Active Substances from the Micro-Immunotherapy Medicine 2LC1® Show In Vitro Anti-Cancer Properties in Colon, Prostate, and Breast Cancer Models and Immune-Enhancing Capabilities in Human Macrophages
Abstract
1. Introduction
2. Results
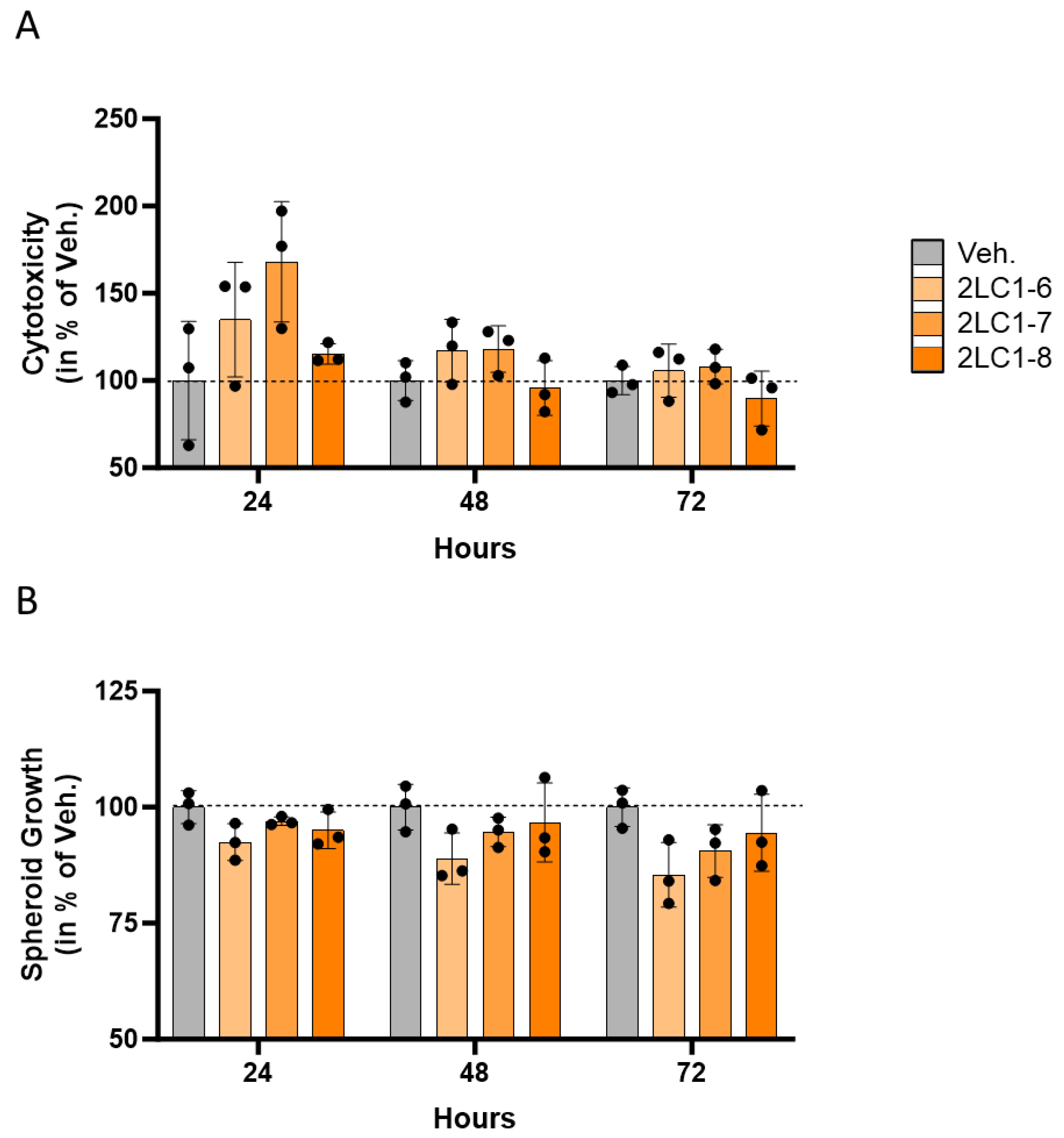
2.1. Actives from 2LC1 Display Cytotoxic and Anti-Proliferative Effects in a Three-Dimensional In Vitro Model of Colon Cancer Spheroids
2.2. Actives from 2LC1 Reduced the Clonogenic Capabilities of Cancer Cells
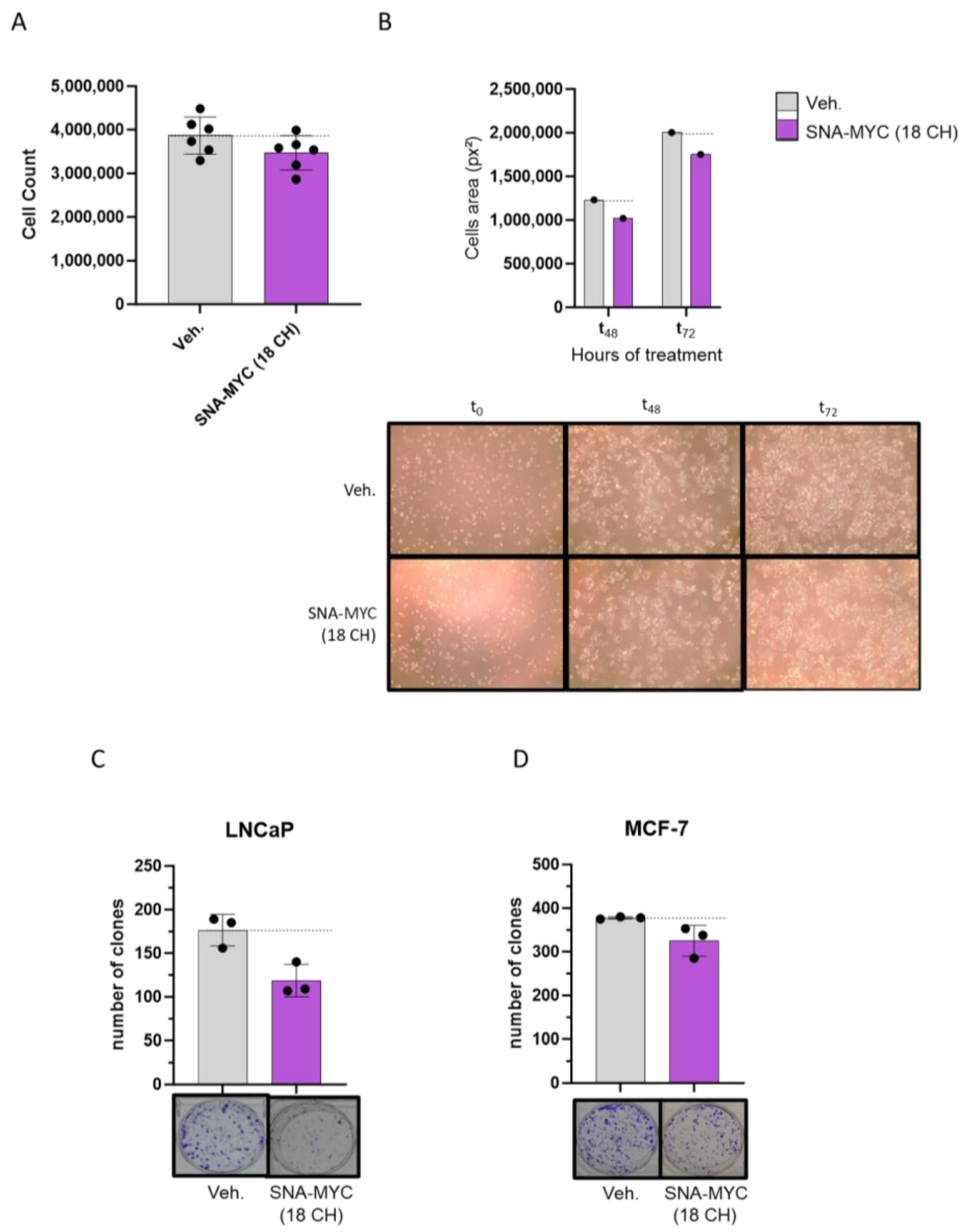
2.3. SNA-MYC (18 CH), Displayed Anti-Proliferative and Anti-Clonogenic Properties in Cancer Cells
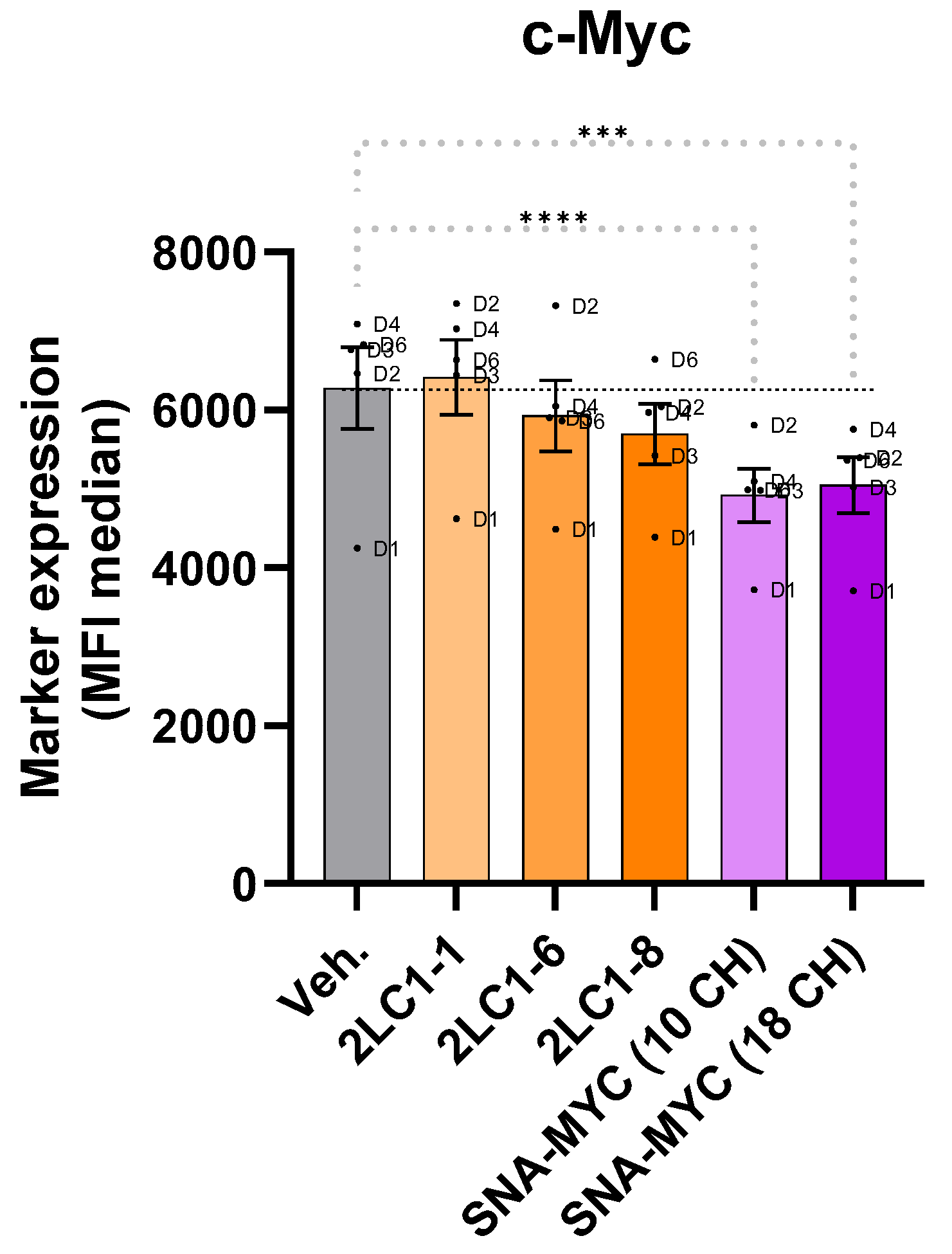
2.4. Actives from 2LC1 Reduced the Expression of C-MYC in a Model of Human CD14+-Derived M2c Macrophages
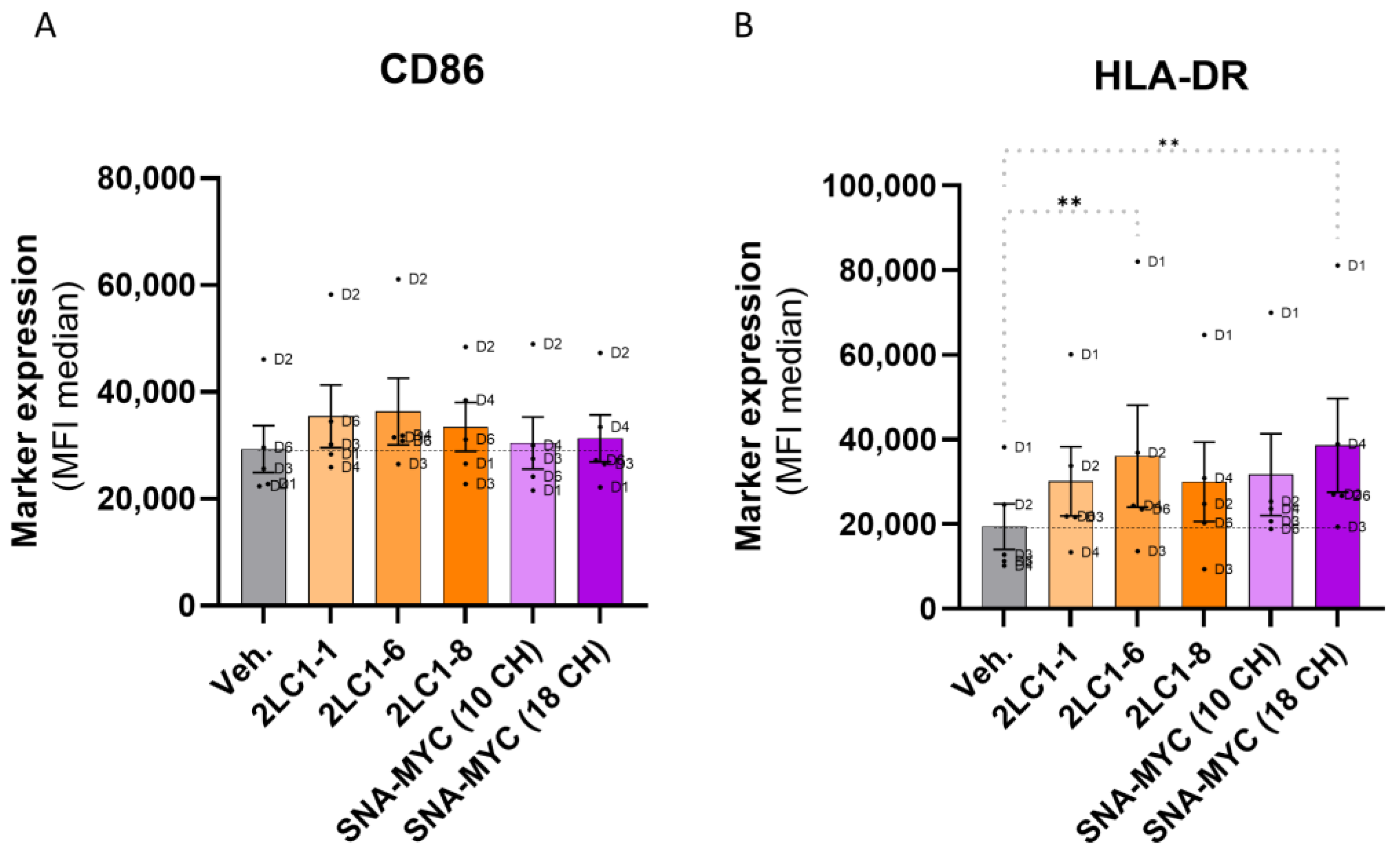
2.5. Actives from 2LC1 Increased the Expression of CD86 and HLA-DR
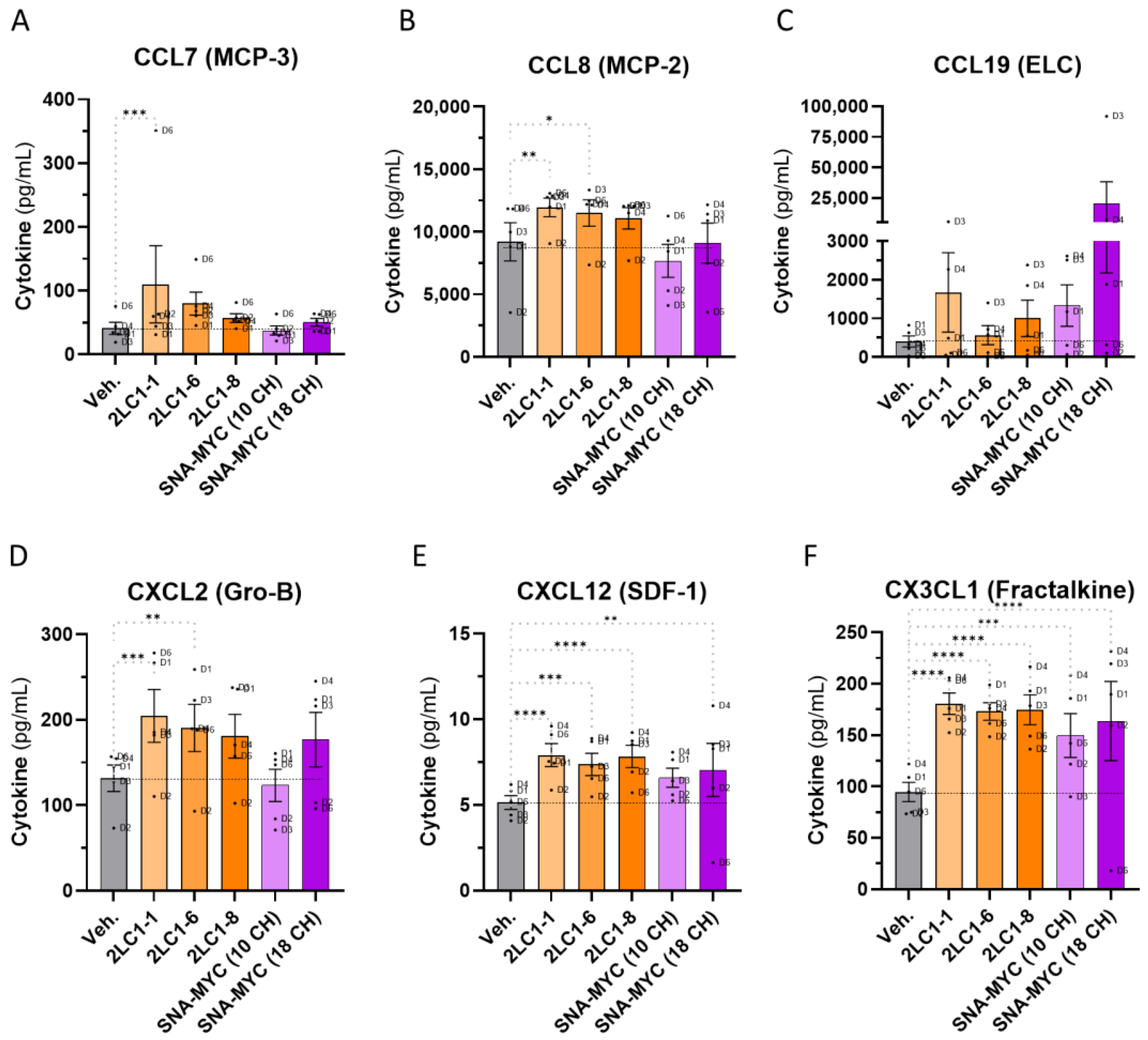
2.6. Actives from 2LC1 Increased the Secretion of Chemokines in a Model of Human CD14+-Derived M2c Macrophages
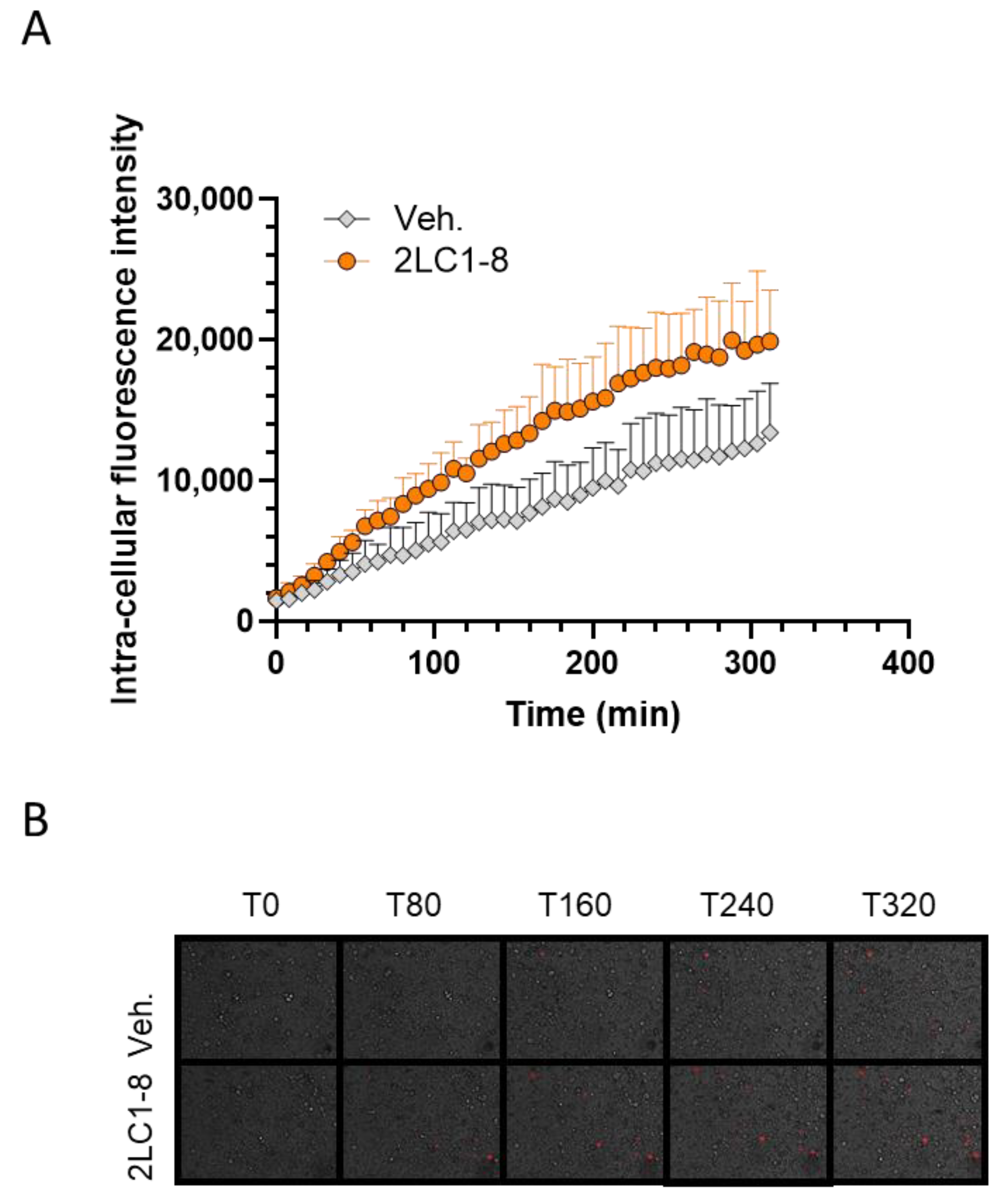
2.7. 2LC1-8 Enhances the Phagocytic Capabilities of Macrophages In Vitro
3. Discussion
4. Materials and Methods
4.1. Tested Items and Experimental Controls
4.2. Colon Cancer In Vitro Model
4.2.1. Initiation of the Spheroid Model
4.2.2. Kinetics of 3D Spheroid Cytotoxicity and Growth
4.2.3. Data Analysis
4.3. Colon Cancer Animal Model
4.4. Evaluation of the Clonogenic Capabilities
4.5. Evaluation of the Anti-Proliferative Activity of the SNA-MYC (18 CH) in Colon Cancer Cells
4.6. Evaluation of the Macrophages Profiles
4.7. Phagocytosis Capabilities Assessment
4.7.1. In Human Monocytes-Derived Macrophages
4.7.2. In Human Granulocytes
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Shurin, M.R. Cancer as an immune-mediated disease. ImmunoTargets Ther. 2012, 1, 1–6. [Google Scholar] [CrossRef]
- Song, K. Current Development Status of Cytokines for Cancer Immunotherapy. Biomol. Ther. 2024, 32, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Fyfe, G.; Fisher, R.I.; Rosenberg, S.A.; Sznol, M.; Parkinson, D.R.; Louie, A.C. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1995, 13, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, A.; Lindenmann, J. Virus interference: I. The interferon. By Alick Isaacs and Jean Lindenmann, 1957. CA Cancer J. Clin. 1988, 38, 280–290. [Google Scholar] [CrossRef]
