Oxidation and Reduction of Hydrazones—Risk Factors Related to the Manufacture and Stability of the Drugs
Abstract
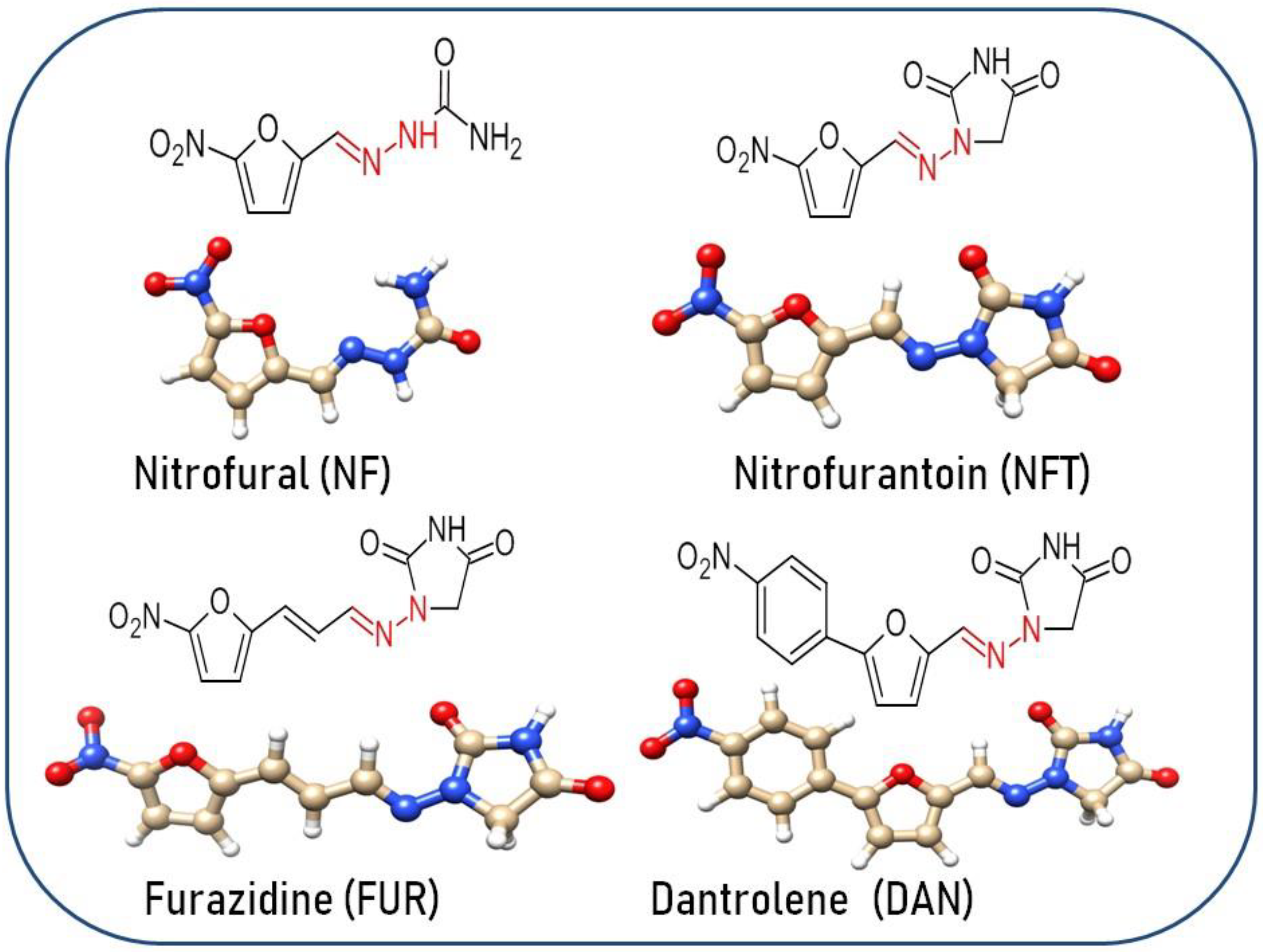
1. Introduction
2. Results and Discussion
