Molecular Mechanisms of Tumor Progression and Novel Therapeutic and Diagnostic Strategies in Mesothelioma
Abstract
1. Introduction
2. Frequent Inactivation of Tumor Suppressor Genes in Mesothelioma
2.1. Genetic Landscape and Clinical Implications of Interpatient Heterogeneity
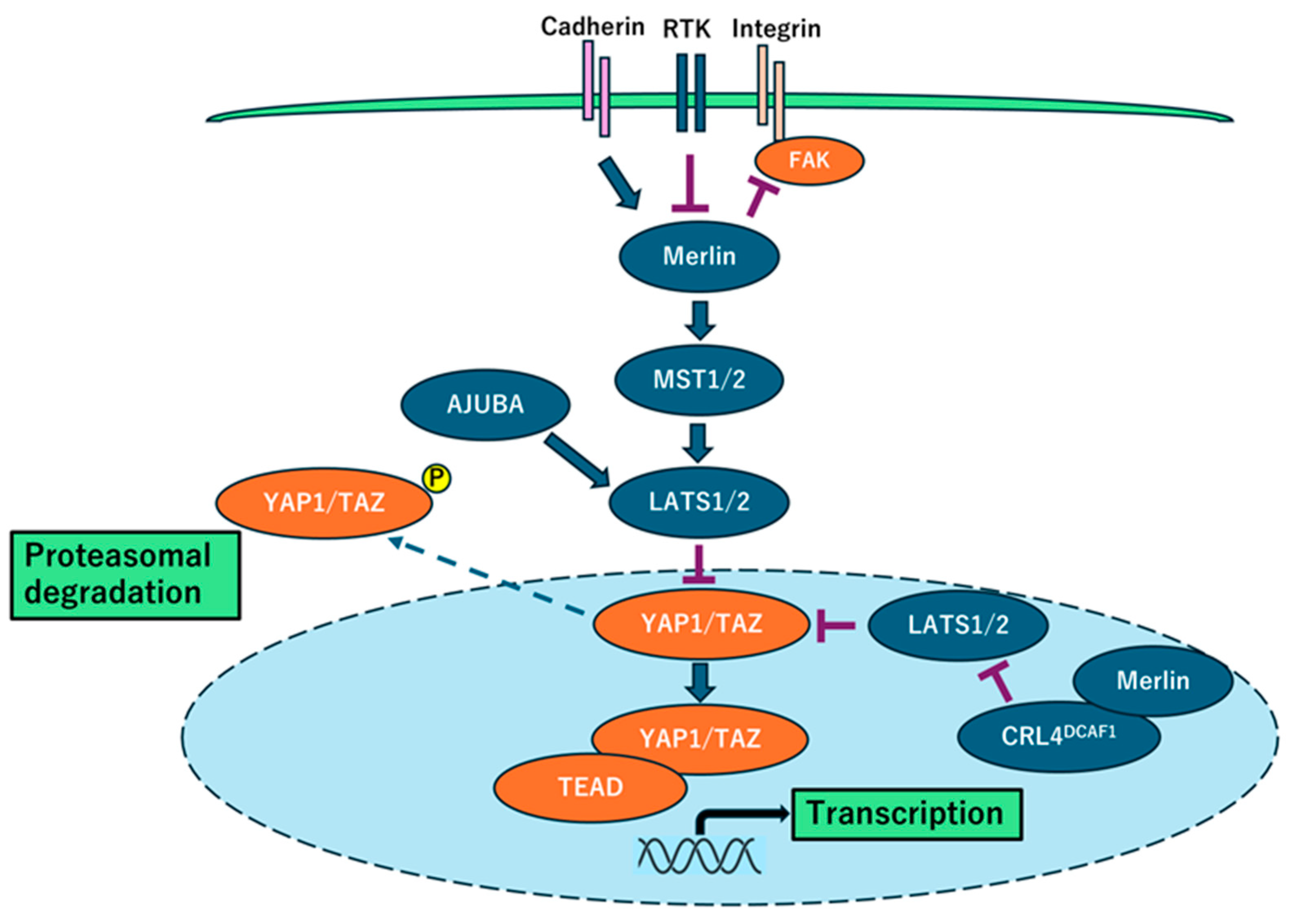
2.2. NF2 Inactivation and Hippo Signaling Pathway
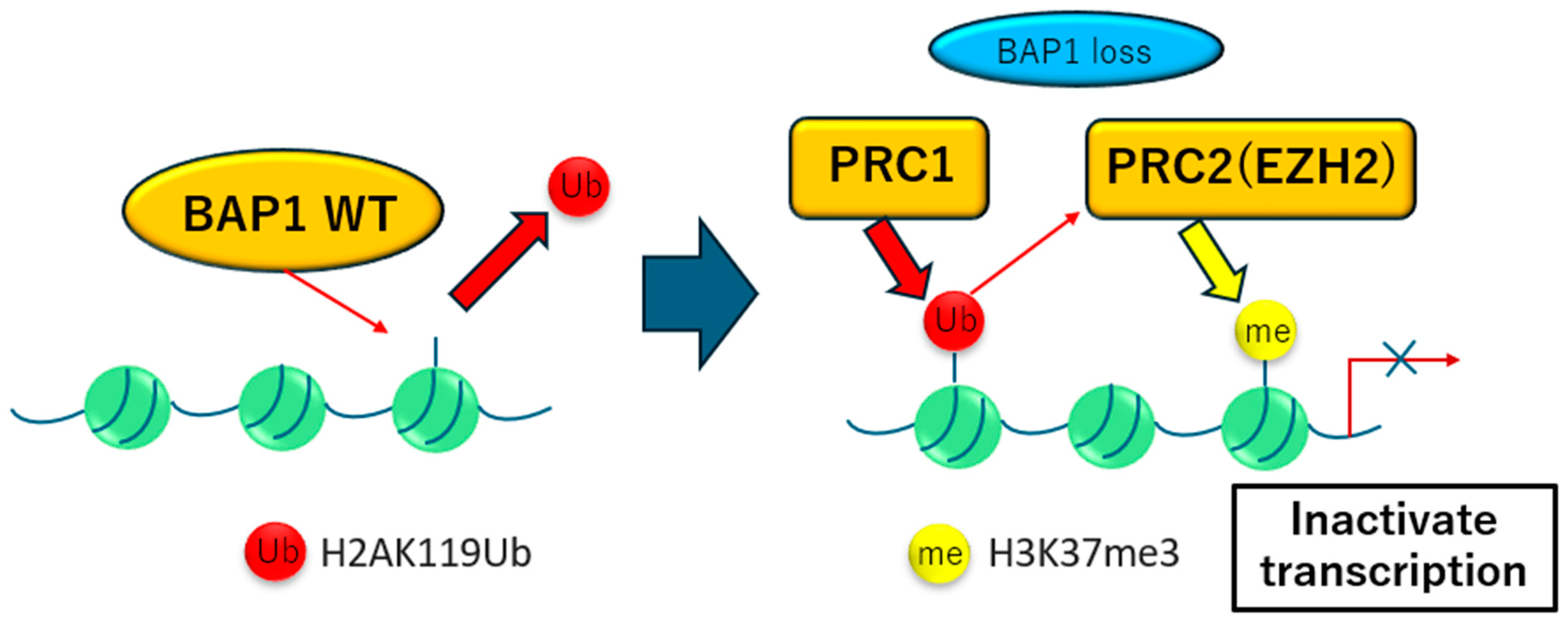
2.3. BAP1 Inactivation and Therapeutic Potential
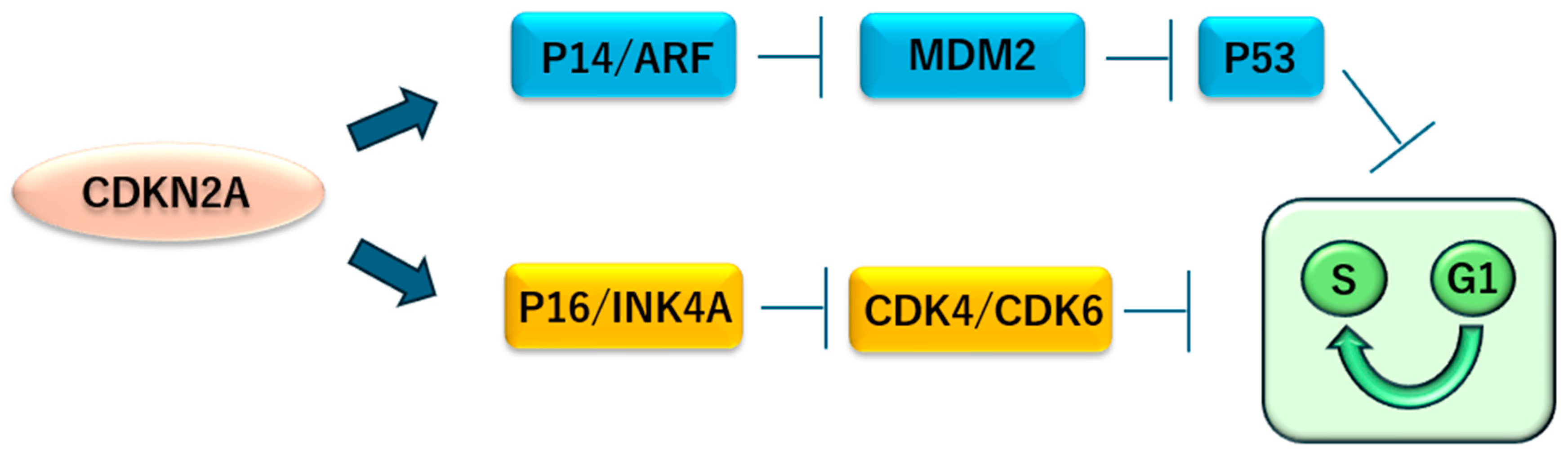
2.4. CDKN2A Inactivation in Mesothelioma
3. Other Targets in Mesothelioma
3.1. Mesothelin
3.2. Oxytocin Receptor
3.3. PRMT5
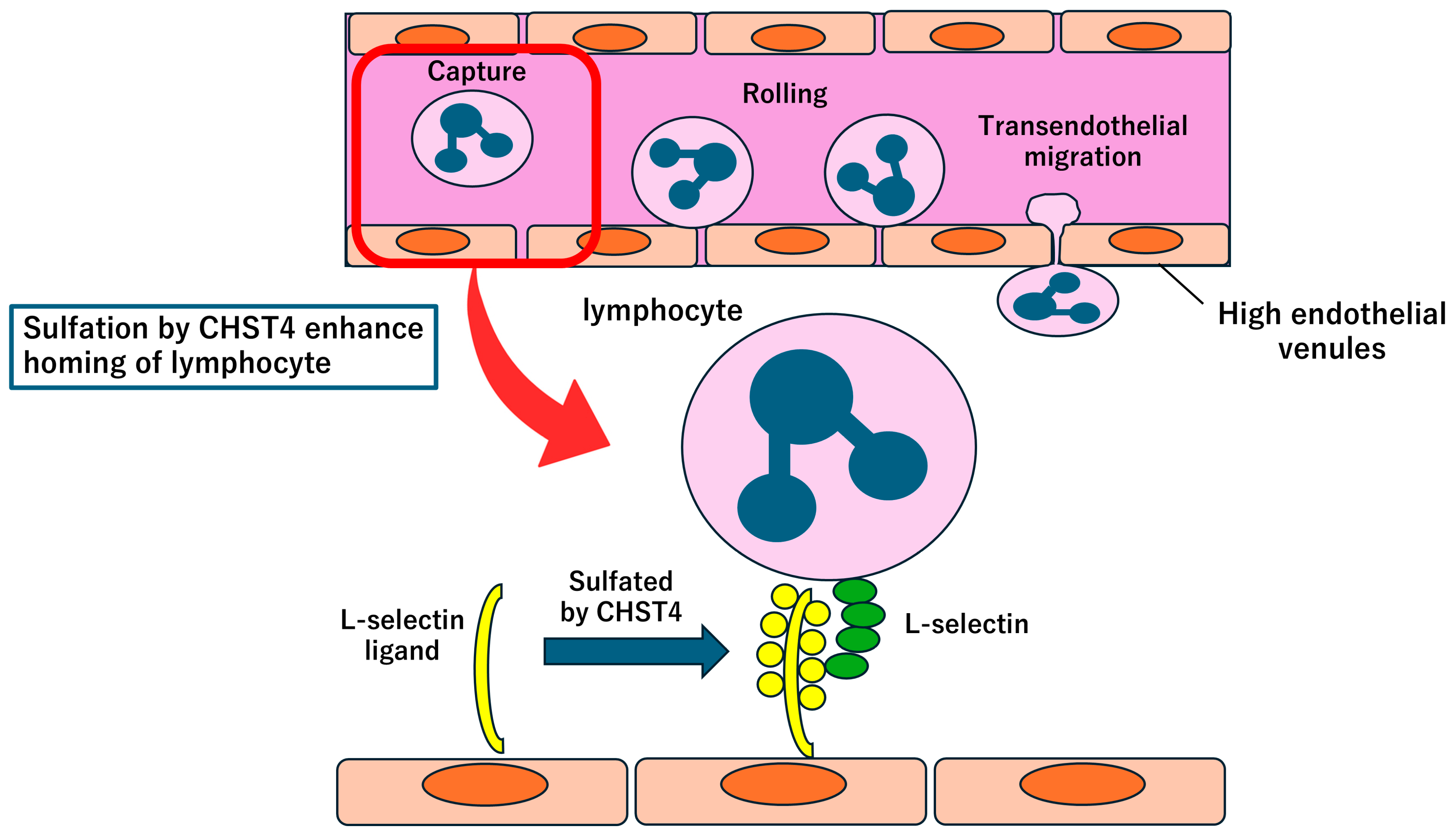
3.4. CHST4
4. Effect of Immunotherapy on Mesothelioma
4.1. Tumor Microenvironment in Mesothelioma
4.2. Chemoimmunotherapy
4.3. Cellular Therapy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhai, Z.; Ruan, J.; Zheng, Y.; Xiang, D.; Li, N.; Hu, J.; Shen, J.; Deng, Y.; Yao, J.; Zhao, P.; et al. Assessment of Global Trends in the Diagnosis of Mesothelioma From 1990 to 2017. JAMA Netw. Open 2021, 4, e2120360. [Google Scholar] [CrossRef]
- Huang, J.; Chan, S.C.; Pang, W.S.; Chow, S.H.; Lok, V.; Zhang, L.; Lin, X.; Lucero-Prisno, D.E., 3rd; Xu, W.; Zheng, Z.J.; et al. Global Incidence, Risk Factors, and Temporal Trends of Mesothelioma: A Population-Based Study. J. Thorac. Oncol. 2023, 18, 792–802. [Google Scholar] [CrossRef]
- Milano, M.T.; Zhang, H. Malignant pleural mesothelioma: A population-based study of survival. J. Thorac. Oncol. 2010, 5, 1841–1848. [Google Scholar] [CrossRef]
- Vogelzang, N.J.; Rusthoven, J.J.; Symanowski, J.; Denham, C.; Kaukel, E.; Ruffie, P.; Gatzemeier, U.; Boyer, M.; Emri, S.; Manegold, C.; et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J. Clin. Oncol. 2003, 21, 2636–2644. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.A.; Aerts, J.G.; Popat, S.; Fennell, D.A. Novel insights into mesothelioma biology and implications for therapy. Nat. Rev. Cancer 2017, 17, 475–488. [Google Scholar] [CrossRef]
- Lim, E.; Waller, D.; Lau, K.; Steele, J.; Pope, A.; Ali, C.; Bilancia, R.; Keni, M.; Popat, S.; O’Brien, M.; et al. Extended pleurectomy decortication and chemotherapy versus chemotherapy alone for pleural mesothelioma (MARS 2): A phase 3 randomised controlled trial. Lancet Respir. Med. 2024, 12, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Sekido, Y. NF2/Merlin Inactivation and Potential Therapeutic Targets in Mesothelioma. Int. J. Mol. Sci. 2018, 19, 988. [Google Scholar] [CrossRef] [PubMed]
- Scherpereel, A.; Mazieres, J.; Greillier, L.; Lantuejoul, S.; Do, P.; Bylicki, O.; Monnet, I.; Corre, R.; Audigier-Valette, C.; Locatelli-Sanchez, M.; et al. Nivolumab or nivolumab plus ipilimumab in patients with relapsed malignant pleural mesothelioma (IFCT-1501 MAPS2): A multicentre, open-label, randomised, non-comparative, phase 2 trial. Lancet Oncol. 2019, 20, 239–253. [Google Scholar] [CrossRef]
- Baas, P.; Scherpereel, A.; Nowak, A.K.; Fujimoto, N.; Peters, S.; Tsao, A.S.; Mansfield, A.S.; Popat, S.; Jahan, T.; Antonia, S.; et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): A multicentre, randomised, open-label, phase 3 trial. Lancet 2021, 397, 375–386. [Google Scholar] [CrossRef]
- Tsao, A.S.; Lindwasser, O.W.; Adjei, A.A.; Adusumilli, P.S.; Beyers, M.L.; Blumenthal, G.M.; Bueno, R.; Burt, B.M.; Carbone, M.; Dahlberg, S.E.; et al. Current and Future Management of Malignant Mesothelioma: A Consensus Report from the National Cancer Institute Thoracic Malignancy Steering Committee, International Association for the Study of Lung Cancer, and Mesothelioma Applied Research Foundation. J. Thorac. Oncol. 2018, 13, 1655–1667. [Google Scholar] [CrossRef]
- Peters, S.; Scherpereel, A.; Cornelissen, R.; Oulkhouir, Y.; Greillier, L.; Kaplan, M.A.; Talbot, T.; Monnet, I.; Hiret, S.; Baas, P.; et al. First-line nivolumab plus ipilimumab versus chemotherapy in patients with unresectable malignant pleural mesothelioma: 3-year outcomes from CheckMate 743. Ann. Oncol. 2022, 33, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Bylicki, O.; Guisier, F.; Scherpereel, A.; Daniel, C.; Swalduz, A.; Grolleau, E.; Bernardi, M.; Hominal, S.; Prevost, J.B.; Pamart, G.; et al. Real-World efficacy and safety of combination nivolumab plus ipilimumab for Untreated, Unresectable, pleural Mesothelioma: The Meso-Immune (GFPC 04-2021) trial. Lung Cancer 2024, 194, 107866. [Google Scholar] [CrossRef]