- Jacques, C.; Marchesi, I.; Fiorentino, F.P.; Chatelais, M.; Lilli, N.L.; Appel, K.; Lejeune, B.; Floris, I. A Micro-Immunotherapy Sequential Medicine MIM-seq Displays Immunomodulatory Effects on Human Macrophages and Anti-Tumor Properties towards In Vitro 2D and 3D Models of Colon Carcinoma and in an In Vivo Subcutaneous Xenograft Colon Carcinoma Model. Int. J. Mol. Sci. 2022, 23, 6059. [Google Scholar] [CrossRef]
- Jacques, C.; Chatelais, M.; Fekir, K.; Brulefert, A.; Floris, I. The Unitary Micro-Immunotherapy Medicine Interferon-γ (4 CH) Displays Similar Immunostimulatory and Immunomodulatory Effects than Those of Biologically Active Human Interferon-γ on Various Cell Types. Int. J. Mol. Sci. 2022, 23, 2314. [Google Scholar] [CrossRef]
- Jacques, C.; Marchand, F.; Chatelais, M.; Brulefert, A.; Riffault, M.; Floris, I. In Vitro Study of Interleukin-6 when Used at Low Dose and Ultra-Low Dose in Micro-Immunotherapy. Life 2024, 14, 375. [Google Scholar] [CrossRef] [PubMed]
- Elbadawy, M.; Usui, T.; Yamawaki, H.; Sasaki, K. Emerging Roles of C-Myc in Cancer Stem Cell-Related Signaling and Resistance to Cancer Chemotherapy: A Potential Therapeutic Target Against Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 2340. [Google Scholar] [CrossRef]
- Rochlitz, C.F.; Herrmann, R.; de Kant, E. Overexpression and amplification of c-myc during progression of human colorectal cancer. Oncology 1996, 53, 448–454. [Google Scholar] [CrossRef]
- Strippoli, A.; Cocomazzi, A.; Basso, M.; Cenci, T.; Ricci, R.; Pierconti, F.; Cassano, A.; Fiorentino, V.; Barone, C.; Bria, E.; et al. c-MYC Expression Is a Possible Keystone in the Colorectal Cancer Resistance to EGFR Inhibitors. Cancers 2020, 12, 638. [Google Scholar] [CrossRef]
- Gao, F.; Li, X.; Xu, K.; Wang, R.; Guan, X. c-MYC mediates the crosstalk between breast cancer cells and tumor microenvironment. Cell Commun. Signal. 2023, 21, 28. [Google Scholar] [CrossRef] [PubMed]
- Fallah, Y.; Brundage, J.; Allegakoen, P.; Shajahan-Haq, A.N. MYC-Driven Pathways in Breast Cancer Subtypes. Biomolecules 2017, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, D.; Brambillasca, C.S.; Talens, F.; Bhin, J.; Linstra, R.; Romanens, L.; Bhattacharya, A.; Joosten, S.E.P.; Da Silva, A.M.; Padrao, N.; et al. MYC promotes immune-suppression in triple-negative breast cancer via inhibition of interferon signaling. Nat. Commun. 2022, 13, 6579. [Google Scholar] [CrossRef]
- Qiu, X.; Boufaied, N.; Hallal, T.; Feit, A.; de Polo, A.; Luoma, A.M.; Alahmadi, W.; Larocque, J.; Zadra, G.; Xie, Y.; et al. MYC drives aggressive prostate cancer by disrupting transcriptional pause release at androgen receptor targets. Nat. Commun. 2022, 13, 2559. [Google Scholar] [CrossRef] [PubMed]
