Peumus boldus Extract Inhibits Lipid Accumulation in 3T3-L1 Adipocytes
Abstract
1. Introduction
2. Results
2.1. Preparation and Characterization of EXTs
2.1.1. Acidity Measurements
2.1.2. Antioxidant Activity and Polyphenol and Flavonoid Content
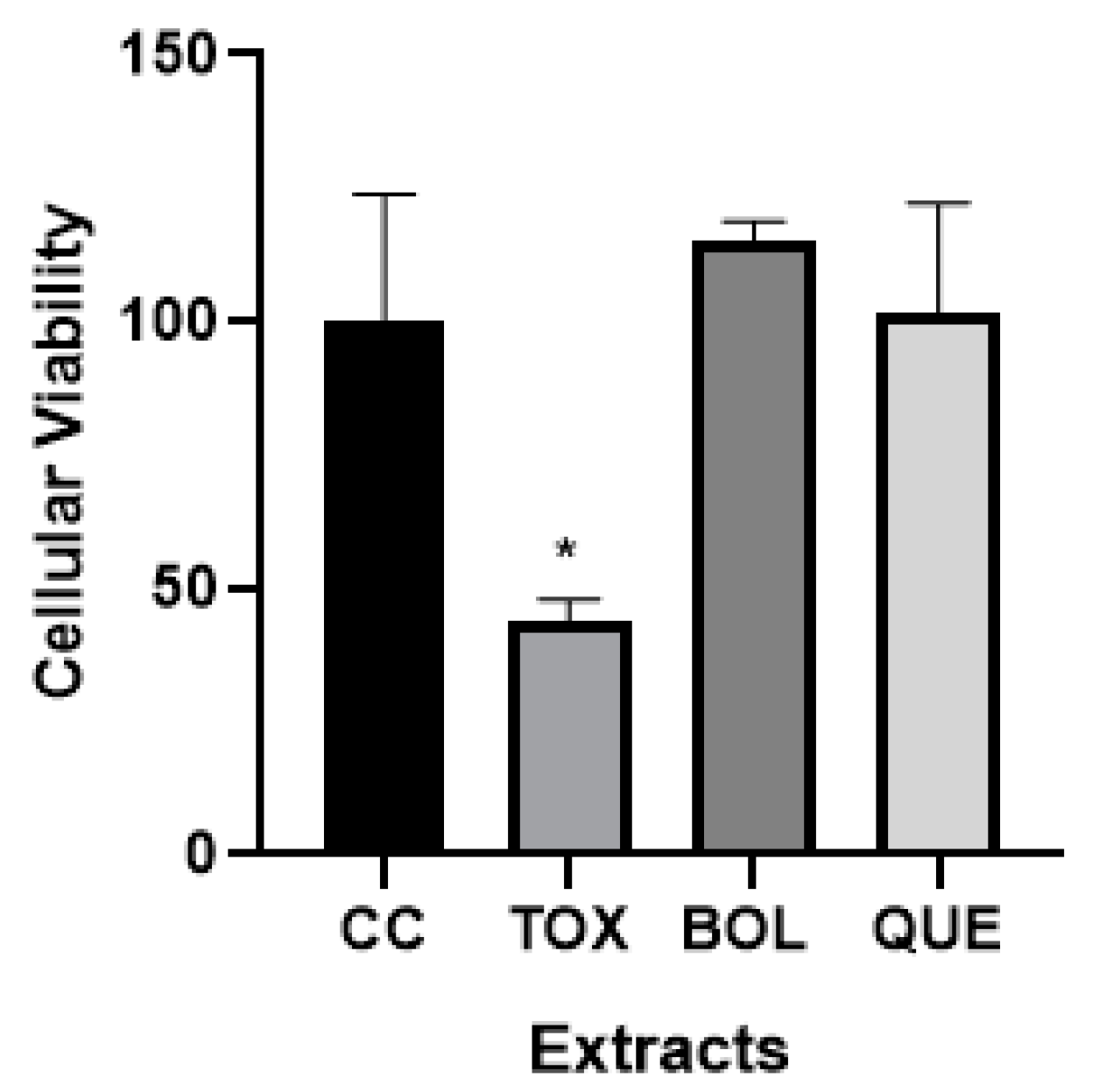
2.2. Cellular Viability
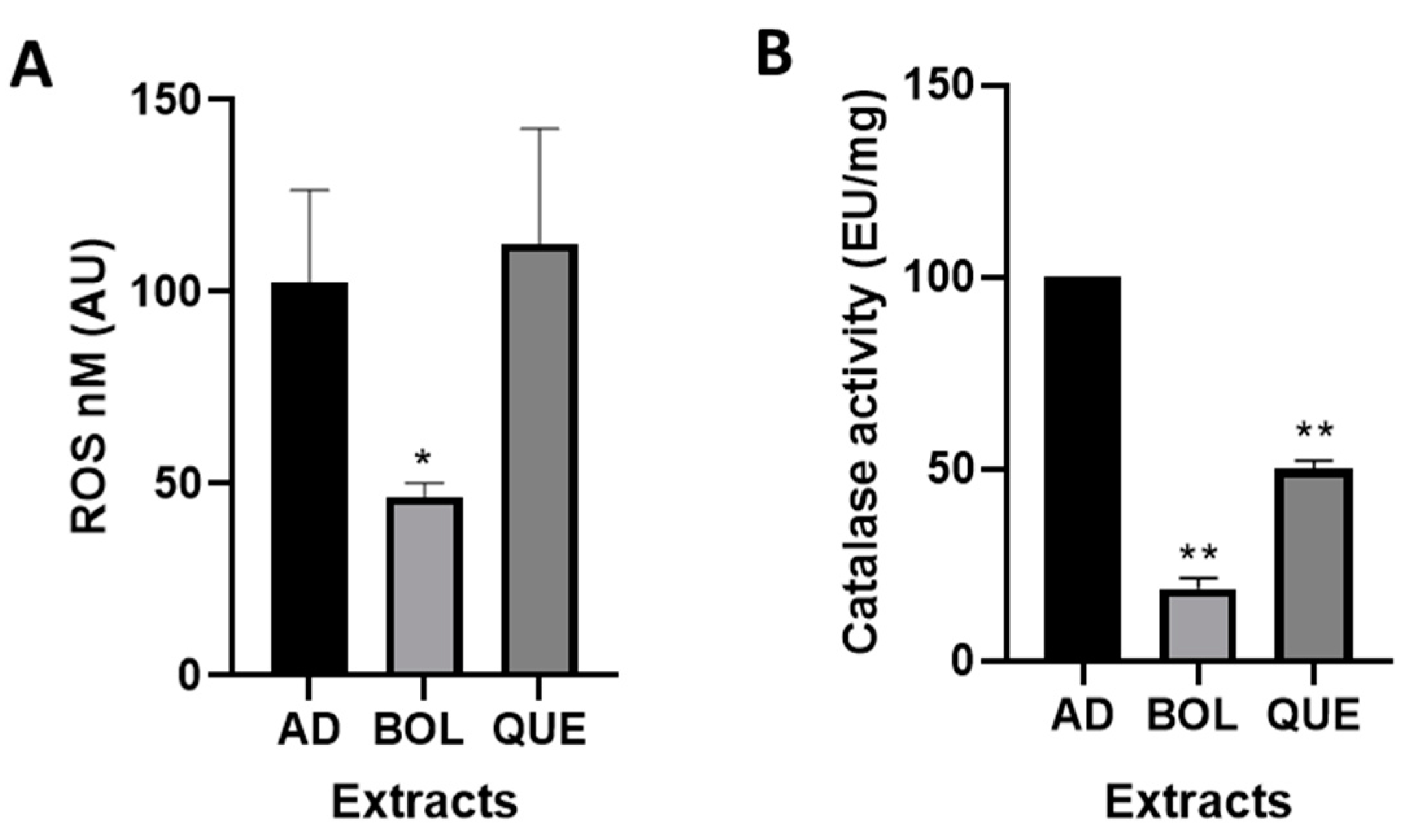
2.3. In Vitro Antioxidant Effect in 3T3-L1 Cells
Intracellular ROS and Catalase Activity
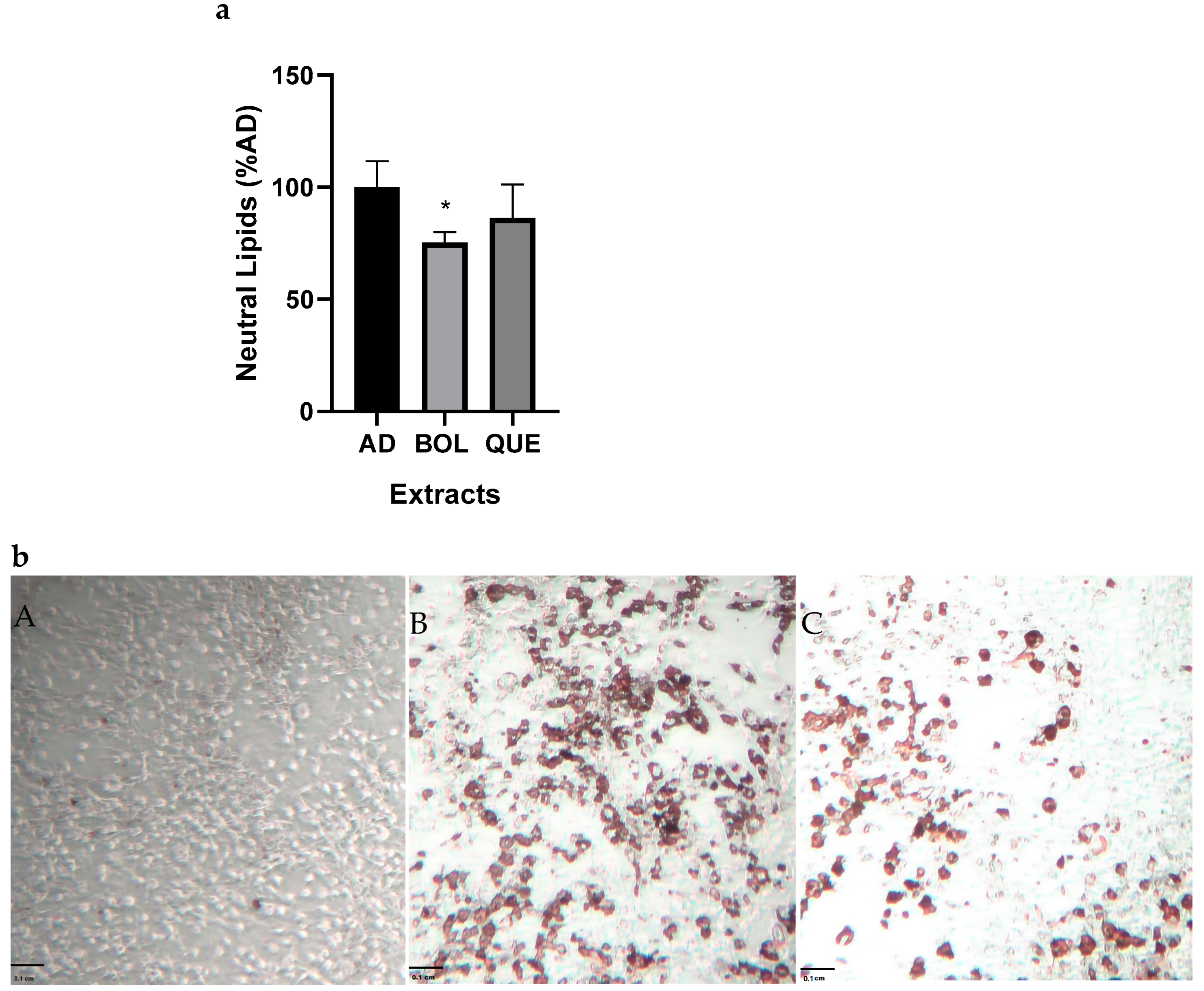
2.4. In Vitro Anti-Adipogenic Effect
2.4.1. Neutral Lipid Content
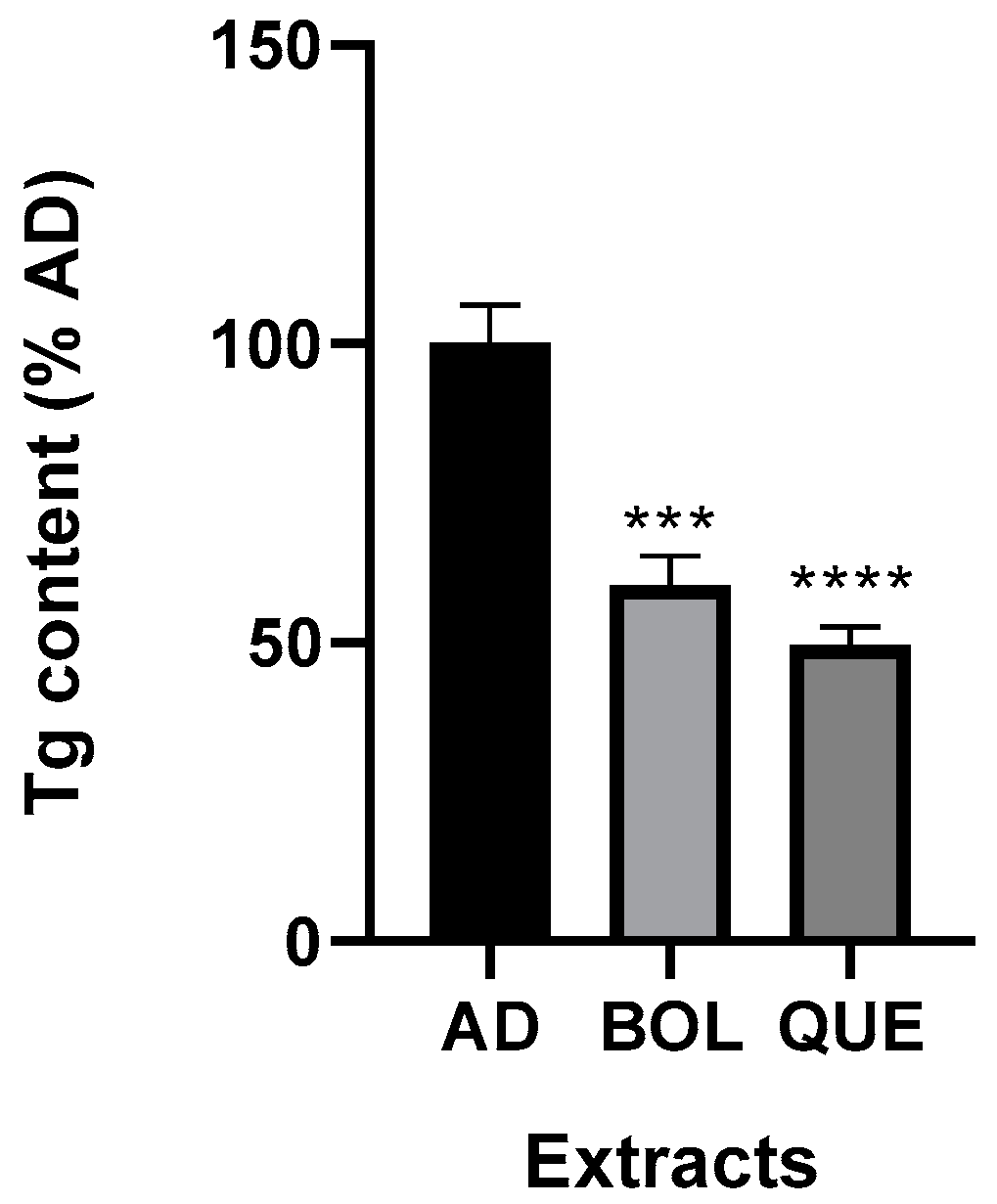
2.4.2. Intracellular Triglyceride Content
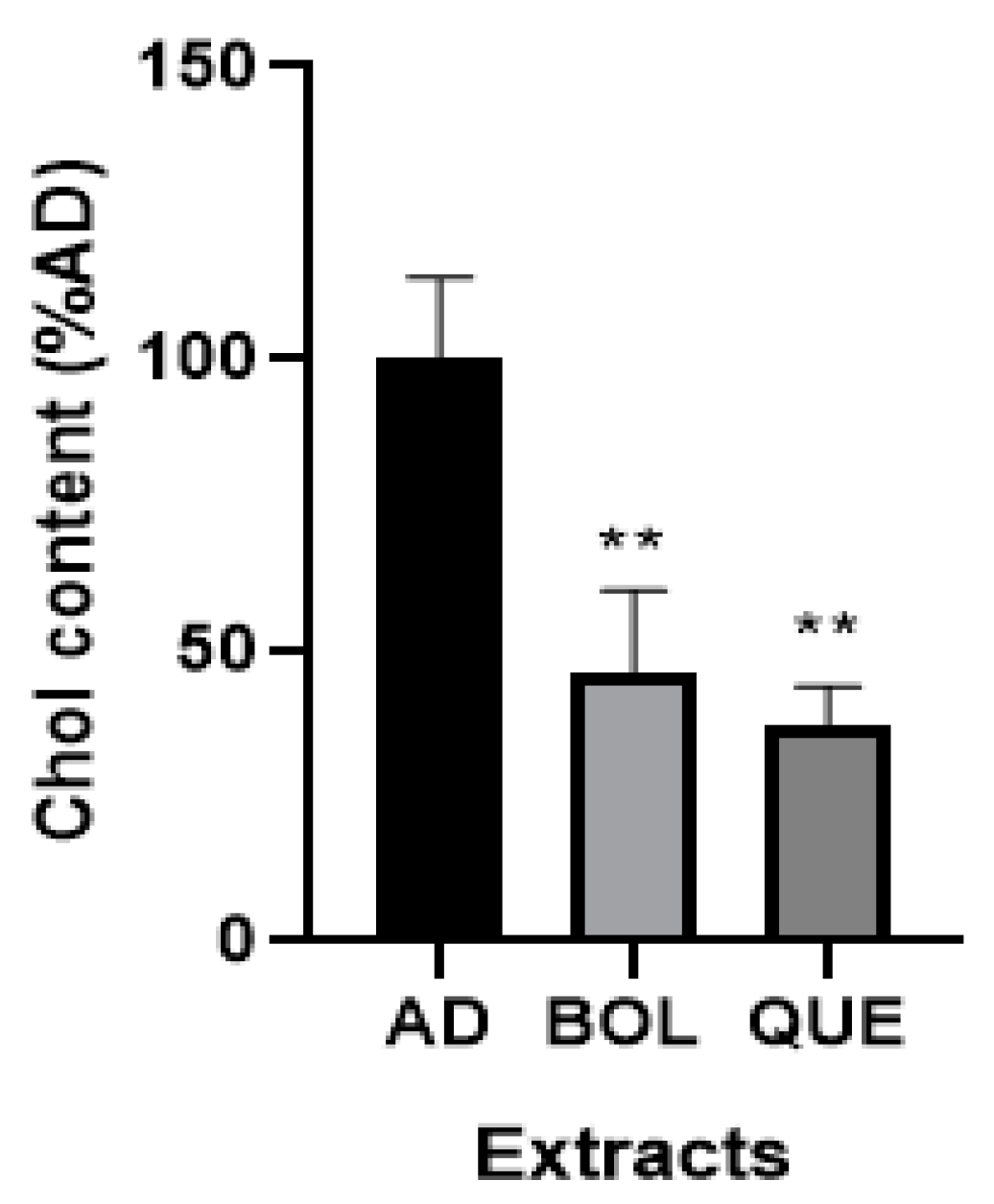
2.4.3. Intracellular Cholesterol Content
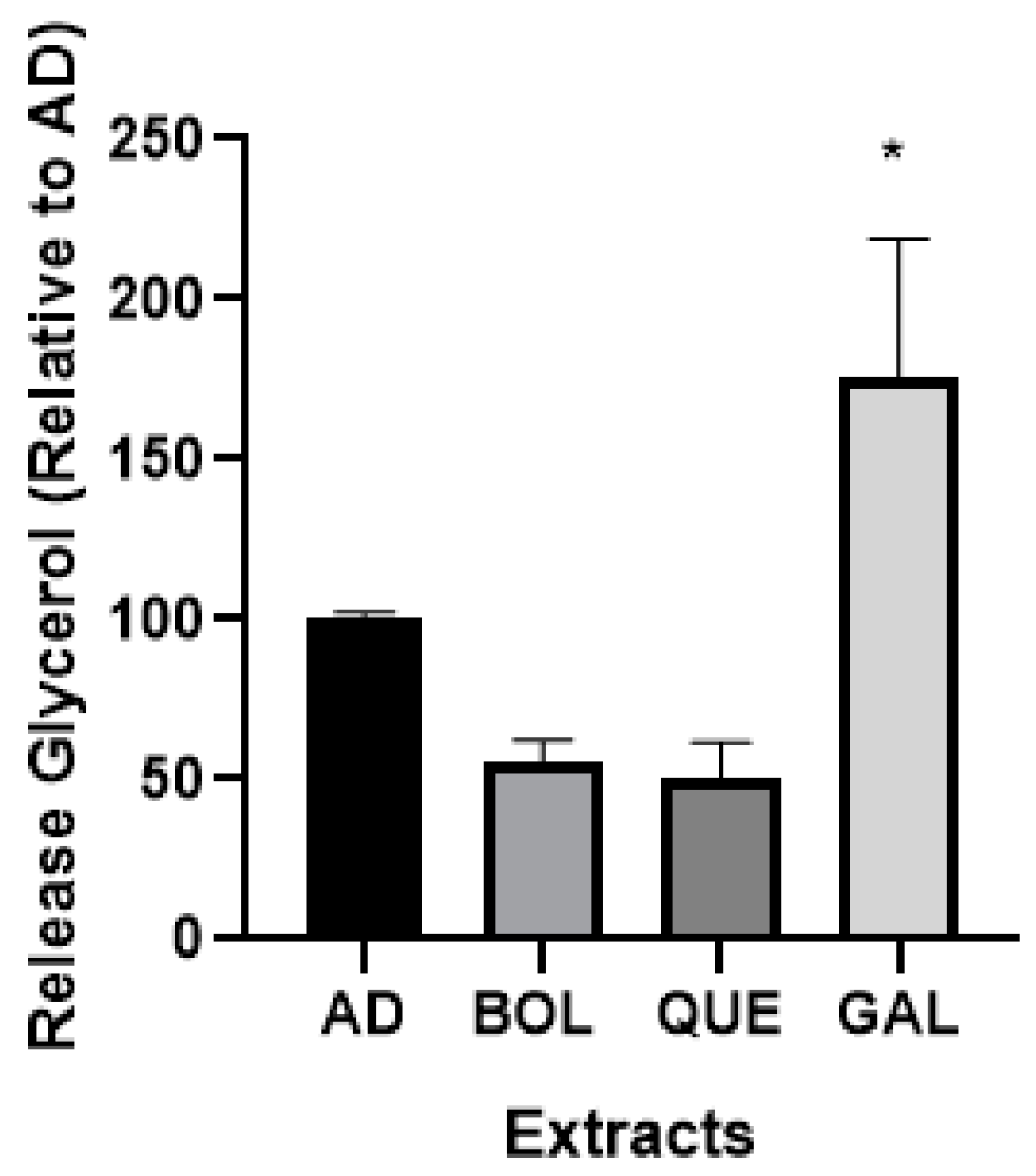
2.4.4. Glycerol Release
2.4.5. Nitrite Production
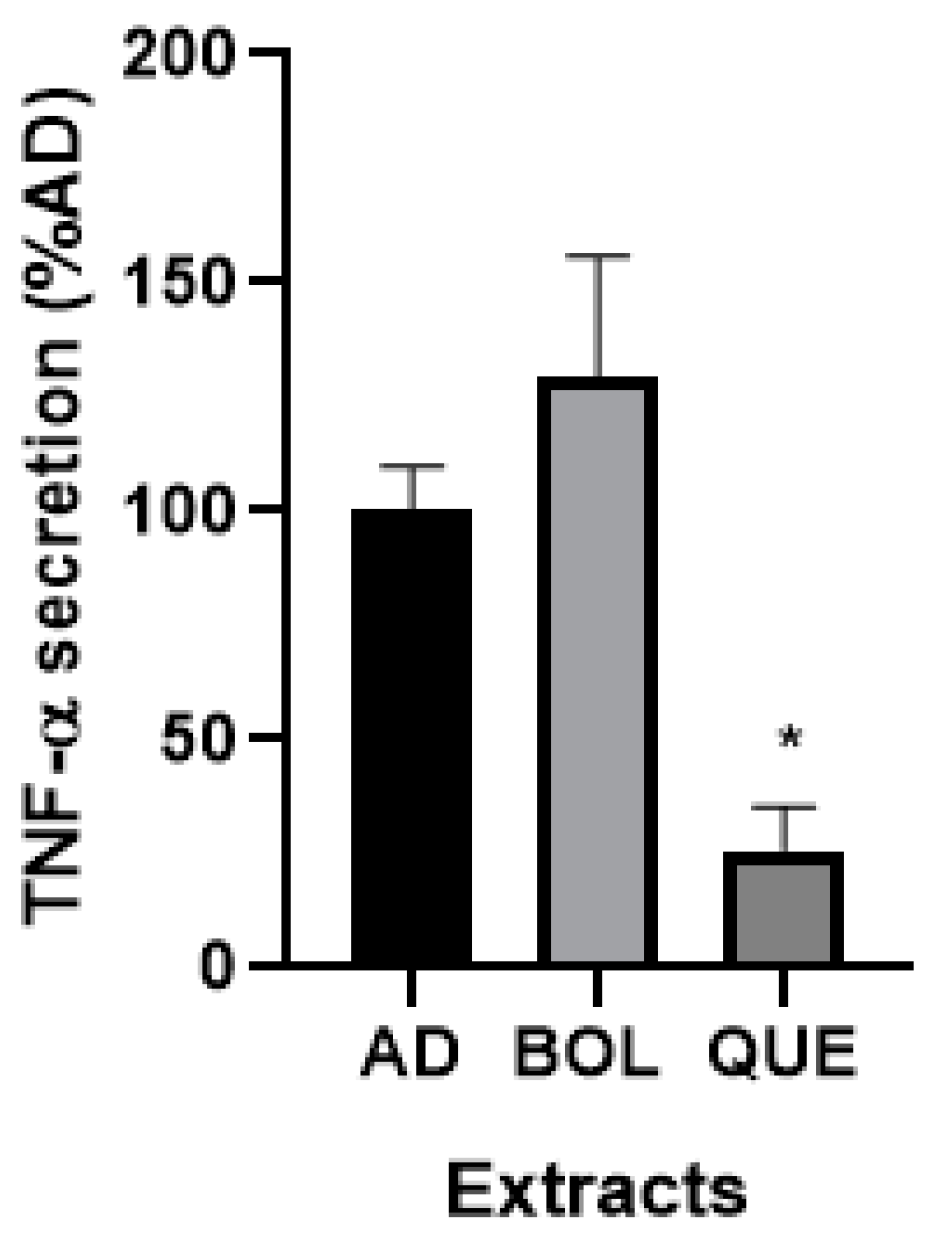
2.4.6. TNF-α Secretion
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Extract Preparation
4.3. EXT Characterization
4.4. Cell Culture and Differentiation of 3T3-L1 Preadipocytes
4.5. In Vitro Treatment
4.6. Cytotoxicity Evaluation
4.7. Determination of Intracellular ROS
4.8. Determination of Catalase Activity
4.9. Oil Red O Staining
4.10. Determination of Triglycerides and Cholesterol
4.11. Determination of Glycerol Released to Culture Medium
4.12. Nitrite Determination
4.13. TNF-α Determination
4.14. Statistical Analysis
5. Conclusions and Future Directions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Engin, A. The Definition and Prevalence of Obesity and Metabolic Syndrome. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2017; Volume 960, pp. 1–17. [Google Scholar]
- Green, H.; Kehinde, O. Sublines of Mouse 3T3 Cells That Accumulate Lipid. Cell 1974, 1, 113–116. [Google Scholar] [CrossRef]
- Poulos, S.P.; Dodson, M.V.; Hausman, G.J. Cell Line Models for Differentiation: Preadipocytes and Adipocytes. Exp. Biol. Med. 2010, 235, 1185–1193. [Google Scholar] [CrossRef]
