneration (AMD). Cells 2021, 10, 2483. [Google Scholar] [CrossRef] [PubMed]
- Pennesi, M.E.; Neuringer, M.; Courtney, R.J. Animal models of age related macular degeneration. Mol. Asp. Med. 2012, 33, 487–509. [Google Scholar] [CrossRef] [PubMed]
- Curcio, C.A.; Millican, C.L. Basal linear deposit and large drusen are specific for early age-related maculopathy. Arch. Ophthalmol. 1999, 117, 329–339. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Boulton, M. RPE lipofuscin and its role in retinal pathobiology. Exp. Eye Res. 2005, 80, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Elizabeth Rakoczy, P.; Yu, M.J.; Nusinowitz, S.; Chang, B.; Heckenlively, J.R. Mouse models of age-related macular degeneration. Exp. Eye Res. 2006, 82, 741–752. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Gregory-Roberts, E.; Yamamoto, K.; Blonska, A.; Ghosh, S.K.; Ueda, K.; Zhou, J. The bisretinoids of retinal pigment epithelium. Prog. Retin. Eye Res. 2012, 31, 121–135. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Kim, H.J. Bisretinoid lipofuscin, fundus autofluorescence and retinal disease. Prog. Retin. Eye Res. 2025, 108, 101388. [Google Scholar] [CrossRef]
- Deng, Y.; Qiao, L.; Du, M.; Qu, C.; Wan, L.; Li, J.; Huang, L. Age-related macular degeneration: Epidemiology, genetics, pathophysiology, diagnosis, and targeted therapy. Genes Dis. 2022, 9, 62–79. [Google Scholar] [CrossRef]
- Chen, Y.; Bedell, M.; Zhang, K. Age-related macular degeneration: Genetic and environmental factors of disease. Mol. Interv. 2010, 10, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.J.; Zeiss, C.; Chew, E.Y.; Tsai, J.Y.; Sackler, R.S.; Haynes, C.; Henning, A.K.; SanGiovanni, J.P.; Mane, S.M.; Mayne, S.T.; et al. Complement factor H polymorphism in age-related macular degeneration. Science 2005, 308, 385–389. [Google Scholar] [CrossRef]
- Kaur, I.; Katta, S.; Reddy, R.K.; Narayanan, R.; Mathai, A.; Majji, A.B.; Chakrabarti, S. The involvement of complement factor B and complement component C2 in an Indian cohort with age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2010, 51, 59–63. [Google Scholar] [CrossRef]
- Ennis, S.; Gibson, J.; Cree, A.J.; Collins, A.; Lotery, A.J. Support for the involvement of complement factor I in age-related macular degeneration. Eur. J. Hum. Genet. 2010, 18, 15–16. [Google Scholar] [CrossRef]
- Ramkumar, H.L.; Zhang, J.; Chan, C.C. Retinal ultrastructure of murine models of dry age-related macular degeneration (AMD). Prog. Retin. Eye Res. 2010, 29, 169–190. [Google Scholar] [CrossRef]
- Holliday, E.G.; Smith, A.V.; Cornes, B.K.; Buitendijk, G.H.; Jensen, R.A.; Sim, X.; Aspelund, T.; Aung, T.; Baird, P.N.; Boerwinkle, E.; et al. Insights into the genetic architecture of early stage age-related macular degeneration: A genome-wide association study meta-analysis. PLoS ONE 2013, 8, e53830. [Google Scholar] [CrossRef] [PubMed]
- Ferris, F.L., 3rd; Wilkinson, C.P.; Bird, A.; Chakravarthy, U.; Chew, E.; Csaky, K.; Sadda, S.R. Clinical classification of age-related macular degeneration. Ophthalmology 2013, 120, 844–851. [Google Scholar] [CrossRef] [PubMed]
- García-Layana, A.; Cabrera-López, F.; García-Arumí, J.; Arias-Barquet, L.; Ruiz-Moreno, J.M. Early and intermediate age-related macular degeneration: Update and clinical review. Clin. Interv. Aging 2017, 12, 1579–1587. [Google Scholar] [CrossRef]
- The Age-Related Eye Disease Study system for classifying age-related macular degeneration from stereoscopic color fundus photographs: The Age-Related Eye Disease Study Report Number 6. Am. J. Ophthalmol. 2001, 132, 668–681. [CrossRef]
- Sergejeva, O.; Botov, R.; Liutkevičienė, R.; Kriaučiūnienė, L. Genetic factors associated with the development of age-related macular degeneration. Medicine 2016, 52, 79–88. [Google Scholar] [CrossRef]
- de Breuk, A.; Heesterbeek, T.J.; Bakker, B.; Verzijden, T.; Lechanteur, Y.T.E.; Klaver, C.C.W.; den Hollander, A.I.; Hoyng, C.B. Evaluating the Occurrence of Rare Variants in the Complement Factor H Gene in Patients with Early-Onset Drusen Maculopathy. JAMA Ophthalmol. 2021, 139, 1218–1226. [Google Scholar] [CrossRef]
- Saksens, N.T.M.; Geerlings, M.J.; Bakker, B.; Schick, T.; Daha, M.R.; Fauser, S.; Boon, C.J.F.; de Jong, E.K.; Hoyng, C.B.; den Hollander, A.I. Rare Genetic Variants Associated with Development of Age-Related Macular Degeneration. JAMA Ophthalmol. 2016, 134, 287–293. [Google Scholar] [CrossRef]
- Stradiotto, E.; Allegrini, D.; Fossati, G.; Raimondi, R.; Sorrentino, T.; Tripepi, D.; Barone, G.; Inforzato, A.; Romano, M.R. Genetic Aspects of Age-Related Macular Degeneration and Their Therapeutic Potential. Int. J. Mol. Sci. 2022, 23, 13280. [Google Scholar] [CrossRef]
- Fritsche, L.G.; Fleckenstein, M.; Fiebig, B.S.; Schmitz-Valckenberg, S.; Bindewald-Wittich, A.; Keilhauer, C.N.; Renner, A.B.; Mackensen, F.; Mößner, A.; Pauleikhoff, D.; et al. A subgroup of age-related macular degeneration is associated with mono-allelic sequence variants in the ABCA4 gene. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2112–2118. [Google Scholar] [CrossRef]
- Radu, R.A.; Hu, J.; Jiang, Z.; Bok, D. Bisretinoid-mediated Complement Activation on Retinal Pigment Epithelial Cells Is Dependent on Complement Factor H Haplotype*. J. Biol. Chem. 2014, 289, 9113–9120. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.L.; Bowes Rickman, C.; Katsanis, N. AMD and the alternative complement pathway: Genetics and functional implications. Human Genom. 2016, 10, 23. [Google Scholar] [CrossRef]
- Geerlings, M.J. Studies of Rare Genetic Variants in Age-Related Macular Degeneration. Doctoral Dissertation, Radboud University Nijmegen, Nijmegen, The Netherlands, 2018. [Google Scholar]
- Strunz, T.; Lauwen, S.; Kiel, C.; Fritsche, L.G.; Igl, W.; Bailey, J.N.C.; Grassmann, F.; Sengupta, S.; Bragg-Gresham, J.L.; Burdon, K.P.; et al. A transcriptome-wide association study based on 27 tissues identifies 106 genes potentially relevant for disease pathology in age-related macular degeneration. Sci. Rep. 2020, 10, 1584. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, L.G.; Igl, W.; Bailey, J.N.C.; Grassmann, F.; Sengupta, S.; Bragg-Gresham, J.L.; Burdon, K.P.; Hebbring, S.J.; Wen, C.; Gorski, M.; et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat. Genet. 2016, 48, 134–143. [Google Scholar] [CrossRef]
- Seddon, J.M.; Reynolds, R.; Maller, J.; Fagerness, J.A.; Daly, M.J.; Rosner, B. Prediction model for prevalence and incidence of advanced age-related macular degeneration based on genetic, demographic, and environmental variables. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2044–2053. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.; Brantley, M.A.; Schwartz, S.G. Genetics and Age-Related Macular Degeneration: A Practical Review for Clinicians. Front. Biosci. 2024, 16, 3. [Google Scholar] [CrossRef]
- Fritsche, L.G.; Loenhardt, T.; Janssen, A.; Fisher, S.A.; Rivera, A.; Keilhauer, C.N.; Weber, B.H.F. Age-related macular degeneration is associated with an unstable ARMS2 (LOC387715) mRNA. Nat. Genet. 2008, 40, 892–896. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.G.; Agarwal, A.; Kovach, J.L.; Gallins, P.J.; Cade, W.; Postel, E.A.; Wang, G.; Ayala-Haedo, J.; Spencer, K.M.; Haines, J.L.; et al. The Arms2 A69s Variant And Bilateral Advanced Age-Related Macular Degeneration. RETINA 2012, 32, 1486–1491. [Google Scholar] [CrossRef]
