Effects of Left Ventricular Unloading on Cardiac Function, Heart Failure Markers, and Autophagy in Rat Hearts with Acute Myocardial Infarction
Abstract
1. Introduction
2. Results
2.1. Partial LV Unloading Model Effectively Reduced LV Size
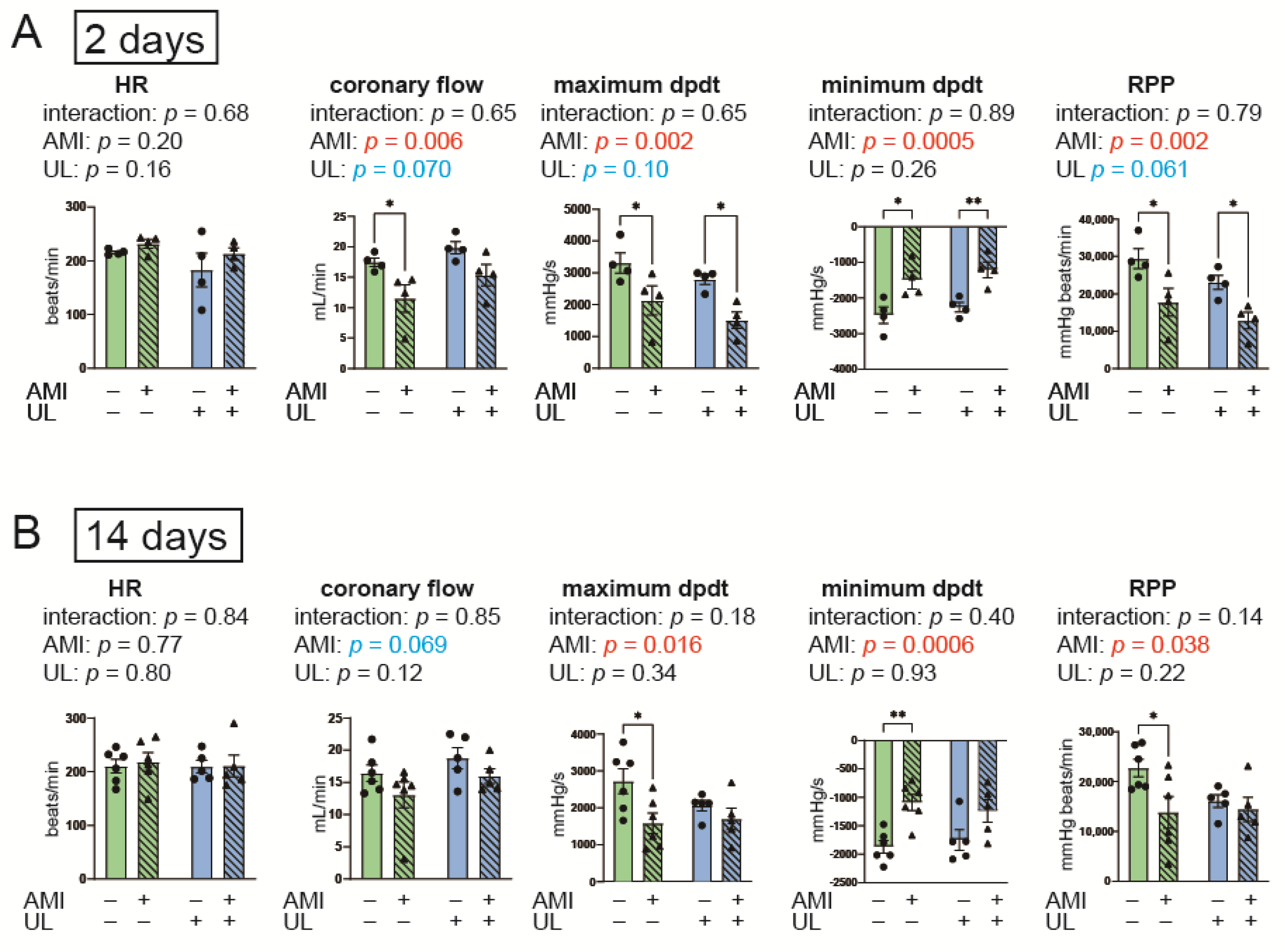
2.2. LV Unloading Tended to Attenuate Cardiac Functional Deterioration After AMI
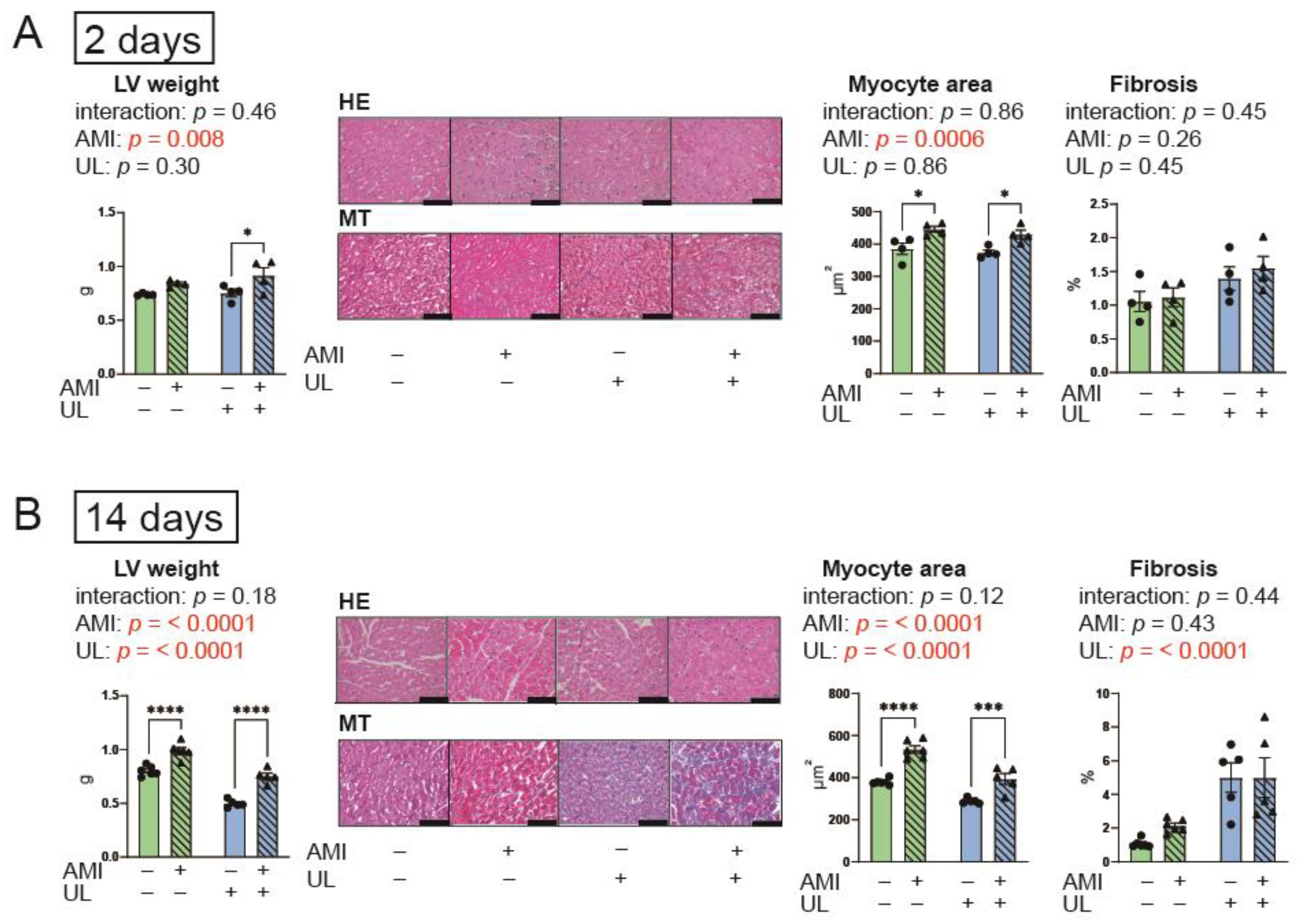
2.3. LV Unloading Did Not Affect Myocardial Remodeling After AMI
2.4. LV Unloading Tended to Attenuate the Increased Fetal Gene Expression After AMI
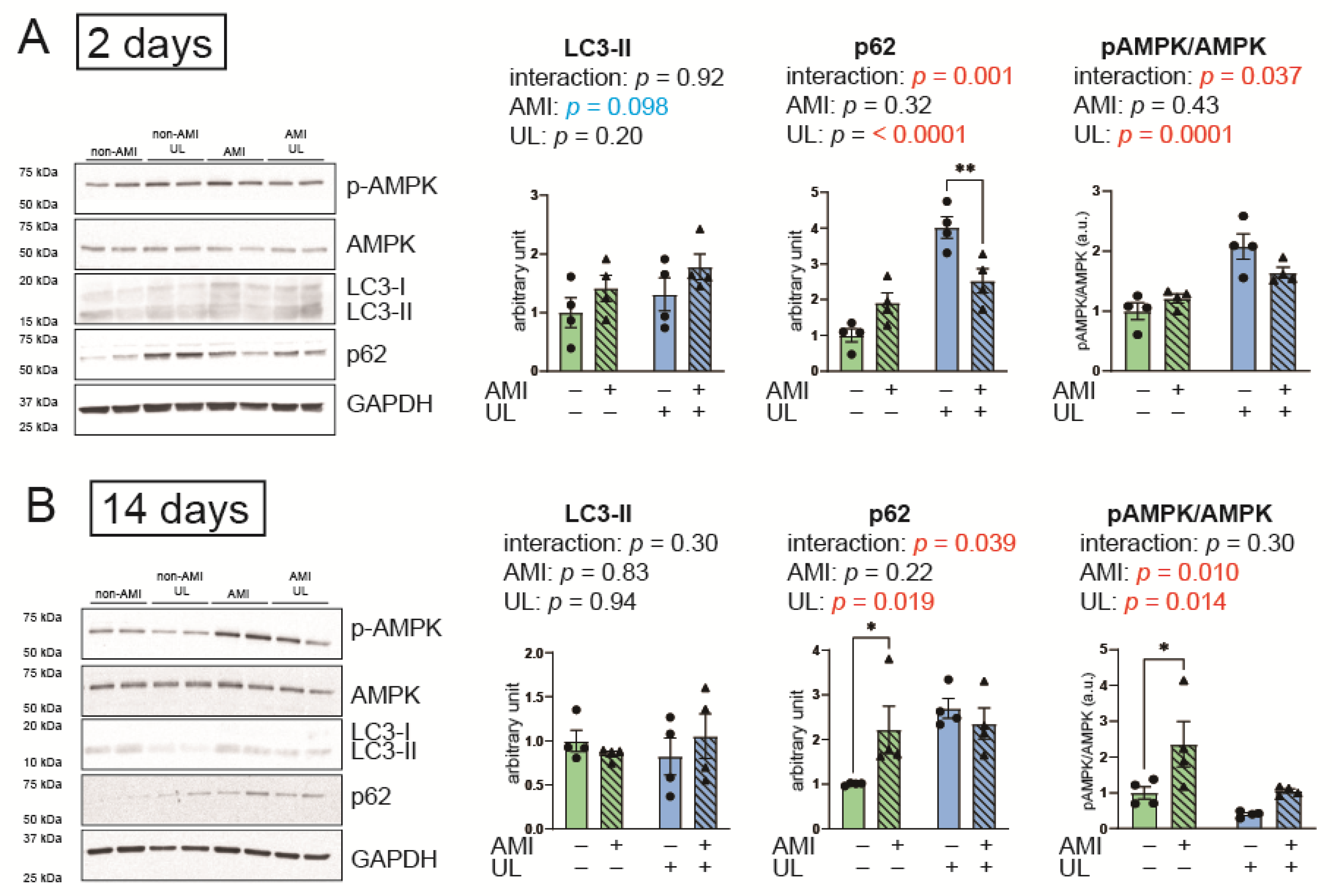
2.5. LV Unloading Attenuated Expressions of an Autophagy-Related Protein, p62 After AMI
2.6. LV Unloading Did Not Affect the Parameters of the UPS and Protein Synthesis
3. Discussion
3.1. Autophagy in AMI and LV Unloading
3.2. Cardiac Remodeling and Ubiquitin Ligases
3.3. Cardiac Fibrosis After LV Unloading
3.4. Limitations
3.5. Conclusions
4. Materials and Methods
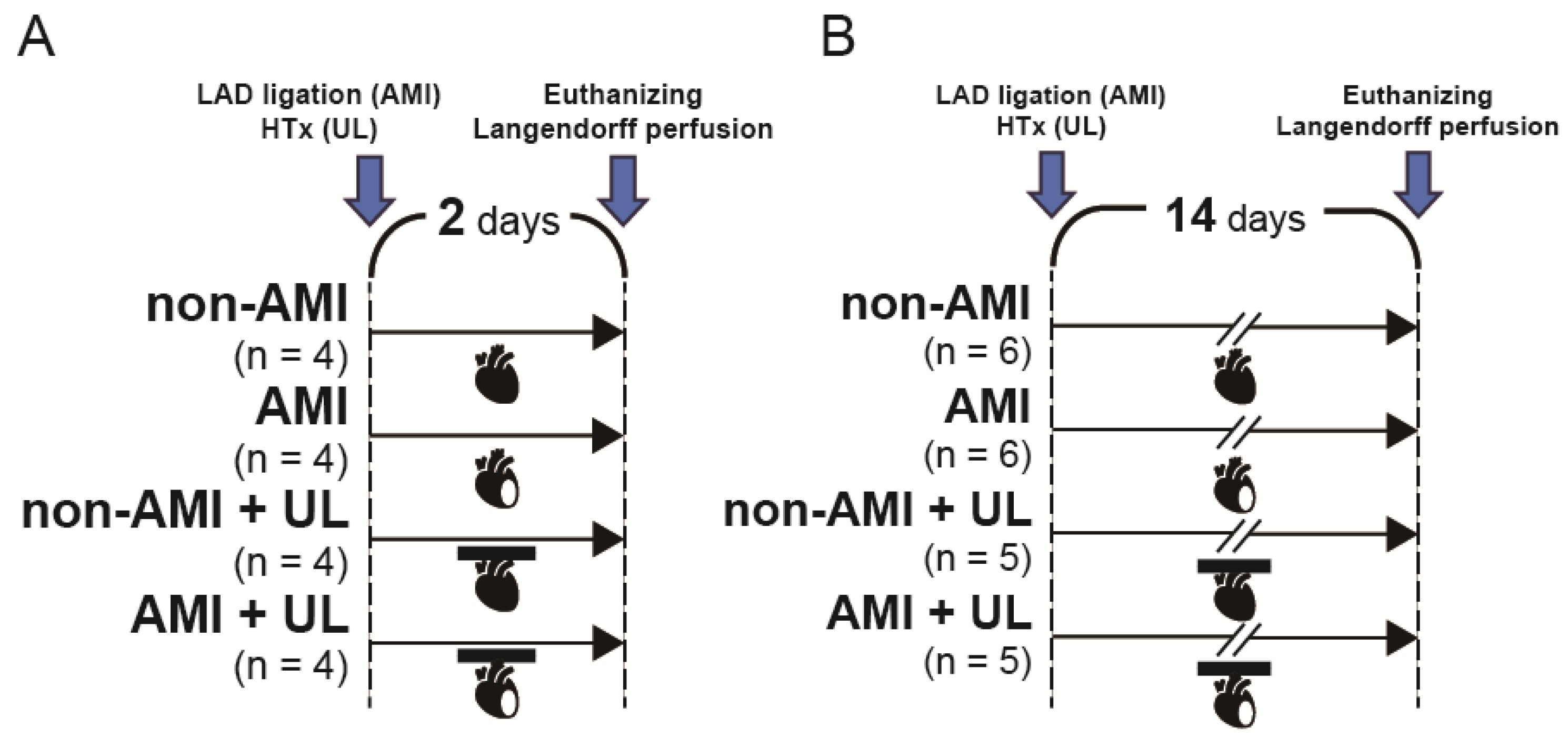
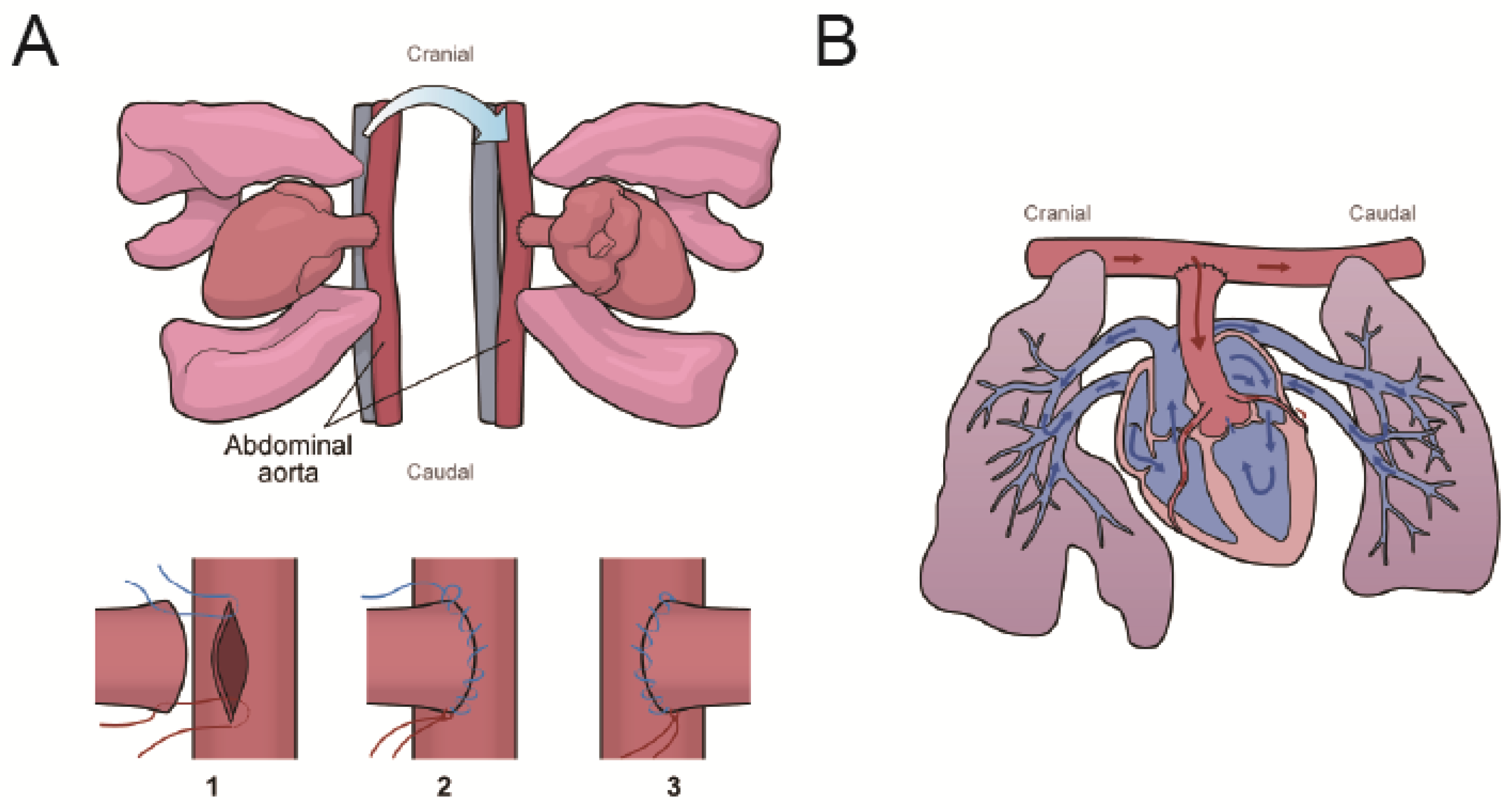
4.1. Experimental Design
4.2. AMI and Heterotopic Heart and Lung Transplantation
4.3. Echocardiography
4.4. Langendorff Heart Perfusion
4.5. Histological Examination
4.6. RT-PCR
4.7. Western Blotting
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar] [CrossRef] [PubMed]
