CCN2 Activates Cellular Senescence Leading to Kidney Fibrosis in Folic Acid-Induced Experimental Nephropathy
Abstract
1. Introduction
2. Results
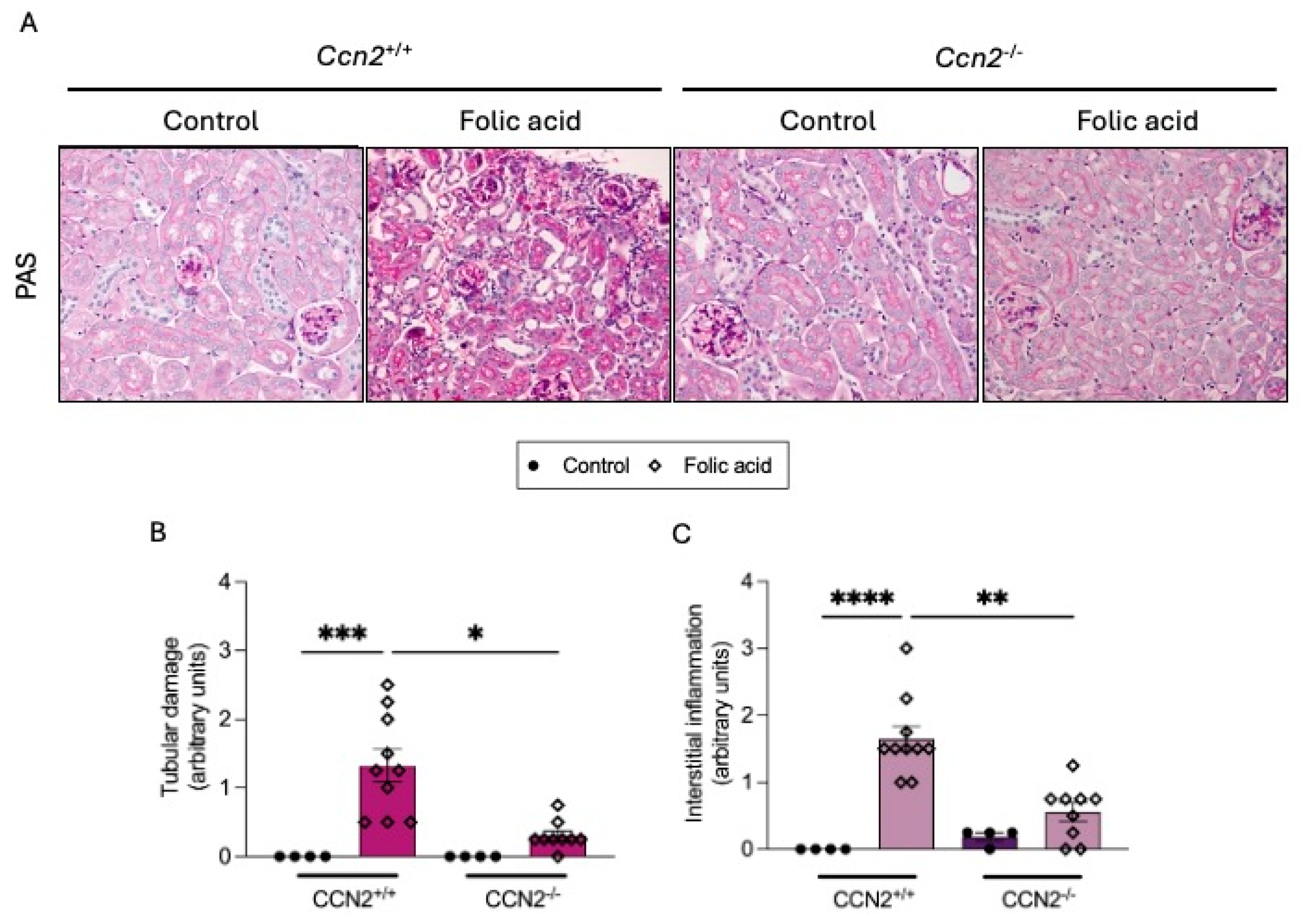
2.1. CCN2 Deficiency Diminishes Kidney Damage in Chronic Folic Acid Nephropathy
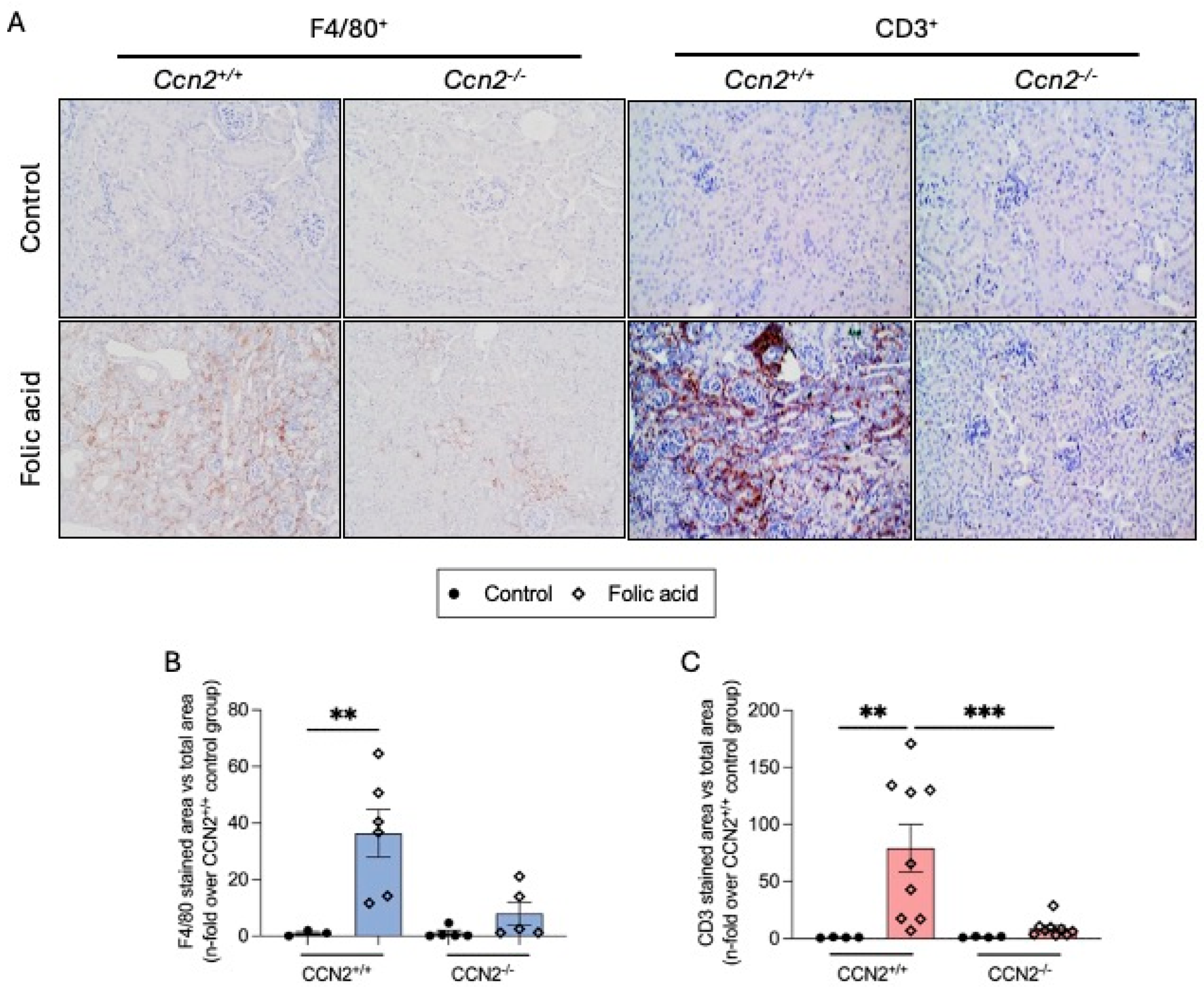
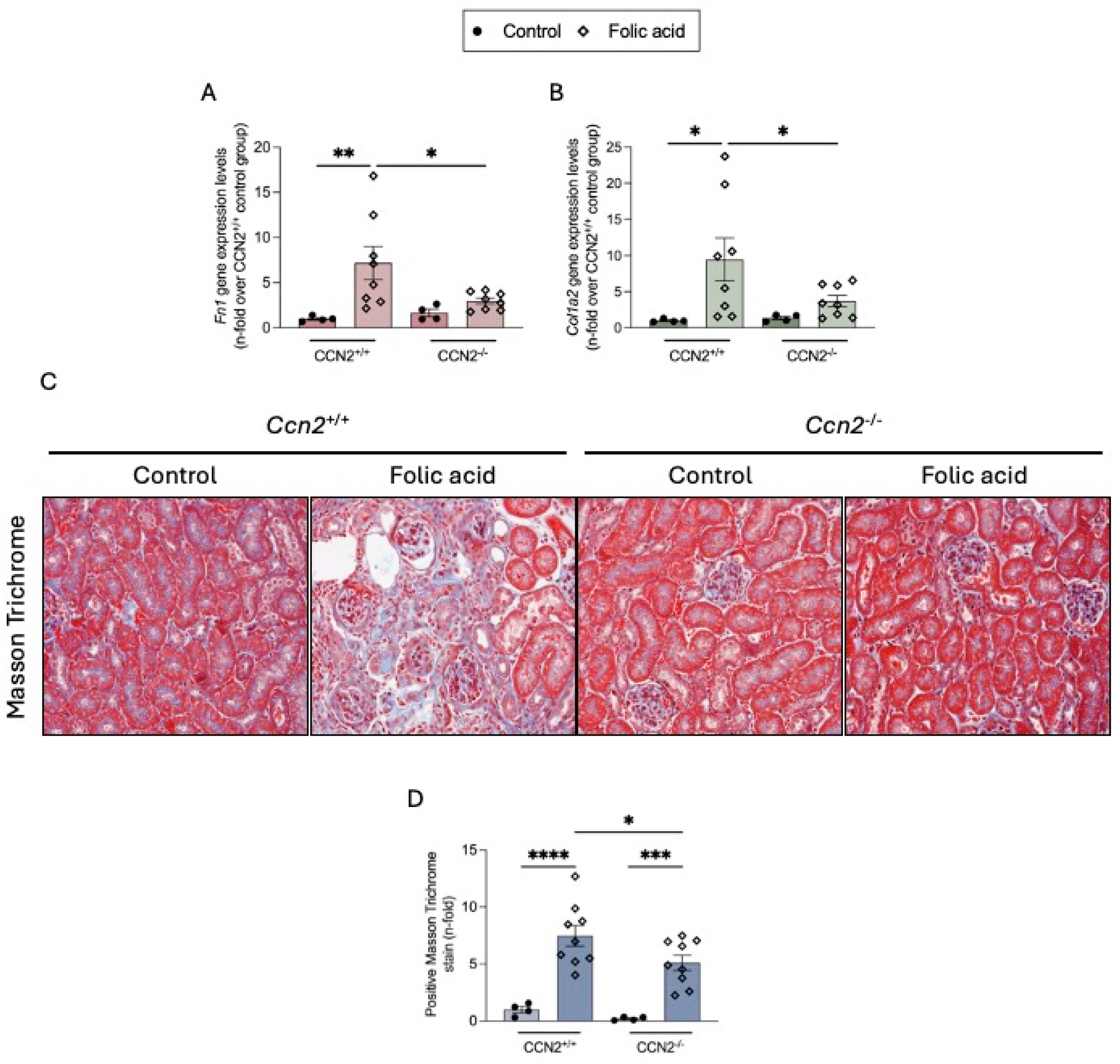
2.2. CCN2 Deficiency Diminishes Renal Inflammation and Fibrosis in Chronic Folic Acid Nephropathy
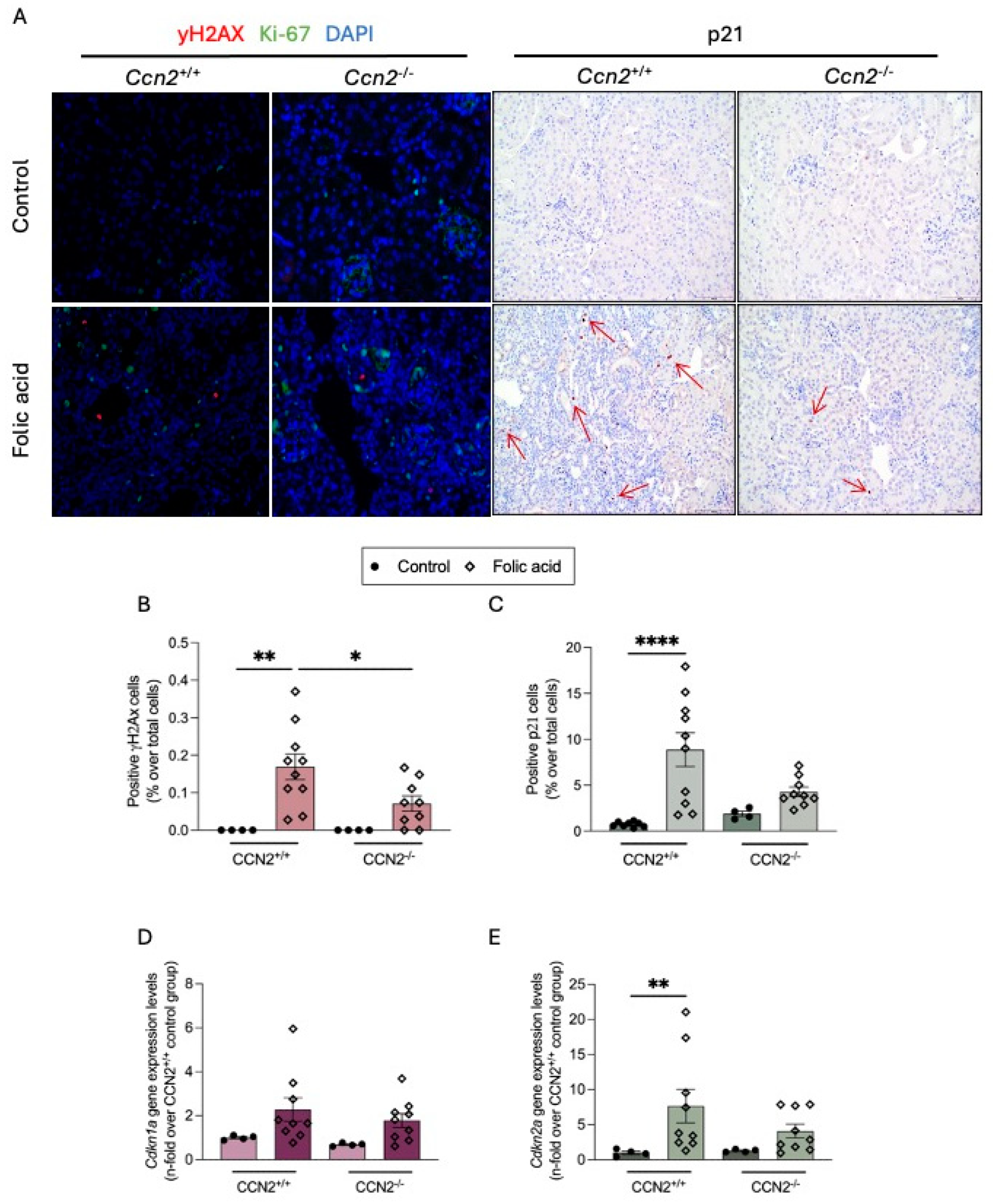
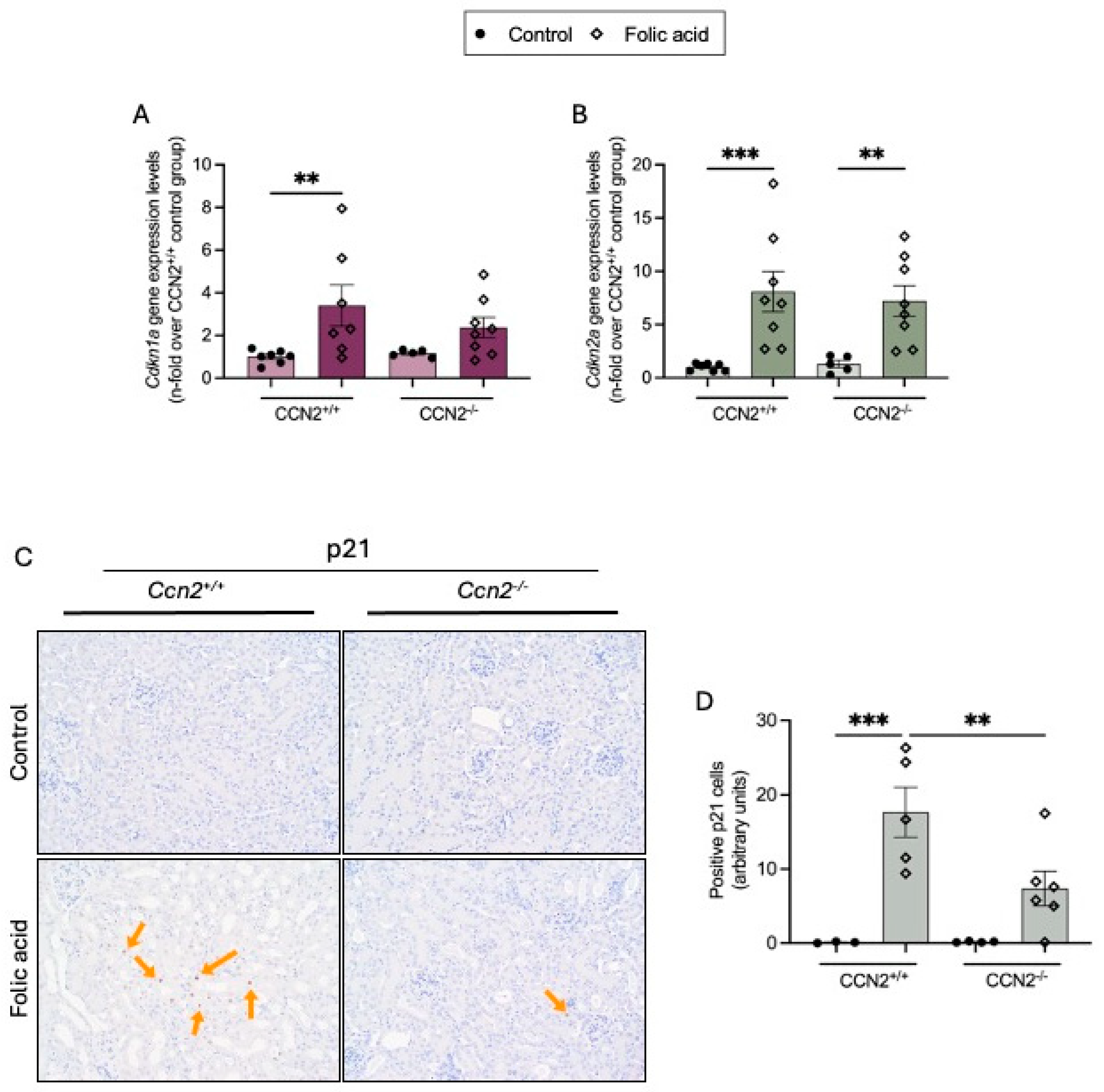
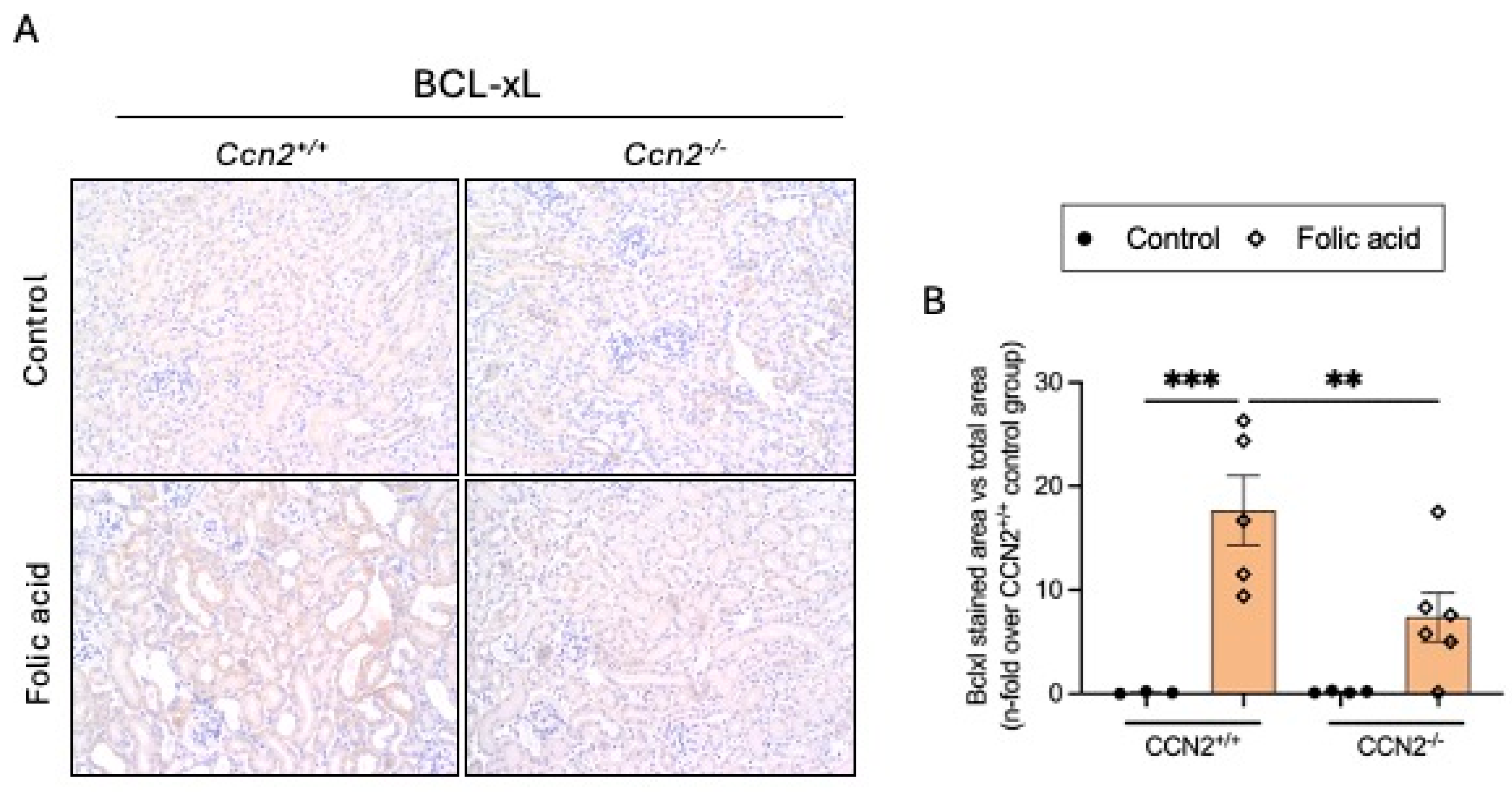
2.3. CCN2 Regulates Cellular Senescence in Chronic Folic Acid Nephropathy
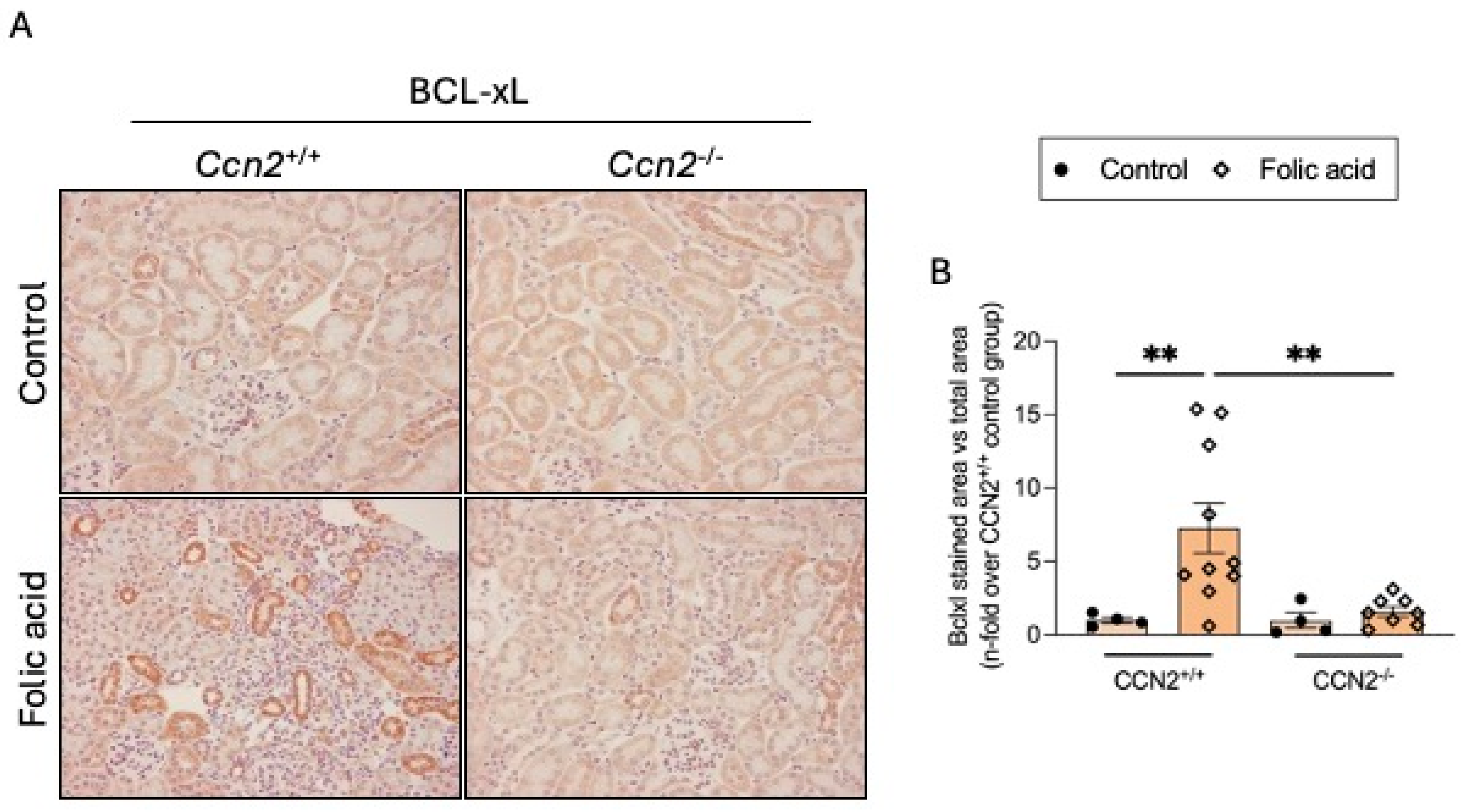
2.4. CCN2 Regulates Cellular Senescence in the Acute Phase of Folic Acid Nephropathy
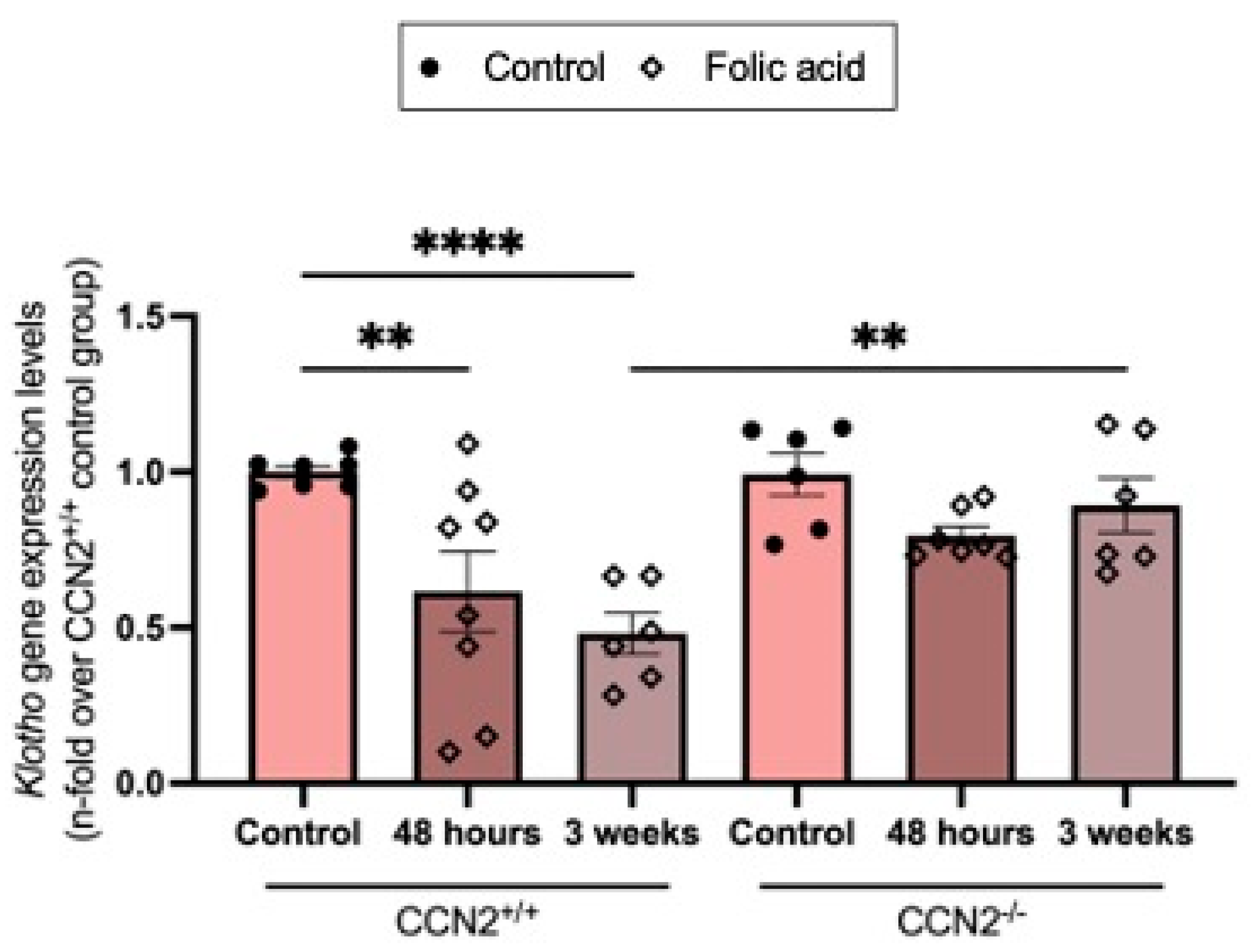
2.5. CCN2 Deficiency Prevents the Loss of the Nephroprotective Factor Klotho in Acute and Chronic Folic Acid Nephropathy
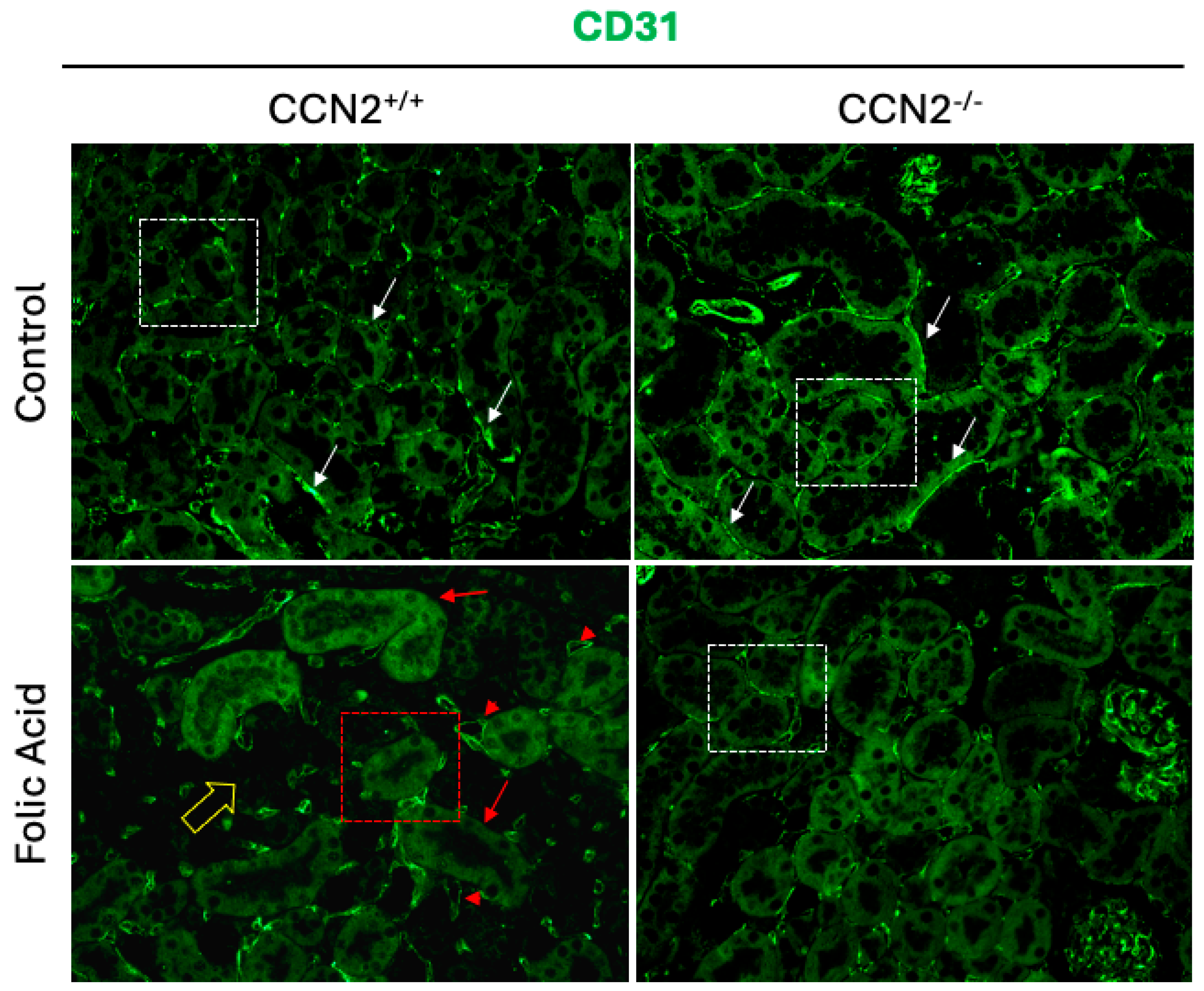
2.6. CCN2 Deficiency Prevents Capillary Rarefaction in Chronic Folic Acid Nephropathy
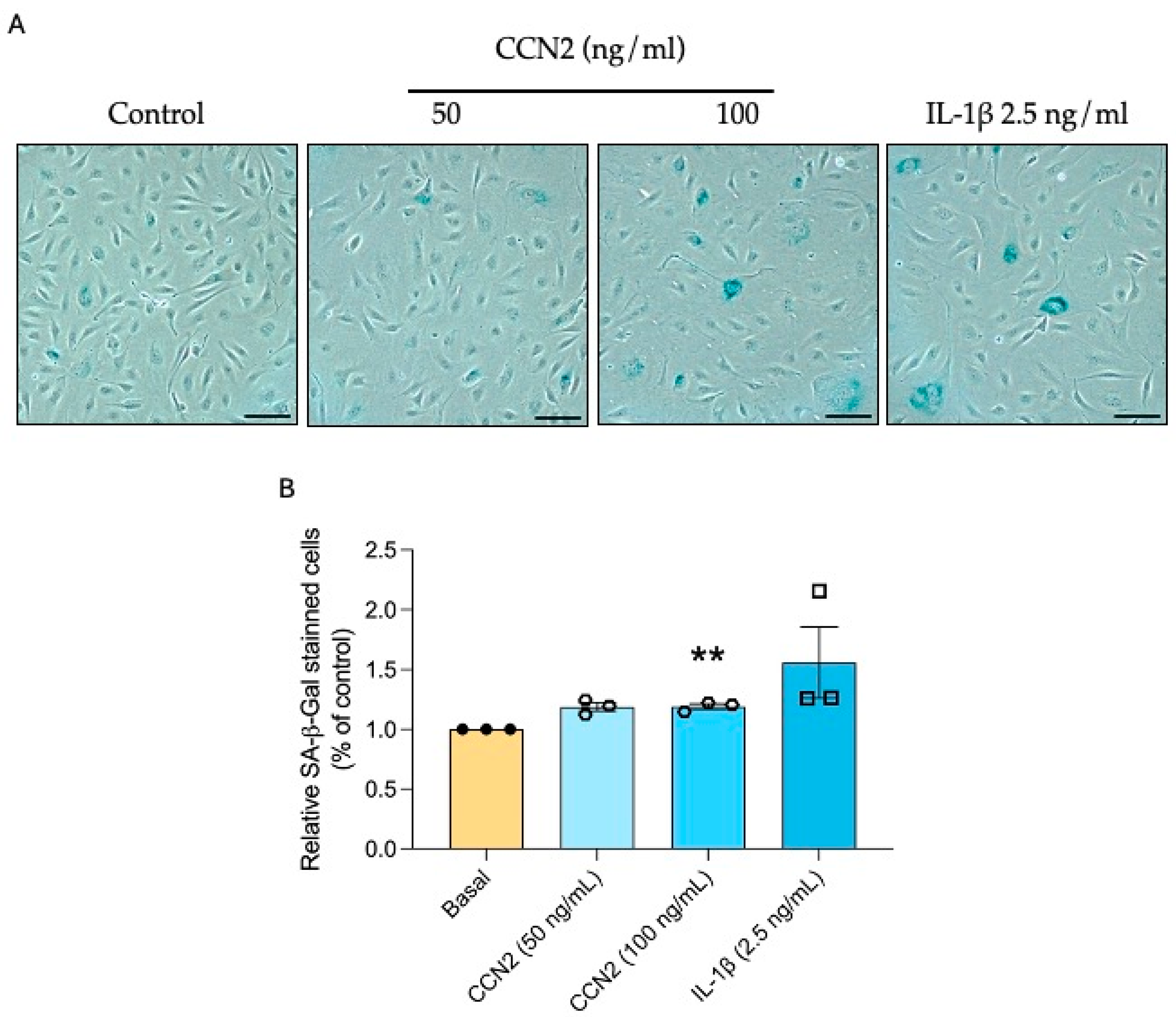
2.7. CCN2 Induces Cellular Senescence in HUVECs
2.8. CCN2 Induces the Priming and Activation of the NLRP3 Inflammasome in HUVECs
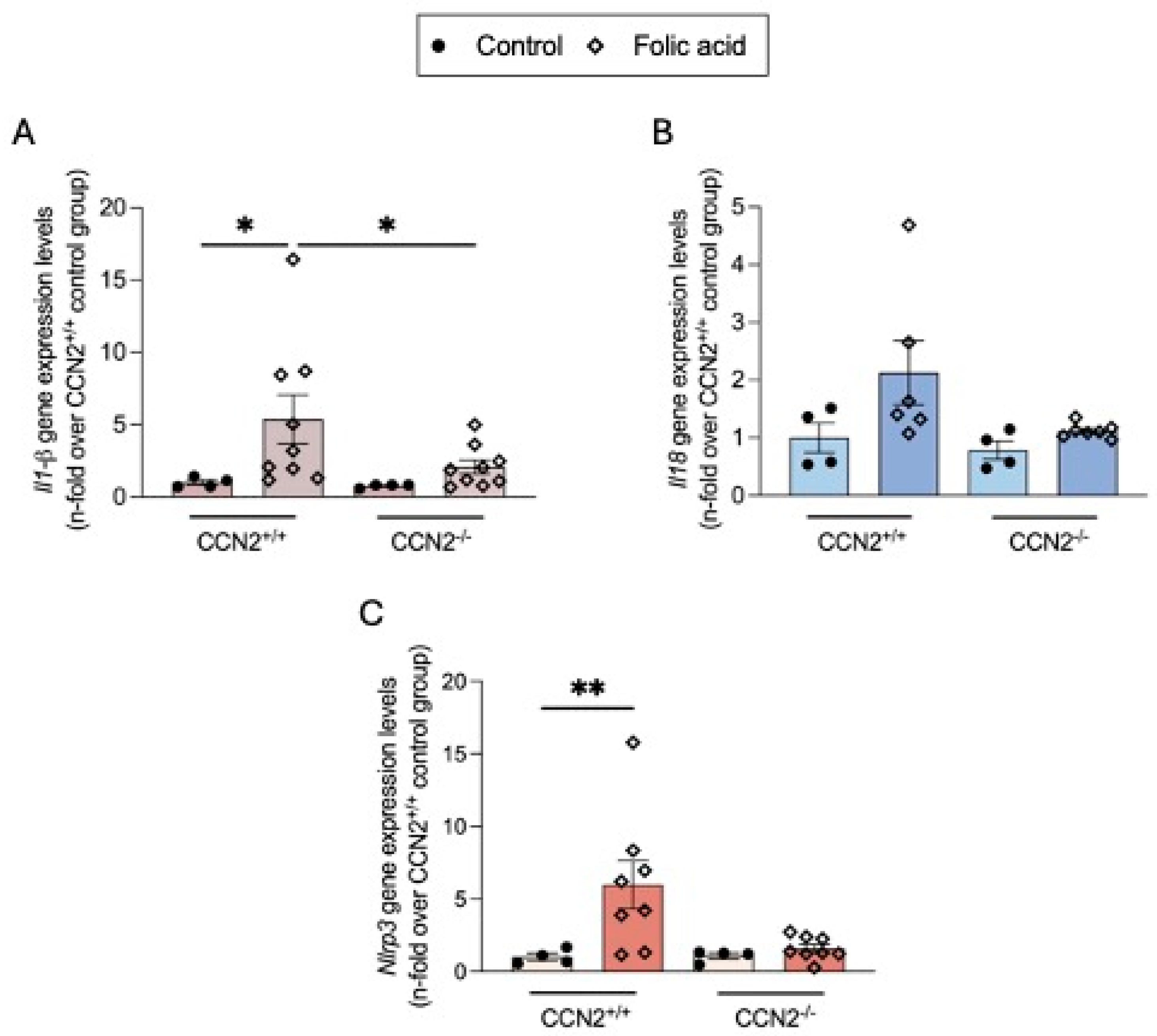
2.9. CCN2 Modulates the NLRP3 Inflammasome in Chronic Folic Acid Nephropathy
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Cell Cultures
4.3. Senescence-Associated-β-Galactosidase Activity
4.4. Cellular Protein Studies
4.5. Histology and Immunohistochemistry
4.6. Gene Expression Studies
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKI | Acute Kidney Injury |
| BCL-xL | B-cell lymphoma-extra large |
| CCA | Cell cycle arrest |
| Ccl2 | Chemokine (C-C motif) ligand 2 |
| CCN2 | Cellular communication network factor 2 |
| Cdkn1a | Cyclin-dependent kinase inhibitor 1A (p21) |
| Cdkn2a | Cyclin-dependent kinase inhibitor 2A (p16) |
| CKD | Chronic Kidney Disease |
| Col1a2 | Collagen type I alpha 2 chain |
| DDR | DNA Damage Response |
| ECM | Extracellular Matrix |
| FA | Folic acid |
| FAN | Folic-acid nephropathy |
| Fn1 | Fibronectin 1 |
| Havcr1 | Hepatitis A Virus Cellular Receptor 1 |
| HUVEC | Human Umbilical Vein Endothelial Cells |
| IL1-β | Interleukin 1 β |
| Il6 | Interleukin 6 |
| IRI | Ischemia-Reperfusion Injury |
| Ki-67 | Marker of proliferation Ki-67 |
| KIM-1 | Kidney Injury Molecule-1 |
| Lcn2 | Lipocalin 2 |
| NF-KB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NGAL | Neutrophil Gelatinase-Associated Lipocalin |
| NLRP3 | NOD-like receptor protein 3 |
| SASP | Senescence-Associated Secretory Phenotype |
| Serpine1 | Serpin Family E Member 1 (also known as PAI-1) |
| TECs | Tubular Epithelial Cells |
| γH2AX | Phosphorylated Histone H2AX (marker of DNA damage) |
References
- Ortiz, A.; Roger, M.; Jiménez, V.M.; Perez, J.C.R.; Furlano, M.; Atxer, L.S.; Zurro, D.G.; Casabona, C.M.R.; Zurro, D.G.; Gómez, C.G.; et al. RICORS2040: The Need for Collaborative Research in Chronic Kidney Disease. Clin. Kidney J. 2022, 15, 372–387. [Google Scholar] [CrossRef]
- Ruiz-Ortega, M.; Rayego-Mateos, S.; Lamas, S.; Ortiz, A.; Rodrigues-Diez, R.R. Targeting the Progression of Chronic Kidney Disease. Nat. Rev. Nephrol. 2020, 16, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Rayego-Mateos, S.; Rodrigues-Diez, R.R.; Fernandez-Fernandez, B.; Mora-Fernández, C.; Marchant, V.; Donate-Correa, J.; Navarro-González, J.F.; Ortiz, A.; Ruiz-Ortega, M. Targeting Inflammation to Treat Diabetic Kidney Disease: The Road to 2030. Kidney Int. 2023, 103, 282–296. [Google Scholar] [CrossRef] [PubMed]
- Rayego-Mateos, S.; Campillo, S.; Rodrigues-Diez, R.R.; Tejera-Muñoz, A.; Marquez-Exposito, L.; Goldschmeding, R.; Rodríguez-Puyol, D.; Calleros, L.; Ruiz-Ortega, M. Interplay between Extracellular Matrix Components and Cellular and Molecular Mechanisms in Kidney Fibrosis. Clin. Sci. 2021, 135, 1999–2029. [Google Scholar] [CrossRef] [PubMed]