- Shaheen, A.R.; Uhr, J.H.; Sridhar, J.; Yannuzzi, N.A. Limitations of direct-to-consumer genetic testing for age-related macular degeneration. Eur. J. Ophthalmol. 2023, 33, 2059–2061. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.K.; Yang, J.; Hsu, C.W.; Gore, A.; Bassuk, A.G.; Brown, L.M.; Colligan, R.; Sengillo, J.D.; Mahajan, V.B.; Tsang, S.H. HTRA1, an age-related macular degeneration protease, processes extracellular matrix proteins EFEMP1 and TSP1. Aging Cell 2018, 17, e12710. [Google Scholar] [CrossRef]
- Newman, A.M.; Gallo, N.B.; Hancox, L.S.; Miller, N.J.; Radeke, C.M.; Maloney, M.A.; Cooper, J.B.; Hageman, G.S.; Anderson, D.H.; Johnson, L.V.; et al. Systems-level analysis of age-related macular degeneration reveals global biomarkers and phenotype-specific functional networks. Genome Med. 2012, 4, 16. [Google Scholar] [CrossRef]
- Chong, V. Genetic influences on biomarkers in AMD. Acta Ophthalmol. 2009, 87. [Google Scholar] [CrossRef]
- Hjelmeland, L.M. Dark Matters in AMD Genetics: Epigenetics and Stochasticity. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1622–1631. [Google Scholar] [CrossRef]
- Chen, L.J. Age-Related Macular Degeneration: From Genetics to Epigenetics. Asia-Pac. J. Ophthalmol. 2013, 2, 211–212. [Google Scholar] [CrossRef]
- Feigl, B.; Morris, C.P. The challenge of predicting macular degeneration. Curr. Med. Res. Opin. 2011, 27, 1745–1748. [Google Scholar] [CrossRef]
- Cano, M.; Thimmalappula, R.; Fujihara, M.; Nagai, N.; Sporn, M.; Wang, A.L.; Neufeld, A.H.; Biswal, S.; Handa, J.T. Cigarette smoking, oxidative stress, the anti-oxidant response through Nrf2 signaling, and Age-related Macular Degeneration. Vision. Res. 2010, 50, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Cousins, S.W.; Espinosa-Heidmann, D.G.; Alexandridou, A.; Sall, J.; Dubovy, S.; Csaky, K. The role of aging, high fat diet and blue light exposure in an experimental mouse model for basal laminar deposit formation. Exp. Eye Res. 2002, 75, 543–553. [Google Scholar] [CrossRef]
- Kim, H.J.; Zhao, J.; Walewski, J.L.; Sparrow, J.R. A high fat diet fosters elevated bisretinoids. J. Biol. Chem. 2023, 299, 104784. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Zhou, J.; Ben-Shabat, S.; Vollmer, H.; Itagaki, Y.; Nakanishi, K. Involvement of oxidative mechanisms in blue-light-induced damage to A2E-laden RPE. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1222–1227. [Google Scholar]
- Li, X.; He, S.; Zhao, M. An Updated Review of the Epigenetic Mechanism Underlying the Pathogenesis of Age-related Macular Degeneration. Aging Dis. 2020, 11, 1219–1234. [Google Scholar] [CrossRef]
- Ach, T.; Huisingh, C.; McGwin, G., Jr.; Messinger, J.D.; Zhang, T.; Bentley, M.J.; Gutierrez, D.B.; Ablonczy, Z.; Smith, R.T.; Sloan, K.R.; et al. Quantitative autofluorescence and cell density maps of the human retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4832–4841. [Google Scholar] [CrossRef] [PubMed]
- Starnes, A.C.; Huisingh, C.; McGwin, G., Jr.; Sloan, K.R.; Ablonczy, Z.; Smith, R.T.; Curcio, C.A.; Ach, T. Multi-nucleate retinal pigment epithelium cells of the human macula exhibit a characteristic and highly specific distribution. Vis. Neurosci. 2016, 33, e001. [Google Scholar] [CrossRef] [PubMed]
- Sarks, S.H. Ageing and degeneration in the macular region: A clinico-pathological study. Br. J. Ophthalmol. 1976, 60, 324–341. [Google Scholar] [CrossRef]
- Handa, J.T.; Bowes Rickman, C.; Dick, A.D.; Gorin, M.B.; Miller, J.W.; Toth, C.A.; Ueffing, M.; Zarbin, M.; Farrer, L.A. A systems biology approach towards understanding and treating non-neovascular age-related macular degeneration. Nat. Commun. 2019, 10, 3347. [Google Scholar] [CrossRef]
- Lutty, G.A.; McLeod, D.S.; Bhutto, I.A.; Edwards, M.M.; Seddon, J.M. Choriocapillaris dropout in early age-related macular degeneration. Exp. Eye Res. 2020, 192, 107939. [Google Scholar] [CrossRef]
- Lee, M.Y.; Yoon, J.; Ham, D.I. Clinical characteristics of reticular pseudodrusen in Korean patients. Am. J. Ophthalmol. 2012, 153, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Mimura, T.; Noma, H. Oxidative Stress in Age-Related Macular Degeneration: From Molecular Mechanisms to Emerging Therapeutic Targets. Antioxidantss 2025, 14, 1251. [Google Scholar] [CrossRef]
- van Lookeren Campagne, M.; LeCouter, J.; Yaspan, B.L.; Ye, W. Mechanisms of age-related macular degeneration and therapeutic opportunities. J. Pathol. 2014, 232, 151–164. [Google Scholar] [CrossRef]
- Beatty, S.; Koh, H.; Phil, M.; Henson, D.; Boulton, M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol. 2000, 45, 115–134. [Google Scholar] [CrossRef]
- Kim, H.J.; Montenegro, D.; Zhao, J.; Sparrow, J.R. Bisretinoids of the Retina: Photo-Oxidation, Iron-Catalyzed Oxidation, and Disease Consequences. Antioxidants 2021, 10, 1382. [Google Scholar] [CrossRef]
- Wang, L.; Clark, M.E.; Crossman, D.K.; Kojima, K.; Messinger, J.D.; Mobley, J.A.; Curcio, C.A. Abundant lipid and protein components of drusen. PLoS ONE 2010, 5, e10329. [Google Scholar] [CrossRef] [PubMed]
- Tserentsoodol, N.; Gordiyenko, N.V.; Pascual, I.; Lee, J.W.; Fliesler, S.J.; Rodriguez, I.R. Intraretinal lipid transport is dependent on high density lipoprotein-like particles and class B scavenger receptors. Mol. Vis. 2006, 12, 1319–1333. [Google Scholar]
- Fujihara, M.; Cano, M.; Handa, J.T. Mice that produce ApoB100 lipoproteins in the RPE do not develop drusen yet are still a valuable experimental system. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7285–7295. [Google Scholar] [CrossRef]
- Yamada, Y.; Tian, J.; Yang, Y.; Cutler, R.G.; Wu, T.; Telljohann, R.S.; Mattson, M.P.; Handa, J.T. Oxidized low density lipoproteins induce a pathologic response by retinal pigmented epithelial cells. J. Neurochem. 2008, 105, 1187–1197. [Google Scholar] [CrossRef]
- Zeiss, C.J. Animals as models of age-related macular degeneration: An imperfect measure of the truth. Vet. Pathol. 2010, 47, 396–413. [Google Scholar] [CrossRef]
- Bhutto, I.A.; Baba, T.; Merges, C.; Juriasinghani, V.; McLeod, D.S.; Lutty, G.A. C-reactive protein and complement factor H in aged human eyes and eyes with age-related macular degeneration. Br. J. Ophthalmol. 2011, 95, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Parente, R.; Clark, S.J.; Inforzato, A.; Day, A.J. Complement factor H in host defense and immune evasion. Cell Mol. Life Sci. 2017, 74, 1605–1624. [Google Scholar] [CrossRef]
- Seddon, J.M.; Gensler, G.; Milton, R.C.; Klein, M.L.; Rifai, N. Association between C-reactive protein and age-related macular degeneration. JAMA 2004, 291, 704–710. [Google Scholar] [CrossRef]
- Brown, E.E.; Lewin, A.S.; Ash, J.D. Mitochondria: Potential Targets for Protection in Age-Related Macular Degeneration. Adv. Exp. Med. Biol. 2018, 1074, 11–17. [Google Scholar] [CrossRef]
- Itoh, K.; Nakamura, K.; Iijima, M.; Sesaki, H. Mitochondrial dynamics in neurodegeneration. Trends Cell Biol. 2013, 23, 64–71. [Google Scholar] [CrossRef]
- Terluk, M.R.; Kapphahn, R.J.; Soukup, L.M.; Gong, H.; Gallardo, C.; Montezuma, S.R.; Ferrington, D.A. Investigating mitochondria as a target for treating age-related macular degeneration. J. Neurosci. 2015, 35, 7304–7311. [Google Scholar] [CrossRef]
