Regulation of Immune-Related Gene Expression by Salinity-Induced HPI Axis in Large Yellow Croaker, Larimichthys crocea
Abstract
1. Introduction
2. Results
2.1. Sequence Analysis and De Novo Assembly for Large Yellow Croaker
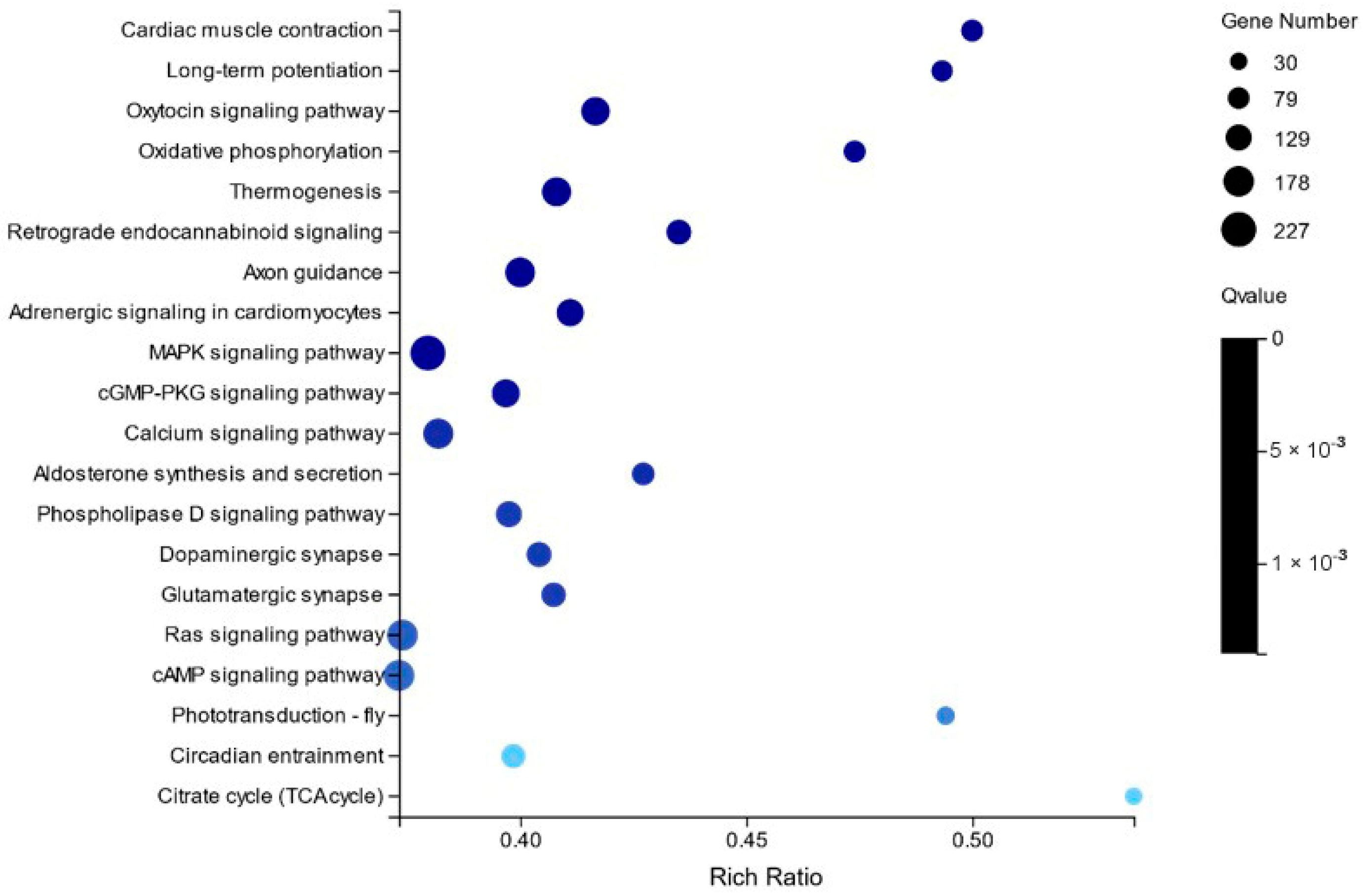
2.2. Differentially Expressed Gene Analysis
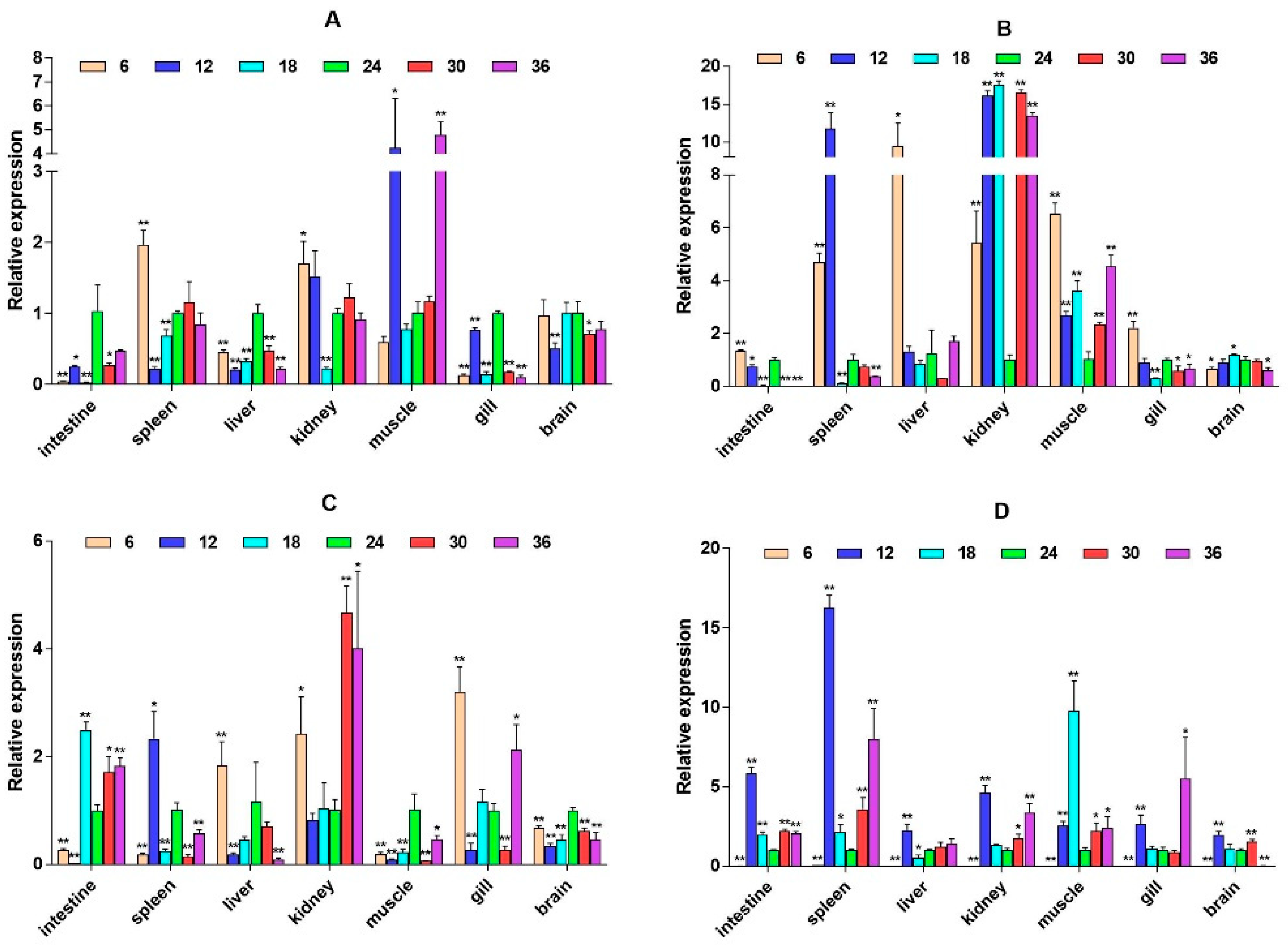
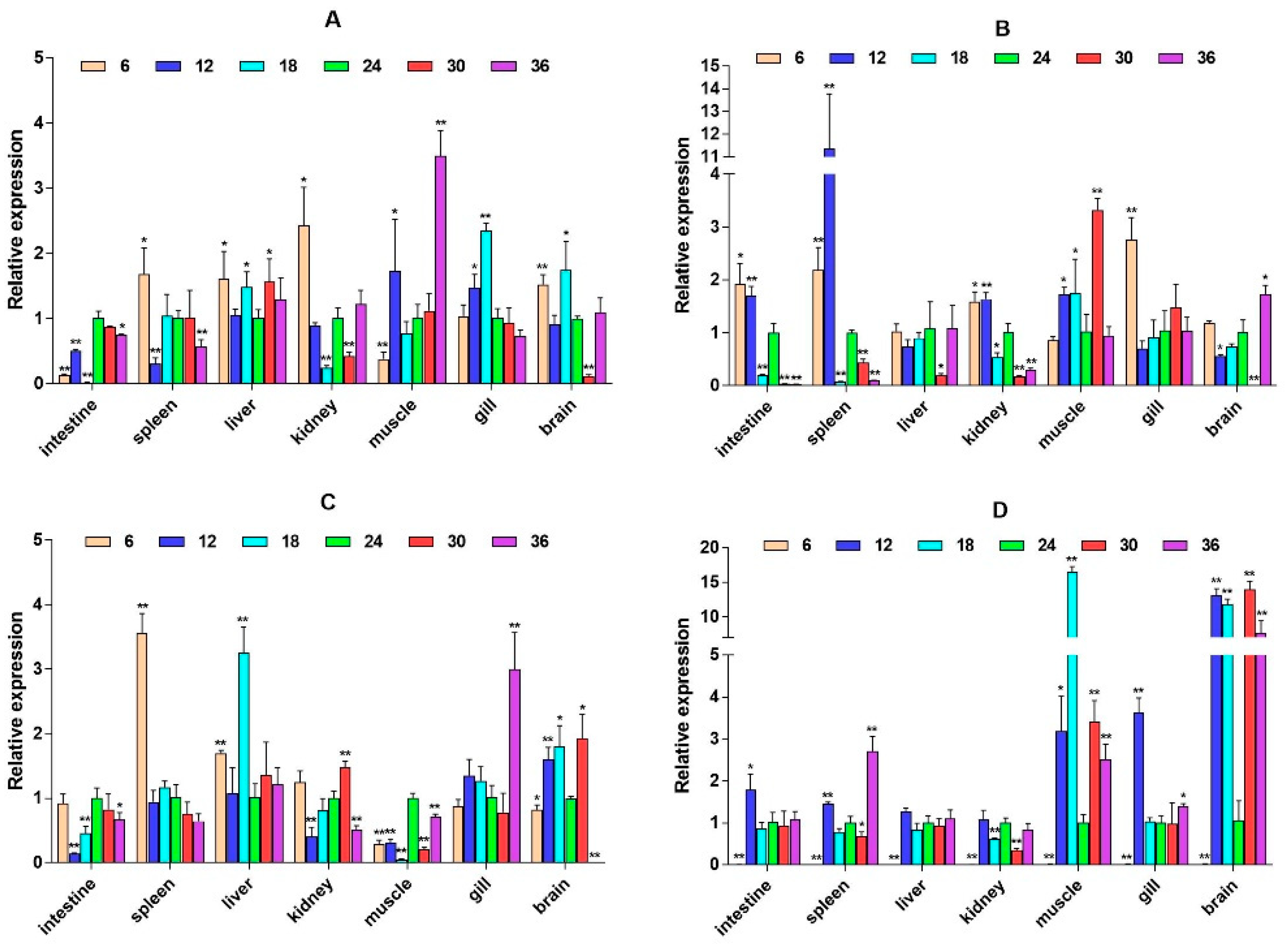
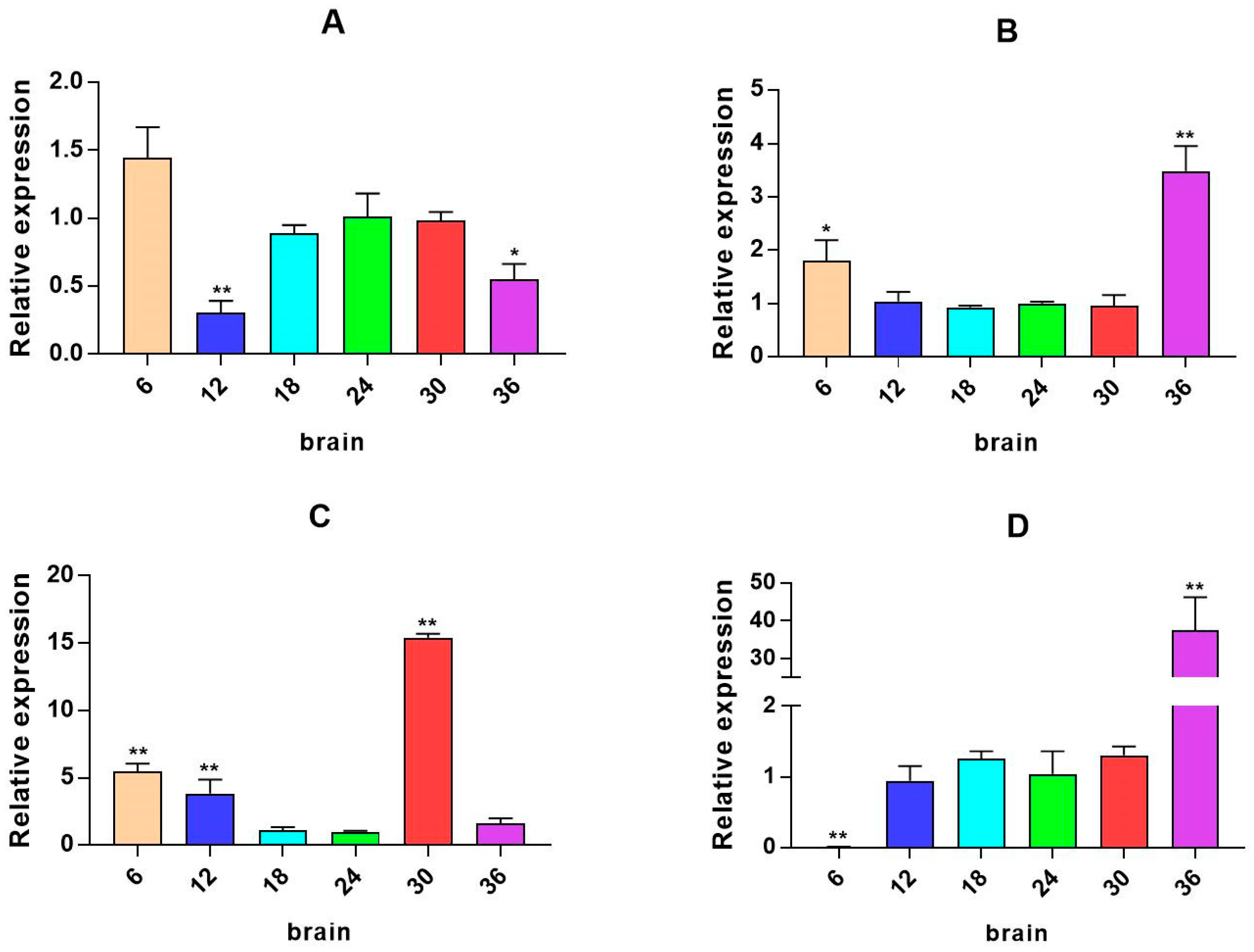
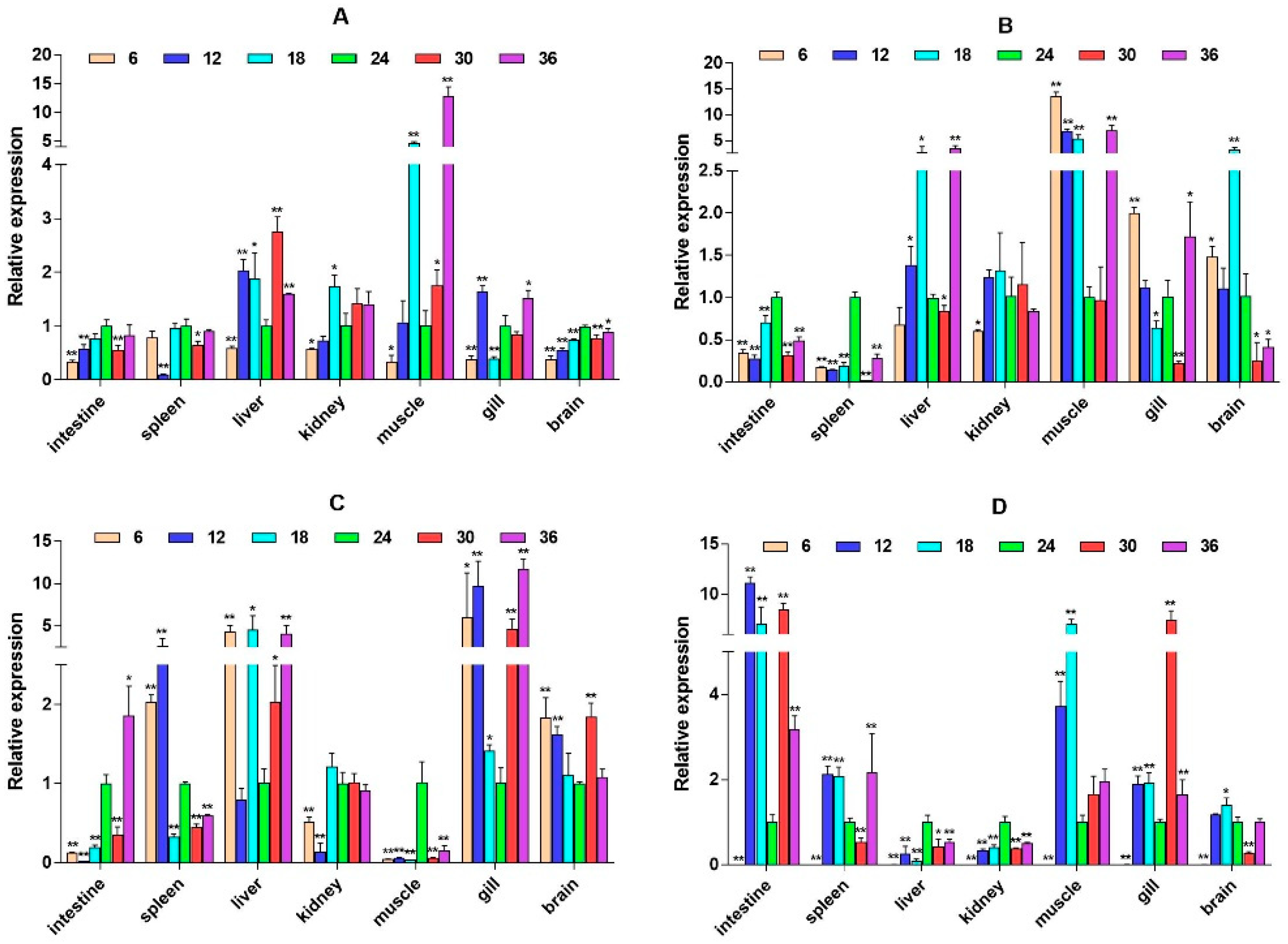
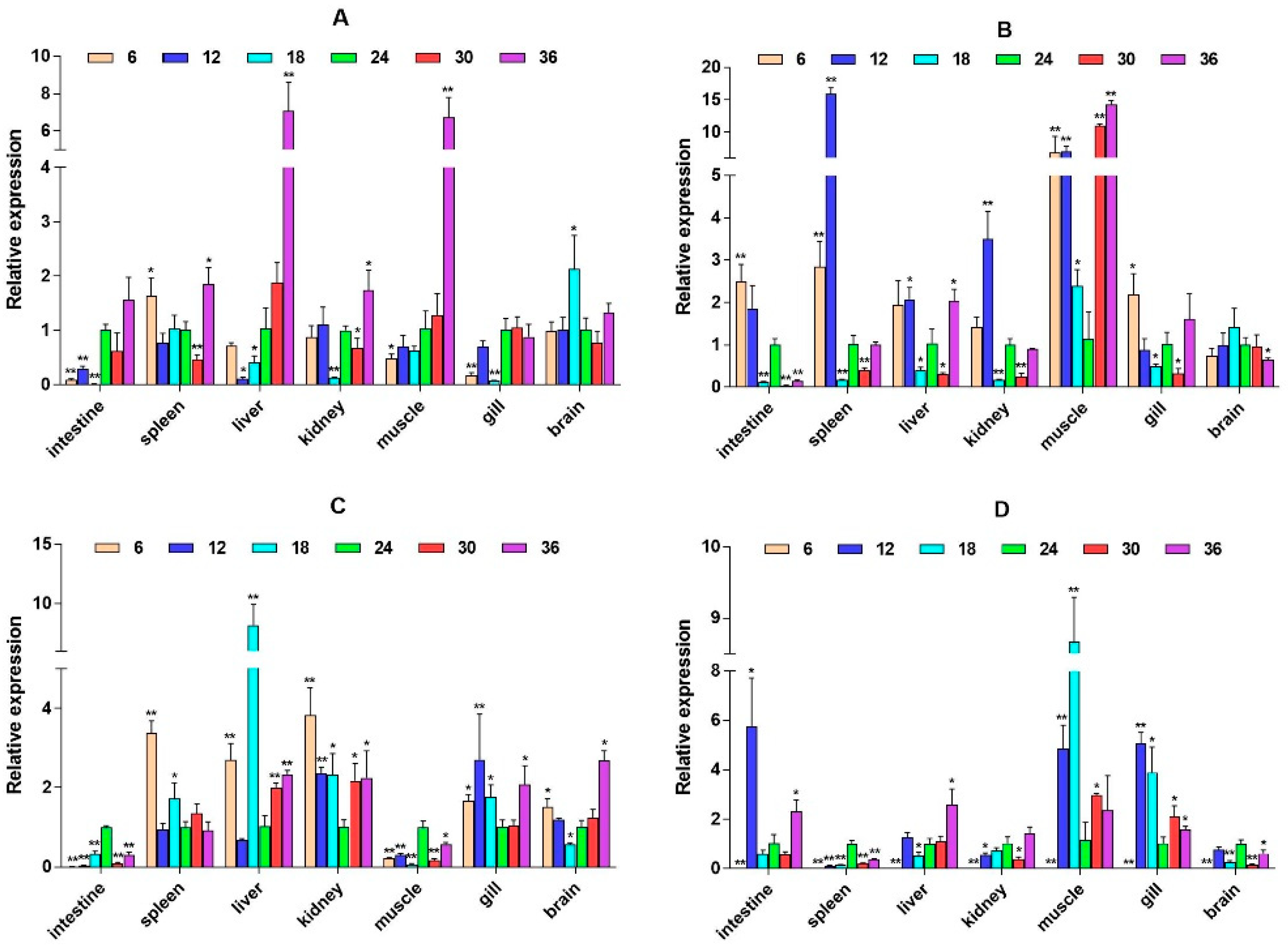
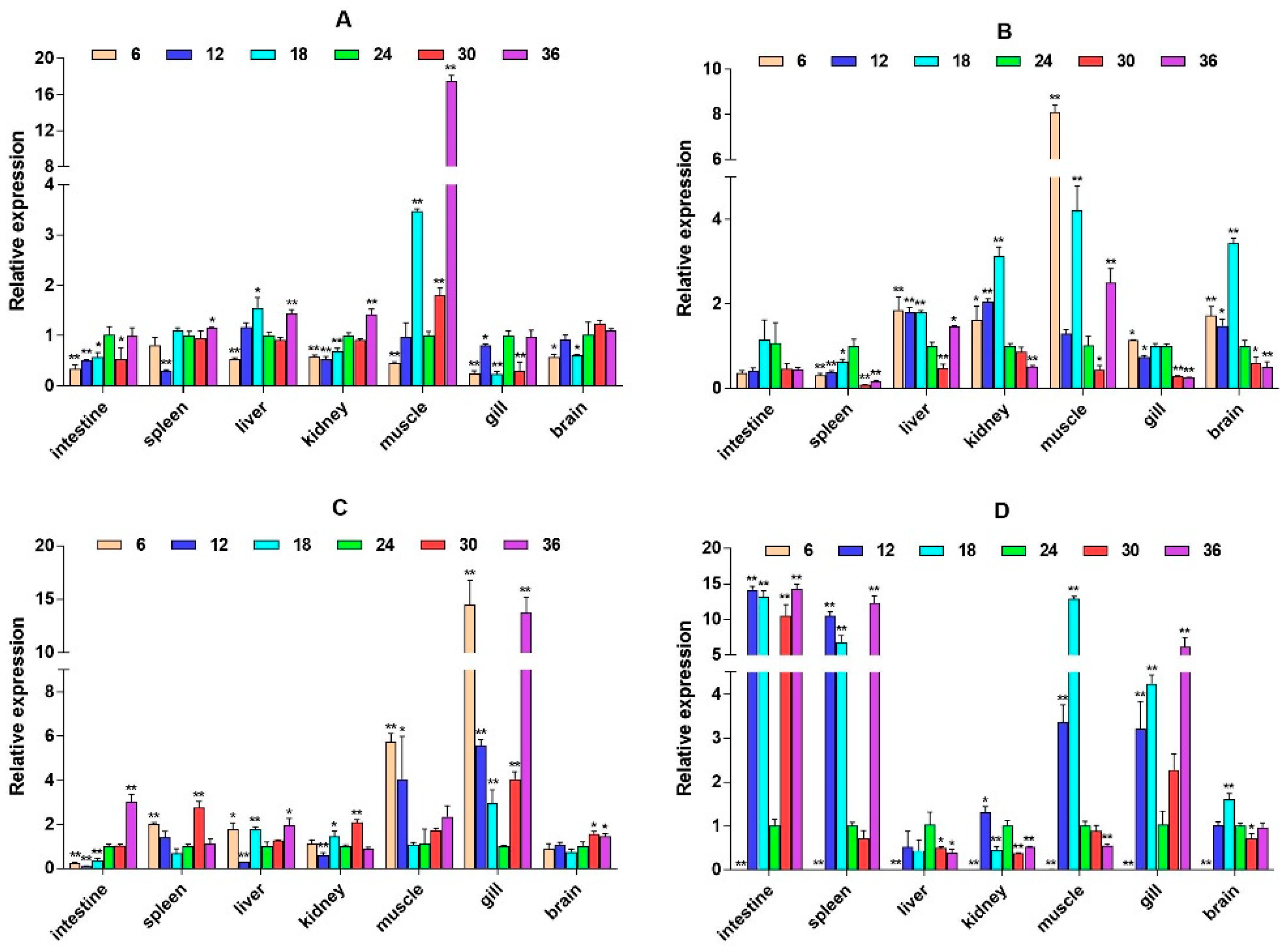
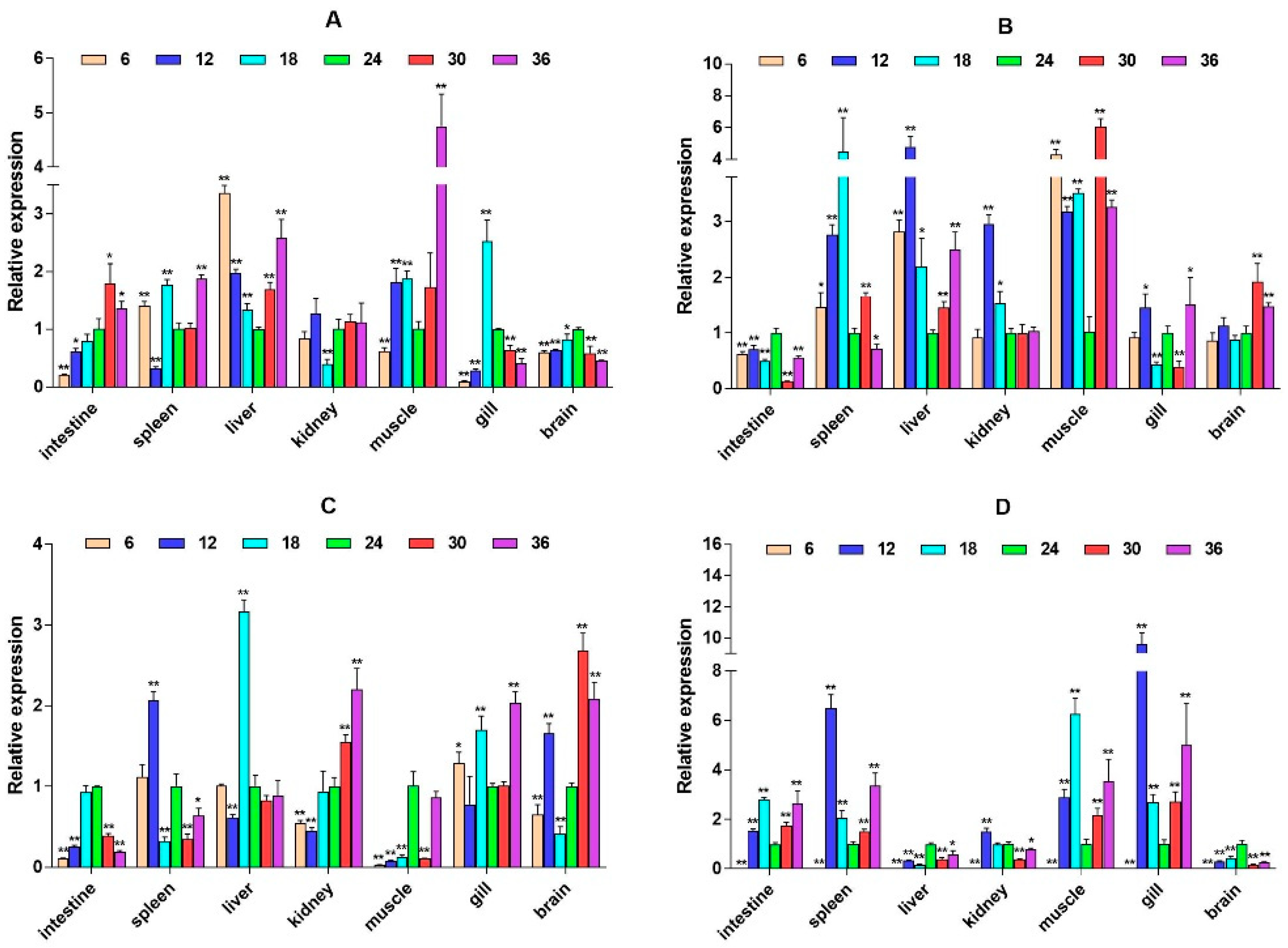
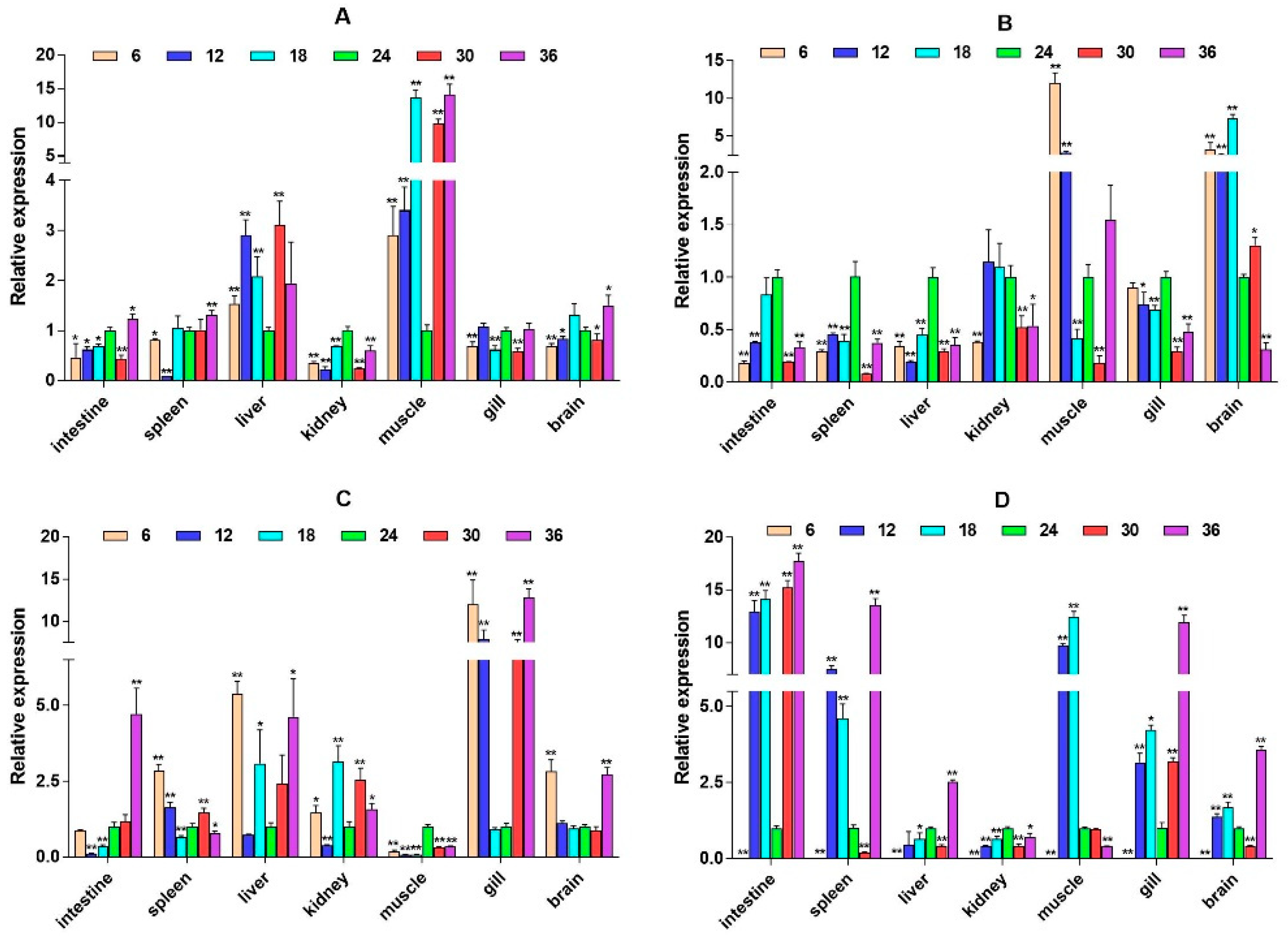
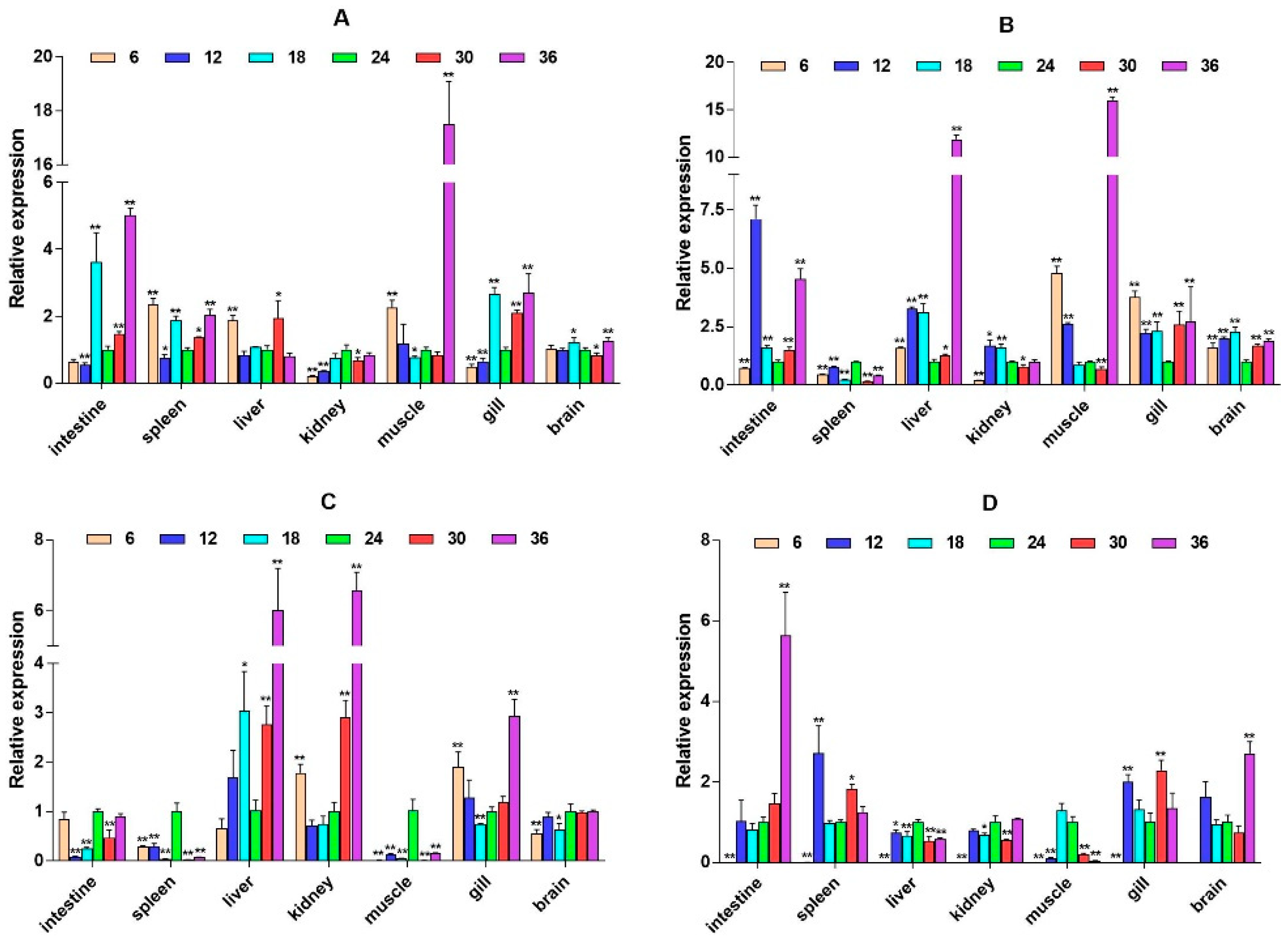
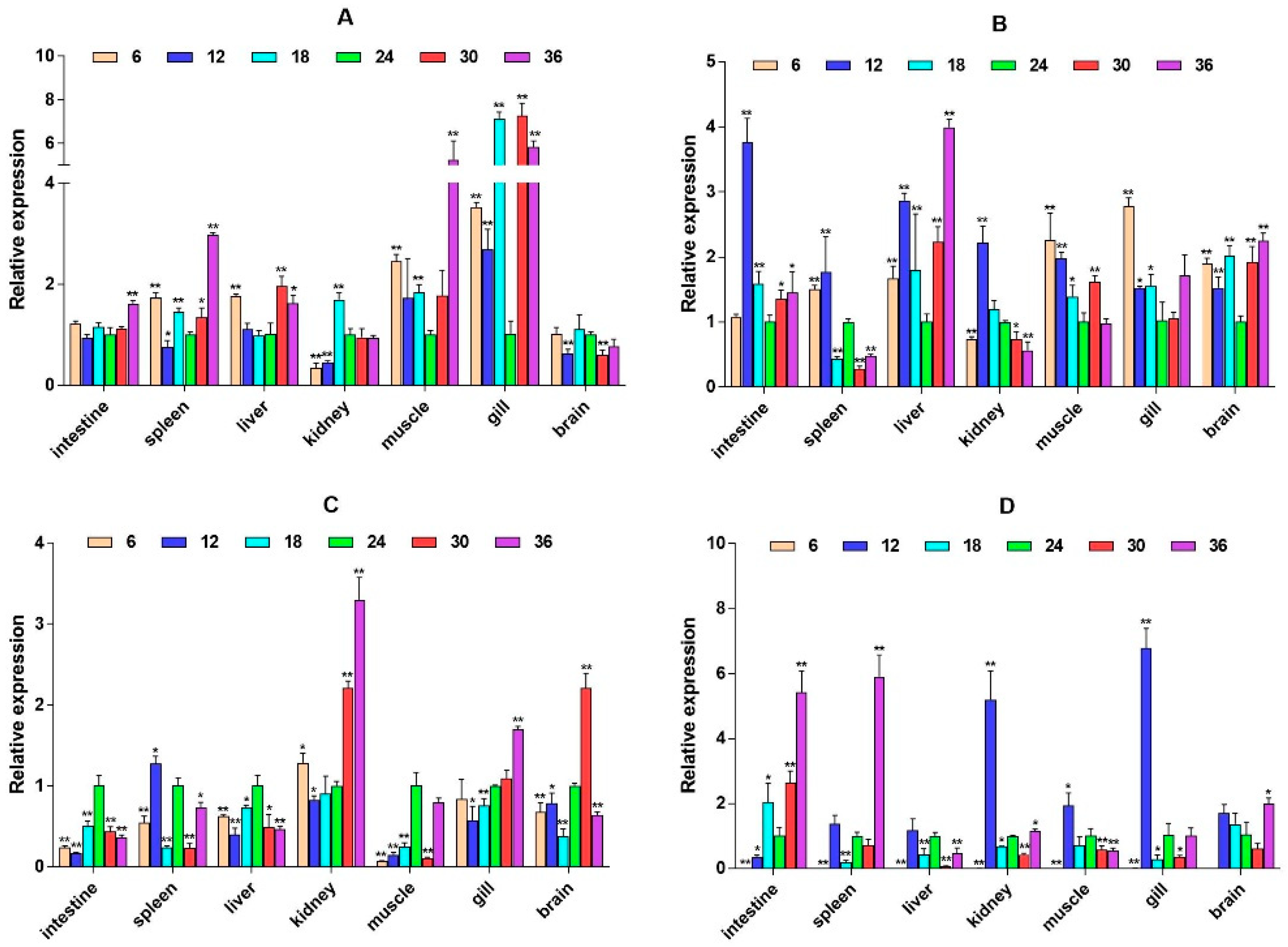
2.3. mRNA Expression Levels of HPI Axis and Immune-Related Genes
3. Discussion
4. Materials and Methods
4.1. Treatment of Fish Salinity Stress
4.2. RNA Extraction and Quantification
4.3. Quantitative Real-Time PCR Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wendelaar Bonga, S.E. The stress response in fish. Physiol. Rev. 1997, 77, 591–625. [Google Scholar] [CrossRef] [PubMed]
- Tort, L. Stress and immune modulation in fish. Dev. Comp. Immunol. 2011, 35, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Reid, S.G.; Bernier, N.J.; Perry, S.F. The adrenergic stress response in fish: Control of catecholamine storage and release. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1998, 120, 1–27. [Google Scholar] [CrossRef]
- Romero, L.M.; Butler, L.K. Endocrinology of Stress. Int. J. Comp. Psychol. 2007, 20, 89–95. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, L.; Shi, X.; Zhang, H.; Gao, Y.; Wang, M.; Kong, P.; Qiu, L.; Song, L. The modulation of catecholamines to the immune response against bacteria Vibrio anguillarum challenge in scallop Chlamys farreri. Fish Shellfish Immunol. 2011, 31, 1065–1071. [Google Scholar] [CrossRef]
- Sangiao-Alvarellos, S.; Miguez, J.M.; Soengas, J.L. Actions of growth hormone on carbohydrate metabolism and osmoregulation of rainbow trout (Oncorhynchus mykiss). Gen. Comp. Endocrinol. 2005, 141, 214–225. [Google Scholar] [CrossRef]
- Prunet, P.; Sandra, O.; Le Rouzic, P.; Marchand, O.; Laudet, V. Molecular characterization of the prolactin receptor in two fish species, tilapia Oreochromis niloticus and rainbow trout, Oncorhynchus mykiss: A comparative approach. Can. J. Physiol. Pharmacol. 2000, 78, 1086–1096. [Google Scholar] [CrossRef] [PubMed]
