Current State and Advances in Antimicrobial Strategies for Burn Wound Dressings: From Metal-Based Antimicrobials and Natural Bioactive Agents to Future Perspectives
Abstract
1. Introduction
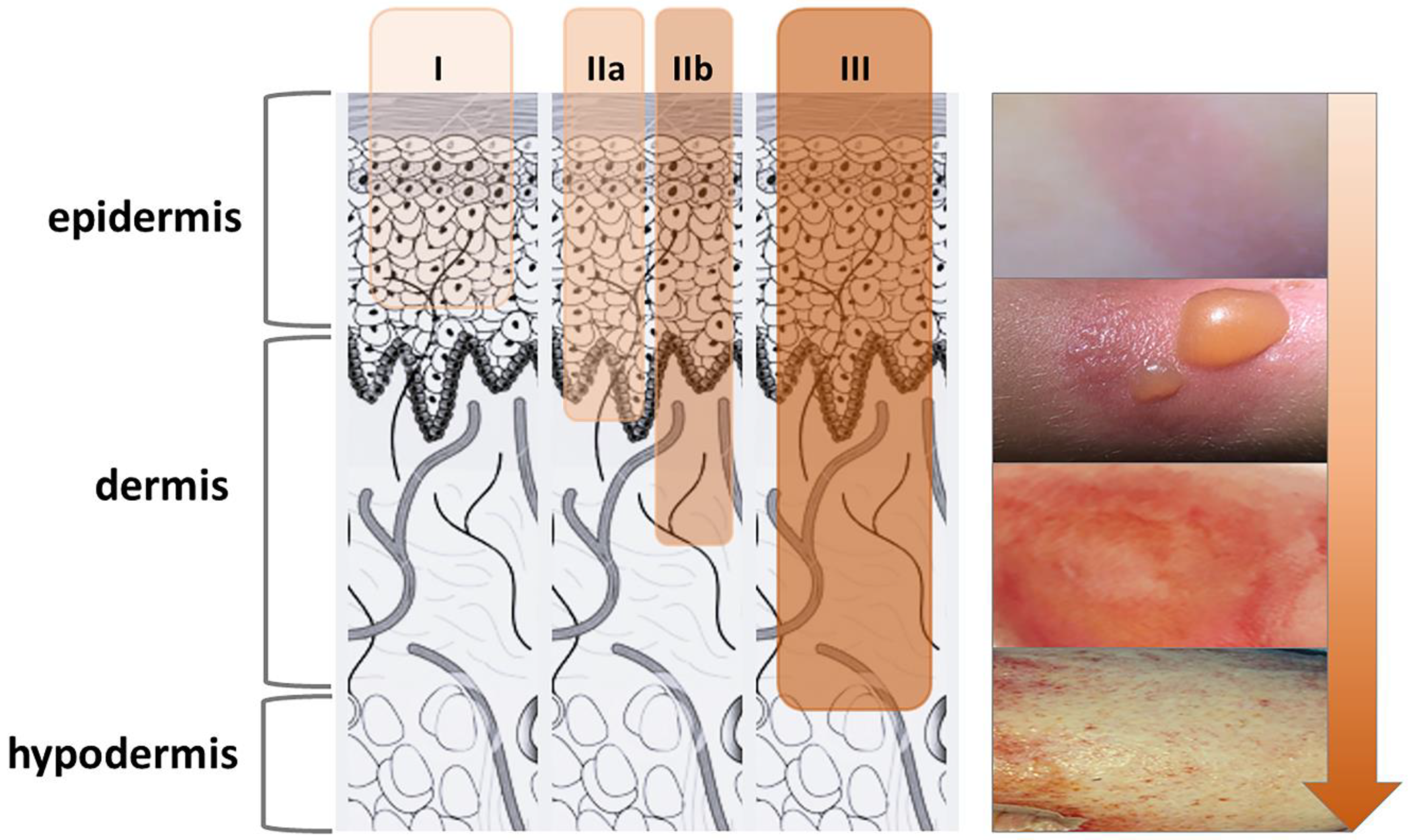
2. Burns and Infection
3. Antimicrobial Wound Dressings: Traditional vs. Advanced
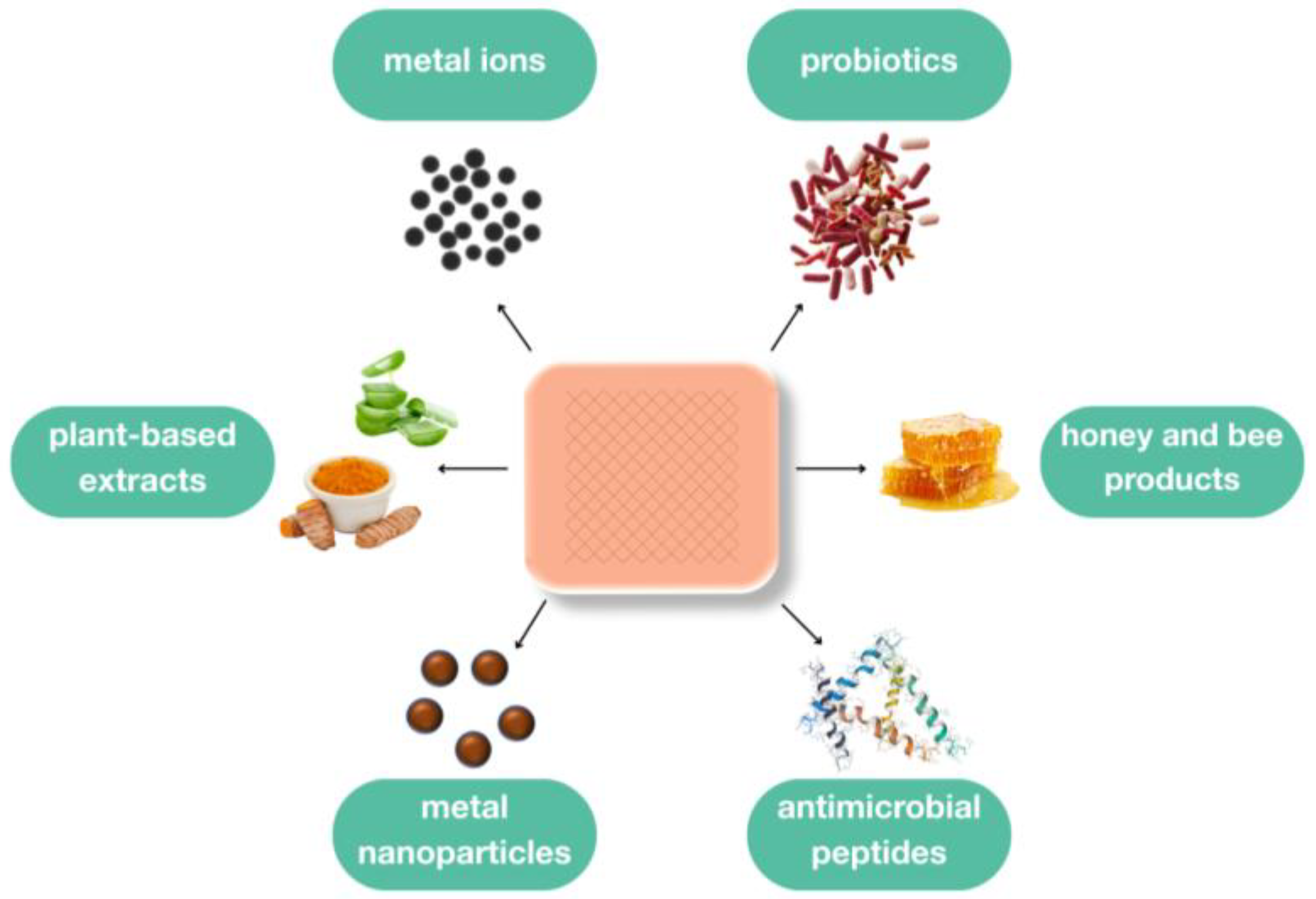
4. Antimicrobial Agents in Wound Dressings
4.1. Metals and Metal-Based Antimicrobials
4.1.1. Silver
4.1.2. Zinc
4.1.3. Copper
4.1.4. Metal Nanoparticles
4.2. Bee Products
4.2.1. Honey
4.2.2. Propolis
4.3. Plant-Based Compounds
4.4. Antimicrobial Peptides
4.5. Probiotics
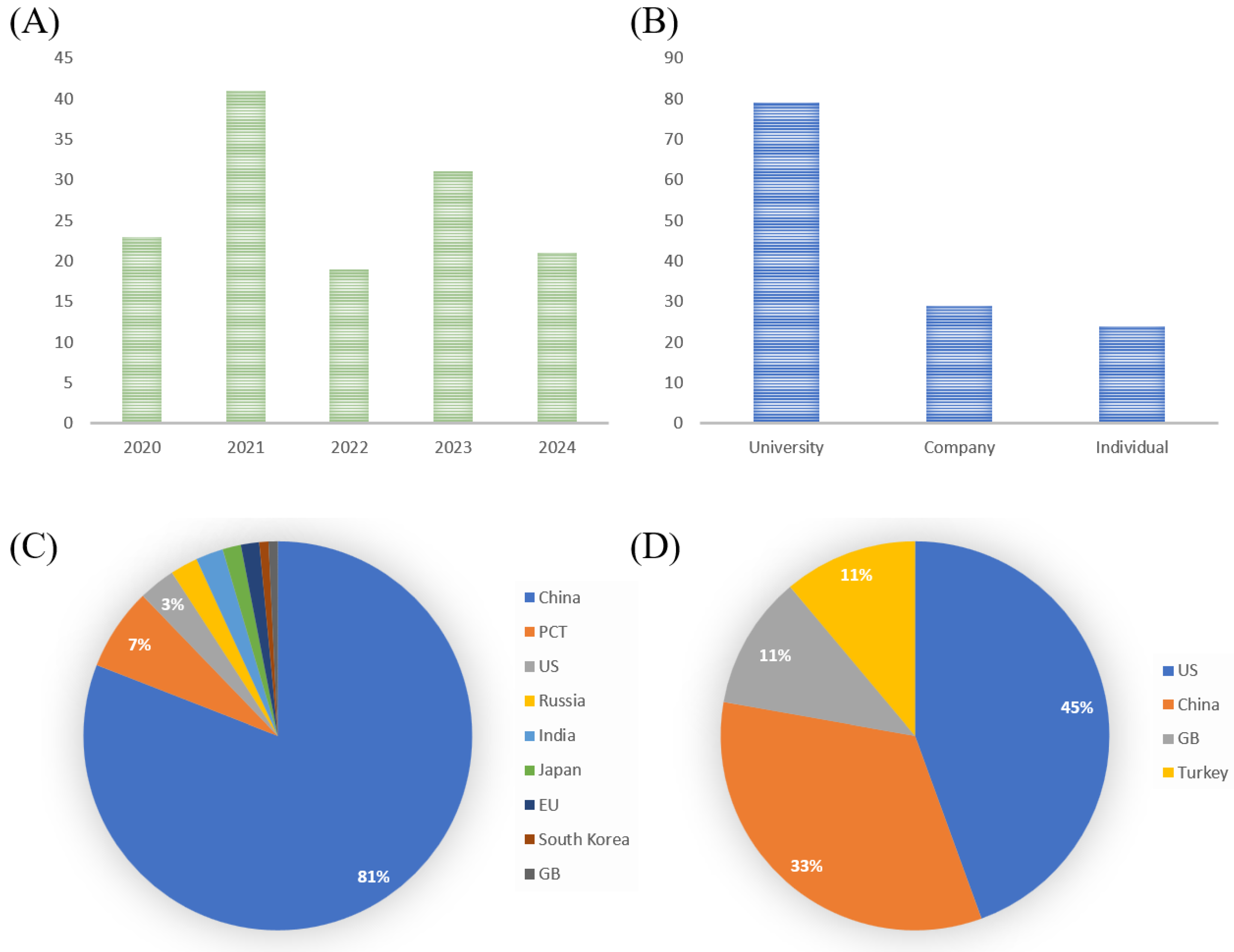
5. Recent Patents and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Available online: https://www.who.int/news-room/fact-sheets/detail/burns (accessed on 28 February 2025).
- Maslova, E.; Eisaiankhongi, L.; Sjöberg, F.; McCarthy, R.R. Burns and biofilms: Priority pathogens and in vivo models. npj Biofilms Microbiomes 2021, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Kopecki, Z. Development of next-generation antimicrobial hydrogel dressing to combat burn wound infection. Biosci. Rep. 2021, 41, BSR20203404. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Sarkar, S.; Das, B.; Bhattacharjee, S.; Tribedi, P. Biofilm, pathogenesis and prevention—A journey to break the wall: A review. Arch. Microbiol. 2015, 198, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Bjarnsholt, T.; Moser, C.; Bassi, G.L.; Coenye, T.; Donelli, G.; Hall-Stoodley, L.; Holá, V.; Imbert, C.; Kirketerp-Møller, K.; et al. ESCMID∗ guideline for the diagnosis and treatment of biofilm infections 2014. Clin. Microbiol. Infect. 2015, 21, S1–S25. [Google Scholar] [CrossRef]
- Uruén, C.; Chopo-Escuin, G.; Tommassen, J.; Mainar-Jaime, R.C.; Arenas, J. Biofilms as Promoters of Bacterial Antibiotic Resistance and Tolerance. Antibiotics 2021, 10, 3. [Google Scholar] [CrossRef]
- Shenkutie, A.M.; Yao, M.Z.; Siu, G.K.-H.; Wong, B.K.C.; Leung, P.H.-M. Biofilm-Induced Antibiotic Resistance in Clinical Acinetobacter baumannii Isolates. Antibiotics 2020, 9, 817. [Google Scholar] [CrossRef]
- Bowler, P.; Murphy, C.; Wolcott, R. Biofilm exacerbates antibiotic resistance: Is this a current oversight in antimicrobial stewardship? Antimicrob. Resist. Infect. Control 2020, 9, 162. [Google Scholar] [CrossRef]
- Michaelis, C.; Grohmann, E. Horizontal Gene Transfer of Antibiotic Resistance Genes in Biofilms. Antibiotics 2023, 12, 328. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Available online: https://www.grandviewresearch.com/horizon/outlook/antimicrobial-wound-care-dressings-market-size/global (accessed on 1 March 2025).
- Indrakumar, S.; Dash, T.K.; Mishra, V.; Tandon, B.; Chatterjee, K. Silk Fibroin and Its Nanocomposites for Wound Care: A Comprehensive Review. ACS Polym. Au 2024, 4, 168–188. [Google Scholar] [CrossRef]
- Jung, K.; Corrigan, N.; Wong, E.H.H.; Boyer, C. Bioactive Synthetic Polymers. Adv. Mater. 2022, 34, 2105063. [Google Scholar] [CrossRef] [PubMed]
- Medici, S.; Peana, M.F.; Nurchi, V.M.; Zoroddu, M.A. Medical Uses of Silver: History, Myths, and Scientific Evidence. J. Med. Chem. 2019, 62, 5923–5943. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.databridgemarketresearch.com/reports/global-silver-wound-dressing-market?srsltid=AfmBOoqddtQMI-2uwNXzwsIu-BK_bn-YLt35l8rBGPcG9upq3LGz1hFd (accessed on 1 March 2025).
- Shrestha, S.; Wang, B.; Dutta, P.K. Commercial Silver-Based Dressings: In Vitro and Clinical Studies in Treatment of Chronic and Burn Wounds. Antibiotics 2024, 13, 910. [Google Scholar] [CrossRef]