- Gaudino, G.; Xue, J.; Yang, H. How asbestos and other fibers cause mesothelioma. Transl. Lung Cancer Res. 2020, 9, S39–S46. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Chmielecki, J.; Goparaju, C.; Heguy, A.; Dolgalev, I.; Carbone, M.; Seepo, S.; Meyerson, M.; Pass, H.I. Whole-exome sequencing reveals frequent genetic alterations in BAP1, NF2, CDKN2A, and CUL1 in malignant pleural mesothelioma. Cancer Res. 2015, 75, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Lo Iacono, M.; Monica, V.; Righi, L.; Grosso, F.; Libener, R.; Vatrano, S.; Bironzo, P.; Novello, S.; Musmeci, L.; Volante, M.; et al. Targeted next-generation sequencing of cancer genes in advanced stage malignant pleural mesothelioma: A retrospective study. J. Thorac. Oncol. 2015, 10, 492–499. [Google Scholar] [CrossRef]
- Bueno, R.; Stawiski, E.W.; Goldstein, L.D.; Durinck, S.; De Rienzo, A.; Modrusan, Z.; Gnad, F.; Nguyen, T.T.; Jaiswal, B.S.; Chirieac, L.R.; et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat. Genet. 2016, 48, 407–416. [Google Scholar] [CrossRef]
- Christensen, B.C.; Houseman, E.A.; Poage, G.M.; Godleski, J.J.; Bueno, R.; Sugarbaker, D.J.; Wiencke, J.K.; Nelson, H.H.; Marsit, C.J.; Kelsey, K.T. Integrated profiling reveals a global correlation between epigenetic and genetic alterations in mesothelioma. Cancer Res. 2010, 70, 5686–5694. [Google Scholar] [CrossRef]
- Fennell, D.A.; Parmar, A.; Shamash, J.; Evans, M.T.; Sheaff, M.T.; Sylvester, R.; Dhaliwal, K.; Gower, N.; Steele, J.; Rudd, R. Statistical validation of the EORTC prognostic model for malignant pleural mesothelioma based on three consecutive phase II trials. J. Clin. Oncol. 2005, 23, 184–189. [Google Scholar] [CrossRef]
- Muruganandan, S.; Alfonso, H.; Franklin, P.; Shilkin, K.; Segal, A.; Olsen, N.; Reid, A.; de Klerk, N.; Musk, A.B.; Brims, F. Comparison of outcomes following a cytological or histological diagnosis of malignant mesothelioma. Br. J. Cancer. 2017, 116, 703–708. [Google Scholar] [CrossRef]
- Sekido, Y. Targeting the Hippo Pathway Is a New Potential Therapeutic Modality for Malignant Mesothelioma. Cancers 2018, 10, 90. [Google Scholar] [CrossRef]
- Yu, F.X.; Zhao, B.; Guan, K.L. Hippo Pathway in Organ Size Control, Tissue Homeostasis, and Cancer. Cell 2015, 163, 811–828. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, T.; Murakami, H.; Fujii, M.; Ishiguro, F.; Tanaka, I.; Kondo, Y.; Akatsuka, S.; Toyokuni, S.; Yokoi, K.; Osada, H.; et al. YAP induces malignant mesothelioma cell proliferation by upregulating transcription of cell cycle-promoting genes. Oncogene 2012, 31, 5117–5122. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Minikes, A.M.; Gao, M.; Bian, H.; Li, Y.; Stockwell, B.R.; Chen, Z.N.; Jiang, X. Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature 2019, 572, 402–406. [Google Scholar] [CrossRef]
- Murakami, H.; Mizuno, T.; Taniguchi, T.; Fujii, M.; Ishiguro, F.; Fukui, T.; Akatsuka, S.; Horio, Y.; Hida, T.; Kondo, Y.; et al. LATS2 is a tumor suppressor gene of malignant mesothelioma. Cancer Res. 2011, 71, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Miyanaga, A.; Masuda, M.; Tsuta, K.; Kawasaki, K.; Nakamura, Y.; Sakuma, T.; Asamura, H.; Gemma, A.; Yamada, T. Hippo pathway gene mutations in malignant mesothelioma: Revealed by RNA and targeted exon sequencing. J. Thorac. Oncol. 2015, 10, 844–851. [Google Scholar] [CrossRef]
- Tanaka, I.; Osada, H.; Fujii, M.; Fukatsu, A.; Hida, T.; Horio, Y.; Kondo, Y.; Sato, A.; Hasegawa, Y.; Tsujimura, T.; et al. LIM-domain protein AJUBA suppresses malignant mesothelioma cell proliferation via Hippo signaling cascade. Oncogene 2015, 34, 73–83. [Google Scholar] [CrossRef]
- Altomare, D.A.; Vaslet, C.A.; Skele, K.L.; De Rienzo, A.; Devarajan, K.; Jhanwar, S.C.; McClatchey, A.I.; Kane, A.B.; Testa, J.R. A mouse model recapitulating molecular features of human mesothelioma. Cancer Res. 2005, 65, 8090–8095. [Google Scholar] [CrossRef]
- Fleury-Feith, J.; Lecomte, C.; Renier, A.; Matrat, M.; Kheuang, L.; Abramowski, V.; Levy, F.; Janin, A.; Giovannini, M.; Jaurand, M.C. Hemizygosity of Nf2 is associated with increased susceptibility to asbestos-induced peritoneal tumours. Oncogene 2003, 22, 3799–3805. [Google Scholar] [CrossRef]
- Yap, T.A.; Kwiatkowski, D.J.; Desai, J.; Dangogo-Jack, I.; Millward, M.; Kindler, H.L.; Tolcher, A.W.; Frentzas, S.; Thurston, A.W.; Post, L.; et al. First-in-class, first-in-human phase 1 trial of VT3989, an inhibitor of yes-associated protein (YAP)/transcriptional enhancer activator domain (TEAD), in patients (pts) with advanced solid tumors enriched for malignant mesothelioma and other tumors with neurofibromatosis 2 (NF2) mutations. Cancer Res. 2023, 83 (Suppl. 8), CT006. [Google Scholar]
