Body Fluid-Derived Stem Cells: Powering Innovative, Less-Invasive Cell Therapies
Abstract
1. Introduction
2. Classifying Stem Cells
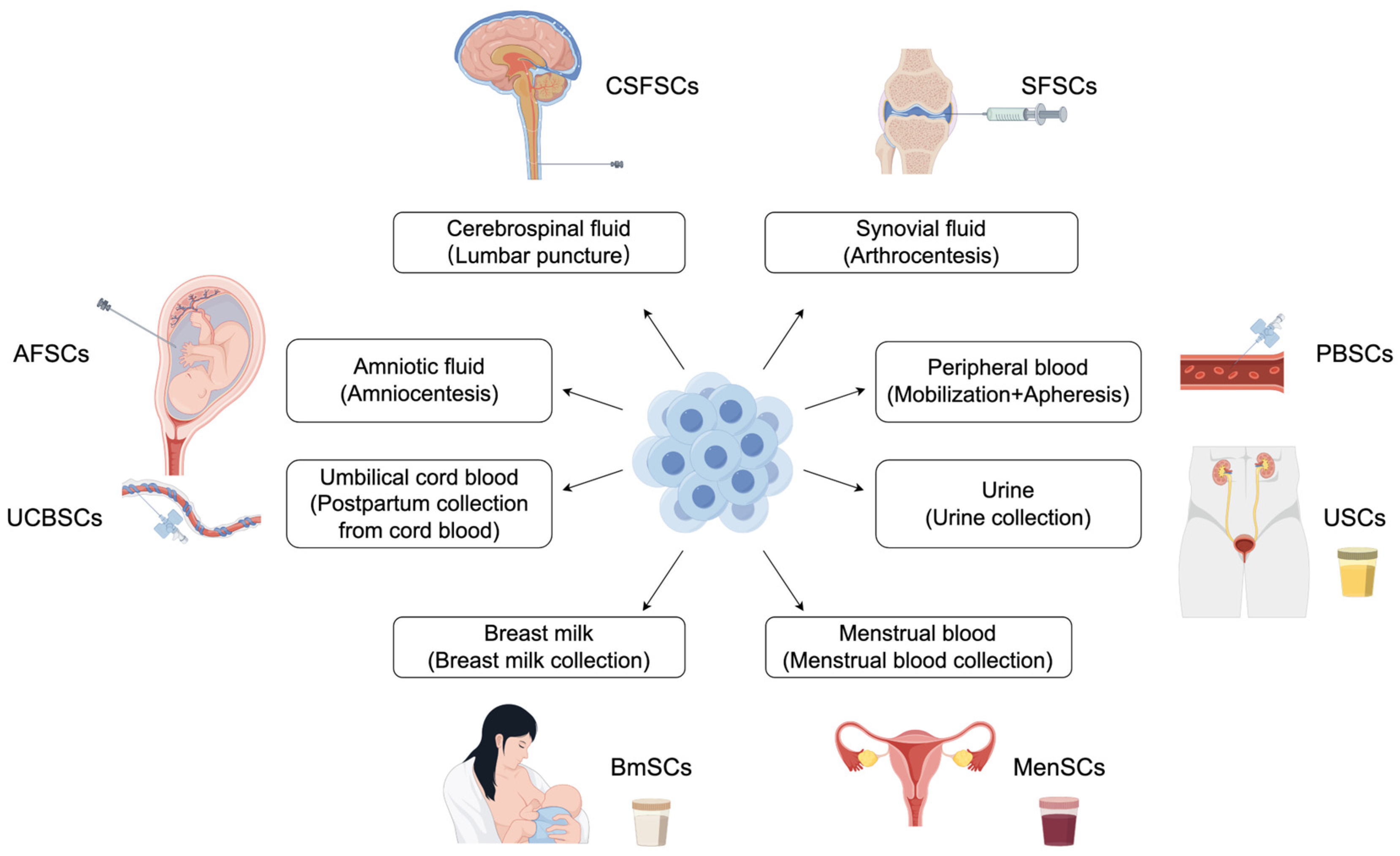
3. Body Fluid-Derived Stem Cells
3.1. Fetal or Neonatal Sources
3.1.1. Amniotic Fluid-Derived Stem Cells
3.1.2. Umbilical Cord Blood-Derived Stem Cells
3.2. Adult Sources
3.2.1. Peripheral Blood-Derived Stem Cells (PBSCs)
3.2.2. Menstrual Blood-Derived Stem Cells (MenSCs)
3.2.3. Urine-Derived Stem Cells (USCs)
3.2.4. Synovial Fluid-Derived Stem Cells (SFSCs)
3.2.5. Breast Milk-Derived Stem Cells (BmSCs)
3.2.6. Cerebrospinal Fluid-Derived Neural Stem Cells
4. Potential Applications of Body Fluid-Derived Stem Cells
4.1. Musculoskeletal System (Bone, Cartilage, Tendons, and Ligaments)
4.2. Cardiovascular System (Heart and Blood Vessels)
4.3. Hematopoietic System (Blood and Bone Marrow)
4.4. Nervous System (Brain and Spinal Cord)
4.5. Urinary System (Kidneys and Urinary Tract)
4.6. Reproductive System (Endometrium)
4.7. Liver
4.8. Skin and Wound Healing
4.9. Lungs
4.10. Intestinal System
4.11. Retina
5. Summary
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AFSCs | Amniotic fluid-derived stem cells |
| ASC | Adult stem cell |
| BMSCs | Breast milk-derived stem cells |
| BFSCs | Body fluid-derived stem cells |
| BM | Bone marrow |
| BM-MSCs | Bone marrow-derived mesenchymal stem cells |
| CSF | Cerebrospinal fluid |
| CSFSCs | Cerebrospinal fluid-derived stem cells |
| ESCs | Embryonic stem cells |
| FSCs | Fetal stem cells |
| HSCs | Hematopoietic stem cells |
| hBM | Human breast milk |
| hBmSCs | Human breast milk-derived stem cells |
| hESCs | Human embryonic stem cells |
| iPSCs | Induced pluripotent stem cells |
| MenSCs | Menstrual blood-derived stem cells |
| MSCs | Mesenchymal stem cells |
| NSCs | Neural stem cells |
| PB | Peripheral blood |
| PBSCs | Peripheral blood-derived stem cells |
| SFSCs | Synovial fluid-derived stem cells |
| UCBSCs | Umbilical cord blood-derived stem cells |
| USCs | Urine-derived stem cells |
| VEGF | Vascular Endothelial Growth Factor |
References
- Weissman, I.L. Stem cells: Units of development, units of regeneration, and units in evolution. Cell 2000, 100, 157–168. [Google Scholar] [CrossRef]
- Dai, R.; Wang, Z.; Samanipour, R.; Koo, K.I.; Kim, K. Adipose-Derived Stem Cells for Tissue Engineering and Regenerative Medicine Applications. Stem Cells Int. 2016, 2016, 6737345. [Google Scholar] [CrossRef]
- Foo, J.B.; Looi, Q.H.; Chong, P.P.; Hassan, N.H.; Yeo, G.E.C.; Ng, C.Y.; Koh, B.; How, C.W.; Lee, S.H.; Law, J.X. Comparing the Therapeutic Potential of Stem Cells and their Secretory Products in Regenerative Medicine. Stem Cells Int. 2021, 2021, 2616807. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Calabrese, C.; De Angelis, B.; Pizzicannella, J.; Kothari, A.; Garcovich, S. Impact of the Different Preparation Methods to Obtain Human Adipose-Derived Stromal Vascular Fraction Cells (AD-SVFs) and Human Adipose-Derived Mesenchymal Stem Cells (AD-MSCs): Enzymatic Digestion Versus Mechanical Centrifugation. Int. J. Mol. Sci. 2019, 20, 5471. [Google Scholar] [CrossRef]
- Walter, S.G.; Randau, T.M.; Hilgers, C.; Haddouti, E.M.; Masson, W.; Gravius, S.; Burger, C.; Wirtz, D.C.; Schildberg, F.A. Molecular and Functional Phenotypes of Human Bone Marrow-Derived Mesenchymal Stromal Cells Depend on Harvesting Techniques. Int. J. Mol. Sci. 2020, 21, 4382. [Google Scholar] [CrossRef]
- Alstrup, T.; Eijken, M.; Brunbjerg, M.E.; Hammer-Hansen, N.; Moller, B.K.; Damsgaard, T.E. Measured Levels of Human Adipose Tissue-Derived Stem Cells in Adipose Tissue Is Strongly Dependent on Harvesting Method and Stem Cell Isolation Technique. Plast. Reconstr. Surg. 2020, 145, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Amouzegar, A.; Dey, B.R.; Spitzer, T.R. Peripheral Blood or Bone Marrow Stem Cells? Practical Considerations in Hematopoietic Stem Cell Transplantation. Transfus. Med. Rev. 2019, 33, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.T.; Phuong, T.N.T.; Tien, N.L.B.; Tran, D.K.; Thanh, V.V.; Quang, T.L.; Truong, D.T.; Pham, V.H.; Ngoc, V.T.N.; Chu-Dinh, T.; et al. An Update on the Progress of Isolation, Culture, Storage, and Clinical Application of Human Bone Marrow Mesenchymal Stem/Stromal Cells. Int. J. Mol. Sci. 2020, 21, 708. [Google Scholar] [CrossRef]
- Mazini, L.; Rochette, L.; Amine, M.; Malka, G. Regenerative Capacity of Adipose Derived Stem Cells (ADSCs), Comparison with Mesenchymal Stem Cells (MSCs). Int. J. Mol. Sci. 2019, 20, 2523. [Google Scholar] [CrossRef]
- Huang, R.L.; Li, Q.; Ma, J.X.; Atala, A.; Zhang, Y. Body fluid-derived stem cells—An untapped stem cell source in genitourinary regeneration. Nat. Rev. Urol. 2023, 20, 739–761. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef]
- Mitalipov, S.; Wolf, D. Totipotency, pluripotency and nuclear reprogramming. Adv. Biochem. Eng. Biotechnol. 2009, 114, 185–199. [Google Scholar]
- Rajabzadeh, N.; Fathi, E.; Farahzadi, R. Stem cell-based regenerative medicine. Stem Cell Investig. 2019, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Guillot, P.V.; O’Donoghue, K.; Kurata, H.; Fisk, N.M. Fetal stem cells: Betwixt and between. Semin. Reprod. Med. 2006, 24, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Rosner, M.; Horer, S.; Feichtinger, M.; Hengstschläger, M. Multipotent fetal stem cells in reproductive biology research. Stem Cell Res. Ther. 2023, 14, 157. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Torre, P.; Flores, A.I. Current Status and Future Prospects of Perinatal Stem Cells. Genes 2020, 12, 6. [Google Scholar] [CrossRef]
- Yang, C.; Wu, M.; You, M.; Chen, Y.; Luo, M.; Chen, Q. The therapeutic applications of mesenchymal stromal cells from human perinatal tissues in autoimmune diseases. Stem Cell Res. Ther. 2021, 12, 103. [Google Scholar] [CrossRef]