- Soto, D.; Martini, C.N.; Frontera, E.M.; Montaldo, L.A.; Vila, M.D.C.; Calvo, J.C.; Guerra, L.N. N-Acetylcysteine Inhibits Lipids Production in Mature Adipocytes Through the Inhibition of Peroxisome Proliferator-Activated Receptor γ. Int. J. Biochem. Res. Rev. 2020, 17–29. [Google Scholar] [CrossRef]
- Swamy, M.K. Plant-Derived Bioactives: Production, Properties and Therapeutic Applications, 1st ed.; Mallappa, K., Ed.; Springer: Singapore, 2020. [Google Scholar]
- Sobhani, M.; Farzaei, M.H.; Kiani, S.; Khodarahmi, R. Immunomodulatory; Anti-Inflammatory/antioxidant Effects of Polyphenols: A Comparative Review on the Parental Compounds and Their Metabolites. Food Rev. Int. 2021, 37, 759–811. [Google Scholar] [CrossRef]
- Kiokias, S.; Proestos, C.; Oreopoulou, V. Phenolic Acids of Plant Origin-A Review on Their Antioxidant Activity in Vitro (O/W Emulsion Systems) along with Their in Vivo Health Biochemical Properties. Foods 2020, 9, 534. [Google Scholar] [CrossRef]
- John, C.M.; Arockiasamy, S. 3,5-Dimethoxy-4-Benzoic Acid (syringic Acid) a Natural Phenolic Acid Reduces Reactive Oxygen Species in Differentiated 3T3-L1 Adipocytes. Vitr. Cell. Dev. Biol. Anim. 2021, 57, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, S.; Cho, E.J. Flavonoids from Acer Okamotoanum Inhibit Adipocyte Differentiation and Promote Lipolysis in the 3T3-L1 Cells. Molecules 2020, 25, 1920. [Google Scholar] [CrossRef] [PubMed]
- Gosmann, G.; Barlette, A.G.; Dhamer, T.; Arçari, D.P.; Santos, J.C.; de Camargo, E.R.; Acedo, S.; Gambero, A.; Gnoatto, S.C.B.; Ribeiro, M.L. Phenolic Compounds from Maté (Ilex paraguariensis) Inhibit Adipogenesis in 3T3-L1 Preadipocytes. Plant Foods Hum. Nutr. 2012, 67, 156–161. [Google Scholar] [CrossRef]