- Provenzano, L.; Ryan, Y.; Hilton, D.A.; Lyons-Rimmer, J.; Dave, F.; Maze, E.A.; Adams, C.L.; Rigby-Jones, R.; Ammoun, S.; Hanemann, C.O. Cellular prion protein (PrP(C)) in the development of Merlin-deficient tumours. Oncogene 2017, 36, 6132–6142. [Google Scholar] [CrossRef]
- Kato, T.; Sato, T.; Yokoi, K.; Sekido, Y. E-cadherin expression is correlated with focal adhesion kinase inhibitor resistance in Merlin-negative malignant mesothelioma cells. Oncogene 2017, 36, 5522–5531. [Google Scholar] [CrossRef]
- Shapiro, I.M.; Kolev, V.N.; Vidal, C.M.; Kadariya, Y.; Ring, J.E.; Wright, Q.; Weaver, D.T.; Menges, C.; Padval, M.; McClatchey, A.I.; et al. Merlin deficiency predicts FAK inhibitor sensitivity: A synthetic lethal relationship. Sci. Transl. Med. 2014, 6, 237ra268. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.C.; Gan, H.K.; Blagden, S.P.; Plummer, R.; Arkenau, H.T.; Ranson, M.; Evans, T.R.; Zalcman, G.; Bahleda, R.; Hollebecque, A.; et al. A phase I, pharmacokinetic and pharmacodynamic study of GSK2256098, a focal adhesion kinase inhibitor, in patients with advanced solid tumors. Ann. Oncol. 2016, 27, 2268–2274. [Google Scholar] [CrossRef] [PubMed]
- Fennell, D.A.; Baas, P.; Taylor, P.; Nowak, A.K.; Gilligan, D.; Nakano, T.; Pachter, J.A.; Weaver, D.T.; Scherpereel, A.; Pavlakis, N.; et al. Maintenance Defactinib Versus Placebo After First-Line Chemotherapy in Patients With Merlin-Stratified Pleural Mesothelioma: COMMAND-A Double-Blind, Randomized, Phase II Study. J. Clin. Oncol. 2019, 37, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Seshasayee, D.; Noubade, R.; French, D.M.; Liu, J.; Chaurushiya, M.S.; Kirkpatrick, D.S.; Pham, V.C.; Lill, J.R.; Bakalarski, C.E.; et al. Loss of the tumor suppressor BAP1 causes myeloid transformation. Science 2012, 337, 1541–1546. [Google Scholar] [CrossRef]
- Kadariya, Y.; Cheung, M.; Xu, J.; Pei, J.; Sementino, E.; Menges, C.W.; Cai, K.Q.; Rauscher, F.J.; Klein-Szanto, A.J.; Testa, J.R. Bap1 Is a Bona Fide Tumor Suppressor: Genetic Evidence from Mouse Models Carrying Heterozygous Germline Bap1 Mutations. Cancer Res. 2016, 76, 2836–2844. [Google Scholar] [CrossRef]
- Ismail, I.H.; Davidson, R.; Gagne, J.P.; Xu, Z.Z.; Poirier, G.G.; Hendzel, M.J. Germline mutations in BAP1 impair its function in DNA double-strand break repair. Cancer Res. 2014, 74, 4282–4294. [Google Scholar] [CrossRef]
- Yu, H.; Pak, H.; Hammond-Martel, I.; Ghram, M.; Rodrigue, A.; Daou, S.; Barbour, H.; Corbeil, L.; Hebert, J.; Drobetsky, E.; et al. Tumor suppressor and deubiquitinase BAP1 promotes DNA double-strand break repair. Proc. Natl. Acad. Sci. USA 2014, 111, 285–290. [Google Scholar] [CrossRef]
- Kemp, C.D.; Rao, M.; Xi, S.; Inchauste, S.; Mani, H.; Fetsch, P.; Filie, A.; Zhang, M.; Hong, J.A.; Walker, R.L.; et al. Polycomb repressor complex-2 is a novel target for mesothelioma therapy. Clin. Cancer Res. 2012, 18, 77–90. [Google Scholar] [CrossRef]
- Zauderer, M.G.; Szlosarek, P.W.; Le Moulec, S.; Popat, S.; Taylor, P.; Planchard, D.; Scherpereel, A.; Koczywas, M.; Forster, M.; Cameron, R.B.; et al. EZH2 inhibitor tazemetostat in patients with relapsed or refractory, BAP1-inactivated malignant pleural mesothelioma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2022, 23, 758–767. [Google Scholar] [CrossRef]
- Marshall, K.; Jackson, S.; Jones, J.; Holme, J.; Lyons, J.; Barrett, E.; Taylor, P.; Bishop, P.; Hodgson, C.; Green, M.; et al. Homozygous deletion of CDKN2A in malignant mesothelioma: Diagnostic utility, patient characteristics and survival in a UK mesothelioma centre. Lung Cancer 2020, 150, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Vandenhoeck, J.; van Meerbeeck, J.P.; Fransen, E.; Raskin, J.; Van Camp, G.; Op de Beeck, K.; Lamote, K. DNA Methylation as a Diagnostic Biomarker for Malignant Mesothelioma: A Systematic Review and Meta-Analysis. J. Thorac. Oncol. 2021, 16, 1461–1478. [Google Scholar] [CrossRef]
- Hida, T.; Matsumoto, S.; Hamasaki, M.; Kawahara, K.; Tsujimura, T.; Hiroshima, K.; Kamei, T.; Taguchi, K.; Iwasaki, A.; Oda, Y.; et al. Deletion status of p16 in effusion smear preparation correlates with that of underlying malignant pleural mesothelioma tissue. Cancer Sci. 2015, 106, 1635–1641. [Google Scholar] [CrossRef]
- Jennings, C.J.; Murer, B.; O’Grady, A.; Hearn, L.M.; Harvey, B.J.; Kay, E.W.; Thomas, W. Differential p16/INK4A cyclin-dependent kinase inhibitor expression correlates with chemotherapy efficacy in a cohort of 88 malignant pleural mesothelioma patients. Br. J. Cancer 2015, 113, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Jongsma, J.; van Montfort, E.; Vooijs, M.; Zevenhoven, J.; Krimpenfort, P.; van der Valk, M.; van de Vijver, M.; Berns, A. A conditional mouse model for malignant mesothelioma. Cancer Cell 2008, 13, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.; Zhou, J.; Anderson, D.; Kratzke, R.A. Inactivation of p16INK4a expression in malignant mesothelioma by methylation. Lung Cancer 2002, 38, 131–136. [Google Scholar] [CrossRef]