- Hombach-Klonisch, S.; Panigrahi, S.; Rashedi, I.; Seifert, A.; Alberti, E.; Pocar, P.; Kurpisz, M.; Schulze-Osthoff, K.; Mackiewicz, A.; Los, M. Adult stem cells and their trans-differentiation potential—perspectives and therapeutic applications. J. Mol. Med. 2008, 86, 1301–1314. [Google Scholar] [CrossRef]
- Sun, X.; Joost, S.; Kasper, M. Plasticity of Epithelial Cells during Skin Wound Healing. Cold Spring Harb. Perspect. Biol. 2023, 15, a041232. [Google Scholar] [CrossRef]
- Arwert, E.N.; Hoste, E.; Watt, F.M. Epithelial stem cells, wound healing and cancer. Nat. Rev. Cancer 2012, 12, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gao, J.; Liang, Z.; Gao, C.; Niu, Q.; Wu, F.; Zhang, L. Mesenchymal stem cells and their microenvironment. Stem Cell Res. Ther. 2022, 13, 429. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, A.C.; Igarashi, K.J.; Nakauchi, H. Haematopoietic stem cell self-renewal in vivo and ex vivo. Nat. Rev. Genet. 2020, 21, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Muñoz, B.; Garcia-Delgado, A.B.; Arribas-Arribas, B.; Sanchez-Pernaute, R. Human Neural Stem Cells for Cell-Based Medicinal Products. Cells 2021, 10, 2377. [Google Scholar] [CrossRef]
- Mangialardi, G.; Madeddu, P. Bone Marrow-Derived Stem Cells: A Mixed Blessing in the Multifaceted World of Diabetic Complications. Curr. Diab. Rep. 2016, 16, 43. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, J.; Cang, Z.; Pei, J.; Zhang, X.; Song, B.; Fan, X.; Ma, X.; Li, Y. Hair follicle stem cells promote epidermal regeneration under expanded condition. Front. Physiol. 2024, 15, 1306011. [Google Scholar] [CrossRef]
- Romani, R.; Manni, G.; Donati, C.; Pirisinu, I.; Bernacchioni, C.; Gargaro, M.; Pirro, M.; Calvitti, M.; Bagaglia, F.; Sahebkar, A.; et al. S1P promotes migration, differentiation and immune regulatory activity in amniotic-fluid-derived stem cells. Eur. J. Pharmacol. 2018, 833, 173–182. [Google Scholar] [CrossRef]
- Furuoka, H.; Endo, K.; Sekiya, I. Mesenchymal stem cells in synovial fluid increase in number in response to synovitis and display more tissue-reparative phenotypes in osteoarthritis. Stem Cell Res. Ther. 2023, 14, 244. [Google Scholar] [CrossRef]
- Kun-Varga, A.; Guban, B.; Miklos, V.; Parvaneh, S.; Guba, M.; Szucs, D.; Monostori, T.; Varga, J.; Varga, A.; Razga, Z.; et al. Herpes Simplex Virus Infection Alters the Immunological Properties of Adipose-Tissue-Derived Mesenchymal-Stem Cells. Int. J. Mol. Sci. 2023, 24, 11989. [Google Scholar] [CrossRef]
- Galvez, P.; Clares, B.; Bermejo, M.; Hmadcha, A.; Soria, B. Standard requirement of a microbiological quality control program for the manufacture of human mesenchymal stem cells for clinical use. Stem Cells Dev. 2014, 23, 1074–1083. [Google Scholar] [CrossRef]
- Kim, E.Y.; Lee, K.B.; Kim, M.K. The potential of mesenchymal stem cells derived from amniotic membrane and amniotic fluid for neuronal regenerative therapy. BMB Rep. 2014, 47, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Pozzobon, M.; Piccoli, M.; Schiavo, A.A.; Atala, A.; De Coppi, P. Isolation of c-Kit+ human amniotic fluid stem cells from second trimester. Methods Mol. Biol. 2013, 1035, 191–198. [Google Scholar]
- Alessio, N.; Pipino, C.; Mandatori, D.; Di Tomo, P.; Ferone, A.; Marchiso, M.; Melone, M.A.B.; Peluso, G.; Pandolfi, A.; Galderisi, U. Mesenchymal stromal cells from amniotic fluid are less prone to senescence compared to those obtained from bone marrow: An in vitro study. J. Cell Physiol. 2018, 233, 8996–9006. [Google Scholar] [CrossRef] [PubMed]
- Roubelakis, M.G.; Bitsika, V.; Zagoura, D.; Trohatou, O.; Pappa, K.I.; Makridakis, M.; Antsaklis, A.; Vlahou, A.; Anagnou, N.P. In vitro and in vivo properties of distinct populations of amniotic fluid mesenchymal progenitor cells. J. Cell Mol. Med. 2011, 15, 1896–1913. [Google Scholar] [CrossRef] [PubMed]
- Lazzarini, R.; Sorgentoni, G.; Caffarini, M.; Sayeed, M.A.; Olivieri, F.; Di Primio, R.; Orciani, M. New miRNAs network in human mesenchymal stem cells derived from skin and amniotic fluid. Int. J. Immunopathol. Pharmacol. 2016, 29, 523–528. [Google Scholar] [CrossRef]
- Liu, N.; Cheng, Y.; Wang, D.; Guan, H.; Chen, D.; Zeng, J.; Lu, D.; Li, Y.; Yang, Y.; Luo, Q.; et al. Tissue-specific populations from amniotic fluid-derived mesenchymal stem cells manifest variant in vitro and in vivo properties. Hum. Cell 2024, 37, 408–419. [Google Scholar] [CrossRef]
- Spinelli, V.; Guillot, P.V.; De Coppi, P. Induced pluripotent stem (iPS) cells from human fetal stem cells (hFSCs). Organogenesis 2013, 9, 101–110. [Google Scholar] [CrossRef][Green Version]
- Spitzhorn, L.S.; Rahman, M.S.; Schwindt, L.; Ho, H.T.; Wruck, W.; Bohndorf, M.; Wehrmeyer, S.; Ncube, A.; Beyer, I.; Hagenbeck, C.; et al. Isolation and Molecular Characterization of Amniotic Fluid-Derived Mesenchymal Stem Cells Obtained from Caesarean Sections. Stem Cells Int. 2017, 2017, 5932706. [Google Scholar] [CrossRef]
- Cananzi, M.; Atala, A.; De Coppi, P. Stem cells derived from amniotic fluid: New potentials in regenerative medicine. Reprod. Biomed. Online 2009, 18 (Suppl. S1), 17–27. [Google Scholar] [CrossRef]
- Luo, H.; Wang, Z.; Qi, F.; Wang, D. Applications of human amniotic fluid stem cells in wound healing. Chin. Med. J. 2022, 135, 2272–2281. [Google Scholar] [CrossRef]
- Frändberg, S.; Boreström, C.; Li, S.; Fogelstrand, L.; Palmqvist, L. Exploring the heterogeneity of the hematopoietic stem and progenitor cell pool in cord blood: Simultaneous staining for side population, aldehyde dehydrogenase activity, and CD34 expression. Transfusion 2015, 55, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Baksh, D.; Yao, R.; Tuan, R.S. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells 2007, 25, 1384–1392. [Google Scholar] [CrossRef]
- Weiss, M.L.; Troyer, D.L. Stem cells in the umbilical cord. Stem Cell Rev. 2006, 2, 155–162. [Google Scholar] [CrossRef]
- Lund, T.C.; Boitano, A.E.; Delaney, C.S.; Shpall, E.J.; Wagner, J.E. Advances in umbilical cord blood manipulation-from niche to bedside. Nat. Rev. Clin. Oncol. 2015, 12, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Hinchly, T.; Bonnet, D.; Anjos-Afonso, F. Methodologic considerations on how to identify human hematopoietic stem cells. Exp. Hematol. 2025, 144, 104729. [Google Scholar] [CrossRef] [PubMed]