- Lara-Fernández, L.; de la Garza-Toledo, H.; Wong-Paz, J.E.; Belmares, R.; Rodríguez-Herrera, R.; Aguilar, C.N. Separation Conditions and Evaluation of Antioxidant Properties of Boldo (Peumus boldus) Extracts. J. Med. Plants Res. 2013, 7, 911–917. [Google Scholar]
- Simirgiotis, M.J.; Schmeda-Hirschmann, G. Direct Identification of Phenolic Constituents in Boldo Folium (Peumus boldus Mol.) Infusions by High-Performance Liquid Chromatography with Diode Array Detection and Electrospray Ionization Tandem Mass Spectrometry. J. Chromatogr. A 2010, 1217, 443–449. [Google Scholar] [CrossRef]
- Almeida, E.R.; Melo, A.M.; Xavier, H. Toxicological Evaluation of the Hydro-Alcohol Extract of the Dry Leaves of Peumus boldus and Boldine in Rats. Phytother. Res. PTR 2000, 14, 99–102. [Google Scholar] [CrossRef]
- Fernandez, J.; Lagos, P.; Rivera, P.; Zamorano-Ponce, E. Effect of boldo (Peumus boldus Molina) infusion on lipoperoxidation induced by cisplatin in mice liver. Phytother. Res. 2009, 23, 1024–1027. [Google Scholar] [CrossRef]
- Lee, H.; Lee, Y.J.; Choi, H.; Ko, E.H.; Kim, J.-W. Reactive Oxygen Species Facilitate Adipocyte Differentiation by Accelerating Mitotic Clonal Expansion. J. Biol. Chem. 2009, 284, 10601–10609. [Google Scholar] [CrossRef] [PubMed]
- Baret, P.; Septembre-Malaterre, A.; Rigoulet, M.; d’Hellencourt, C.L.; Priault, M.; Gonthier, M.P.; Devin, A. Dietary Polyphenols Preconditioning Protects 3T3-L1 Preadipocytes from Mitochondrial Alterations Induced by Oxidative Stress. Int. J. Biochem. Cell Biol. 2013, 45, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-S.; Chan, E.C.; Higuchi, M.; Dusting, G.J.; Jiang, F. Redox Mechanisms in Regulation of Adipocyte Differentiation: Beyond a General Stress Response. Cells 2012, 1, 976–993. [Google Scholar] [CrossRef] [PubMed]
- Mayer, B. Nitric Oxide; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Kawachi, H.; Moriya, N.H.; Korai, T.; Tanaka, S.-Y.; Watanabe, M.; Matsui, T.; Kawada, T.; Yano, H. Nitric Oxide Suppresses Preadipocyte Differentiation in 3T3-L1 Culture. Mol. Cell. Biochem. 2007, 300, 61–67. [Google Scholar] [CrossRef]