- Koh, C.M.; Bieberich, C.J.; Dang, C.V.; Nelson, W.G.; Yegnasubramanian, S.; De Marzo, A.M. MYC and Prostate Cancer. Genes Cancer. 2010, 1, 617–628. [Google Scholar] [CrossRef]
- Witsch, E.; Sela, M.; Yarden, Y. Roles for Growth Factors in Cancer Progression. Physiology 2010, 25, 85–101. [Google Scholar] [CrossRef]
- Thompson-Elliott, B.; Johnson, R.; Khan, S.A. Alterations in TGFβ signaling during prostate cancer progression. Am. J. Clin. Exp. Urol. 2021, 9, 318–328. [Google Scholar]
- de Kruijf, E.M.; Dekker, T.J.A.; Hawinkels, L.J.A.C.; Putter, H.; Smit, V.T.H.B.M.; Kroep, J.R.; Kuppen, P.J.K.; van de Velde, C.J.H.; ten Dijke, P.; Tollenaar, R.A.E.M.; et al. The prognostic role of TGF-β signaling pathway in breast cancer patients. Ann. Oncol. 2013, 24, 384–390. [Google Scholar] [CrossRef]
- Tauriello, D.V.F.; Palomo-Ponce, S.; Stork, D.; Berenguer-Llergo, A.; Badia-Ramentol, J.; Iglesias, M.; Sevillano, M.; Ibiza, S.; Cañellas, A.; Hernando-Momblona, X.; et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 2018, 554, 538–543. [Google Scholar] [CrossRef]
- Uttamsingh, S.; Bao, X.; Nguyen, K.T.; Bhanot, M.; Gong, J.; Chan, J.L.-K.; Liu, F.; Chu, T.T.; Wang, L.-H. Synergistic effect between EGF and TGF-beta1 in inducing oncogenic properties of intestinal epithelial cells. Oncogene 2008, 27, 2626–2634. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, J.; Fan, Y.; Wang, Z.; Tian, R.; Ji, W.; Zhang, F.; Niu, R. TGF-β transactivates EGFR and facilitates breast cancer migration and invasion through canonical Smad3 and ERK/Sp1 signaling pathways. Mol. Oncol. 2018, 12, 305–321. [Google Scholar] [CrossRef]
- Masuda, H.; Zhang, D.; Bartholomeusz, C.; Doihara, H.; Hortobagyi, G.N.; Ueno, N.T. Role of Epidermal Growth Factor Receptor in Breast Cancer. Breast Cancer Res. Treat. 2012, 136, 331–345. [Google Scholar] [CrossRef]
- Tang, L.-Y.; Heller, M.; Meng, Z.; Yu, L.-R.; Tang, Y.; Zhou, M.; Zhang, Y.E. Transforming Growth Factor-β (TGF-β) Directly Activates the JAK1-STAT3 Axis to Induce Hepatic Fibrosis in Coordination with the SMAD Pathway. J. Biol. Chem. 2017, 292, 4302–4312. [Google Scholar] [CrossRef]