- Sturmlechner, I.; Durik, M.; Sieben, C.J.; Baker, D.J.; van Deursen, J.M. Cellular Senescence in Renal Ageing and Disease. Nat. Rev. Nephrol. 2017, 13, 77–89. [Google Scholar] [CrossRef]
- Valentijn, F.A.; Falke, L.L.; Nguyen, T.Q.; Goldschmeding, R. Cellular Senescence in the Aging and Diseased Kidney. J. Cell Commun. Signal 2018, 12, 69–82. [Google Scholar] [CrossRef]
- Krtolica, A.; Parrinello, S.; Lockett, S.; Desprez, P.-Y.; Campisi, J. Senescent Fibroblasts Promote Epithelial Cell Growth and Tumorigenesis: A Link between Cancer and Aging. Proc. Natl. Acad. Sci. USA 2001, 98, 12072–12077. [Google Scholar] [CrossRef]
- Coppé, J.-P.; Patil, C.K.; Rodier, F.; Sun, Y.; Muñoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.-Y.; Campisi, J. Senescence-Associated Secretory Phenotypes Reveal Cell-Nonautonomous Functions of Oncogenic RAS and the P53 Tumor Suppressor. PLoS Biol. 2008, 6, e301. [Google Scholar] [CrossRef]
- Hernandez-Segura, A.; de Jong, T.V.; Melov, S.; Guryev, V.; Campisi, J.; Demaria, M. Unmasking Transcriptional Heterogeneity in Senescent Cells. Curr. Biol. 2017, 27, 2652–2660.e4. [Google Scholar] [CrossRef]
- Campisi, J. The Biology of Replicative Senescence. Eur. J. Cancer 1997, 33, 703–709. [Google Scholar] [CrossRef]
- Toussaint, O.; Dumont, P.; Dierick, J.; Pascal, T.; Frippiat, C.; Chainiaux, F.; Sluse, F.; Eliaers, F.; Remacle, J. Stress-Induced Premature Senescence: Essence of Life, Evolution, Stress, and Aging. Ann. N. Y Acad. Sci. 2000, 908, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Datto, M.B.; Li, Y.; Panus, J.F.; Howe, D.J.; Xiong, Y.; Wang, X.F. Transforming Growth Factor Beta Induces the Cyclin-Dependent Kinase Inhibitor P21 through a P53-Independent Mechanism. Proc. Natl. Acad. Sci. USA 1995, 92, 5545–5549. [Google Scholar] [CrossRef]
- Perbal, B. CCN Proteins: A Centralized Communication Network. J. Cell Commun. Signal 2013, 7, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Kubota, S.; Kawata, K.; Hattori, T.; Nishida, T. Molecular and Genetic Interactions between CCN2 and CCN3 behind Their Yin–Yang Collaboration. Int. J. Mol. Sci. 2022, 23, 5887. [Google Scholar] [CrossRef] [PubMed]
- Frazier, K.; Williams, S.; Kothapalli, D.; Klapper, H.; Grotendorst, G.R. Stimulation of Fibroblast Cell Growth, Matrix Production, and Granulation Tissue Formation by Connective Tissue Growth Factor. J. Investig. Dermatol. 1996, 107, 404–411. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Rayego, S.; Rodrigues-Díez, R.; Rodriguez, J.S.; Rodrigues-Díez, R.; Rodríguez-Vita, J.; Carvajal, G.; Aroeira, L.S.; Selgas, R.; Mezzano, S.A.; et al. CTGF Promotes Inflammatory Cell Infiltration of the Renal Interstitium by Activating NF-ΚB. J. Am. Soc. Nephrol. 2009, 20, 1513–1526. [Google Scholar] [CrossRef]
- Babic, A.M.; Chen, C.-C.; Lau, L.F. Fisp12/Mouse Connective Tissue Growth Factor Mediates Endothelial Cell Adhesion and Migration through Integrin αvβ3, Promotes Endothelial Cell Survival, and Induces Angiogenesis In Vivo. Mol. Cell Biol. 1999, 19, 2958–2966. [Google Scholar] [CrossRef]
- Shimo, T.; Nakanishi, T.; Nishida, T.; Asano, M.; Kanyama, M.; Kuboki, T.; Tamatani, T.; Tezuka, K.; Takemura, M.; Matsumura, T.; et al. Connective Tissue Growth Factor Induces the Proliferation, Migration, and Tube Formation of Vascular Endothelial Cells In Vitro, and Angiogenesis In Vivo. J. Biochem. 1999, 126, 137–145. [Google Scholar] [CrossRef]
- Valentijn, F.A.; Knoppert, S.N.; Marquez-Exposito, L.; Rodrigues-Diez, R.R.; Pissas, G.; Tang, J.; Tejedor-Santamaria, L.; Broekhuizen, R.; Samarakoon, R.; Eleftheriadis, T.; et al. Cellular Communication Network 2 (Connective Tissue Growth Factor) Aggravates Acute DNA Damage and Subsequent DNA Damage Response-Senescence-Fibrosis Following Kidney Ischemia Reperfusion Injury. Kidney Int. 2022, 102, 1305–1319. [Google Scholar] [CrossRef]
- Rayego-Mateos, S.; Morgado-Pascual, J.L.; Rodrigues-Diez, R.R.; Rodrigues-Diez, R.; Falke, L.L.; Mezzano, S.; Ortiz, A.; Egido, J.; Goldschmeding, R.; Ruiz-Ortega, M. Connective Tissue Growth Factor Induces Renal Fibrosis via Epidermal Growth Factor Receptor Activation. J. Pathol. 2018, 244, 227–241. [Google Scholar] [CrossRef]