- Cawthorn, W.P.; Sethi, J.K. TNF-Alpha and Adipocyte Biology. FEBS Lett. 2008, 582, 117–131. [Google Scholar] [CrossRef]
- Bradley, R.L.; Fisher, F.M.; Maratos-Flier, E. Dietary Fatty Acids Differentially Regulate Production of TNF-Alpha and IL-10 by Murine 3T3-L1 Adipocytes. Obesity 2008, 16, 938–944. [Google Scholar] [CrossRef]
- Hoareau, L.; Bencharif, K.; Rondeau, P.; Murumalla, R.; Ravanan, P.; Tallet, F.; Delarue, P.; Cesari, M.; Roche, R.; Festy, F. Signaling Pathways Involved in LPS Induced TNFalpha Production in Human Adipocytes. J. Inflamm. 2010, 7, 1. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Y.; Xiong, Z.; Yu, S.; Zhou, B.; Ling, Y.; Zheng, Z.; Shi, G.; Wu, Y.; Qian, X. Ginsenoside Rb1 Inhibits Free Fatty Acids-induced Oxidative Stress and Inflammation in 3T3-L1 Adipocytes. Mol. Med. Rep. 2017, 16, 9165–9172. [Google Scholar] [CrossRef]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An Overview of Plant Phenolic Compounds and Their Importance in Human Nutrition and Management of Type 2 Diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, K.; Olejnik, A.; Szwajgier, D.; Olkowicz, M. Inhibitory Activity of Chokeberry, Bilberry, Raspberry and Cranberry Polyphenol-Rich Extract Towards Adipogenesis and Oxidative Stress in Differentiated 3T3-L1 Adipose Cells. PLoS ONE 2017, 12, e0188583. [Google Scholar] [CrossRef]
- Montaldo, L.; Gallo, A.; Rocha, G.; Csernoch, C.; De Marzi, M.; Guerra, L.N. Anthocyanin-Enriched Extract from Ribes Nigrum Inhibits Triglyceride and Cholesterol Accumulation in Adipocytes. Ther. Deliv. 2023, 14, 675–687. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH Antioxidant Assay Revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Udomwasinakun, N.; Pirak, T.; Chanput, W.P. Identification of Polyphenols in White Mugwort (Artemisia lactiflora Wall.) Ethanolic Extracts and Their Anti-Inflammatory and Anti-Adipogenic Activity Potential. Food Biosci. 2022, 47, 101761. [Google Scholar] [CrossRef]
