Compounds Derived from Tryptophan Metabolism in Torulaspora delbrueckii CBS1146T and Zygosaccharomyces bailii ATCC36947T
Abstract
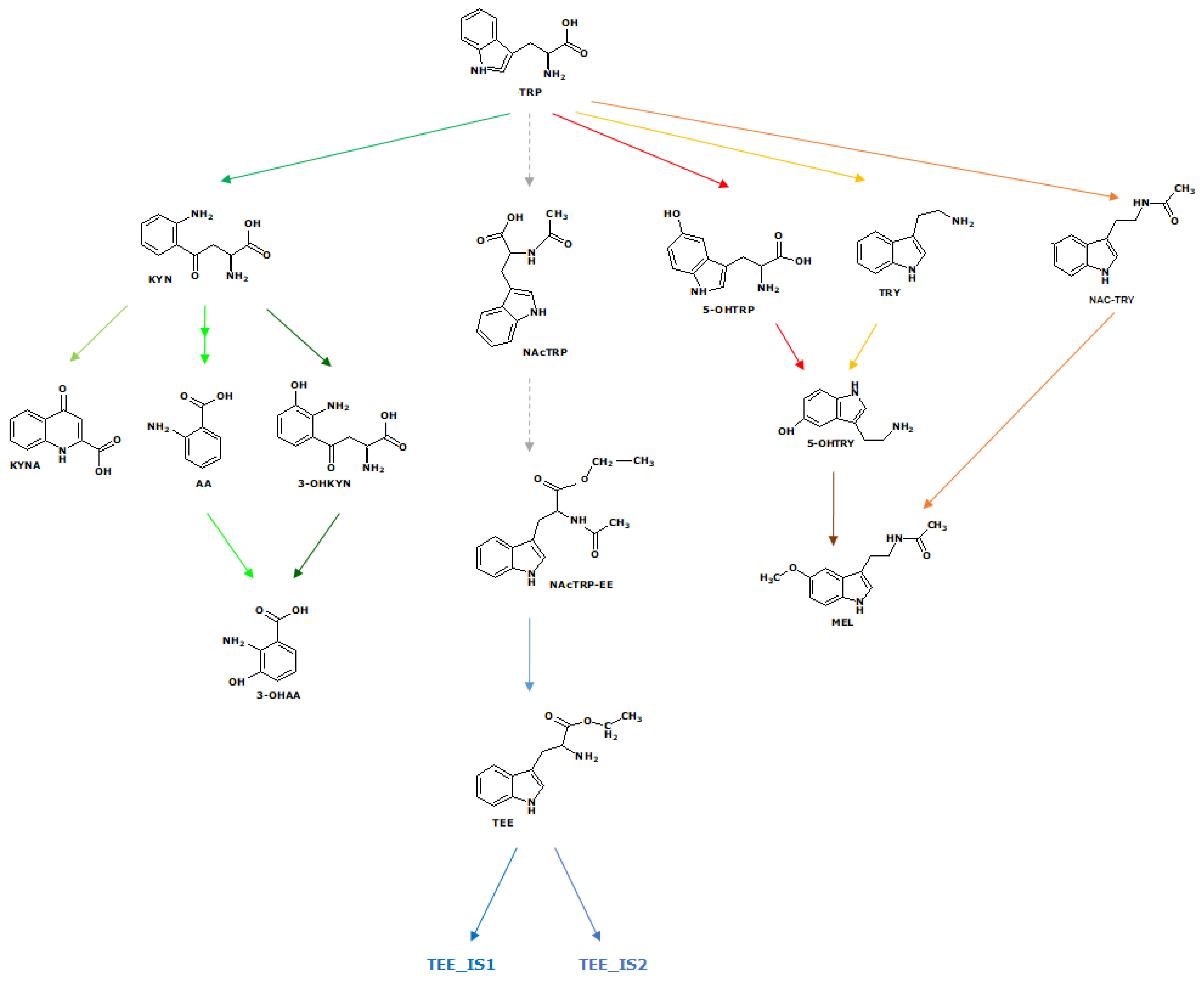
1. Introduction
2. Results and Discussion
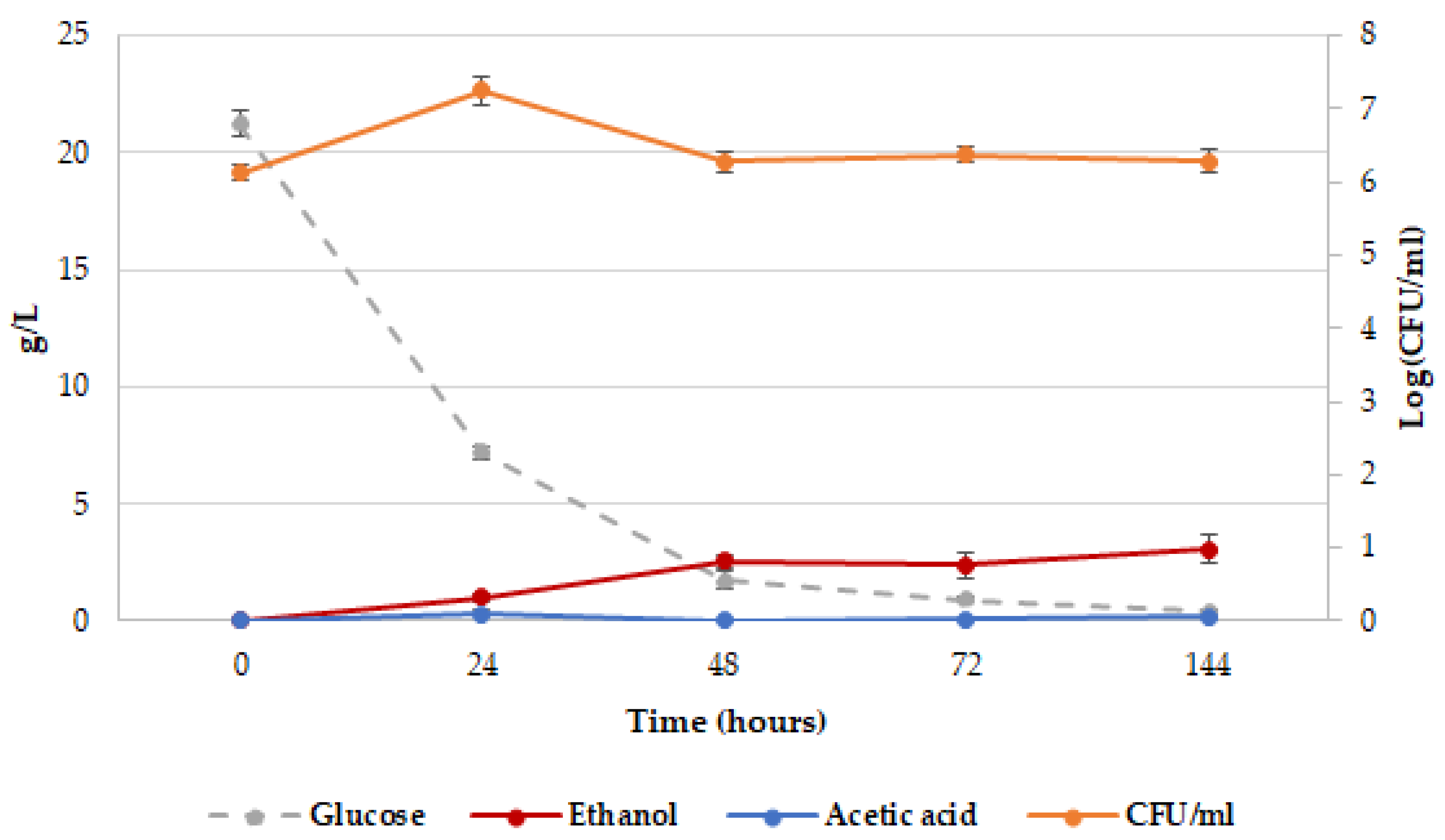
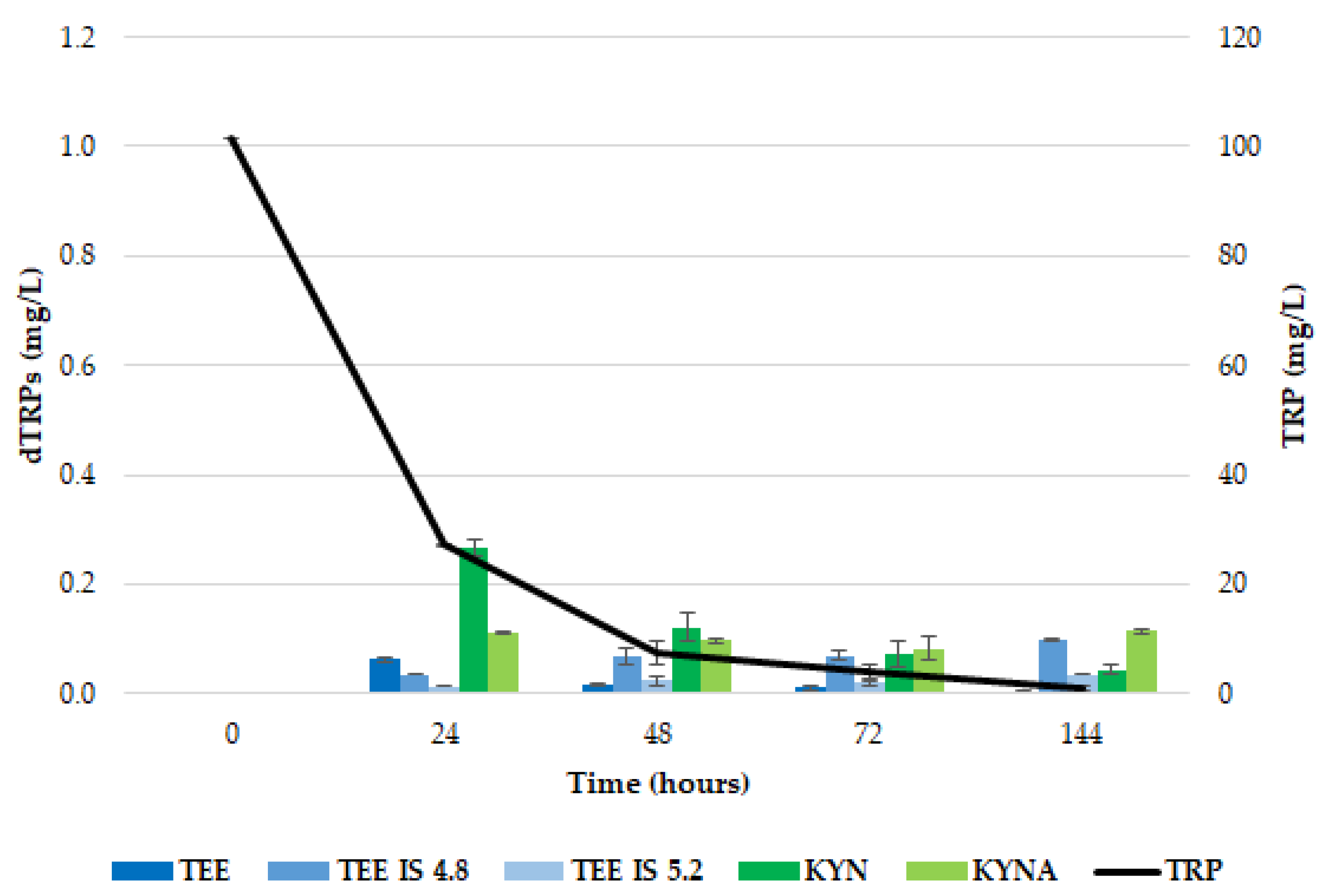
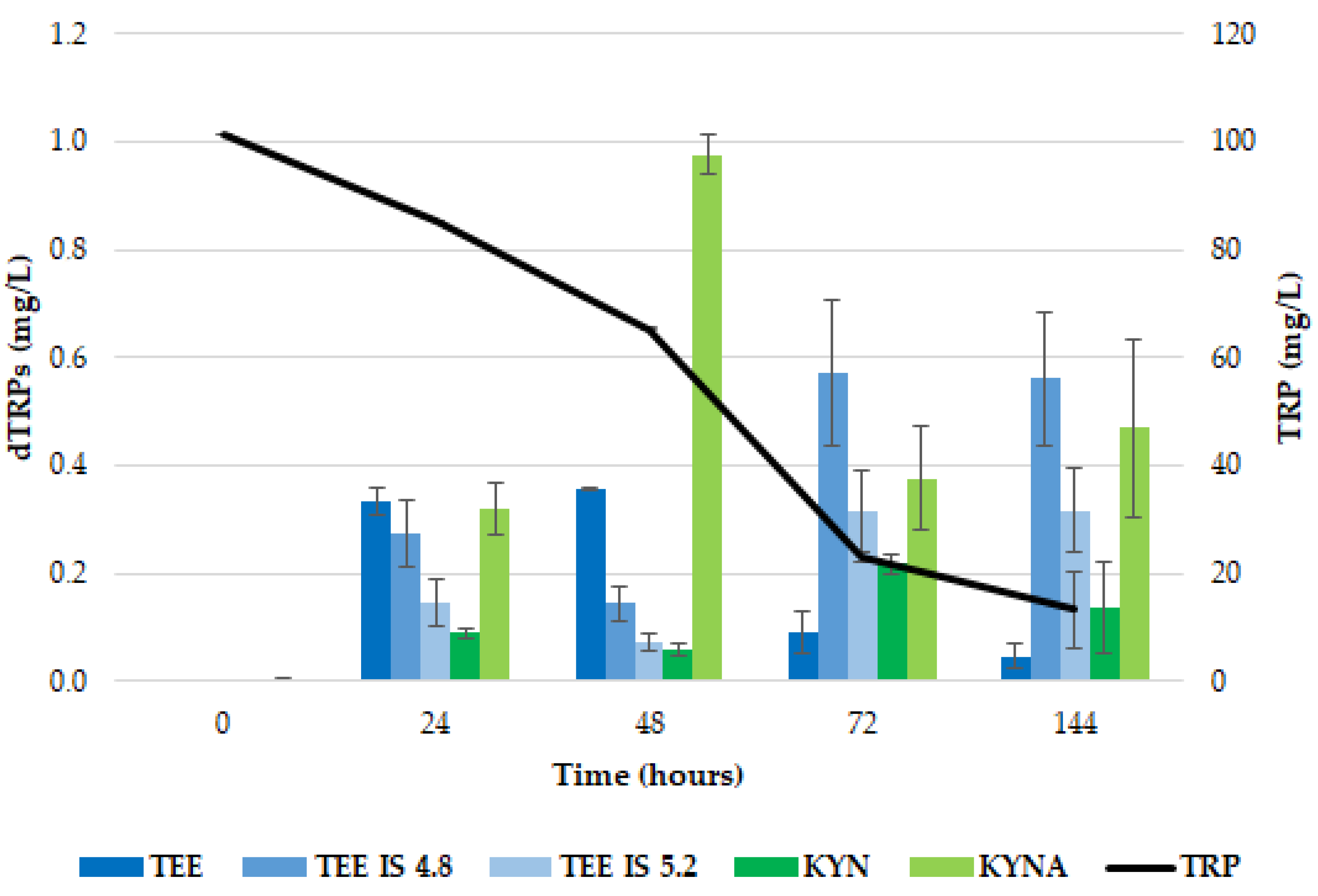
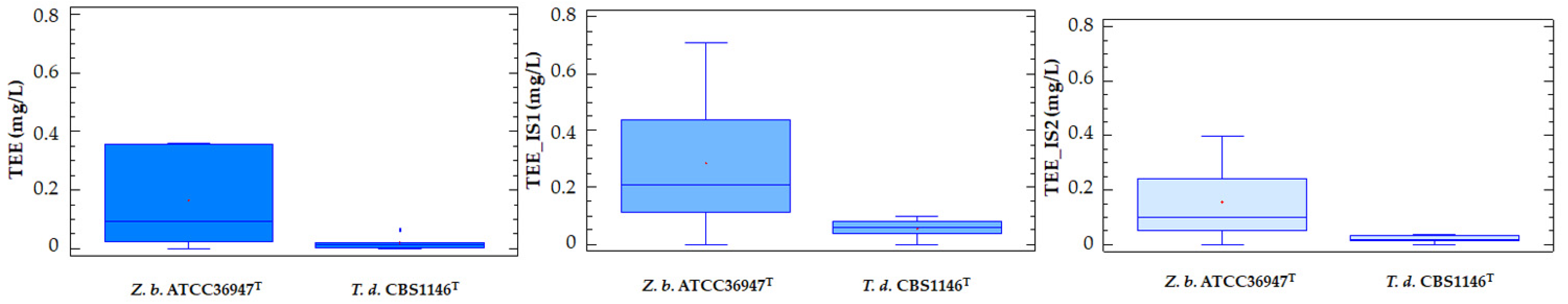
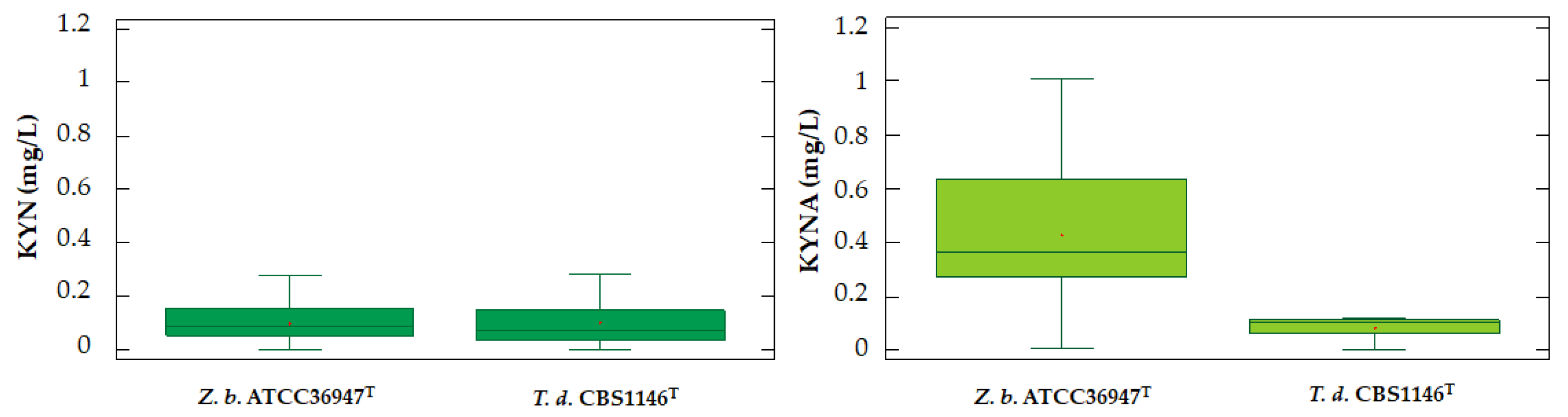
2.1. Release of dTRPs by T. delbrueckii CBS1146T
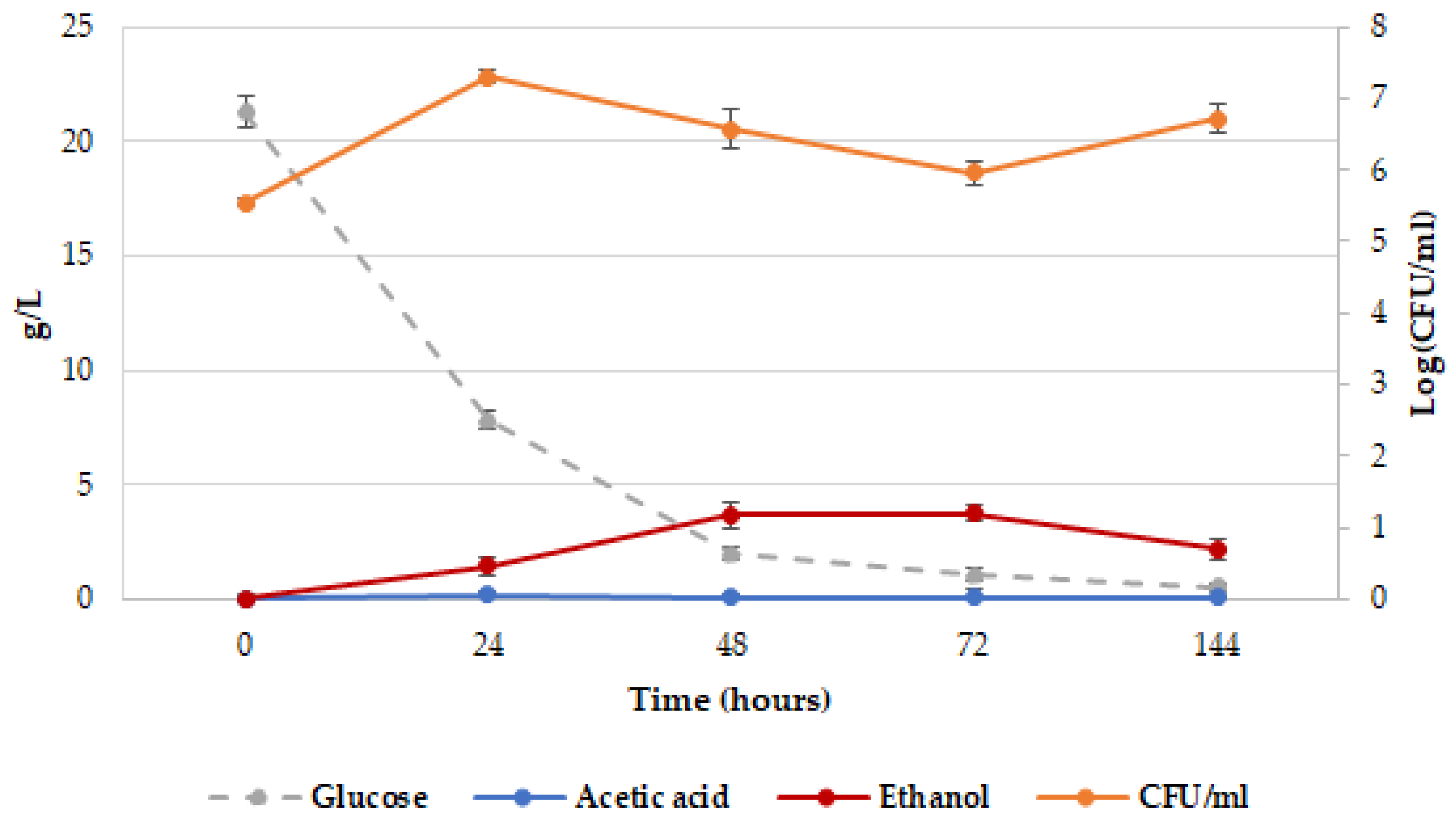
2.2. Release of dTRPs by Z. bailii ATCC36947T
2.3. Different Behavior of the Analyzed Yeasts
2.4. Accumulation of dTRPs in Grape Must
3. Materials and Methods
3.1. Yeast Strain
3.2. Growth Conditions in YNB-Based Media
3.3. Growth Conditions in Grape Must
3.4. TRP Conversion Tests
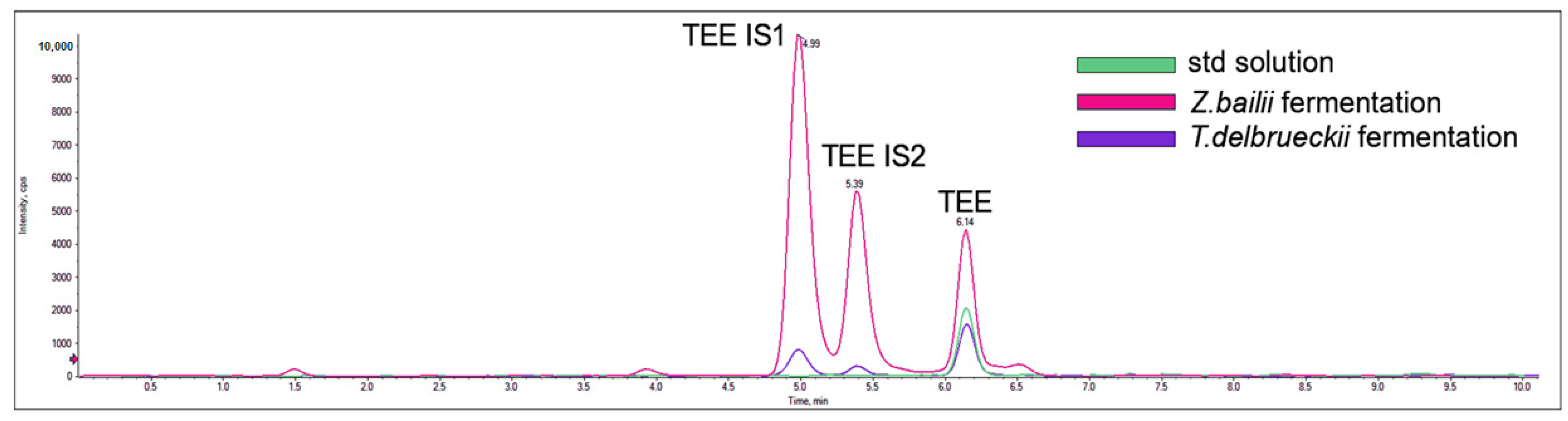
3.5. Chemical Analysis of TRP Derivatives
3.5.1. Purification of Supernatants Deriving from Yeast Fermentation
3.5.2. LC-MS/MS Analysis for the Detection of dTRPs
3.5.3. Validation of the Analytical Method
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TRY | Tryptamine |
| NAC TRY | N-Ac Tryptamine |
| NAcTRP | N-Ac Tryptophan |
| NAcTRP-EE | N-Ac Tryptophan Ethyl Ester |
| 5OH TRY | 5-OH Tryptamine or Serotonin |
| NAC 5OH TRY | N-Ac-5-OH Tryptamine |
| 5OH TRP | 5-OH Tryptophan |
| TRP | Tryptophan |
| TEE | Tryptophan Ethyl Ester |
| TEE_IS1 | TEE isomer 4.99 |
| TEE_IS2 | TEE isomer 5.39 |
| AA | Anthranilic acid |
| 3OH AA | 3-OH Anthranilic acid |
| KYNA | Kynurenic acid |
| KYN | Kynurenine |
| 3OH KYN | 3-OH Kynurenine |
| MEL MEL ISO4.8 | Melatonin or N-Ac-5- OCH3 Tryptamine Melatonin isomer (Rt 4.8 min) |
References
- Salmerón, I. Fermented cereal beverages: From probiotic, prebiotic and synbiotic towards nanoscience designed healthy drinks. Lett. Appl. Microbiol. 2017, 65, 114–124. [Google Scholar] [CrossRef]
- Gupta, A.; Sanwal, N.; Bareen, M.A.; Barua, S.; Sharma, N.; Olatunji, O.J.; Nirmal, N.P.; Sahu, J.K. Trends in functional beverages: Functional ingredients, processing technologies, stability, health benefits, and consumer perspective. Food Res. Int. 2023, 170, 113046. [Google Scholar] [CrossRef] [PubMed]
- The European Parliament and the Council of the European Union. Regulation (ec) no 1924/2006 of the European parliament and of the council on nutrition and health claims made on foods. Off. J. Eur. Union 2006, 404, 9–25. [Google Scholar]