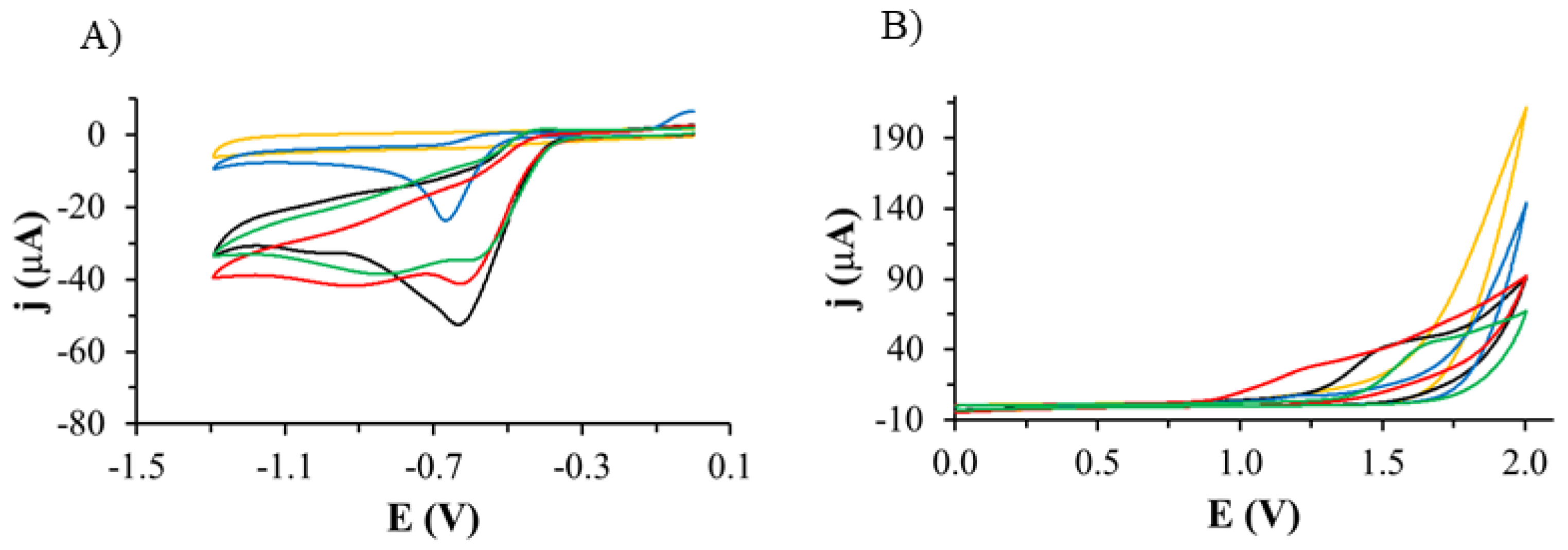
2.1. Electrochemical Measurements
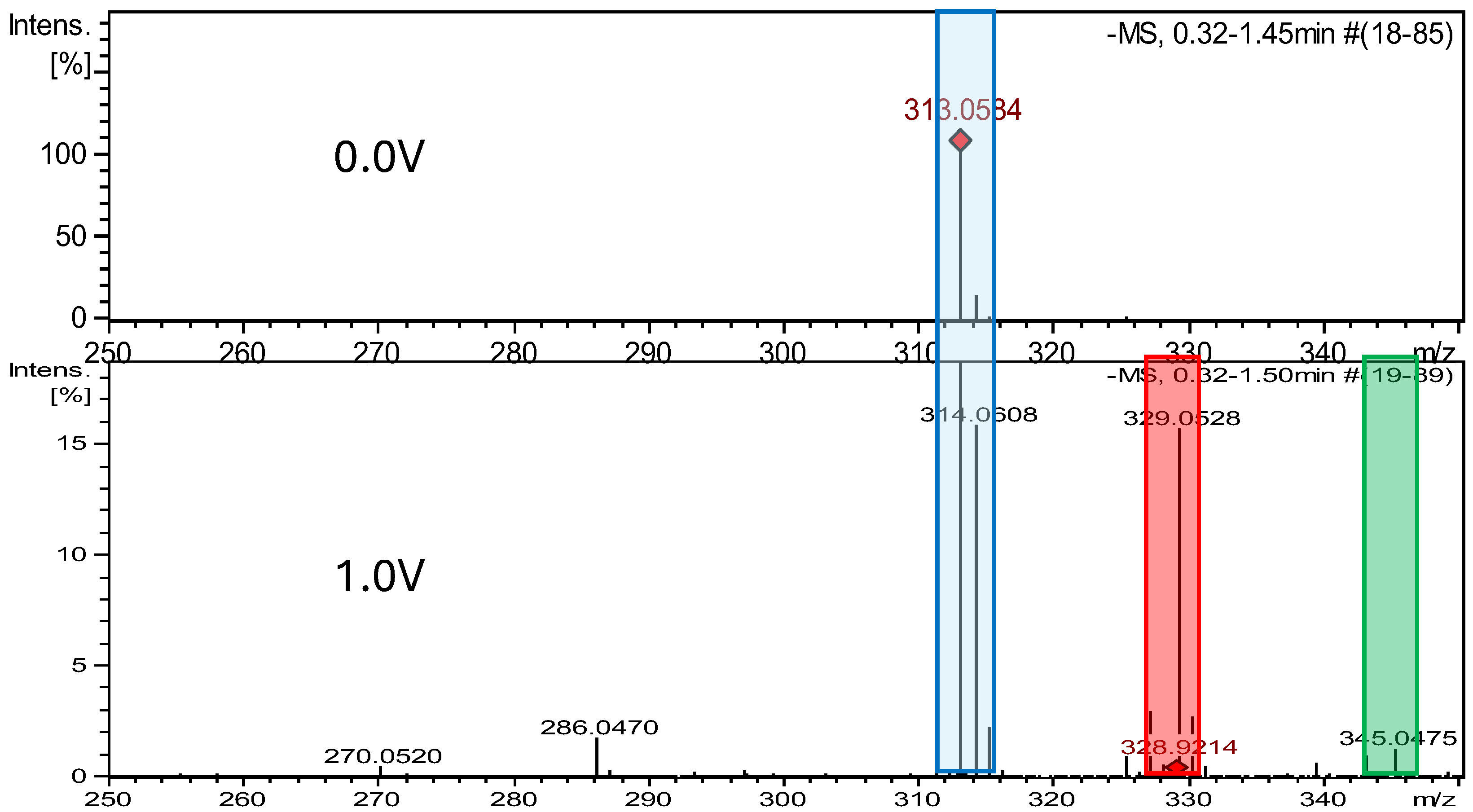
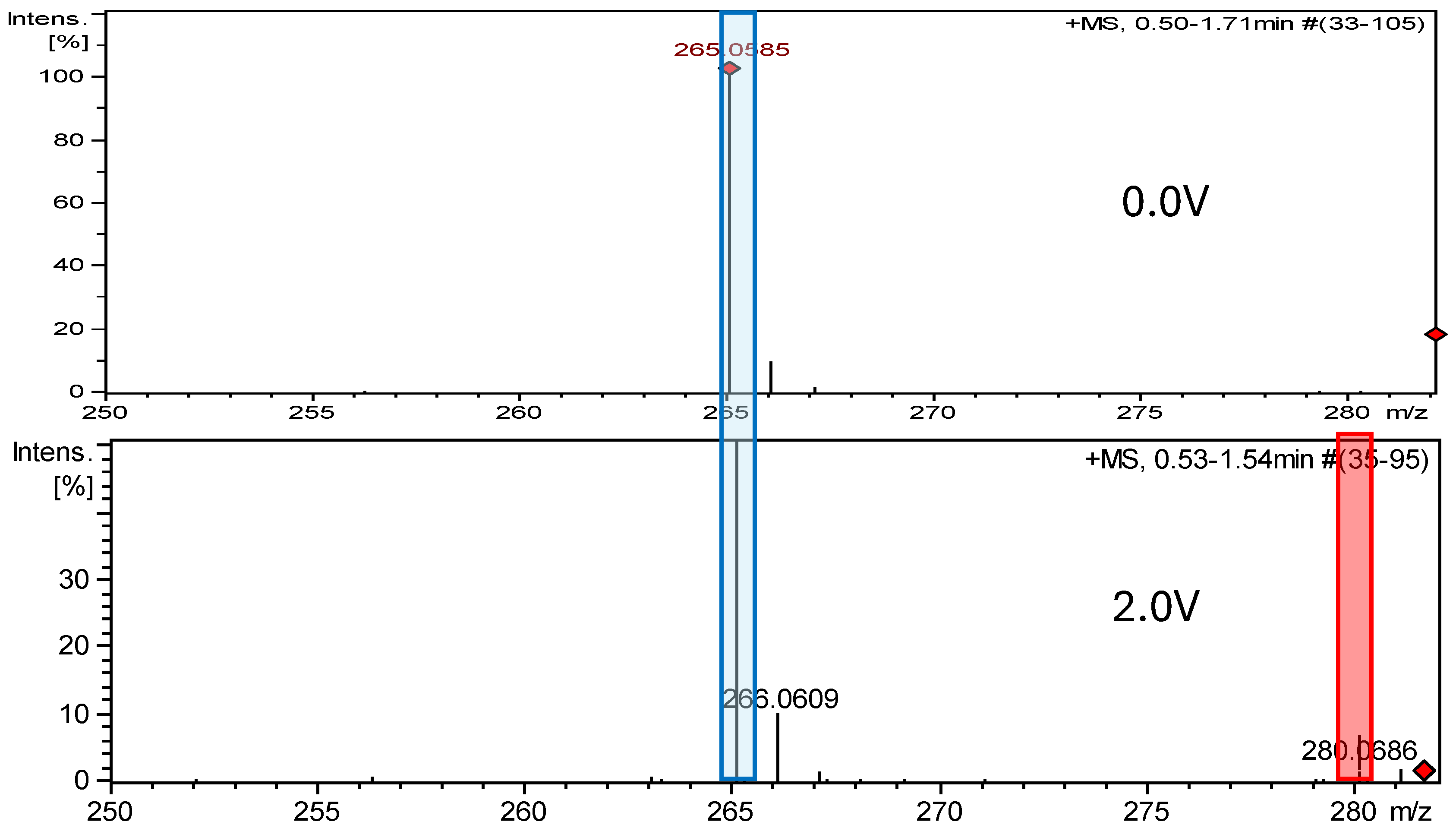
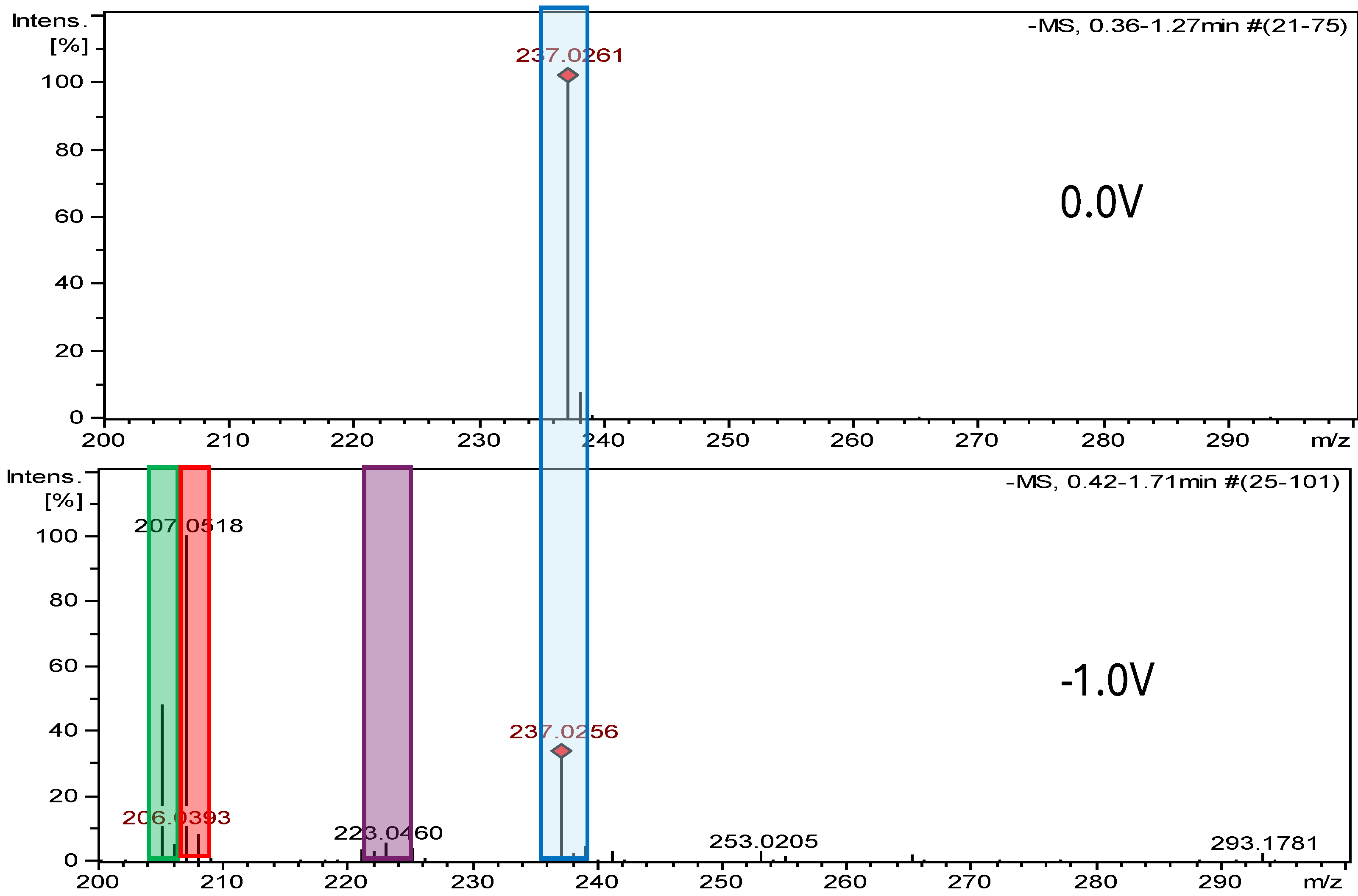
