NAT10 Regulates LPS-Induced Inflammation via Stabilization of N4-Acetylated PTX3 mRNA in Human Dental Pulp Stem Cells
Abstract
1. Introduction
2. Results
2.1. Expression of NAT10 Is Upregulated in Inflamed Dental Pulp and LPS-Treated hDPSCs
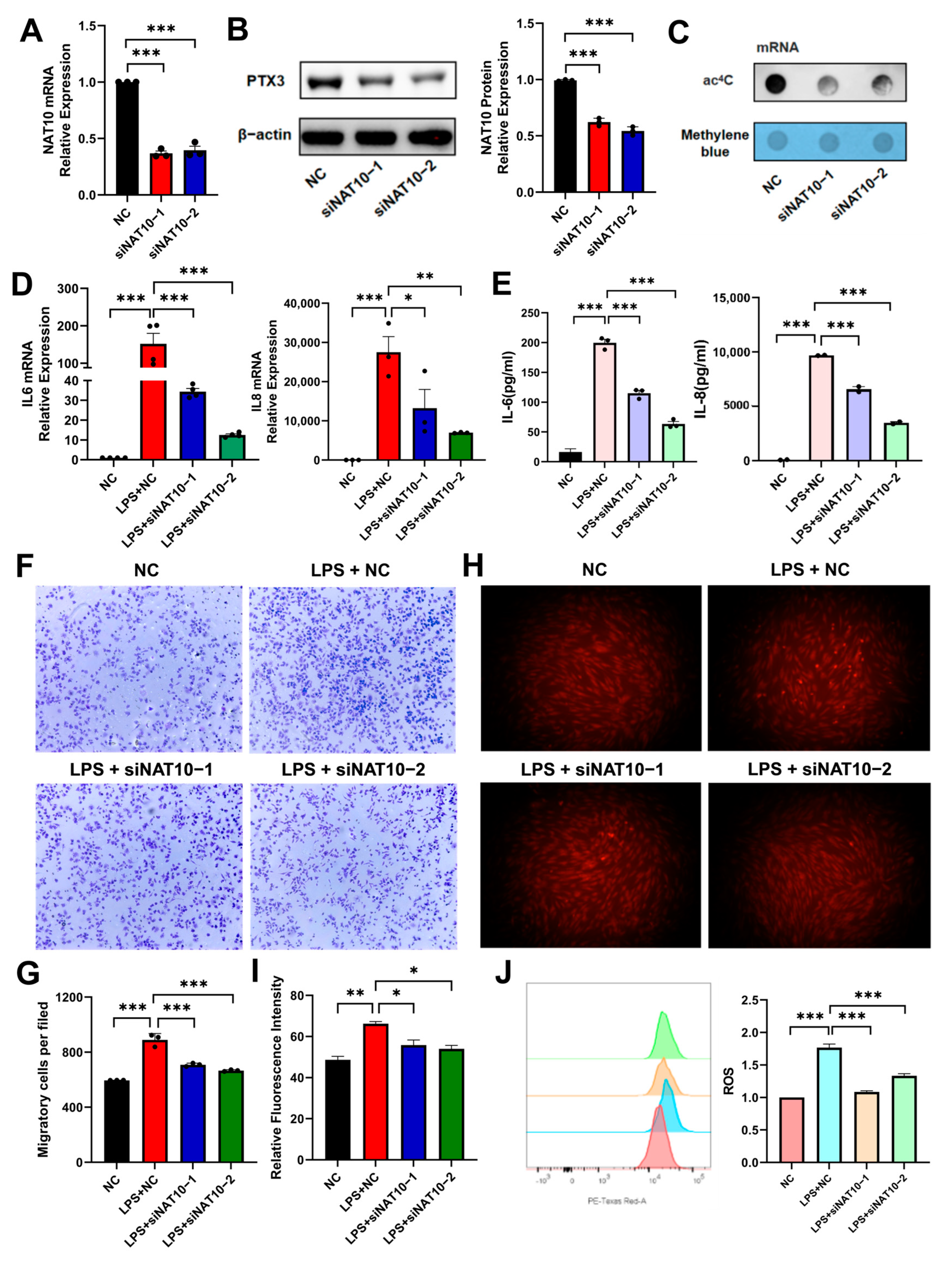
2.2. NAT10 Knockdown Attenuates LPS-Induced Inflammation in hDPSCs
2.3. Inhibition of NAT10 Represses LPS-Activated NF-Kappa B and MAPK Signaling Pathways in hDPSCs
2.4. PTX3 May Be an ac4C -Modified Target of NAT10 in hDPSC Inflammation
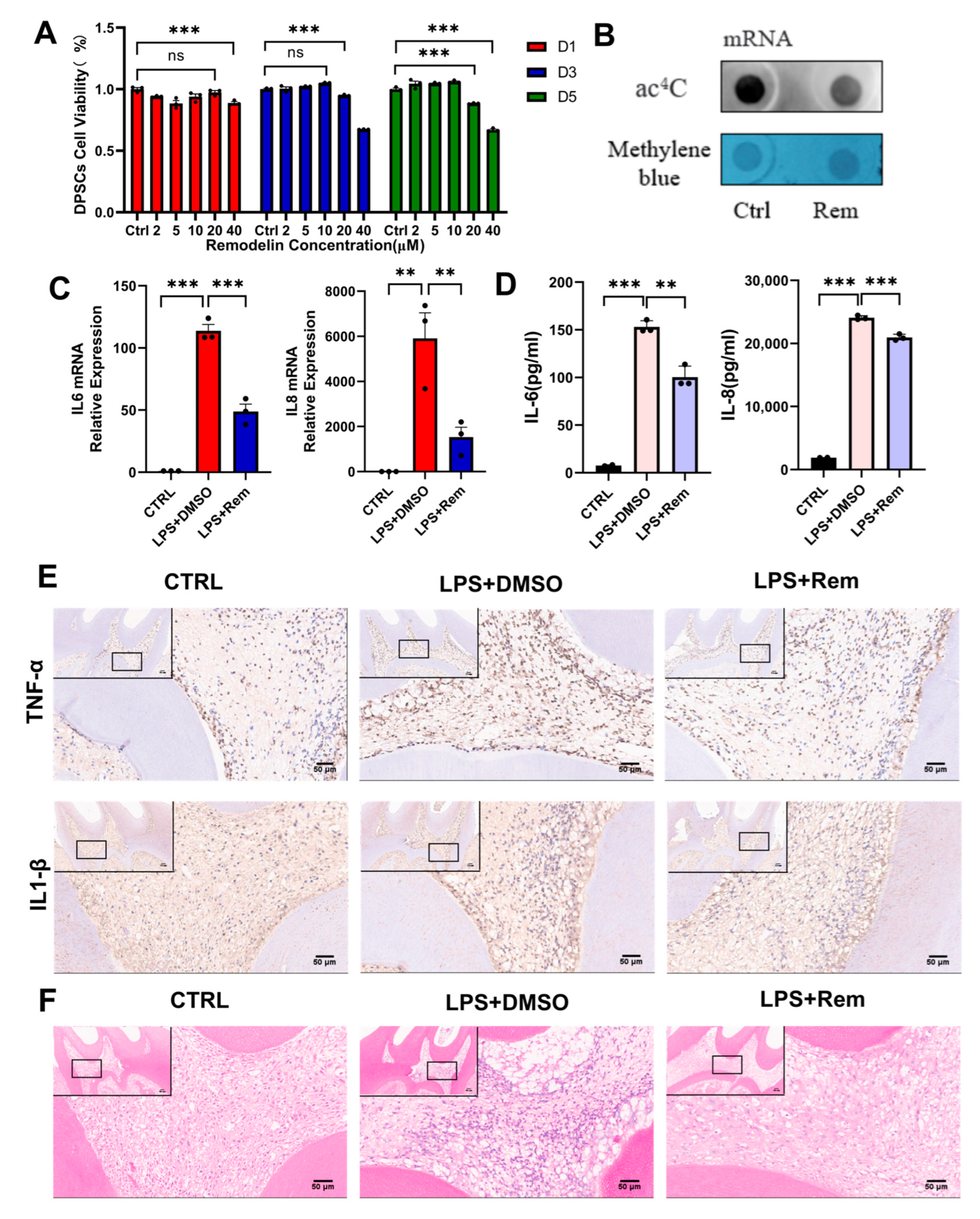
2.5. Remodelin Alleviates hDPSC Inflammation Induced by LPS and Dental Pulp Inflammation of Rats with Pulpitis
3. Discussion
4. Materials and Methods
4.1. Collection of Clinical Samples
4.2. Culture of Primary Human Dental Pulp Stem Cells (hDPSCs)
4.3. Induction of Osteogenic and Adipogenic Differentiation of hDPSCs
4.4. Alizarin Red (ARS) and Oil Red O Staining
4.5. Flow Cytometric Analysis
4.6. Immunohistochemistry
4.7. Cell Proliferation Assay
4.8. Cell Transfection
4.9. Real-Time Quantitative Polymerase Chain Reaction (qRT-PCR)
4.10. Western Blot
4.11. Enzyme-Linked Immunosorbent Assay (ELISA)
4.12. Transwell Chemotactic Migration Assay
4.13. Measurement of Reactive Oxygen Species (ROS) Expression
4.14. RNA Sequencing
4.15. RNA ac4C Dot Blot Assay
4.16. RNA-Binding Protein Immunoprecipitation (RIP)
4.17. Assessment of the mRNA Stability
4.18. Pulpitis Model in Vivo
4.19. H&E, Immunohistochemistry and Immunofluorescence Staining
4.20. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, D.; Dong, W.; Cong, Y.; Liu, Y.; Liang, Y.; Ye, Z.; Zhang, J.; Zhou, Y. LIF Aggravates Pulpitis by Promoting Inflammatory Response in Macrophages. Inflammation 2024, 47, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C.; Chang, S.H.; Tsai, Y.L.; Pan, Y.H.; Yeung, S.Y.; Chang, H.H.; Jeng, J.H. Inducing phospholipase A2 and cyclooxygenase-2 expression and prostaglandins’ production of human dental pulp cells by activation of NOD receptor and its downstream signaling. Int. J. Biol. Macromol. 2025, 292, 139193. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.; Li, Q.; Cai, L.; Zhan, M.; Xu, Q. The effect of DNA methylation on the miRNA expression pattern in lipopolysaccharide-induced inflammatory responses in human dental pulp cells. Mol. Immunol. 2019, 111, 11–18. [Google Scholar] [CrossRef]