- Corbo, M.R.; Bevilacqua, A.; Petruzzi, L.; Casanova, F.P.; Sinigaglia, M. Functional beverages: The emerging side of functional foods: Commercial trends, research, and health implications. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1192–1206. [Google Scholar] [CrossRef]
- Irkin, R. Natural Fermented Beverages. In Natural Beverages: Volume 13: The Science of Beverages; Academic Press: Cambridge, MA, USA, 2019; pp. 399–425. [Google Scholar]
- Misihairabgwi, J.; Cheikhyoussef, A. Traditional fermented foods and beverages of Namibia. J. Ethn. Foods 2017, 4, 145–153. [Google Scholar] [CrossRef]
- Cong, L.; Bremer, P.; Mirosa, M. Functional beverages in selected countries of asia pacific region: A review. Beverages 2020, 6, 21. [Google Scholar] [CrossRef]
- Yılmaz, C.; Gökmen, V. Neuroactive compounds in foods: Occurrence, mechanism and potential health effects. Food Res. Int. 2020, 128, 108744. [Google Scholar] [CrossRef]
- Asti, A.L.; Crespi, S.; Rampino, T.; Zelini, P.; Gregorini, M.; Pascale, A.; Marchesi, N.; Saccucci, S.; Colombani, C.; Vitalini, S.; et al. Yet another in vitro evidence that natural compounds introduced by diet have anti-amyloidogenic activities and can counteract neurodegenerative disease depending on aging. Nat. Prod. Res. 2024, 38, 861–866. [Google Scholar] [CrossRef]
- Isa, M.H.M.; Shamsudin, N.H.; Al-Shorgani, N.K.N.; Alsharjabi, F.A.; Kalil, M.S. Evaluation of antibacterial potential of biosurfactant produced by surfactin-producing Bacillus isolated from selected Malaysian fermented foods. Food Biotechnol. 2020, 34, 1–24. [Google Scholar]
- Ashalou, T.J. A review on selection of fermentative microorganisms for functional foods and beverages: The production and future perspectives. Food Sci. Technol. 2019, 54, 2511–2519. [Google Scholar] [CrossRef]
- Tofalo, R.; Fusco, V.; Böhnlein, C.; Kabisch, J.; Logrieco, A.F.; Habermann, D.; Cho, G.S.; Benomar, N.; Abriouel, H.; Schmidt-Heydt, M.; et al. The life and times of yeasts in traditional food fermentations. Crit. Rev. Food Sci. Nutr. 2020, 60, 3103–3132. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Cruz, E.; Carrasco-Galàn, F.; Cerezo-Lòpez, A.B.; Valero, E.; Morcillo-Parra, M.A.; Beltran, G.; Torija, M.J.; Troncoso, A.M.; Garcìa-Parrilla, M.C. Occurrence of melatonin and indolic compounds derived from L-tryptophan yeast metabolism in fermented wort and commercial beers. Food Chem. 2020, 331, 127192. [Google Scholar] [CrossRef]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef]
