Unraveling the COVID-19 Severity Hubs and Interplays in Inflammatory-Related RNA–Protein Networks
Abstract
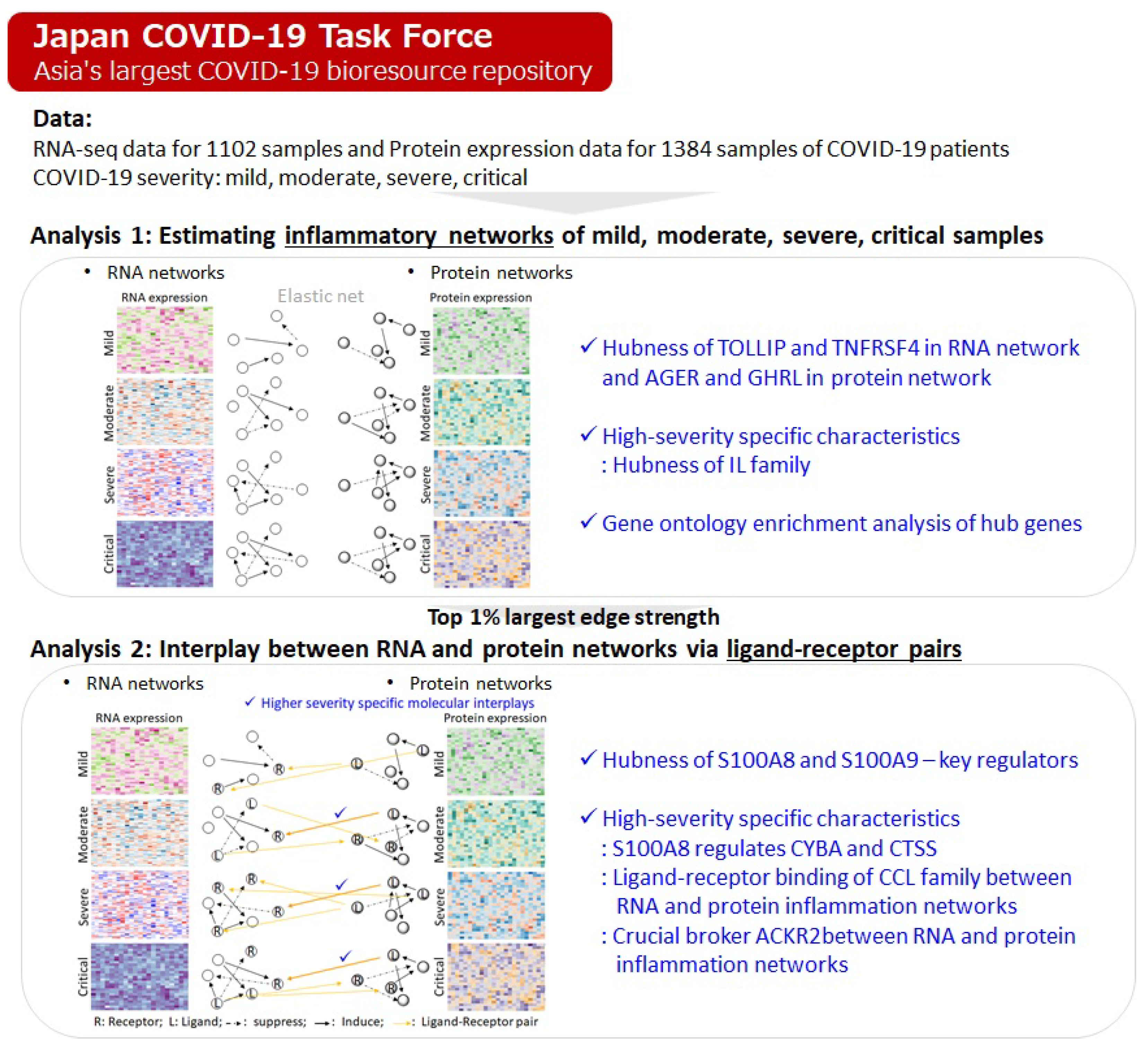
1. Introduction
2. Results
2.1. Characteristics of Inflammation Networks Estimated from RNA and Protein Expressions
2.1.1. Hubness of TOLLIP and TNFRSF4 in RNA Network and AGER and GHRL in Protein Network
2.1.2. High-Severity Specific Characteristics: Hubness of IL Family
2.1.3. Gene Ontology Enrichment Analysis of Hub Genes Provides Clear Distinction of Molecular Interplays
2.2. Uncovering Interplays Between RNA and Protein Networks via Ligand–Receptor Pairs
2.2.1. Hubness of S100A8 and S100A9—Key Regulators
2.2.2. Specific Characteristics of High-Severity Samples: S100A8 Regulates CYBA and CTSS
2.2.3. Specific Characteristics of High-Severity Samples: Ligand–Receptor Binding of CCL Family Between RNA and Protein Inflammation Networks
2.2.4. Specific Characteristics of High-Severity Samples: ACKR2 Is a Crucial Broker Between RNA and Protein Inflammation Networks
2.2.5. Summary of Specific Molecular Interplays in High-Severity Samples
- ACKR2 is a crucial broker between the RNA and protein networks.