- Singhi, A.D.; Krasinskas, A.M.; Choudry, H.A.; Bartlett, D.L.; Pingpank, J.F.; Zeh, H.J.; Luvison, A.; Fuhrer, K.; Bahary, N.; Seethala, R.R.; et al. The prognostic significance of BAP1, NF2, and CDKN2A in malignant peritoneal mesothelioma. Mod. Pathol. 2016, 29, 14–24. [Google Scholar] [CrossRef]
- Chernova, T.; Murphy, F.A.; Galavotti, S.; Sun, X.M.; Powley, I.R.; Grosso, S.; Schinwald, A.; Zacarias-Cabeza, J.; Dudek, K.M.; Dinsdale, D.; et al. Long-Fiber Carbon Nanotubes Replicate Asbestos-Induced Mesothelioma with Disruption of the Tumor Suppressor Gene Cdkn2a (Ink4a/Arf). Curr. Biol. 2017, 27, 3302–3314. [Google Scholar] [CrossRef]
- Frizelle, S.P.; Kratzke, M.G.; Carreon, R.R.; Engel, S.C.; Youngquist, L.; Klein, M.A.; Fourre, L.; Shekels, L.L.; Kratzke, R.A. Inhibition of both mesothelioma cell growth and Cdk4 activity following treatment with a TATp16INK4a peptide. Anticancer Res. 2008, 28, 1–7. [Google Scholar]
- Nguyen, T.T.T.; Shingyoji, M.; Hanazono, M.; Zhong, B.; Morinaga, T.; Tada, Y.; Shimada, H.; Hiroshima, K.; Tagawa, M. An MDM2 inhibitor achieves synergistic cytotoxic effects with adenoviruses lacking E1B55kDa gene on mesothelioma with the wild-type p53 through augmenting NFI expression. Cell Death Dis. 2021, 12, 663. [Google Scholar] [CrossRef]
- Singh, A.; Bhattacharyya, N.; Srivastava, A.; Pruett, N.; Ripley, R.T.; Schrump, D.S.; Hoang, C.D. MicroRNA-215-5p Treatment Suppresses Mesothelioma Progression via the MDM2-p53-Signaling Axis. Mol. Ther. 2019, 27, 1665–1680. [Google Scholar] [CrossRef] [PubMed]
- Ou, W.B.; Lu, M.; Eilers, G.; Li, H.; Ding, J.; Meng, X.; Wu, Y.; He, Q.; Sheng, Q.; Zhou, H.M.; et al. Co-targeting of FAK and MDM2 triggers additive anti-proliferative effects in mesothelioma via a coordinated reactivation of p53. Br. J. Cancer 2016, 115, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Walter, R.F.; Mairinger, F.D.; Ting, S.; Vollbrecht, C.; Mairinger, T.; Theegarten, D.; Christoph, D.C.; Schmid, K.W.; Wohlschlaeger, J. MDM2 is an important prognostic and predictive factor for platin-pemetrexed therapy in malignant pleural mesotheliomas and deregulation of P14/ARF (encoded by CDKN2A) seems to contribute to an MDM2-driven inactivation of P53. Br. J. Cancer 2015, 112, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Sui, G.; Affar el, B.; Shi, Y.; Brignone, C.; Wall, N.R.; Yin, P.; Donohoe, M.; Luke, M.P.; Calvo, D.; Grossman, S.R.; et al. Yin Yang 1 is a negative regulator of p53. Cell 2004, 117, 859–872. [Google Scholar] [CrossRef]
- Fennell, D.A.; King, A.; Mohammed, S.; Greystoke, A.; Anthony, S.; Poile, C.; Nusrat, N.; Scotland, M.; Bhundia, V.; Branson, A.; et al. Abemaciclib in patients with p16ink4A-deficient mesothelioma (MiST2): A single-arm, open-label, phase 2 trial. Lancet Oncol. 2022, 23, 374–381. [Google Scholar] [CrossRef]
- Servais, E.L.; Colovos, C.; Rodriguez, L.; Bograd, A.J.; Nitadori, J.; Sima, C.; Rusch, V.W.; Sadelain, M.; Adusumilli, P.S. Mesothelin overexpression promotes mesothelioma cell invasion and MMP-9 secretion in an orthotopic mouse model and in epithelioid pleural mesothelioma patients. Clin. Cancer Res. 2012, 18, 2478–2489. [Google Scholar] [CrossRef]
- Pastan, I.; Hassan, R. Discovery of mesothelin and exploiting it as a target for immunotherapy. Cancer Res. 2014, 74, 2907–2912. [Google Scholar] [CrossRef]
- Kushitani, K.; Takeshima, Y.; Amatya, V.J.; Furonaka, O.; Sakatani, A.; Inai, K. Immunohistochemical marker panels for distinguishing between epithelioid mesothelioma and lung adenocarcinoma. Pathol. Int. 2007, 57, 190–199. [Google Scholar] [CrossRef]
- Pantazopoulos, I.; Boura, P.; Xanthos, T.; Syrigos, K. Effectiveness of mesothelin family proteins and osteopontin for malignant mesothelioma. Eur. Respir. J. 2013, 41, 706–715. [Google Scholar] [CrossRef]
- Hollevoet, K.; Reitsma, J.B.; Creaney, J.; Grigoriu, B.D.; Robinson, B.W.; Scherpereel, A.; Cristaudo, A.; Pass, H.I.; Nackaerts, K.; Rodriguez Portal, J.A.; et al. Serum mesothelin for diagnosing malignant pleural mesothelioma: An individual patient data meta-analysis. J. Clin. Oncol. 2012, 30, 1541–1549. [Google Scholar] [CrossRef]
