Bovine Milk Protein-Derived Preparations and Their Hydrolysates as Sources of ACE-Inhibitory, DPP-IV-Inhibitory, and Antioxidative Peptides Analyzed Using in Silico and in Vitro Protocols
Abstract
1. Introduction
2. Results and Discussion
2.1. Bovine Milk Proteins as the Sources of Biopeptides—An In Silico Analysis Results
2.2. Progress in the Hydrolysis of Bovine Milk Protein Preparations (MPPs)
2.3. Bioactivity of MPPs and Their Respective Hydrolysates
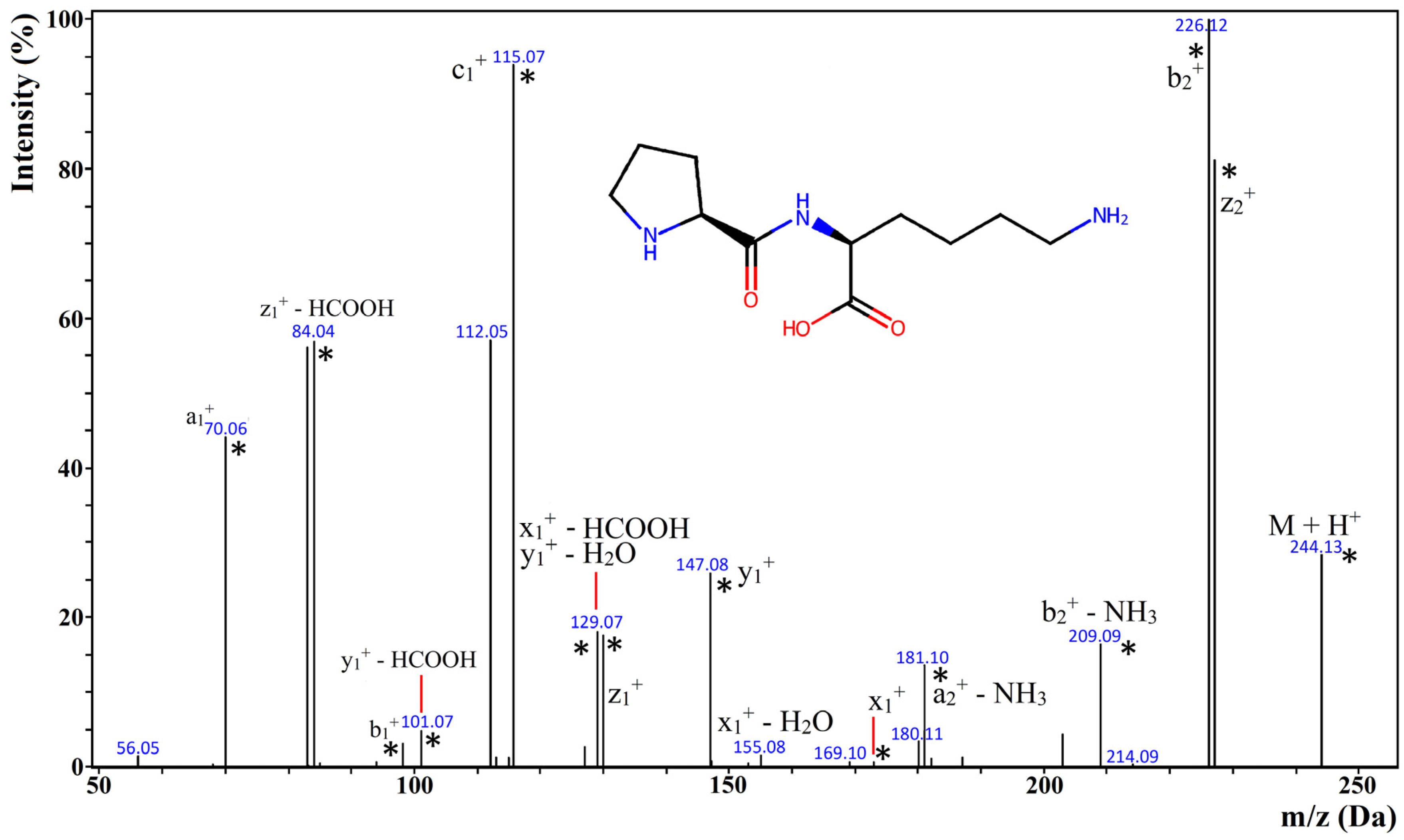
2.4. Identification of Biopeptides in MPPs and Their Respective Hydrolysates
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. In Silico Analysis of Bovine Milk Protein Sequences for Enzymatic Release of Selected Enzyme Inhibitors and Antioxidative Peptides
3.2.2. Laboratory Analyses of Bovine Milk Protein Preparations (MPPs) Laboratory Analyses of Bovine Milk Protein Preparations (MPPs)
Milk Protein Preparations (MPPs)
Simulation of Human Digestion of MPPs Using the INFOGEST Protocol
Protein Content
The Monitoring of MPPs Hydrolysis Processes by Means of Reversed-Phase High-Performance Liquid Chromatography with UV-Visible Spectroscopy (RP-HPLC UV/Vis)
Bioactivities of MPPs and Their Hydrolysates
- (1)
- ACE inhibition
- (2)
- DPP-IV inhibition
- (3)
- DPPH• (i.e., 2,2-diphenyl-β-picrylhydrazyl) radical scavenging activity
- (4)
- ABTS•+ (i.e., 2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt) radical scavenging effect
- (5)
- Ferric Reducing Antioxidant Power (FRAP)
- (6)
- IC50 values for specific bioactivity of MPPs and their hydrolysates
- (7)
- Identification of Peptides in MPPs and Their Hydrolysates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| αs1-CN, B | αs1-casein, genetic variant B |
| αs2-CN, A | αs2-casein, genetic variant A |
| β-CN, A1 | β-casein, genetic variant A1 |
| κ-CN, A | κ-casein, genetic variant A |
| α-La, A | α-lactalbumin, genetic variant A |
| β-Lg, A | β-lactoglobulin, genetic variant A |
| ABTS | 2,2′-azino-bis(3-ethylbenzotialozline-6-sulfonic acid |
| ACE | Angiotensin converting enzyme (EC 3.4.15.1) |
| ACN | Acetonitrile |
| BSA | Bovine serum albumin |
| IC50 | Tantamount to the sample concentrations (mg/mL) corresponding to their half-maximal activity |
| BIOPEP-UWM | BIOPEP-UWM database of protein and bioactive peptide sequences |
| DPP-IV | Dipeptidylpeptidase-IV (EC 3.4.14.5) |
| DPPH | 2,2-diphenyl-β-picrylhydrazyl |
| FA | Fluoroacetic acid |
| FRAP | Ferric Reducing Antioxidant Power |
| Lf | Lactoferrin |
| MBP | MCC with ultrafiltrated buttermilk permeate |
| MBP D | MBP subjected to the duodenal phase of simulated digestion |
| MCC | Micellar casein concentrate |
| MCC D | MCC subjected to the duodenal phase of simulated digestion |
| SPC | Serum protein concentrate |
| SPC D | SPC subjected to the duodenal phase of simulated digestion |
| MPP | Milk Protein Preparation |
| MS | Mass spectrometry |
| MS/MS | Tandem mass spectrometry |
| PEPT1 | Peptide Transporter 1 |
| PEPT2 | Peptide Transporter 2 |
| RP-HPLC UV/Vis | Reversed-phase high-performance liquid chromatography with UV-visible Spectroscopy |
| SM | skim milk |
| SM D | SM subjected to the duodenal phase of simulated digestion |
References
- Ross, R.P.; FitzGerald, R.F.; Stanton, C. Unraveling the digestion of milk protein. Am. J. Clin. Nutr. 2013, 97, 1161–1162. [Google Scholar] [CrossRef] [PubMed]
- Guha, S.; Sharma, H.; Deshwal, G.K.; Rao, P.S. A comprehensive review on bioactive peptides derived from milk and milk products of minor dairy species. Food Prod. Process. Nutr. 2021, 3, 2. [Google Scholar] [CrossRef]
