Organoids-on-Chips Technology: Unveiling New Perspectives in Rare-Disease Research
Abstract
1. Introduction
2. Challenges and the Need for Preclinical Models
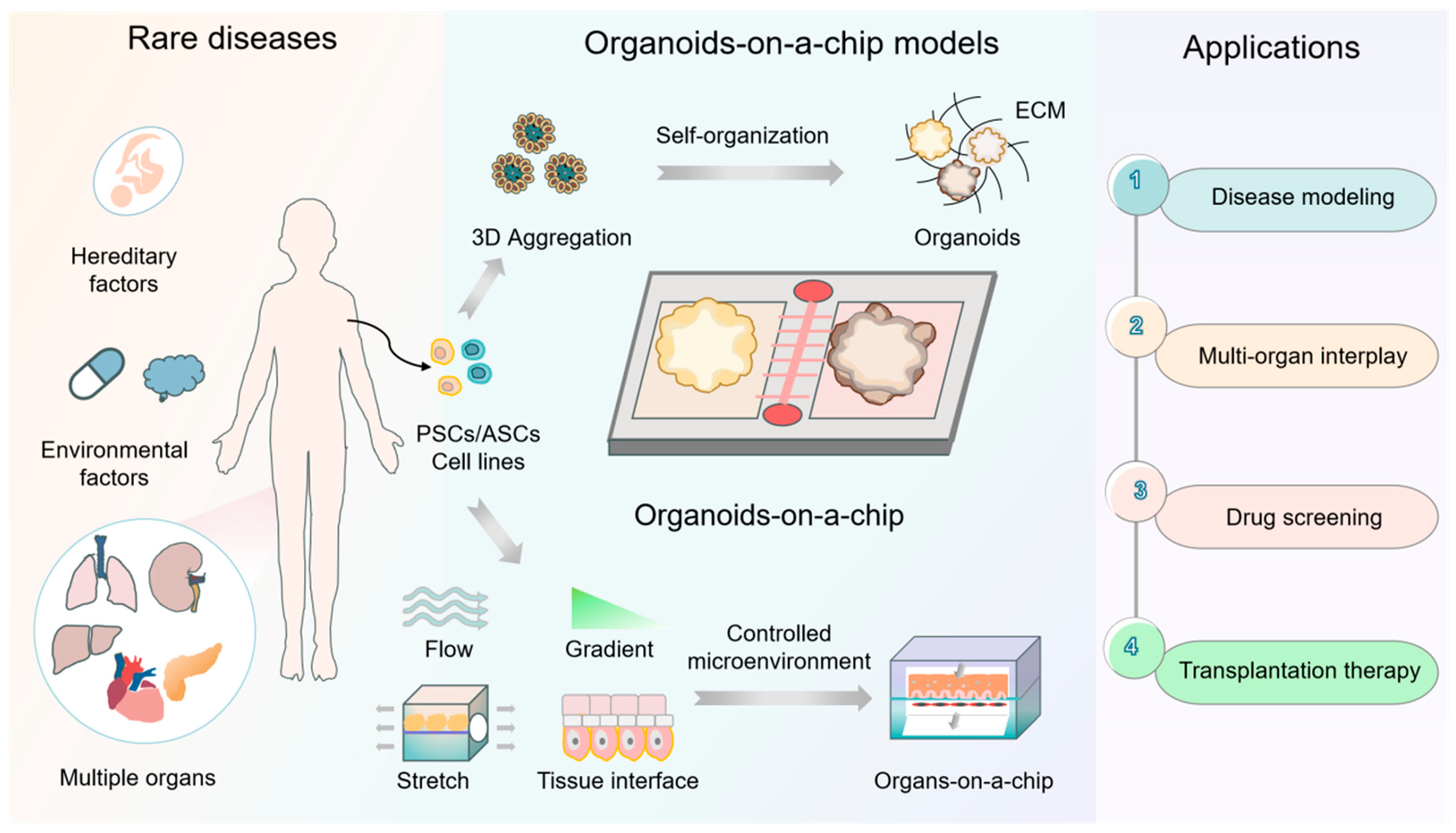
3. Organoid and Organ Chip Models: An Overview

4. Development of Organoid-on-a-Chip Models for Rare-Disease Modeling, Drug Development, and Transplantation Therapy
5. Conclusions and Perspectives
6. Clinical Translation and Ethical Compliance
Author Contributions
Funding
Conflicts of Interest
References
- The Lancet. Hope for rare diseases. The lancet. 2024, 404, 10464. [CrossRef]
- The Lancet Global Health. The landscape for rare diseases in 2024. Lancet Glob. Heal. 2024, 12, e341. [Google Scholar] [CrossRef]
- Ozkan, A.; LoGrande, N.T.; Feitor, J.F.; Goyal, G.; Ingber, D.E. Intestinal organ chips for disease modelling and personalized medicine. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 751–773. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; You, X.Y.; Zhao, G.P. Microbial volatile communication in human 3D intestinal organotypic models. Sci. Bull. 2023, 68, 1353–1358. [Google Scholar] [CrossRef]
- Zushin, P.H.; Mukherjee, S.; Wu, J.C. FDA Modernization Act 2.0: Transitioning beyond animal models with human cells, organoids, and AI/ML-based approaches. J. Clin. Investig. 2023, 133, e175824. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Shi, P.; You, X.; Zhao, G. Establishment and evaluation of on-chip intestinal barrier biosystems based on microfluidic techniques. Mater. Today Bio 2024, 26, 101079. [Google Scholar] [CrossRef]
- Fang, G.; Chen, Y.C.; Lu, H.; Jin, D. Advances in Spheroids and Organoids on a Chip. Adv. Funct. Mater. 2023, 33, 2215043. [Google Scholar] [CrossRef]
- Selimovic, S.; Dokmeci, M.R.; Khademhosseini, A. Organs-on-a-chip for drug discovery. Curr. Opin. Pharmacol. 2013, 13, 829–833. [Google Scholar] [CrossRef]
- Wadman, M. FDA no longer has to require animal testing for new drugs. Science 2023, 379, 127–128. [Google Scholar] [CrossRef]
- Park, S.E.; Georgescu, A.; Huh, D. Organoids-on-a-chip. Science 2019, 364, 960–965. [Google Scholar] [CrossRef]
- Ahadian, S.; Civitarese, R.; Bannerman, D.; Mohammadi, M.H.; Lu, R.; Wang, E.; Davenport-Huyer, L.; Lai, B.; Zhang, B.; Zhao, Y.; et al. Organ-On-A-Chip Platforms: A Convergence of Advanced Materials, Cells, and Microscale Technologies. Adv. Heal. Mater. 2018, 7, 1700506. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-J.; Li, J.-Y.; Wang, Y.-R.; Li, L.-J.; Li, H.-Y. Progress in construction and application of skin tissue engineering. Biomed. Eng. Commun. 2023, 2, 5. [Google Scholar] [CrossRef]
- Liu, H.; Gan, Z.; Qin, X.; Wang, Y.; Qin, J. Advances in Microfluidic Technologies in Organoid Research. Adv. Heal. Mater. 2023, 13, e2302686. [Google Scholar] [CrossRef]