- Sun, G.; Ren, Q.; Bai, L.; Zhang, L. Phoenixin-20 suppresses lipopolysaccharide-induced inflammation in dental pulp cells. Chem.-Biol. Interact. 2020, 318, 108971. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, X.; Wang, Y.; Yan, Y.; Wang, Y.; Su, M.; Lv, H.; Li, K.; Hao, X.; Xing, X.; et al. Application of lipopolysaccharide in establishing inflammatory models. Int. J. Biol. Macromol. 2024, 279, 135371. [Google Scholar] [CrossRef]
- Bindal, P.; Ramasamy, T.S.; Kasim, N.H.A.; Gnanasegaran, N.; Chai, W.L. Immune responses of human dental pulp stem cells in lipopolysaccharide-induced microenvironment. Cell Biol. Int. 2018, 42, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Nara, K.; Kawashima, N.; Noda, S.; Fujii, M.; Hashimoto, K.; Tazawa, K.; Okiji, T. Anti-inflammatory roles of microRNA 21 in lipopolysaccharide-stimulated human dental pulp cells. J. Cell. Physiol. 2019, 234, 21331–21341. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, C.; Chen, Z.; Yue, L.; Yu, Q.; Hou, B.; Ling, J.; Liang, J.; Wei, X.; Chen, W.; et al. Expert consensus on pulpotomy in the management of mature permanent teeth with pulpitis. Int. J. Oral. Sci. 2025, 17, 4. [Google Scholar] [CrossRef]
- Bucchi, C.; Bucchi, A.; Martínez-Rodríguez, P. Biological Properties of Dental Pulp Stem Cells Isolated from Inflamed and Healthy Pulp and Cultured in an Inflammatory Microenvironment. J. Endod. 2023, 49, 395–401.e6. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, X.; Dai, R.; Li, Q.; Cao, C.Y. LL-37 regulates odontogenic differentiation of dental pulp stem cells in an inflammatory microenvironment. Stem. Cell Res. Ther. 2024, 15, 469. [Google Scholar] [CrossRef] [PubMed]
- Sonmez Kaplan, S.; Sazak Ovecoglu, H.; Genc, D.; Akkoc, T. TNF-α, IL-1B and IL-6 affect the differentiation ability of dental pulp stem cells. BMC Oral. Health 2023, 23, 555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, T.; Li, X.; Zhang, Y.; Zou, X.; Chen, F.; Yue, L. Single-cell atlas of dental pulp stem cells exposed to the oral bacteria Porphyromonas gingivalis and Enterococcus faecalis. Front. Cell Dev. Biol. 2023, 11, 1166934. [Google Scholar] [CrossRef] [PubMed]