- The RNA and protein inflammation networks interact via ligand–receptor binding of the CCL family.
- CCL family interplay is present in the protein network.
- S100A8 regulates CYBA and CTSS.
- GO term of common hub genes include “GO:0004896 cytokine receptor activity” and “GO:0007155 cell adhesion”.
- The IL family shows active interplay.
3. Data Source
3.1. RNA-Seq Data and Protein Expression Data
3.2. Networks Estimated for Mild/Moderate/Severe/Critical Phases to Analyze the Interplays
4. Discussion
5. Method for Gene Network Estimation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, Y.; Zhao, T.; Zhou, X.; Xiang, Y.; Gutierrez-Castrellon, P.; Ma, X. Inflammatory pathways in COVID-19: Mechanisms and therapeutic interventions. MedComm 2020, 3, e154. [Google Scholar] [CrossRef] [PubMed]
- Manjili, R.H.; Zarei, M.; Habibi, M.; Manjili, M.H. COVID-19 as an Acute Inflammatory Disease. J. Immunol. 2020, 205, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Park, J.; Im, H.J.; Na, K.J.; Choi, H. Discovery of potential imaging and therapeutic targets for severe inflammation in COVID-19 patients. Sci. Rep. 2021, 11, 14151. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Yang, Y.; Zhang, X.; Cai, J.J. Association between pyroptosis and severeness of COVID-19 as revealed by integrated single-cell transcriptome data analysis. Immunoinformatics 2022, 6, 100013. [Google Scholar] [CrossRef]
- Powell, T.R.; Hotopf, M.; Hatch, S.L.; Breen, G.; Duarte, R.R.R.; Nixon, D.F. Genetic risk of severe COVID-19 correlates with lower levels of inflammatory markers in a SARS-CoV-2-negative cohort. Clin. Transl. Immunol. 2021, 10, e1292. [Google Scholar] [CrossRef]
- Namkoong, H.; Edahiro, R.; Takano, T.; Nishihara, H.; Shirai, Y.; Sonehara, K.; Tanakam, H.; Azekawa, S.; Mikami, Y.; Lee, H.; et al. DOCK2 is involved in the host genetics and biology of severe COVID-19. Nature 2022, 609, 754–760. [Google Scholar] [CrossRef]
- Lourda, M.; Dzidic, M.; Hertwig, L.; Bergsten, H.; Palma Medina, L.M.; Sinha, I.; Kvedaraite, E.; Chen, P.; Muvva, J.R.; Gorin, J.B.; et al. Karolinska KI/K COVID-19 Study Group. High-dimensional profiling revealed phenotypic heterogeneity and disease-specific alterations in granulocytes in COVID-19. Proc. Natl. Acad. Sci. USA 2021, 118, e2109123118. [Google Scholar] [CrossRef]
- Lee, J.S.; Park, S.; Jeong, H.W.; Ahn, J.Y.; Choi, S.J.; Lee, H.; Choi, B.; Nam, S.K.; Sa, M.; Kwon, J.S.; et al. Immunophenotyping of COVID-19 and influenza highlights the role of type I interferons in the development of severe COVID-19. Sci. Immunol. 2020, 5, eabd1554. [Google Scholar] [CrossRef]
- Zhou, Z.; Ren, L.; Zhang, L.; Zhong, J.; Xiao, Y.; Jia, Z.; Guo, L.; Yang, J.; Wang, C.; Jiang, S.; et al. Heightened innate immune responses in the respiratory tract of COVID-19 patients. Cell Host Microbe 2020, 27, 883–890.e2. [Google Scholar] [CrossRef]
- COVID-19 Host Genetics Initiative. A second update on mapping the human genetic architecture of COVID-19. COVID-19 Host Genetics Initiative. Nature 2023, 621, E7–E26. [Google Scholar] [CrossRef]
- Wang, Q.S.; Edahiro, R.; Namkoong, H.; Hasegawa, T.; Shirai, Y.; Sonehara, K.; Tanaka, H.; Lee, H.; Saiki, R.; Hyugaji, T.; et al. The whole blood transcriptional regulation landscape in 465 COVID-19 infected samples from Japan COVID-19 Task Force. Nat. Commun. 2022, 13, 4830. [Google Scholar] [CrossRef]