- Segunda, M.N.; Díaz, C.; Torres, C.G.; Parraguez, V.H.; De Los Reyes, M.; Peralta, O.A. Bovine Peripheral Blood-Derived Mesenchymal Stem Cells (PB-MSCs) and Spermatogonial Stem Cells (SSCs) Display Contrasting Expression Patterns of Pluripotency and Germ Cell Markers under the Effect of Sertoli Cell Conditioned Medium. Animals 2024, 14, 803. [Google Scholar] [CrossRef]
- Kuwana, M.; Okazaki, Y.; Kodama, H.; Izumi, K.; Yasuoka, H.; Ogawa, Y.; Kawakami, Y.; Ikeda, Y. Human circulating CD14+ monocytes as a source of progenitors that exhibit mesenchymal cell differentiation. J. Leukoc. Biol. 2003, 74, 833–845. [Google Scholar] [CrossRef]
- Li, S.; Huang, K.J.; Wu, J.C.; Hu, M.S.; Sanyal, M.; Hu, M.; Longaker, M.T.; Lorenz, H.P. Peripheral blood-derived mesenchymal stem cells: Candidate cells responsible for healing critical-sized calvarial bone defects. Stem Cells Transl. Med. 2015, 4, 359–368. [Google Scholar] [CrossRef]
- Anasetti, C.; Logan, B.R.; Lee, S.J.; Waller, E.K.; Weisdorf, D.J.; Wingard, J.R.; Cutler, C.S.; Westervelt, P.; Woolfrey, A.; Couban, S.; et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N. Engl. J. Med. 2012, 367, 1487–1496. [Google Scholar] [CrossRef]
- Liu, Y.; Niu, R.; Yang, F.; Yan, Y.; Liang, S.; Sun, Y.; Shen, P.; Lin, J. Biological characteristics of human menstrual blood-derived endometrial stem cells. J. Cell Mol. Med. 2018, 22, 1627–1639. [Google Scholar] [CrossRef]
- Rodrigues, M.C.; Lippert, T.; Nguyen, H.; Kaelber, S.; Sanberg, P.R.; Borlongan, C.V. Menstrual Blood-Derived Stem Cells: In Vitro and In Vivo Characterization of Functional Effects. Adv. Exp. Med. Biol. 2016, 951, 111–121. [Google Scholar] [PubMed]
- Sadiasa, A.; Werkmeister, J.A.; Gurung, S.; Gargett, C.E. Steps towards the clinical application of endometrial and menstrual fluid mesenchymal stem cells for the treatment of gynecological disorders. Expert. Opin. Biol. Ther. 2025, 25, 285–307. [Google Scholar] [CrossRef]
- Rahman, M.S.; Wruck, W.; Spitzhorn, L.S.; Nguyen, L.; Bohndorf, M.; Martins, S.; Asar, F.; Ncube, A.; Erichsen, L.; Graffmann, N.; et al. The FGF, TGFβ and WNT axis Modulate Self-renewal of Human SIX2(+) Urine Derived Renal Progenitor Cells. Sci. Rep. 2020, 10, 739. [Google Scholar] [CrossRef] [PubMed]
- Culenova, M.; Nicodemou, A.; Novakova, Z.V.; Debreova, M.; Smolinská, V.; Bernatova, S.; Ivanisova, D.; Novotna, O.; Vasicek, J.; Varga, I.; et al. Isolation, Culture and Comprehensive Characterization of Biological Properties of Human Urine-Derived Stem Cells. Int. J. Mol. Sci. 2021, 22, 12503. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Sun, Y.; Li, W.; Bi, Y. Hypoxia improves self-renew and migration of urine-derived stem cells by upregulating autophagy and mitochondrial function through ERK signal pathway. Mitochondrion 2023, 73, 1–9. [Google Scholar] [CrossRef]
- Burdeyron, P.; Giraud, S.; Hauet, T.; Steichen, C. Urine-derived stem/progenitor cells: A focus on their characterization and potential. World J. Stem Cells 2020, 12, 1080–1096. [Google Scholar] [CrossRef]
- Zhang, Y.; McNeill, E.; Tian, H.; Soker, S.; Andersson, K.E.; Yoo, J.J.; Atala, A. Urine derived cells are a potential source for urological tissue reconstruction. J. Urol. 2008, 180, 2226–2233. [Google Scholar] [CrossRef]
- Xiong, G.; Tang, W.; Zhang, D.; He, D.; Wei, G.; Atala, A.; Liang, X.J.; Bleyer, A.J.; Bleyer, M.E.; Yu, J.; et al. Impaired Regeneration Potential in Urinary Stem Cells Diagnosed from the Patients with Diabetic Nephropathy. Theranostics 2019, 9, 4221–4232. [Google Scholar] [CrossRef]
- Garcia, J.; Wright, K.; Roberts, S.; Kuiper, J.H.; Mangham, C.; Richardson, J.; Mennan, C. Characterisation of synovial fluid and infrapatellar fat pad derived mesenchymal stromal cells: The influence of tissue source and inflammatory stimulus. Sci. Rep. 2016, 6, 24295. [Google Scholar] [CrossRef]
- Xu, X.; Xu, L.; Xia, J.; Wen, C.; Liang, Y.; Zhang, Y. Harnessing knee joint resident mesenchymal stem cells in cartilage tissue engineering. Acta Biomater. 2023, 168, 372–387. [Google Scholar] [CrossRef]
- Amemiya, M.; Tsuji, K.; Katagiri, H.; Miyatake, K.; Nakagawa, Y.; Sekiya, I.; Muneta, T.; Koga, H. Synovial fluid-derived mesenchymal cells have non-inferior chondrogenic potential and can be utilized for regenerative therapy as substitute for synovium-derived cells. Biochem. Biophys. Res. Commun. 2020, 523, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Sani, M.; Hosseini, S.M.; Salmannejad, M.; Aleahmad, F.; Ebrahimi, S.; Jahanshahi, S.; Talaei-Khozani, T. Origins of the breast milk-derived cells; an endeavor to find the cell sources. Cell Biol. Int. 2015, 39, 611–618. [Google Scholar] [CrossRef]
- Hassiotou, F.; Beltran, A.; Chetwynd, E.; Stuebe, A.M.; Twigger, A.J.; Metzger, P.; Trengove, N.; Lai, C.T.; Filgueira, L.; Blancafort, P.; et al. Breastmilk is a novel source of stem cells with multilineage differentiation potential. Stem Cells 2012, 30, 2164–2174. [Google Scholar] [CrossRef]
- Kumari, P.; Raval, A.; Rana, P.; Mahto, S.K. Regenerative Potential of Human Breast Milk: A Natural Reservoir of Nutrients, Bioactive Components and Stem cells. Stem Cell Rev. Rep. 2023, 19, 1307–1327. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Chong, Y.S.; Choolani, M.A.; Cregan, M.D.; Chan, J.K. Unravelling the mystery of stem/progenitor cells in human breast milk. PLoS ONE 2010, 5, e14421. [Google Scholar] [CrossRef]
- Marotta, M.; Fernandez-Martin, A.; Oria, M.; Fontecha, C.G.; Gine, C.; Martinez-Ibanez, V.; Carreras, E.; Belfort, M.A.; Pelizzo, G.; Peiro, J.L. Isolation, characterization, and differentiation of multipotent neural progenitor cells from human cerebrospinal fluid in fetal cystic myelomeningocele. Stem Cell Res. 2017, 22, 33–42. [Google Scholar] [CrossRef]