- Wounds International. International Consensus. Appropriate use of Silver Dressings in Wounds. An Expert Working Group Consensus; Wounds International: London, UK, 2012; Available online: www.woundsinternational.com (accessed on 1 March 2025).
- Frei, A.; Verderosa, A.D.; Elliott, A.G.; Zuegg, J.; Blaskovich, M.A.T. Metals to combat antimicrobial resistance. Nat. Rev. Chem. 2023, 7, 202–224. [Google Scholar] [CrossRef]
- Norton, R.; Finley, P.J. Clinically isolated bacteria resistance to silver-based wound dressings. J. Wound Care 2021, 30, 238–247. [Google Scholar] [CrossRef]
- McNeilly, O.; Mann, R.; Hamidian, M.; Gunawan, C. Emerging Concern for Silver Nanoparticle Resistance in Acinetobacter baumannii and Other Bacteria. Front. Microbiol. 2021, 12, 652863. [Google Scholar] [CrossRef]
- Caron, A.J.; Ali, I.J.; Delgado, M.J.; Johnson, D.; Reeks, J.M.; Strzhemechny, Y.M.; McGillivray, S.M. Zinc oxide nanoparticles mediate bacterial toxicity in Mueller-Hinton Broth via Zn2+. Front. Microbiol. 2024, 15, 1394078. [Google Scholar] [CrossRef]
- Agren, M.S.; Ostenfeld, U.; Kallehave, F.; Gong, Y.; Raffn, K.; Crawford, M.E.; Kiss, K.; Friis-Møller, A.; Gluud, C.; Jorgensen, L.N. A randomized, double-blind, placebo-controlled multicenter trial evaluating topical zinc oxide for acute open wounds following pilonidal disease excision. Wound Repair Regen. 2006, 14, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T. Antibacterial Mechanism of Bacteriolyses of Bacterial Cell Walls by Zinc(II) Ion Induced Activations of PGN Autolysins, and DNA damages. J. Genes Proteins 2017, 1, 1–7. [Google Scholar]
- Jarosz, M.; Olbert, M.; Wyszogrodzka, G.; Młyniec, K.; Librowski, T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology 2017, 25, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Castro, C.; Machado, R.A.; Vaz-Moreira, I.; Manaia, C.M. Assessment of copper and zinc salts as selectors of antibiotic resistance in Gram-negative bacteria. Sci. Total. Environ. 2015, 530, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Osmokrovic, A.; Jancic, I.; Zizak, Z.; Milenkovic, M.; Obradovic, B. Activated Charcoal-Alginate Platform for Simultaneous Local Delivery of Bioactive Agents: At the Nexus of Antimicrobial and Cytotoxic Activity of Zn2+ Ions. Gels 2024, 10, 724. [Google Scholar] [CrossRef] [PubMed]
- van Alen, S.; Kaspar, U.; Idelevich, E.A.; Köck, R.; Becker, K. Increase of zinc resistance in German human derived livestock-associated MRSA between 2000 and 2014. Vet. Microbiol. 2018, 214, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Huang, Z.; Li, Y.; Ren, X.; Zhou, C.; Liu, R.; Zhang, P.; Lei, G.; Lyu, J.; Li, J.; et al. Copper exerts cytotoxicity through inhibition of iron-sulfur cluster biogenesis on ISCA1/ISCA2/ISCU assembly proteins. Free. Radic. Biol. Med. 2023, 204, 359–373. [Google Scholar] [CrossRef]
- Salvo, J.; Sandoval, C. Role of copper nanoparticles in wound healing for chronic wounds: Literature review. Burn. Trauma 2022, 10, tkab047. [Google Scholar] [CrossRef]
- Pickart, L. The human tri-peptide GHK and tissue remodeling. J. Biomater. Sci. Polym. Ed. 2008, 19, 969–988. [Google Scholar] [CrossRef]
- Sen, C.K.; Khanna, S.; Venojarvi, M.; Trikha, P.; Ellison, E.C.; Hunt, T.K.; Roy, S. Copper-induced vascular endothelial growth factor expression and wound healing. Am. J. Physiol. Circ. Physiol. 2002, 282, H1821–H1827. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://medcu.com/medcu-2/ (accessed on 10 April 2025).