- Masoudi, F.A.; Ponirakis, A.; de Lemos, J.A.; Jollis, J.G.; Kremers, M.; Messenger, J.C.; Moore, J.W.M.; Moussa, I.; Oetgen, W.J.; Varosy, P.D.; et al. Trends in U.S. Cardiovascular Care: 2016 Report From 4 ACC National Cardiovascular Data Registries. J. Am. Coll. Cardiol. 2017, 69, 1427–1450. [Google Scholar] [CrossRef] [PubMed]
- Thiele, H.; Ohman, E.M.; de Waha-Thiele, S.; Zeymer, U.; Desch, S. Management of cardiogenic shock complicating myocardial infarction: An update 2019. Eur. Heart J. 2019, 40, 2671–2683. [Google Scholar] [CrossRef] [PubMed]
- Manzi, L.; Sperandeo, L.; Forzano, I.; Castiello, D.S.; Florimonte, D.; Paolillo, R.; Santoro, C.; Mancusi, C.; Di Serafino, L.; Esposito, G.; et al. Contemporary Evidence and Practice on Right Heart Catheterization in Patients with Acute or Chronic Heart Failure. Diagnostics 2024, 14, 136. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- De Luca, L.; Mistrulli, R.; Scirpa, R.; Thiele, H.; De Luca, G. Contemporary Management of Cardiogenic Shock Complicating Acute Myocardial Infarction. J. Clin. Med. 2023, 12, 2184. [Google Scholar] [CrossRef]
- Tavazzi, G.; Morrow, D.A. Efficacy and safety of mechanical circulatory support in acute myocardial infarction complicated by cardiogenic shock: DanGer trial in perspective. Eur. Heart J. Acute Cardiovasc. Care 2024, 13, 365–367. [Google Scholar] [CrossRef]