2.2. Electrochemical Measurements ROXY-QTOF
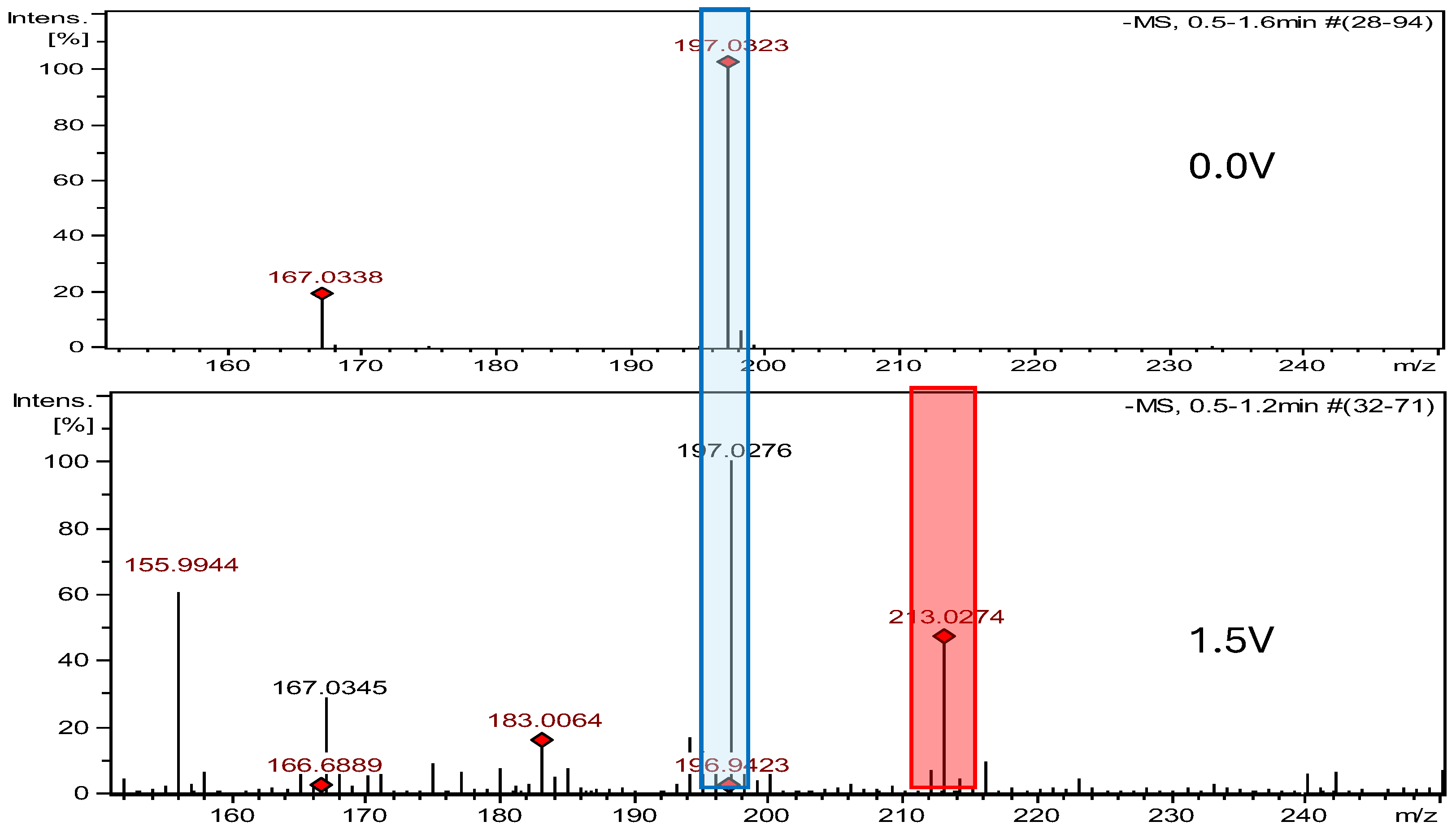
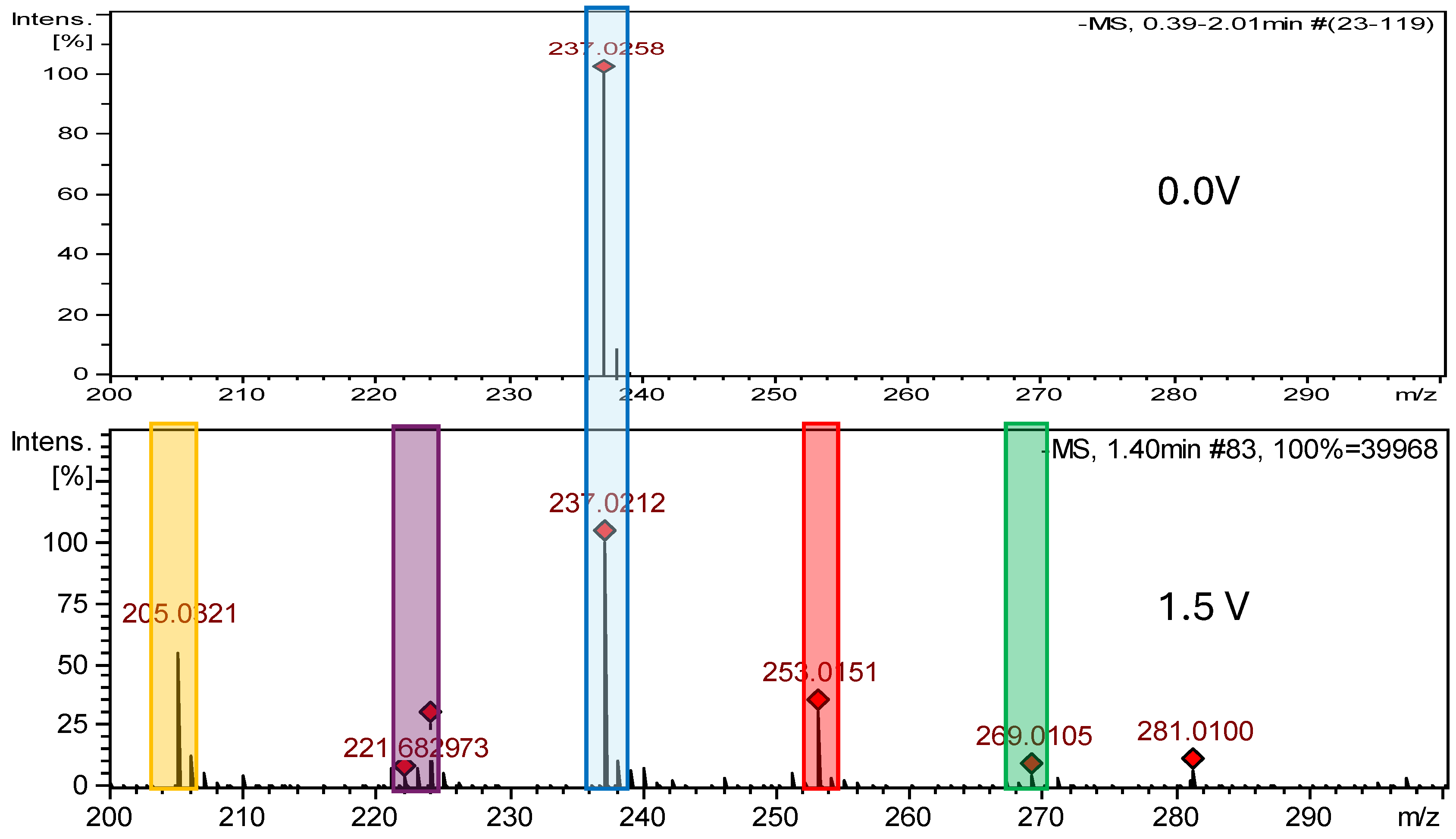
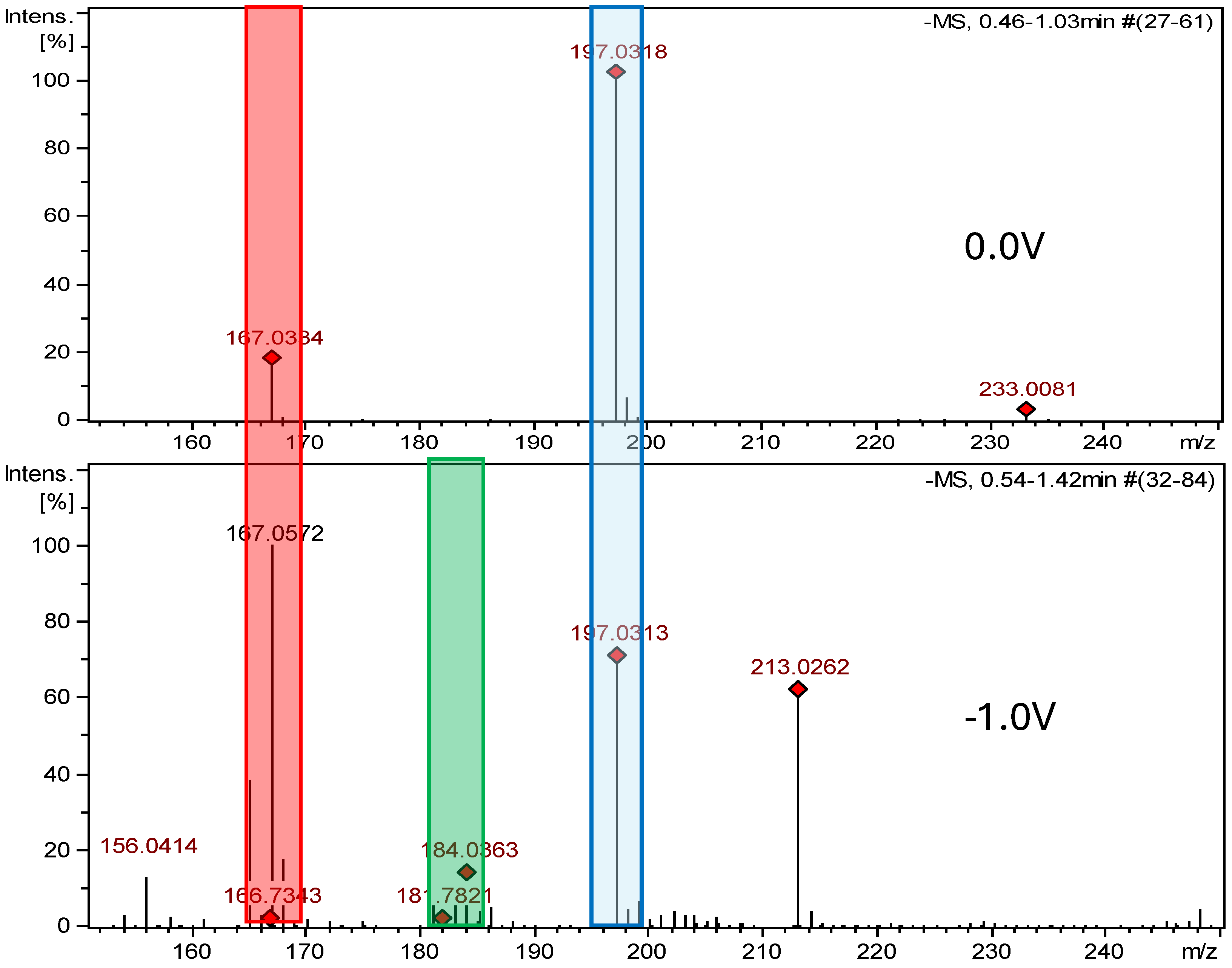
2.2.1. Electrochemical Oxidation
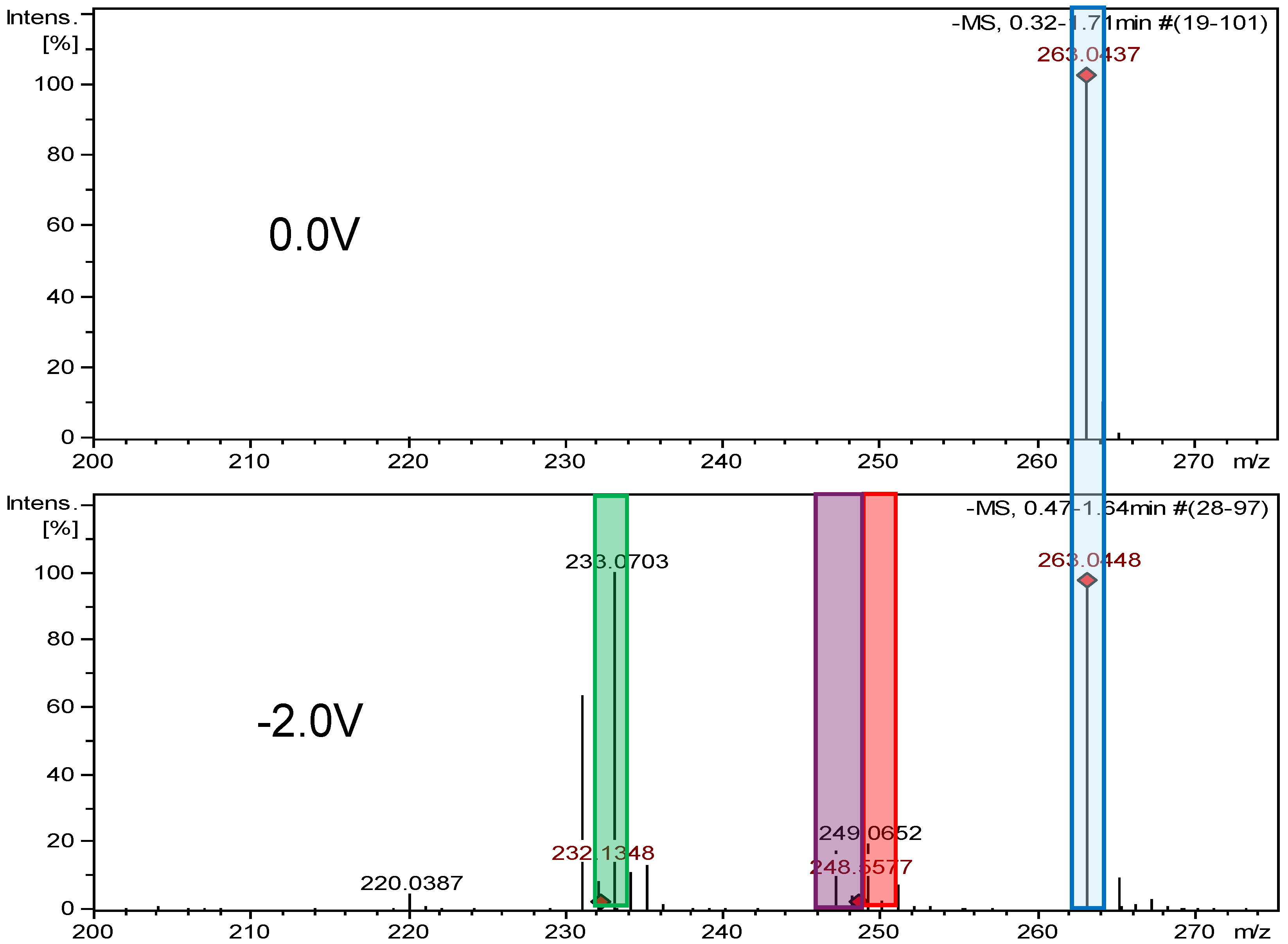
2.2.2. Electrochemical Reduction
2.3. Quantum Mechanical DFT Calculations
2.3.1. Oxidation
2.3.2. Reduction
- NF (nitrofural) undergoes first the NO2- group reduction up to the amino -NH2 group.