- Garavaglia, J.; Schneider, R.C.; Camargo Mendes, S.D.; Welke, J.E.; Zini, C.A.; Caramão, E.B.; Valente, P. Evaluation of Zygosaccharomyces bailii BCV 08 as a co-starter in wine fermentation for the improvement of ethyl esters production. Microbiol. Res. 2015, 173, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, C.; Gökmen, V. Perspective on the formation, analysis, and health effects of neuroactive compounds in foods. J. Agric. Food Chem. 2021, 69, 13364–13372. [Google Scholar] [CrossRef]
- Fernández-Cruz, E.; Álvarez-Fernández, M.A.; Valero, E.; Troncoso, A.M.; García-Parrilla, M.C. Melatonin and derived l-tryptophan metabolites produced during alcoholic fermentation by different wine yeast strains. Food Chem. 2017, 217, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Fracassetti, D.; Bottelli, P.; Corona, O.; Foschino, R.; Vigentini, I. Innovative alcoholic drinks obtained by co-fermenting grape must and fruit juice. Metabolites 2019, 9, 86. [Google Scholar] [CrossRef]
- Li, S.; Bi, P.; Sun, N.; Gao, Z.; Chen, X.; Guo, J. Characterization of different non-Saccharomyces yeasts via mono-fermentation to produce polyphenol-enriched and fragrant kiwi wine. Food Microbiol. 2022, 103, 103867. [Google Scholar] [CrossRef]
- Roudil, L.; Russo, P.; Berbegal, C.; Albertin, W.; Spano, G.; Capozzi, V. Non-Saccharomyces commercial starter cultures: Scientific trends, recent patents and innovation in the wine sector. Recent Pat. Food Nutr. Agric. 2020, 11, 27–39. [Google Scholar] [CrossRef]
- Xu, Y.; Zhi, Y.; Wu, Q.; Du, R.; Xu, Y. Zygosaccharomyces bailii is a potential producer of various flavor compounds in Chinese maotai-flavor liquor fermentation. Front. Microbiol. 2017, 8, 2609. [Google Scholar] [CrossRef]
- Freire, A.L.; Lacerda Ramos, C.; da Costa Souza, P.N.; Barros Cardoso, M.G.; Freitas Schwan, R. Non-dairy beverage produced by controlled fermentation with potential probiotic starter cultures of lactic acid bacteria and yeast. Int. J. Food Microb. 2017, 248, 39–46. [Google Scholar] [CrossRef]
- Dei Cas, M.; Vigentini, I.; Vitalini, S.; Laganaro, A.; Iriti, M.; Paroni, R.; Foschino, R. Tryptophan derivatives by Saccharomyces cerevisiae EC1118: Evaluation, optimization, and production in a soybean-based medium. Int. J. Mol. Sci. 2021, 22, 472. [Google Scholar] [CrossRef]
- Terefe, S.N.; Augustin, M.A. Fermentation for tailoring the technological and health related functionality of food products. Crit. Rev. Food Sci. Nutr. 2020, 60, 2887–2913. [Google Scholar] [CrossRef] [PubMed]