- Meyns, B.; Stolinski, J.; Leunens, V.; Verbeken, E.; Flameng, W. Left ventricular support by catheter-mounted axial flow pump reduces infarct size. J. Am. Coll. Cardiol. 2003, 41, 1087–1095. [Google Scholar] [CrossRef]
- Saku, K.; Kakino, T.; Arimura, T.; Sunagawa, G.; Nishikawa, T.; Sakamoto, T.; Kishi, T.; Tsutsui, H.; Sunagawa, K. Left Ventricular Mechanical Unloading by Total Support of Impella in Myocardial Infarction Reduces Infarct Size, Preserves Left Ventricular Function, and Prevents Subsequent Heart Failure in Dogs. Circ.-Heart Fail. 2018, 11, e004397. [Google Scholar] [CrossRef]
- Yalta, K.; Yilmaz, M.B.; Yalta, T.; Palabiyik, O.; Taylan, G.; Zorkun, C. Late Versus Early Myocardial Remodeling After Acute Myocardial Infarction: A Comparative Review on Mechanistic Insights and Clinical Implications. J. Cardiovasc. Pharmacol. Ther. 2020, 25, 15–26. [Google Scholar] [CrossRef]
- Razeghi, P.; Sharma, S.; Ying, J.; Li, Y.P.; Stepkowski, S.; Reid, M.B.; Taegtmeyer, H. Atrophic remodeling of the heart in vivo simultaneously activates pathways of protein synthesis and degradation. Circulation 2003, 108, 2536–2541. [Google Scholar] [CrossRef] [PubMed]