- Naemi, F.M.A.; Al-Adwani, S.; Al-Khatabi, H.; Al-Nazawi, A. Association between the HLA genotype and the severity of COVID-19 infection among South Asians. J. Med. Virol. 2021, 93, 4430–4437. [Google Scholar] [CrossRef]
- Pairo-Castineira, E.; Clohisey, S.; Klaric, L.; Bretherick, A.D.; Rawlik, K.; Pasko, D.; Walker, S.; Parkinson, N.; Fourman, M.H.; Russell, C.D.; et al. Genetic mechanisms of critical illness in COVID-19. Nature 2021, 591, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Huang, S.; Gao, R.; Zhou, Y.; Lai, C.; Li, Z.; Xian, W.; Qian, X.; Li, Z.; Huang, Y.; et al. Initial whole-genome sequencing and analysis of the host genetic contribution to COVID-19 severity and susceptibility. Cell Discov. 2020, 6, 83. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Ding, L.; Li, X.; Liu, S.; Cheng, F.; He, Q.; Xiao, M.; Wu, P.; Hou, H.; Jiang, M.; et al. Trans-ethnic genome-wide association study of severe COVID-19. Commun. Biol. 2021, 4, 1034. [Google Scholar] [CrossRef] [PubMed]
- Su, C.Y.; Zhou, S.; Gonzalez-Kozlova, E.; Butler-Laporte, G.; Brunet-Ratnasingham, E.; Nakanishi, T.; Jeon, W.; Morrison, D.R.; Laurent, L.; Afilalo, J.; et al. Circulating Proteins for Predicting COVID-19 Severity. Sci. Rep. 2023, 13, 6236. [Google Scholar]
- Gisby, J.; Clarke, C.L.; Medjeral-Thomas, N.; Malik, T.H.; Papadaki, A.; Mortimer, P.M.; Buang, N.B.; Lewis, S.; Pereira, M.; Toulza, F.; et al. Longitudinal proteomic profiling of dialysis patients with COVID-19 revealed markers of disease severity and predictors of death. Elife 2021, 10, e64827. [Google Scholar] [CrossRef]
- George, P.M.; Reed, A.; Desai, S.R.; Devaraj, A.; Faiez, T.S.; Laverty, S.; Kanwal, A.; Esneau, C.; Liu, M.K.C.; Kamal, F.; et al. A persistent neutrophil-associated immune signature characterizes post-COVID-19 pulmonary sequelae. Sci. Transl. Med. 2022, 14, eabo5795. [Google Scholar] [CrossRef]
- Brionne, A.; Juanchich, A.; Hennequet-Antier, C. ViSEAGO: A Bioconductor package for clustering biological functions using Gene Ontology and semantic similarity. BioData Min. 2019, 12, 16. [Google Scholar] [CrossRef]
- Li, D.; Chen, R.; Huang, C.; Zhang, G.; Li, Z.; Xu, X.; Wang, B.; Li, B.; Chu, X.M. Comprehensive bioinformatics analyses and systems biology approaches have been used to identify the interplay between COVID-19 and pericarditis. Front. Immunol. 2024, 15, 1264856. [Google Scholar]
- Qi, P.; Huang, M.; Zhu, H. Exploring potential biomarkers and therapeutic targets of long COVID-associated inflammatory cardiomyopathy. Front. Med. 2023, 10, 1191354. [Google Scholar] [CrossRef] [PubMed]
- Vastrad, B.; Vastrad, C.; Tengli, A. Identification of potential mRNA panels for the diagnosis and treatment of severe acute respiratory syndrome coronavirus 2 (COVID-19) diagnosis and treatment using microarray datasets and bioinformatics methods. 3 Biotech 2020, 10, 422. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.; Ciordia, S.; Fátima, S.M.; Jiménez, D.; Martínez-Sanz, J.; Vizcarra, P.; Ron, R.; Sánchez-Conde, M.; Bargiela, R.; Sanchez-Carrillo, S.; et al. Proteomic snapshots of saliva samples predicted new pathways implicated in SARS-CoV-2 pathogenesis. Clin. Proteomics 2024, 21, 37. [Google Scholar] [CrossRef] [PubMed]