- Ruddick, J.P.; Evans, A.K.; Nutt, D.J.; Lightman, S.L.; Rook, G.A.; Lowry, C.A. Tryptophan metabolism in the central nervous system: Medical implications. Expert. Rev. Mol. Med. 2006, 8, 1–27. [Google Scholar] [CrossRef]
- Kocadağlı, T.; Yılmaz, C.; Gökmen, V. Determination of melatonin and its isomer in foods by liquid chromatography tandem mass spectrometry. Food Chemist. 2014, 153, 151–156. [Google Scholar] [CrossRef]
- Yılmaz, C.; Gökmen, V. Determination of tryptophan derivatives in kynurenine pathway in fermented foods using liquid chromatography tandem mass spectrometry. Food Chem. 2018, 243, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, K.; Kawai, S.; Murata, K. Secretion of quinolinic acid, an intermediate in the kynurenine pathway, for utilization in NAD+ biosynthesis in the yeast Saccharomyces cerevisiae. Eukaryot. Cell 2013, 12, 648–653. [Google Scholar] [CrossRef]
- Shin, M.; Sano, K.; Umezawa, C. Metabolism of tryptophan to niacin in Saccharomyces uvarum. J. Nutr. Sci. Vitaminol. 1991, 37, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, K.; Chaleckis, R.; Takaine, M.; Wheelock, C.E.; Yoshida, S. Kynurenine aminotransferase activity of Aro8/Aro9 engage tryptophan degradation by producing kynurenic acid in Saccharomyces cerevisiae. Sci. Rep. 2017, 7, 12180. [Google Scholar] [CrossRef]
- Chen, Y.; Guillemin, G.J. Kynurenine pathway metabolites in humans: Disease and healthy states. Int. J. Tryptophan Res. 2009, 2, 1–19. [Google Scholar] [CrossRef]
- Schwarcz, R.; Bruno, J.P.; Muchowski, P.J.; Wu, H.Q. Kynurenines in the mammalian brain: When physiology meets pathology. Nat. Rev. Neurosci. 2012, 13, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Sedlmayr, P.; Blaschitz, A.; Stocker, R. The role of placental tryptophan catabolism. Front. Immunol. 2014, 5, 230. [Google Scholar] [CrossRef] [PubMed]
- Widner, B.; Leblhuber, F.; Frick, B.; Laich, A.; Artner-Dworzak, E.; Fuchs, D. Moderate hyperhomocysteinaemia and immune activation in Parkinson’s disease. J. Neural Transm. 2002, 109, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, J.; Hardeland, R.; Fuhrberg, B.; Han, S.Z. Melatonin and other 5-methoxylated indoles in yeast: Presence in high concentrations and dependence on tryptophan availability. Cytologia 1999, 64, 209–213. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a potent and inducible endogenous antioxidant: Synthesis and metabolism. Molecules 2015, 20, 18886–18906. [Google Scholar] [CrossRef]