- Rohrer, B.; Bandyopadhyay, M.; Beeson, C. Reduced Metabolic Capacity in Aged Primary Retinal Pigment Epithelium (RPE) is Correlated with Increased Susceptibility to Oxidative Stress. Adv. Exp. Med. Biol. 2016, 854, 793–798. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Tombran-Tink, J. Mitochondrial decay and impairment of antioxidant defenses in aging RPE cells. Adv. Exp. Med. Biol. 2010, 664, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Hyttinen, J.M.T.; Koskela, A.; Blasiak, J.; Kaarniranta, K. Autophagy in drusen biogenesis secondary to age-related macular degeneration. Acta Ophthalmol. 2024, 102, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.L.; Lukas, T.J.; Yuan, M.; Du, N.Y.; Tso, M.O.M.; Neufeld, A.H. Autophagy, exosomes and drusen formation in age-related macular degeneration. Autophagy 2009, 5, 563–564. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.L.; Lukas, T.J.; Yuan, M.; Du, N.Y.; Tso, M.O.M.; Neufeld, A.H. Autophagy and Exosomes in the Aged Retinal Pigment Epithelium: Possible Relevance to Drusen Formation and Age-Related Macular Degeneration. PLoS ONE 2009, 4, e4160. [Google Scholar] [CrossRef]
- Yao, J.; Jia, L.; Khan, N.; Lin, C.; Mitter, S.K.; Boulton, M.E.; Dunaief, J.L.; Klionsky, D.J.; Guan, J.L.; Thompson, D.A.; et al. Deletion of autophagy inducer RB1CC1 results in degeneration of the retinal pigment epithelium. Autophagy 2015, 11, 939–953. [Google Scholar] [CrossRef]
- Mitter, S.K.; Song, C.; Qi, X.; Mao, H.; Rao, H.; Akin, D.; Lewin, A.; Grant, M.; Dunn, W., Jr.; Ding, J.; et al. Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD. Autophagy 2014, 10, 1989–2005. [Google Scholar] [CrossRef] [PubMed]
- McLeod, D.S.; Grebe, R.; Bhutto, I.; Merges, C.; Baba, T.; Lutty, G.A. Relationship between RPE and choriocapillaris in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4982–4991. [Google Scholar] [CrossRef]
- Moreira-Neto, C.A.; Moult, E.M.; Fujimoto, J.G.; Waheed, N.K.; Ferrara, D. Choriocapillaris Loss in Advanced Age-Related Macular Degeneration. J. Ophthalmol. 2018, 2018, 8125267. [Google Scholar] [CrossRef]
- Saint-Geniez, M.; Kurihara, T.; Sekiyama, E.; Maldonado, A.E.; D’Amore, P.A. Soluble VEGF isoforms are required for the maintenance of the retinal pigment epithelium (RPE)-choriocapillaris complex in the adult. FASEB J. 2008, 23, 1537. [Google Scholar]
- Huang, K.T.; Lin, C.C.; Tsai, M.C.; Chen, K.D.; Chiu, K.W. Pigment epithelium-derived factor in lipid metabolic disorders. Biomed. J. 2018, 41, 102–108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cao, W.; Tombran-tink, J.; Elias, R.V.; Sezate, S.A.; Mrazek, D.A.; McGinnis, J.F. In vivo protection of photoreceptors from light damage by pigment epithelium-derived factor. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1646–1652. [Google Scholar]
- Jakobsen, T.S.; Adsersen, R.L.; Askou, A.L.; Corydon, T.J. Functional Roles of Pigment Epithelium-Derived Factor in Retinal Degenerative and Vascular Disorders: A Scoping Review. Investig. Ophthalmol. Vis. Sci. 2024, 65, 41. [Google Scholar] [CrossRef] [PubMed]
- Bennis, A.; Gorgels, T.G.; Ten Brink, J.B.; van der Spek, P.J.; Bossers, K.; Heine, V.M.; Bergen, A.A. Comparison of Mouse and Human Retinal Pigment Epithelium Gene Expression Profiles: Potential Implications for Age-Related Macular Degeneration. PLoS ONE 2015, 10, e0141597. [Google Scholar] [CrossRef] [PubMed]
- Volland, S.; Esteve-Rudd, J.; Hoo, J.; Yee, C.; Williams, D.S. A comparison of some organizational characteristics of the mouse central retina and the human macula. PLoS ONE 2015, 10, e0125631. [Google Scholar] [CrossRef]
- Marmorstein, A.D.; Marmorstein, L.Y. The challenge of modeling macular degeneration in mice. Trends Genet. 2007, 23, 225–231. [Google Scholar] [CrossRef]
- Rabin, D.M.; Temple, S.; Stern, J.H. Chronic oxidative stress up-regulates drusen-related protein synthesis in primary human RPE cells: A novel culture model for the study of age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3912. [Google Scholar]
- Kushwah, N.; Bora, K.; Maurya, M.; Pavlovich, M.C.; Chen, J. Oxidative Stress and Antioxidants in Age-Related Macular Degeneration. Antioxidants 2023, 12, 1379. [Google Scholar] [CrossRef]
- Toma, C.; De Cillà, S.; Palumbo, A.; Garhwal, D.P.; Grossini, E. Oxidative and Nitrosative Stress in Age-Related Macular Degeneration: A Review of Their Role in Different Stages of Disease. Antioxidants 2021, 10, 653. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Taskintuna, K.; Hum, J.; Gulati, J.; Olaya, S.; Steinman, J.; Golestaneh, N. PGC-1α repression dysregulates lipid metabolism and induces lipid droplet accumulation in retinal pigment epithelium. Cell Death Dis. 2024, 15, 385. [Google Scholar] [CrossRef]
- Guo, X.; Dason, E.S.; Zanon-Moreno, V.; Jiang, Q.; Nahirnyj, A.; Chan, D.; Flanagan, J.G.; Sivak, J.M. PGC-1α signaling coordinates susceptibility to metabolic and oxidative injury in the inner retina. Am. J. Pathol. 2014, 184, 1017–1029. [Google Scholar] [CrossRef] [PubMed]
- Abu Shelbayeh, O.; Arroum, T.; Morris, S.; Busch, K.B. PGC-1α Is a Master Regulator of Mitochondrial Lifecycle and ROS Stress Response. Antioxidants 2023, 12, 1075. [Google Scholar] [CrossRef]
- Zhang, M.; Chu, Y.; Mowery, J.; Konkel, B.; Galli, S.; Theos, A.C.; Golestaneh, N. Pgc-1α repression and high-fat diet induce age-related macular degeneration-like phenotypes in mice. Dis. Model. Mech. 2018, 11, dmm032698. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Chen, Y.; Wang, J.; Sternberg, P.; Freeman, M.L.; Grossniklaus, H.E.; Cai, J. Age-related retinopathy in NRF2-deficient mice. PLoS ONE 2011, 6, e19456. [Google Scholar] [CrossRef]
- Rakoczy, P.E.; Zhang, D.; Robertson, T.; Barnett, N.L.; Papadimitriou, J.; Constable, I.J.; Lai, C.M. Progressive age-related changes similar to age-related macular degeneration in a transgenic mouse model. Am. J. Pathol. 2002, 161, 1515–1524. [Google Scholar] [CrossRef]
- Wang, K.; Zheng, M.; Lester, K.L.; Han, Z. Light-induced Nrf2(-/-) mice as atrophic age-related macular degeneration model and treatment with nanoceria laden injectable hydrogel. Sci. Rep. 2019, 9, 14573. [Google Scholar] [CrossRef]
- Sridevi Gurubaran, I.; Viiri, J.; Koskela, A.; Hyttinen, J.M.T.; Paterno, J.J.; Kis, G.; Antal, M.; Urtti, A.; Kauppinen, A.; Felszeghy, S.; et al. Mitophagy in the Retinal Pigment Epithelium of Dry Age-Related Macular Degeneration Investigated in the NFE2L2/PGC-1α(-/-) Mouse Model. Int. J. Mol. Sci. 2020, 21, 1976. [Google Scholar] [CrossRef]
- Leinonen, H.; Rossi, M.; Salo, A.M.; Tiainen, P.; Hyvärinen, J.; Pitkänen, M.; Sormunen, R.; Miinalainen, I.; Zhang, C.; Soininen, R.; et al. Lack of P4H-TM in mice results in age-related retinal and renal alterations. Hum. Mol. Genet. 2016, 25, 3810–3823. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Yang, J.; Xiao, B.; Wang, Y.; Lei, Y.; Lai, D.; Qiu, Q. Aberrant Lipid Metabolism and Complement Activation in Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2024, 65, 20. [Google Scholar] [CrossRef]
- Choudhary, M.; Ding, J.D.; Qi, X.; Boulton, M.E.; Yao, P.L.; Peters, J.M.; Malek, G. PPARβ/δ selectively regulates phenotypic features of age-related macular degeneration. Aging 2016, 8, 1952–1978. [Google Scholar] [CrossRef]
- Saadane, A.; Petrov, A.; Mast, N.; El-Darzi, N.; Dao, T.; Alnemri, A.; Song, Y.; Dunaief, J.L.; Pikuleva, I.A. Mechanisms that minimize retinal impact of apolipoprotein E absence. J. Lipid Res. 2018, 59, 2368–2382. [Google Scholar] [CrossRef]
- Dithmar, S.; Curcio, C.A.; Le, N.A.; Brown, S.; Grossniklaus, H.E. Ultrastructural changes in Bruch’s membrane of apolipoprotein E-deficient mice. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2035–2042. [Google Scholar]