- Panou, V.; Vyberg, M.; Weinreich, U.M.; Meristoudis, C.; Falkmer, U.G.; Roe, O.D. The established and future biomarkers of malignant pleural mesothelioma. Cancer Treat. Rev. 2015, 41, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Johnen, G.; Burek, K.; Raiko, I.; Wichert, K.; Pesch, B.; Weber, D.G.; Lehnert, M.; Casjens, S.; Hagemeyer, O.; Taeger, D.; et al. Prediagnostic detection of mesothelioma by circulating calretinin and mesothelin—A case-control comparison nested into a prospective cohort of asbestos-exposed workers. Sci. Rep. 2018, 8, 14321. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.; Kindler, H.L.; Jahan, T.; Bazhenova, L.; Reck, M.; Thomas, A.; Pastan, I.; Parno, J.; O’Shannessy, D.J.; Fatato, P.; et al. Phase II clinical trial of amatuximab, a chimeric antimesothelin antibody with pemetrexed and cisplatin in advanced unresectable pleural mesothelioma. Clin. Cancer Res. 2014, 20, 5927–5936. [Google Scholar] [CrossRef] [PubMed]
- Kindler, H.L.; Novello, S.; Bearz, A.; Ceresoli, G.L.; Aerts, J.; Spicer, J.; Taylor, P.; Nackaerts, K.; Greystoke, A.; Jennens, R.; et al. Anetumab ravtansine versus vinorelbine in patients with relapsed, mesothelin-positive malignant pleural mesothelioma (ARCS-M): A randomised, open-label phase 2 trial. Lancet Oncol. 2022, 23, 540–552. [Google Scholar] [CrossRef]
- Hassan, R.; Sharon, E.; Thomas, A.; Zhang, J.; Ling, A.; Miettinen, M.; Kreitman, R.J.; Steinberg, S.M.; Hollevoet, K.; Pastan, I. Phase 1 study of the antimesothelin immunotoxin SS1P in combination with pemetrexed and cisplatin for front-line therapy of pleural mesothelioma and correlation of tumor response with serum mesothelin, megakaryocyte potentiating factor, and cancer antigen 125. Cancer 2014, 120, 3311–3319. [Google Scholar] [CrossRef]
- Baldo, P.; Cecco, S. Amatuximab and novel agents targeting mesothelin for solid tumors. Onco Targets Ther. 2017, 10, 5337–5353. [Google Scholar] [CrossRef]
- Golfier, S.; Kopitz, C.; Kahnert, A.; Heisler, I.; Schatz, C.A.; Stelte-Ludwig, B.; Mayer-Bartschmid, A.; Unterschemmann, K.; Bruder, S.; Linden, L.; et al. Anetumab ravtansine: A novel mesothelin-targeting antibody-drug conjugate cures tumors with heterogeneous target expression favored by bystander effect. Mol. Cancer Ther. 2014, 13, 1537–1548. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.; Thomas, A.; Alewine, C.; Le, D.T.; Jaffee, E.M.; Pastan, I. Mesothelin Immunotherapy for Cancer: Ready for Prime Time? J. Clin. Oncol. 2016, 34, 4171–4179. [Google Scholar] [CrossRef]
- Hassan, R.; Blumenschein, G.R., Jr.; Moore, K.N.; Santin, A.D.; Kindler, H.L.; Nemunaitis, J.J.; Seward, S.M.; Thomas, A.; Kim, S.K.; Rajagopalan, P.; et al. First-in-Human, Multicenter, Phase I Dose-Escalation and Expansion Study of Anti-Mesothelin Antibody-Drug Conjugate Anetumab Ravtansine in Advanced or Metastatic Solid Tumors. J. Clin. Oncol. 2020, 38, 1824–1835. [Google Scholar] [CrossRef]
- Mansfield, A.S.; Vivien Yin, J.; Bradbury, P.; Kwiatkowski, D.J.; Patel, S.; Bazhenova, L.A.; Forde, P.; Lou, Y.; Dizona, P.; Villaruz, L.C.; et al. Randomized trial of anetumab ravtansine and pembrolizumab compared to pembrolizumab for mesothelioma. Lung Cancer 2024, 195, 107928. [Google Scholar] [CrossRef]
- Kodama, Y.; Tanaka, I.; Sato, T.; Hori, K.; Gen, S.; Morise, M.; Matsubara, D.; Sato, M.; Sekido, Y.; Hashimoto, N. Oxytocin receptor is a promising therapeutic target of malignant mesothelioma. Cancer Sci. 2021, 112, 3520–3532. [Google Scholar] [CrossRef]
- Jurek, B.; Neumann, I.D. The Oxytocin Receptor: From Intracellular Signaling to Behavior. Physiol. Rev. 2018, 98, 1805–1908. [Google Scholar] [CrossRef]
- Horie, K.; Inoue, K.; Suzuki, S.; Adachi, S.; Yada, S.; Hirayama, T.; Hidema, S.; Young, L.J.; Nishimori, K. Oxytocin receptor knockout prairie voles generated by CRISPR/Cas9 editing show reduced preference for social novelty and exaggerated repetitive behaviors. Horm. Behav. 2019, 111, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Schmid, B.; Wong, S.; Mitchell, B.F. Transcriptional regulation of oxytocin receptor by interleukin-1beta and interleukin-6. Endocrinology 2001, 142, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
- Marjon, K.; Cameron, M.J.; Quang, P.; Clasquin, M.F.; Mandley, E.; Kunii, K.; McVay, M.; Choe, S.; Kernytsky, A.; Gross, S.; et al. MTAP Deletions in Cancer Create Vulnerability to Targeting of the MAT2A/PRMT5/RIOK1 Axis. Cell Rep. 2016, 15, 574–587. [Google Scholar] [CrossRef] [PubMed]
- Guccione, E.; Richard, S. The regulation, functions and clinical relevance of arginine methylation. Nat. Rev. Mol. Cell Biol. 2019, 20, 642–657. [Google Scholar] [CrossRef]