- Ymam, S.D.; Ahmed, S.N.; Raid, J.M. Role of serum cathepsin level as a predictor of COVID-19 severity. IV Nurs. Artic. 2023, 23, 551–555. [Google Scholar]
- Huang, I.C.; Bosch, B.J.; Li, F.; Li, W.; Lee, K.H.; Ghiran, S.; Vasilieva, N.; Dermody, T.S.; Harrison, S.C.; Dormitzer, P.R.; et al. SARS coronavirus, but not the human coronavirus NL63, uses cathepsin L to infect ACE2-expressing cells. J. Biol. Chem. 2006, 281, 3198–3203. [Google Scholar] [CrossRef]
- Gomes, A.M.C.; Farias, G.B.; Dias-Silva, M.; Laia, J.; Trombetta, A.C.; Godinho-Santos, A.; Rosmaninho, P.; Santos, D.F.; Conceição, C.M.; Costa-Reis, R.; et al. SARS-CoV2 pneumonia recovery is linked to the expansion of type 2 innate lymphoid cells expressing CCR10. Eur. J. Immunol. 2021, 51, 3194–3201. [Google Scholar] [CrossRef]
- Khalil, B.A.; Elemam, N.M.; Maghazachi, A.A. Chemokines and chemokine receptors during COVID-19 infection. Comput. Struct. Biotechnol. J. 2021, 19, 976–988. [Google Scholar] [CrossRef]
- Tveita, A.; Murphy, S.L.; Holter, J.C.; Kildal, A.B.; Michelsen, A.E.; Lerum, T.V.; Kaarbø, M.; Heggelund, L.; Holten, A.R.; Finbråten, A.K.; et al. The NOR-SOLIDARITY Consortium and the Norwegian SARS-CoV-2 Study Group Investigators. High circulating levels of Homeostatic Chemokines CCL19 and CCL21 predict mortality and disease severity in COVID-19. J. Infect. Dis. 2022, 226, 2150–2160. [Google Scholar] [CrossRef]
- Coperchini, F.; Chiovato, L.; Ricci, G.; Croce, L.; Magri, F.; Rotondi, M. The cytokine storm in COVID-19: Further advances in our understanding of the role of specific chemokines involved. Cytokine Growth Factor Rev. 2021, 28, 82–91. [Google Scholar] [CrossRef]
- Muri, J.; Cecchinato, V.; Cavalli, A.; Shanbhag, A.A.; Matkovic, M.; Biggiogero, M.; Maida, P.A.; Moritz, J.; Toscano, C.; Ghovehoud, E.; et al. Anti-chemokine antibodies after SARS-CoV-2 infection correlate with favorable disease course. Nat. Immunol. 2023, 24, 604–611. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, H.; Zhan, M.; Jiang, J.; Yin, H.; Dauphars, D.J.; Li, S.Y.; Li, Y.; He, Y.W. Preventing Mortality in COVID-19 Patients: Which Cytokine Targets in a Raging Storm? Front. Cell Dev. Biol. 2020, 8, 677. [Google Scholar] [CrossRef] [PubMed]
- OBrien, T.E.; Silcox, J.W. Nonlinear Regression Modelling: A Primer with Applications and Caveats. Bull. Math. Biol. 2024, 86, 40. [Google Scholar] [CrossRef] [PubMed]
- Ronkko, M.; Aalto, E.; Tenhunen, H.; Aguirre-Urreta, M.I. Eight simple guidelines for improved understanding of transformations and nonlinear effects. Organ. Res. Methods 2021, 25, 48–87. [Google Scholar] [CrossRef]
- Jarantow, S.W.; Pisors, E.D.; Chiu, M.L. Introduction to the use of linear and nonlinear regression analysis in quantitative biological assays. Curr. Protoc. 2023, 3, e801. [Google Scholar] [CrossRef]
- Dong, Z.; Song, T.; Yuan, C. Inference of gene regulatory networks from genetic perturbations with linear regression model. PLoS ONE 2013, 8, e83263. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Ching, W.K.; Tsing, N.K.; Leung, H.Y.; Guo, D. A new multiple regression approach for the construction of genetic regulatory networks. Artif. Intell. Med. 2010, 48, 153–160. [Google Scholar] [CrossRef][Green Version]