- Stobiecka, M.; Król, J.; Brodziak, A.; Wajs, J. Characteristics of milk from different species of farm animals with special emphasis on health-promoting ingredients. Acta Sci. Pol. Zootech. 2021, 20, 85–96. [Google Scholar] [CrossRef]
- Górska-Warsewicz, H.; Rejman, K.; Laskowski, W.; Czeczotko, M. Milk and Dairy Products and Their Nutritional Contribution to the Average Polish Diet. Nutrients 2019, 11, 1771. [Google Scholar] [CrossRef] [PubMed]
- McBean, L.D.; Miller, G.D.; Heaney, R.P. Effect of Cow’s Milk on Human Health. In Beverages in Nutrition and Health; Wilson, T., Temple, N.J., Eds.; Humana Press Inc.: Totowa, NJ, USA, 2004; pp. 205–221. [Google Scholar] [CrossRef]
- Dallas, D.C.; Murray, N.M.; Gan, J. Proteolytic Systems in Milk: Perspectives on the Evolutionary Function within the Mammary Gland and the Infant. J. Mammary Gland. Biol. Neoplasia 2015, 20, 133–147. [Google Scholar] [CrossRef]
- Marcone, S.; Belton, O.; Fitzgerald, D.J. Milk-derived bioactive peptides and their health promoting effects: A potential role in atherosclerosis. Br. J. Clin. Pharmacol. 2017, 83, 152–162. [Google Scholar] [CrossRef]
- Chelliah, R.; Wei, S.; Daliri, E.B.; Elahi, F.; Yeon, S.J.; Tyagi, A.; Liu, S.; Madar, I.H.; Sultan, G.; Oh, D.H. The Role of Bioactive Peptides in Diabetes and Obesity. Foods 2021, 10, 2220. [Google Scholar] [CrossRef]
- De Leo, F.; Panarese, S.; Gallerani, R.; Ceci, L.R. Angiotensin converting enzyme (ACE) inhibitory peptides: Production and implementation of functional food. Curr. Pharm. Des. 2009, 15, 3622–3643. [Google Scholar] [CrossRef]
- Carrera-Alvarado, G.; Toldrá, F.; Mora, L. DPP-IV Inhibitory Peptides GPF, IGL, and GGGW Obtained from Chicken Blood Hydrolysates. Int. J. Mol. Sci. 2022, 23, 14140. [Google Scholar] [CrossRef]
- Antony, P.; Vijayan, R. Bioactive Peptides as Potential Nutraceuticals for Diabetes Therapy: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 9059. [Google Scholar] [CrossRef]
- Chia, T.Y.; Gan, C.-Y.; Shafie, M.H.; Yap, P.G.; Mohd Rodhi, A.; Ahmad, A.; Murugaiyah, V.; Abdulla, M.H.; Johns, E.J. A comprehensive review on the pancreatic lipase inhibitory peptides: A future anti-obesity strategy. Electron. J. Gen. Med. 2003, 20, em470. [Google Scholar] [CrossRef] [PubMed]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef]
- Iwaniak, A.; Minkiewicz, P.; Darewicz, M. BIOPEP-UWM database—Present and future. Curr. Opin. Food Sci. 2024, 55, 101108. [Google Scholar] [CrossRef]
- Marvin, H.J.; Janssen, E.M.; Bouzembrak, Y.; Hendriksen, P.J.; Staats, M. Big data in food safety: An overview. Crit. Rev. Food Sci. Nutr. 2017, 57, 2286–2295. [Google Scholar] [CrossRef] [PubMed]
- Pripp, A.H.; Isaksson, T.; Stepaniak, L.; Sørhaug, T. Quantitative structure-activity relationship modeling of ACE-inhibitory peptides derived from milk proteins. Eur. Food Res. Technol. 2004, 219, 579–583. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Structure activity relationship modelling of milk protein-derived peptides with dipeptidyl peptidase IV (DPP-IV) inhibitory activity. Peptides 2016, 79, 1–7. [Google Scholar] [CrossRef]
- Mudgil, P.; Al Dhaheri, M.K.O.; Alsubousi, M.S.M.; Khan, H.; Redha, A.A.; Yap, P.-G.; Gan, C.-Y.; Maqsood, S. Molecular docking studies on α-amylase inhibitory peptides from milk of different farm animals. J. Dairy Sci. 2024, 107, 2633–2652. [Google Scholar] [CrossRef]
- Iwaniak, A.; Mogut, D.; Minkiewicz, P.; Żulewska, J.; Darewicz, M. Gouda Cheese with Modified Content of β-Casein as a Source of Peptides with ACE- and DPP-IV-Inhibiting Bioactivity: A Study Based on In Silico and In Vitro Protocol. Int. J. Mol. Sci. 2021, 22, 2949. [Google Scholar] [CrossRef]