- Espinosa-Heidmann, D.G.; Sall, J.; Hernandez, E.P.; Cousins, S.W. Basal laminar deposit formation in APO B100 transgenic mice: Complex interactions between dietary fat, blue light, and vitamin E. Investig. Ophthalmol. Vis. Sci. 2004, 45, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, M.; Bartels, E.; Nielsen, L.B.; Handa, J.T. A human apoB100 transgenic mouse expresses human apoB100 in the RPE and develops features of early AMD. Exp. Eye Res. 2009, 88, 1115–1123. [Google Scholar] [CrossRef]
- Marneros, A.G.; Keene, D.R.; Hansen, U.; Fukai, N.; Moulton, K.; Goletz, P.L.; Moiseyev, G.; Pawlyk, B.S.; Halfter, W.; Dong, S.; et al. Collagen XVIII/endostatin is essential for vision and retinal pigment epithelial function. Embo J 2004, 23, 89–99. [Google Scholar] [CrossRef]
- Chuang, J.Z.; Yang, N.; Nakajima, N.; Otsu, W.; Fu, C.; Yang, H.H.; Lee, M.P.; Akbar, A.F.; Badea, T.C.; Guo, Z.; et al. Retinal pigment epithelium-specific CLIC4 mutant is a mouse model of dry age-related macular degeneration. Nat. Commun. 2022, 13, 374. [Google Scholar] [CrossRef] [PubMed]
- Ascunce, K.; Dhodapkar, R.M.; Huang, D.; Hafler, B.P. Innate immune biology in age-related macular degeneration. Front. Cell Dev. Biol. 2023, 11, 1118524. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, A.; Aragona, E.; Bandello, F. The Role of Inflammation in Age-Related Macular Degeneration: Updates and Possible Therapeutic Approaches. Asia Pac. J. Ophthalmol. 2023, 12, 158–167. [Google Scholar] [CrossRef]
- Chen, M.; Xu, H. Parainflammation, chronic inflammation, and age-related macular degeneration. J. Leukoc. Biol. 2015, 98, 713–725. [Google Scholar] [CrossRef]
- Rowan, S.; Weikel, K.; Chang, M.L.; Nagel, B.A.; Thinschmidt, J.S.; Carey, A.; Grant, M.B.; Fliesler, S.J.; Smith, D.; Taylor, A. Cfh genotype interacts with dietary glycemic index to modulate age-related macular degeneration-like features in mice. Investig. Ophthalmol. Vis. Sci. 2014, 55, 492–501. [Google Scholar] [CrossRef]
- Ufret-Vincenty, R.L.; Aredo, B.; Liu, X.; McMahon, A.; Chen, P.W.; Sun, H.; Niederkorn, J.Y.; Kedzierski, W. Transgenic mice expressing variants of complement factor H develop AMD-like retinal findings. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5878–5887. [Google Scholar] [CrossRef]
- Combadière, C.; Feumi, C.; Raoul, W.; Keller, N.; Rodéro, M.; Pézard, A.; Lavalette, S.; Houssier, M.; Jonet, L.; Picard, E.; et al. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J. Clin. Investig. 2007, 117, 2920–2928. [Google Scholar] [CrossRef]
- Tuo, J.; Bojanowski, C.M.; Zhou, M.; Shen, D.; Ross, R.J.; Rosenberg, K.I.; Cameron, D.J.; Yin, C.; Kowalak, J.A.; Zhuang, Z.; et al. Murine ccl2/cx3cr1 deficiency results in retinal lesions mimicking human age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3827–3836. [Google Scholar] [CrossRef]
- Lyzogubov, V.; Wu, X.; Jha, P.; Tytarenko, R.; Triebwasser, M.; Kolar, G.; Bertram, P.; Bora, P.S.; Atkinson, J.P.; Bora, N.S. Complement regulatory protein CD46 protects against choroidal neovascularization in mice. Am. J. Pathol. 2014, 184, 2537–2548. [Google Scholar] [CrossRef]
- Lyzogubov, V.V.; Bora, P.S.; Wu, X.; Horn, L.E.; de Roque, R.; Rudolf, X.V.; Atkinson, J.P.; Bora, N.S. The Complement Regulatory Protein CD46 Deficient Mouse Spontaneously Develops Dry-Type Age-Related Macular Degeneration-Like Phenotype. Am. J. Pathol. 2016, 186, 2088–2104. [Google Scholar] [CrossRef] [PubMed]
- Lyzogubov, V.; Wu, X.; Kolar, G.; Bora, P.; Atkinson, J.; Bora, N. Cd46-/-Mouse as a Model of Dry-type Age-related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4591. [Google Scholar]
- Su, N.; Hansen, U.; Plagemann, T.; Gäher, K.; Leclaire, M.D.; König, J.; Höhn, A.; Grune, T.; Uhlig, C.E.; Eter, N.; et al. Sub-Retinal Injection of Human Lipofuscin in the Mouse—A Model of “Dry” Age-Related Macular Degeneration? Aging Dis. 2023, 14, 184–203. [Google Scholar] [CrossRef]
- Wu, L.; Ueda, K.; Nagasaki, T.; Sparrow, J.R. Light Damage in Abca4 and Rpe65rd12 Mice. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1910–1918. [Google Scholar] [CrossRef] [PubMed]
- Charbel Issa, P.; Barnard, A.R.; Herrmann, P.; Washington, I.; MacLaren, R.E. Rescue of the Stargardt phenotype in Abca4 knockout mice through inhibition of vitamin A dimerization. Proc. Natl. Acad. Sci. USA 2015, 112, 8415–8420. [Google Scholar] [CrossRef] [PubMed]
- Farnoodian, M.; Bose, D.; Barone, F.; Nelson, L.M.; Boyle, M.; Jun, B.; Do, K.; Gordon, W.; Guerin, M.K.; Perera, R.; et al. Retina and RPE lipid profile changes linked with ABCA4 associated Stargardt’s maculopathy. Pharmacol. Ther. 2023, 249, 108482. [Google Scholar] [CrossRef]
- Ueda, K.; Zhao, J.; Kim, H.J.; Sparrow, J.R. Photodegradation of retinal bisretinoids in mouse models and implications for macular degeneration. Proc. Natl. Acad. Sci. USA 2016, 113, 6904–6909. [Google Scholar] [CrossRef]
- Conley, S.M.; Cai, X.; Makkia, R.; Wu, Y.; Sparrow, J.R.; Naash, M.I. Increased cone sensitivity to ABCA4 deficiency provides insight into macular vision loss in Stargardt’s dystrophy. Biochim. Biophys. Acta 2012, 1822, 1169–1179. [Google Scholar] [CrossRef]
- Maeda, A.; Maeda, T.; Golczak, M.; Palczewski, K. Retinopathy in mice induced by disrupted all-trans-retinal clearance. J. Biol. Chem. 2008, 283, 26684–26693. [Google Scholar] [CrossRef]
- Karan, G.; Lillo, C.; Yang, Z.; Cameron, D.J.; Locke, K.G.; Zhao, Y.; Thirumalaichary, S.; Li, C.; Birch, D.G.; Vollmer-Snarr, H.R.; et al. Lipofuscin accumulation, abnormal electrophysiology, and photoreceptor degeneration in mutant ELOVL4 transgenic mice: A model for macular degeneration. Proc. Natl. Acad. Sci. USA 2005, 102, 4164–4169. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.L.; LaVail, M.M.; Yasumura, D.; Matthes, M.T.; Yang, H.; Trautmann, N.; Chappelow, A.V.; Feng, W.; Earp, H.S.; Matsushima, G.K.; et al. An RCS-like retinal dystrophy phenotype in mer knockout mice. Investig. Ophthalmol. Vis. Sci. 2003, 44, 826–838. [Google Scholar] [CrossRef]
- Keeling, E.; Lynn, S.A.; Koh, Y.M.; Scott, J.A.; Kendall, A.; Gatherer, M.; Page, A.; Cagampang, F.R.; Lotery, A.J.; Ratnayaka, J.A. A High Fat “Western-style” Diet Induces AMD-Like Features in Wildtype Mice. Mol. Nutr. Food Res. 2022, 66, e2100823. [Google Scholar] [CrossRef]
- Hollyfield, J.G.; Bonilha, V.L.; Rayborn, M.E.; Yang, X.; Shadrach, K.G.; Lu, L.; Ufret, R.L.; Salomon, R.G.; Perez, V.L. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat. Med. 2008, 14, 194–198. [Google Scholar] [CrossRef]
- Azuma, K.; Suzuki, T.; Kobayashi, K.; Nagahara, M.; Imai, H.; Suga, A.; Iwata, T.; Shiraya, T.; Aihara, M.; Ueta, T. Retinal pigment epithelium-specific ablation of GPx4 in adult mice recapitulates key features of geographic atrophy in age-related macular degeneration. Cell Death Dis. 2024, 15, 763. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowski, A.M.; Bell, B.A.; Song, Y.; Anderson, B.D.; Conomikes, A.; Petruconis, C.; Dunaief, J.L. Inducible RPE-specific GPX4 knockout causes oxidative stress and retinal degeneration with features of age-related macular degeneration. Exp. Eye Res. 2024, 247, 110028. [Google Scholar] [CrossRef]
- Cruz-Guilloty, F.; Echegaray, J.; Perez, V.L. The Pathogenesis of Dry Age-Related Macular Degeneration. Available online: https://retinatoday.com/articles/2011-apr/the-pathogenesis-of-dry-age-related-macular-degeneration (accessed on 2 February 2025).