- Kim, H.; Lee, J.K.; Park, T. Inference of large-scale gene regulatory networks using regression-based network approach. J. Bioinform. Comput. Biol. 2009, 7, 717–735. [Google Scholar] [CrossRef]
- Zou, H.; Hastie, T. Regularization and variable selection using elastic net. J. R. Stat. Soc. Ser. B 2005, 67, 301–320. [Google Scholar] [CrossRef]
- Ledru, N.; Wilson, P.C.; Muto, Y.; Yoshimura, Y.; Wu, H.; Li, D.; Asthana, A.; Tullius, S.G.; Waikar, S.S.; Orlando, G.; et al. Predicting proximal tubule failed repair drivers through regularized regression analysis of single cell multiomic sequencing. Nat. Commun. 2024, 15, 1291. [Google Scholar] [CrossRef]
- Yuan, Q.; Duren, Z. Inferring gene regulatory networks from single-cell multiome data using atlas-scale external data. Nat. Biotechnol. 2025, 43, 247–257. [Google Scholar] [CrossRef]
- Li, X.; Goobie, G.C.; Gregory, A.D.; Kass, D.J.; Zhang, Y. Toll-interacting proteins in pulmonary diseases. Abidding by Goldilock principle. Am. J. Respir. Cell Mol. Biol. 2021, 64, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; He, X.; Ma, L.; Zhuang, Z.; Wang, Y.; Lin, M.; Cai, S.; Wei, L.; Wang, Z.; Zhao, Z.; et al. Suppression of ACE2 SUMOylation protects against SARS-CoV-2 infection via TOLLIP-mediated selective autophagy. Nat. Commun. 2022, 13, 5204. [Google Scholar] [CrossRef] [PubMed]
- Sellegounder, D.; Zafari, P.; Rajabinejad, M.; Taghadosi, M.; Kapahi, P. Advanced glycation end products (AGEs) and their receptor RAGE modulate age-dependent COVID-19 morbidity and mortality. Review and hypotheses. Int. Immunopharmacol. 2021, 98, 107806. [Google Scholar] [CrossRef] [PubMed]
- Salehi, M.; Amiri, S.; Ilghari, D.; Hasham, L.F.A.; Piri, H. Role of the receptor for Advanced Glycation End products (RAGE) and Its Soluble Isoforms in COVID-19: The Importance of RAGE Pathway in the Lung Injuries. Indian J. Clin. Biochem. 2023, 38, 159–171. [Google Scholar] [CrossRef]
- Waraich, R.S.; Sohail, F.A.; Khan, G.; Durr-E-Shahwar, S.; Khan, B.; Rafi, S.; Nasir, S. Enhanced RAGE AXIS expression was associated with Severity of COVID-19 in patients with Comorbidities. Metab. Syndr. Relat. Disord. 2023, 21, 141–147. [Google Scholar] [CrossRef]
- Angioni, R.; Bonfanti, M.; Caporale, N.; Sánchez-Rodríguez, R.; Munari, F.; Savino, A.; Pasqualato, S.; Buratto, D.; Pagani, I.; Bertoldi, N.; et al. RAGE engagement by SARS-CoV-2 enables monocyte infection, underlies COVID-19 severity. Cell Rep. Med. 2023, 4, 101266. [Google Scholar] [CrossRef]
- Binayke, A.; Zaheer, A.; Vishwakarma, S.; Singh, S.; Sharma, P.; Chandwaskar, R.; Gosain, M.; Raghavan, S.; Murugesan, D.R.; Kshetrapal, P.; et al. A quest for universal anti-SARS-CoV-2 T cell assay: Systematic review, meta-analysis, and experimental validation. NPJ Vaccines 2024, 9, 3. [Google Scholar] [CrossRef]
- van der Vorst, E.P.C.; Peters, L.J.F.; Müller, M.; Gencer, S.; Yan, Y.; Weber, C.; Döring, Y. G-Protein Coupled Receptor Targeting on Myeloid Cells in Atherosclerosis. Front. Pharmacol. 2019, 10, 531. [Google Scholar] [CrossRef]
- Qi, Z.; Jiang, Y.; Holland, J.W.; Nie, P.; Secombes, C.J.; Wang, T. Identification and expression analysis of an atypical chemokine receptor-2 (ACKR2)/CC chemokine binding protein-2 (CCBP2) in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2015, 44, 389–398. [Google Scholar] [CrossRef]