- Pérez-Torres, I.; Castrejón-Téllez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative Stress, Plant Natural Antioxidants, and Obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef]
- Mozaffarian, D. Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity: A Comprehensive Review. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef]
- Shabbir, U.; Rubab, M.; Daliri EB, M.; Chelliah, R.; Javed, A.; Oh, D.H. Curcumin, Quercetin, Catechins and Metabolic Diseases: The Role of Gut Microbiota. Nutrients 2021, 13, 206. [Google Scholar] [CrossRef]
- Rinella, M.E.; Sookoian, S. From NAFLD to MASLD: Updated Naming and Diagnosis Criteria for Fatty Liver Disease. J. Lipid Res. 2024, 65, 100485. [Google Scholar] [CrossRef]
- Tarantino, G.; Balsano, C.; Santini, S.J.; Brienza, G.; Clemente, I.; Cosimini, B.; Sinatti, G. It Is High Time Physicians Thought of Natural Products for Alleviating NAFLD. Is There Sufficient Evidence to Use Them? Int. J. Mol. Sci. 2021, 22, 13424. [Google Scholar] [CrossRef]
- Soto, C.; Caballero, E.; Pérez, E.; Zúñiga, M.E. Effect of Extraction Conditions on Total Phenolic Content and Antioxidant Capacity of Pretreated Wild Peumus boldus Leaves from Chile. Food Bioprod. Process. 2014, 92, 328–333. [Google Scholar] [CrossRef]
- Quezada, N.; Asencio, M.; Del Valle, J.M.; Aguilera, J.M.; Gómez, B. Antioxidant Activity of Crude Extract, Alkaloid Fraction, and Flavonoid Fraction from Boldo (Peumus boldus Molina) Leaves. J. Food Sci. 2004, 69, C371–C376. [Google Scholar] [CrossRef]