- Muniz-Calvo, S.; Bisquert, R.; Fernandez-Cruz, E.; García-Parrilla, M.C.; Guillamón, J.M. Deciphering the melatonin metabolism in Saccharomyces cerevisiae by the bioconversion of related metabolites. J. Pineal Res. 2019, 66, e12554. [Google Scholar] [CrossRef]
- Bisquert, R.; Planells-Cárcel, A.; Alonso-del-Real, J.; Muñiz-Calvo, S.; Guillamón, J.M. The Role of the PAA1 Gene on Melatonin Biosynthesis in Saccharomyces cerevisiae: A Search of New Arylalkylamine N-Acetyltransferases. Microorganisms 2023, 11, 1115. [Google Scholar] [CrossRef]
- Vitalini, S.; Gardana, C.; Simonetti, P.; Fico, G.; Iriti, M. Melatonin, melatonin isomers and stilbenes in Italian traditional grape products and their antiradical capacity. J. Pineal Res. 2013, 54, 322–333. [Google Scholar] [CrossRef]
- Mor, M.; Spadoni, G.; Diamantini, G.; Bedini, A.; Tarzia, G.; Silva, C.; Vacondio, F.; Rivara, M.; Plazzi, P.V.; Franceschini, D.; et al. Antioxidant and cytoprotective activity of indole derivatives related to melatonin. Adv. Exp. Med. Biol. 2003, 527, 567–575. [Google Scholar]
- Mor, M.; Silva, C.; Vacondio, F.; Plazzi, P.V.; Bertoni, S.; Spadoni, G.; Diamantini, G.; Bedini, A.; Tarzia, G.; Zusso, M.; et al. Indole-based analogs of melatonin: In vitro antioxidant and cytoprotective activities. J. Pineal Res. 2004, 36, 95–102. [Google Scholar] [CrossRef]
- Spadoni, G.; Diamantini, G.; Bedini, A.; Tarzia, G.; Vacondio, F.; Silva, C.; Rivara, M.; Mor, M.; Plazzi, P.V.; Zusso, M.; et al. Synthesis, antioxidant activity and structure-activity relationships for a new series of 2-(N-acylaminoethyl)indoles with melatoninlike cytoprotective activity. J. Pineal Res. 2006, 40, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Wang, Z.; Wang, B.; Hou, B.; Cheng, J.; Bai, T.; Zhang, Y.; Wang, W.; Yan, L.; Zhang, J. Expanding the application of tryptophan: Industrial biomanufacturing of tryptophan derivatives. Front. Microbiol. 2023, 14, 1099098. [Google Scholar] [CrossRef]
- Vigentini, I.; Gardana, C.; Fracassetti, D.; Gabrielli, M.; Foschino, R.; Simonetti, P.; Tirelli, A.; Iriti, M. Yeast contribution to melatonin, melatonin isomers and tryptophan ethyl ester during alcoholic fermentation of grape musts. J. Pineal Res. 2015, 58, 388–396. [Google Scholar] [CrossRef]
- Bianchi, F.; van’t Klooster, J.S.; Ruiz, S.J.; Poolman, B. Regulation of amino acid transport in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2019, 83, e00024-19. [Google Scholar] [CrossRef] [PubMed]