- Motohashi, E.; Hasegawa, S.; Mishiro, K.; Ando, H. Osmoregulatory responses of expression of vasotocin, isotocin, prolactin and growth hormone genes following hypoosmotic challenge in a stenohaline marine teleost, tiger puffer (Takifugu rubripes). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2009, 154, 353–359. [Google Scholar] [CrossRef]
- Choi, Y.J.; Kim, N.N.; Choi, C.Y. Profiles of hypothalamus-pituitary-interrenal axis gene expression in the parr and smolt stages of rainbow trout, Oncorhynchus mykiss: Effects of recombinant aquaporin 3 and seawater acclimation. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 182, 14–21. [Google Scholar] [CrossRef]
- Mohammed-Geba, K.; González, A.A.; Suárez, R.A.; Galal-Khallaf, A.; Martos-Sitcha, J.A.; Ibrahim, H.M.; Martínez-Rodríguez, G.; Mancera, J.M. Molecular performance of Prl and Gh/Igf1 axis in the Mediterranean meager, Argyrosomus regius, acclimated to different rearing salinities. Fish Physiol. Biochem. 2017, 43, 203–216. [Google Scholar] [CrossRef]
- Mohammed-Geba, K.; Yúfera, M.; Martínez-Rodríguez, G.; Mancera, J.M. Molecular endocrine changes of Gh/Igf1 axis in gilthead sea bream (Sparus aurata L.) exposed to different environmental salinities during larvae to post-larvae stages. Fish Physiol. Biochem. 2016, 42, 1177–1186. [Google Scholar] [CrossRef]
- Gu, J.; Dai, S.; Liu, H.; Cao, Q.; Yin, S.; Lai, K.P.; Tse, W.K.F.; Wong, C.K.C.; Shi, H. Identification of immune-related genes in gill cells of Japanese eels (Anguilla japonica) in adaptation to water salinity changes. Fish Shellfish Immunol. 2018, 73, 288–296. [Google Scholar] [CrossRef]
- Wang, J.; He, R.Z.; Lu, G.L.; Luo, H.L.; Lu, D.Q.; Li, A.X. Vaccine-induced antibody level as the parameter of the influence of environmental salinity on vaccine efficacy in Nile tilapia. Fish Shellfish Immunol. 2018, 82, 522–530. [Google Scholar] [CrossRef]
- Suo, Y.T. Effects of Salinity Stress on Immune Damage and Modulation Effects of Dietary β-Glucan in Nile tilapia (Oreochromis niloticus). Master. Thesis, East China Normal University, Shanghai, China, 2018. [Google Scholar]