- Lin, S.-R.; Yeh, H.-L.; Liu, Y.-N. Interplay of Epidermal Growth Factor Receptor and Signal Transducer and Activator of Transcription 3 in Prostate Cancer: Beyond Androgen Receptor Transactivation. Cancers 2021, 13, 3452. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, U.; Cerasi, D.; Taylor, I.; Kocherginsky, M.; Tekin, U.; Badal, S.; Aluri, L.; Sehdev, A.; Cerda, S.; Mustafi, R.; et al. EGFR is required for Colonic Tumor Promotion by Dietary Fat in the Azoxymethane/Dextran Sulfate Sodium Model: Roles of TGF-α and PTGS2. Clin. Cancer Res. 2009, 15, 6780–6789. [Google Scholar] [CrossRef]
- Furgiuele, S.; Descamps, G.; Cascarano, L.; Boucq, A.; Dubois, C.; Journe, F.; Saussez, S. Dealing with Macrophage Plasticity to Address Therapeutic Challenges in Head and Neck Cancers. Int. J. Mol. Sci. 2022, 23, 6385. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Geng, X.; Hou, J.; Wu, G. New insights into M1/M2 macrophages: Key modulators in cancer progression. Cancer Cell Int. 2021, 21, 389. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Cui, K.; Zeng, L.; Li, M.; Fan, Y.; Shi, P.; Wang, Z.; Su, W.; Wang, H. Advance in the role of chemokines/chemokine receptors in carcinogenesis: Focus on pancreatic cancer. Eur. J. Pharmacol. 2024, 967, 176357. [Google Scholar] [CrossRef]
- Feng, X.; Zhu, S.; Qiao, J.; Ji, Z.; Zhou, B.; Xu, W. CX3CL1 promotes M1 macrophage polarization and osteoclast differentiation through NF-κB signaling pathway in ankylosing spondylitis in vitro. J. Transl. Med. 2023, 21, 573. [Google Scholar] [CrossRef]
- Pello, O.M.; De Pizzol, M.; Mirolo, M.; Soucek, L.; Zammataro, L.; Amabile, A.; Doni, A.; Nebuloni, M.; Swigart, L.B.; Evan, G.I.; et al. Role of c-MYC in alternative activation of human macrophages and tumor-associated macrophage biology. Blood 2012, 119, 411–421. [Google Scholar] [CrossRef]
- Tan, J.; Yang, L.; Zhao, H.; Ai, Y.; Ren, L.; Zhang, F.; Dong, W.; Shi, R.; Sun, D.; Feng, Y. The role of NFATc1/c-myc/PKM2/IL-10 axis in activating cervical cancer tumor-associated M2 macrophage polarization to promote cervical cancer progression. Exp. Cell Res. 2022, 413, 113052. [Google Scholar] [CrossRef]
- Zhang, Q.; Sioud, M. Tumor-Associated Macrophage Subsets: Shaping Polarization and Targeting. Int. J. Mol. Sci. 2023, 24, 7493. [Google Scholar] [CrossRef]
- Hung, C.-H.; Chen, F.-M.; Lin, Y.-C.; Tsai, M.-L.; Wang, S.-L.; Chen, Y.-C.; Chen, Y.-T.; Hou, M.-F. Altered monocyte differentiation and macrophage polarization patterns in patients with breast cancer. BMC Cancer 2018, 18, 366. [Google Scholar] [CrossRef]
- Kunz-Schughart, L.A.; Freyer, J.P.; Hofstaedter, F.; Ebner, R. The use of 3-D cultures for high-throughput screening: The multicellular spheroid model. J. Biomol. Screen. 2004, 9, 273–285. [Google Scholar] [CrossRef]