- Cooper, P.R.; Takahashi, Y.; Graham, L.W.; Simon, S.; Imazato, S.; Smith, A.J. Inflammation–regeneration interplay in the dentine–pulp complex. J. Dent. 2010, 38, 687–697. [Google Scholar] [CrossRef]
- Wang, F.; Han, Y.; Xi, S.; Lu, Y. Catechins reduce inflammation in lipopolysaccharide-stimulated dental pulp cells by inhibiting activation of the NF-κB pathway. Oral. Dis. 2020, 26, 815–821. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhang, Y.; She, Y.; Yuan, G.; Yang, G. IPO7 promotes lipopolysaccharide-induced inflammatory responses in human dental pulp cells via p38 MAPK and NF-κB signaling pathways. Mol. Immunol. 2023, 163, 116–126. [Google Scholar] [CrossRef]
- Cooper, P.R.; Chicca, I.J.; Holder, M.J.; Milward, M.R. Inflammation and Regeneration in the Dentin-pulp Complex: Net Gain or Net Loss? J. Endod. 2017, 43, S87–S94. [Google Scholar] [CrossRef]
- Zhu, M.; Ding, Q.; Lin, Z.; Chen, X.; Chen, S.; Zhu, Y. New insights of epigenetics in vascular and cellular senescence. J. Transl. Intern. Med. 2021, 9, 239–248. [Google Scholar] [CrossRef]
- Feng, J.; Liu, Y.; Li, K.; Wu, Y. Challenges and opportunities in targeting epigenetic mechanisms for pulmonary arterial hypertension treatment. Int. J. Pharm. 2025, 672, 125332. [Google Scholar] [CrossRef]
- Li, P.; Lin, Y.; Ma, H.; Zhang, J.; Zhang, Q.; Yan, R.; Fan, Y. Epigenetic regulation in female reproduction: The impact of m6A on maternal-fetal health. Cell Death Discov. 2025, 11, 43. [Google Scholar] [CrossRef]
- Zhang, Y.; Lei, Y.; Dong, Y.; Chen, S.; Sun, S.; Zhou, F.; Zhao, Z.; Chen, B.; Wei, L.; Chen, J.; et al. Emerging roles of RNA ac4C modification and NAT10 in mammalian development and human diseases. Pharmacol. Ther. 2024, 253, 108576. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Xu, Y.; Wu, P.; Lu, Y.; Tao, Y.; Zhou, C.; Cui, R.; Li, J.; Han, R. NAT10 promotes osteogenic differentiation of periodontal ligament stem cells by regulating VEGFA-mediated PI3K/AKT signaling pathway through ac4C modification. Odontology 2023, 111, 870–882. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jing, Y.; Wang, Y.; Tang, J.; Zhu, X.; Jin, W.-L.; Wang, Y.; Yuan, W.; Li, X.; Li, X. NAT10 promotes gastric cancer metastasis via N4-acetylated COL5A1. Sig. Transduct. Target. Ther. 2021, 6, 173. [Google Scholar] [CrossRef]
- Wei, R.; Cui, X.; Min, J.; Lin, Z.; Zhou, Y.; Guo, M.; An, X.; Liu, H.; Janz, S.; Gu, C.; et al. NAT10 promotes cell proliferation by acetylating CEP170 mRNA to enhance translation efficiency in multiple myeloma. Acta. Pharm. Sin. B 2022, 12, 3313–3325. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Z.; Zhou, J.; Gu, J.; Wu, H.; Jiang, Y.; Gao, S.; Liao, Y.; Shen, R.; Miao, C.; et al. NAT10 regulates neutrophil pyroptosis in sepsis via acetylating ULK1 RNA and activating STING pathway. Commun. Biol. 2022, 5, 916. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Cai, Y.; Li, D.; He, J.; Feng, Z.; Xu, Q. NAT10 regulates the LPS-induced inflammatory response via the NOX2-ROS-NF-κB pathway in macrophages. Biochim. Biophys. Acta (BBA)—Mol. Cell Research 2023, 1870, 119521. [Google Scholar] [CrossRef] [PubMed]
- Galler, K.M.; Weber, M.; Korkmaz, Y.; Widbiller, M.; Feuerer, M. Inflammatory Response Mechanisms of the Dentine–Pulp Complex and the Periapical Tissues. IJMS 2021, 22, 1480. [Google Scholar] [CrossRef]
- Giraud, T. Pulp capping materials modulate the balance between inflammation and regeneration. Dent. Mater. 2019, 35, 24–35. [Google Scholar] [CrossRef]
- Chen, J.; Xu, H.; Xia, K.; Cheng, S.; Zhang, Q. Resolvin E1 accelerates pulp repair by regulating inflammation and stimulating dentin regeneration in dental pulp stem cells. Stem. Cell Res. Ther. 2021, 12, 75. [Google Scholar] [CrossRef]
- Arango, D.; Sturgill, D.; Alhusaini, N.; Dillman, A.A.; Sweet, T.J.; Hanson, G.; Hosogane, M.; Sinclair, W.R.; Nanan, K.K.; Mandler, M.D.; et al. Acetylation of Cytidine in mRNA Promotes Translation Efficiency. Cell 2018, 175, 1872–1886. [Google Scholar] [CrossRef]
- Luo, J.; Cao, J.; Chen, C.; Xie, H. Emerging role of RNA acetylation modification ac4C in diseases: Current advances and future challenges. Biochem. Pharmacol. 2023, 213, 115628. [Google Scholar] [CrossRef]