- Kaur, I.; Purves, J.; Harwood, M.; Ketley, J.M.; Andrew, P.W.; Waldron, K.J.; Morrissey, J.A. Role of horizontally transferred copper resistance genes in Staphylococcus aureus and Listeria monocytogenes. Microbiology 2022, 168, 001162. [Google Scholar] [CrossRef]
- Pal, C.; Asiani, K.; Arya, S.; Rensing, C.; Stekel, D.J.; Larsson, D.J.; Hobman, J.L. Metal Resistance and Its Association With Antibiotic Resistance. Adv. Microb. Physiol. 2017, 70, 261–313. [Google Scholar] [CrossRef]
- Stojkovska, J.; Kostić, D.; Jovanović, Ž.; Vukašinović-Sekulić, M.; Mišković-Stanković, V.; Obradović, B. A comprehensive approach to in vitro functional evaluation of Ag/alginate nanocomposite hydrogels. Carbohydr. Polym. 2014, 111, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Batool, Z.; Muhammad, G.; Iqbal, M.M.; Aslam, M.S.; Raza, M.A.; Sajjad, N.; Abdullah, M.; Akhtar, N.; Syed, A.; Elgorban, A.M.; et al. Hydrogel assisted synthesis of gold nanoparticles with enhanced microbicidal and in vivo wound healing potential. Sci. Rep. 2022, 12, 6575. [Google Scholar] [CrossRef] [PubMed]
- Batool, M.; Khurshid, S.; Qureshi, Z.; Daoush, W.M. Adsorption, antimicrobial and wound healing activities of biosynthesised zinc oxide nanoparticles. Chem. Pap. 2021, 75, 893–907. [Google Scholar] [CrossRef]
- Khan, A.; Rashid, A.; Younas, R.; Chong, R. A chemical reduction approach to the synthesis of copper nanoparticles. Int. Nano Lett. 2016, 6, 21–26. [Google Scholar] [CrossRef]
- Alotaibi, B.; Elekhnawy, E.; El-Masry, T.A.; Saleh, A.; El-Bouseary, M.M.; Alosaimi, M.E.; Alotaibi, K.N.; Abdelkader, D.H.; Negm, W.A. Green synthetized Cu-Oxide Nanoparticles: Properties and applications for enhancing healing of wounds infected with Staphylococcus aureus. Int. J. Pharm. 2023, 645, 123415. [Google Scholar] [CrossRef]
- Raja, F.N.S.; Worthington, T.; Martin, R.A. The antimicrobial efficacy of copper, cobalt, zinc and silver nanoparticles: Alone and in combination. Biomed. Mater. 2023, 18, 045003. [Google Scholar] [CrossRef]
- Stojkovska, J.; Djurdjevic, Z.; Jancic, I.; Bufan, B.; Milenkovic, M.; Jankovic, R.; Miskovic-Stankovic, V.; Obradovic, B. Comparative in vivo evaluation of novel formulations based on alginate and silver nanoparticles for wound treatments. J. Biomater. Appl. 2018, 32, 1197–1211. [Google Scholar] [CrossRef]
- Lok, C.-N.; Ho, C.-M.; Chen, R.; He, Q.-Y.; Yu, W.-Y.; Sun, H.; Tam, P.K.-H.; Chiu, J.-F.; Che, C.-M. Proteomic Analysis of the Mode of Antibacterial Action of Silver Nanoparticles. J. Proteome Res. 2006, 5, 916–924. [Google Scholar] [CrossRef]
- Padmavathy, N.; Vijayaraghavan, R. Enhanced bioactivity of ZnO nanoparticles—An antimicrobial study. Sci. Technol. Adv. Mater. 2008, 9, 035004. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle? A Study of the Gram-Negative Bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadegan, A.; Ghahramani, Y.; Gholami, A.; Hemmateenejad, B.; Dorostkar, S.; Nabavizadeh, M.; Sharghi, H. The Effect of Charge at the Surface of Silver Nanoparticles on Antimicrobial Activity against Gram-Positive and Gram-Negative Bacteria: A Preliminary Study. J. Nanomater. 2015, 2015, 720654. [Google Scholar] [CrossRef]
- Ali, A.Y.; Alani, A.-A.K.; Ahmed, B.O.; Hamid, L.L. Effect of biosynthesized silver nanoparticle size on antibacterial and anti-biofilm activity against pathogenic multi-drug resistant bacteria. OpenNano 2024, 20, 100213. [Google Scholar] [CrossRef]
- Cheon, J.Y.; Kim, S.J.; Rhee, Y.H.; Kwon, O.H.; Park, W.H. Shape-dependent antimicrobial activities of silver nanoparticles. Int. J. Nanomed. 2019, 14, 2773–2780. [Google Scholar] [CrossRef]
- Panáček, A.; Kvítek, L.; Prucek, R.; Kolář, M.; Večeřová, R.; Pizúrová, N.; Sharma, V.K.; Nevĕčná, T.; Zbořil, R. Silver colloid nanoparticles: Synthesis, characterization, and their antibacterial activity. J. Phys. Chem. B 2006, 110, 16248–16253. [Google Scholar] [CrossRef] [PubMed]
- Selim, M.I.; Sonbol, F.I.; El-Banna, T.E.; Negm, W.A.; Elekhnawy, E. Antibacterial and wound healing potential of biosynthesized zinc oxide nanoparticles against carbapenem-resistant Acinetobacter baumannii: An in vitro and in vivo study. Microb. Cell Factories 2024, 23, 281. [Google Scholar] [CrossRef]
- Karimi, B.; Mardani, M.; Kaboutari, J.; Javdani, M.; Albadi, J.; Shirian, S. Green synthesis of copper oxide nanoparticles using Artemisia annua aqueous extract and its characterization, antioxidant, and burn wound healing activities. Chem. Pap. 2024, 78, 231–243. [Google Scholar] [CrossRef]
- Chen, W.; Chu, R.; Li, H.; Hua, T.; Chen, H.; Li, R.; Zhou, D.; Cao, S.; Ye, S.; Li, H. A novel wound dressing based on a gold nanoparticle self-assembled hydrogel to promote wound healing. Mater. Adv. 2023, 4, 2918–2925. [Google Scholar] [CrossRef]
- Alizadeh, S.; Seyedalipour, B.; Shafieyan, S.; Kheime, A.; Mohammadi, P.; Aghdami, N. Copper nanoparticles promote rapid wound healing in acute full thickness defect via acceleration of skin cell migration, proliferation, and neovascularization. Biochem. Biophys. Res. Commun. 2019, 517, 684–690. [Google Scholar] [CrossRef]
- Balderrama-González, A.-S.; Piñón-Castillo, H.-A.; Ramírez-Valdespino, C.-A.; Landeros-Martínez, L.-L.; Orrantia-Borunda, E.; Esparza-Ponce, H.-E. Antimicrobial Resistance and Inorganic Nanoparticles. Int. J. Mol. Sci. 2021, 22, 12890. [Google Scholar] [CrossRef]
- Ribeiro, A.I.; Dias, A.M.; Zille, A. Synergistic Effects Between Metal Nanoparticles and Commercial Antimicrobial Agents: A Review. ACS Appl. Nano Mater. 2022, 5, 3030–3064. [Google Scholar] [CrossRef] [PubMed]
- Rogowska, A.; Rafińska, K.; Pomastowski, P.; Walczak, J.; Railean-Plugaru, V.; Buszewska-Forajta, M.; Buszewski, B. Silver nanoparticles functionalized with ampicillin. Special Issue:Focus on Electrochemistry in (Bio)-Nanoanalysis. Electromigr. Liq. Phase Sep. 2017, 38, 2757–2764. [Google Scholar]
- Rónavári, A.; Bélteky, P.; Boka, E.; Zakupszky, D.; Igaz, N.; Szerencsés, B.; Pfeiffer, I.; Kónya, Z.; Kiricsi, M. Polyvinyl-Pyrrolidone-Coated Silver Nanoparticles—The Colloidal, Chemical, and Biological Consequences of Steric Stabilization under Biorelevant Conditions. Int. J. Mol. Sci. 2021, 22, 8673. [Google Scholar] [CrossRef]
- Bengalli, R.; Colantuoni, A.; Perelshtein, I.; Gedanken, A.; Collini, M.; Mantecca, P.; Fiandra, L. In vitro skin toxicity of CuO and ZnO nanoparticles: Application in the safety assessment of antimicrobial coated textiles. NanoImpact 2021, 21, 100282. [Google Scholar] [CrossRef]
- Saweres-Argüelles, C.; Ramírez-Novillo, I.; Vergara-Barberán, M.; Carrasco-Correa, E.J.; Lerma-García, M.J.; Simó-Alfonso, E.F. Skin absorption of inorganic nanoparticles and their toxicity: A review. Eur. J. Pharm. Biopharm. 2022, 182, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Stojkovska, J.; Zvicer, J.; Obradovic, B. Preclinical functional characterization methods of nanocomposite hydrogels containing silver nanoparticles for biomedical applications. Appl. Microbiol. Biotechnol. 2020, 104, 4643–4658. [Google Scholar] [CrossRef]
- Sojka, M.; Valachova, I.; Bucekova, M.; Majtan, J. Antibiofilm efficacy of honey and bee-derived defensin-1 on multispecies wound biofilm. J. Med. Microbiol. 2016, 65, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Molan, P.C. Re-introducing honey in the management of wounds and ulcers—Theory and practice. Ostomy Wound Manag. 2002, 48, 28–40. [Google Scholar]
- Molan, P.; Rhodes, T. Honey: A Biologic Wound Dressing. Wounds 2015, 27, 141–151. [Google Scholar]
- Majtan, J. Honey: An immunomodulator in wound healing. Wound Repair Regen. 2014, 22, 187–192. [Google Scholar] [CrossRef]
- Available online: https://www.grandviewresearch.com/industry-analysis/honey-dressing-market-report (accessed on 1 March 2025).