- Mellett, L.; Khader, S.A. S100A8/A9 in COVID-19 pathogenesis: Impact on clinical outcomes. Cytokine Growth Factor Rev. 2022, 63, 90–97. [Google Scholar] [CrossRef]
- Guo, Q.; Zhao, Y.; Li, J.; Liu, J.; Yang, X.; Guo, X.; Kuang, M.; Xia, H.; Zhang, Z.; Cao, L.; et al. Induction of alarmin S100A8/A9 mediates the activation of aberrant neutrophils during the pathogenesis of COVID-19. Cell Host Microbe 2021, 29, 222–235.e4. [Google Scholar] [CrossRef] [PubMed]
- Oguariri, R.M.; Brann, T.W.; Adelsberger, J.W.; Chen, Q.; Goswami, S.; Mele, A.R.; Imamichi, T. Short Communication: S100A8 and S100A9, Biomarkers of SARS-CoV-2 Infection and other diseases, suppress HIV replication in primary macrophages. AIDS Res. Hum. Retroviruses 2022, 38, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Bagheri-Hosseinabadi, Z.; Abbasi, M.; Kahnooji, M.; Ghorbani, Z.; Abbasifard, M. Prognostic value of S100A calcium-binding proteins in predicting severe forms of COVID-19. Inflamm. Res. 2022, 71, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Hoerl, A.E.; Kennard, R.W. Ridge regression: Biased estimation for nonorthogonal problems. Techonometrics 2000, 12, 55–67. [Google Scholar] [CrossRef]
- Tibshirani, R. Regression shrinkage and selection via the lasso. J. R. Stat. Soc. Ser. Stat. Methodol. 1996, 28, 267–288. [Google Scholar] [CrossRef]



| RNA | Protein | |||||||
|---|---|---|---|---|---|---|---|---|
| Rank | Mild | Moderate | Severe | Critical | Mild | Moderate | Severe | Critical |
| 1 | BRD4 | * IL22RA1 | MEP1B | * PGLYRP2 | PTN | * TLR2 | * AHSG | * AHSG |
| 2 | TOLLIP | * PGLYRP2 | GPR33 | * IL20RB | IL1R1 | * SMPDL3B | * TLR2 | * SMPDL3B |
| 3 | CCL1 | * IL20RB | * MSMP | * IL22RA1 | RARRES2 | * AHSG | GHRL | GHRL |
| 4 | PSEN1 | CRHBP | CRHBP | CELA1 | CXCL10 | GHRL | * SMPDL3B | * TLR2 |
| 5 | TICAM2 | * TRIM55 | * IL20RB | * MSMP | CRHBP | * NDST1 | * IFNGR2 | * IFNGR2 |
| 6 | TUSC2 | * CASP12 | BDKRB2 | ACKR2 | CD200R1 | * IFNGR2 | * DPEP1 | * NDST1 |
| 7 | CLEC7A | * IL17D | * PGLYRP2 | * CASP12 | ATRN | PBXIP1 | * NDST1 | * DPEP1 |
| 8 | P2RX7 | SCN11A | * IL22RA1 | CD5L | MIF | * DPEP1 | PBXIP1 | PBXIP1 |
| 9 | PTGES | * MVK | * IL17D | * IL17D | SNAP23 | * HMOX1 | * IL20RB | * IL20RB |
| 10 | FFAR2 | SPINK7 | * CASP12 | TOLLIP | BCR | * IL20RB | CD5L | SHPK |
| 11 | CD200 | TOLLIP | * MVK | PRKCZ | PPBP | * ZP3 | * VWF | * ZP3 |
| 12 | CST7 | PRKCZ | SMO | * TRIM55 | PTPN6 | AGER | * HMOX1 | * VWF |
| 13 | TNFRSF4 | ZEB2 | ACKR2 | CRLF2 | GATA3 | * VWF | * IL36A | * HMOX1 |
| 14 | NFKBIZ | CLEC7A | CDH5 | * MVK | MGLL | TLR3 | AGER | FCER1A |
| 15 | PTGER3 | * MSMP | TOLLIP | SMO | AGER | * EPHA2 | ITGAV | TLR3 |
| 16 | CERS6 | TNFRSF4 | FBXL2 | KDM4D | VAMP8 | NPY | * AGT | AGER |
| 17 | PXK | CD5L | SPINK7 | RIPK1 | IL1RAP | * AGT | * ZP3 | SIRPA |
| 18 | STAP1 | C1QTNF3 | * TRIM55 | TNFRSF4 | TNFRSF1A | FCER1A | SIRPA | * EPHA2 |
| 19 | ACKR2 | RIPK1 | TNFRSF4 | CCL22 | GHRL | * IL36A | * EPHA2 | * IL36A |
| 20 | CCL26 | F8 | CRLF2 | COL6A1 | PARK7 | ITGAV | REG3G | * AGT |
| ♯ edges | 23,922 | 36,447 | 39,024 | 34,755 | 6460 | 14,066 | 16,216 | 16,017 |
| Network | Severity | Term | p. Value | Genes |
|---|---|---|---|---|