- Hale, A.N.; Ledbetter, D.J.; Gawriluk, T.R.; Rucker, E.B., III. Autophagy: Regulation and role in development. Autophagy 2013, 9, 951–972. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Otsu, K. Autophagy during cardiac remodeling. J. Mol. Cell. Cardiol. 2016, 95, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Barac, Y.D.; Emrich, F.; Krutzwakd-Josefson, E.; Schrepfer, S.; Sampaio, L.C.; Willerson, J.T.; Robbins, R.C.; Ciechanover, A.; Mohr, F.-W.; Aravot, D.; et al. The ubiquitin-proteasome system: A potential therapeutic target for heart failure. J. Heart Lung Transpl. 2017, 36, 708–714. [Google Scholar] [CrossRef]
- Baskin, K.K.; Rodriguez, M.R.; Kansara, S.; Chen, W.H.; Carranza, S.; Frazier, O.H.; Glass, D.J.; Taegtmeyer, H. MAFbx/Atrogin-1 is required for atrophic remodeling of the unloaded heart. J. Mol. Cell. Cardiol. 2014, 72, 168–176. [Google Scholar] [CrossRef]
- Cao, D.J.; Jiang, N.; Blagg, A.; Johnstone, J.L.; Gondalia, R.; Oh, M.; Luo, X.; Yang, K.-C.; Shelton, J.M.; Rothermel, B.A.; et al. Mechanical unloading activates FoxO3 to trigger Bnip3-dependent cardiomyocyte atrophy. J. Am. Heart Assoc. 2013, 2, e000016. [Google Scholar] [CrossRef]
- Kanamori, H.; Takemura, G.; Goto, K.; Maruyama, R.; Ono, K.; Nagao, K.; Tsujimoto, A.; Ogino, A.; Takeyama, T.; Kawaguchi, T.; et al. Autophagy limits acute myocardial infarction induced by permanent coronary artery occlusion, American journal of physiology. Heart Circ. Physiol. 2011, 300, H2261–H2271. [Google Scholar] [CrossRef]