- Iwaniak, A.; Mogut, D.; Minkiewicz, P.; Żulewska, J.; Darewicz, M. An integrated approach to the analysis of antioxidative peptides derived from Gouda cheese with a modified β-casein content. Sci. Rep. 2022, 12, 13314. [Google Scholar] [CrossRef]
- Iwaniak, A.; Minkiewicz, P.; Hrynkiewicz, M.; Bucholska, J.; Darewicz, M. Hybrid Approach in the Analysis of Bovine Milk Protein Hydrolysates as a Source of Peptides Containing Di- and Tripeptide Bitterness Indicators. Pol. J. Food Nutr. Sci. 2020, 70, 139–150. [Google Scholar] [CrossRef]
- Peredo-Lovillo, A.; Hernández-Mendoza, A.; Vallejo-Cordoba, B.; Romero-Luna, H.E. Conventional and in silico approaches to select promising food-derived bioactive peptides: A review. Food Chem. X 2021, 13, 100183. [Google Scholar] [CrossRef] [PubMed]
- Kiełczewska, K.; Dąbrowska, A.; Bielecka, M.M.; Dec, B.; Baranowska, M.; Ziajka, J.; Zhennai, Y.; Żulewska, J. Protein Preparations as Ingredients for the Enrichment of Non-Fermented Milks. Foods 2022, 11, 1817. [Google Scholar] [CrossRef]
- Ziarno, M.; Hasalliu, R.; Cwalina, A. Effect of the Addition of Milk Protein Preparations on Selected Quality Parameters and Nutritional Characteristics of Kefir. Appl. Sci. 2021, 11, 966. [Google Scholar] [CrossRef]
- Jeewanthi, R.K.; Kim, M.H.; Lee, N.K.; Yoon, Y.C.; Paik, H.D. Peptide Analysis and the Bioactivity of Whey Protein Hydrolysates from Cheese Whey with Several Enzymes. Korean J. Food Sci. Anim. Resour. 2017, 37, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Hussein, F.A.; Chay, S.Y.; Zarei, M.; Auwal, S.M.; Hamid, A.A.; Wan Ibadullah, W.Z.; Saari, N. Whey Protein Concentrate as a Novel Source of Bifunctional Peptides with Angiotensin-I Converting Enzyme Inhibitory and Antioxidant Properties: RSM Study. Foods 2020, 9, 64. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Balance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Balance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Atallah, N.; Deracinois, B.; Boulier, A.; Baniel, A.; Jouan-Rimbaud Bouveresse, D.; Ravallec, R.; Flahaut, C.; Cudennec, B. In Vitro Assessment of the Impact of Industrial Processes on the Gastrointestinal Digestion of Milk Protein Matrices Using the INFOGEST Protocol. Foods 2020, 9, 1580. [Google Scholar] [CrossRef]
- Web of Science. Available online: https://www.webofscience.com/ (accessed on 17 June 2024).
- Akbarian, M.; Khani, A.; Eghbalpour, S.; Uversky, V.N. Bioactive Peptides: Synthesis, Sources, Applications, and Proposed Mechanisms of Action. Int. J. Mol. Sci. 2022, 23, 1445. [Google Scholar] [CrossRef]
- Xu, Q.; Hong, H.; Wy, J.; Han, Y. Bioavailability of bioactive peptides derived from food proteins across the intestinal epithelial membrane: A review. Trends Food Sci. Technol. 2019, 86, 399–411. [Google Scholar] [CrossRef]
- Darewicz, M.; Pliszka, M.; Borawska-Dziadkiewicz, J.; Minkiewicz, P.; Iwaniak, A. Multi-Bioactivity of Protein Digests and Peptides from Oat (Avena sativa L.) Kernels in the Prevention of the Cardiometabolic Syndrome. Molecules 2022, 27, 7907. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Mariscal, C.; Carrera-Alvarado, G.; Mora, L.; Toldrá, F. Neprilysin (NEP) and Angiotensin Converting Enzyme-I (ACE-I) inhibitory dipeptides from chicken carcass hydrolysates. LWT 2025, 221, 117591. [Google Scholar] [CrossRef]
- BIOPEP-UWM Database. May–September 2024. Available online: https://biochemia.uwm.edu.pl/biopep-uwm/ (accessed on 1 May 2024).