- Tang, X.; Deng, P.; Li, L.; He, Y.; Wang, J.; Hao, D.; Yang, H. Advances in genetically modified neural stem cell therapy for central nervous system injury and neurological diseases. Stem Cell Res. Ther. 2024, 15, 482. [Google Scholar] [CrossRef]
- Kita, K.; Lee, J.O.; Finnerty, C.C.; Herndon, D.N. Cord blood-derived hematopoietic stem/progenitor cells: Current challenges in engraftment, infection, and ex vivo expansion. Stem Cells Int. 2011, 2011, 276193. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.K.; Kuo, T.K.; Chen, W.-M.; Lee, K.-D.; Hsieh, S.-L.; Chen, T.-H. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood 2004, 103, 1669–1675. [Google Scholar] [CrossRef]
- Thomaidou, A.C.; Goulielmaki, M.; Tsintarakis, A.; Zoumpourlis, P.; Toya, M.; Christodoulou, I.; Zoumpourlis, V. miRNA-Guided Regulation of Mesenchymal Stem Cells Derived from the Umbilical Cord: Paving the Way for Stem-Cell Based Regeneration and Therapy. Int. J. Mol. Sci. 2023, 24, 9189. [Google Scholar] [CrossRef]
- Xing, W.; Yang, J.; Zheng, Y.; Yao, L.; Peng, X.; Chen, Y.; Yang, C. The Role of the Notch Signaling Pathway in the Differentiation of Human Umbilical Cord-Derived Mesenchymal Stem Cells. Front. Biosci. 2024, 29, 74. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, B. Peripheral blood stem cells: Phenotypic diversity and potential clinical applications. Stem Cell Rev. Rep. 2012, 8, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Anjos-Afonso, F.; Bonnet, D. Human CD34+ hematopoietic stem cell hierarchy: How far are we with its delineation at the most primitive level? Blood 2023, 142, 509–518. [Google Scholar] [CrossRef]
- Morhayim, J.; Ghebes, C.A.; Erkeland, S.J.; Ter Borg, M.N.D.; Hoogenboezem, R.M.; Bindels, E.M.J.; van Alphen, F.P.J.; Kassem, M.; van Wijnen, A.J.; Cornelissen, J.J.; et al. Identification of osteolineage cell-derived extracellular vesicle cargo implicated in hematopoietic support. FASEB J. 2020, 34, 5435–5452. [Google Scholar] [CrossRef] [PubMed]
- Branco, A.; Rayabaram, J.; Miranda, C.C.; Fernandes-Platzgummer, A.; Fernandes, T.G.; Sajja, S.; da Silva, C.L.; Vemuri, M.C. Advances in ex vivo expansion of hematopoietic stem and progenitor cells for clinical applications. Front. Bioeng. Biotechnol. 2024, 12, 1380950. [Google Scholar] [CrossRef] [PubMed]
- Berebichez-Fridman, R.; Gomez-Garcia, R.; Granados-Montiel, J.; Berebichez-Fastlicht, E.; Olivos-Meza, A.; Granados, J.; Velasquillo, C.; Ibarra, C. The Holy Grail of Orthopedic Surgery: Mesenchymal Stem Cells-Their Current Uses and Potential Applications. Stem Cells Int. 2017, 2017, 2638305. [Google Scholar] [CrossRef]
- Cundell, T.; Atkins, J.W.; Lau, A.F. Sterility Testing for Hematopoietic Stem Cells. J. Clin. Microbiol. 2023, 61, e0165422. [Google Scholar] [CrossRef]
- Faramarzi, H.; Mehrabani, D.; Fard, M.; Akhavan, M.; Zare, S.; Bakhshalizadeh, S.; Manafi, A.; Kazemnejad, S.; Shirazi, R. The Potential of Menstrual Blood-Derived Stem Cells in Differentiation to Epidermal Lineage: A Preliminary Report. World J. Plast. Surg. 2016, 5, 26–31. [Google Scholar]
- Sanchez-Mata, A.; Gonzalez-Muñoz, E. Understanding menstrual blood-derived stromal/stem cells: Definition and properties Are we rushing into their therapeutic applications? iScience 2021, 24, 103501. [Google Scholar] [CrossRef]
- Santos, R.A.; Asensi, K.D.; de Barros, J.H.O.; de Menezes, R.C.S.; Cordeiro, I.R.; Neto, J.M.B.; Kasai-Brunswick, T.H.; Goldenberg, R. Intrinsic Angiogenic Potential and Migration Capacity of Human Mesenchymal Stromal Cells Derived from Menstrual Blood and Bone Marrow. Int. J. Mol. Sci. 2020, 21, 9563. [Google Scholar] [CrossRef]
- Li, T.; Li, R.H.W.; Ng, E.H.Y.; Yeung, W.S.B.; Chiu, P.C.N.; Chan, R.W.S. Interleukin 6 at menstruation promotes the proliferation and self-renewal of endometrial mesenchymal stromal/stem cells through the WNT/beta-catenin signaling pathway. Front. Immunol. 2024, 15, 1378863. [Google Scholar]
- Bozorgmehr, M.; Gurung, S.; Darzi, S.; Nikoo, S.; Kazemnejad, S.; Zarnani, A.H.; Gargett, C.E. Endometrial and Menstrual Blood Mesenchymal Stem/Stromal Cells: Biological Properties and Clinical Application. Front. Cell Dev. Biol. 2020, 8, 497. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.J.; Pi, J.K.; Hu, J.G.; Huang, Y.Z.; Gao, H.W.; Li, S.F.; Li-Ling, J.; Xie, H.Q. Identification and characterization of two morphologically distinct stem cell subpopulations from human urine samples. Sci. China Life Sci. 2020, 63, 712–723. [Google Scholar] [CrossRef]
- Qiao, Y.; Shen, L.; Zhang, Y.; Zhou, M.; Sun, Z. Boldine promotes stemness of human urine-derived stem cells by activating the Wnt/beta-catenin signaling pathway. Mol. Cell Biochem. 2024, 479, 243–254. [Google Scholar] [CrossRef]
- Yu, P.; Bosholm, C.C.; Zhu, H.; Duan, Z.; Atala, A.; Zhang, Y. Beyond waste: Understanding urine’s potential in precision medicine. Trends Biotechnol. 2024, 42, 953–969. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhao, H.; Yang, S.; Wang, G.; Zhu, L.; Sun, C.; An, Y. Urine-derived stem cells: Promising advancements and applications in regenerative medicine and beyond. Heliyon 2024, 10, e27306. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, S.H.; Jin, J.A.; So, H.J.; Lee, J.U.; Ji, M.J.; Kwon, E.J.; Han, P.S.; Lee, H.K.; Kang, T.W. In vivo safety and biodistribution profile of Klotho-enhanced human urine-derived stem cells for clinical application. Stem Cell Res. Ther. 2023, 14, 355. [Google Scholar] [CrossRef]
- Li, F.; Chen, J.; Gong, M.; Bi, Y.; Hu, C.; Zhang, Y.; Li, M. Isolation and Characterization of Human Synovial Fluid-Derived Mesenchymal Stromal Cells from Popliteal Cyst. Stem Cells Int. 2020, 2020, 7416493. [Google Scholar] [CrossRef]
- Sekiya, I.; Katano, H.; Ozeki, N. Characteristics of MSCs in Synovial Fluid and Mode of Action of Intra-Articular Injections of Synovial MSCs in Knee Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 2838. [Google Scholar] [CrossRef]