- Lee, O.-H.; Kwon, Y.-I.; Hong, H.-D.; Park, C.-S.; Lee, B.-Y.; Kim, Y.-C. Production of Reactive Oxygen Species and Changes in Antioxidant Enzyme Activities During Differentiation of 3T3-L1 Adipocyte. J. Korean Soc. Appl. Biol. Chem. 2009, 52, 70–75. [Google Scholar] [CrossRef]
- Calzadilla, P.; Gómez-Serrano, M.; García-Santos, E.; Schiappacasse, A.; Abalde, Y.; Calvo, J.; Peral, B.; Guerra, L. N-Acetylcysteine Affects Obesity-Related Protein Expression in 3T3-L1 Adipocytes. Redox Rep. Commun. Free Radic. Res. 2013, 18, 210–218. [Google Scholar] [CrossRef]
- Zhao, L.; Zheng, M.; Cai, H.; Chen, J.; Lin, Y.; Wang, F.; Wang, L.; Zhang, X.; Liu, J. The Activity Comparison of Six Dietary Flavonoids Identifies That Luteolin Inhibits 3T3-L1 Adipocyte Differentiation through Reducing ROS Generation. J. Nutr. Biochem. 2023, 112, 109208. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, L. Autofluorescence in Plants. Molecules 2020, 25, 2393. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Mazibuko-Mbeje, S.E.; Nyambuya, T.M.; Mxinwa, V.; Tiano, L.; Marcheggiani, F.; Cirilli, I.; Louw, J.; Nkambule, B.B. The Beneficial Effects of N-Acetyl Cysteine (NAC) Against Obesity Associated Complications: A Systematic Review of Pre-Clinical Studies. Pharmacol. Res. 2019, 146, 104332. [Google Scholar] [CrossRef]
- Calzadilla, P.; Sapochnik, D.; Cosentino, S.; Diz, V.; Dicelio, L.; Calvo, J.C.; Guerra, L.N. N-Acetylcysteine Reduces Markers of Differentiation in 3T3-L1 Adipocytes. Int. J. Mol. Sci. 2011, 12, 6936–6951. [Google Scholar] [CrossRef]
- Choi, D.-H.; Han, J.-H.; Yu, K.-H.; Min, H.; Lee, S.Y.; Park, K.-H.; Lee, S.-U.; Kwon, T.-H. Antioxidant and Anti-Obesity Activities of Polygonum cuspidatum Extract Through Alleviation of Lipid Accumulation on 3T3-L1 Adipocytes. J. Microbiol. Biotechnol. 2020, 30, 21–30. [Google Scholar] [CrossRef]