- Huang, W.Q.; Zhang, Y.; Ke, Q.Z.; Lin, P.H.; Chen, M.H.; Han, K.H. Growth traits research of the breeding group sub 2 generation of large yellow croaker (Larimichthys crocea). South China Fish. Sci. 2013, 9, 14–19. [Google Scholar]
- Wang, G.L.; Zhu, J.L.; Jin, S. Molecular identification and phylogenetics of pathogenic vibrios in cultured large yellow croaker (Pseudosciaena crocea) Richardson. Oceanologia. Limnologia. Sinica 2008, 2, 162–167. [Google Scholar]
- Wang, Y.B.; Hu, Z.H.; Zhu, Y.H.; Chai, X.J. Effects of salinity on growth and non-specific immunity of Nibea japonica. J. Zhejiang Ocean. Univ. (Nat. Sci.) 2015, 34, 26–31. [Google Scholar]
- Kato, H.; Kawaguchi, M.; Inoue, S.; Hirai, K.; Furuya, H. The effects of beta-adrenoceptor antagonists on proinflammatory cytokine concentrations after subarachnoid hemorrhage in rats. Anesth. Analg. 2009, 108, 288–295. [Google Scholar] [CrossRef]
- Yin, F.; Wang, Y.Y.; Du, J.H.; Li, C.; Lu, Z.Z.; Han, C.; Zhang, Y.Y. Noncanonical cAMP pathway and p38 MAPK mediate β2-adrenergic receptor-induced IL-6 production in neonatal mouse cardiac fibroblasts. J. Mol. Cell Cardiol. 2006, 40, 384–393. [Google Scholar] [CrossRef]
- Chikazawa, M.; Sato, R. Identification of functional food factors as β2-adrenergic receptor agonists and their potential roles in skeletal muscle. J. Nutr. Sci. Vitaminol. 2018, 64, 68–74. [Google Scholar] [CrossRef]
- Sato, S.; Nomura, S.; Kawano, F.; Tanihata, J.; Tachiyashiki, K.; Imaizumi, K. Effects of the beta2-agonist clenbuterol on beta1- and beta2-adrenoceptor mRNA expressions of rat skeletal and left ventricle muscles. J. Pharmacol. Sci. 2008, 107, 393–400. [Google Scholar] [CrossRef]
- Li, W.S.; Chen, D.; Wong, A.O.; Lin, H.R. Molecular cloning, tissue distribution, and ontogeny of mRNA expression of growth hormone in orange-spotted grouper (Epinephelus coioides). Gen. Comp. Endocrinol. 2005, 144, 78–89. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, L.; Duan, D.; Zhang, H.; Wang, Y.; Li, W.; Lin, H. Growth hormone and two forms of insulin-like growth factors I in the giant grouper (Epinephelus lanceolatus): Molecular cloning and characterization of tissue distribution. Fish Physiol. Biochem. 2010, 36, 201–212. [Google Scholar] [CrossRef]
- Rousseau, K.; Dufour, S. Comparative aspects of GH and metabolic regulation in lower vertebrates. Neuroendocrinology 2007, 86, 165–174. [Google Scholar] [CrossRef]