- van den Berg, A.J.; van den Worm, E.; van Ufford, H.Q.; Halkes, S.; Hoekstra, M.; Beukelman, C. An in vitro examination of the antioxidant and anti-inflammatory properties of buckwheat honey. J. Wound Care 2008, 17, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Ranzato, E.; Martinotti, S.; Burlando, B. Honey exposure stimulates wound repair of human dermal fibroblasts. Burn. Trauma 2013, 1, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Bucekova, M.; Buriova, M.; Pekarik, L.; Majtan, V.; Majtan, J. Phytochemicals-mediated production of hydrogen peroxide is crucial for high antibacterial activity of honeydew honey. Sci. Rep. 2018, 8, 9061. [Google Scholar] [CrossRef]
- Combarros-Fuertes, P.; Fresno, J.M.; Estevinho, M.M.; Sousa-Pimenta, M.; Tornadijo, M.E.; Estevinho, L.M. Honey: Another Alternative in the Fight against Antibiotic-Resistant Bacteria? Antibiotics 2020, 9, 774. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.A.; Jenkins, L. A comparison between medical grade honey and table honeys. Wounds 2009, 21, 29–36. [Google Scholar]
- Nolan, V.C.; Harrison, J.; Wright, J.E.E.; Cox, J.A.G. Clinical Significance of Manuka and Medical-Grade Honey for Antibiotic-Resistant Infections: A Systematic Review. Antibiotics 2020, 9, 766. [Google Scholar] [CrossRef]
- Luca, L.; Pauliuc, D.; Oroian, M. Honey microbiota, methods for determining the microbiological composition and the antimicrobial effect of honey—A review. Food Chem. 2024, 23, 101524. [Google Scholar] [CrossRef]
- SanMelix Laboratories, Inc., FL, USA, Buckwheat Honey and Bacitracin Wound-Healing Dressing. U.S. Patent US20180236009A1, 23 August 2018.
- SanMelix Laboratories, Inc., FL, USA, Buckwheat Honey and Povidone-Iodine Wound-Healing Dressing. WOIP 2019/040185, 28 February 2019.
- Stojkovska, J.; Petrovic, P.; Jancic, I.; Milenkovic, M.T.; Obradovic, B. Novel nano-composite hydrogels with honey effective against multi-resistant clinical strains of Acinetobacter baumannii and Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2019, 103, 8529–8543. [Google Scholar] [CrossRef]
- Schuhladen, K.; Raghu, S.N.V.; Liverani, L.; Neščáková, Z.; Boccaccini, A.R. Production of a novel poly(ɛ-caprolactone)-methylcellulose electrospun wound dressing by incorporating bioactive glass and Manuka honey. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 180–192. [Google Scholar] [CrossRef]
- Mele, E. Electrospinning of honey and propolis for wound care. Biotechnol. Bioeng. 2023, 120, 1229–1240. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Potential role of propolis in wound healing: Biological properties and therapeutic activities. Biomed. Pharmacother. 2018, 98, 469–483. [Google Scholar] [CrossRef]
- Yang, J.; Pi, A.; Yan, L.; Li, J.; Nan, S.; Zhang, J.; Hao, Y. Research Progress on Therapeutic Effect and Mechanism of Propolis on Wound Healing. Evidence-Based Complement. Altern. Med. 2022, 2022, 5798941. [Google Scholar] [CrossRef] [PubMed]
- Han, M.C.; Durmus, A.S.; Karabulut, E.; Yaman, I. Effects of Turkish Propolis and Silver Sulfadiazine on Burn Wound Healing in Rats. Rev. De Médecine Vétérinaire 2005, 156, 624–627. [Google Scholar]
- Hilaria, M.; Elisma, E.; Uhe, A.L. Effect of Propolis Extract to Heal The Burns in New Zealand Rabbit. Indones. J. Cancer Chemoprevention 2017, 7, 54–59. [Google Scholar] [CrossRef]
- El-Kersh, D.M.; El-Ezz, R.F.A.; Ramadan, E.; El-Kased, R.F. Correction: In vitro and in vivo burn healing study of standardized propolis: Unveiling its antibacterial, antioxidant and anti-inflammatory actions in relation to its phytochemical profiling. PLoS ONE 2025, 20, e0319204. [Google Scholar] [CrossRef]
- Atiyeh, B.; Masellis, A.; Conte, C. Optimizing burn treatment in developing low- and middle-income countries with limited health care resources (Part 2). Ann. Burn. Fire Disasters 2009, 22, 189–195. [Google Scholar]
- Ejiohuo, O.; Folami, S.; Maigoro, A.Y. Calendula in modern medicine: Advancements in wound healing and drug delivery applications. Eur. J. Med. Chem. Rep. 2024, 12, 100199. [Google Scholar] [CrossRef]
- Akaberi, M.; Sobhani, Z.; Javadi, B.; Sahebkar, A.; Emami, S.A. Therapeutic effects of Aloe spp. in traditional and modern medicine: A review. Biomed. Pharmacother. 2016, 84, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Yadav, J. Ethnobotanical and pharmacological properties of Aloe vera: A review. J. Med. Plant Res. 2014, 8, 1387–1398. [Google Scholar]
- Maenthaisong, R.; Chaiyakunapruk, N.; Niruntraporn, S.; Kongkaew, C. The efficacy of aloe vera used for burn wound healing: A systematic review. Burns 2007, 33, 713–718. [Google Scholar] [CrossRef]
- Khorasani, G.; Hosseinimehr, S.J.; Azadbakht, M.; Zamani, A.; Mahdavi, M.R. Aloe versus silver sulfadiazine creams for second-degree burns: A randomized controlled study. Surg. Today 2009, 39, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Atiba, A.; Abdo, W.; Ali, E.K.; Abd-Elsalam, M.; Amer, M.; Monsef, A.A.; Taha, R.; Antar, S.; Mahmoud, A. Topical and oral applications of Aloe vera improve healing of deep second-degree burns in rats via modulation of growth factors. Biomarkers 2022, 27, 608–617. [Google Scholar] [CrossRef]
- Lievre, M.; Marichy, J.; Baux, S.; Foyatier, J.L.; Perrot, J.; Boissel, J.P. Controlled study of three ointments for the local management of 2nd and 3rd degree burns. Clin. Trials Meta-Anal. 1992, 28, 9–12. [Google Scholar]
- Rezai, S.; Rahzani, K.; Hekmatpou, D.; Rostami, A. Effect of oral Calendula officinalis on second-degree burn wound healing. Scars Burn. Heal. 2023, 9, 20595131221134053. [Google Scholar] [CrossRef]
- Chandran, P.K.; Kuttan, R. Effect of Calendula officinalis Flower Extract on Acute Phase Proteins, Antioxidant Defense Mechanism and Granuloma Formation During Thermal Burns. J. Clin. Biochem. Nutr. 2008, 43, 58–64. [Google Scholar] [CrossRef]
- Wölfle, U.; Seelinger, G.; Schempp, C.M. Topical Application of St. John’s Wort (Hypericum perforatum). Planta Med. 2013, 80, 109–120. [Google Scholar] [CrossRef]
- Celik, A.; Ersoy, O.; Kayaoglu, H.; Ozkan, N.; Lortlar, N.; Ömeroğlu, S.; Cakir, E. The Effects of Hypericum Perforatum Extract on Topical Burn Injury: A Comparative Study with Iodine. J. Clin. Anal. Med. 2010, 1, 4–7. [Google Scholar] [CrossRef]
- Kıyan, S.; Uyanikgil, Y.; Altunci, Y.A.; Cavusoglu, T.; Cetin Uyanikgil, E.O.; Karabey, F. Investigation of Acute Effects of Hypericum Perforatum (Kantaron) Treatment in Experimental Thermal Burns and Comparison with Silver Sulfadiazine Treatment. Turk. J. Trauma Emerg. Surg. 2015, 21, 323–336. [Google Scholar] [CrossRef]
- Farhan, M. The Promising Role of Polyphenols in Skin Disorders. Molecules 2024, 29, 865. [Google Scholar] [CrossRef]
- Zhao, H.; Lou, Z.; Chen, Y.; Cheng, J.; Wu, Y.; Li, B.; He, P.; Tu, Y.; Liu, J. Tea polyphenols (TPP) as a promising wound healing agent: TPP exerts multiple and distinct mechanisms at different phases of wound healing in a mouse model. Biomed. Pharmacother. 2023, 166, 115437. [Google Scholar] [CrossRef]
- Pipelzadeh, M.; Siahpoosh, A.; Sheikhi, A.R.; Jafarzadeh, E. Effectiveness of green tea cream in comparison with silver sulfadiazine cream in the treatment of second degree burn in human subjects. J. Herb. Med. 2022, 32, 100533. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Curcumin, a Natural Antimicrobial Agent with Strain-Specific Activity. Pharmaceuticals 2020, 13, 153. [Google Scholar] [CrossRef] [PubMed]
- Rolfe, V.; Mackonochie, M.; Mills, S.; MacLennan, E. Turmeric / curcumin and health outcomes: A meta-review of systematic reviews. Eur. J. Integr. Med. 2020, 40, 101252. [Google Scholar] [CrossRef]