- Altaie, A.; Baboolal, T.G.; Wall, O.; Pandit, H.; Jones, E.; McGonagle, D. Device-Based Enrichment of Knee Joint Synovial Cells to Drive MSC Chondrogenesis Without Prior Culture Expansion In Vitro: A Step Closer to 1-Stage Orthopaedic Procedures. Am. J. Sports Med. 2022, 50, 152–161. [Google Scholar] [CrossRef]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. North. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [PubMed]
- Bode, L.; McGuire, M.; Rodriguez, J.M.; Geddes, D.T.; Hassiotou, F.; Hartmann, P.E.; McGuire, M.K. It’s alive: Microbes and cells in human milk and their potential benefits to mother and infant. Adv. Nutr. 2014, 5, 571–573. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C.; Stremmel, W.; Weiskirchen, R.; John, S.M.; Schmitz, G. Exosome-Derived MicroRNAs of Human Milk and Their Effects on Infant Health and Development. Biomolecules 2021, 11, 851. [Google Scholar] [CrossRef]
- Gialeli, G.; Panagopoulou, O.; Liosis, G.; Siahanidou, T. Potential Epigenetic Effects of Human Milk on Infants’ Neurodevelopment. Nutrients 2023, 15, 3614. [Google Scholar] [CrossRef] [PubMed]
- de Sonnaville, S.; van Strien, M.E.; Middeldorp, J.; Sluijs, J.A.; van den Berge, S.A.; Moeton, M.; Donega, V.; van Berkel, A.; Deering, T.; De Filippis, L.; et al. The adult human subventricular zone: Partial ependymal coverage and proliferative capacity of cerebrospinal fluid. Brain Commun. 2020, 2, fcaa150. [Google Scholar] [CrossRef]
- Wang, S.; He, Y.; Zhang, H.; Chen, L.; Cao, L.; Yang, L.; Wang, C.; Pan, Y.; Tang, Q.; Tan, W.; et al. The Neural Stem Cell Properties of PKD2L1(+) Cerebrospinal Fluid-Contacting Neurons in vitro. Front. Cell Neurosci. 2021, 15, 630882. [Google Scholar] [CrossRef]
- Ren, C.; Yin, P.; Ren, N.; Wang, Z.; Wang, J.; Zhang, C.; Ge, W.; Geng, D.; Wang, X. Cerebrospinal fluid-stem cell interactions may pave the path for cell-based therapy in neurological diseases. Stem Cell Res. Ther. 2018, 9, 66. [Google Scholar] [CrossRef]
- Singh, G.; van Laarhoven, A.; Adams, R.; Reid, T.D.; Combrinck, J.; van Dorp, S.; Riou, C.; Thango, N.; Enslin, J.; Kruger, S.; et al. The influence of fixation and cryopreservation of cerebrospinal fluid on antigen expression and cell percentages by flow cytometric analysis. Sci. Rep. 2024, 14, 2463. [Google Scholar] [CrossRef]
- Wang, B.; Iriguchi, S.; Waseda, M.; Ueda, N.; Ueda, T.; Xu, H.; Minagawa, A.; Ishikawa, A.; Yano, H.; Ishi, T.; et al. Generation of hypoimmunogenic T cells from genetically engineered allogeneic human induced pluripotent stem cells. Nat. Biomed. Eng. 2021, 5, 429–440. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Li, S.; Xue, H.; Schmitt, K.; Hergenroeder, G.W.; Wu, J.; Zhang, Y.; Kim, D.H.; Cao, Q. Human neural progenitors derived from integration-free iPSCs for SCI therapy. Stem Cell Res. 2017, 19, 55–64. [Google Scholar] [CrossRef]
- Guan, J.; Niu, X.; Gong, F.; Hu, B.; Guo, S.; Lou, Y.; Zhang, C.; Deng, Z.; Wang, Y. Biological characteristics of human Urine derived Stem Cells: Potential for cell-based therapy in neurology. Tissue Eng. Part A 2014, 20, 1794–1806. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Li, Q.; Shu, Y.; Wang, H.; Thomas, B.; Maxwell, J.T.; Zhang, Y. Exploiting urine-derived induced pluripotent stem cells for advancing precision medicine in cell therapy, disease modeling, and drug testing. J. Biomed. Sci. 2024, 31, 47. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.L.; Zhou, C.Y.; Yu, J.K. A new source of mesenchymal stem cells for articular cartilage repair: MSCs derived from mobilized peripheral blood share similar biological characteristics in vitro and chondrogenesis in vivo as MSCs from bone marrow in a rabbit model. Am. J. Sports Med. 2014, 42, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Vallmajo-Martin, Q.; Kivelio, A.S.; Metzger, S.; Milleret, V.; Lienemann, P.S.; Carrara, B.M.; Millan, C.; Ghayor, C.; Ochsenbein-Koelble, N.; Ehrbar, M. Undifferentiated Human Amniotic Fluid Progenitor Cells Promote Bone Regeneration in Vivo. Adv. Healthc. Mater. 2025, 14, e2300843. [Google Scholar] [CrossRef]
- Zuliani, C.C.; Damas, I.I.; Andrade, K.C.; Westin, C.B.; Moraes, A.M.; Coimbra, I.B. Chondrogenesis of human amniotic fluid stem cells in Chitosan-Xanthan scaffold for cartilage tissue engineering. Sci. Rep. 2021, 11, 3063. [Google Scholar] [CrossRef]
- Ren, H.; Sang, Y.; Zhang, F.; Liu, Z.; Qi, N.; Chen, Y. Comparative Analysis of Human Mesenchymal Stem Cells from Umbilical Cord, Dental Pulp, and Menstrual Blood as Sources for Cell Therapy. Stem Cells Int. 2016, 2016, 3516574. [Google Scholar] [CrossRef]
- Khanmohammadi, M.; Khanjani, S.; Bakhtyari, M.S.; Zarnani, A.H.; Edalatkhah, H.; Akhondi, M.M.; Mirzadegan, E.; Kamali, K.; Alimoghadam, K.; Kazemnejad, S. Proliferation and chondrogenic differentiation potential of menstrual blood- and bone marrow-derived stem cells in two-dimensional culture. Int. J. Hematol. 2012, 95, 484–493. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, J.; Huang, Y.; Wu, C.; Xie, H. Urine-derived stem cells: Applications in skin, bone and articular cartilage repair. Burn. Trauma. 2021, 9, tkab039. [Google Scholar] [CrossRef]
- Wu, C.; Chen, L.; Huang, Y.Z.; Huang, Y.; Parolini, O.; Zhong, Q.; Tian, X.; Deng, L. Comparison of the Proliferation and Differentiation Potential of Human Urine-, Placenta Decidua Basalis-, and Bone Marrow-Derived Stem Cells. Stem Cells Int. 2018, 2018, 7131532. [Google Scholar] [CrossRef]
- Qin, H.; Zhu, C.; An, Z.; Jiang, Y.; Zhao, Y.; Wang, J.; Liu, X.; Hui, B.; Zhang, X.; Wang, Y. Silver nanoparticles promote osteogenic differentiation of human urine-derived stem cells at noncytotoxic concentrations. Int. J. Nanomed. 2014, 9, 2469–2478. [Google Scholar] [CrossRef]
- Jia, Z.; Zhu, F.; Li, X.; Liang, Q.; Zhuo, Z.; Huang, J.; Duan, L.; Xiong, J.; Wang, D. Repair of osteochondral defects using injectable chitosan-based hydrogel encapsulated synovial fluid-derived mesenchymal stem cells in a rabbit model. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Petsche Connell, J.; Camci-Unal, G.; Khademhosseini, A.; Jacot, J.G. Amniotic fluid-derived stem cells for cardiovascular tissue engineering applications. Tissue Eng. Part B Rev. 2013, 19, 368–379. [Google Scholar] [CrossRef]