- Kinashi, H.; Falke, L.L.; Nguyen, T.Q.; Bovenschen, N.; Aten, J.; Leask, A.; Ito, Y.; Goldschmeding, R. Connective Tissue Growth Factor Regulates Fibrosis-Associated Renal Lymphangiogenesis. Kidney Int. 2017, 92, 850–863. [Google Scholar] [CrossRef] [PubMed]
- Rayego-Mateos, S.; Marquez-Exposito, L.; Basantes, P.; Tejedor-Santamaria, L.; Sanz, A.B.; Nguyen, T.Q.; Goldschmeding, R.; Ortiz, A.; Ruiz-Ortega, M. CCN2 Activates RIPK3, NLRP3 Inflammasome, and NRF2/Oxidative Pathways Linked to Kidney Inflammation. Antioxidants 2023, 12, 1541. [Google Scholar] [CrossRef]
- Rodrigues-Díez, R.; Rodrigues-Díez, R.R.; Rayego-Mateos, S.; Suarez-Alvarez, B.; Lavoz, C.; Stark Aroeira, L.; Sánchez-López, E.; Orejudo, M.; Alique, M.; Lopez-Larrea, C.; et al. The C-Terminal Module IV of Connective Tissue Growth Factor Is a Novel Immune Modulator of the Th17 Response. Lab. Investig. 2013, 93, 812–824. [Google Scholar] [CrossRef]
- Phanish, M.K.; Winn, S.K.; Dockrell, M.E.C. Connective Tissue Growth Factor-(CTGF, CCN2)—A Marker, Mediator and Therapeutic Target for Renal Fibrosis. Nephron Exp. Nephrol. 2009, 114, e83–e92. [Google Scholar] [CrossRef] [PubMed]
- Leask, A. CCN2: A Novel, Specific and Valid Target for Anti-Fibrotic Drug Intervention. Expert. Opin. Ther. Targets 2013, 17, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Clarkson, M.R.; Duggan, J.; Brady, H.R. Connective Tissue Growth Factor: Potential Role in Glomerulosclerosis and Tubulointerstitial Fibrosis. Kidney Int. 2000, 58, 1389–1399. [Google Scholar] [CrossRef]
- Jun, J.-I.; Lau, L.F. CCN2 Induces Cellular Senescence in Fibroblasts. J. Cell Commun. Signal 2017, 11, 15–23. [Google Scholar] [CrossRef]
- Wahab, N.; Cox, D.; Witherden, A.; Mason, R.M. Connective Tissue Growth Factor (CTGF) Promotes Activated Mesangial Cell Survival via up-Regulation of Mitogen-Activated Protein Kinase Phosphatase-1 (MKP-1). Biochem. J. 2007, 406, 131–138. [Google Scholar] [CrossRef]
- Valentijn, F.A.; Knoppert, S.N.; Pissas, G.; Rodrigues-Diez, R.R.; Marquez-Exposito, L.; Broekhuizen, R.; Mokry, M.; Kester, L.A.; Falke, L.L.; Goldschmeding, R.; et al. CCN2 Aggravates the Immediate Oxidative Stress–DNA Damage Response Following Renal Ischemia–Reperfusion Injury. Antioxidants 2021, 10, 2020. [Google Scholar] [CrossRef]
- Rayego-Mateos, S.; Morgado-Pascual, J.L.; Lavoz, C.; Rodrigues-Díez, R.R.; Márquez-Expósito, L.; Tejera-Muñoz, A.; Tejedor-Santamaría, L.; Rubio-Soto, I.; Marchant, V.; Ruiz-Ortega, M. CCN2 Binds to Tubular Epithelial Cells in the Kidney. Biomolecules 2022, 12, 252. [Google Scholar] [CrossRef]
- Inoue, T.; Kusano, T.; Amano, H.; Nakamoto, H.; Okada, H. Cellular Communication Network Factor 2 (CCN2) Promotes the Progression of Acute Kidney Injury to Chronic Kidney Disease. Biochem. Biophys. Res. Commun. 2019, 517, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Polichnowski, A.J. Microvascular Rarefaction and Hypertension in the Impaired Recovery and Progression of Kidney Disease Following AKI in Preexisting CKD States. Am. J. Physiol.-Ren. Physiol. 2018, 315, F1513–F1518. [Google Scholar] [CrossRef]
- Kramann, R.; Wongboonsin, J.; Chang-Panesso, M.; Machado, F.G.; Humphreys, B.D. Gli1+ Pericyte Loss Induces Capillary Rarefaction and Proximal Tubular Injury. J. Am. Soc. Nephrol. 2017, 28, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Arefin, S.; Mudrovcic, N.; Hobson, S.; Pietrocola, F.; Ebert, T.; Ward, L.J.; Witasp, A.; Hernandez, L.; Wennberg, L.; Lundgren, T.; et al. Early Vascular Aging in Chronic Kidney Disease: Focus on Microvascular Maintenance, Senescence Signature and Potential Therapeutics. Transl. Res. 2025, 275, 32–47. [Google Scholar] [CrossRef]