- Cheppudira, B.; Fowler, M.; McGhee, L.; Greer, A.; Mares, A.; Petz, L.; Devore, D.; Loyd, D.R.; Clifford, J.L. Curcumin: A novel therapeutic for burn pain and wound healing. Expert Opin. Investig. Drugs 2013, 22, 1295–1303. [Google Scholar] [CrossRef]
- Zhi, L.; Dong, L.; Kong, D.; Sun, B.; Sun, Q.; Grundy, D.; Zhang, G.; Rong, W. Curcumin acts via transient receptor potential vanilloid-1 receptors to inhibit gut nociception and reverses visceral hyperalgesia. Neurogastroenterol. Motil. 2013, 25, e429–e440. [Google Scholar] [CrossRef] [PubMed]
- Yeon, K.; Kim, S.; Kim, Y.; Lee, M.; Ahn, D.; Kim, H.; Kim, J.; Jung, S.; Oh, S. Curcumin Produces an Antihyperalgesic Effect via Antagonism of TRPV1. J. Dent. Res. 2009, 89, 170–174. [Google Scholar] [CrossRef]
- Azani, A.; Fawzy, A. Reviewing the Potential Use of Curcumin Extract for Topical Therapy Supporting Burn Wound Healing. Int. J. Med. Sci. Clin. Res. Stud. 2025, 05, 68–73. [Google Scholar] [CrossRef]
- Dai, X.; Liu, J.; Zheng, H.; Wichmann, J.; Hopfner, U.; Sudhop, S.; Prein, C.; Shen, Y.; Machens, H.-G.; Schilling, A.F. Nano-formulated curcumin accelerates acute wound healing through Dkk-1-mediated fibroblast mobilization and MCP-1-mediated anti-inflammation. NPG Asia Mater. 2017, 9, e368. [Google Scholar] [CrossRef]
- Afshar, M.; Jafari, M.; Hassanzadeh Taheri, M.; Khorashadizadeh, M.; Taheri Olyayie, H. Evaluation of the effects of curcumin nanoliposomes on viability and motility of fibroblast cells and burn wound healing in mice: An in vivo and in vitro study. Iran. J. Dermatol. 2022, 25, 210–220. [Google Scholar] [CrossRef]
- Mehrabani, D.; Farjam, M.; Geramizadeh, B.; Tanideh, N.; Amini, M.; Panjehshahin, M.R. The Healing Effect of Curcumin on Burn Wounds in Rat. World J. Plast. Surg. 2015, 4, 29–35. [Google Scholar]
- Jopke, K.; Sanders, H.; White-Traut, R. Use of Essential Oils Following Traumatic Burn Injury: A Case Study. J. Pediatr. Nurs. 2017, 34, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Ang, L.; Kim, J.T.; Lee, M.S. Aromatherapy for Symptom Relief in Patients with Burn: A Systematic Review and Meta-Analysis. Medicina 2021, 58, 1. [Google Scholar] [CrossRef] [PubMed]
- Amini, M.; Kherad, M.; Mehrabani, D.; Azarpira, N.; Panjehshahin, M.R.; Tanideh, N. Effect of Plantago major on Burn Wound Healing in Rat. J. Appl. Anim. Res. 2010, 37, 53–56. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Ali, M.F.; Mohamed, N.M.; Bayoumi, S.A.; Zahran, A.M.; Elsayh, K.I. Exploring the efficacy of various wheat bran extracts in promoting burn wound healing: A comparative analysis. J. Ethnopharmacol. 2024, 319, 117174. [Google Scholar] [CrossRef]
- Skowrońska, W.; Bazylko, A. The Potential of Medicinal Plants and Natural Products in the Treatment of Burns and Sunburn—A Review. Pharmaceutics 2023, 15, 633. [Google Scholar] [CrossRef]
- Mora, L.; Aristoy, M.-C.; Toldrá, F. Bioactive Peptides. In Encyclopedia of Food Chemistry; Laurence, M., Fereidoon, S., Peter, V., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 381–389. [Google Scholar] [CrossRef]
- Veldkamp, T.; Dong, L.; Paul, A.; Govers, C. Bioactive properties of insect products for monogastric animals—A review. J. Insects Food Feed. 2022, 8, 1027–1040. [Google Scholar] [CrossRef]
- Miao, H.; Wang, L.; Wu, Q.; Huang, Z. Antimicrobial Peptides: Mechanism, Expressions, and Optimization Strategies. Probiotics Antimicrob. Proteins 2024, 17, 857–872. [Google Scholar] [CrossRef]
- Krunic, T.; Rakin, M.; Bulatovic, M.; Zaric, D. The contribution of bioactive peptides of whey to quality of food products. In Food Processing for Increased Quality and Consumption; Grumezescu, A.M., Holban, A.M., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 251–285. [Google Scholar] [CrossRef]
- Embiriekah, S.M.; Bulatović, M.L.; Gnjatović, M.L.; Vukašinović-Sekulić, M.S.; Krunić, T.Ž.; Zarić, D.B.; Rakin, M.B. Comparative analysis of functionality of spray dried whey protein hydrolysates obtained by enzymatic and microbial hydrolysis. Chem. Ind. 2018, 72, 265–274. [Google Scholar] [CrossRef]
- Nordström, R.; Malmsten, M. Delivery systems for antimicrobial peptides. Adv. Colloid Interface Sci. 2017, 242, 17–34. [Google Scholar] [CrossRef]
- Krunic, T.; Rakin, M. FTIR analysis of protein/peptide-based biopolymer used for probiotic encapsulation. In Probiotics and Their Role of Health and Disease; Olsen, Y., Ed.; Nova Science Publisher: Hauppauge, NY, USA, 2021; pp. 211–234. [Google Scholar]
- Krunić, T.Ž.; Rakin, M.B. Enriching alginate matrix used for probiotic encapsulation with whey protein concentrate or its trypsin-derived hydrolysate: Impact on antioxidant capacity and stability of fermented whey-based beverages. Food Chem. 2022, 370, 130931. [Google Scholar] [CrossRef]
- Wang, G.; Huang, Y.; Shi, Y.; Han, Q.; Zhang, J.; Song, Y.; Li, C. The novel cathelicidin-DM antimicrobial peptide conjugated carbomer and thermosensitive chitosan hydrogel speeds up wound-healing in both non-infected and S. aureus-infected wounds. Int. J. Biol. Macromol. 2025, 288, 138659. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Feng, C.; Yin, S.; Feng, Z.; Tang, J.; Liu, N.; Yang, F.; Yang, X.; Wang, Y. A novel peptide from the skin of amphibian Rana limnocharis with potency to promote skin wound repair. Nat. Prod. Res. 2020, 35, 3514–3518. [Google Scholar] [CrossRef]
- Bădăluță, V.A.; Curuțiu, C.; Dițu, L.M.; Holban, A.M.; Lazăr, V. Probiotics in Wound Healing. Int. J. Mol. Sci. 2024, 25, 5723. [Google Scholar] [CrossRef]
- Lukic, J.; Chen, V.; Strahinic, I.; Begovic, J.; Lev-Tov, H.; Davis, S.C.; Tomic-Canic, M.; Pastar, I. Probiotics or pro-healers: The role of beneficial bacteria in tissue repair. Wound Repair Regen. 2018, 25, 912–922. [Google Scholar] [CrossRef]
- Knackstedt, R.; Knackstedt, T.; Gatherwright, J. The role of topical probiotics on wound healing: A review of animal and human studies. Int. Wound J. 2020, 17, 1687–1694. [Google Scholar] [CrossRef] [PubMed]
- Tsiouris, C.G.; Kelesi, M.; Vasilopoulos, G.; Kalemikerakis, I.; Papageorgiou, E.G. The efficacy of probiotics as pharmacological treatment of cutaneous wounds: Meta-analysis of animal studies. Eur. J. Pharm. Sci. 2017, 104, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Valdéz, J.C.; Peral, M.C.; Rachid, M.; Santana, M.; Perdigón, G. Interference of Lactobacillus plantarum with Pseudomonas aeruginosa in vitro and in infected burns: The potential use of probiotics in wound treatment. Clin. Microbiol. Infect. 2005, 11, 472–479. [Google Scholar] [CrossRef]
- Rodrigues, K.L.; Caputo, L.R.G.; Carvalho, J.C.T.; Evangelista, J.; Schneedorf, J.M. Antimicrobial and healing activity of kefir and kefiran extract. Int. J. Antimicrob. Agents 2005, 25, 404–408. [Google Scholar] [CrossRef]
- Huseini, H.F.; Rahimzadeh, G.; Fazeli, M.R.; Mehrazma, M.; Salehi, M. Evaluation of wound healing activities of kefir products. Burns 2012, 38, 719–723. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E.; Eskandari, M.H. Kefir Accelerates Burn Wound Healing Through Inducing Fibroblast Cell Migration In Vitro and Modulating the Expression of IL-1ß, TGF-ß1, and bFGF Genes In Vivo. Probiotics Antimicrob. Proteins 2019, 11, 874–886. [Google Scholar] [CrossRef]
- Ong, J.S.; Taylor, T.D.; Yong, C.C.; Khoo, B.Y.; Sasidharan, S.; Choi, S.B.; Ohno, H.; Liong, M.T. Lactobacillus plantarum USM8613 Aids in Wound Healing and Suppresses Staphylococcus aureus Infection at Wound Sites. Probiotics Antimicrob. Proteins 2020, 12, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Satish, L.; Gallo, P.H.; Johnson, S.; Yates, C.C.; Kathju, S. Local Probiotic Therapy with Lactobacillus plantarum Mitigates Scar Formation in Rabbits after Burn Injury and Infection. Surg. Infect. 2017, 18, 119–127. [Google Scholar] [CrossRef]