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM database of bioactive peptides: Current opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef]
- Darewicz, M.; Borawska, J.; Vegarud, G.E.; Minkiewicz, P.; Iwaniak, A. Angiotensin I-converting enzyme (ACE) inhibitory activity and ACE inhibitory peptides of salmon (Salmo salar) protein hydrolysates obtained by human and porcine gastrointestinal enzymes. Int. J. Mol. Sci. 2014, 15, 14077–14101. [Google Scholar] [CrossRef] [PubMed]
- Borawska, J.; Darewicz, M.; Vegarud, G.E.; Minkiewicz, P. Antioxidant properties of carp (Cyprinus carpio L.) protein ex vivo and in vitro hydrolysates. Food Chem. 2016, 194, 770–779. [Google Scholar] [CrossRef]
- Iwaniak, A.; Hrynkiewicz, M.; Minkiewicz, P.; Bucholska, J.; Darewicz, M. Soybean (Glycine max) Protein Hydrolysates as Sources of Peptide Bitter-Tasting Indicators: An Analysis Based on Hybrid and Fragmentomic Approaches. Appl. Sci. 2020, 10, 2514. [Google Scholar] [CrossRef]
- Silvestre, M.P.C.; Morais, H.A.; Silva, V.D.M.; Silva, M.R. Degree of hydrolysis and peptide profile of whey proteins using pancreatin. Rev. Soc. Bras. Aliment. Nutr. 2013, 38, 278–290. [Google Scholar] [CrossRef]
- Akimov, M.; Bezuglov, V. Methods of Protein Digestive Stability Assay—State of the Art. In New Advances in the Basic and Clinical Gastroenterology; Brzozowski, T., Ed.; Intech: Rijeka, Croatia, 2012; pp. 211–234. [Google Scholar] [CrossRef]
- Delgado, M.C.O.; Nardo, A.; Pavlovic, M.; Rogniaux, H.; Añón, M.C.; Tironi, V.A. Identification and characterization of antioxidant peptides obtained by gastrointestinal digestion of amaranth proteins. Food Chem. 2016, 197, 1160–1167. [Google Scholar] [CrossRef]
- Baugreet, S.; Gomez, C.; Auty, M.A.; Kerry, J.P.; Hamill, R.M.; Brodkorb, A. In vitro digestion of protein-enriched restructured beef steaks with pea protein isolate, rice protein and lentil flour following sous vide processing. Innov. Food Sci. Emerg. Technol. 2019, 54, 152–161. [Google Scholar] [CrossRef]
- Mazloomi, S.N.; Mahoonak, A.S.; Mora, L.; Ghorban, I.M.; Houshmand, G.; Toldrá, F. Pepsin hydrolysis of orange by-products for the production of bioactive peptides with gastrointestinal resistant properties. Foods 2021, 10, 679. [Google Scholar] [CrossRef]
- Sheng, B.; Nielsen, S.D.; Poulsen, N.A.; Larsen, L.B. Differential in vitro digestion rates in gastric phase of bovine milk with different κ-casein phenotypes. J. Dairy Sci. 2021, 104, 10462–10472. [Google Scholar] [CrossRef] [PubMed]
- Visser, S.; Slangen, C.J.; Rollema, H.S. Phenotyping of bovine milk poteins by reversed-phase high-performance liquid chromatography. J. Chromatogr. 1991, 548, 361–370. [Google Scholar] [CrossRef]
- Forstedt, T.; Forssén, P.; Westerlund, D. System peaks and their impact in liquid chromatography. Trends Anal. Chem. 2016, 81, 42–50. [Google Scholar] [CrossRef]
- Szymczak, M.; Kamiński, P.; Turło, M.; Bucholska, J.; Mogut, D.; Minkiewicz, P.; Mizielińska, M.; Stobińska, M. Effect of Marinating Temperature of Atlantic Herring on Meat Ripening, Peptide Fractions Proportion, and Antioxidant Activity of Meat and Brine. Appl. Sci. 2023, 13, 7225. [Google Scholar] [CrossRef]
- Dontha, S. A review on antioxidant methods. Asian J. Pharm. Clin. Res. 2016, 9, 14–32. [Google Scholar] [CrossRef]
- Di Bernardini, R.; Harnedy, P.; Bolton, D.; Kerry, J.; O’Neill, E.; Mullen, A.M.; Hayes, M. Antioxidant and antimicrobial peptidic hydrolysates from muscle protein sources and by-products. Food Chem. 2011, 124, 1296–1307. [Google Scholar] [CrossRef]
- Perna, A.; Intaglietta, I.; Simonetti, A.; Gambacorta, E. Short communication: Effect of genetic type on antioxidant activity of Caciocavallo cheese during ripening. J. Dairy Sci. 2015, 98, 3690–3694. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D.D. Antioxidant property of coffee components: Assessment of methods to define the mechanisms of action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef]
- Zhang, Q.; Zheng, L.; Luo, D.; Zhao, M. In vitro simulated gastrointestinal digestion stability of a neuroprotective octapeptide WCPFSRSF and prediction of potential bioactive peptides in its digestive fragments by multiple bioinformatics tools. J. Agric. Food Chem. 2023, 71, 6987–6998. [Google Scholar] [CrossRef]