- Yang, W.; Li, H.Y.; Wu, Y.F.; Mi, R.; Liu, W.; Shen, X.; Lu, Y.; Jiang, Y.; Ma, M.; Shen, H. ac4C acetylation of RUNX2 catalyzed by NAT10 spurs osteogenesis of BMSCs and prevents ovariectomy-induced bone loss. Mol. Ther.—Nucleic Acids 2021, 26, 135–147. [Google Scholar] [CrossRef]
- Li, J.; Yushanjiang, F.; Fang, Z.; Liu, W. NAT10-mediated RNA ac4C acetylation contributes to the myocardial infarction-induced cardiac fibrosis. J. Cell. Mol. Med. 2024, 28, e70141. [Google Scholar] [CrossRef] [PubMed]
- Xing, P.; Zhou, M.; Sun, J.; Wang, D.; Huang, W.; An, P. NAT10-mediated ac4C acetylation of TFRC promotes sepsis-induced pulmonary injury through regulating ferroptosis. Mol. Med. 2024, 30, 140. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Zhang, Y.; Wang, D.; Xu, L.; Li, Z.; Bai, X.; Wang, Y. Targeting NAT10 protects against sepsis-induced skeletal muscle atrophy by inhibiting ROS/NLRP3. Life Sci. 2023, 330, 121948. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-P.; Mao, X.-T.; Xie, J.-H.; Li, J.-Y.; Liu, B.-Q.; Wu, L.-X.; Yang, B.; Li, Y.-Y.; Jin, J. N-acetyltransferase 10 is implicated in the pathogenesis of cycling T cell-mediated autoimmune and inflammatory disorders in mice. Nat. Commun. 2024, 15, 9388. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, C.-L.; Li, R.-M.; Hui, T.-Q.; Su, Y.-Y.; Yuan, Q.; Zhou, X.-D.; Ye, L. Wnt5a Promotes Inflammatory Responses via Nuclear Factor κB (NF-κB) and Mitogen-activated Protein Kinase (MAPK) Pathways in Human Dental Pulp Cells J. Biol. Chem. 2014, 289, 21028–21039. [Google Scholar] [CrossRef]
- Le Fournis, C.; Jeanneau, C.; Giraud, T.; El Karim, I.; Lundy, F.T.; About, I. Fibroblasts Control Macrophage Differentiation during Pulp Inflammation. J. Endod. 2021, 47, 1427–1434. [Google Scholar] [CrossRef]
- Chen, A.; Huang, H.; Fang, S.; Hang, Q. ROS: A “booster” for chronic inflammation and tumor metastasis. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2024, 1879, 189175. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, Y.; Chen, J.; Hu, D. Revolutionizing oral care: Reactive oxygen species (ROS)-Regulating biomaterials for combating infection and inflammation. Redox Biol. 2025, 79, 103451. [Google Scholar] [CrossRef]