- Hager, C.L.; Isham, N.; Schrom, K.P.; Chandra, J.; McCormick, T.; Miyagi, M.; Ghannoum, M.A. Effects of a Novel Probiotic Combination on Pathogenic Bacterial-Fungal Polymicrobial Biofilms. MBio J. 2019, 10, 10–1128. [Google Scholar] [CrossRef]
- Nezamdoost-Sani, N.; Khaledabad, M.A.; Amiri, S.; Khaneghah, A.M. Alginate and derivatives hydrogels in encapsulation of probiotic bacteria: An updated review. Food Biosci. 2023, 52, 102433. [Google Scholar] [CrossRef]
- Farahani, F.H.; Moraffah, F.; Samadi, N.; Sharifzadeh, M.; Motasadizadeh, H.; Vatanara, A. Improved infectious burn wound healing by applying lyophilized particles containing probiotics and prebiotics. Int. J. Pharm. 2023, 636, 122800. [Google Scholar] [CrossRef] [PubMed]
- Dou, Z.; Li, B.; Wu, L.; Qiu, T.; Wang, X.; Zhang, X.; Shen, Y.; Lu, M.; Yang, Y. Probiotic-Functionalized Silk Fibroin/Sodium Alginate Scaffolds with Endoplasmic Reticulum Stress-Relieving Properties for Promoted Scarless Wound Healing. ACS Appl. Mater. Interfaces 2023, 15, 6297–6311. [Google Scholar] [CrossRef]
- Mojsilovic, S.; Krunic, T.; Lazic, V.; Djuknic, M.; Osmokrovic, A. Activated charcoal as a carrier of probiotics: A new approach for pathogen elimination in wounds. Chem. Ind. 2024, 78, 63. [Google Scholar]
- Ming, Z.; Han, L.; Bao, M.; Zhu, H.; Qiang, S.; Xue, S.; Liu, W. Living Bacterial Hydrogels for Accelerated Infected Wound Healing. Adv. Sci. 2021, 8, 2102545. [Google Scholar] [CrossRef]
- Yang, L.; Han, Z.; Chen, C.H.; Li, Z.Y.; Yu, S.P.; Qu, Y.; Zeng, R. Novel probiotic-bound oxidized Bletilla striata polysaccharide-chitosan composite hydrogel. Mater. Sci. Eng. C 2020, 117, 111265. [Google Scholar] [CrossRef]
- Mei, L.; Zhang, D.; Shao, H.; Hao, Y.; Zhang, T.; Zheng, W.; Ji, Y.; Ling, P.; Lu, Y.; Zhou, Q. Injectable and Self-Healing Probiotics-Loaded Hydrogel for Promoting Superbacteria-Infected Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 20538–20550. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, J.; Lee, S.M.; Woo, M.R.; Kim, D.W.; Kim, J.O.; Choi, H.G.; Jin, S.G. Development of guar gum-based dual-layer wound dressing containing Lactobacillus plantarum: Rapid recovery and mechanically flexibility. Int. J. Biol. Macromol. 2022, 221, 1572–1579. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, Y.; Song, J.; Zhou, L.; Wu, K.; Lu, X.; Zhai, X.; Wan, Z.; Gao, J. Highly active probiotic hydrogels matrixed on bacterial EPS accelerate wound healing via maintaining stable skin microbiota and reducing inflammation. Bioact. Mater. 2024, 35, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Yu, H.; Woo, M.R.; Kim, D.W.; Kim, J.O.; Ku, S.K.; Jin, S.G.; Choi, H.-G. Influence of hydrophilic polymers on mechanical property and wound recovery of hybrid bilayer wound dressing system for delivering thermally unstable probiotic. Biomater. Adv. 2022, 135, 112696. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, S.K.; Raut, J.; Kumar, S.; Singh, M.; Ahmed, B.; Singh, J.; Rana, V.; Rishi, P.; Ganesh, N.; Dua, K.; et al. Nanocurcumin and viable Lactobacillus plantarum based sponge dressing for skin wound healing. Int. J. Pharm. 2023, 643, 123187. [Google Scholar] [CrossRef]
- Brightwake Ltd., UK. Wound Dressings. WIPO 2024170896, 8 August 2024. [Google Scholar]
- The Curators of the University of Missouri, Columbia, MO, USA. Dressings and Methods for Wound Healing. U.S. Patent 20240165296, 23 May 2024. [Google Scholar]
- Taizhou Roosin Medical Product Co., Ltd., China. Bismuth-Containing Burn Dressing and Preparation Method Thereof. CNIPA 116672488, 10 October 2023. [Google Scholar]
- Beijing University of Chemical Technology, China. A Nanofiber Dressing. CNIPA 116870231, 13 October 2023. [Google Scholar]
- Zhuhai Weimei Biotechnology Co., ltd., China. Dressing Containing Polysaccharide as Well as Preparation Method and Application of Dressing. CNIPA 117045844, 14 November 2023. [Google Scholar]
- Fujian Tongantang Biotechnology Co., ltd., China. Production Process of Polyethylene Glycol Paste Dressing. CNIPA 117338990, 5 January 2024. [Google Scholar]
- Axenoll Life Sciences Ag, Switzerland. A 3D Biocompatible Matrix and Its Uses in Wound Management. EP 4201439, 28 June 2023. [Google Scholar]
- Matoke Holdings Ltd., UK. Antimicrobial Superabsorbant Compositions. U.S. Patent 12225905, 28 December 2023. [Google Scholar]
- Shaanxi University of Science and Technology, China. Use of Ferrous Ions in Preparation of Burn Infection Treatment Drugs and Burn Care Products. WOIP 2025030704, 13 February 2025. [Google Scholar]
- Zhejiang Pharmaceutical Vocational University, China. Bio-Drug for Treating Large-Area Burn, Engineering Skin Substitute and large-Area Burn Treatment Dressing. CNIPA 118526573, 23 August 2024. [Google Scholar]
- Dalian University of Technology, China. Preparation Method of Multifunctional Double-Layer Heterogeneous Hydrogel Dressing. 118384318, 26 July 2024. [Google Scholar]
- Brunel University London, UK. Prevention and/or Treatment of Wound Infection. WOIP 2024074803, 11 April 2024. [Google Scholar]
- China Pharmaceutical University, China. Nitric Oxide Dressing Capable of Directionally Draining Exudate as Well as Preparation Process and Application of Nitric Oxide Dressing. CNIPA 117398496, 16 January 2024. [Google Scholar]
- Sichuan University, China. Hydrogel Attached with Antibacterial Coating and Application Thereof. CNIPA 116966335, 31 October 2023. [Google Scholar]
- Vladimirovich, S.A.; Vladimirovich, Z.E.; Valerevich, K.D.; Konstantinovich, K.P.; Olegovich, Z.O.; Anatolevich, M.V. Method of Treating Borderline Dermal Skin Burns by Applying a Gel of Rarely Cross-Linked Acrylic Polymers With a Complex of Natural Antimicrobial Peptides FLIP7. RU 0002800302, 20 July 2023. [Google Scholar]
- Zhongkai University of Agriculture and Engineering, China. Drug-Loaded Liposome Hydrogel as Well as Preparation and Application Thereof. CNIPA 115887744, 4 April 2023. [Google Scholar]
- Ninth Peoples Hospital Shanghai Jiaotong University School of Medicine, China. Curcumin-Containing Polymer and Application Thereof in Promoting Healing of Burns. CNIPA 115737838, 7 March 2023. [Google Scholar]
- Jadavpur University, India. Fabrication of Green Silver Nanoparticle-Embedded Microsphere and Therapeutic Activity Against Bacteria Infected Burn and Excision Wound. IN 202331006688, 10 February 2023. [Google Scholar]
- Shantou No 2 People S Hospital, China. Antibacterial Dressing for Burn Wounds. CNIPA 115501041, 23 December 2022. [Google Scholar]
- Zhejiang Sci Tech University ZSTU, China. Preparation Method of Silk-Spider Silk Composite Silk Fibroin Nano-Microspheres Containing Chitosan Modified Graphene Oxide. CNIPA 115105621, 27 September 2022. [Google Scholar]
- Shenzhen Bay Laboratory, China. Composition for Preparing Burn and Wound Dressing as Well as Preparation and Preparation Method Thereof. CNIPA 114748682, 15 July 2022. [Google Scholar]
- Kim, S.H. Composition for Treating Wound or Burn Comprising Aged Mink Oil by Addition of Snake Venom as Active Ingredient. KR 1020220059777, 10 May 2022. [Google Scholar]
- Shaanxi Provincial Peoples Hospital, China. Alginate Encapsulated Bacterial Cellulose Composite Photo-Thermal Antibacterial Medical Dressing and Preparation Method Thereof. CNIPA 114000349, 1 February 2022. [Google Scholar]
- Nanjing Zeheng Pharmaceutical Science; Technology Co., Ltd., China. Preparation and Antibacterial Property Research of Silver Sulfadiazine Spray. CNIPA 113952302, 21 January 2022. [Google Scholar]
- Taiyuan University of Technology, China. Double-Sustained-Release Drug-Loaded Hydrogel Dressing with Semi-Interpenetrating Network Entrapped Double-Layer Microspheres as Well as Preparation Method and Application Thereof. CNIPA 113648455, 16 November 2021. [Google Scholar]