- Jiménez-Munoz, L.; Nielsen, S.D.-H.; Corredig, M. Changes in potato peptide bioactivity after gastrointestinal digestion. An in silico and in vitro study of different patatin-rich potato protein isolate matrices. Food Chem. Adv. 2024, 4, 100679. [Google Scholar] [CrossRef]
- Sharma, D.; Gite, S.; Tuohy, M.G. Exploring the physicochemical characteristics of marine protein hydrolysates and the impact of in vitro gastrointestinal digestion on their bioactivity. Mar. Drugs 2024, 22, 452. [Google Scholar] [CrossRef] [PubMed]
- Daliri, E.B.-M.; Oh, D.H.; Lee, B.H. Bioactive Peptides. Foods 2017, 6, 32. [Google Scholar] [CrossRef]
- Searle, B.C.; Pino, L.K.; Egertson, J.D.; Ting, Y.S.; Lawrence, R.T.; MacLean, B.X.; Villén, J.; MacCoss, M.J. Chromatogram libraries improve peptide detection and quantification by data independent acquisition mass spectrometry. Nature Commun. 2018, 9, 5128. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Ahadi, S.; Zhou, W.; Röst, H. DIAlignR provides precise retention time alignment across distant runs in DIA and targeted proteomics. Mol. Cell. Proteom. 2019, 18, 806–817. [Google Scholar] [CrossRef] [PubMed]
- Tóth, G.; Panić-Janković, T.; Mitulović, G. Pillar array columns for peptide separations in nanoscale reversed-phase chromatography. J. Chromatogr. A 2019, 1603, 426–432. [Google Scholar] [CrossRef]
- Roepstorff, P.; Fohlman, J. Proposal for a common nomenclature for sequence ions in mass spectra of peptides. Biomed. Mass Spectrom. 1984, 11, 601. [Google Scholar] [CrossRef]
- Paizs, B.; Suhai, S. Fragmentation pathways of protonated peptides. Mass Spectrom. Rev. 2005, 24, 508–548. [Google Scholar] [CrossRef]
- Medzihradszky, K.F.; Chalkley, R.J. Lessons in de novo peptide sequencing by tandem mass spectrometry. Mass Spectrom. Rev. 2015, 34, 43–63. [Google Scholar] [CrossRef]
- Poliseli, C.B.; Tonin, A.P.P.; Martinez, F.C.; do Nascimento, N.C.; Braz Junior, V.; Maluf, J.; Ribeiro, V.M.S.; Della Rosa, F.A.; Souza, G.H.M.F.; Eberline, M.N.; et al. Tri- and dipeptides identification in whey protein and porcine liver protein hydrolysates by fast LC-MS/MS neutral loss screening and de novo sequencing. J. Mass Spectrom. 2021, 56, e4701. [Google Scholar] [CrossRef]
- Lan, V.T.T.; Ito, K.; Ohno, M.; Motoyama, T.; Ito, S.; Kawarasaki, Y. Analyzing a dipeptide library to identify human dipeptidyl peptidase IV inhibitor. Food Chem. 2015, 175, 66–73. [Google Scholar] [CrossRef]
- Domingo-Almenara, X.J.; Montenegro-Burke, R.; Ivanisevic, J.; Thomas, A.; Sidibé, J.; Teav, T.; Guijas, C.; Aisporna, A.E.; Rinehart, D.; Hoang, L.; et al. XCMS-MRM and METLIN-MRM: A cloud library and public resource for targeted analysis of small molecules. Nat. Methods 2018, 15, 681–684. [Google Scholar] [CrossRef] [PubMed]
- METLIN Database. May–September 2024. Available online: https://metlin.scripps.edu/landing_page.php?pgcontent=mainPage (accessed on 1 May 2024).
- Evans, J.; Zulewska, J.; Newbold, M.; Drake, M.A.; Barbano, D.M. Comparison of composition, sensory, and volatile components of thirty-four percent whey protein and milk serum protein concentrates. J. Dairy Sci. 2009, 92, 4773–4791. [Google Scholar] [CrossRef] [PubMed]
- Hammam, A.R.A.; Martínez-Monteagudo, S.I.; Metzger, L.E. Progress in micellar casein concentrate: Production and applications. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4426–4449. [Google Scholar] [CrossRef] [PubMed]
- Minkiewicz, P.; Darewicz, M.; Iwaniak, A.; Sokołowska, J.; Starowicz, P.; Bucholska, J.; Hrynkiewicz, M. Common Amino Acid Subsequences in a Universal Proteome—Relevance for Food Science. Int. J. Mol. Sci. 2015, 16, 20748–20773. [Google Scholar] [CrossRef]
- Żulewska, J.; Baranowska, M.; Bielecka, M.M.; Dąbrowska, A.Z.; Tarapata, J.; Kiełczewska, K.; Łobacz, A. Effect of Fortification with High-Milk-Protein Preparations on Yogurt Quality. Foods 2025, 14, 80. [Google Scholar] [CrossRef]
- University of Warmia and Mazury in Olsztyn, Poland. Method of Producing a High-Protein Preparation Containing Milk Serum Proteins and Buttermilk Retentate Proteins. Republic of. Poland Patent Application No. P.450274, 15 November 2024.
- University of Warmia and Mazury in Olsztyn, Poland. Method of Producing a High-Protein Preparation from Milk and Buttermilk. Republic of. Poland Patent Application No. P.450275, 15 November 2024.