- Mavrakis, K.J.; McDonald, E.R., 3rd; Schlabach, M.R.; Billy, E.; Hoffman, G.R.; deWeck, A.; Ruddy, D.A.; Venkatesan, K.; Yu, J.; McAllister, G.; et al. Disordered methionine metabolism in MTAP/CDKN2A-deleted cancers leads to dependence on PRMT5. Science 2016, 351, 1208–1213. [Google Scholar] [CrossRef]
- Hmeljak, J.; Sanchez-Vega, F.; Hoadley, K.A.; Shih, J.; Stewart, C.; Heiman, D.; Tarpey, P.; Danilova, L.; Drill, E.; Gibb, E.A.; et al. Integrative Molecular Characterization of Malignant Pleural Mesothelioma. Cancer Discov. 2018, 8, 1548–1565. [Google Scholar] [CrossRef]
- Kryukov, G.V.; Wilson, F.H.; Ruth, J.R.; Paulk, J.; Tsherniak, A.; Marlow, S.E.; Vazquez, F.; Weir, B.A.; Fitzgerald, M.E.; Tanaka, M.; et al. MTAP deletion confers enhanced dependency on the PRMT5 arginine methyltransferase in cancer cells. Science 2016, 351, 1214–1218. [Google Scholar] [CrossRef]
- Barbarino, M.; Cesari, D.; Bottaro, M.; Luzzi, L.; Namagerdi, A.; Bertolino, F.M.; Bellan, C.; Proietti, F.; Somma, P.; Micheli, M.; et al. PRMT5 silencing selectively affects MTAP-deleted mesothelioma: In vitro evidence of a novel promising approach. J. Cell Mol. Med. 2020, 24, 5565–5577. [Google Scholar] [CrossRef]
- Engstrom, L.D.; Aranda, R.; Waters, L.; Moya, K.; Bowcut, V.; Vegar, L.; Trinh, D.; Hebbert, A.; Smith, C.R.; Kulyk, S.; et al. MRTX1719 Is an MTA-Cooperative PRMT5 Inhibitor That Exhibits Synthetic Lethality in Preclinical Models and Patients with MTAP-Deleted Cancer. Cancer Discov. 2023, 13, 2412–2431. [Google Scholar] [CrossRef] [PubMed]
- Okado, S.; Kato, T.; Hanamatsu, Y.; Emoto, R.; Imamura, Y.; Watanabe, H.; Kawasumi, Y.; Kadomatsu, Y.; Ueno, H.; Nakamura, S.; et al. CHST4 Gene as a Potential Predictor of Clinical Outcome in Malignant Pleural Mesothelioma. Int. J. Mol. Sci. 2024, 25, 2270. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Tosolini, M.; Kirilovsky, A.; Waldner, M.; Obenauf, A.C.; Angell, H.; Fredriksen, T.; Lafontaine, L.; Berger, A.; et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 2013, 39, 782–795. [Google Scholar] [CrossRef] [PubMed]
- Veerman, K.; Tardiveau, C.; Martins, F.; Coudert, J.; Girard, J.P. Single-Cell Analysis Reveals Heterogeneity of High Endothelial Venules and Different Regulation of Genes Controlling Lymphocyte Entry to Lymph Nodes. Cell Rep. 2019, 26, 3116–3131. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, H.; Qiu, G.; Li, Z.; Wang, Y. The LINC00452/miR-204/CHST4 Axis Regulating Thymic Tregs Might Be Involved in the Progression of Thymoma-Associated Myasthenia Gravis. Front. Neurol. 2022, 13, 828970. [Google Scholar] [CrossRef]
- Nowak, A.K.; Giroux, D.J.; Eisele, M.; Rosenthal, A.; Bille, A.; Gill, R.R.; Kindler, H.L.; Pass, H.I.; Rice, D.; Ripley, R.T.; et al. The International Association for the Study of Lung Cancer Pleural Mesothelioma Staging Project: Proposal for Revision of the TNM Stage Groupings in the Forthcoming (Ninth) Edition of the TNM Classification for Pleural Mesothelioma. J. Thorac. Oncol. 2024, 19, 1339–1351. [Google Scholar] [CrossRef]
- Ito, T.; Nakamura, S.; Kadomatsu, Y.; Ueno, H.; Kato, T.; Ozeki, N.; Fukumoto, K.; Chen-Yoshikawa, T.F. Impact of Pleural Thickness on Occurrence of Postoperative Complications in Patients with Malignant Pleural Mesothelioma. Ann. Surg. Oncol. 2023, 30, 1574–1583. [Google Scholar] [CrossRef]
- Cersosimo, F.; Barbarino, M.; Lonardi, S.; Vermi, W.; Giordano, A.; Bellan, C.; Giurisato, E. Mesothelioma Malignancy and the Microenvironment: Molecular Mechanisms. Cancers 2021, 13, 5664. [Google Scholar] [CrossRef]
- Izzi, V.; Chiurchiu, V.; D’Aquilio, F.; Palumbo, C.; Tresoldi, I.; Modesti, A.; Baldini, P.M. Differential effects of malignant mesothelioma cells on THP-1 monocytes and macrophages. Int. J. Oncol. 2009, 34, 543–550. [Google Scholar] [CrossRef][Green Version]
- Chu, G.J.; van Zandwijk, N.; Rasko, J.E.J. The Immune Microenvironment in Mesothelioma: Mechanisms of Resistance to Immunotherapy. Front. Oncol. 2019, 9, 1366. [Google Scholar] [CrossRef]
- Hasko, G.; Pacher, P. Regulation of macrophage function by adenosine. Arter. Thromb. Vasc. Biol. 2012, 32, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Blum, Y.; Meiller, C.; Quetel, L.; Elarouci, N.; Ayadi, M.; Tashtanbaeva, D.; Armenoult, L.; Montagne, F.; Tranchant, R.; Renier, A.; et al. Dissecting heterogeneity in malignant pleural mesothelioma through histo-molecular gradients for clinical applications. Nat. Commun. 2019, 10, 1333. [Google Scholar] [CrossRef]
- Ujiie, H.; Kadota, K.; Nitadori, J.I.; Aerts, J.G.; Woo, K.M.; Sima, C.S.; Travis, W.D.; Jones, D.R.; Krug, L.M.; Adusumilli, P.S. The tumoral and stromal immune microenvironment in malignant pleural mesothelioma: A comprehensive analysis reveals prognostic immune markers. Oncoimmunology 2015, 4, e1009285. [Google Scholar] [CrossRef] [PubMed]