- Botero, T.M.; Son, J.S.; Vodopyanov, D.; Hasegawa, M.; Shelburne, C.E.; Nör, J.E. MAPK Signaling Is Required for LPS-induced VEGF in Pulp Stem Cells. J. Dent. Res. 2010, 89, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Chen, L.; Wu, J.; Lin, Z.; Huang, S. Long noncoding RNA MEG3 expressed in human dental pulp regulates LPS-Induced inflammation and odontogenic differentiation in pulpitis. Exp. Cell Res. 2021, 400, 112495. [Google Scholar] [CrossRef]
- Ye, X.; Wang, Z.; Lei, W.; Shen, M.; Tang, J.; Xu, X.; Yang, Y.; Zhang, H. Pentraxin 3: A promising therapeutic target for cardiovascular diseases. Ageing Res. Rev. 2024, 93, 102163. [Google Scholar] [CrossRef]
- Massimino, A.M.; Colella, F.E.; Bottazzi, B.; Inforzato, A. Structural insights into the biological functions of the long pentraxin PTX3. Front. Immunol. 2023, 14, 1274634. [Google Scholar] [CrossRef]
- Bozza, S.; Bistoni, F.; Gaziano, R.; Pitzurra, L.; Zelante, T.; Bonifazi, P.; Perruccio, K.; Bellocchio, S.; Neri, M.; Iorio, A.M.; et al. Pentraxin 3 protects from MCMV infection and reactivation through TLR sensing pathways leading to IRF3 activation. Blood 2006, 108, 3387–3396. [Google Scholar] [CrossRef] [PubMed]
- Deban, L.; Russo, R.C.; Sironi, M.; Moalli, F.; Scanziani, M.; Zambelli, V.; Cuccovillo, I.; Bastone, A.; Gobbi, M.; Valentino, S.; et al. Regulation of leukocyte recruitment by the long pentraxin PTX3. Nat. Immunol. 2010, 11, 328–334. [Google Scholar] [CrossRef]
- Kim, Y.; Park, J.-S.; Park, H.-J.; Kim, M.-K.; Kim, Y.-I.; Bae, S.-K.; Kim, H.J.; Jeong, C.-H.; Bae, M.-K. Pentraxin 3 Modulates the Inflammatory Response in Human Dental Pulp Cells. J. Endod. 2018, 44, 1826–1831. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Kuang, Y.; Chen, S.; Li, R.; Su, F.; Zhang, S.; Qiu, Q.; Lin, S.; Shen, C.; Liu, Y.; et al. NAT10 promotes synovial aggression by increasing the stability and translation of N4-acetylated PTX3 mRNA in rheumatoid arthritis. Ann. Rheum. Dis. 2024, 83, 1118–1131. [Google Scholar] [CrossRef]