- Gardana, C.; Iriti, M.; Stuknytė, M.; De Noni, I.; Simonetti, P. ‘Melatonin isomer’ in wine is not an isomer of the melatonin but tryptophan-ethylester. J. Pineal Res. 2014, 57, 435–441. [Google Scholar] [CrossRef]
- Jonas, A.J.; Butler, I.J. Circumvention of defective neutral amino acid transport in Hartnup disease using tryptophan ethyl ester. J. Clin. Investig. 1989, 84, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Ramprasath, T.; Wang, H.; Zou, M.H. Abnormal kynurenine pathway of tryptophan catabolism in cardiovascular diseases. Cell. Mol. Life Sci. 2017, 74, 2899–2916. [Google Scholar] [CrossRef]
- O’Connor-Cox, E.S.C.; Ingledew, W.M. Wort nitrogenous sources-Their use by brewing yeasts: A review. J. Am. Soc. Brew. Chem. 1989, 47, 102–108. [Google Scholar] [CrossRef]
- Yılmaz, C.; Gökmen, V. Kinetic evaluation of the formation of tryptophan derivatives in the kynurenine pathway during wort fermentation using Saccharomyces pastorianus and Saccharomyces cerevisiae. Food Chem. 2019, 297, 124975. [Google Scholar] [CrossRef]
- Regenberg, B.; During-Olsen, L.; Kielland-Brandt, M.C.; Holmberg, S. Substrate specificity and gene expression of the amino acid permeases in Saccharomyces cerevisiae. Curr. Genet. 1999, 36, 317–328. [Google Scholar] [CrossRef]
- Kumari, S.; Kumar, M.; Gaur, N.A.; Prasad, R. Multiple roles of ABC transporters in yeast. Fungal Genet. Biol. 2021, 150, 103550. [Google Scholar] [CrossRef] [PubMed]
- Li, S. Modulation of immunity by tryptophan microbial metabolites. Front. Nutr. 2023, 10, 1209613. [Google Scholar] [CrossRef] [PubMed]
- Pinu, F.R.; Granucci, N.; Daniell, J.; Han, T.L.; Carneiro, S.; Rocha, I.; Nielsen, J.; Villas-Boas, S.G. Metabolite secretion in microorganisms: The theory of metabolic overflow put to the test. Metabolomics 2018, 14, 43. [Google Scholar] [CrossRef]
- Yılmaz, C.; Gökmen, V. Formation of amino acid derivatives in white and red wines during fermentation: Effects of non-Saccharomyces yeasts and Oenococcus oeni. Food Chem. 2021, 343, 128415. [Google Scholar] [CrossRef] [PubMed]
- Planells-Carcel, A.; Kazakova, J.; Pérez, C.; Gonzalez-Ramirez, M.; Garcia-Parrilla, M.C.; Guillamon, J.M. A consortium of different Saccharomyces species enhances the content of bioactive tryptophan-derived compounds in wine fermentations. Int. J. Food Microbiol. 2024, 416, 110681. [Google Scholar] [CrossRef]
- Planells-Carcel, A.; Quintas, G.; Pardo, J.; Garcia-Rios, E.; Guillamon, J.M. Exploring Proteomic and Metabolomic Interactions in a Yeast Consortium Designed to Enhance Bioactive Compounds in Wine Fermentations. Food Front. 2025, 1, 14. [Google Scholar] [CrossRef]