- Rodrigues-Diez, R.R.; Garcia-Redondo, A.B.; Orejudo, M.; Rodrigues-Diez, R.; Briones, A.M.; Bosch-Panadero, E.; Kery, G.; Pato, J.; Ortiz, A.; Salaices, M.; et al. The C-Terminal Module IV of Connective Tissue Growth Factor, Through EGFR/Nox1 Signaling, Activates the NF-ΚB Pathway and Proinflammatory Factors in Vascular Smooth Muscle Cells. Antioxid. Redox Signal 2015, 22, 29–47. [Google Scholar] [CrossRef]
- Marquez-Exposito, L.; Tejedor-Santamaria, L.; Santos-Sanchez, L.; Valentijn, F.A.; Cantero-Navarro, E.; Rayego-Mateos, S.; Rodrigues-Diez, R.R.; Tejera-Muñoz, A.; Marchant, V.; Sanz, A.B.; et al. Acute Kidney Injury Is Aggravated in Aged Mice by the Exacerbation of Proinflammatory Processes. Front. Pharmacol. 2021, 12, 662020. [Google Scholar] [CrossRef] [PubMed]
- Podhorecka, M.; Skladanowski, A.; Bozko, P. H2AX Phosphorylation: Its Role in DNA Damage Response and Cancer Therapy. J. Nucleic Acids 2010, 2010, 920161. [Google Scholar] [CrossRef]
- Bullwinkel, J.; Baron-Lühr, B.; Lüdemann, A.; Wohlenberg, C.; Gerdes, J.; Scholzen, T. Ki-67 Protein Is Associated with Ribosomal RNA Transcription in Quiescent and Proliferating Cells. J. Cell Physiol. 2006, 206, 624–635. [Google Scholar] [CrossRef]
- Megyesi, J.; Safirstein, R.L.; Price, P.M. Induction of P21WAF1/CIP1/SDI1 in Kidney Tubule Cells Affects the Course of Cisplatin-Induced Acute Renal Failure. J. Clin. Investig. 1998, 101, 777–782. [Google Scholar] [CrossRef]
- Hodeify, R.; Tarcsafalvi, A.; Megyesi, J.; Safirstein, R.L.; Price, P.M. Cdk2-Dependent Phosphorylation of P21 Regulates the Role of Cdk2 in Cisplatin Cytotoxicity. Am. J. Physiol.-Ren. Physiol. 2011, 300, F1171–F1179. [Google Scholar] [CrossRef][Green Version]
- Nishioka, S.; Nakano, D.; Kitada, K.; Sofue, T.; Ohsaki, H.; Moriwaki, K.; Hara, T.; Ohmori, K.; Kohno, M.; Nishiyama, A. The Cyclin-Dependent Kinase Inhibitor P21 Is Essential for the Beneficial Effects of Renal Ischemic Preconditioning on Renal Ischemia/Reperfusion Injury in Mice. Kidney Int. 2014, 85, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Ebert, T.; Pawelzik, S.-C.; Witasp, A.; Arefin, S.; Hobson, S.; Kublickiene, K.; Shiels, P.G.; Bäck, M.; Stenvinkel, P. Inflammation and Premature Ageing in Chronic Kidney Disease. Toxins 2020, 12, 227. [Google Scholar] [CrossRef]
- Kida, Y.; Duffield, J.S. Pivotal Role of Pericytes in Kidney Fibrosis. Clin. Exp. Pharmacol. Physiol. 2011, 38, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Kida, Y.; Ieronimakis, N.; Schrimpf, C.; Reyes, M.; Duffield, J.S. EphrinB2 Reverse Signaling Protects against Capillary Rarefaction and Fibrosis after Kidney Injury. J. Am. Soc. Nephrol. 2013, 24, 559–572. [Google Scholar] [CrossRef]
- Choi, Y. Peritubular Capillary Loss Is Associated with Chronic Tubulointerstitial Injury in Human Kidney: Altered Expression of Vascular Endothelial Growth Factor. Hum. Pathol. 2000, 31, 1491–1497. [Google Scholar] [CrossRef]
- Romero, A.; Dongil, P.; Valencia, I.; Vallejo, S.; Hipólito-Luengo, Á.S.; Díaz-Araya, G.; Bartha, J.L.; González-Arlanzón, M.M.; Rivilla, F.; de la Cuesta, F.; et al. Pharmacological Blockade of NLRP3 Inflammasome/IL-1β-Positive Loop Mitigates Endothelial Cell Senescence and Dysfunction. Aging Dis. 2022, 13, 284. [Google Scholar] [CrossRef] [PubMed]
- Shamoon, L.; Espitia-Corredor, J.A.; Dongil, P.; Menéndez-Ribes, M.; Romero, A.; Valencia, I.; Díaz-Araya, G.; Sánchez-Ferrer, C.F.; Peiró, C. Resolvin E1 Attenuates Doxorubicin-Induced Endothelial Senescence by Modulating NLRP3 Inflammasome Activation. Biochem. Pharmacol. 2022, 201, 115078. [Google Scholar] [CrossRef]
- Villacampa, A.; Shamoon, L.; Valencia, I.; Morales, C.; Figueiras, S.; de la Cuesta, F.; Sánchez-Niño, D.; Díaz-Araya, G.; Sánchez-Pérez, I.; Sánchez-Ferrer, C.F.; et al. SARS-CoV-2 S Protein Reduces Cytoprotective Defenses and Promotes Human Endothelial Cell Senescence. Aging Dis. 2024. [Google Scholar] [CrossRef]
- Shi, L.; Zhao, Y.; Fei, C.; Guo, J.; Jia, Y.; Wu, D.; Wu, L.; Chang, C. Cellular Senescence Induced by S100A9 in Mesenchymal Stromal Cells through NLRP3 Inflammasome Activation. Aging 2019, 11, 9626–9642. [Google Scholar] [CrossRef]