- Ribière, C.; Jaubert, A.M.; Sabourault, D.; Lacasa, D.; Giudicelli, Y. Insulin Stimulates Nitric Oxide Production in Rat Adipocytes. Biochem. Biophys. Res. Commun. 2002, 291, 394–399. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Georgé, S.; Brat, P.; Alter, P.; Amiot, M.J. Rapid Determination of Polyphenols and Vitamin C in Plant-Derived Products. J. Agric. Food Chem. 2005, 53, 1370–1373. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, L.M.; Almeida, M.I.G.S.; Barreiros, L.; Reis, S.; Segundo, M.A. Automatic Aluminum Chloride Method for Routine Estimation of Total Flavonoids in Red Wines and Teas. Food Anal. Methods 2011, 5, 530–539. [Google Scholar] [CrossRef]
- Soto, D.; Gomez-Serrano, M.; Pieralisi, A.; Calvo, J.C.; Peral, B.; Guerra, L.N. N-Acetylcysteine Inhibits Kinase Phosphorylation during 3T3-L1 Adipocyte Differentiation. Redox Rep. Commun. Free Radic. Res. 2017, 22, 265–271. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Martini, C.; Sosa, F.N.; Malvicini, R.; Pacienza, N.; Yannarelli, G.; Vila, M.D.C. Alendronate Inhibits Triglyceride Accumulation and Oxidative Stress in Adipocytes and the Inflammatory Response of Macrophages Which Are Associated with Adipose Tissue Dysfunction. J. Physiol. Biochem. 2021, 77, 601–611. [Google Scholar] [CrossRef]
- Frontera, E.; Desimone, M.F.; De Marzi, M.C.; Guerra, L.N. N-Acetylcysteine Delivery with Silica Nanoparticles into 3T3-L1 Adipocytes. Ther. Deliv. 2021, 12, 287–296. [Google Scholar] [CrossRef]
- Miler, E.A.; Ríos de Molina, M.D.C.; Domínguez, G.; Guerra, L.N. Thyroid Hormone Effect in Human Hepatocytes. Redox Rep. Commun. Free Radic. Res. 2008, 13, 185–191. [Google Scholar] [CrossRef]
- Pieralisi, A.; Martini, C.; Soto, D.; Vila, M.; Calvo, J.; Guerra, L. N-Acetylcysteine Inhibits Lipid Accumulation in Mouse Embryonic Adipocytes. Redox Biol. 2016, 9, 39–44. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, X.; Broderick, M.; Fein, H. Measurement of Nitric Oxide Production in Biological Systems by Using Griess Reaction Assay. Sensors 2003, 3, 276–284. [Google Scholar] [CrossRef]