- Hofer, M.; Lutolf, M.P. Engineering organoids. Nat. Rev. Mater. 2021, 6, 402–420. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, X.; Dowbaj, A.M.; Sljukic, A.; Bratlie, K.; Lin, L.; Fong, E.L.S.; Balachander, G.M.; Chen, Z.; Soragni, A.; et al. Organoids. Nat. Rev. Methods Prim. 2022, 2, 94. [Google Scholar] [CrossRef] [PubMed]
- Haendel, M.; Vasilevsky, N.; Unni, D.; Bologa, C.; Harris, N.; Rehm, H.; Hamosh, A.; Baynam, G.; Groza, T.; McMurry, J.; et al. How many rare diseases are there? Nat. Rev. Drug Discov. 2019, 19, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Means, J.C.; Martinez-Bengochea, A.L.; Louiselle, D.A.; Nemechek, J.M.; Perry, J.M.; Farrow, E.G.; Pastinen, T.; Younger, S.T. Rapid and scalable personalized ASO screening in patient-derived organoids. Nature 2025, 638, 237–243. [Google Scholar] [CrossRef]
- Mellerio, J.E. The challenges of clinical trials in rare diseases. Br. J. Dermatol. 2022, 187, 453–454. [Google Scholar] [CrossRef]
- Liu, K.; Fang, X.; Aazmi, A.; Wang, Q.; Gong, X.; Chen, Z.; Qin, M.; Pu, C.; Zhao, Y.; Qiu, X.; et al. Organoids: Principle, application and perspective. Innov. Life 2024, 2, 100088-1–100088-30. [Google Scholar] [CrossRef]
- Grass, T.; Dokuzluoglu, Z.; Buchner, F.; Rosignol, I.; Thomas, J.; Caldarelli, A.; Dalinskaya, A.; Becker, J.; Rost, F.; Marass, M.; et al. Isogenic patient-derived organoids reveal early neurodevelopmental defects in spinal muscular atrophy initiation. Cell Rep. Med. 2024, 5, 101659. [Google Scholar] [CrossRef]
- Gerli, M.F.M.; Calà, G.; Beesley, M.A.; Sina, B.; Tullie, L.; Sun, K.Y.; Panariello, F.; Michielin, F.; Davidson, J.R.; Russo, F.M.; et al. Single-cell guided prenatal derivation of primary fetal epithelial organoids from human amniotic and tracheal fluids. Nat. Med. 2024, 30, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Dayton, T.L.; Alcala, N.; Moonen, L.; Hartigh, L.D.; Geurts, V.; Mangiante, L.; Lap, L.; Dost, A.F.; Beumer, J.; Levy, S.; et al. Druggable growth dependencies and tumor evolution analysis in patient-derived organoids of neuroendocrine neoplasms from multiple body sites. Cancer Cell 2023, 41, 2083–2099.e9. [Google Scholar] [CrossRef] [PubMed]
- Omran, M.M.; Vafaei, S.; Alkhrait, S.; Ali, F.L.; Bariani, M.V.; Bai, T.; Thompson, W.E.; Yang, Q.; Ali, M.; Al-Hendy, A. Utilising Human Myometrial and Uterine Fibroid Stem Cell-Derived Three Dimentional Organoids as a Robust Model System for Understanding the Pathophysiology of Uterine Fibroids. Cell Prolif. 2025, 0, e70025. [Google Scholar] [CrossRef]
- Fang, X.; Shu, L.; Chen, T.; Zhao, X.; Yang, L.; Dou, T.; Yang, L.; Li, X.; Feng, M. Organoids derived from patients provide a new opportunity for research and individualized treatment of malignant peritoneal mesothelioma. Mol. Cancer 2024, 23, 12. [Google Scholar] [CrossRef]