- Liu, W.-B.; Wang, S.-S.; Zhang, X.; Ke, Z.-Z.; Wen, X.-Y.; Zhao, J.; Zhuang, X.-D.; Liao, L.-Z. Enhanced Cardiomyocyte NLRP3 Inflammasome-Mediated Pyroptosis Promotes d-Galactose-Induced Cardiac Aging. J. Am. Heart Assoc. 2024, 13, e032904. [Google Scholar] [CrossRef]
- Vanhove, T.; Kinashi, H.; Nguyen, T.Q.; Metalidis, C.; Poesen, K.; Naesens, M.; Lerut, E.; Goldschmeding, R.; Kuypers, D.R.J. Tubulointerstitial Expression and Urinary Excretion of Connective Tissue Growth Factor 3 Months after Renal Transplantation Predict Interstitial Fibrosis and Tubular Atrophy at 5 Years in a Retrospective Cohort Analysis. Transplant. Int. 2017, 30, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Wahab, N.A.; Weston, B.S.; Roberts, T.; Mason, R.M. Connective Tissue Growth Factor and Regulation of the Mesangial Cell Cycle. J. Am. Soc. Nephrol. 2002, 13, 2437–2445. [Google Scholar] [CrossRef]
- Jang, J.-H.; Chand, H.S.; Bruse, S.; Doyle-Eisele, M.; Royer, C.; McDonald, J.; Qualls, C.; Klingelhutz, A.J.; Lin, Y.; Mallampalli, R.; et al. Connective Tissue Growth Factor Promotes Pulmonary Epithelial Cell Senescence and Is Associated with COPD Severity. COPD J. Chronic Obstr. Pulm. Dis. 2017, 14, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Apelt, K.; Bijkerk, R.; Lebrin, F.; Rabelink, T.J. Imaging the Renal Microcirculation in Cell Therapy. Cells 2021, 10, 1087. [Google Scholar] [CrossRef]
- Kim, H.D.; Kim, E.N.; Lim, J.H.; Kim, Y.; Ban, T.H.; Lee, H.; Kim, Y.S.; Park, C.W.; Choi, B.S. Phosphodiesterase Inhibitor Ameliorates Senescent Changes of Renal Interstitial Pericytes in Aging Kidney. Aging Cell 2024, 23, e14075. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, F.; Sun, D. The Renal Microcirculation in Chronic Kidney Disease: Novel Diagnostic Methods and Therapeutic Perspectives. Cell Biosci. 2021, 11, 90. [Google Scholar] [CrossRef]
- Rojas, A.; Chang, F.-C.; Lin, S.-L.; Duffield, J.S. The Role Played by Perivascular Cells in Kidney Interstitial Injury. Clin. Nephrol. 2012, 77, 400–408. [Google Scholar] [CrossRef]
- Kramann, R.; Humphreys, B.D. Kidney Pericytes: Roles in Regeneration and Fibrosis. Semin. Nephrol. 2014, 34, 374–383. [Google Scholar] [CrossRef]
- Kida, Y. Peritubular Capillary Rarefaction: An Underappreciated Regulator of CKD Progression. Int. J. Mol. Sci. 2020, 21, 8255. [Google Scholar] [CrossRef]
- Goligorsky, M.S. Permissive Role of Vascular Endothelium in Fibrosis: Focus on the Kidney. Am. J. Physiol.-Cell Physiol. 2024, 326, C712–C723. [Google Scholar] [CrossRef]
- Gogiraju, R.; Xu, X.; Bochenek, M.L.; Steinbrecher, J.H.; Lehnart, S.E.; Wenzel, P.; Kessel, M.; Zeisberg, E.M.; Dobbelstein, M.; Schäfer, K. Endothelial P53 Deletion Improves Angiogenesis and Prevents Cardiac Fibrosis and Heart Failure Induced by Pressure Overload in Mice. J. Am. Heart Assoc. 2015, 4, e001770. [Google Scholar] [CrossRef] [PubMed]
- Ellis, L.V.; Bywaters, J.D.; Chen, J. Endothelial Deletion of P53 Generates Transitional Endothelial Cells and Improves Lung Development during Neonatal Hyperoxia. bioRxiv 2024. [Google Scholar] [CrossRef]
- Tejera-Muñoz, A.; Marquez-Exposito, L.; Tejedor-Santamaría, L.; Rayego-Mateos, S.; Orejudo, M.; Suarez-Álvarez, B.; López-Larrea, C.; Ruíz-Ortega, M.; Rodrigues-Díez, R.R. CCN2 Increases TGF-β Receptor Type II Expression in Vascular Smooth Muscle Cells: Essential Role of CCN2 in the TGF-β Pathway Regulation. Int. J. Mol. Sci. 2021, 23, 375. [Google Scholar] [CrossRef]
- Romeo, S.G.; Secco, I.; Schneider, E.; Reumiller, C.M.; Santos, C.X.C.; Zoccarato, A.; Musale, V.; Pooni, A.; Yin, X.; Theofilatos, K.; et al. Human Blood Vessel Organoids Reveal a Critical Role for CTGF in Maintaining Microvascular Integrity. Nat. Commun. 2023, 14, 5552. [Google Scholar] [CrossRef]