| RNA | Mild | Early endosome | 0.047 | PXK, TICAM2, TOLLIP, ACKR2 |
| Severe | Cytokine receptor activity | 0.041 | IL22RA1, IL20RB, CRLF2 | |
| Critical | Cytokine receptor activity | 0.041 | IL22RA1, IL20RB, CRLF2 | |
| Protein | Mild | Extracellular region | 0.001 | IL1R1, RARRES2, PPBP, |
| IL1RAP, PTN, MIF, AGER, | ||||
| TNFRSF1A, CXCL10,CRHBP, | ||||
| CD200R1, PTPN6, GHRL | ||||
| Schaffer collateral—CA1 synapse | 0.047 | BCR, GHRL, PTN | ||
| Moderate | Extracellular matrix | 0.012 | VWF, AHSG, ZP3, TLR3 | |
| Transmembrane signaling receptor activity | 0.021 | FCER1A, AGER, TLR3, TLR2 | ||
| IL36A, VWF, AHSG, NPY, | ||||
| Extracellular space | 0.022 | DPEP1, HMOX1, GHRL, ZP3 | ||
| SMPDL3B, AGT, TLR3 | ||||
| Microglial cell activation | 0.026 | IFNGR2, AGER, TLR3, TLR2 | ||
| Cell adhesion | 0.040 | VWF, ITGAV, PBXIP1, | ||
| AGER, EPHA2 | ||||
| Severe | Cell adhesion | 0.008 | VWF, SIRPA, ITGAV, PBXIP1, | |
| AGER, EPHA2 | ||||
| Cell migration | 0.016 | SIRPA, ITGAV, PBXIP1, EPHA2 | ||
| IL36A, VWF, AHSG, CD5L, | ||||
| Extracellular space | 0.022 | DPEP1, HMOX1, GHRL, REG3G, | ||
| ZP3, SMPDL3B, AGT | ||||
| Critical | Extracellular matrix | 0.012 | VWF, AHSG, ZP3, TLR3 | |
| Transmembrane signaling receptor activity | 0.021 | FCER1A, AGER, TLR3, TLR2 | ||
| Microglial cell activation | 0.026 | IFNGR2, AGER, TLR3, TLR2 | ||
| Cell adhesion | 0.040 | VWF, SIRPA, PBXIP1 | ||
| AGER, EPHA2 |
| Mild | Moderate | Severe | Critical | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Gene | Network | Gene | Network | Gene | Network | Gene | Network | ||
| S100A8 | Child | RPS19 | RNA | RPS19 | RNA | RPS19 | RNA | RPS19 | RNA |
| S100A9 | RNA | S100A9 | RNA | S100A9 | RNA | S100A9 | RNA | ||
| ITGB2 | L-R | ITGB2 | L-R | ITGB2 | L-R | ITGB2 | L-R | ||
| CYBA | RNA | CYBA | RNA | CYBA | RNA | ||||
| Grand child | S100A8 | RNA | S100A8 | RNA | S100A8 | RNA | S100A8 | RNA | |
| ITGAM | Protein | ITGAM | Protein | CTSS | RNA | CTSS | RNA | ||
| ITGB2 | L-R | ITGB2 | L-R | S100A8 | RNA | ITGAM | Protein | ||
| ITGAM | Protein | ITGB2 | L-R | ||||||
| ITGB2 | L-R | ||||||||
| Grand parents | CCL5 | LR | NAMPT | Protein | TNC | RNA | C3AR1 | RNA | |
| EPHB6 | Protein | FFAR2 | RNA | LDLR | RNA | ||||
| CCL11 | L-R | CCR7 | RNA | MVK | RNA | ||||
| CCL17 | L-R | IL17B | RNA | PLA2G7 | RNA | ||||
| CCL19 | L-R | ANXA1 | RNA | MUC19 | RNA | ||||
| CCL22 | L-R | S100A8 | RNA | SERPINE1 | Protein | ||||
| CCL24 | L-R | IL22RA2 | RNA | EPHB6 | Protein | ||||
| CCL7 | L-R | HSPA4 | RNA | TNFAIP8L2 | Protein | ||||
| LPL | RNA | SNCA | Protein | ||||||
| MIF | RNA | CCL11 | L-R | ||||||
| SERPINE1 | RNA | CCL17 | L-R | ||||||
| IL18RAP | RNA | CCL19 | L-R | ||||||
| CD14 | RNA | CCL22 | L-R | ||||||
| NAMPT | Protein | CCL24 | L-R | ||||||
| EPHB6 | Protein | CCL7 | L-R | ||||||
| C3 | L-R | ||||||||
| CCL11 | L-R | ||||||||
| CCL17 | L-R | ||||||||
| CCL19 | L-R | ||||||||
| CCL22 | L-R | ||||||||
| CCL24 | L-R | ||||||||
| CCL3 | L-R | ||||||||
| CCL4 | L-R | ||||||||
| CCL5 | L-R | ||||||||
| CCL7 | L-R | ||||||||
| S100A8 | Child | S100A8 | RNA | S100A8 | RNA | S100A8 | RNA | S100A8 | RNA |
| ITGB2 | L-R | ITGB2 | L-R | ITGB2 | L-R | ITGB2 | L-R | ||
| Grand child | RPS19 | RNA | CYBA | RNA | CYBA | RNA | CYBA | RNA | |