- Zhang, H.-L.; Wang, P.; Lu, M.-Z.; Zhang, S.-D.; Zheng, L. c-Myc maintains the self-renewal and chemoresistance properties of colon cancer stem cells. Oncol. Lett. 2019, 17, 4487–4493. [Google Scholar] [CrossRef] [PubMed]
- Pello, O.M.; Andrés, V. Role of c-MYC in tumor-associated macrophages and cancer progression. Oncoimmunology 2013, 2, e22984. [Google Scholar] [CrossRef]
- Aras, S.; Zaidi, M.R. TAMeless traitors: Macrophages in cancer progression and metastasis. Br. J. Cancer. 2017, 117, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P.; Marchesi, F.; Garlanda, C. Macrophages as tools and targets in cancer therapy. Nat. Rev. Drug Discov. 2022, 21, 799–820. [Google Scholar] [CrossRef] [PubMed]
- Jacques, C.; Chatelais, M.; Fekir, K.; Fauconnier, L.; Mellier, M.; Togbe, D.; Floris, I. The Micro-Immunotherapy Medicine 2LEID Exhibits an Immunostimulant Effect by Boosting Both Innate and Adaptive Immune Responses. Int. J. Mol. Sci. 2021, 23, 110. [Google Scholar] [CrossRef]
- Ma, W.; Liu, Y.; Ellison, N.; Shen, J. Induction of C-X-C chemokine receptor type 7 (CXCR7) switches stromal cell-derived factor-1 (SDF-1) signaling and phagocytic activity in macrophages linked to atherosclerosis. J. Biol. Chem. 2013, 288, 15481–15494. [Google Scholar] [CrossRef]
- Tsai, W.-H.; Chang, S.-C.; Lin, Y.-C.; Hsu, H.-C. CX3CL1(+) Microparticles-Induced MFG-E8 Enhances Apoptotic Cell Clearance by Alveolar Macrophages. Cells 2021, 10, 2583. [Google Scholar] [CrossRef] [PubMed]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Anfray, C.; Ummarino, A.; Torres Andón, F.; Allavena, P. Current Strategies to Target Tumor-Associated-Macrophages to Improve Anti-Tumor Immune Responses. Cells 2019, 9, 46. [Google Scholar] [CrossRef]
- Dang, C.V. MYC, metabolism, cell growth, and tumorigenesis. Cold Spring Harb. Perspect. Med. 2013, 3, a014217. [Google Scholar] [CrossRef] [PubMed]
- Napoli, S.; Negri, U.; Arcamone, F.; Capobianco, M.L.; Carbone, G.M.; Catapano, C.V. Growth inhibition and apoptosis induced by daunomycin-conjugated triplex-forming oligonucleotides targeting the c-myc gene in prostate cancer cells. Nucleic Acids Res. 2006, 34, 734–744. [Google Scholar] [CrossRef]