- Hall-Glenn, F.; De Young, R.A.; Huang, B.-L.; van Handel, B.; Hofmann, J.J.; Chen, T.T.; Choi, A.; Ong, J.R.; Benya, P.D.; Mikkola, H.; et al. CCN2/Connective Tissue Growth Factor Is Essential for Pericyte Adhesion and Endothelial Basement Membrane Formation during Angiogenesis. PLoS ONE 2012, 7, e30562. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.-H.; Spierings, J.; van Laar, J.M.; de Vries-Bouwstra, J.K.; van Dijk, M.; Goldschmeding, R. Association of Endothelial to Mesenchymal Transition and Cellular Senescence with Fibrosis in Skin Biopsies of Systemic Sclerosis Patients: A Cross-Sectional Study. Clin. Exp. Rheumatol. 2022, 4, 1612–1617. [Google Scholar] [CrossRef]
- Trogisch, F.A.; Abouissa, A.; Keles, M.; Birke, A.; Fuhrmann, M.; Dittrich, G.M.; Weinzierl, N.; Wink, E.; Cordero, J.; Elsherbiny, A.; et al. Endothelial Cells Drive Organ Fibrosis in Mice by Inducing Expression of the Transcription Factor SOX9. Sci. Transl. Med. 2024, 16, eabq4581. [Google Scholar] [CrossRef]
- Mulay, S.R.; Kulkarni, O.P.; Rupanagudi, K.V.; Migliorini, A.; Darisipudi, M.N.; Vilaysane, A.; Muruve, D.; Shi, Y.; Munro, F.; Liapis, H.; et al. Calcium Oxalate Crystals Induce Renal Inflammation by NLRP3-Mediated IL-1β Secretion. J. Clin. Investig. 2013, 123, 236–246. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Andrade, L.; Rodrigues, C.E.; Gomes, S.A.; Noronha, I.L. Acute Kidney Injury as a Condition of Renal Senescence. Cell Transplant. 2018, 27, 739–753. [Google Scholar] [CrossRef]
- Li, C.; Shen, Y.; Huang, L.; Liu, C.; Wang, J. Senolytic Therapy Ameliorates Renal Fibrosis Postacute Kidney Injury by Alleviating Renal Senescence. FASEB J. 2021, 35, e21229. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Livingston, M.J.; Ma, Z.; Hu, X.; Wen, L.; Ding, H.-F.; Zhou, D.; Dong, Z. Tubular Cell Senescence Promotes Maladaptive Kidney Repair and Chronic Kidney Disease after Cisplatin Nephrotoxicity. JCI Insight 2023, 8, e166643. [Google Scholar] [CrossRef] [PubMed]
- Hejazian, S.M.; Hejazian, S.S.; Mostafavi, S.M.; Hosseiniyan, S.M.; Montazersaheb, S.; Ardalan, M.; Zununi Vahed, S.; Barzegari, A. Targeting Cellular Senescence in Kidney Diseases and Aging: A Focus on Mesenchymal Stem Cells and Their Paracrine Factors. Cell Commun. Signal. 2024, 22, 609. [Google Scholar] [CrossRef]
- Chien, Y.; Scuoppo, C.; Wang, X.; Fang, X.; Balgley, B.; Bolden, J.E.; Premsrirut, P.; Luo, W.; Chicas, A.; Lee, C.S.; et al. Control of the Senescence-Associated Secretory Phenotype by NF-ΚB Promotes Senescence and Enhances Chemosensitivity. Genes Dev. 2011, 25, 2125–2136. [Google Scholar] [CrossRef]
- Xu, M.; Pirtskhalava, T.; Farr, J.N.; Weigand, B.M.; Palmer, A.K.; Weivoda, M.M.; Inman, C.L.; Ogrodnik, M.B.; Hachfeld, C.M.; Fraser, D.G.; et al. Senolytics Improve Physical Function and Increase Lifespan in Old Age. Nat. Med. 2018, 24, 1246–1256. [Google Scholar] [CrossRef]
- Yan, L. Folic Acid-induced Animal Model of Kidney Disease. Anim. Model. Exp. Med. 2021, 4, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Martin-Sanchez, D.; Guerrero-Mauvecin, J.; Fontecha-Barriuso, M.; Mendez-Barbero, N.; Saiz, M.L.; Lopez-Diaz, A.M.; Sanchez-Niño, M.D.; Carrasco, S.; Cannata-Ortiz, P.; Ruiz-Ortega, M.; et al. Bone Marrow–Derived RIPK3 Mediates Kidney Inflammation in Acute Kidney Injury. J. Am. Soc. Nephrol. 2022, 33, 357–373. [Google Scholar] [CrossRef]
- Vitalone, M.J.; O’Connell, P.J.; Wavamunno, M.; Fung, C.L.-S.; Chapman, J.R.; Nankivell, B.J. Transcriptome Changes of Chronic Tubulointerstitial Damage in Early Kidney Transplantation. Transplantation 2010, 89, 537–547. [Google Scholar] [CrossRef]
- Leask, A.; Parapuram, S.K.; Shi-wen, X.; Abraham, D.J. Connective Tissue Growth Factor (CTGF, CCN2) Gene Regulation: A Potent Clinical Bio-marker of Fibroproliferative Disease? J. Cell Commun. Signal 2009, 3, 89–94. [Google Scholar] [CrossRef]
- Ramazani, Y.; Knops, N.; Elmonem, M.A.; Nguyen, T.Q.; Arcolino, F.O.; van den Heuvel, L.; Levtchenko, E.; Kuypers, D.; Goldschmeding, R. Connective Tissue Growth Factor (CTGF) from Basics to Clinics. Matrix Biol. 2018, 68–69, 44–66. [Google Scholar] [CrossRef]