- Sciarretta, S.; Yee, D.; Nagarajan, N.; Bianchi, F.; Saito, T.; Valenti, V.; Tong, M.; Del Re, D.P.; Vecchione, C.; Schirone, L.; et al. Trehalose-Induced Activation of Autophagy Improves Cardiac Remodeling After Myocardial Infarction. J. Am. Coll. Cardiol. 2018, 71, 1999–2010. [Google Scholar] [CrossRef]
- Martin, T.G.; Juarros, M.A.; Cleveland, J.C., Jr.; Bristow, M.R.; Ambardekar, A.V.; Buttrick, P.M.; Leinwand, L.A. Assessment of Autophagy Markers Suggests Increased Activity Following LVAD Therapy. JACC Basic Transl. Sci. 2023, 8, 1043–1056. [Google Scholar] [CrossRef]
- Kassiotis, C.; Ballal, K.; Wellnitz, K.; Vela, D.; Gong, M.; Salazar, R.; Frazier, O.H.; Taegtmeyer, H. Markers of autophagy are downregulated in failing human heart after mechanical unloading. Circulation 2009, 120, S191–S197. [Google Scholar] [CrossRef]
- Ono, K.; Lindsey, E.S. Improved technique of heart transplantation in rats. J. Thorac. Cardiovasc. Surg. 1969, 57, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Brinks, H.; Giraud, M.N.; Segiser, A.; Ferrie, C.; Longnus, S.; Ullrich, N.D.; Koch, W.J.; Most, P.; Carrel, T.P.; Tevaearai, H.T. Dynamic patterns of ventricular remodeling and apoptosis in hearts unloaded by heterotopic transplantation. J. Heart Lung Transpl. 