- Zhengzhou University, China. Preparation Method and Application of Drug-Loaded Hydrogel. CNIPA 113425893, 24 September 2021. [Google Scholar]
- Dingxi Chinese Medicine And Traditional Chinese Medicine And Western Medicine Hospital, China. Traditional Chinese Medicine Composition for Treating Burn and Scald Wounds, Traditional Chinese Medicine Preparation and Preparation Method. CNIPA 113209253, 6 August 2021. [Google Scholar]
- Sobha, K.; Pradeep, D.; Subrahmanyam, C.V. SMART-DRE-M: Biopolymer Composite Based Nano-Fibrous Wound Dressing Material. IN 202041052202, 11 December 2020. [Google Scholar]
- Woodroof, E.A. Improved Biosynthetic Wound and Burn Dressing with Silver-Based Broad Antimicrobial Activity. WOIP 2020232456, 19 November 2020. [Google Scholar]
- Shandong Branden Medical Devices Co., Ltd., China. Preparation Method of Hemostatic and Antibacterial Dressing Containing Field Thistle Herb Extract. CNIPA 111921005, 13 November 2020. [Google Scholar]
- Beihang University, China. Preparation and Application of Antibacterial Modified Exosome Burn Wound Healing-Promoting Biological Dressing. CNIPA 110975000, 10 April 2020. [Google Scholar]
- Guangping, Y. Coptis Chinensis Skin Healing Paste and Clinical Research Method for Treating Burns by Using Gauze Strips of Paste. CNIPA 111265639, 12 June 2020. [Google Scholar]
- University of South Carolina Aiken, USA. pH Indicator Dressing for Monitoring of Wound and Infection. U.S. Patent 20200069482, 5 March 2020. [Google Scholar]
- Wonbiogen Co., Ltd., China. Polyurethane Foam Dressing Material Containing Silver-Activated Carbon Composite and Producing Method Thereof. KR 1020200013449, 7 February 2020. [Google Scholar]
- Sichuan University, China. Wound Dressing Capable of Rapidly Absorbing Heat as Well as Preparation Method and Application of Wound Dressing. CNIPA 113289054, 24 August 2021. [Google Scholar]
| Type of Study | Polymer | Probiotic Strain (With/Without Prebiotic) | Pathogen Strain | Ref. |
|---|---|---|---|---|
| In vitro and in vivo (Wistar rat model) | gelatin, HA, and LRHA hydrogel | L. reuteri | E. coli S. aureus Salmonella spp. | [139] |
| In vitro and in vivo (mice model) | oxidized Bletilla striata-chitosan composite hydrogel | L. plantarum | E. coli S. aureus P. aeruginosa | [140] |
| In vitro and in vivo (mice model) | hyaluronate-adipic dihydrazide/aldehyde-terminated Pluronic F127/fucoidan hydrogel | L. rhamnosus | multi-resistant P. aeruginosa | [141] |
| In vitro and in vivo animal model | pectin, alginate, and chitosan | L. plantarum (ATCC 1058), fructooligosaccharide (FOS) | P. aeruginosa (ATCC 9027) S. aureus (ATCC 6538) | [136] |
| In vitro and in vivo animal model | Guar gum and PVA | L. plantarum | P. aeruginosa | [142] |
| In vivo animal model | Silk fibroin/sodium alginate | L. casei | E. coli S. aureus | [137] |
| In vitro and in vivo (Wistar rat model) | Probiotic hydrogels | L. paracasei (TYM202), extracellular polysaccharides 9EPS0 from B. velezensis (M76T11B) | E. coli S. aureus | [143] |
| In vivo (rat model) | Hybrid bilayer wound dressing | L. brevis (KCTC 3498) | S. aureus subsp. aureus KCCM 40050 | [144] |
| In vivo (mice model) | Sponge dressings | L. plantarum UBLP-40 (MTCC 5380) | S. aureus 9144 | [145] |
| Patent Title | Composition/Key Features | Ref. |
|---|---|---|
| Wound dressings | An amorphous gel composed of a nitrite layer with nitrite salts and an acid layer with at least one acid, along with a solid conjugate base to create a buffer system with a pH of 3.8 to 6.0; the dressing generates nitric oxide through the acidification of the nitrite salts | [146] |
| Dressings and methods for wound healing | 3D-printed wound dressing comprising a hydrogel matrix (alginate, gelatin, gelMA, cellulose, or chitosan) with up to 50 w/v % bioactive borate glass (BBG) containing boron | [147] |
| Bismuth-containing burn dressing and preparation method thereof | Bismuth-containing burn dressing with three layers: a back paste layer, an antibacterial matrix layer made of a bismuth-infused polyurethane sponge, and a releasing layer | [148] |
| Nanofiber dressing | A core-shell structure drug-loaded nanofiber burn wound dressing, prepared by electrostatic spinning using polylactic acid (PLA), 2-hydroxypropyl-alpha-cyclodextrin (HP-alpha-CD), and curcumin (12.4–13.4%) | [149] |
| Dressing containing polysaccharides, as well as preparation method and application of the dressing | Ganoderma lucidum beta-glucan, Ganoderma lucidum chitosan, glycerol, and carbomer | [150] |
| Production process of polyethylene glycol paste dressing | Polyethylene glycol paste dressing incorporating beta-glucose, magnolia flower extract, and Herba houttuyniae extract in the water phase, and sesame oil with Melaleuca alternifolia essential oil in the oil phase | [151] |
| A 3D biocompatible matrix and its uses in wound management | A 3D scaffold based on natural (e.g., chitosan, collagen, alginate) or synthetic polymers (e.g., PLA, PGA, PLGA, polysiloxanes), incorporating non-opioid analgesics, extracellular vesicles (preferably human-derived), or artificial lipid vesicles (20–150 nm in size) | [152] |
| Antimicrobial superabsorbent compositions | A powder containing an enzyme that converts a substrate (e.g., honey) to release hydrogen peroxide, a precursor-substrate or substrate for the enzyme, and a superabsorbent component; forming a gel upon contact with water | [153] |
| Use of ferrous ions in the preparation of burn infection treatment drugs and burn care products | A ferrous compound (e.g., ferrous sulfate), a protective agent, a dressing matrix (such as sodium alginate or hyaluronic acid), and a solvent; these components are mixed and heated to form a ferrous compound hydrogel, which addresses burn infections caused by Pseudomonas aeruginosa, reduces antibiotic resistance risks | [154] |
| Bio-drug for treating large-area burns, engineering skin substitutes, and large-area burn treatment dressing | Dressing composed of eupatorin, fibroblast growth factors, curcumin, mulberry leaf extract, Ranunculus polysaccharide extract, and sulfadiazine silver for large-area burn wounds | [155] |
| Preparation method of multifunctional double-layer heterogeneous hydrogel dressing | Multifunctional double-layer heterogeneous hydrogel dressing for large-area burn wound treatment prepared using acrylamide, N-isopropylacrylamide, chitosan, sodium alginate, polyvinyl alcohol, an antibacterial agent, and a growth factor, cross-linked by ultraviolet light | [156] |
| Prevention and/or treatment of wound infection | A probiotic composition for treating and/or preventing wound infections, containing Cutibacterium acnes cells, cellular contents, cell-free supernatant, or bioactive components derived from the supernatant; it can be formulated as an ointment, gel, or cream for application to burns or skin wounds | [157] |
| Nitric oxide dressing capable of directionally draining exudate, as well as the preparation process and application of nitric oxide dressing | Multilayer dressing containing nitric oxide and an activating agent | [158] |
| Hydrogel attached with antibacterial coating and its application | A macroporous polysaccharide hydrogel with an antibacterial coating of catechol compounds and antibacterial peptides | [159] |
| Method of treating borderline dermal skin burns by applying a gel of rarely cross-linked acrylic polymers with a complex of natural antimicrobial peptides FLIP7 | An antimicrobial gel made from cross-linked natural acrylic polymers carrying FLIP7 natural peptides | [160] |