- Guerrero-Mauvecin, J.; Villar-Gómez, N.; Rayego-Mateos, S.; Ramos, A.M.; Ruiz-Ortega, M.; Ortiz, A.; Sanz, A.B. Regulated Necrosis Role in Inflammation and Repair in Acute Kidney Injury. Front. Immunol. 2023, 14, 1324996. [Google Scholar] [CrossRef] [PubMed]
- Rayego-Mateos, S.; Marquez-Expósito, L.; Rodrigues-Diez, R.; Sanz, A.B.; Guiteras, R.; Doladé, N.; Rubio-Soto, I.; Manonelles, A.; Codina, S.; Ortiz, A.; et al. Molecular Mechanisms of Kidney Injury and Repair. Int. J. Mol. Sci. 2022, 23, 1542. [Google Scholar] [CrossRef] [PubMed]
- Fontecha-Barriuso, M.; Lopez-Diaz, A.M.; Guerrero-Mauvecin, J.; Miguel, V.; Ramos, A.M.; Sanchez-Niño, M.D.; Ruiz-Ortega, M.; Ortiz, A.; Sanz, A.B. Tubular Mitochondrial Dysfunction, Oxidative Stress, and Progression of Chronic Kidney Disease. Antioxidants 2022, 11, 1356. [Google Scholar] [CrossRef] [PubMed]
- Martin-Sanchez, D.; Fontecha-Barriuso, M.; Carrasco, S.; Sanchez-Niño, M.D.; von Mässenhausen, A.; Linkermann, A.; Cannata-Ortiz, P.; Ruiz-Ortega, M.; Egido, J.; Ortiz, A.; et al. TWEAK and RIPK1 Mediate a Second Wave of Cell Death during AKI. Proc. Natl. Acad. Sci. USA 2018, 115, 4182–4187. [Google Scholar] [CrossRef]
- Hu, M.-C.; Moe, O.W. Klotho as a Potential Biomarker and Therapy for Acute Kidney Injury. Nat. Rev. Nephrol. 2012, 8, 423–429. [Google Scholar] [CrossRef]
- Zhou, L.; Li, Y.; Zhou, D.; Tan, R.J.; Liu, Y. Loss of Klotho Contributes to Kidney Injury by Derepression of Wnt/β-Catenin Signaling. J. Am. Soc. Nephrol. 2013, 24, 771–785. [Google Scholar] [CrossRef]
- Ray, S.K.; Masarkar, N.; Mukherjee, S. Implications of Klotho Protein for Managing Kidney Disease—An Emerging Role in Therapeutics and Molecular Medicine. Curr. Mol. Med. 2021, 21, 484–494. [Google Scholar] [CrossRef]
- Gifford, C.C.; Lian, F.; Tang, J.; Costello, A.; Goldschmeding, R.; Samarakoon, R.; Higgins, P.J. PAI-1 Induction during Kidney Injury Promotes Fibrotic Epithelial Dysfunction via Deregulation of Klotho, P53, and TGF-β1-receptor Signaling. FASEB J. 2021, 35, e21725. [Google Scholar] [CrossRef]
- Baar, M.P.; Brandt, R.M.C.; Putavet, D.A.; Klein, J.D.D.; Derks, K.W.J.; Bourgeois, B.R.M.; Stryeck, S.; Rijksen, Y.; van Willigenburg, H.; Feijtel, D.A.; et al. Targeted Apoptosis of Senescent Cells Restores Tissue Homeostasis in Response to Chemotoxicity and Aging. Cell 2017, 169, 132–147.e16. [Google Scholar] [CrossRef]
- Zhang, L.; Pitcher, L.E.; Prahalad, V.; Niedernhofer, L.J.; Robbins, P.D. Targeting Cellular Senescence with Senotherapeutics: Senolytics and Senomorphics. FEBS J. 2023, 290, 1362–1383. [Google Scholar] [CrossRef]
- Adler, S.G.; Schwartz, S.; Williams, M.E.; Arauz-Pacheco, C.; Bolton, W.K.; Lee, T.; Li, D.; Neff, T.B.; Urquilla, P.R.; Sewell, K.L. Phase 1 Study of Anti-CTGF Monoclonal Antibody in Patients with Diabetes and Microalbuminuria. Clin. J. Am. Soc. Nephrol. 2010, 5, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.S.; Gravning, J.; Martinov, V.N.; von Lueder, T.G.; Edvardsen, T.; Czibik, G.; Moe, I.T.; Vinge, L.E.; Øie, E.; Valen, G.; et al. Mechanisms of Novel Cardioprotective Functions of CCN2/CTGF in Myocardial Ischemia-Reperfusion Injury. Am. J. Physiol.-Heart Circ. Physiol. 2011, 300, H1291–H1302. [Google Scholar] [CrossRef]
- Krizhanovsky, V.; Yon, M.; Dickins, R.A.; Hearn, S.; Simon, J.; Miething, C.; Yee, H.; Zender, L.; Lowe, S.W. Senescence of Activated Stellate Cells Limits Liver Fibrosis. Cell 2008, 134, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Demaria, M.; Ohtani, N.; Youssef, S.A.; Rodier, F.; Toussaint, W.; Mitchell, J.R.; Laberge, R.-M.; Vijg, J.; Van Steeg, H.; Dollé, M.E.T.; et al. An Essential Role for Senescent Cells in Optimal Wound Healing through Secretion of PDGF-AA. Dev. Cell 2014, 31, 722–733. [Google Scholar] [CrossRef]
- Fontes, M.S.C.; Kessler, E.L.; van Stuijvenberg, L.; Brans, M.A.; Falke, L.L.; Kok, B.; Leask, A.; van Rijen, H.V.M.; Vos, M.A.; Goldschmeding, R.; et al. CTGF Knockout Does Not Affect Cardiac Hypertrophy and Fibrosis Formation upon Chronic Pressure Overload. J. Mol. Cell Cardiol. 2015, 88, 82–90. [Google Scholar] [CrossRef]