| TRP | TEE | TEE_IS1 | TEE_IS2 | KYN | KYNA | |
|---|---|---|---|---|---|---|
| TRP | 1 | |||||
| TEE | −0.1893 | 1 | ||||
| TEE_IS1 | −0.9007 | −0.1562 | 1 | |||
| TEE_IS2 | −0.8600 | −0.1324 | 0.9886 | 1 | ||
| KYN | −0.3234 | 0.9747 | −0.0279 | −0.0133 | 1 | |
| KYNA | −0.8943 | 0.4824 | 0.7826 | 0.7939 | 0.5888 | 1 |
| dTRPs (mg/L) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Time (h) | AA | 3OH AA | 3OH KYN | 5OH TRP | TRY | 5OH TRY | NAC-5OH TRY | MEL ISO 4.8 |
| 0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 24 | 0.006 ± 0.002 | 0.002 ± 0.000 | 0.003 ± 0.002 | 0.006 ± 0.002 | 0.001 ± 0.001 | 0.010 ± 0.004 | 0.007 ± 0.000 | 0.274 ± 0.064 |
| 48 | 0.011 ± 0.002 | 0.011 ± 0.009 | 0.020 ± 0.002 | 0.003 ± 0.001 | 0.003 ± 0.001 | 0.015 ± 0.000 | 0.011 ± 0.002 | 0.144 ± 0.033 |
| 72 | 0.006 ± 0.001 | 0.002 ± 0.000 | 0.013 ± 0.002 | 0.007 ± 0.004 | 0.002 ± 0.001 | 0.004 ± 0.001 | 0.002 ± 0.000 | 0.572 ± 0.134 |
| 144 | 0.006 ± 0.003 | 0.001 ± 0.001 | 0.022 ± 0.013 | 0.007 ± 0.004 | 0.001 ± 0.000 | 0.012 ± 0.000 | 0.002 ± 0.001 | 0.439 ± 0.244 |
| TRP | TEE | TEE_IS1 | TEE_IS2 | KYN | KYNA | AA | 3OH AA | 3OH KYN | 5OH TRP | TRY | 5OH TRY | NAC-5OH TRY | MEL ISO 4.8 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TRP | 1 | |||||||||||||
| TEE | 0.2647 | 1 | ||||||||||||
| TEE_IS1 | −0.7434 | −0.0869 | 1 | |||||||||||
| TEE_IS2 | −0.7222 | −0.1052 | 0.9984 | 1 | ||||||||||
| KYN | −0.7184 | −0.0569 | 0.9835 | 0.9792 | 1 | |||||||||
| KYNA | −0.2967 | 0.6576 | 0.1853 | 0.1700 | 0.2209 | 1 | ||||||||
| AA | −0.2909 | 0.7152 | 0.2491 | 0.2288 | 0.2713 | 0.9091 | 1 | |||||||
| 3OH AA | −0.0127 | 0.5219 | −0.1305 | −0.1482 | −0.1237 | 0.6713 | 0.7101 | 1 | ||||||
| 3OH KYNA | −0.6451 | 0.1443 | 0.5631 | 0.5646 | 0.5520 | 0.7708 | 0.7058 | 0.3937 | 1 | |||||
| 5OH TRP | −0.5570 | 0.1546 | 0.8518 | 0.8461 | 0.8569 | 0.2814 | 0.3449 | −0.1445 | 0.5473 | 1 | ||||
| TRY | −0.2192 | 0.6963 | 0.2101 | 0.1839 | 0.3107 | 0.8391 | 0.7527 | 0.4008 | 0.5246 | 0.4555 | 1 | |||
| 5OH TRY | −0.2534 | 0.6965 | 0.0521 | 0.0265 | 0.0364 | 0.8222 | 0.8298 | 0.5636 | 0.5872 | 0.3225 | 0.7200 | 1 | ||
| NAC-5OH TRY | 0.1981 | 0.9422 | −0.0877 | −0.1015 | −0.0736 | 0.7648 | 0.8183 | 0.7314 | 0.3017 | 0.0425 | 0.6346 | 0.7084 | 1 | |
| MEL ISO 4.8 | −0.7436 | −0.0868 | 1 | 0.9984 | 0.9835 | 0.1854 | 0.2493 | −0.1301 | 0.5632 | 0.8518 | 0.2102 | 0.0522 | −0.0876 | 1 |
| Cabernet Must | T. delbrueckii CBS1146T | Z. bailii ATCC36947T | |