- Tanambell, H.; Danielsen, M.; Devold, T.G.; Møller, A.H.; Dalsgaard, T.K. In vitro protein digestibility of RuBisCO from alfalfa obtained from different processing histories: Insights from free N-terminal and mass spectrometry study. Food. Chem. 2024, 434, 137301. [Google Scholar] [CrossRef]
- Tanambell, H.; Møller, A.H.; Roman, L.; Corredig, M.; Dalsgaard, T.K. Supramolecular structure modification of RuBisCO from alfalfa during removal of chloroplastic materials. Innov. Food Sci. Emerg. Technol. 2023, 87, 103408. [Google Scholar] [CrossRef]
- Shivanna, S.K.; Nataraj, B.H. Revisiting therapeutic and toxicological fingerprints of milk-derived bioactive peptides: An overview. Food Biosci. 2020, 38, 100771. [Google Scholar] [CrossRef]
- Baptista, D.P.; Gigante, M.L. Bioactive peptides in ripened cheeses: Release during technological processes and resistance to the gastrointestinal tract. J. Sci. Food Agric. 2021, 101, 4010–4017. [Google Scholar] [CrossRef]
- Bielecka, M.; Cichosz, G.; Czeczot, H. Antioxidant, antimicrobial and anticarcinogenic activities of bovine milk proteins and their hydrolysates—A review. Int. Dairy J. 2022, 127, 105208. [Google Scholar] [CrossRef]
- Mudgil, P.; Kamal, H.; Kilari, B.P.; Salim, M.A.S.M.; Gan, C.-Y.; Maqsood, S. Simulated gastrointestinal digestion of camel and bovine casein hydrolysates: Identification and characterization of novel anti-diabetic bioactive peptides. Food Chem. 2021, 353, 129374. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.H.; Zhang, Y.; Liu, J.; Tu, Z.C. Investigation into predominant peptide and potential allergenicity of ultrasonicated β-lactoglobulin digestion products. Food Chem. 2021, 361, 130099. [Google Scholar] [CrossRef]
- The UniProt Consortium. UniProt: The Universal Protein Knowledgebase in 2023. Nucleic Acids Res. 2023, 51, D523–D531. [Google Scholar] [CrossRef]
- UniProtKB. May–September 2024. Available online: http://www.uniprot.org (accessed on 1 May 2024).
- Caroli, A.M.; Chessa, S.; Erhardt, G.J. Invited review: Milk protein polymorphisms in cattle: Effect on animal breeding and human nutrition. J. Dairy Sci. 2009, 92, 5335–5352. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Jimsheena, V.K.; Gowda, L.R. Colorimetric, high-throughput assay for screening Angiotensin I-converting enzyme inhibitors. Anal. Chem. 2009, 81, 9388–9394. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yamamoto, N.; Sakai, K.; Okubo, A.; Yamazaki, S.; Takano, T. Purification and characterization of angiotensin I-converting enzyme inhibitors from sour milk. J. Dairy Sci. 1995, 78, 777–783. [Google Scholar] [CrossRef]
- Lacroix, I.M.E.E.; Li-Chan, E.C.Y.Y. Dipeptidyl peptidase-IV inhibitory activity of dairy protein hydrolysates. Int. Dairy J. 2012, 25, 97–102. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.; Wang, Z.; Chen, S.; Luo, Y. Production and identification of antioxidant and angiotensin-converting enzyme inhibition and dipeptidyl peptidase IV inhibitory peptides from bighead carp (Hypophthalmichthys nobilis) muscle hydrolysate. J. Funct. Foods 2017, 35, 224–235. [Google Scholar] [CrossRef]
- Wu, H.C.; Chen, H.M.; Shiau, C.Y. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus). Food Res. Int. 2003, 36, 949–957. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Borawska, J.; Darewicz, M.; Vegarud, G.E.; Iwaniak, A.; Minkiewicz, P. Ex vivo digestion of carp muscle tissue—ACE inhibitory and antioxidant activities of the obtained hydrolysates. Food Funct. 2015, 6, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, T.K.; Issoufou, A.; Zhou, H. Antioxidant activity of fractionated foxtail millet protein hydrolysate. Int. Food Res. J. 2012, 19, 207–213. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Venskutonis, R.; Gruzdienè, G.; Tirzite, D.; Tirzitis, G. Assessment of antioxidant activity of plant extracts by different methods. Acta Hortic. 2005, 677, 99–107. [Google Scholar] [CrossRef]