- Liu, S.; Shi-Wen, X.; Abraham, D.J.; Leask, A. CCN2 Is Required for Bleomycin-Induced Skin Fibrosis in Mice. Arthritis Rheumatol. 2011, 63, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Metz-Kurschel, U.; Kurschel, E.; Wagner, K.; Aulbert, E.; Graben, N.; Philipp, T. Folate Nephropathy Occurring During Cytotoxic Chemotherapy with High-Dose Folinic Acid and 5-Fluorouracil. Ren. Fail. 1990, 12, 93–97. [Google Scholar] [CrossRef]
- Valencia, I.; Vallejo, S.; Dongil, P.; Romero, A.; San Hipólito-Luengo, Á.; Shamoon, L.; Posada, M.; García-Olmo, D.; Carraro, R.; Erusalimsky, J.D.; et al. DPP4 Promotes Human Endothelial Cell Senescence and Dysfunction via the PAR2-COX-2-TP Axis and NLRP3 Inflammasome Activation. Hypertension 2022, 79, 1361–1373. [Google Scholar] [CrossRef]
- Romero, A.; San Hipólito-Luengo, Á.; Villalobos, L.A.; Vallejo, S.; Valencia, I.; Michalska, P.; Pajuelo-Lozano, N.; Sánchez-Pérez, I.; León, R.; Bartha, J.L.; et al. The Angiotensin-(1-7)/Mas Receptor Axis Protects from Endothelial Cell Senescence via Klotho and Nrf2 Activation. Aging Cell 2019, 18, e12913. [Google Scholar] [CrossRef]
- Marquez-Exposito, L.; Tejedor-Santamaria, L.; Valentijn, F.A.; Tejera-Muñoz, A.; Rayego-Mateos, S.; Marchant, V.; Rodrigues-Diez, R.R.; Rubio-Soto, I.; Knoppert, S.N.; Ortiz, A.; et al. Oxidative Stress and Cellular Senescence Are Involved in the Aging Kidney. Antioxidants 2022, 11, 301. [Google Scholar] [CrossRef]
- Zoja, C.; Corna, D.; Camozzi, D.; Cattaneo, D.; Rottoli, D.; Batani, C.; Zanchi, C.; Abbate, M.; Remuzzi, G. How To Fully Protect the Kidney in a Severe Model of Progressive Nephropathy. J. Am. Soc. Nephrol. 2002, 13, 2898–2908. [Google Scholar] [CrossRef] [PubMed]
- Rayego-Mateos, S.; Rodrigues-Diez, R.; Morgado-Pascual, J.L.; Valentijn, F.; Valdivielso, J.M.; Goldschmeding, R.; Ruiz-Ortega, M. Role of Epidermal Growth Factor Receptor (EGFR) and Its Ligands in Kidney Inflammation and Damage. Mediat. Inflamm. 2018, 2018, 8739473. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.M. FEll-M Uir Lecture: Connective Tissue Growth Factor (CCN2)—A Pernicious and Pleiotropic Player in the Development of Kidney Fibrosis. Int. J. Exp. Pathol. 2013, 94, 1–16. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tejedor-Santamaria, L.; Marquez-Exposito, L.; Villacampa, A.; Marchant, V.; Battaglia-Vieni, A.; Rayego-Mateos, S.; Rodrigues-Diez, R.R.; Santos, F.M.; Valentijn, F.A.; Knoppert, S.N.; et al. CCN2 Activates Cellular Senescence Leading to Kidney Fibrosis in Folic Acid-Induced Experimental Nephropathy. Int. J. Mol. Sci. 2025, 26, 4401. https://doi.org/10.3390/ijms26094401
Tejedor-Santamaria L, Marquez-Exposito L, Villacampa A, Marchant V, Battaglia-Vieni A, Rayego-Mateos S, Rodrigues-Diez RR, Santos FM, Valentijn FA, Knoppert SN, et al. CCN2 Activates Cellular Senescence Leading to Kidney Fibrosis in Folic Acid-Induced Experimental Nephropathy. International Journal of Molecular Sciences. 2025; 26(9):4401. https://doi.org/10.3390/ijms26094401
Chicago/Turabian StyleTejedor-Santamaria, Lucia, Laura Marquez-Exposito, Alicia Villacampa, Vanessa Marchant, Antonio Battaglia-Vieni, Sandra Rayego-Mateos, Raul R. Rodrigues-Diez, Fatima Milhano Santos, Floris A. Valentijn, Sebastian N. Knoppert, and et al. 2025. "CCN2 Activates Cellular Senescence Leading to Kidney Fibrosis in Folic Acid-Induced Experimental Nephropathy" International Journal of Molecular Sciences 26, no. 9: 4401. https://doi.org/10.3390/ijms26094401
APA StyleTejedor-Santamaria, L., Marquez-Exposito, L., Villacampa, A., Marchant, V., Battaglia-Vieni, A., Rayego-Mateos, S., Rodrigues-Diez, R. R., Santos, F. M., Valentijn, F. A., Knoppert, S. N., Broekhuizen, R., Ruiz-Torres, M. P., Goldschmeding, R., Ortiz, A., Peiró, C., Nguyen, T. Q., Ramos, A. M., & Ruiz-Ortega, M. (2025). CCN2 Activates Cellular Senescence Leading to Kidney Fibrosis in Folic Acid-Induced Experimental Nephropathy. International Journal of Molecular Sciences, 26(9), 4401. https://doi.org/10.3390/ijms26094401