| S100A9 | RNA | RPS19 | RNA | RPS19 | RNA | RPS19 | RNA | ||
| ITGAM | Protein | S100A9 | RNA | CTSS | RNA | CTSS | RNA | ||
| ITGB2 | L-R | ITGAM | Protein | S100A9 | RNA | S100A9 | RNA | ||
| ITGB2 | L-R | ITGAM | Protein | ITGAM | Protein | ||||
| ITGB2 | L-R | ITGB2 | L-R | ||||||
| Grand parents | CD14 | RNA | CD14 | RNA | CD14 | RNA | CD14 | RNA | |
| FPR2 | RNA | GRN | RNA | GRN | RNA | GRN | RNA | ||
| AXL | RNA | TNFRSF1A | RNA | TNFRSF1A | RNA | TNFRSF1A | RNA | ||
| SELP | Protein | FPR2 | RNA | FPR2 | RNA | FPR2 | RNA | ||
| ANXA1 | LR | IFNG | RNA | ZDHHC12 | RNA | IFNG | RNA | ||
| CCL5 | LR | ZDHHC12 | RNA | AXL | RNA | ZDHHC12 | RNA | ||
| IL18 | LR | AXL | RNA | TNFRSF1A | Protein | AXL | RNA | ||
| TNFRSF1A | Protein | GRN | Protein | TNFRSF1A | Protein | ||||
| SELP | Protein | ANXA1 | L-R | SELP | Protein | ||||
| GRN | Protein | C3 | L-R | TNFRSF1A | Protein | ||||
| HAVCR2 | Protein | GRN | Protein | ||||||
| ANXA1 | L-R | ANXA1 | L-R | ||||||
| C3 | L-R | C3 | L-R | ||||||
| CCL3 | L-R | CCL2 | L-R | ||||||
| CCL4 | L-R | CCL3 | L-R | ||||||
| CCL5 | L-R | CCL4 | L-R | ||||||
| IL18 | L-R | CCL5 | L-R | ||||||
| Age Group | Gender | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20 s | 30 s | 40 s | 50 s | 60 s | 70 s | 80 s | 90 s | 100 s | Male | Female | ||
| RNA | Mild | 7 | 16 | 7 | 4 | 8 | 12 | 13 | 4 | 0 | 33 | 38 |
| Moderate | 29 | 48 | 52 | 34 | 51 | 21 | 6 | 0 | 0 | 162 | 79 | |
| Severe | 4 | 14 | 38 | 70 | 99 | 75 | 79 | 24 | 1 | 287 | 117 | |
| Critical | 0 | 6 | 20 | 54 | 86 | 75 | 52 | 10 | 0 | 239 | 64 | |
| Protein | Mild | 7 | 17 | 7 | 4 | 8 | 16 | 13 | 4 | 0 | 35 | 41 |
| Moderate | 35 | 55 | 60 | 45 | 62 | 34 | 24 | 8 | 0 | 218 | 105 | |
| Severe | 5 | 14 | 43 | 76 | 115 | 93 | 106 | 33 | 2 | 345 | 142 | |
| Critical | 1 | 8 | 28 | 78 | 140 | 137 | 92 | 14 | 0 | 389 | 109 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.; Wang, Q.S.; Hasegawa, T.; Namkoong, H.; Tanaka, H.; Koike, R.; Kitagawa, Y.; Kimura, A.; Imoto, S.; Kanai, T.; et al. Unraveling the COVID-19 Severity Hubs and Interplays in Inflammatory-Related RNA–Protein Networks. Int. J. Mol. Sci. 2025, 26, 4412. https://doi.org/10.3390/ijms26094412
Park H, Wang QS, Hasegawa T, Namkoong H, Tanaka H, Koike R, Kitagawa Y, Kimura A, Imoto S, Kanai T, et al. Unraveling the COVID-19 Severity Hubs and Interplays in Inflammatory-Related RNA–Protein Networks. International Journal of Molecular Sciences. 2025; 26(9):4412. https://doi.org/10.3390/ijms26094412
Chicago/Turabian StylePark, Heewon, Qingbo S. Wang, Takanori Hasegawa, Ho Namkoong, Hiroko Tanaka, Ryuji Koike, Yuko Kitagawa, Akinori Kimura, Seiya Imoto, Takanori Kanai, and et al. 2025. "Unraveling the COVID-19 Severity Hubs and Interplays in Inflammatory-Related RNA–Protein Networks" International Journal of Molecular Sciences 26, no. 9: 4412. https://doi.org/10.3390/ijms26094412
APA StylePark, H., Wang, Q. S., Hasegawa, T., Namkoong, H., Tanaka, H., Koike, R., Kitagawa, Y., Kimura, A., Imoto, S., Kanai, T., Fukunaga, K., Ogawa, S., Okada, Y., & Miyano, S. (2025). Unraveling the COVID-19 Severity Hubs and Interplays in Inflammatory-Related RNA–Protein Networks. International Journal of Molecular Sciences, 26(9), 4412. https://doi.org/10.3390/ijms26094412