- Oliveira Sá, A; Pimentel, T; Oliveira, N. Boldo-Induced Hepatotoxicity: A Case of Unexplained Jaundice. Eur. J. Case Rep. Intern. Med. 2020, 7, 002116. [CrossRef]
- Izzo, A.A.; Di Carlo, G.; Borrelli, F.; Ernst, E. Cardiovascular pharmacotherapy and herbal medicines: The risk of drug interaction. Int. J. Cardiol. 2005, 98, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Carbajal, R.; Yisfalem, A.; Pradhan, N.; Baumstein, D.; Chaudhari, A. Case report: Boldo (Peumus boldus) and tacrolimus interaction in a renal transplant patient. Transpl. Proc. 2014, 46, 2400–2402. [Google Scholar] [CrossRef]

| Extracts | Antioxidant Activity | Polyphenols (mg GAE/mL) | Flavonoids (mg RE/mL) |
|---|---|---|---|
| Boldo | 82.83 ± 2.86 | 0.20 ± 0.00 | 1.30 ± 0.02 |
| Quebracho | 77.07 ± 0.74 | 7.82 ± 0.17 | 5.32 ± 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montaldo, L.; Bendezu Meza, L.; De Marzi, M.; Guerra, L.N. Peumus boldus Extract Inhibits Lipid Accumulation in 3T3-L1 Adipocytes. Int. J. Mol. Sci. 2025, 26, 4326. https://doi.org/10.3390/ijms26094326
Montaldo L, Bendezu Meza L, De Marzi M, Guerra LN. Peumus boldus Extract Inhibits Lipid Accumulation in 3T3-L1 Adipocytes. International Journal of Molecular Sciences. 2025; 26(9):4326. https://doi.org/10.3390/ijms26094326
Chicago/Turabian StyleMontaldo, Laura, Llerson Bendezu Meza, Mauricio De Marzi, and Liliana Noemi Guerra. 2025. "Peumus boldus Extract Inhibits Lipid Accumulation in 3T3-L1 Adipocytes" International Journal of Molecular Sciences 26, no. 9: 4326. https://doi.org/10.3390/ijms26094326
APA StyleMontaldo, L., Bendezu Meza, L., De Marzi, M., & Guerra, L. N. (2025). Peumus boldus Extract Inhibits Lipid Accumulation in 3T3-L1 Adipocytes. International Journal of Molecular Sciences, 26(9), 4326. https://doi.org/10.3390/ijms26094326