- Dvela-Levitt, M.; Shaw, J.L.; Greka, A. A Rare Kidney Disease To Cure Them All? Towards Mechanism-Based Therapies for Proteinopathies. Trends Mol. Med. 2021, 27, 394–409. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, J. Advances in human organoids-on-chips in biomedical research. Life Med. 2023, 2, lnad007. [Google Scholar] [CrossRef]
- Wang, P.; Jin, L.; Zhang, M.; Wu, Y.; Duan, Z.; Guo, Y.; Wang, C.; Guo, Y.; Chen, W.; Liao, Z.; et al. Blood-brain barrier injury and neuroinflammation induced by SARS-CoV-2 in a lung-brain microphysiological system. Nat. Biomed. Eng. 2023, 8, 1053–1068. [Google Scholar] [CrossRef]
- Al-Hilal, T.A.; Keshavarz, A.; Kadry, H.; Lahooti, B.; Al-Obaida, A.; Ding, Z.; Li, W.; Kamm, R.; McMurtry, I.F.; Lahm, T.; et al. Pulmonary-arterial-hypertension (PAH)-on-a-chip: Fabrication, validation and application. Lab Chip 2020, 20, 3334–3345. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.; Wood, A.R.; Ongenaert, M.; Saidiyan, P.; Elstak, E.D.; Lanz, H.L.; Stallen, J.; Janssen, R.; Smythe, E.; Erdmann, K.S. A 3D Renal Proximal Tubule on Chip Model Phenocopies Lowe Syndrome and Dent II Disease Tubulopathy. Int. J. Mol. Sci. 2021, 22, 5361. [Google Scholar] [CrossRef]
- Rumsey, J.W.; Lorance, C.; Jackson, M.; Sasserath, T.; McAleer, C.W.; Long, C.J.; Goswami, A.; Russo, M.A.; Raja, S.M.; Gable, K.L.; et al. Classical Complement Pathway Inhibition in a “Human-On-A-Chip” Model of Autoimmune Demyelinating Neuropathies. Adv. Ther. 2022, 5, 2200030. [Google Scholar] [CrossRef]
- Hiratsuka, K.; Miyoshi, T.; Kroll, K.T.; Gupta, N.R.; Valerius, M.T.; Ferrante, T.; Yamashita, M.; Lewis, J.A.; Morizane, R. Organoid-on-a-chip model of human ARPKD reveals mechanosensing pathomechanisms for drug discovery. Sci. Adv. 2022, 8, eabq0866. [Google Scholar] [CrossRef] [PubMed]
- Afting, C.; Walther, T.; Drozdowski, O.M.; Schlagheck, C.; Schwarz, U.S.; Wittbrodt, J.; Gopfrich, K. DNA microbeads for spatio-temporally controlled morphogen release within organoids. Nat. Nanotechnol. 2024, 19, 1849–1857. [Google Scholar] [CrossRef]
- Zhao, Y.; Landau, S.; Okhovatian, S.; Liu, C.; Lu, R.X.Z.; Lai, B.F.L.; Wu, Q.; Kieda, J.; Cheung, K.; Rajasekar, S.; et al. Integrating organoids and organ-on-a-chip devices. Nat. Rev. Bioeng. 2024, 2, 588–608. [Google Scholar] [CrossRef]
- Achberger, K.; Probst, C.; Haderspeck, J.; Bolz, S.; Rogal, J.; Chuchuy, J.; Nikolova, M.; Cora, V.; Antkowiak, L.; Haq, W.; et al. Merging organoid and organ-on-a-chip technology to generate complex multi-layer tissue models in a human retina-on-a-chip platform. eLife 2019, 8, e46188. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Cui, K.; Guo, Y.; Zhang, X.; Qin, J. Advances in Hydrogels in Organoids and Organs-on-a-Chip. Adv. Mater. 2019, 31, e1902042. [Google Scholar] [CrossRef]