- Quirós, A.; Hernández-Ledesma, B.; Ramos, M.; Amigo, L.; Recio, I. Angiotensin-Converting Enzyme Inhibitory Activity of Peptides Derived from Caprine Kefir. J. Dairy Sci. 2005, 88, 3480–3487. [Google Scholar] [CrossRef]

| Protein | AE * | W | BE | V | ||||
|---|---|---|---|---|---|---|---|---|
| Inhibitory Activity Against | ||||||||
| ACE | DPP-IV | ACE | DPP-IV | ACE | DPP-IV | ACE | DPP-IV | |
| αs1-CN, B | 0.0452 | 0.0804 | 0.0825 | 0.1311 | 0.0018 | 0.0004 | 0.0765 | 0.8077 |
| αs2-CN, A | 0.0676 | 0.0991 | 0.1471 | 0.1419 | 0.0043 | 0.0001 | 0.1587 | 0.0298 |
| β-CN, A1 | 0.0526 | 0.1675 | 0.082 | 0.2084 | 0.0012 | 0.0002 | 0.0263 | 0.1385 |
| κ-CN, A | 0.1105 | 0.1579 | 0.2165 | 0.2027 | 0.0047 | 0.0002 | 0.0996 | 0.1429 |
| α-La, A | 0.0775 | 0.0915 | 0.1865 | 0.1368 | 0.0049 | 0 | 0.2235 | 0 |
| β-Lg, A | 0.0678 | 0.1186 | 0.1186 | 0.181 | 0.0018 | 0.0003 | 0.0195 | 0.3353 |
| BSA | 0.0428 | 0.0939 | 0.1125 | 0.1588 | 0.0029 | 0.0001 | 0.1373 | 0.5195 |
| Lf | 0.0452 | 0.0876 | 0.1026 | 0.1423 | 0.0058 | 0.0002 | 0.2706 | 0.4435 |
| Average Percentage of Protein 1 in: | |||||
|---|---|---|---|---|---|
| MCC 2 | MBP 3 | SPC 4 | |||
| 0 5 ± SD 6 | D 7 ± SD | 0 ± SD | D ± SD | 0 ± SD | D ± SD |
| 68.67 ± 4.50 | 32.33 ± 1.89 | 56.67 ± 1.70 | 26.33 ± 0.94 | 53.67 ± 0.94 | 27.33 ± 0.47 |
| Sample | Percentage of Areas Under Peaks in the Time Segments [%] | |||
|---|---|---|---|---|
| Retention Times [min] | 0.00–9.99 | 10.00–19.99 | 20.00–54.99 | 55.00–70.00 |
| MCC * | 95.421 | 1.110 | 1.107 | 2.363 |
| MCC D | 95.977 | 2.505 | 1.175 | 0.343 |
| MBP | 96.871 | 1.305 | 0.860 | 0.964 |
| MBP D | 95.538 | 2.821 | 1.341 | 0.300 |
| SPC | 92.570 | 1.131 | 0.505 | 5.794 |
| SPC D | 95.576 | 3.007 | 1.082 | 0.335 |
| SM | 91.064 | 0.000 | 5.121 | 3.815 |
| SM D | 91.253 | 0.029 | 7.941 | 0.777 |
| Sample | IC50 [mg/mL] * | ||||
|---|---|---|---|---|---|
| Inhibition of | Antioxidative Power Towards | ||||
| ACE | DPP-IV | ABTS•+ | DPPH• | FRAP | |
| MCC | 1.856 | n.d. | 3.444 | 1.645 | 16.880 |
| MCC D | 2.897 | 0.404 | 4.673 | 1.481 | 14.990 |
| MBP | 2.307 | 0.561 | 4.144 | 1.342 | 31.120 |
| MBP D | 7.627 | 0.0067 | 2.754 | 1.687 | 20.140 |
| SPC | 3.738 | 1.693 | 6.244 | 1.246 | 14.470 |
| SPC D | 4.143 | 0.512 | 4.616 | 1.238 | 13.720 |
| m/z (M + H)+ | Sequence 1 | Retention Time [min] of Peptide Identified in: | Protein Source 7 | |||||
|---|---|---|---|---|---|---|---|---|
| MCC 2 | MBP 3 | SPC 4 | ||||||
| 0 5 | D 6 | 0 | D | 0 | D | |||
| 163.0714 | SG | - | - | 1.28 | - | 1.25 | - | αs1-CN; κ-CN; α-La |
| 175.1078 | VG | - | 1.27 | - | 1.27 | - | 1.27 | α-La; Lf; BSA |
| 187.1078 | PA | - | 1.45 | - | 1.45 | - | 1.45 | κ-CN; β-Lg |
| 189.1234 | IG | - | 2.19 | - | 2.19 | - | 2.19 | αs1-CN; αs2-CN |
| 189.1234 | VA | 4.52 | 4.52 | 4.52 | 4.52 | - | 4.52 | αs1-CN; αs2-CN; β-CN; κ-CN; β-Lg; Lf; BSA |
| 205.1183 | VS | 4.58 | 4.53 | 4.63 | 4.62 | - | 4.62 | BSA |
| 207.0976 | ST | - | 2.83 | - | 2.87 | - | 2.87 | αs1-CN; αs2-CN; κ-CN; α-La; Lf; BSA |
| 215.1391 | VP | - | - | 2.05 | 1.9 | - | - | αs2-CN; Lf |
| 217.1183 | PT | - | - | - | - | - | 1.52 | αs2-CN; β-CN; κ-CN; β-Lg; Lf; BSA |
| 219.1340 | SL | - | 2.04 | - | 2.03 | - | 2.01 | β-CN; β-Lg; Lf; BSA |
| 219.1340 | VT | - | 1.17 | - | 1.12 | - | - | κ-CN; β-Lg; Lf; BSA |
| 229.1547 | PL | - | 3.89 | - | 3.82 | - | 3.77 | αs1-CN; β-CN; β-Lg; Lf; BSA |
| 229.1547 | IP | - | 3.89 | - | 3.82 | - | 3.77 | κ-CN |
| 230.1136 | PN | - | 1.31 | - | 1.31 | - | 1.31 | Lf; BSA |
| 231.1704 | VL | - | 2.68 | - | 2.61 | - | - | αs1-CN; αs2-CN; β-CN; κ-CN; β-Lg; Lf; BSA |
| 234.1449 | SK | - | 1.89 | - | 1.90 | - | - | αs1-CN; αs2-CN; β-CN; Lf |