- Bollini, S.; Cheung, K.K.; Riegler, J.; Dong, X.; Smart, N.; Ghionzoli, M.; Loukogeorgakis, S.P.; Maghsoudlou, P.; Dube, K.N.; Riley, P.R.; et al. Amniotic fluid stem cells are cardioprotective following acute myocardial infarction. Stem Cells Dev. 2011, 20, 1985–1994. [Google Scholar] [CrossRef]
- Golpanian, S.; Wolf, A.; Hatzistergos, K.E.; Hare, J.M. Rebuilding the Damaged Heart: Mesenchymal Stem Cells, Cell-Based Therapy, and Engineered Heart Tissue. Physiol. Rev. 2016, 96, 1127–1168. [Google Scholar] [CrossRef]
- Greco, N.; Laughlin, M.J. Umbilical cord blood stem cells for myocardial repair and regeneration. Methods Mol. Biol. 2010, 660, 29–52. [Google Scholar] [PubMed]
- Liu, Y.; Niu, R.; Li, W.; Lin, J.; Stamm, C.; Steinhoff, G.; Ma, N. Therapeutic potential of menstrual blood-derived endometrial stem cells in cardiac diseases. Cell Mol. Life Sci. 2019, 76, 1681–1695. [Google Scholar] [CrossRef]
- Orlando, N.; Pellegrino, C.; Valentini, C.G.; Bianchi, M.; Barbagallo, O.; Sparnacci, S.; Forni, F.; Fontana, T.M.; Teofili, L. Umbilical cord blood: Current uses for transfusion and regenerative medicine. Transfus. Apher. Sci. 2020, 59, 102952. [Google Scholar] [CrossRef] [PubMed]
- Sakuragawa, N.; Thangavel, R.; Mizuguchi, M.; Hirasawa, M.; Kamo, I. Expression of markers for both neuronal and glial cells in human amniotic epithelial cells. Neurosci. Lett. 1996, 209, 9–12. [Google Scholar] [CrossRef]
- Liang, C.C.; Shaw, S.W.; Huang, Y.H.; Lee, T.H. Human amniotic fluid stem cells can improve cerebral vascular remodelling and neurological function after focal cerebral ischaemia in diabetic rats. J. Cell Mol. Med. 2021, 25, 10185–10196. [Google Scholar] [CrossRef]
- Corcelli, M.; Hawkins, K.; Vlahova, F.; Hunjan, A.; Dowding, K.; De Coppi, P.; David, A.L.; Peebles, D.; Gressens, P.; Hagberg, H.; et al. Neuroprotection of the hypoxic-ischemic mouse brain by human CD117(+)CD90(+)CD105(+) amniotic fluid stem cells. Sci. Rep. 2018, 8, 2425. [Google Scholar] [CrossRef]
- Zarrabi, M.; Akbari, M.G.; Amanat, M.; Majmaa, A.; Moaiedi, A.R.; Montazerlotfelahi, H.; Nouri, M.; Hamidieh, A.A.; Badv, R.S.; Karimi, H.; et al. The safety and efficacy of umbilical cord blood mononuclear cells in individuals with spastic cerebral palsy: A randomized double-blind sham-controlled clinical trial. BMC Neurol. 2022, 22, 123. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Zhang, G.; Xia, Y.; Zhu, Q.; Zhang, J.; Li, Q.; Niu, X.; Hu, G.; Yang, Y.; Wang, Y.; et al. Exosomes from human urine-derived stem cells enhanced neurogenesis via miR-26a/HDAC6 axis after ischaemic stroke. J. Cell. Mol. Med. 2020, 24, 640–654. [Google Scholar] [CrossRef] [PubMed]
- Monteiro Carvalho Mori da Cunha, M.G.; Zia, S.; Oliveira Arcolino, F.; Carlon, M.S.; Beckmann, D.V.; Pippi, N.L.; Luhers Graca, D.; Levtchenko, E.; Deprest, J.; Toelen, J. Amniotic Fluid Derived Stem Cells with a Renal Progenitor Phenotype Inhibit Interstitial Fibrosis in Renal Ischemia and Reperfusion Injury in Rats. PLoS ONE 2015, 10, e0136145. [Google Scholar]
- Minocha, E.; Chaturvedi, C.P.; Nityanand, S. Renogenic characterization and in vitro differentiation of rat amniotic fluid stem cells into renal proximal tubular- and juxtaglomerular-like cells. In Vitr. Cell. Dev. Biol. Anim. 2019, 55, 138–147. [Google Scholar] [CrossRef]
- Kang, H.H.; Kang, J.J.; Kang, H.G.; Chung, S.S. Urothelial differentiation of human amniotic fluid stem cells by urothelium specific conditioned medium. Cell Biol. Int. 2014, 38, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, Y.; Bharadwaj, S.; Atala, A.; Zhang, Y. Human urine-derived stem cells seeded in a modified 3D porous small intestinal submucosa scaffold for urethral tissue engineering. Biomaterials 2011, 32, 1317–1326. [Google Scholar] [CrossRef]
- Gao, W.W.; Chun, S.Y.; Kim, B.S.; Ha, Y.S.; Lee, J.N.; Lee, E.H.; Kim, I.Y.; You, S.; Kwon, T.G. Locally transplanted human urine-induced nephron progenitor cells contribute to renal repair in mice kidney with diabetic nephropathy. Biochem. Biophys. Res. Commun. 2022, 629, 128–134. [Google Scholar] [CrossRef]
- Liu, T.; He, B.; Xu, X. Repairing and Regenerating Injured Endometrium Methods. Reprod. Sci. 2023, 30, 1724–1736. [Google Scholar] [CrossRef]
- Chen, K.; Wang, H.; Zhao, X.; Wang, J.; Jin, Q.; Tong, X.; Zheng, S. A Novel Method to Repair Thin Endometrium and Restore Fertility Based on Menstruation-Derived Stem Cell. Reprod. Sci. 2024, 31, 1662–1673. [Google Scholar] [CrossRef]
- Tan, J.; Li, P.; Wang, Q.; Li, Y.; Li, X.; Zhao, D.; Xu, X.; Kong, L. Autologous menstrual blood-derived stromal cells transplantation for severe Asherman’s syndrome. Hum. Reprod. 2016, 31, 2723–2729. [Google Scholar] [CrossRef]
- Zhang, S.; Chang, Q.; Li, P.; Tong, X.; Feng, Y.; Hao, X.; Zhang, X.; Yuan, Z.; Tan, J. Concentrated small extracellular vesicles from menstrual blood-derived stromal cells improve intrauterine adhesion, a pre-clinical study in a rat model. Nanoscale 2021, 13, 7334–7347. [Google Scholar] [CrossRef]
- Chen, H.; Tang, S.; Liao, J.; Liu, M.; Lin, Y. Therapeutic effect of human umbilical cord blood mesenchymal stem cells combined with G-CSF on rats with acute liver failure. Biochem. Biophys. Res. Commun. 2019, 517, 670–676. [Google Scholar] [CrossRef]
- Chen, H.; Tang, S.; Liao, J.; Liu, M.; Lin, Y. VEGF(165) gene-modified human umbilical cord blood mesenchymal stem cells protect against acute liver failure in rats. J. Gene Med. 2021, 23, e3369. [Google Scholar] [CrossRef]