- Duluc, D.; Delneste, Y.; Tan, F.; Moles, M.-P.; Grimaud, L.; Lenoir, J.; Preisser, L.; Anegon, I.; Catala, L.; Ifrah, N.; et al. Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells. Blood 2007, 110, 4319–4330. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Yang, D.; Shen, L.; Fang, J.; Khan, R.; Liu, D. Overexpression of CD86 enhances the ability of THP-1 macrophages to defend against Talaromyces marneffei. Immun. Inflamm. Dis. 2022, 10, e740. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, Y.; Liu, L.; Wu, Y.; Xiong, X. Crucial biological functions of CCL7 in cancer. PeerJ 2018, 6, e4928. [Google Scholar] [CrossRef]
- Ji, J.; Wang, P.; Zhou, Q.; Zhu, L.; Zhang, H.; Zhang, Y.; Zheng, Z.; Bhatta, A.K.; Zhang, G.; Wang, X. CCL8 enhances sensitivity of cutaneous squamous cell carcinoma to photodynamic therapy by recruiting M1 macrophages. Photodiagnosis Photodyn. Ther. 2019, 26, 235–243. [Google Scholar] [CrossRef]
- Xuan, W.; Qu, Q.; Zheng, B.; Xiong, S.; Fan, G.-H. The chemotaxis of M1 and M2 macrophages is regulated by different chemokines. J. Leukoc. Biol. 2015, 97, 61–69. [Google Scholar] [CrossRef]
- Rouault, C.; Pellegrinelli, V.; Schilch, R.; Cotillard, A.; Poitou, C.; Tordjman, J.; Sell, H.; Clément, K.; Lacasa, D. Roles of Chemokine Ligand-2 (CXCL2) and Neutrophils in Influencing Endothelial Cell Function and Inflammation of Human Adipose Tissue. Endocrinology 2013, 154, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Karin, N. The multiple faces of CXCL12 (SDF-1α) in the regulation of immunity during health and disease. J. Leukoc. Biol. 2010, 88, 463–473. [Google Scholar] [CrossRef]
- dos Reis, F.D.; Jerónimo, C.; Correia, M.P. Epigenetic modulation and prostate cancer: Paving the way for NK cell anti-tumor immunity. Front. Immunol. 2023, 14, 1152572. [Google Scholar] [CrossRef]
- Mozaffari, F.; Lindemalm, C.; Choudhury, A.; Granstam-Björneklett, H.; Helander, I.; Lekander, M.; Mikaelsson, E.; Nilsson, B.; Ojutkangas, M.-L.; Österborg, A.; et al. NK-cell and T-cell functions in patients with breast cancer: Effects of surgery and adjuvant chemo- and radiotherapy. Br. J. Cancer 2007, 97, 105–111. [Google Scholar] [CrossRef]
- Gong, Q.; Jiang, Y.; Pan, X.; You, Y. Fractalkine aggravates LPS-induced macrophage activation and acute kidney injury via Wnt/β-catenin signalling pathway. J. Cell Mol. Med. 2021, 25, 6963–6975. [Google Scholar] [CrossRef] [PubMed]
- Ohta, M.; Tanaka, F.; Yamaguchi, H.; Sadanaga, N.; Inoue, H.; Mori, M. The high expression of Fractalkine results in a better prognosis for colorectal cancer patients. Int. J. Oncol. 2005, 26, 41–47. [Google Scholar] [CrossRef]