2014, 33, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Diakos, N.A.; Selzman, C.H.; Sachse, F.B.; Stehlik, J.; Kfoury, A.G.; Wever-Pinzon, O.; Catino, A.; Alharethi, R.; Reid, B.B.; Miller, D.V.; et al. Myocardial Atrophy and Chronic Mechanical Unloading of the Failing Human Heart. J. Am. Coll. Cardiol. 2014, 64, 1602–1612. [Google Scholar] [CrossRef]
- Bruggink, A.H.; van Oosterhout, M.F.M.; de Jonge, N.; Ivangh, B.; van Kuik, J.; Voorbij, R.H.A.M.; Cleutjens, J.P.M.; Gmelig-Meyling, F.H.J.; de Weger, R.A. Reverse remodeling of the myocardial extracellular matrix after prolonged left ventricular assist device support follows a biphasic pattern. J. Heart Lung Transpl. 2006, 25, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, A.; Schneeberger, Y.; Schulz, S.; Krasemann, S.; Werner, T.; Piasecki, A.; Höppner, G.; Müller, C.; Morhenn, K.; Lorenz, K.; et al. Analysis of fibrosis in control or pressure overloaded rat hearts after mechanical unloading by heterotopic heart transplantation. Sci. Rep. 2019, 9, 5710. [Google Scholar] [CrossRef]
- Zaglia, T.; Milan, G.; Franzoso, M.; Bertaggia, E.; Pianca, N.; Piasentini, E.; Voltarelli, V.A.; Chiavegato, D.; Brum, P.C.; Glass, D.J.; et al. Cardiac sympathetic neurons provide trophic signal to the heart via beta2-adrenoceptor-dependent regulation of proteolysis. Cardiovasc. Res. 2013, 97, 240–250. [Google Scholar] [CrossRef]