- Yin, F.; Zhang, X.; Wang, L.; Wang, Y.; Zhu, Y.; Li, Z.; Tao, T.; Chen, W.; Yu, H.; Qin, J. HiPSC-derived multi-organoids-on-chip system for safety assessment of antidepressant drugs. Lab Chip 2021, 21, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Shirure, V.S.; Hughes, C.C.W.; George, S.C. Engineering Vascularized Organoid-on-a-Chip Models. Annu. Rev. Biomed. Eng. 2021, 23, 141–167. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Man, K.; Liu, J.; Liu, Y.; Chen, Q.; Zhou, Y.; Yang, Y. Microphysiological Systems: Design, Fabrication, and Applications. ACS Biomater. Sci. Eng. 2020, 6, 3231–3257. [Google Scholar] [CrossRef]
- Ingber, D.E. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat. Rev. Genet. 2022, 23, 467–491. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, W.; Xu, C.; Su, W.; Li, Z. Engineering organoids-on-chips for drug testing and evaluation. Metabolism 2024, 162, 156065. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Aleman, J.; Shin, S.R.; Kilic, T.; Kim, D.; Shaegh, S.A.M.; Massa, S.; Riahi, R.; Chae, S.; Hu, N.; et al. Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. Proc. Natl. Acad. Sci. USA 2017, 114, E2293–E2302. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.K.; Ginhoux, F. Advancements in 3D models for studying human iPSC-microglia: Insights into neurodevelopment and neurological disorders. hLife 2025, in press. [Google Scholar] [CrossRef]
- Spitz, S.; Ko, E.; Ertl, P.; Kamm, R.D. How Organ-on-a-Chip Technology Can Assist in Studying the Role of the Glymphatic System in Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 2171. [Google Scholar] [CrossRef] [PubMed]
- Spitz, S.; Bolognin, S.; Brandauer, K.; Füßl, J.; Schuller, P.; Schobesberger, S.; Jordan, C.; Schädl, B.; Grillari, J.; Wanzenboeck, H.D.; et al. Development of a multi-sensor integrated midbrain organoid-on-a-chip platform for studying Parkinson’s disease. bioRxiv 2022, in press. [Google Scholar] [CrossRef]
- Hu, Y.; Sui, X.; Song, F.; Li, Y.; Li, K.; Chen, Z.; Yang, F.; Chen, X.; Zhang, Y.; Wang, X.; et al. Lung cancer organoids analyzed on microwell arrays predict drug responses of patients within a week. Nat. Commun. 2021, 12, 2581. [Google Scholar] [CrossRef]
- Cui, Y.; Xiao, R.; Zhou, Y.; Liu, J.; Wang, Y.; Yang, X.; Shen, Z.; Liang, B.; Shen, K.; Li, Y.; et al. Establishment of organoid models based on a nested array chip for fast and reproducible drug testing in colorectal cancer therapy. Bio-Des. Manuf. 2022, 5, 674–686. [Google Scholar] [CrossRef]
- Tao, T.; Deng, P.; Wang, Y.; Zhang, X.; Guo, Y.; Chen, W.; Qin, J. Microengineered Multi-Organoid System from hiPSCs to Recapitulate Human Liver-Islet Axis in Normal and Type 2 Diabetes. Adv. Sci. 2022, 9, e2103495. [Google Scholar] [CrossRef]