- Park, M.H.; Lee, J.S.; Yoon, J.H. High expression of CX3CL1 by tumor cells correlates with a good prognosis and increased tumor-infiltrating CD8+ T cells, natural killer cells, and dendritic cells in breast carcinoma. J. Surg. Oncol. 2012, 106, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Hoki, T.; Oba, T.; Saito, H.; Attwood, K.; Sabel, M.S.; Chang, A.E.; Odunsi, K.; Ito, F. CX3CR1-CD8+ T cells are critical in antitumor efficacy but functionally suppressed in the tumor microenvironment. JCI Insight 2020, 5, e133920. [Google Scholar] [CrossRef]
- Tang, L.; Hu, H.-D.; Hu, P.; Lan, Y.-H.; Peng, M.-L.; Chen, M.; Ren, H. Gene therapy with CX3CL1/Fractalkine induces antitumor immunity to regress effectively mouse hepatocellular carcinoma. Gene Ther. 2007, 14, 1226–1234. [Google Scholar] [CrossRef]
- Nukiwa, M.; Andarini, S.; Zaini, J.; Xin, H.; Kanehira, M.; Suzuki, T.; Fukuhara, T.; Mizuguchi, H.; Hayakawa, T.; Saijo, Y.; et al. Dendritic cells modified to express fractalkine/CX3CL1 in the treatment of preexisting tumors. Eur. J. Immunol. 2006, 36, 1019–1027. [Google Scholar] [CrossRef]
- Siddiqui, I.; Erreni, M.; van Brakel, M.; Debets, R.; Allavena, P. Enhanced recruitment of genetically modified CX3CR1-positive human T cells into Fractalkine/CX3CL1 expressing tumors: Importance of the chemokine gradient. J. Immunother. Cancer 2016, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Floris, I.; Appel, K.; Rose, T.; Lejeune, B. 2LARTH®, a micro-immunotherapy medicine, exerts anti-inflammatory effects in vitro and reduces TNF-α and IL-1β secretion. J. Inflamm. Res. 2018, 11, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Floris, I.; Rose, T.; Rojas, J.A.C.; Appel, K.; Roesch, C.; Lejeune, B. Pro-Inflammatory Cytokines at Ultra-Low Dose Exert Anti-Inflammatory Effect In Vitro: A Possible Mode of Action Involving Sub-Micron Particles? Dose-Response 2020, 18, 1559325820961723. [Google Scholar] [CrossRef]
- Floris, I.; García-González, V.; Palomares, B.; Appel, K.; Lejeune, B. The Micro-Immunotherapy Medicine 2LARTH® Reduces Inflammation and Symptoms of Rheumatoid Arthritis In Vivo. Int. J. Rheumatol. 2020, 2020, 1594573. [Google Scholar] [CrossRef] [PubMed]
- Obinu, A.; Rassu, G.; Corona, P.; Maestri, M.; Riva, F.; Miele, D.; Giunchedi, P.; Gavini, E. Poly (ethyl 2-cyanoacrylate) nanoparticles (PECA-NPs) as possible agents in tumor treatment. Colloids Surf. B Biointerfaces 2019, 177, 520–528. [Google Scholar] [CrossRef]
- Fiorentino, F.P.; Marchesi, I.; Schröder, C.; Schmidt, R.; Yokota, J.; Bagella, L. BET-Inhibitor I-BET762 and PARP-Inhibitor Talazoparib Synergy in Small Cell Lung Cancer Cells. Int. J. Mol. Sci. 2020, 21, 9595. [Google Scholar] [CrossRef]