- Abass, N.Y.; Elwakil, H.E.; Hemeida, A.A.; Abdelsalam, N.R.; Ye, Z.; Su, B.; Alsaqufi, A.S.; Weng, C.C.; Trudeau, V.L.; Dunham, R.A. Genotype–environment interactions for survival at low and sub-zero temperatures at varying salinity for channel catfish, hybrid catfish and transgenic channel catfish. Aquaculture 2016, 458, 140–148. [Google Scholar] [CrossRef]
- Marano, R.J.; Ben-Jonathan, N. Minireview: Extrapituitary prolactin: An update on the distribution, regulation, and functions. Mol. Endocrinol. 2014, 28, 622–633. [Google Scholar] [CrossRef]
- Makino, K.; Onuma, T.A.; Kitahashi, T.; Ando, H.; Ban, M.; Urano, A. Expression of hormone genes and osmoregulation in homing chum salmon: A minireview. Gen. Comp. Endocrinol. 2007, 152, 304–309. [Google Scholar] [CrossRef]
- Onuma, T.; Kitahashi, T.; Taniyama, S.; Saito, D.; Ando, H.; Urano, A. Changes in expression of genes encoding gonadotropin subunits and growth hormone/prolactin/somatolactin family hormones during final maturation and freshwater adaptation in prespawning chum salmon. Endocrine 2003, 20, 23–33. [Google Scholar] [CrossRef]
- Freeman, M.E.; Kanyicska, B.; Lerant, A.; Nagy, G. Prolactin: Structure, function, and regulation of secretion. Physiol. Rev. 2000, 80, 1523–1631. [Google Scholar] [CrossRef]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015, 16, 448–457. [Google Scholar] [CrossRef]
- Ataie-Kachoie, P.; Pourgholami, M.H.; Richardson, D.R.; Morris, D.L. Gene of the month: Interleukin 6 (IL-6). J. Clin. Pathol. 2014, 67, 932–937. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Y.; Wen, C.; Lv, M.; Gan, N.; Zhou, H.; Zhang, A.; Yang, K. Molecular characterization of grass carp interleukin-6 receptor and the agonistic activity of its soluble form in head kidney leucocytes. Fish Shellfish Immunol. 2019, 86, 1072–1080. [Google Scholar] [CrossRef]
- Engelsma, M.Y.; Huising, M.O.; van Muiswinkel, W.B.; Flik, G.; Kwang, J.; Savelkoul, H.F.; Verburg-van Kemenade, B.L. Neuroendocrine-immune interactions in fish: A role for interleukin-1. Vet. Immunol. Immunopathol. 2002, 87, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Thulasitha, W.S.; Umasuthan, N.; Whang, I.; Lim, B.S.; Jung, H.B.; Noh, J.K.; Lee, J. A CXC chemokine gene, CXCL12, from rock bream, Oplegnathus fasciatus: Molecular characterization and transcriptional profile. Fish Shellfish Immunol. 