- Chen, D.; Zeng, R.; Teng, G.; Cai, C.; Pan, T.; Tu, H.; Lin, H.; Du, Q.; Wang, H.; Chen, Y. Menstrual blood-derived mesenchymal stem cells attenuate inflammation and improve the mortality of acute liver failure combining with A2AR agonist in mice. J. Gastroenterol. Hepatol. 2021, 36, 2619–2627. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, C.; Chen, L.; Wang, X.; Xiang, B.; Wu, X.; Guo, Y.; Mou, X.; Yuan, L.; Chen, B.; et al. Human Menstrual Blood-Derived Stem Cells Ameliorate Liver Fibrosis in Mice by Targeting Hepatic Stellate Cells via Paracrine Mediators. Stem Cells Transl. Med. 2017, 6, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhao, L.; Liu, D.; Hu, C.; Wang, Y.; He, T.; Bi, Y.; He, Y. Characterization of Urine-Derived Stem Cells from Patients with End-Stage Liver Diseases and Application to Induced Acute and Chronic Liver Injury of Nude Mice Model. Stem Cells Dev. 2021, 30, 1126–1138. [Google Scholar] [CrossRef]
- Fukutake, M.; Ochiai, D.; Masuda, H.; Abe, Y.; Sato, Y.; Otani, T.; Sakai, S.; Aramaki-Hattori, N.; Shimoda, M.; Matsumoto, T.; et al. Human amniotic fluid stem cells have a unique potential to accelerate cutaneous wound healing with reduced fibrotic scarring like a fetus. Hum. Cell 2019, 32, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Park, K.; Jang, J.; Son, D.; Park, J.; Kim, J.; Yoo, J.E.; You, S.; Kim, I.Y. Utilizing stem cell-secreted molecules as a versatile toolbox for skin regenerative medicine. J. Control Release 2024, 370, 583–599. [Google Scholar] [CrossRef]
- Al-Zahrani, M.; Bauthman, N.M.; Alzahrani, Y.A.; Almohaimeed, H.M.; Alsolami, K.; Al-Sarraj, F.; Hakeem, G.H.; Alahmari, M.A.; Azher, Z.A.; Makhlof, R.T.M. Transplantation of hyaluronic acid and menstrual blood-derived stem cells accelerated wound healing in a diabetic rat model. Tissue Cell 2024, 89, 102442. [Google Scholar] [CrossRef]
- Dalirfardouei, R.; Jamialahmadi, K.; Jafarian, A.H.; Mahdipour, E. Promising effects of exosomes isolated from menstrual blood-derived mesenchymal stem cell on wound-healing process in diabetic mouse model. J. Tissue Eng. Regen. Med. 2019, 13, 555–568. [Google Scholar] [CrossRef]
- Edström, D.; Niroomand, A.; Stenlo, M.; Broberg, E.; Hirdman, G.; Ghaidan, H.; Hyllén, S.; Pierre, L.; Olm, F.; Lindstedt, S. Amniotic fluid-derived mesenchymal stem cells reduce inflammation and improve lung function following transplantation in a porcine model. J. Heart Lung Transplant. 2024, 43, 2018–2030. [Google Scholar] [CrossRef]
- Solaiman, A.; Mehanna, R.A.; Meheissen, G.A.; Elatrebi, S.; Said, R.; Elsokkary, N.H. Potential effect of amniotic fluid-derived stem cells on hyperoxia-induced pulmonary alveolar injury. Stem Cell Res. Ther. 2022, 13, 145. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Jiang, W.; Chen, L.; Xu, Z.; Zhang, Q.; Zhu, M.; Ye, P.; Li, H.; Yu, L.; Zhou, X.; et al. Evaluation of the safety and efficacy of using human menstrual blood-derived mesenchymal stromal cells in treating severe and critically ill COVID-19 patients: An exploratory clinical trial. Clin. Transl. Med. 2021, 11, e297. [Google Scholar] [CrossRef]
- Lian, J.; Zhu, X.; Du, J.; Huang, B.; Zhao, F.; Ma, C.; Guo, R.; Zhang, Y.; Ji, L.; Yahaya, B.H.; et al. Extracellular vesicle-transmitted miR-671-5p alleviates lung inflammation and injury by regulating the AAK1/NF-κB axis. Mol. Ther. 2023, 31, 1365–1382. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.M.; Zhang, Y.; Yang, M.F.; Xu, H.M.; Zhu, M.Z.; Yao, J.; Wang, L.S.; Liang, Y.J.; Li, D.F. Stem Cell Therapy in Inflammatory Bowel Disease: A Review of Achievements and Challenges. J. Inflamm. Res. 2023, 16, 2089–2119. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Ma, A.; Sun, C.; Qin, H.; Zhu, Y.; Li, G.; Wang, H.; Wang, H. Melatonin pretreatment improves endometrial regenerative cell-mediated therapeutic effects in experimental colitis. Int. Immunopharmacol. 2024, 133, 112092. [Google Scholar] [CrossRef]
- Xu, H.; Fu, J.; Chen, L.; Zhou, S.; Fang, Y.; Zhang, Q.; Chen, X.; Yuan, L.; Li, Y.; Xu, Z.; et al. TNF-α Enhances the Therapeutic Effects of MenSC-Derived Small Extracellular Vesicles on Inflammatory Bowel Disease through Macrophage Polarization by miR-24-3p. Stem Cells Int. 2023, 2023, 2988907. [Google Scholar] [CrossRef]
- Li, B.; Lee, C.; O’Connell, J.S.; Antounians, L.; Ganji, N.; Alganabi, M.; Cadete, M.; Nascimben, F.; Koike, Y.; Hock, A.; et al. Activation of Wnt signaling by amniotic fluid stem cell-derived extracellular vesicles attenuates intestinal injury in experimental necrotizing enterocolitis. Cell Death Dis. 2020, 11, 750. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, X.R.; Liu, H.S.; Liu, X.H.; Liu, G.H.; Zheng, X.B.; Hu, T.; Liang, Z.X.; He, X.W.; Wu, X.J.; et al. Immunomodulatory Effect of Urine-derived Stem Cells on Inflammatory Bowel Diseases via Downregulating Th1/Th17 Immune Responses in a PGE2-dependent Manner. J. Crohns Colitis 2020, 14, 654–668. [Google Scholar] [CrossRef]
- Reider, S.; Binder, L.; Fürst, S.; Hatzl, S.; Blesl, A. Hematopoietic Stem Cell Transplantation in Refractory Crohn’s Disease: Should It Be Considered? Cells 2022, 11, 3463. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, J.; Guo, M.; Sung, T.C.; Wang, T.; Yu, T.; Tian, Z.; Fan, G.; Wu, W.; Higuchi, A. Comparison of retinal degeneration treatment with four types of different mesenchymal stem cells, human induced pluripotent stem cells and RPE cells in a rat retinal degeneration model. J. Transl. Med. 2023, 21, 910. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.L.; Li, N.; Zhang, M.Y.; Liu, J.; Wang, T.H.; Liu, H. Urine-derived stem cells ameliorates the aging of retinal pigment epithelial cells. Tissue Cell 2022, 79, 101926. [Google Scholar] [CrossRef]
- Dan, Q.Q.; Chen, L.; Shi, L.L.; Zhou, X.; Wang, T.H.; Liu, H. Urine-derived mesenchymal stem cells-derived exosomes enhances survival and proliferation of aging retinal ganglion cells. BMC Mol. Cell Biol. 2023, 24, 8. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Liu, Q.; Yin, H.; Wang, K.; Diao, N.; Zhang, Y.; Lin, J.; Guo, A. Prevalence Trends of Site-Specific Osteoarthritis From 1990 to 2019: Findings From the Global Burden of Disease Study 2019. Arthritis Rheumatol. 2022, 74, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- van der Linde, D.; Konings, E.E.; Slager, M.A.; Witsenburg, M.; Helbing, W.A.; Takkenberg, J.J.; Roos-Hesselink, J.W. Birth prevalence of congenital heart disease worldwide: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2011, 58, 2241–2247. [Google Scholar] [CrossRef]