- Chen, S.-J.; Lin, T.-B.; Peng, H.-Y.; Liu, H.-J.; Lee, A.-S.; Lin, C.-H.; Tseng, K.-W. Cytoprotective potential of fucoxanthin in oxidative stress-induced age-related macular degeneration and retinal pigment epithelial cell senescence in vivo and in vitro. Mar. Drugs 2021, 19, 114. [Google Scholar]
- Moriguchi, M.; Nakamura, S.; Inoue, Y.; Nishinaka, A.; Nakamura, M.; Shimazawa, M.; Hara, H. Irreversible Photoreceptors and RPE Cells Damage by Intravenous Sodium Iodate in Mice Is Related to Macrophage Accumulation. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3476–3487. [Google Scholar] [CrossRef]
- Lyzogubov, V.V.; Bora, N.S.; Tytarenko, R.G.; Bora, P.S. Polyethylene glycol induced mouse model of retinal degeneration. Exp. Eye Res. 2014, 127, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.; Lako, M. Retinal organoids provide unique insights into molecular signatures of inherited retinal disease throughout retinogenesis. J. Anat. 2023, 243, 186–203. [Google Scholar] [CrossRef] [PubMed]
- Mesch, L.; Pashkovskaia, N.; Cora, V.; Pars, S.; Corti, S.; Cipriano, M.; Loskill, P.; Koertvely, E.; Kustermann, S.; Mesquida, M. Aged human iPSC-RPE organoid cultures display hallmarks of drusen formation. bioRxiv 2021. [Google Scholar] [CrossRef]
- Lako, M.; Buskin, A.; Zhu, L.; Chichagova, V.; Basu, B.; Mozaffari-Jovin, S.; Dolan, D.; Droop, A.P.; Collin, J.; Hilgen, G.; et al. Human iPSC-derived RPE and retinal organoids reveal impaired alternative splicing of genes involved in pre-mRNA splicing in PRPF31 autosomal dominant retinitis pigmentosa. bioRxiv 2017. [Google Scholar] [CrossRef]
- Galloway, C.A.; Dalvi, S.; Hung, S.S.C.; MacDonald, L.A.; Latchney, L.R.; Wong, R.C.B.; Guymer, R.H.; Mackey, D.A.; Williams, D.S.; Chung, M.M.; et al. Drusen in patient-derived hiPSC-RPE models of macular dystrophies. Proc. Natl. Acad. Sci. USA 2017, 114, E8214–E8223. [Google Scholar] [CrossRef] [PubMed]
- Saini, J.S.; Corneo, B.; Miller, J.D.; Kiehl, T.R.; Wang, Q.; Boles, N.C.; Blenkinsop, T.A.; Stern, J.H.; Temple, S. Nicotinamide Ameliorates Disease Phenotypes in a Human iPSC Model of Age-Related Macular Degeneration. Cell Stem Cell 2017, 20, 635–647.E7. [Google Scholar] [CrossRef]
- Farrer, L.A.; DeAngelis, M.M. Human induced pluripotent stem cells illuminate pathways and novel treatment targets for age-related macular degeneration. Stem Cell Investig. 2017, 4, 92. [Google Scholar] [CrossRef]
- Zhong, X.; Gutierrez, C.; Xue, T.; Hampton, C.; Vergara, M.N.; Cao, L.H.; Peters, A.; Park, T.S.; Zambidis, E.T.; Meyer, J.S.; et al. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat. Commun. 2014, 5, 4047. [Google Scholar] [CrossRef]
- Moy, A.B.; Kamath, A.; Ternes, S.; Kamath, J. The Challenges to Advancing Induced Pluripotent Stem Cell-Dependent Cell Replacement Therapy. Med. Res. Arch. 2023, 11, 4784. [Google Scholar] [CrossRef] [PubMed]
- Moshiri, A.; Chen, R.; Chen, R.; Kim, S.; Harris, R.A.; Li, Y.; Raveendran, M.; Davis, S.; Liang, Q.; Pomerantz, O.; et al. A nonhuman primate model of inherited retinal disease. J. Clin. Investig. 2019, 129, 863–874. [Google Scholar] [CrossRef]
- Picaud, S.; Dalkara, D.; Marazova, K.; Goureau, O.; Roska, B.; Sahel, J.A. The primate model for understanding and restoring vision. Proc. Natl. Acad. Sci. USA 2019, 116, 26280–26287. [Google Scholar] [CrossRef]
- Uyhazi, K.E.; Bennett, J. Blinded by the light: A nonhuman primate model of achromatopsia. J. Clin. Investig. 2019, 129, 513–515. [Google Scholar] [CrossRef]
- Yi, W.; Xu, M.; Xue, Y.; Cao, Y.; Yang, Z.; Zhou, L.; Zhou, Y.; Shi, L.; Mai, X.; Sun, Z.; et al. A spontaneous nonhuman primate model of inherited retinal degeneration. JCI Insight 2025, 10, e190807. [Google Scholar] [CrossRef]
- Pan, L.; Wang, N.; Wu, J. Non-human primates as preclinical models for optic nerve research: Advancing insights into their application and potential. Eye 2025, 39, 1254–1263. [Google Scholar] [CrossRef]
- Ail, D.; Nava, D.P.; Hwang, I.P.; Brazhnikova, E.; Nouvel-Jaillard, C.; Dentel, A.; Joffrois, C.; Rousseau, L.; Dégardin, J.; Bertin, S.; et al. Inducible nonhuman primate models of retinal degeneration for testing end-stage therapies. Sci. Adv. 2022, 9, eadg8163. [Google Scholar] [CrossRef]
- Takamatsu, H. Non-Human Primate Model of Age-Related Macular Degeneration and Method for Producing Same. U.S. Patent 10,532,112, 14 January 2020. [Google Scholar]
- Peraramelli, S.; Chen, J.; Yu, A.; Chen, X.; Santillan, E.; Huynh, D.; Luo, N.; Li, S.; Li, J.; Liu, S. Protective Efficacy of ABI-201 in a Sodium Iodate-Induced Retinal Damage Model of Dry AMD in Non-Human Primates. Investig. Ophthalmol. Vis. Sci. 2025, 66, 1898. [Google Scholar]
- Rajagopalan, L.; Ghosn, C.; Tamhane, M.; Almazan, A.; Andrews-Jones, L.; Kulkarni, A.; Christie, L.A.; Burke, J.; López, F.J.; Engles, M. A nonhuman primate model of blue light-induced progressive outer retina degeneration showing brimonidine drug delivery system-mediated cyto- and neuroprotection. Exp. Eye Res. 2021, 209, 108678. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Lin, Q.; He, H.L.; Liu, Y.X.; Li, M.; Zhang, X.H.; Jin, Z.B. Juvenile macular degeneration in nonhuman primates generated by germline knockout of ABCA4 gene. Sci. Bull. 2025, 70, 2237–2240. [Google Scholar] [CrossRef]
- Cai, H.; Gong, J.; Del Priore, L.V.; Tezel, T.H.; Fields, M.A. Culturing of Retinal Pigment Epithelial Cells on an Ex Vivo Model of Aged Human Bruch’s Membrane. J. Vis. Exp. 2018, 57084. [Google Scholar] [CrossRef]
- Ablonczy, Z.; Dahrouj, M.; Tang, P.H.; Liu, Y.; Sambamurti, K.; Marmorstein, A.D.; Crosson, C.E. Human retinal pigment epithelium cells as functional models for the RPE in vivo. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8614–8620. [Google Scholar] [CrossRef]
- Wagner, N.; Safaei, A.; Vogt, P.A.; Gammel, M.R.; Dick, H.B.; Schnichels, S.; Joachim, S.C. Coculture of ARPE-19 Cells and Porcine Neural Retina as an Ex Vivo Retinal Model. Altern. Lab. Anim. 2022, 50, 27–44. [Google Scholar] [CrossRef]
- Lynn, S.A.; Keeling, E.; Dewing, J.M.; Johnston, D.A.; Page, A.; Cree, A.J.; Tumbarello, D.A.; Newman, T.A.; Lotery, A.J.; Ratnayaka, J.A. A convenient protocol for establishing a human cell culture model of the outer retina. F1000Research 2018, 7, 1107. [Google Scholar] [CrossRef] [PubMed]
- Huber, G.; Heynen, S.; Imsand, C.; vom Hagen, F.; Muehlfriedel, R.; Tanimoto, N.; Feng, Y.; Hammes, H.P.; Grimm, C.; Peichl, L.; et al. Novel rodent models for macular research. PLoS ONE 2010, 5, e13403. [Google Scholar] [CrossRef]
- Schnichels, S.; Kiebler, T.; Hurst, J.; Maliha, A.M.; Löscher, M.; Dick, H.B.; Bartz-Schmidt, K.U.; Joachim, S.C. Retinal Organ Cultures as Alternative Research Models. Altern. Lab. Anim. 2019, 47, 19–29. [Google Scholar] [CrossRef]
- Wu, A.; Lu, R.; Lee, E. Tissue engineering in age-related macular degeneration: A mini-review. J. Biol. Eng. 2022, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Lide Biotech Staff. Mouse Models: Applications, Types, Advantages, and Disadvantages; Lide Biotech Staff: Shanghai, China, 2023. [Google Scholar]
- Emulate. In Vitro vs In Vivo: A History of Modern Cell Culture; Emulate: Boston, MA, USA, 2024. [Google Scholar]
- National Research Council. Summary of Advantages and Disadvantages of In Vitro and In Vivo Methods; National Research Council: Ottawa, ON, Canada, 1999. [Google Scholar]
- Guimarães, A.I. Are Animal Models Necessary? Exploring (Dis)advantages and Alternatives. Eur. J. Neurosci. 2025, 61, e16651. [Google Scholar] [CrossRef] [PubMed]
- Baxter, V.K.; Griffin, D.E. Chapter 10—Animal Models: No Model Is Perfect, but Many Are Useful. In Viral Pathogenesis, 3rd ed.; Katze, M.G., Korth, M.J., Law, G.L., Nathanson, N., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 125–138. [Google Scholar]
- Long, X.; Zeng, J. Why non-human primates are needed in stroke preclinical research. Stroke Vasc. Neurol. 2024, 10. [Google Scholar] [CrossRef]
- VandeBerg, J.L.; Williams-Blangero, S. Advantages and limitations of nonhuman primates as animal models in genetic research on complex diseases. J. Med. Primatol. 1997, 26, 113–119. [Google Scholar] [CrossRef]
- Animal Models. 2025. Available online: https://hms.harvard.edu/research/animal-research/animal-models (accessed on 2 December 2025).