2015, 45, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Foord, S.M.; Bonner, T.I.; Neubig, R.R.; Rosser, E.M.; Pin, J.P.; Davenport, A.P.; Spedding, M.; Harmar, A.J. International union of pharmacology. XLVI. G protein-coupled receptor list. Pharmacol. Rev. 2005, 57, 279–288. [Google Scholar] [CrossRef]
- Vassart, G.; Costagliola, S. G protein-coupled receptors: Mutations and endocrine diseases. Nat. Rev. Endocrinol. 2011, 7, 362–372. [Google Scholar] [CrossRef]
- Shao, C.; Niu, Y.; Rastas, P.; Liu, Y.; Xie, Z.; Li, H.; Wang, L.; Jiang, Y.; Tai, S.; Tian, Y. Genome-wide SNP identification for the construction of a high-resolution genetic map of Japanese flounder (Paralichthys olivaceus): Applications to QTL mapping of Vibrio anguillarum disease resistance and comparative genomic analysis. DNA Res. 2015, 22, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Nie, P.; Thompson, K.D.; Adams, A.; Wang, T.; Secombes, C.J.; Zou, J. The search for the IFN-γ receptor in fish: Functional and expression analysis of putative binding and signalling chains in rainbow trout Oncorhynchus mykiss. Dev. Comp. Immunol. 2009, 33, 920–931. [Google Scholar] [CrossRef]
- Sun, B.; Greiner-Tollersrud, L.; Koop, B.F.; Robertsen, B. Atlantic salmon possesses two clusters of type I interferon receptor genes on different chromosomes, which allows for a larger repertoire of interferon receptors than in zebrafish and mammals. Dev. Comp. Immunol. 2014, 47, 275–286. [Google Scholar] [CrossRef]
- Zou, J.; Secombes, C.J. Teleost fish interferons and their role in immunity. Dev. Comp. Immunol. 2011, 35, 1376–1387. [Google Scholar] [CrossRef]
- Hoffmann, H.H.; Schneider, W.M.; Rice, C.M. Interferons and viruses: An evolutionary arms race of molecular interactions. Trends Immunol. 2015, 36, 124–138. [Google Scholar] [CrossRef]
- Yao, C.L.; Kong, P.; Huang, X.N.; Wang, Z.Y. Molecular cloning and expression of IRF1 in large yellow croaker, Pseudosciaena crocea. Fish Shellfish Immunol. 2010, 28, 654–660. [Google Scholar] [CrossRef]
- Yabu, T.; Hirose, H.; Hirono, I.; Katagiri, T.; Aoki, T.; Yamamoto, E. Molecular cloning of a novel interferon regulatory factor in Japanese flounder, Paralichthys olivaceus. Mol. Mar. Biol. Biotechnol. 1998, 7, 138–144. [Google Scholar]