- NFT (nitrofurantoin): here, reduction of the NO2 group to the -NH2 group becomes the most probable degradation path.
- FUR (furadizine): here too, the reduction of the NO2 group to the -NH2 group becomes the most probable degradation path.
- DAN (dantrolene): the degradation of the NO2 group to -NH-OH or =NH residues becomes the most likely path. Here, the reduction does not terminate at the -NH2 group.
3. Materials and Methods
3.1. Chemicals
3.2. Electrochemical Measurements
3.3. EC/ESI-HRMS Measurements
3.4. DFT Calculations
3.5. In Silico Prediction of Metabolism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vikram, H.P.R.; Kumar, T.P.; Kumar, G.; Beeraka, N.M.; Deka, R.; Suhail, S.M.; Jat, S.; Bannimath, N.; Padmanabhan, G.; Chandan, R.S.; et al. Nitrosamines Crisis in Pharmaceuticals—Insights on Toxicological Implications, Root Causes and Risk Assessment: A Systematic Review. J. Pharm. Anal. 2024, 14, 100919. [Google Scholar] [CrossRef]
- Torres, S.; Brown, R.; Szucs, R.; Hawkins, J.M.; Zelesky, T.; Scrivens, G.; Pettman, A.; Taylor, M.R. The Application of Electrochemistry to Pharmaceutical Stability Testing—Comparison with in Silico Prediction and Chemical Forced Degradation Approaches. J. Pharm. Biomed. Anal. 2015, 115, 487–501. [Google Scholar] [CrossRef] [PubMed]
- Jahn, S.; Karst, U. Electrochemistry Coupled to (Liquid Chromatography/) Mass Spectrometry—Current State and Future Perspectives. J. Chromatogr. A 2012, 1259, 16–49. [Google Scholar] [CrossRef]
- Baumann, A.; Karst, U. Online Electrochemistry/Mass Spectrometry in Drug Metabolism Studies: Principles and Applications. Expert. Opin. Drug Metab. Toxicol. 2010, 6, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Jurva, U.; Wikström, H.V.; Weidolf, L.; Bruins, A.P. Comparison between Electrochemistry/Mass Spectrometry and Cytochrome P450 Catalyzed Oxidation Reactions. Rapid Commun. Mass. Spectrom. 2003, 17, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, W.; Karst, U. Electrochemistry Meets Enzymes: Instrumental on-Line Simulation of Oxidative and Conjugative Metabolism Reactions of Toremifene. Anal. Bioanal. Chem. 2009, 394, 1341–1348. [Google Scholar] [CrossRef]
- Jahn, S.; Baumann, A.; Roscher, J.; Hense, K.; Zazzeroni, R.; Karst, U. Investigation of the Biotransformation Pathway of Verapamil Using Electrochemistry/Liquid Chromatography/Mass Spectrometry—A Comparative Study with Liver Cell Microsomes. J. Chromatogr. A 2011, 1218, 9210–9220. [Google Scholar] [CrossRef]
- Johansson, T.; Weidolf, L.; Jurva, U. Mimicry of Phase I Drug Metabolism—Novel Methods for Metabolite Characterization and Synthesis. Rapid Commun. Mass. Spectrom. 2007, 21, 2323–2331. [Google Scholar] [CrossRef]
- Blankert, B.; Hayen, H.; van Leeuwen, S.M.; Karst, U.; Bodoki, E.; Lotrean, S.; Sandulescu, R.; Diez, N.M.; Dominguez, O.; Arcos, J.; et al. Electrochemical, Chemical and Enzymatic Oxidations of Phenothiazines. Electroanalysis 2005, 17, 1501–1510. [Google Scholar] [CrossRef]
- Lohmann, W.; Karst, U. Generation and Identification of Reactive Metabolites by Electrochemistry and Immobilized Enzymes Coupled On-Line to Liquid Chromatography/Mass Spectrometry. Anal. Chem. 2007, 79, 6831–6839. [Google Scholar] [CrossRef]
- van Leeuwen, S.M.; Hayen, H.; Karst, U. Liquid Chromatography–Electrochemistry–Mass Spectrometry of Polycyclic Aromatic Hydrocarbons. Anal. Bioanal. Chem. 2004, 378, 917–925. [Google Scholar] [CrossRef]
- Baumann, A.; Lohmann, W.; Schubert, B.; Oberacher, H.; Karst, U. Metabolic Studies of Tetrazepam Based on Electrochemical Simulation in Comparison to in Vivo and in Vitro Methods. J. Chromatogr. A 2009, 1216, 3192–3198. [Google Scholar] [CrossRef] [PubMed]
- Portychová, L.; Schug, K.A. Instrumentation and Applications of Electrochemistry Coupled to Mass Spectrometry for Studying Xenobiotic Metabolism: A Review. Anal. Chim. Acta 2017, 993, 1–21. [Google Scholar] [CrossRef]
- Jurva, U.; Weidolf, L. Electrochemical Generation of Drug Metabolites with Applications in Drug Discovery and Development. TrAC Trends Anal. Chem. 2015, 70, 92–99. [Google Scholar] [CrossRef]
- Plattner, S.; Erb, R.; Chervet, J.P.; Oberacher, H. Studying the Reducing Potencies of Antioxidants with the Electrochemistry Inherently Present in Electrospray Ionization-Mass Spectrometry. Anal. Bioanal. Chem. 2014, 406, 213–224. [Google Scholar] [CrossRef]
- Torres, S.; Brown, R.; Szucs, R.; Hawkins, J.M.; Scrivens, G.; Pettman, A.; Kraus, D.; Taylor, M.R. Rapid Synthesis of Pharmaceutical Oxidation Products Using Electrochemistry: A Systematic Study of N-Dealkylation Reactions of Fesoterodine Using a Commercially Available Synthesis Cell. Org. Process Res. Dev. 2015, 19, 1596–1603. [Google Scholar] [CrossRef]
- Permentier, H.; Bruins, A.; Bischoff, R. Electrochemistry-Mass Spectrometry in Drug Metabolism and Protein Research. Mini-Rev. Med. Chem. 2008, 8, 46–56. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, H.; Wu, S.; Chen, H. Probing Protein 3D Structures and Conformational Changes Using Electrochemistry-Assisted Isotope Labeling Cross-Linking Mass Spectrometry. J. Am. Soc. Mass. Spectrom. 2016, 27, 864–875. [Google Scholar] [CrossRef]
- Büter, L.; Frensemeier, L.M.; Vogel, M.; Karst, U. Dual Reductive/Oxidative Electrochemistry/Liquid Chromatography/Mass Spectrometry: Towards Peptide and Protein Modification, Separation and Identification. J. Chromatogr. A 2017, 1479, 153–160. [Google Scholar] [CrossRef]