- Fabre, M.; Mateo, L.; Lamaa, D.; Baillif, S.; Pagès, G.; Demange, L.; Ronco, C.; Benhida, R. Recent Advances in Age-Related Macular Degeneration Therapies. Molecules 2022, 27, 5089. [Google Scholar] [CrossRef]
- Group, A.-R.E.D.S.R. A Randomized, Placebo-Controlled, Clinical Trial of High-Dose Supplementation with Vitamins C and E, Beta Carotene, and Zinc for Age-Related Macular Degeneration and Vision Loss: AREDS Report No. 8. Arch. Ophthalmol. 2001, 119, 1417–1436. [Google Scholar] [CrossRef]
- Ruskin, D. The Legacy of AREDS: AREDS-2 raised eyebrows, but could also open doors to ‘pharmaco-genomics’ and more meaningful use of personalized genetic testing. Rev. Optom. 2014, 151, 36–41. [Google Scholar]
- Keenan, T.D.L.; Agrón, E.; Keane, P.A.; Domalpally, A.; Chew, E.Y. Oral Antioxidant and Lutein/Zeaxanthin Supplements Slow Geographic Atrophy Progression to the Fovea in Age-Related Macular Degeneration. Ophthalmology 2025, 132, 14–29. [Google Scholar] [CrossRef]
- Nadeem, A.; Malik, I.A.; Shariq, F.; Afridi, E.K.; Taha, M.; Raufi, N.; Naveed, A.K.; Iqbal, J.; Habte, A. Advancements in the treatment of geographic atrophy: Focus on pegcetacoplan in age-related macular degeneration. Ann. Med. Surg. 2023, 85, 6067–6077. [Google Scholar] [CrossRef]
- Letter, M. Pegcetacoplan (Syfovre) for geographic atrophy in age-related macular degeneration. Med. Lett. Drugs Ther. 2023, 65, 49–50. [Google Scholar] [CrossRef]
- Bio, I. Iveric Bio Receives U.S. FDA Approval for IZERVAY™ (Avacincaptad Pegol Intravitreal Solution), a New Treatment for Geographic Atrophy. 2023. Available online: https://www.astellas.com/en/news/28281 (accessed on 2 December 2025).
- Yuan, X.; Gavriilaki, E.; Thanassi, J.A.; Yang, G.; Baines, A.C.; Podos, S.D.; Huang, Y.; Huang, M.; Brodsky, R.A. Small-molecule factor D inhibitors selectively block the alternative pathway of complement in paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome. Haematologica 2017, 102, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Boyer, D.D.; Ko, Y.P.; Podos, S.D.; Cartwright, M.E.; Gao, X.; Wiles, J.A.; Huang, M. Danicopan, an Oral Complement Factor D Inhibitor, Exhibits High and Sustained Exposure in Ocular Tissues in Preclinical Studies. Transl. Vis. Sci. Technol. 2022, 11, 37. [Google Scholar] [CrossRef]
- Jaffe, G.J.; Sahni, J.; Fauser, S.; Geary, R.S.; Schneider, E.; McCaleb, M. Development of IONIS-FB-LRx to treat geographic atrophy associated with AMD. Investig. Ophthalmol. Vis. Sci. 2020, 61, 4305. [Google Scholar]
- Patel, P.N.; Patel, P.A.; Land, M.R.; Bakerkhatib-Taha, I.; Ahmed, H.; Sheth, V. Targeting the Complement Cascade for Treatment of Dry Age-Related Macular Degeneration. Biomedicines 2022, 10, 1884. [Google Scholar] [CrossRef]
- Furfine, E.; Rao, A.; Baker, S.; Connacher, M.K.; Kazanstev, A.; Kumar, B.; Blouse, G.; Stanton, M.; Traylor, M. Pegylated CB2782: A complement factor C3-inactivating protease and potential long-acting treatment for dry AMD. Investig. Ophthalmol. Vis. Sci. 2019, 60, 374. [Google Scholar]
- Hong, T.; Chang, A.; Maddess, T.; Provis, J.; Penfold, P. Phase 1B study of the safety and tolerability of the mineralocorticoid fludrocortisone acetate in patients with geographical atrophy. BMJ Open Ophthalmol. 2022, 7, e001032. [Google Scholar] [CrossRef] [PubMed]
- Therapeutics, I. InflammX Clinical Candidates and Indications. Available online: https://inflammx.com/xiflam (accessed on 30 June 2025).
- Charters, L. Xiflam inhibits initiation of the inflammatory cascade. Ophthalmology Times, 11 February 2022. [Google Scholar]
- Ma, L.; Kaufman, Y.; Zhang, J.; Washington, I. C20-D3-vitamin A slows lipofuscin accumulation and electrophysiological retinal degeneration in a mouse model of Stargardt disease. J. Biol. Chem. 2011, 286, 7966–7974. [Google Scholar] [CrossRef]
- Filkins, K. FDA grants Breakthrough Therapy Designation for Tinlarebant. Ophthalmology Times, 21 May 2025. [Google Scholar]
- Stargazer Pharmaceuticals, I. Stargazer Pharmaceuticals, Inc. Announces $57 Million Series A Financing and Initiation of a Phase 2a Clinical Study of STG-001 in Stargardt Disease Patients. RD FUND, 9 November 2020. [Google Scholar]
- Bavik, C.; Henry, S.H.; Zhang, Y.; Mitts, K.; McGinn, T.; Budzynski, E.; Pashko, A.; Lieu, K.L.; Zhong, S.; Blumberg, B.; et al. Visual Cycle Modulation as an Approach toward Preservation of Retinal Integrity. PLoS ONE 2015, 10, e0124940. [Google Scholar] [CrossRef]
- Rosenfeld, P.J.; Dugel, P.U.; Holz, F.G.; Heier, J.S.; Pearlman, J.A.; Novack, R.L.; Csaky, K.G.; Koester, J.M.; Gregory, J.K.; Kubota, R. Emixustat hydrochloride for geographic atrophy secondary to age-related macular degeneration: A randomized clinical trial. Ophthalmology 2018, 125, 1556–1567. [Google Scholar] [CrossRef]
- Kocab, A.J.; Cano, M.; Bacellar-Galdino, M.; Jamison, J.A.; Brock, W.J.; Zacks, D.N.; Handa, J.T. In Vivo Characterization of ONL1204, a Small Peptide Inhibitor of the Fas Receptor, as a Potential Neuroprotective Therapy for Geographic Atrophy and Dry Age-Related Macular Degeneration. Biomedicines 2025, 13, 2052. [Google Scholar] [CrossRef]
- Kauper, K.; McGovern, C.; Sherman, S.; Heatherton, P.; Rapoza, R.; Stabila, P.; Dean, B.; Lee, A.; Borges, S.; Bouchard, B.; et al. Two-year intraocular delivery of ciliary neurotrophic factor by encapsulated cell technology implants in patients with chronic retinal degenerative diseases. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7484–7491. [Google Scholar] [CrossRef]
- Lin, J.B.; Murakami, Y.; Miller, J.W.; Vavvas, D.G. Neuroprotection for Age-Related Macular Degeneration. Ophthalmol. Sci. 2022, 2, 100192. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, Y.; Duan, Y.; Zhang, X.; Li, X. Mesenchymal-Stem-Cell-Based Strategies for Retinal Diseases. Genes 2022, 13, 1901. [Google Scholar] [CrossRef]
- Sivapathasuntharam, C.; Sivaprasad, S.; Hogg, C.; Jeffery, G. Aging retinal function is improved by near infrared light (670 nm) that is associated with corrected mitochondrial decline. Neurobiol. Aging 2017, 52, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Girgis, S.; Lee, L.R. Treatment of dry age-related macular degeneration: A review. Clin. Exp. Ophthalmol. 2023, 51, 835–852. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, S.N.; Devenyi, R.G.; Munk, M.R.; Croissant, C.L.; Tedford, S.E.; Rückert, R.; Walker, M.G.; Patino, B.E.; Chen, L.; Nido, M.; et al. A Double-Masked, Randomized, Sham-Controlled, Single-Center Study with Photobiomodulation For The Treatment Of Dry Age-Related Macular Degeneration. Retina 2020, 40, 1471–1482. [Google Scholar] [CrossRef] [PubMed]