- Wang, T.; Secombes, C.J. Rainbow trout suppressor of cytokine signalling (SOCS)-1, 2 and 3: Molecular identification, expression and modulation. Mol. Immunol. 2008, 45, 1449–1457. [Google Scholar] [CrossRef]
- Thanasaksiri, K.; Hirono, I.; Kondo, H. Identification and expression analysis of suppressors of cytokine signaling (SOCS) of Japanese flounder Paralichthys olivaceus. Fish Shellfish Immunol. 2016, 58, 145–152. [Google Scholar] [CrossRef]
- Wang, T.; Gao, Q.; Nie, P.; Secombes, C.J. Identification of suppressor of cytokine signalling (SOCS) 6, 7, 9 and CISH in rainbow trout Oncorhynchus mykiss and analysis of their expression in relation to other known trout SOCS. Fish Shellfish Immunol. 2010, 29, 656–667. [Google Scholar] [CrossRef]
- Philip, A.M.; Daniel Kim, S.; Vijayan, M.M. Cortisol modulates the expression of cytokines and suppressors of cytokine signaling (SOCS) in rainbow trout hepatocytes. Dev. Comp. Immunol. 2012, 38, 360–367. [Google Scholar] [CrossRef]
- Kolmus, K.; Tavernier, J.; Gerlo, S. β2-Adrenergic receptors in immunity and inflammation: Stressing NF-kB. Brain Behav. Immun. 2015, 45, 297–310. [Google Scholar] [CrossRef]
- Barnett Jr, C.C.; Moore, E.E.; Partrick, D.A.; Silliman, C.C. β-adrenergic stimulation down-regulates neutrophil priming for superoxide generation, but not elastase release. J. Surg. Res. 1997, 70, 166–170. [Google Scholar] [CrossRef]
- Haskó, G.; Szabó, C.; Németh, Z.H.; Salzman, A.L.; Vizi, E.S. Stimulation of β-adrenoceptors inhibits endotoxin-induced IL-12 production in normal and IL-10 deficient mice. J. Neuroimmunol. 1998, 88, 57–61. [Google Scholar] [CrossRef]
- Szelényi, J.; Kiss, J.P.; Vizi, E.S. Differential involvement of sympathetic nervous system and immune system in the modulation of TNF-α production by α2- and β-adrenoceptors in mice. J. Neuroimmunol. 2000, 103, 34–40. [Google Scholar] [CrossRef]
- Van der Poll, T.; Lowry, S.F. Epinephrine inhibits endotoxin-induced IL-1β production: Roles of tumor necrosis factor-α and IL-10. Am. J. Physiol.-Reg. I 1997, 273, R1885–R1890. [Google Scholar] [CrossRef]
- Hostager, B.S.; Bishop, G.A. CD40-mediated activation of the NF-kB2 pathway. Front. Immunol. 2013, 4, 376. [Google Scholar] [CrossRef]