- Zhang, Q.; He, J.; Zhu, D.; Chen, Y.; Fu, M.; Lu, S.; Qiu, Y.; Zhou, G.; Yang, G.; Jiang, Z. Genetically modified organoids for tissue engineering and regenerative medicine. Adv. Colloid Interface Sci. 2024, 335, 103337. [Google Scholar] [CrossRef]
- Pode-Shakked, N.; Slack, M.; Sundaram, N.; Schreiber, R.; McCracken, K.W.; Dekel, B.; Helmrath, M.; Kopan, R. RAAS-deficient organoids indicate delayed angiogenesis as a possible cause for autosomal recessive renal tubular dysgenesis. Nat. Commun. 2023, 14, 8159. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, L.; Fu, X.; Liu, J.; Liang, Z.; Tan, H.; Li, W.; Zhao, Y. Engineering microcapsules to construct vascularized human brain organoids. Chem. Eng. J. 2021, 424, 130427. [Google Scholar] [CrossRef]
- Berishvili, E.; Casiraghi, F.; Amarelli, C.; Scholz, H.; Piemonti, L.; Berney, T.; Montserrat, N. Mini-organs forum: How to advance organoid technology to organ transplant community. Transpl. Int. 2021, 34, 1588–1593. [Google Scholar] [CrossRef]
- Juraski, A.C.; Sharma, S.; Sparanese, S.; da Silva, V.A.; Wong, J.; Laksman, Z.; Flannigan, R.; Rohani, L.; Willerth, S.M. 3D bioprinting for organ and organoid models and disease modeling. Expert Opin. Drug Discov. 2023, 18, 1043–1059. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Mao, X.; Wang, W.; Wang, X.; Li, S.; Wang, Z. Bioprinted research models of urological malignancy. Exploration 2024, 4, 20230126. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, X.; You, X.; Zhao, G. Harnessing the power of artificial intelligence for human living organoid research. Bioact. Mater. 2024, 42, 140–164. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Wu, Y.; Li, G.; Zhang, W.; Zhang, H.; Su, J. AI-enabled organoids: Construction, analysis, and application. Bioact. Mater. 2024, 31, 525–548. [Google Scholar] [CrossRef]
- Shi, X.; Li, Y.; Yuan, Q.; Tang, S.; Guo, S.; Zhang, Y.; He, J.; Zhang, X.; Han, M.; Liu, Z.; et al. Integrated profiling of human pancreatic cancer organoids reveals chromatin accessibility features associated with drug sensitivity. Nat. Commun. 2022, 13, 2169. [Google Scholar] [CrossRef]
- De Jongh, D.; Massey, E.K.; Consortium, V.; Bunnik, E.M. Organoids: A systematic review of ethical issues. Stem Cell Res. Ther. 2022, 13, 337. [Google Scholar] [CrossRef]
- Bredenoord, A.L.; Clevers, H.; Knoblich, J.A. Human tissues in a dish: The research and ethical implications of organoid technology. Science 2017, 355, eaaf9414. [Google Scholar] [CrossRef]
| Category | Key Information |
|---|---|