- Khanani, A.M.; Thomas, M.J.; Aziz, A.A.; Weng, C.Y.; Danzig, C.J.; Yiu, G.; Kiss, S.; Waheed, N.K.; Kaiser, P.K. Review of gene therapies for age-related macular degeneration. Eye 2022, 36, 303–311. [Google Scholar] [CrossRef]
- Rudnick, N.D.; Kim, L.A.; Comander, J. Adeno-associated Viral Vectors in the Retina: Delivering Gene Therapy to the Right Destination. Int. Ophthalmol. Clin. 2022, 62, 215–229. [Google Scholar] [CrossRef]
- Keefe, D.; Munye, M.; Hasan, R.; Rathi, S.; Bishop, P.; Clark, S. CTx001, a gene therapy for the treatment of geographic atrophy in aged-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2023, 64, 3852. [Google Scholar]
- Jensen, E.G.; Jakobsen, T.S.; Schnabolk, G.; Wilson, K.; Rask-Pedersen, M.; Jensen, N.; Andersen, G.R.; Thiel, S.; Aagaard, L.; Rohrer, B.; et al. Nanobody-based gene therapy targeting complement component C3 reduces choroidal neovascularization in mice. Mol. Ther. Methods Clin. Dev. 2025, 33, 101620. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Zhang, L.; Luo, L.; Yang, M.; Chen, Y.; Lin, F. A nanobody-based complement inhibitor targeting complement component 2 reduces hemolysis in a complement humanized mouse model of autoimmune hemolytic anemia. Clin. Immunol. 2023, 253, 109678. [Google Scholar] [CrossRef]
- Takita, H.; Yoneya, S.; Gehlbach, P.L.; Duh, E.J.; Wei, L.L.; Mori, K. Retinal Neuroprotection against Ischemic Injury Mediated by Intraocular Gene Transfer of Pigment Epithelium-Derived Factor. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4497–4504. [Google Scholar] [CrossRef]
- Osborne, A.; Boyd, K.; Vaux, L.; Widdowson, P.S.; Binley, K.; Warner, E. Evaluation of a novel bi-cistronic gene therapy for the treatment of geographic atrophy. Investig. Ophthalmol. Vis. Sci. 2023, 64, 799. [Google Scholar]
- Dhooge, P.P.A.; Möller, P.T.; Boon, C.J.F.; Lotery, A.J.; Herrmann, P.; Battaglia Parodi, M.; Klein, W.; Fsadni, M.G.; Wheeler-Schilling, T.H.; Jungmann, O.; et al. The STArgardt Remofuscin Treatment Trial (STARTT): Design and baseline characteristics of enrolled Stargardt patients. Open Res. Eur. 2021, 1, 96. [Google Scholar] [CrossRef] [PubMed]
- Young, A. RISUTEGANIB Improves Visual Acuity in Patients with Dry AMD in Phase 2A Study. Healio, 18 November 2021. [Google Scholar]
- Wright, C.B.; Ambati, J. Dry Age-Related Macular Degeneration Pharmacology. Handb. Exp. Pharmacol. 2017, 242, 321–336. [Google Scholar] [CrossRef]
- Abidi, M.; Karrer, E.; Csaky, K.; Handa, J.T. A Clinical and Preclinical Assessment of Clinical Trials for Dry Age-Related Macular Degeneration. Ophthalmol. Sci. 2022, 2, 100213. [Google Scholar] [CrossRef]
- Schmetterer, L.; Scholl, H.; Garhöfer, G.; Janeschitz-Kriegl, L.; Corvi, F.; Sadda, S.R.; Medeiros, F.A. Endpoints for clinical trials in ophthalmology. Prog. Retin. Eye Res. 2023, 97, 101160. [Google Scholar] [CrossRef]
- Yehoshua, Z.; de Amorim Garcia Filho, C.A.; Nunes, R.P.; Gregori, G.; Penha, F.M.; Moshfeghi, A.A.; Zhang, K.; Sadda, S.; Feuer, W.; Rosenfeld, P.J. Systemic complement inhibition with eculizumab for geographic atrophy in age-related macular degeneration: The COMPLETE study. Ophthalmology 2014, 121, 693–701. [Google Scholar] [CrossRef]
- Yaspan, B.L.; Williams, D.F.; Holz, F.G.; Regillo, C.D.; Li, Z.; Dressen, A.; van Lookeren Campagne, M.; Le, K.N.; Graham, R.R.; Beres, T.; et al. Targeting factor D of the alternative complement pathway reduces geographic atrophy progression secondary to age-related macular degeneration. Sci. Transl. Med. 2017, 9, eaaf1443. [Google Scholar] [CrossRef] [PubMed]
- Holz, F.G.; Sadda, S.R.; Busbee, B.; Chew, E.Y.; Mitchell, P.; Tufail, A.; Brittain, C.; Ferrara, D.; Gray, S.; Honigberg, L.; et al. Efficacy and Safety of Lampalizumab for Geographic Atrophy Due to Age-Related Macular Degeneration: Chroma and Spectri Phase 3 Randomized Clinical Trials. JAMA Ophthalmol. 2018, 136, 666–677. [Google Scholar] [CrossRef]
- Radu, R.A.; Han, Y.; Bui, T.V.; Nusinowitz, S.; Bok, D.; Lichter, J.; Widder, K.; Travis, G.H.; Mata, N.L. Reductions in Serum Vitamin A Arrest Accumulation of Toxic Retinal Fluorophores: A Potential Therapy for Treatment of Lipofuscin-Based Retinal Diseases. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4393–4401. [Google Scholar] [CrossRef]
- Cabral de Guimaraes, T.A.; Daich Varela, M.; Georgiou, M.; Michaelides, M. Treatments for dry age-related macular degeneration: Therapeutic avenues, clinical trials and future directions. Br. J. Ophthalmol. 2022, 106, 297–304. [Google Scholar] [CrossRef]
- Heesterbeek, T.J.; Lorés-Motta, L.; Hoyng, C.B.; Lechanteur, Y.T.E.; den Hollander, A.I. Risk factors for progression of age-related macular degeneration. Ophthalmic Physiol. Opt. 2020, 40, 140–170. [Google Scholar] [CrossRef]
- Achberger, K.; Probst, C.; Haderspeck, J.C.; Bolz, S.; Rogal, J.; Chuchuy, J.; Nikolova, M.; Cora, V.; Antkowiak, L.; Haq, W.; et al. Merging organoid and organ-on-a-chip technology to generate complex multi-layer tissue models in a human retina-on-a-chip platform. eLife 2019, 8, e46188. [Google Scholar] [CrossRef]
- Dorgau, B.; Georgiou, M.; Chaudhary, A.; Moya-Molina, M.; Collin, J.; Queen, R.; Hilgen, G.; Davey, T.; Hewitt, P.; Schmitt, M.; et al. Human Retinal Organoids Provide a Suitable Tool for Toxicological Investigations: A Comprehensive Validation Using Drugs and Compounds Affecting the Retina. Stem Cells Transl. Med. 2022, 11, 159–177. [Google Scholar] [CrossRef]
- Hao, X.; Du, L.; Liu, G.; Li, Z.; Wang, S. Human retinal organoids for modelling dry age-related macular degeneration and screening drugs. Genes. Dis. 2025, 12, 101593. [Google Scholar] [CrossRef] [PubMed]
- Parween, S.; Howell, A.; Varghese, S.; Vergara, M.N. Human Retinal Organoid Model of Photoreceptor Cell Death Amenable to Drug Screening for AMD. Investig. Ophthalmol. Vis. Sci. 2024, 65, 4508. [Google Scholar]
- Yuan, L.Y.; Li, L.P.; Hua, X.; Yuan, X.Y. Tracing global progress: Two decades of age-related macular degeneration research. Int. J. Ophthalmol. 2025, 18, 925–936. [Google Scholar] [CrossRef]
- Shetty, S.; Singh, K.; Barve, K. Therapeutic Management and New Upcoming Approaches for Age Related Macular Degeneration. Curr. Drug Res. Rev. 2025, 17, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Jay, C.M.; John, A. Next-Generation Therapies to Slow or Halt AMD Vision Loss. Available online: https://eyeandear.org/2025/07/next-generation-therapies-to-slow-or-halt-amd-vision-loss/ (accessed on 23 September 2025).