- Verburg-van Kemenade, B.M.; Ribeiro, C.M.; Chadzinska, M. Neuroendocrine-immune interaction in fish: Differential regulation of phagocyte activity by neuroendocrine factors. Gen. Comp. Endocrinol. 2011, 172, 31–38. [Google Scholar] [CrossRef]
- Wang, P.; Gao, C.; Guo, N.; Zhang, S.D.; Wang, W.; Yao, L.P.; Zhang, J.; Efferth, T.; Fu, Y.J. 2′-O-Galloylhyperin isolated from Pyrola incarnata fisch. Attenuates LPS-induced inflammatory response by activation of SIRT1/Nrf2 and inhibition of the NF-kB pathways in vitro and vivo. Front. Pharmacol. 2018, 9, 679. [Google Scholar] [CrossRef]
- Dejkhamron, P.; Thimmarayappa, J.; Kotlyarevska, K.; Sun, J.; Lu, C.; Bonkowski, E.L.; Denson, L.A.; Menon, R.K. Lipopolysaccharide (LPS) directly suppresses growth hormone receptor (GHR) expression through MyD88-dependent and -independent Toll-like receptor-4/MD2 complex signaling pathways. Mol. Cell Endocrinol. 2007, 274, 35–42. [Google Scholar] [CrossRef]
- Batista, C.R.; Figueiredo, M.A.; Almeida, D.V.; Romano, L.A.; Marins, L.F. Impairment of the immune system in GH-overexpressing transgenic zebrafish (Danio rerio). Fish Shellfish Immunol. 2014, 36, 519–524. [Google Scholar] [CrossRef]
- Batista, C.R.; Figueiredo, M.A.; Almeida, D.V.; Romano, L.A.; Marins, L.F. Effects of somatotrophic axis (GH/GHR) double transgenesis on structural and molecular aspects of the zebrafish immune system. Fish Shellfish Immunol. 2015, 45, 725–732. [Google Scholar] [CrossRef]
- Reinhardt-Poulin, P.; McLean, J.R.; Deslauriers, Y.; Gorman, W.; Cabat, S.; Rouabhia, M. The use of silver-stained “comets” to visualize DNA damage and repair in normal and Xeroderma pigmentosum fibroblasts after exposure to simulated solar radiation. Photochem. Photobiol. 2000, 71, 422–425. [Google Scholar] [CrossRef]
- Vidal, O.M.; Merino, R.; Rico-Bautista, E.; Fernandez-Perez, L.; Chia, D.J.; Woelfle, J.; Ono, M.; Lenhard, B.; Norstedt, G. In vivo transcript profiling and phylogenetic analysis identifies suppressor of cytokine signaling 2 as a direct signal transducer and activator of transcription 5b target in liver. Mol. Endocrinol. 2007, 21, 293–311. [Google Scholar] [CrossRef]