- Lievense, L.A.; Sterman, D.H.; Cornelissen, R.; Aerts, J.G. Checkpoint Blockade in Lung Cancer and Mesothelioma. Am. J. Respir. Crit. Care Med. 2017, 196, 274–282. [Google Scholar] [CrossRef]
- Maio, M.; Scherpereel, A.; Calabro, L.; Aerts, J.; Perez, S.C.; Bearz, A.; Nackaerts, K.; Fennell, D.A.; Kowalski, D.; Tsao, A.S.; et al. Tremelimumab as second-line or third-line treatment in relapsed malignant mesothelioma (DETERMINE): A multicentre, international, randomised, double-blind, placebo-controlled phase 2b trial. Lancet Oncol. 2017, 18, 1261–1273. [Google Scholar] [CrossRef]
- Popat, S.; Curioni-Fontecedro, A.; Dafni, U.; Shah, R.; O’Brien, M.; Pope, A.; Fisher, P.; Spicer, J.; Roy, A.; Gilligan, D.; et al. A multicentre randomised phase III trial comparing pembrolizumab versus single-agent chemotherapy for advanced pre-treated malignant pleural mesothelioma: The European Thoracic Oncology Platform (ETOP 9-15) PROMISE-meso trial. Ann. Oncol. 2020, 31, 1734–1745. [Google Scholar] [CrossRef]
- Ceresoli, G.L.; Zucali, P.A.; De Vincenzo, F.; Gianoncelli, L.; Simonelli, M.; Lorenzi, E.; Ripa, C.; Giordano, L.; Santoro, A. Retreatment with pemetrexed-based chemotherapy in patients with malignant pleural mesothelioma. Lung Cancer 2011, 72, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, L.; Rodriguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Alley, E.W.; Lopez, J.; Santoro, A.; Morosky, A.; Saraf, S.; Piperdi, B.; van Brummelen, E. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): Preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol. 2017, 18, 623–630. [Google Scholar] [CrossRef]
- Chu, Q.; Perrone, F.; Greillier, L.; Tu, W.; Piccirillo, M.C.; Grosso, F.; Lo Russo, G.; Florescu, M.; Mencoboni, M.; Morabito, A.; et al. Pembrolizumab plus chemotherapy versus chemotherapy in untreated advanced pleural mesothelioma in Canada, Italy, and France: A phase 3, open-label, randomised controlled trial. Lancet 2023, 402, 2295–2306. [Google Scholar] [CrossRef]
- Forde, P.M.; Anagnostou, V.; Sun, Z.; Dahlberg, S.E.; Kindler, H.L.; Niknafs, N.; Purcell, T.; Santana-Davila, R.; Dudek, A.Z.; Borghaei, H.; et al. Durvalumab with platinum-pemetrexed for unresectable pleural mesothelioma: Survival, genomic and immunologic analyses from the phase 2 PrE0505 trial. Nat. Med. 2021, 27, 1910–1920. [Google Scholar] [CrossRef]
- Nowak, A.K.; Lesterhuis, W.J.; Kok, P.S.; Brown, C.; Hughes, B.G.; Karikios, D.J.; John, T.; Kao, S.C.; Leslie, C.; Cook, A.M.; et al. Durvalumab with first-line chemotherapy in previously untreated malignant pleural mesothelioma (DREAM): A multicentre, single-arm, phase 2 trial with a safety run-in. Lancet Oncol. 2020, 21, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Kok, P.S.; Forde, P.M.; Hughes, B.; Sun, Z.; Brown, C.; Ramalingam, S.; Cook, A.; Lesterhuis, W.J.; Yip, S.; O’Byrne, K.; et al. Protocol of DREAM3R: DuRvalumab with chEmotherapy as first-line treAtment in advanced pleural Mesothelioma-a phase 3 randomised trial. BMJ Open 2022, 12, e057663. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Zalcman, G.; Mazieres, J.; Margery, J.; Greillier, L.; Audigier-Valette, C.; Moro-Sibilot, D.; Molinier, O.; Corre, R.; Monnet, I.; Gounant, V.; et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): A randomised, controlled, open-label, phase 3 trial. Lancet 2016, 387, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Felip, E.; Popat, S.; Dafni, U.; Ribi, K.; Pope, A.; Cedres, S.; Shah, R.; de Marinis, F.; Cove Smith, L.; Bernabe, R.; et al. A randomised phase III study of bevacizumab and carboplatin-pemetrexed chemotherapy with or without atezolizumab as first-line treatment for advanced pleural mesothelioma: Results of the ETOP 13-18 BEAT-meso trial. Ann. Oncol. 2025, 36, 548–560. [Google Scholar] [CrossRef]
- Okada, M.; Kijima, T.; Aoe, K.; Kato, T.; Fujimoto, N.; Nakagawa, K.; Takeda, Y.; Hida, T.; Kanai, K.; Imamura, F.; et al. Clinical Efficacy and Safety of Nivolumab: Results of a Multicenter, Open-label, Single-arm, Japanese Phase II study in Malignant Pleural Mesothelioma (MERIT). Clin. Cancer Res. 2019, 25, 5485–5492. [Google Scholar] [CrossRef]
- Fennell, D.A.; Ewings, S.; Ottensmeier, C.; Califano, R.; Hanna, G.G.; Hill, K.; Danson, S.; Steele, N.; Nye, M.; Johnson, L.; et al. Nivolumab versus placebo in patients with relapsed malignant mesothelioma (CONFIRM): A multicentre, double-blind, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 1530–1540. [Google Scholar] [CrossRef]