- Grenga, P.L.; Ciancimino, C.; Meduri, A.; Fragiotta, S. Optogenetics: A Novel Therapeutic Avenue for Age-Related Macular Degeneration. Biomolecules 2025, 15, 1286. [Google Scholar] [CrossRef] [PubMed]
- Sahel, J.-A.; Boulanger-Scemama, E.; Pagot, C.; Arleo, A.; Galluppi, F.; Martel, J.N.; Esposti, S.D.; Delaux, A.; de Saint Aubert, J.-B.; de Montleau, C.; et al. Partial recovery of visual function in a blind patient after optogenetic therapy. Nat. Med. 2021, 27, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Campbell, R.E.; Côté, D.C.; Paquet, M.E. Challenges for Therapeutic Applications of Opsin-Based Optogenetic Tools in Humans. Front. Neural Circuits 2020, 14, 41. [Google Scholar] [CrossRef]
- Umulisa, K.J. Statistics in Personalized Medicine: Challenges and Innovations. NEWPORT Int. J. Biol. Appl. Sci. 2024, 5, 39–43. [Google Scholar] [CrossRef]
- S, D.; R, K. A Review of the Regulatory Challenges of Personalized Medicine. Cureus 2024, 16, e67891. [Google Scholar] [CrossRef]
- Enzendorfer, M.L.; Tratnig-Frankl, M.; Eidenberger, A.; Schrittwieser, J.; Kuchernig, L.; Schmidt-Erfurth, U. Rethinking Clinical Trials in Age-Related Macular Degeneration: How AI-Based OCT Analysis Can Support Successful Outcomes. Pharmaceuticals 2025, 18, 284. [Google Scholar] [CrossRef]
- Finger, R.P.; Schmitz-Valckenberg, S.; Schmid, M.; Rubin, G.S.; Dunbar, H.; Tufail, A.; Crabb, D.P.; Binns, A.; Sánchez, C.I.; Margaron, P.; et al. MACUSTAR: Development and Clinical Validation of Functional, Structural, and Patient-Reported Endpoints in Intermediate Age-Related Macular Degeneration. Ophthalmologica 2019, 241, 61–72. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guymer, R.; Wu, Z. Age-related macular degeneration (AMD): More than meets the eye. The role of multimodal imaging in today’s management of AMD—A review. Clin. Exp. Ophthalmol. 2020, 48, 983–995. [Google Scholar] [CrossRef]
- Krader, C.G. No one imaging tool fits all in identifying AMD lesions. Modern Retina, 27 May 2017. [Google Scholar]
- Schmidt-Erfurth, U.; Klimscha, S.; Waldstein, S.M.; Bogunović, H. A view of the current and future role of optical coherence tomography in the management of age-related macular degeneration. Eye 2017, 31, 26–44. [Google Scholar] [CrossRef] [PubMed]
- Eric, H.S.; Oudy, S.; Vittorio, C. Capturing Geographic Atrophy with Multimodal Imaging. Retina Today, 15 April 2024. [Google Scholar]
- Jiskoot, L.C.; Panman, J.L.; Meeter, L.H.; Dopper, E.G.P.; Donker Kaat, L.; Franzen, S.; van der Ende, E.L.; van Minkelen, R.; Rombouts, S.A.R.B.; Papma, J.M.; et al. Longitudinal multimodal MRI as prognostic and diagnostic biomarker in presymptomatic familial frontotemporal dementia. Brain 2018, 142, 193–208. [Google Scholar] [CrossRef]
- Cohn, A.C.; Guymer, R.H. Current advances in multimodal imaging in geographic atrophy secondary to age-related macular degeneration: A review. Taiwan J. Ophthalmol. 2024, 14, 464–472. [Google Scholar] [CrossRef]
- Laíns, I.; Kelly, R.S.; Miller, J.B.; Silva, R.; Vavvas, D.G.; Kim, I.K.; Murta, J.N.; Lasky-Su, J.; Miller, J.W.; Husain, D. Human Plasma Metabolomics Study across All Stages of Age-Related Macular Degeneration Identifies Potential Lipid Biomarkers. Ophthalmology 2018, 125, 245–254. [Google Scholar] [CrossRef]
- Lauwen, S.; de Jong, E.K.; Lefeber, D.J.; den Hollander, A. Omics Biomarkers in Ophthalmology. Investig. Ophthalmol. Vis. Sci. 2017, 58, BIO88–BIO98. [Google Scholar] [CrossRef] [PubMed]
| Mouse Model | Key Pathological Feature | Impact Summary |
|---|---|---|
| Oxidative Stress | ||
| Pgc-1α+/− | Drusen, lipofuscin, mitochondrial dysfunction, RPE migration | Mitochondrial stress model |
| Nrf2−/− | ROS ↑, drusen, RPE degeneration | Oxidative stress-driven AMD |
| P4htm−/− | Drusen, photoreceptor shortening, RPE abnormalities | Age-progressive retinal degeneration |
| Lipid Dysregulation | ||
| Pparβ/δ−/− | Subretinal deposits, RPE degeneration, Apoe upregulation, ERG changes. | Lipid imbalance and RPE loss |
| Apoe−/− | Hypercholesterolemia, Bruch’s membrane alterations | Cholesterol-associated AMD |
| Tg (Apob100) | BrM thickening, BLamD, RPE vacuolization | Diet and light-sensitive model |
| Col18α1−/− | BLamD-like deposits, impaired vitamin A metabolism | Vitamin A dysregulation model |
| Clic4−/− | Fat accumulation, RPE abnormalities | Fatty deposit model |
| Inflammation and Immunity | ||
| Cfh−/− | Complement activation, subretinal deposits, vision loss | Complement-driven AMD |
| Cx3cr1−/− | Subretinal microglia, drusen-like lesions | Microglia-associated drusen |
| Ccl2−/−/Cx3cr1−/− | Drusen, inflammation, photoreceptor atrophy | Early-onset inflammatory AMD |
| Cd46−/− | Drusen formation, PR loss, autophagy dysregulation | Complement and autophagy dysregulation |
| Mouse Model | Key Pathological Feature | Impact Summary |
|---|---|---|
| Lipofuscin Accumulation | ||
| Abca4−/− | Lipofuscin accumulation (↑ A2E); mild rod degeneration | Stargardt disease type1 (STGD1) Model |
| Tg (Elovl4) | Lipofuscin accumulation (↑ A2E); undigested phagosomes; RPE atrophy; photoreceptor loss | Stargardt-like degeneration (STGD3) model |
| Mertk−/− | Lipofuscin accumulation (↑ A2E); impaired phagocytosis; photoreceptor degeneration | Phagocytosis defect model |
| High-fat diet | Lipofuscin accumulation (↑ A2E); RPE vacuolization; disorganized PROS; BlamD-like deposits | Diet-induced AMD features |
| Mouse Model | Inductive Process | Key Pathological Feature | Impact Summary |
|---|---|---|---|
| Oxidative Stress | |||
| NaIO3-induced | Chemical | Macrophage infiltration, PR apoptosis, ↑ ROS/MDA, ONL thinning | Oxidative damage model |
| Gpx4−/− | Genetic | Progressive RPE loss, ↓ visual function | Geographic atrophy model |
| Inflammation | |||
| PEG-induced | Chemical | ONL thinning, RPE hypopigmentation, ↑ autophagy (ATG12), drusen-like deposits | Acute inflammatory model |
| CEP-immunized | Immunological | Anti-CEP antibodies, C3d in BrM, sub-RPE drusen, RPE lesions | Immune-mediated drusen model |
| Drug Name | Target | Status | Phase |
|---|---|---|---|
| Nutraceuticals | |||
| AREDS/AREDS2 | Antioxidants | As a dietary supplement | |
| Complement Inhibitors | |||
| Pegcetacoplan | C3 | FDA approval in April 2023, Recruiting | Phase IV |
| Avacincaptad pegol (ACP) | C5 | Completed FDA approval for the treatment of GA. | Phase III |
| Danicopan | Factor D | Ongoing | Phase II |
| IONIS-FB-LRx | Factor B | Active, not recruiting | Phase II |
| ANX0007 | C1Q | Active, recruiting | Phase II |
| CB2782-PEG | C3 | Development Phase | Preclinical study |
| CB2782 | C3 | Development Phase | Preclinical study |
| Inflammation Modulators | |||
| Fludrocortisone acetate | PLA2 | Completed | Phase Ib |
| Xiflam | NLRP3 inflammasome | No any update | Expected phase II |
| Visual Cycle Modulators | |||
| ALK-001 | Vit-A dimerization | Active, not recruiting | Phase III |
| Tinlarebant | RBP4 | Ongoing | Phase III |
| STG-001 | RBP4 | Completed | Phase II |
| Emuixustat | RPE65 | Completed | Phase III |
| Neuroprotective Agents | |||
| ONL 1204 | CD95 | Completed | Phase I |
| CNTF | Neuroprotection (PRs and RPE cells) | Completed | Phase II |
| Photobiomodulation | |||
| Valeda® Light Delivery System | Cytochrome C oxidase | Active clinical research | Phase II/III |
| Others | |||
| Remofusin | Lipofuscin granules | Completed | Phase II |
| RO7303359 | IL-33 | Completed | Phase I |
| Risuteganib | Integrin heterodimers | Completed | Phase II |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Bhandari, S.K.; Lee, S.; Kim, H.J. Integrative Landscape of Dry AMD Pathogenesis, Models, and Emerging Therapeutic Strategies. Int. J. Mol. Sci. 2026, 27, 202. https://doi.org/10.3390/ijms27010202
Bhandari SK, Lee S, Kim HJ. Integrative Landscape of Dry AMD Pathogenesis, Models, and Emerging Therapeutic Strategies. International Journal of Molecular Sciences. 2026; 27(1):202. https://doi.org/10.3390/ijms27010202
Chicago/Turabian StyleBhandari, Shiva Kumar, Sooyeun Lee, and Hye Jin Kim. 2026. "Integrative Landscape of Dry AMD Pathogenesis, Models, and Emerging Therapeutic Strategies" International Journal of Molecular Sciences 27, no. 1: 202. https://doi.org/10.3390/ijms27010202
APA StyleBhandari, S. K., Lee, S., & Kim, H. J. (2026). Integrative Landscape of Dry AMD Pathogenesis, Models, and Emerging Therapeutic Strategies. International Journal of Molecular Sciences, 27(1), 202. https://doi.org/10.3390/ijms27010202