| 244.1656 | PK | - | 1.32 | - | 1.32 | - | 1.27 | αs1-CN; αs2-CN; β-CN; Lf; BSA |
| 246.1449 | IN | - | - | - | - | - | 2.49 | β-CN; κ-CN; α-La |
| 247.1111 | PM | - | 2.83 | - | 2.83 | - | 2.86 | αs1-CN; β-Lg |
| 249.1268 | VM | - | 1.55 | - | 1.58 | - | 1.57 | β-CN; BSA |
| 253.1296 | PH | - | 1.20 | - | 1.20 | - | 1.21 | β-CN; κ-CN; α-La; BSA |
| 261.1445 | IE | - | 2.37 | - | 2.36 | - | 2.36 | β-CN; κ-CN; BSA |
| 263.1391 | PF | - | 6.96 | - | 6.94 | - | 6.94 | αs1-CN; β-CN; κ-CN |
| 269.1609 | IH | - | 2.22 | - | 2.22 | - | 2.28 | αs1-CN; β-CN; κ-CN |
| 272.1718 | PR | - | - | - | - | - | 1.20 | Lf |
| 274.1874 | VR | - | 3.59 | - | - | - | - | αs2-CN; β-CN; κ-CN; β-Lg; Lf; BSA |
| 279.1340 | PY | - | 3.07 | - | 3.15 | - | 3.17 | κ-CN; Lf; BSA |
| 281.1496 | VY | - | 4.52 | - | 4.50 | - | 4.53 | αs2-CN; β-CN; β-Lg |
| 288.2031 | IR | - | 5.94 | - | 5.96 | - | - | κ-CN; β-Lg; Lf |
| 292.1292 | SW | - | 1.28 | - | 1.31 | - | 1.28 | β-CN; Lf |
| 300.1918 | IPA | - | 3.45 | - | 3.45 | - | - | β-Lg |
| 302.1500 | PW | - | 5.82 | - | 5.82 | - | 5.82 | αs2-CN |
| 318.1813 | IW | - | - | - | - | - | 2.32 | αs2-CN; α-La; Lf |
| 326.2075 | PPL | - | 15.01 | - | 14.95 | - | - | β-CN; BSA |
| 328.2231 | VPL | - | 7.71 | - | 7.76 | - | 7.59 | αs1-CN |
| 360.1952 | IPM | - | 11.39 | - | 10.45 | - | 10.48 | Lf |
| Number of peptides | 36 | 2 | 30 | 4 | 30 | 1 | 28 | |
| Activity | MCC D * | MBP D | SPC D |
|---|---|---|---|
| Total | 30 | 30 | 28 |
| Angiotensin I-converting enzyme (ACE) (EC 3.4.15.1) inhibition | 10 | 11 | 11 |
| Dipeptidyl peptidase IV (DPP-IV) (EC 3.4.14.5) inhibition | 27 | 27 | 23 |
| Antioxidative | 3 | 3 | 2 |
| Stage | Time [min] | Solvent [%] | |
|---|---|---|---|
| A | B | ||
| Separation | 0 | 100 | 0 |
| 60 | 60 | 40 | |
| Washing | 65 | 0 | 100 |
| 70 | 0 | 100 | |
| Column equlibration | 71 | 100 | 0 |
| 80 | 100 | 0 | |
| Stage | Time [min] | Solvent [%] | |
|---|---|---|---|
| A | B | ||
| Separation | 0:00 | 95 | 5 |
| 40:00 | 65 | 35 | |
| Column washing | 40:01 | 65 | 35 |
| 41:00 | 0 | 100 | |
| 46:00 | 0 | 100 | |
| Column equilibration | 46:01 | 0 | 100 |
| 47:00 | 95 | 5 | |
| 60:00 | 95 | 5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwaniak, A.; Minkiewicz, P.; Mogut, D.; Borawska-Dziadkiewicz, J.; Żulewska, J.; Darewicz, M. Bovine Milk Protein-Derived Preparations and Their Hydrolysates as Sources of ACE-Inhibitory, DPP-IV-Inhibitory, and Antioxidative Peptides Analyzed Using in Silico and in Vitro Protocols. Int. J. Mol. Sci. 2025, 26, 4323. https://doi.org/10.3390/ijms26094323
Iwaniak A, Minkiewicz P, Mogut D, Borawska-Dziadkiewicz J, Żulewska J, Darewicz M. Bovine Milk Protein-Derived Preparations and Their Hydrolysates as Sources of ACE-Inhibitory, DPP-IV-Inhibitory, and Antioxidative Peptides Analyzed Using in Silico and in Vitro Protocols. International Journal of Molecular Sciences. 2025; 26(9):4323. https://doi.org/10.3390/ijms26094323
Chicago/Turabian StyleIwaniak, Anna, Piotr Minkiewicz, Damir Mogut, Justyna Borawska-Dziadkiewicz, Justyna Żulewska, and Małgorzata Darewicz. 2025. "Bovine Milk Protein-Derived Preparations and Their Hydrolysates as Sources of ACE-Inhibitory, DPP-IV-Inhibitory, and Antioxidative Peptides Analyzed Using in Silico and in Vitro Protocols" International Journal of Molecular Sciences 26, no. 9: 4323. https://doi.org/10.3390/ijms26094323
APA StyleIwaniak, A., Minkiewicz, P., Mogut, D., Borawska-Dziadkiewicz, J., Żulewska, J., & Darewicz, M. (2025). Bovine Milk Protein-Derived Preparations and Their Hydrolysates as Sources of ACE-Inhibitory, DPP-IV-Inhibitory, and Antioxidative Peptides Analyzed Using in Silico and in Vitro Protocols. International Journal of Molecular Sciences, 26(9), 4323. https://doi.org/10.3390/ijms26094323