- Vaux, D.L. Research methods: Know when your numbers are significant. Nature 2012, 492, 180–191. [Google Scholar] [CrossRef]

| Starting Materials (CH) | 2LC1-1 | 2LC1-6 | 2LC1-7 | 2LC1-8 |
|---|---|---|---|---|
| hr-IL-1β | 6 | 10 | 10 | 10 |
| hr-IL-2 | 10 | 10 | 10 | 10 |
| hr-IL-4 | 10 | 10 | 10 | 10 |
| hr-IL-6 | 6 | 10 | 10 | 10 |
| hr-IL-7 | 10 | 10 | 10 | 10 |
| hr-IFN-α | 10 | 10 | 10 | 10 |
| hr-IFN-γ | 10 | 10 | 10 | 6 |
| Dimethylsulfoxyde | 10 | 10 | 3 | 10 |
| hr-EGF | 10 | 10 | 15 | 10 |
| hr-TGF-β | 10 | 10 | 30 | 10 |
| hr-GM-CSF | 10 | 10 | 10 | 10 |
| hr-TNF-α | 5 | 10 | 10 | 5 |
| SNA-HLA-I | 10 | 10 | 10 | 10 |
| SNA-HLA-II | 10 | 10 | 10 | 10 |
| DNA (K) | 10 | 10 | 12 | 30 |
| RNA (K) | 6 | 10 | 10 | 10 |
| SNA-C1a | 10 | 10 | 10 | 10 |
| SNA-C1b (including SNA-MYC) | 10 | 18 | 10 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacques, C.; Marchesi, I.; Fiorentino, F.P.; Marchand, F.; Chatelais, M.; Floris, I. Active Substances from the Micro-Immunotherapy Medicine 2LC1® Show In Vitro Anti-Cancer Properties in Colon, Prostate, and Breast Cancer Models and Immune-Enhancing Capabilities in Human Macrophages. Int. J. Mol. Sci. 2025, 26, 4300. https://doi.org/10.3390/ijms26094300
Jacques C, Marchesi I, Fiorentino FP, Marchand F, Chatelais M, Floris I. Active Substances from the Micro-Immunotherapy Medicine 2LC1® Show In Vitro Anti-Cancer Properties in Colon, Prostate, and Breast Cancer Models and Immune-Enhancing Capabilities in Human Macrophages. International Journal of Molecular Sciences. 2025; 26(9):4300. https://doi.org/10.3390/ijms26094300
Chicago/Turabian StyleJacques, Camille, Irene Marchesi, Francesco Paolo Fiorentino, Flora Marchand, Mathias Chatelais, and Ilaria Floris. 2025. "Active Substances from the Micro-Immunotherapy Medicine 2LC1® Show In Vitro Anti-Cancer Properties in Colon, Prostate, and Breast Cancer Models and Immune-Enhancing Capabilities in Human Macrophages" International Journal of Molecular Sciences 26, no. 9: 4300. https://doi.org/10.3390/ijms26094300
APA StyleJacques, C., Marchesi, I., Fiorentino, F. P., Marchand, F., Chatelais, M., & Floris, I. (2025). Active Substances from the Micro-Immunotherapy Medicine 2LC1® Show In Vitro Anti-Cancer Properties in Colon, Prostate, and Breast Cancer Models and Immune-Enhancing Capabilities in Human Macrophages. International Journal of Molecular Sciences, 26(9), 4300. https://doi.org/10.3390/ijms26094300