| Research background | 1. Rare diseases: Over 7000 rare diseases affect ~560 million people globally, with 80% being hereditary and 50% of them emerging in childhood. 2. Unmet needs: Only ~700 drugs (addressing 6% of rare diseases) have been approved. A total of 94% of rare diseases lack effective treatments. 3. Model flaws: Traditional models (2D cell cultures and animal models) fail to recapitulate human-specific pathologies, genetic heterogeneity, and complex microenvironments, leading to a high quantity of drug trial failures. |
| Core technologies | 1. Organoids: Organoids have self-assembled 3D structures and are used in organ development studies and the modeling of human physiopathology and drug responses. They also exhibit regenerative potential and allow patient origin models to be constructed. 2. Organ chips: These chips simulate in vivo microenvironment mechanics (shear stress, stretch, etc.), biochemistry (oxygen gradients, extracellular matrix, etc.), etc., and interact with multiple organs through microfluidic systems. Biomaterials and biosensors can easily be integrated into the chips. |
| Combined advantages | Organoids-on-chips technology: 1. Controlled integration of multiple microenvironmental factors recapitulates genetic heterogeneity (e.g., monogenic disorders, chromosomal abnormalities, etc.) and the complex pathologic phenotypes of rare diseases. 2. This multimodal research platform enables disease modeling, drug screening, real-time monitoring (using integrated sensors to detect pH, metabolite changes, etc.), and the simulation of multi-organ interactions. |
| Applications in rare diseases | 1. Disease modeling: Recapitulates development defects; captures intra-disease heterogeneity. 2. Drug development: Can be used in high-throughput screening and multi-organ toxicity/pharmacokinetics modeling. 3. Transplantation therapy: Biomimetic organoids can be used to replace dysfunctional tissues; microencapsulation strategies can be used to mitigate immune rejection. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wang, H.; You, X.; Zhao, G. Organoids-on-Chips Technology: Unveiling New Perspectives in Rare-Disease Research. Int. J. Mol. Sci. 2025, 26, 4367. https://doi.org/10.3390/ijms26094367
Li X, Wang H, You X, Zhao G. Organoids-on-Chips Technology: Unveiling New Perspectives in Rare-Disease Research. International Journal of Molecular Sciences. 2025; 26(9):4367. https://doi.org/10.3390/ijms26094367
Chicago/Turabian StyleLi, Xiangyang, Hui Wang, Xiaoyan You, and Guoping Zhao. 2025. "Organoids-on-Chips Technology: Unveiling New Perspectives in Rare-Disease Research" International Journal of Molecular Sciences 26, no. 9: 4367. https://doi.org/10.3390/ijms26094367
APA StyleLi, X., Wang, H., You, X., & Zhao, G. (2025). Organoids-on-Chips Technology: Unveiling New Perspectives in Rare-Disease Research. International Journal of Molecular Sciences, 26(9), 4367. https://doi.org/10.3390/ijms26094367