|---|---|---|---|
| Fermentation Time | Initial | Final | Final |
| Counts (log CFU/mL) | 6.25 ± 0.2 | 7.9 ± 0.3 | 7.6 ± 0.4 |
| Glucose (g/L) | 106.12 ± 3.58 | 13.53 ± 1.35 | 54.99 ± 9.66 |
| Fructose (g/L) | 110.64 ± 9.19 | 26.30 ± 2.23 | 7.91 ± 0.68 |
| Ethanol (ml/100 mL) | 0.04 ± 0.01 | 10.00 ± 0.45 | 8.45 ± 1.67 |
| Acetic acid (g/L) | 0.56 ± 0.06 | 0.95 ± 0.25 | 0.61 ± 0.03 |
| TRP (mg/L) | 104.2 ± 16.7 | 38.7 ± 2.8 | 0.8 ± 0.6 |
| 5OH-TRP (mg/L) | 18.9 ± 8.9 | 17.2 ± 9.0 | 9.5 ± 1.4 |
| TEE (mg/L) | 1.3 ± 0.3 | 30.5 ± 5.6 | 11.1 ± 0.8 |
| TEE_IS1 (mg/L) | n.d. | 24.0 ± 1.5 | 7.1 ± 0.9 |
| TEE_IS2 (mg/L) | n.d. | 9.7 ± 1.5 | 2.9 ± 0.5 |
| KYN (mg/L) | 63.0 ± 40.9 | 86.4 ± 0.6 | 6.5 ± 0.5 |
| KYNA (mg/L) | 61.2 ± 11.7 | 336.2 ± 11.0 | 299.2 ± 8.4 |
| AA (mg/L) | 7.5 ± 1.2 | 2.5 ± 1.1 | 3.3 ± 0.6 |
| 3OH AA (mg/L) | 1.0 ± 0.6 | 26.8 ± 4.9 | 36.2 ± 1.5 |
| 3OH KYN (mg/L) | 12.4 ± 1.3 | 28.3 ± 5.5 | 22.0 ± 2.9 |
| TRY (mg/L) | 5.1 ± 3.2 | 4.5 ± 2.7 | 24.6 ± 1.7 |
| 5OH TRY (mg/L) | 14.1 ± 2.1 | 15.9 ± 4.0 | 7.0 ± 2.9 |
| NAC-TRY (mg/L) | 1.2 ± 0.6 | 3.8 ± 1.0 | 2.9 ± 2.3 |
| NAC-5OH TRY (mg/L) | 3.1 ± 2.7 | n.d. | n.d. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Canito, A.; Dei Cas, M.; Vitalini, S.; Iriti, M.; Paroni, R.; Vigentini, I.; Foschino, R. Compounds Derived from Tryptophan Metabolism in Torulaspora delbrueckii CBS1146T and Zygosaccharomyces bailii ATCC36947T. Int. J. Mol. Sci. 2025, 26, 4301. https://doi.org/10.3390/ijms26094301
Di Canito A, Dei Cas M, Vitalini S, Iriti M, Paroni R, Vigentini I, Foschino R. Compounds Derived from Tryptophan Metabolism in Torulaspora delbrueckii CBS1146T and Zygosaccharomyces bailii ATCC36947T. International Journal of Molecular Sciences. 2025; 26(9):4301. https://doi.org/10.3390/ijms26094301
Chicago/Turabian StyleDi Canito, Alessandra, Michele Dei Cas, Sara Vitalini, Marcello Iriti, Rita Paroni, Ileana Vigentini, and Roberto Foschino. 2025. "Compounds Derived from Tryptophan Metabolism in Torulaspora delbrueckii CBS1146T and Zygosaccharomyces bailii ATCC36947T" International Journal of Molecular Sciences 26, no. 9: 4301. https://doi.org/10.3390/ijms26094301
APA StyleDi Canito, A., Dei Cas, M., Vitalini, S., Iriti, M., Paroni, R., Vigentini, I., & Foschino, R. (2025). Compounds Derived from Tryptophan Metabolism in Torulaspora delbrueckii CBS1146T and Zygosaccharomyces bailii ATCC36947T. International Journal of Molecular Sciences, 26(9), 4301. https://doi.org/10.3390/ijms26094301