- Mysling, S.; Salbo, R.; Ploug, M.; Jørgensen, T.J.D. Electrochemical Reduction of Disulfide-Containing Proteins for Hydrogen/Deuterium Exchange Monitored by Mass Spectrometry. Anal. Chem. 2014, 86, 340–345. [Google Scholar] [CrossRef]
- Faber, H.; Lutze, H.; Lareo, P.L.; Frensemeier, L.; Vogel, M.; Schmidt, T.C.; Karst, U. Liquid Chromatography/Mass Spectrometry to Study Oxidative Degradation of Environmentally Relevant Pharmaceuticals by Electrochemistry and Ozonation. J. Chromatogr. A 2014, 1343, 152–159. [Google Scholar] [CrossRef]
- Kučerová, P.; Skopalová, J.; Kučera, L.; Táborský, J.; Švecová, H.; Lemr, K.; Cankař, P.; Barták, P. Electrochemical Oxidation of 5-Hydroxymethyl Tolterodine and Identification of Its Oxidation Products Using Liquid Chromatography and Mass Spectrometry. Electrochim. Acta 2016, 215, 617–625. [Google Scholar] [CrossRef]
- Šelešovská, R.; Hlobeňová, F.; Skopalová, J.; Cankař, P.; Janíková, L.; Chýlková, J. Electrochemical Oxidation of Anti-Inflammatory Drug Meloxicam and Its Determination Using Boron Doped Diamond Electrode. J. Electroanal. Chem. 2020, 858, 113758. [Google Scholar] [CrossRef]
- Nowak, P.; Woźniakiewicz, M.; Kościelniak, P. Simulation of Drug Metabolism. TrAC Trends Anal. Chem. 2014, 59, 42–49. [Google Scholar] [CrossRef]
- Özkan, S.A. LC with Electrochemical Detection. Recent Application to Pharmaceuticals and Biological Fluids. Chromatographia 2007, 66, 3–13. [Google Scholar] [CrossRef]
- Karst, U.B.A.W. Electrochemistry and LC–MS for Metabolite Generation and Identification: Tools, Technologies and Trends. LCGC Europe 2010, 23, 8–16. [Google Scholar]
- Pretty, J.R.; Deng, H.; Goeringer, D.E.; Van Berkel, G.J. Electrochemically Modulated Preconcentration and Matrix Elimination for Organic Analytes Coupled On-Line with Electrospray Mass Spectrometry. Anal. Chem. 2000, 72, 2066–2074. [Google Scholar] [CrossRef]
- Mielczarek, P.; Smoluch, M.; Kotlinska, J.H.; Labuz, K.; Gotszalk, T.; Babij, M.; Suder, P.; Silberring, J. Electrochemical Generation of Selegiline Metabolites Coupled to Mass Spectrometry. J. Chromatogr. A 2015, 1389, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Knoche, L.; Lisec, J.; Koch, M. Analysis of Electrochemical and Liver Microsomal Transformation Products of Lasalocid by LC/HRMS. Rapid Commun. Mass. Spectrom. 2022, 36, e9349. [Google Scholar] [CrossRef]
- Kotthoff, L.; Lisec, J.; Schwerdtle, T.; Koch, M. Prediction of Transformation Products of Monensin by Electrochemistry Compared to Microsomal Assay and Hydrolysis. Molecules 2019, 24, 2732. [Google Scholar] [CrossRef]
- Potęga, A.; Żelaszczyk, D.; Mazerska, Z. Electrochemical Simulation of Metabolism for Antitumor-Active Imidazoacridinone C-1311 and in Silico Prediction of Drug Metabolic Reactions. J. Pharm. Biomed. Anal. 2019, 169, 269–278. [Google Scholar] [CrossRef]
- López-Rodríguez, R.; McManus, J.A.; Murphy, N.S.; Ott, M.A.; Burns, M.J. Pathways for N-Nitroso Compound Formation: Secondary Amines and Beyond. Org. Process Res. Dev. 2020, 24, 1558–1585. [Google Scholar] [CrossRef]
- Lunn, G.; Sansone, E.B.; Andrews, A.W. Aerial Oxidation of Hydrazines to Nitrosamines. Environ. Mol. Mutagen. 1991, 17, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Liu, Y.D.; Zhong, R. NDMA Formation Mechanisms from Typical Hydrazines and Hydrazones during Ozonation: A Computational Study. J. Hazard. Mater. 2019, 366, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, V.V.; Ivantsova, N.A.; Kuzin, E.N.; Pirogov, A.V.; Mezhuev, Y.O.; Filatova, E.A.; Averina, Y.M. Study of the Process of Electrochemical Oxidation of Active Pharmaceutical Substances on the Example of Nitrofurazone ((2E)-2-[(5-Nitro-2-Furyl)Methylene]Hydrazine Carboxamide). Water 2023, 15, 3370. [Google Scholar] [CrossRef]
- O’Keeffe, M.; Christodoulidou, A.; Nebbia, C. Presence of Nitrofurans and Their Metabolites in Gelatine. EFSA J. 2021, 19, e06881. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.; Kaplan, E.; Laurieri, N.; Lowe, E.; Sim, E. Activation of Nitrofurazone by Azoreductases: Multiple Activities in One Enzyme. Sci. Rep. 2011, 1, 63. [Google Scholar] [CrossRef]
- Streeter, A.J.; Krueger, T.R.; Hoener, B.A. Oxidative Metabolites of 5-Nitrofurans. Pharmacology 1988, 36, 283–288. [Google Scholar] [CrossRef]
- Hall, B.S.; Bot, C.; Wilkinson, S.R. Nifurtimox Activation by Trypanosomal Type I Nitroreductases Generates Cytotoxic Nitrile Metabolites. J. Biol. Chem. 2011, 286, 13088–13095. [Google Scholar] [CrossRef]
- Jonen, H.G. Reductive and Oxidative Metabolism of Nitrofurantoin in Rat Liver. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1980, 315, 167–175. [Google Scholar] [CrossRef]
- Amano, T.; Fukami, T.; Ogiso, T.; Hirose, D.; Jones, J.P.; Taniguchi, T.; Nakajima, M. Identification of Enzymes Responsible for Dantrolene Metabolism in the Human Liver: A Clue to Uncover the Cause of Liver Injury. Biochem. Pharmacol. 2018, 151, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Ellis, K.O.; Wessels, F.L. Muscle Relaxant Properties of the Identified Metabolites of Dantrolene. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1978, 301, 237–240. [Google Scholar] [CrossRef]
- European Medical Agency Committee for Medicinal Products for Human Use. ICH M7(R2) Guideline on Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk. 2023. Available online: https://www.ema.europa.eu/en/ich-m7-assessment-control-dna-reactive-mutagenic-impurities-pharmaceuticals-limit-potential-carcinogenic-risk-scientific-guideline (accessed on 1 March 2025).