- Huang, H.; Okamoto, M.; Watanabe, M.; Matsumoto, S.; Moriyama, K.; Komichi, S.; Ali, M.; Matayoshi, S.; Nomura, R.; Nakano, K.; et al. Development of Rat Caries-Induced Pulpitis Model for Vital Pulp Therapy. J. Dent. Res. 2023, 102, 574–582. [Google Scholar] [CrossRef]
- Zhai, Y.; Yuan, X.; Zhao, Y.; Ge, L.; Wang, Y. Potential Application of Human β-Defensin 4 in Dental Pulp Repair. Front. Physiol. 2020, 11, 1077. [Google Scholar] [CrossRef]



| siRNA | Sequences (5′–3′) |
|---|---|
| #1 siRNA | GCACCACUGCUGAGAAUAATT |
| UUAUUCUCAGCAGUGGUGCTG | |
| #2 siRNA | GCUGCUGCAGAUGUACUAUTT |
| AUAGUACAUCUGCAGCAGCTG |
| Gene | Forward Primer (5′–3′) | Reverse Primer (3′–5′) |
|---|---|---|
| IL-6 | CCGGGAACGAAAGAGAAGCTC | ACCGAAGGCGCTTGTGGAG |
| IL-8 | TTTCTGATGGAGAGAGCTCTGTCTGG | AGTGGAACAAGACTTGTGGATCCTGG |
| PTX3 | CGAAATAGACAATGGACTCCATCC | CTCATCTGCGAGTTCTCCAGCA |
| NAT10 | ATAGCAGCCACAAACATTCGC | ACACACATGCCGAAGGTATTG |
| β-actin | CCTGGCACCCAGCACAAT | GGGCCGGACTCGTCATACT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, Z.; Cai, L.; Tsai, I.-C.; Ding, W.; Tian, C.; Li, D.; Xu, Q. NAT10 Regulates LPS-Induced Inflammation via Stabilization of N4-Acetylated PTX3 mRNA in Human Dental Pulp Stem Cells. Int. J. Mol. Sci. 2025, 26, 4325. https://doi.org/10.3390/ijms26094325
Ni Z, Cai L, Tsai I-C, Ding W, Tian C, Li D, Xu Q. NAT10 Regulates LPS-Induced Inflammation via Stabilization of N4-Acetylated PTX3 mRNA in Human Dental Pulp Stem Cells. International Journal of Molecular Sciences. 2025; 26(9):4325. https://doi.org/10.3390/ijms26094325
Chicago/Turabian StyleNi, Zihan, Luhui Cai, I-Chen Tsai, Wenqian Ding, Cheng Tian, Di Li, and Qiong Xu. 2025. "NAT10 Regulates LPS-Induced Inflammation via Stabilization of N4-Acetylated PTX3 mRNA in Human Dental Pulp Stem Cells" International Journal of Molecular Sciences 26, no. 9: 4325. https://doi.org/10.3390/ijms26094325
APA StyleNi, Z., Cai, L., Tsai, I.-C., Ding, W., Tian, C., Li, D., & Xu, Q. (2025). NAT10 Regulates LPS-Induced Inflammation via Stabilization of N4-Acetylated PTX3 mRNA in Human Dental Pulp Stem Cells. International Journal of Molecular Sciences, 26(9), 4325. https://doi.org/10.3390/ijms26094325