- Olavarria, V.H.; Figueroa, J.E.; Mulero, V. Prolactin-induced activation of phagocyte NADPH oxidase in the teleost fish gilthead seabream involves the phosphorylation of p47phox by protein kinase C. Dev. Comp. Immunol. 2012, 36, 216–221. [Google Scholar] [CrossRef]
- Paredes, M.; Gonzalez, K.; Figueroa, J.; Montiel-Eulefi, E. Immunomodulatory effect of prolactin on Atlantic salmon (Salmo salar) macrophage function. Fish Physiol. Biochem. 2013, 39, 1215–1221. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]


| Sample | Total Raw Reads (M) | Total Clean Reads (M) | Total Clean Bases (Gb) | Clean Reads Q20 (%) | Clean Reads Q30 (%) | Clean Reads Ratio (%) |
|---|---|---|---|---|---|---|
| HB1 | 43.82 | 43.37 | 6.51 | 98.11 | 94.62 | 98.97 |
| HB2 | 43.82 | 43.4 | 6.51 | 97.88 | 93.97 | 99.05 |
| HB3 | 43.82 | 43.38 | 6.51 | 97.96 | 94.18 | 99 |
| LB1 | 45.57 | 42.69 | 6.4 | 96.82 | 88.07 | 93.66 |
| LB2 | 43.82 | 43.41 | 6.51 | 97.83 | 93.8 | 99.07 |
| LB3 | 43.82 | 43.42 | 6.51 | 97.87 | 93.93 | 99.08 |
| NB1 | 43.82 | 43.38 | 6.51 | 98 | 94.31 | 98.99 |
| NB2 | 43.82 | 43.43 | 6.51 | 97.97 | 94.19 | 99.11 |
| NB3 | 43.82 | 43.37 | 6.51 | 98 | 94.32 | 98.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, J.; Lou, Z.; Feng, H.; Zhang, Y.; Li, H.; Chu, W.; Xue, L. Regulation of Immune-Related Gene Expression by Salinity-Induced HPI Axis in Large Yellow Croaker, Larimichthys crocea. Int. J. Mol. Sci. 2025, 26, 4298. https://doi.org/10.3390/ijms26094298
Cheng J, Lou Z, Feng H, Zhang Y, Li H, Chu W, Xue L. Regulation of Immune-Related Gene Expression by Salinity-Induced HPI Axis in Large Yellow Croaker, Larimichthys crocea. International Journal of Molecular Sciences. 2025; 26(9):4298. https://doi.org/10.3390/ijms26094298
Chicago/Turabian StyleCheng, Jia, Zhengjia Lou, Huijie Feng, Yu Zhang, Honghui Li, Wuying Chu, and Liangyi Xue. 2025. "Regulation of Immune-Related Gene Expression by Salinity-Induced HPI Axis in Large Yellow Croaker, Larimichthys crocea" International Journal of Molecular Sciences 26, no. 9: 4298. https://doi.org/10.3390/ijms26094298
APA StyleCheng, J., Lou, Z., Feng, H., Zhang, Y., Li, H., Chu, W., & Xue, L. (2025). Regulation of Immune-Related Gene Expression by Salinity-Induced HPI Axis in Large Yellow Croaker, Larimichthys crocea. International Journal of Molecular Sciences, 26(9), 4298. https://doi.org/10.3390/ijms26094298