- Flythe, J.E.; Watnick, S. Dialysis for Chronic Kidney Failure: A Review. JAMA 2024, 332, 1559–1573. [Google Scholar] [CrossRef]
- GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 245–266. [Google Scholar] [CrossRef]
- Tao, Y.C.; Chen, E.Q. Clinical application of stem cell in patients with end-stage liver disease: Progress and challenges. Ann. Transl. Med. 2020, 8, 564. [Google Scholar] [CrossRef]
- Chae, J.B.; Jang, H.; Son, C.; Park, C.W.; Choi, H.; Jin, S.; Lee, H.Y.; Lee, H.; Ryu, J.H.; Kim, N.; et al. Targeting senescent retinal pigment epithelial cells facilitates retinal regeneration in mouse models of age-related macular degeneration. Geroscience 2021, 43, 2809–2833. [Google Scholar] [CrossRef]
| Classification Basis | Description | Examples |
|---|---|---|
| Potency | Ability of a stem cell to give rise to various cell types |
|
| Development Stage | When the stem cells arise during an organism’s development | |
| Cell Types | Focuses on the specific lineages or tissues that the stem cells can differentiate into |
|
| Origin | Source of the stem cells within the body |
|
| Source | Key Markers | Advantages | Limitations |
|---|---|---|---|
| Fetal or Neonatal Stem Cells | |||
| Amniotic Fluid | CD73, CD90, CD105, SSEA4, c-Kit, TRA-1-60, TRA-1-81, PSG5, EMX-2, and EVR-3 [38] | Differentiation potential, immunomodulatory properties [39]; lower rate of senescence [33]; minimal ethical concern [40] | Heterogenous population [31]; requires specific gestational timing; risk associated with amniocentesis |
| Umbilical Cord Blood | CD34, CD45, and CD117 (c-Kit) [41] | High and preserved differentiation capacity [42]; low immunogenicity [43] | Limited cell quantity per sample; mostly hematopoietic cells (a few MSCs) [44] |
| Adult Stem Cells | |||
| Peripheral Blood | CD34 and CD90 [45] | Ease of collection [46,47,48]; faster engraftment after transplantation [49] | Low HSC numbers; higher incidence of GVHDs [49]; requires mobilization agent or growth factor injection [46,47,48] |
| Menstrual Blood | -ASC markers: CD29, CD44, CD73, CD90, and CD105 [50]; -ESC markers: OCT-4, SOX2, and SSEA-4 [50] | High proliferative potential; expression of adult and embryonic markers [51]; non-invasive alternative [52] | Affected by donor age, hormonal status, and contraceptive use; optimal isolation and sterilization techniques are yet to be established [52] |
| Urine | -MSC markers: CD73, CD90, and CD105 [53]; -RPC markers: SIX2, CITED1, WT1, CD24, and CD106 [54] | MSC-like properties [55]; high telomerase activity and karyotype levels after in vitro expansion; immunomodulating properties [56]; low tumorgenicity [57]; non-invasive [54] | Vary Isolation efficiency between individuals; regenerative ability reduced in USCs from aged donors or diabetic nephropathy [58]; potential for contamination, particularly in collecting urine samples from females [56] |
| Synovial Fluid | MSC-like markers: CD73, CD90, CD105, and CD44 [59] | Chondrogenic differentiation potential; immunomodulatory properties; anti-inflammatory effects [60] | Lower proliferation potential; limited fluid volume [61]; quality and quantity affected by age and joint disease |
| Breast Milk | -MSC markers: CD90, CD44, CD271, and CD146 [62]; -ESC markers in subpopulation: TRA 60-1, Oct4, Nanog, and Sox2 [62] | ESC gene expression [63]; multilineage differentiation [64]; non-invasive collection [64] | Heterogenicity of cell population [65]; limited cell number and suboptimal culture conditions [65]; cell yield may vary between individuals and lactation stages |
| Cerebro- spinal Fluid | Neural progenitor markers: TBR2, CD15, and SOX2 [66] | Treats neurodegenerative diseases and neuronal ischemic injury [67] | Challenging to harvest and low cell yield [66]; differentiation potential restricted primarily to neural lineages; potential for tumor formation (gliomas) |
| Tissue/Organ System | BFSCs with Applications | References |
|---|---|---|
| Musculoskeletal | AFSCs, PBSCs (MSCs), MenSCs, USCs, SFSCs | [61,76,103,104,105,106,107,108,109,110,111] |
| Cardiovascular | AFSCs, PBSCs (HSCs/MSCs), UCBSCs, MenSCs | [112,113,114,115,116] |
| Hematopoietic | PBSCs (HSCs), UCBSCs | [43,49,117] |
| Nervous | AFSCs, UCBSCs, USCs, CSFSCs | [67,118,119,120,121,122] |
| Urinary | AFSCs, USCs | [123,124,125,126,127] |
| Reproductive (Endometrium) | MenSCs | [128,129,130,131] |
| Liver | MenSCs, UBCSCs, USCs | [132,133,134,135,136] |
| Skin/Wound Healing | AFSCs, MenSCs | [40,137,138,139,140] |
| Lungs | AFSCs, MenSCs | [141,142,143,144] |
| Intestinal | MenSCs, AFSCs, USCs | [145,146,147,148,149,150] |
| Retina | AFSCs, USCs (conditioned medium) | [151,152,153] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goff, A.D.; Zhang, X.; Thomas, B.; Ong, S.S.Y.; Atala, A.; Zhang, Y. Body Fluid-Derived Stem Cells: Powering Innovative, Less-Invasive Cell Therapies. Int. J. Mol. Sci. 2025, 26, 4382. https://doi.org/10.3390/ijms26094382
Goff AD, Zhang X, Thomas B, Ong SSY, Atala A, Zhang Y. Body Fluid-Derived Stem Cells: Powering Innovative, Less-Invasive Cell Therapies. International Journal of Molecular Sciences. 2025; 26(9):4382. https://doi.org/10.3390/ijms26094382
Chicago/Turabian StyleGoff, Adam David, Xinyue Zhang, Biju Thomas, Sally Shin Yee Ong, Anthony Atala, and Yuanyuan Zhang. 2025. "Body Fluid-Derived Stem Cells: Powering Innovative, Less-Invasive Cell Therapies" International Journal of Molecular Sciences 26, no. 9: 4382. https://doi.org/10.3390/ijms26094382
APA StyleGoff, A. D., Zhang, X., Thomas, B., Ong, S. S. Y., Atala, A., & Zhang, Y. (2025). Body Fluid-Derived Stem Cells: Powering Innovative, Less-Invasive Cell Therapies. International Journal of Molecular Sciences, 26(9), 4382. https://doi.org/10.3390/ijms26094382