| Drug-loaded liposome hydrogel, as well as preparation and application thereof | A drug-loaded liposome hydrogel, composed of a carboxymethyl chitosan-sodium alginate hydrogel cross-linked with madecassoside and coated with liposomes carrying acetylshikonin and aloe-emodin, enabling slow, continuous, and sequential drug release | [161] |
| Curcumin-containing polymer and application thereof in promoting healing of burns | ε-poly-L-lysine and γ-polyglutamic polymer matrix with curcumin | [162] |
| Fabrication of green silver nanoparticle-embedded microsphere and therapeutic activity against bacteria-infected burn and excision wound | Green silver nanoparticle-embedded mucilage microspheres with high water absorption capacity | [163] |
| Antibacterial dressing for burn wounds | An antibacterial burn wound dressing made of nano-silver-coated gauze and polyurethane foam connected to a negative pressure drainage device | [164] |
| Preparation method of silk-spider silk composite silk fibroin nano-microspheres containing chitosan-modified graphene oxide | Chitosan-modified graphene oxide silk-spider silk composite fibroin nanospheres with excellent biocompatibility and biodegradability | [165] |
| Composition for preparing burn and wound dressing, as well as preparation and preparation method thereof | A composite burn wound dressing made from silk fibroin (as an antioxidant), active iodine (for broad-spectrum antibacterial action), and a chitosan-based water-absorbing framework, resulting in a porous, asymmetric dressing | [166] |
| Composition for treating wounds or burns comprising aged mink oil by the addition of snake venom as the active ingredient | A composition for treating wounds and burns and promoting skin cell regeneration, using aged mink oil combined with snake venom as the active ingredient | [167] |
| Alginate-encapsulated bacterial cellulose composite photo-thermal antibacterial medical dressing and preparation method thereof | A composite photo-thermal antibacterial medical dressing made of an inner bacterial cellulose/zinc oxide/copper sulfide porous membrane and an outer alginate encapsulation layer | [168] |
| Preparation and antibacterial property research of silver sulfadiazine spray | A silver sulfadiazine spray formulated with a surfactant and polymer, offering a non-greasy, evenly distributed protective film that improves patient comfort and compliance | [169] |
| Double-sustained-release drug-loaded hydrogel dressing with semi-interpenetrating network entrapped double-layer microspheres, as well as preparation method and application thereof | A dual pH- and temperature-sensitive hydrogel incorporates double-layer microspheres (calcium alginate core with bovine serum albumin and a chitosan shell with azithromycin) within a hydrogel matrix containing acrylic acid, methacrylic acid 2-ethyl ester, and oligomeric (ethylene glycol) methyl ether methacrylate, with embedded gentamicin sulfate | [170] |
| Preparation method and application of drug-loaded hydrogel | Drug-loaded injectable hydrogel composed of oxidized dextran (Odex) and gelatin grafted with protocatechuic acid (GT-PCA), prepared by mixing both solutions and cross-linking through a Schiff base reaction to form the GT-PCA/Odex hydrogel | [171] |
| Traditional Chinese medicine composition for treating burn and scald wounds, traditional Chinese medicine preparation, and preparation method | Traditional medicine composition for treating burn and scald wounds, made from Astragalus membranaceus, Rheum officinale, Angelica sinensis, Carthamus tinctorius (safflower), Rhizoma sparganii, Curcuma zedoaria, Cercis chinensis (Chinese redbud bark), Angelica dahurica (Dahurian angelica root), Saposhnikovia divaricata (radix saposhnikoviae), and Endothelium corneum gigeriae galli | [172] |
| Smart-DRE-M: biopolymer composite-based nano-fibrous wound dressing material | Core-shell nanofiber wound dressing fabricated from natural, low-cost biomaterials, including mucilage/gum from Cochlospermum gossypium (Yellow Silk Cotton Tree) and Canthium coromandelicum (Native Indian Herb), along with leaf extract of Chromolaena odorata (Siam weed), fluorine-doped carbon dots loaded with the anti-inflammatory drug Zaltoprofen and taurine as a bio-piezoelectric component | [173] |
| Improved biosynthetic wound and burn dressing with silver-based broad antimicrobial activity | Dressing with silver ion antimicrobial coating | [174] |
| Preparation method of hemostatic and antibacterial dressing containing field thistle herb extract | Hemostatic and antibacterial dressing containing field thistle herb extract, chitosan, growth factors, and hyaluronic acid | [175] |
| Preparation and application of antibacterial-modified exosome burn wound healing-promoting biological dressing | Antibacterial biological dressing composed of chitosan porous material loaded with artificially modified exosomes carrying broad-spectrum antibacterial agents | [176] |
| Coptis chinensis skin healing paste and clinical research method for treating burns by using gauze strips of paste | Skin healing paste (composed of Coptis chinensis, Angelica sinensis, Phellodendron chinense Schneid, Radix Rehmanniae Recens, Rhizoma Wenyujin Concisum, raw Sanguisorba officinalis, frankincense, myrrh, raw rhubarb, beeswax, and sesame oil) is soaked onto multiple layers of gauze strips | [177] |
| pH Indicator dressing for monitoring of wound and infection | A biocompatible polymer integrated with a pH-indicating agent, applied as a fiber, hydrogel, or microsphere on the wound site, enables early detection of infection or chronic wound states by color change, supporting monitoring of acute, chronic, or burn wound healing | [178] |
| Polyurethane foam dressing material containing silver-activated carbon composite and producing method thereof | Three-layered antibacterial polyurethane foam dressing composed of a microporous polyurethane foam layer loaded with a silver-activated carbon composite, a moisture-permeable and waterproof layer, and a perforated polyurethane film layer; it provides consistent, stable release of antimicrobial silver particles regardless of exudate levels, promoting effective wound healing and protection against infection | [179] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osmokrovic, A.; Stojkovska, J.; Krunic, T.; Petrovic, P.; Lazic, V.; Zvicer, J. Current State and Advances in Antimicrobial Strategies for Burn Wound Dressings: From Metal-Based Antimicrobials and Natural Bioactive Agents to Future Perspectives. Int. J. Mol. Sci. 2025, 26, 4381. https://doi.org/10.3390/ijms26094381
Osmokrovic A, Stojkovska J, Krunic T, Petrovic P, Lazic V, Zvicer J. Current State and Advances in Antimicrobial Strategies for Burn Wound Dressings: From Metal-Based Antimicrobials and Natural Bioactive Agents to Future Perspectives. International Journal of Molecular Sciences. 2025; 26(9):4381. https://doi.org/10.3390/ijms26094381
Chicago/Turabian StyleOsmokrovic, Andrea, Jasmina Stojkovska, Tanja Krunic, Predrag Petrovic, Vesna Lazic, and Jovana Zvicer. 2025. "Current State and Advances in Antimicrobial Strategies for Burn Wound Dressings: From Metal-Based Antimicrobials and Natural Bioactive Agents to Future Perspectives" International Journal of Molecular Sciences 26, no. 9: 4381. https://doi.org/10.3390/ijms26094381
APA StyleOsmokrovic, A., Stojkovska, J., Krunic, T., Petrovic, P., Lazic, V., & Zvicer, J. (2025). Current State and Advances in Antimicrobial Strategies for Burn Wound Dressings: From Metal-Based Antimicrobials and Natural Bioactive Agents to Future Perspectives. International Journal of Molecular Sciences, 26(9), 4381. https://doi.org/10.3390/ijms26094381