- Ragab, M.A.A.; Korany, M.A.; Galal, S.M.; Ahmed, A.R. Voltammetric Analysis of Dantrolene and Its Active Metabolite with Indomethacin in Rat Plasma. Bioanalysis 2019, 11, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Ghoneim, E.M. Electroreduction of the Muscle Relaxant Drug Dantrolene Sodium at the Mercury Electrode and Its Determination in Bulk Form and Pharmaceutical Formulation. Chem. Pharm. Bull. 2007, 55, 1483–1488. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, M.; Alagumalai, K.; Chen, T.-W.; Chen, S.-M.; Liu, X.; Selvaganapathy, M. Simultaneous Electrochemical Determination of Nitrofurantoin and Nifedipine with Assistance of Needle-Shaped Perovskite Structure: Barium Stannate Fabricated Glassy Carbon Electrode. Microchimica Acta 2021, 188, 19. [Google Scholar] [CrossRef]
- Rahi, A.; Sattarahmady, N.; Dehdari Vais, R.; Heli, H. Sonoelectrodeposition of Gold Nanorods at a Gold Surface—Application for Electrocatalytic Reduction and Determination of Nitrofurazone. Sens. Actuators B Chem. 2015, 210, 96–102. [Google Scholar] [CrossRef]
- Brito, C.L.; Ferreira, E.I.; La-Scalea, M.A. Application of Multi-Walled Carbon Nanotubes Functionalized with Hemin to Evaluate the Electrochemical Behavior of Nitrofurazone in Aqueous Media. Electrochim. Acta 2023, 459, 142486. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016; Available online: https://gaussian.com (accessed on 1 March 2025).
- Stolarczyk, E.U.; Strzempek, W.; Łaszcz, M.; Leś, A.; Menaszek, E.; Stolarczyk, K. Thiogenistein—Antioxidant Chemistry, Antitumor Activity, and Structure Elucidation of New Oxidation Products. Int. J. Mol. Sci. 2022, 23, 7816. [Google Scholar] [CrossRef]


| Compound | MW | Molecular Formula | Initial Conditions | Suggested Transformation | EC | In Silico | DFT Gibbs Energy [kcal/mol] |
|---|---|---|---|---|---|---|---|