- Poormasjedi-Meibod, M.S.; Mansouri, M.; Fossey, M.; Squair, J.W.; Liu, J.; McNeill, J.H.; West, C.R. Experimental Spinal Cord Injury Causes Left-Ventricular Atrophy and Is Associated with an Upregulation of Proteolytic Pathways. J. Neurotrauma 2019, 36, 950–961. [Google Scholar] [CrossRef]
- Zheng, S.; Li, L.; Liu, L.; Liang, S.; Tao, J.; Wang, J.; Zheng, J. Changes in echocardiographic parameters of the donor’s heart before and after heart transplantation and their relationship with post-transplant survival. Ann. Transl. Med. 2022, 10, 280. [Google Scholar] [CrossRef]
- Didie, M.; Biermann, D.; Buchert, R.; Hess, A.; Wittkopper, K.; Christalla, P.; Döker, S.; Jebran, F.; Schöndube, F.; Reichenspurner, H.; et al. Preservation of left ventricular function and morphology in volume-loaded versus volume-unloaded heterotopic heart transplants. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H533–H541. [Google Scholar] [CrossRef][Green Version]
- Ibrahim, M.; Navaratnarajah, M.; Kukadia, P.; Rao, C.; Siedlecka, U.; Cartledge, J.E.; Soppa, G.K.; Van Doorn, C.; Yacoub, M.H.; Terracciano, C.M. Heterotopic abdominal heart transplantation in rats for functional studies of ventricular unloading. J. Surg. Res. 2013, 179, e31–e39. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abdelmohsen, K.; Abe, A.; Abedin, M.J.; Abeliovich, H.; Arozena, A.A.; Adachi, H.; Adams, C.M.; Adams, P.D.; Adeli, K. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 2016, 12, 1–222. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azuma, R.; Shingu, Y.; Gao, J.; Wakasa, S. Effects of Left Ventricular Unloading on Cardiac Function, Heart Failure Markers, and Autophagy in Rat Hearts with Acute Myocardial Infarction. Int. J. Mol. Sci. 2025, 26, 4422. https://doi.org/10.3390/ijms26094422
Azuma R, Shingu Y, Gao J, Wakasa S. Effects of Left Ventricular Unloading on Cardiac Function, Heart Failure Markers, and Autophagy in Rat Hearts with Acute Myocardial Infarction. International Journal of Molecular Sciences. 2025; 26(9):4422. https://doi.org/10.3390/ijms26094422
Chicago/Turabian StyleAzuma, Ryota, Yasushige Shingu, Jingwen Gao, and Satoru Wakasa. 2025. "Effects of Left Ventricular Unloading on Cardiac Function, Heart Failure Markers, and Autophagy in Rat Hearts with Acute Myocardial Infarction" International Journal of Molecular Sciences 26, no. 9: 4422. https://doi.org/10.3390/ijms26094422
APA StyleAzuma, R., Shingu, Y., Gao, J., & Wakasa, S. (2025). Effects of Left Ventricular Unloading on Cardiac Function, Heart Failure Markers, and Autophagy in Rat Hearts with Acute Myocardial Infarction. International Journal of Molecular Sciences, 26(9), 4422. https://doi.org/10.3390/ijms26094422