- Sterman, D.H.; Alley, E.; Stevenson, J.P.; Friedberg, J.; Metzger, S.; Recio, A.; Moon, E.K.; Haas, A.R.; Vachani, A.; Katz, S.I.; et al. Pilot and Feasibility Trial Evaluating Immuno-Gene Therapy of Malignant Mesothelioma Using Intrapleural Delivery of Adenovirus-IFNalpha Combined with Chemotherapy. Clin. Cancer Res. 2016, 22, 3791–3800. [Google Scholar] [CrossRef]
- Fesnak, A.D.; June, C.H.; Levine, B.L. Engineered T cells: The promise and challenges of cancer immunotherapy. Nat. Rev. Cancer 2016, 16, 566–581. [Google Scholar] [CrossRef]
- Maus, M.V.; Haas, A.R.; Beatty, G.L.; Albelda, S.M.; Levine, B.L.; Liu, X.; Zhao, Y.; Kalos, M.; June, C.H. T cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer Immunol. Res. 2013, 1, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Beatty, G.L.; Haas, A.R.; Maus, M.V.; Torigian, D.A.; Soulen, M.C.; Plesa, G.; Chew, A.; Zhao, Y.; Levine, B.L.; Albelda, S.M.; et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol. Res. 2014, 2, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Adusumilli, P.S.; Zauderer, M.G.; Riviere, I.; Solomon, S.B.; Rusch, V.W.; O’Cearbhaill, R.E.; Zhu, A.; Cheema, W.; Chintala, N.K.; Halton, E.; et al. A Phase I Trial of Regional Mesothelin-Targeted CAR T-cell Therapy in Patients with Malignant Pleural Disease, in Combination with the Anti-PD-1 Agent Pembrolizumab. Cancer Discov. 2021, 11, 2748–2763. [Google Scholar] [CrossRef] [PubMed]
| Trial | ID | Treatment | Status/Result |
|---|---|---|---|
| DREAM3R | NCT04334759 | Randomized phase III trial of durvalumab with chemotherapy as first-line treatment | Active but not recruiting |
| MAPS | NCT00651456 | Randomized phase III trial of bevacizumab plus standard chemotherapy as first-line treatment | Positive |
| BEAT-meso trial | NCT03762018 | Randomized phase III trial comparing atezolizumab plus bevacizumab and standard chemotherapy with bevacizumab and standard chemotherapy as first-line treatment | Negative |
| PROMISE-Meso | NCT02991482 | Randomized phase III trial comparing pembrolizumab with standard chemotherapy for pretreated mesothelioma | Negative |
| CONFIRM | NCT03063450 | Placebo controlled phase III trial to evaluate the efficacy of nivolumab in relapsed mesothelioma | Positive |
| INFINITE | NCT03710876 | Randomized phase III trial of intrapleural administration of adenovirus-delivered interferon alfa-2b in combination with celecoxib and gemcitabine for pretreated mesothelioma | Active but not recruiting |
| CheckMate 743 | NCT0289929 | Randomized phase III trial of nivolumab plus ipilimumab vs. pemetrexed and platinum as first-line treatment | Positive |
| KEYNOTE-028 | NCT02054806 | Single-arm phase Ib trial of pembrolizumab for previous treated mesothelioma | OS 18 months |
| DREAM | ACTRN 12616001170415 | Single-arm phase II trial of durvalumab in combination with cisplatin and pemetrexed as first-line treatment | OS 18.4 months |
| PrECOG0505 | NCT02899195 | Sigle-arm phase II trial of durvalumab in combination with cisplatin and pemetrexed as the first-line treatment | OS 20.5 months |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato, T.; Tanaka, I.; Huang, H.; Okado, S.; Imamura, Y.; Nomata, Y.; Takenaka, H.; Watanabe, H.; Kawasumi, Y.; Nakanishi, K.; et al. Molecular Mechanisms of Tumor Progression and Novel Therapeutic and Diagnostic Strategies in Mesothelioma. Int. J. Mol. Sci. 2025, 26, 4299. https://doi.org/10.3390/ijms26094299
Kato T, Tanaka I, Huang H, Okado S, Imamura Y, Nomata Y, Takenaka H, Watanabe H, Kawasumi Y, Nakanishi K, et al. Molecular Mechanisms of Tumor Progression and Novel Therapeutic and Diagnostic Strategies in Mesothelioma. International Journal of Molecular Sciences. 2025; 26(9):4299. https://doi.org/10.3390/ijms26094299
Chicago/Turabian StyleKato, Taketo, Ichidai Tanaka, Heng Huang, Shoji Okado, Yoshito Imamura, Yuji Nomata, Hirofumi Takenaka, Hiroki Watanabe, Yuta Kawasumi, Keita Nakanishi, and et al. 2025. "Molecular Mechanisms of Tumor Progression and Novel Therapeutic and Diagnostic Strategies in Mesothelioma" International Journal of Molecular Sciences 26, no. 9: 4299. https://doi.org/10.3390/ijms26094299
APA StyleKato, T., Tanaka, I., Huang, H., Okado, S., Imamura, Y., Nomata, Y., Takenaka, H., Watanabe, H., Kawasumi, Y., Nakanishi, K., Kadomatsu, Y., Ueno, H., Nakamura, S., Mizuno, T., & Chen-Yoshikawa, T. F. (2025). Molecular Mechanisms of Tumor Progression and Novel Therapeutic and Diagnostic Strategies in Mesothelioma. International Journal of Molecular Sciences, 26(9), 4299. https://doi.org/10.3390/ijms26094299