| Dantrolene (DAN) | 314.253 | C14H10N4O5 | - | - | - | - | - |
| Dantrolene impurities | |||||||
| DAN-Imp1-Ox(A) DAN-Imp1-Ox(B) | 330.252 | C14H10N4O6 | pH 7.4, +1.0 | +O | √ X | X √ | −52 −47 |
| DAN-Imp2-Ox | 346.252 | C14H10N4O7 | pH 7.4, +1.2 V | +2O | √ | X | −98 |
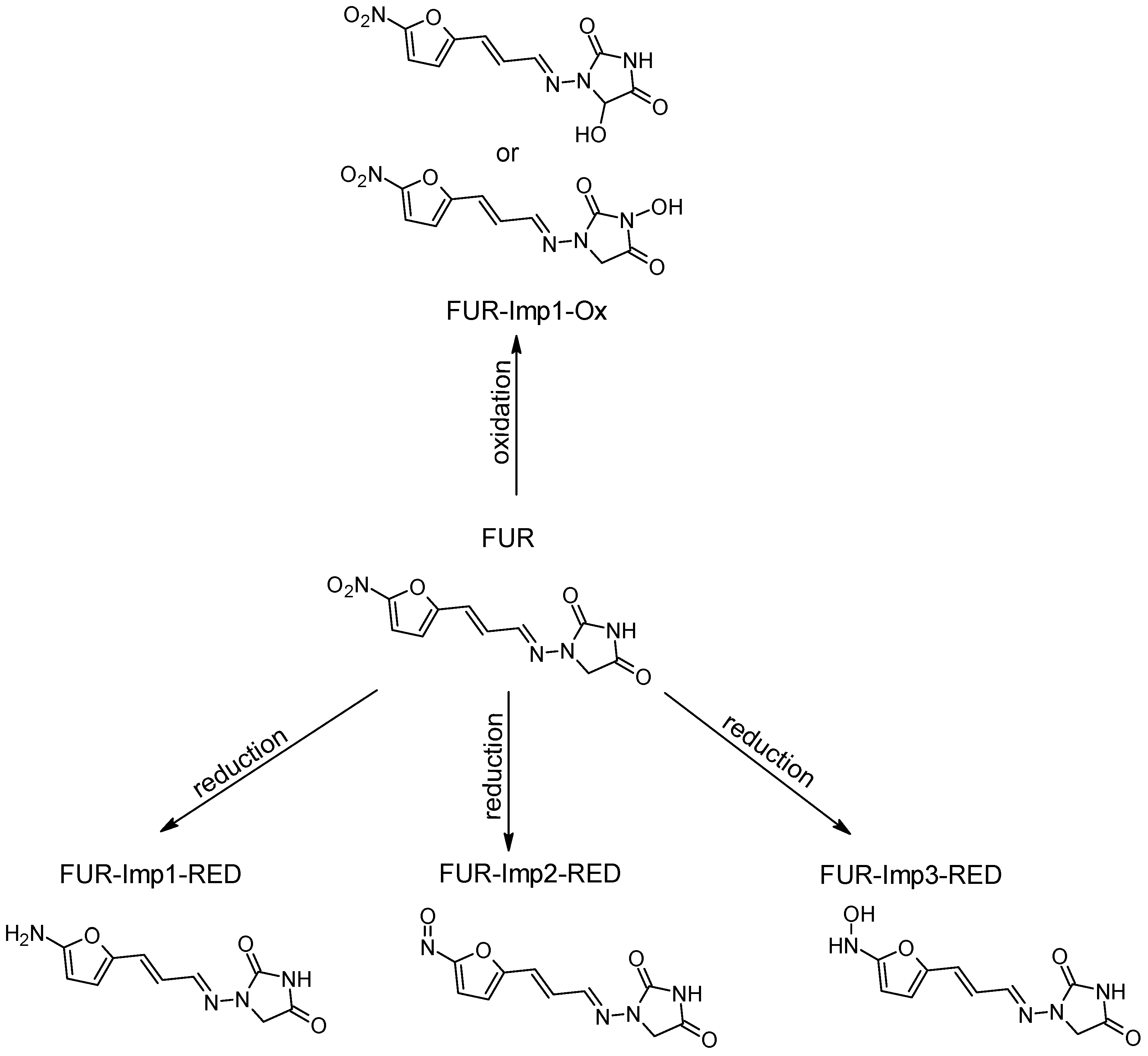
| Furazidine (FUR) | 264.194 | C10H8N4O5 | - | - | - | - | - |
| Furazidine impurities | |||||||
| FUR-Imp1-Ox(A) FUR-Imp1-Ox(B) | 280.194 | C10H8N4O6 | pH 7.4, +2.0 V | +O | X √ | √ X | −47 −9 |
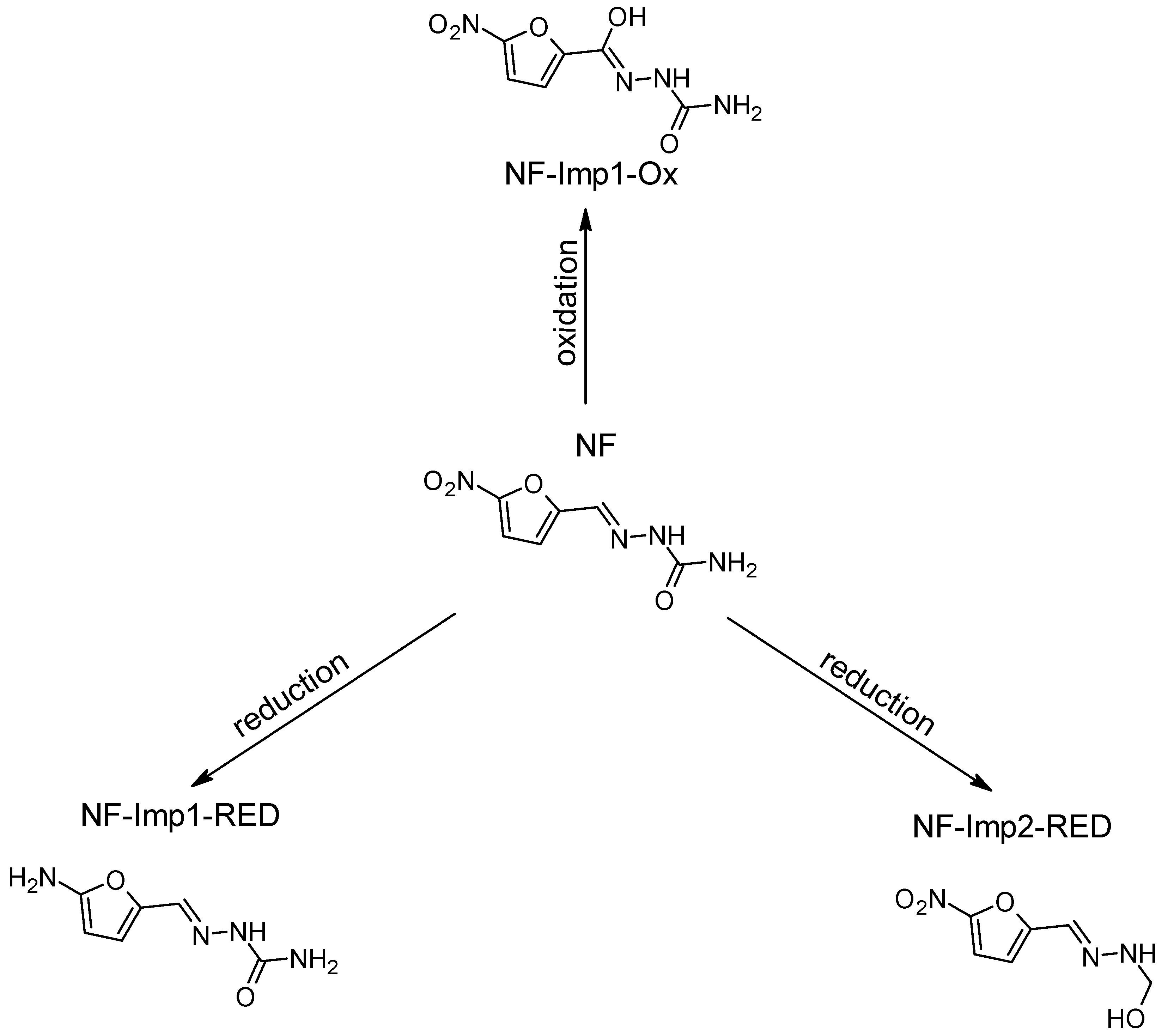
| Nitrofural (NF) | 198.136 | C6H6N4O4 | - | - | - | - | - |
| Nitrofural impurities | |||||||
| NF-Imp1-Ox | 214.136 | C6H5N4O5 | pH 7.4, +1.5 | +O | √ | X | −54 |
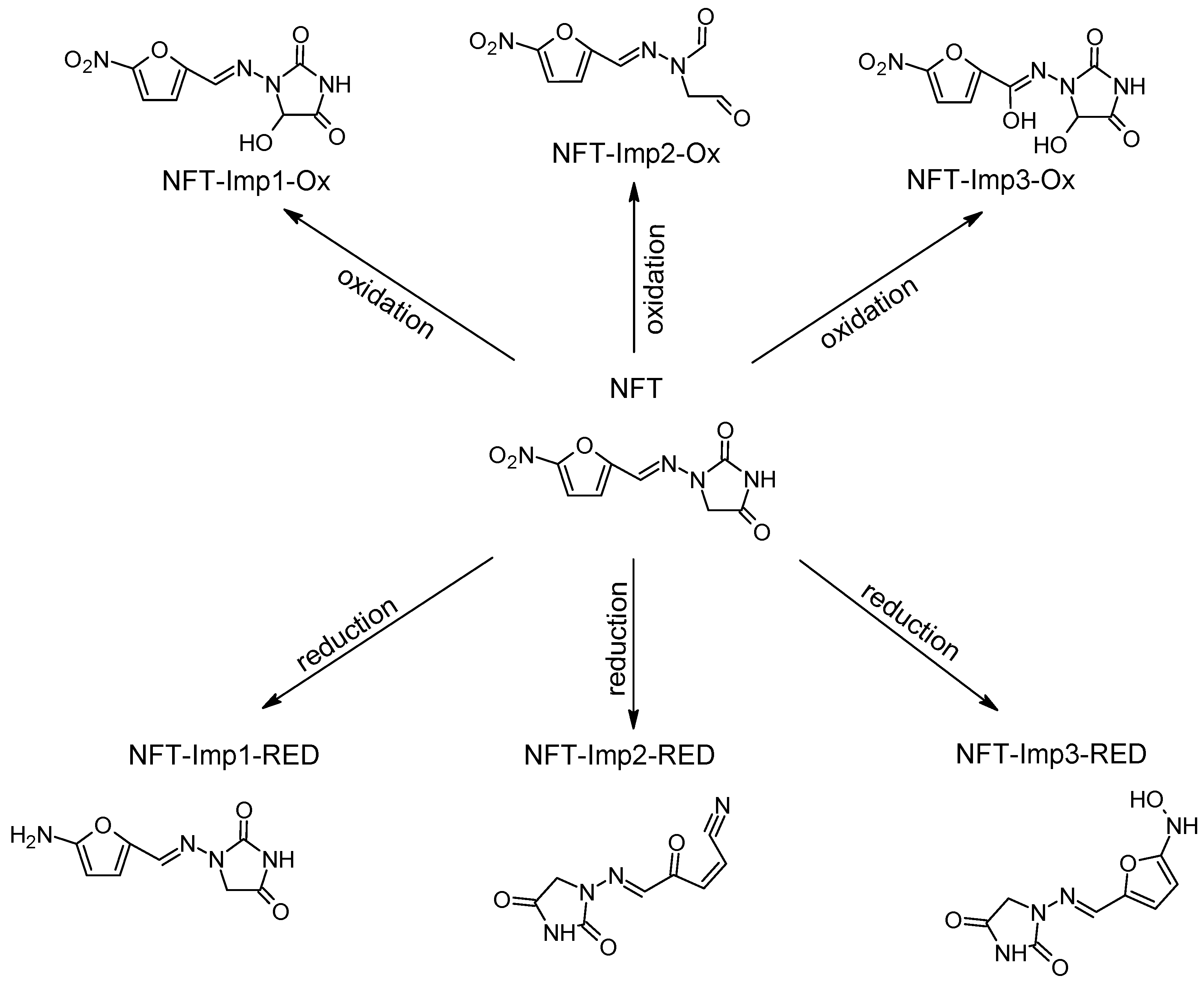
| Nitrofurantoin (NFT) | 238.157 | C8H6N4O5 | - | - | - | - | |
| Nitrofurantoin impurities | |||||||
| NFT-Imp1-Ox | 254.156 | C8H6N4O6 | pH 7.4, +0.8 V | +O | √ | √ | −47 |
| NFT-Imp2-Ox | 225.158 | C8H7N3O5 | pH 7.4, +1.0 V | -N+H | √ | X | −190 |
| NFT-Imp3-Ox | 270.156 | C8H6N4O7 | pH 7.4, +1.2 V | +2O | √ | X | −100 |
| Compound | MW | Molecular Formula | Conditions | Suggested Transformation | EC | In Silico | DFT Gibbs Energy [kcal/mol] |
|---|---|---|---|---|---|---|---|
| Dantrolene (DAN) | 314.253 | C14H10N4O5 | - | - | - | - | - |
| Dantrolene impurities | |||||||
| DAN-Imp1-RED | 300.270 | C14H12N4O4 | pH 7.4, −0.7 V | +2H -O | √ | √ | −239 |
| DAN-Imp2-RED | 282.259 | C14H10N4O3 | pH 7.4, −0.7 V | -2O | √ | X | −204 |
| Furazidine (FUR) | 264.194 | C10H8N4O5 | - | - | - | - | - |
| Furazidine impurities | |||||||
| FUR-Imp1-RED | 234.211 | C10H10N4O3 | pH 7.4, −0.7 V pH 7.4, −2.0 V | +2H -2O | √ | √ | −398 |
| FUR-Imp2-RED | 248.195 | C10H8N4O4 | pH 7.4, −0.7 V | -O | √ | X | −128 |
| FUR-Imp3-RED | 250.211 | C10H10N4O4 | pH 7.4, −0.9 V | +2H -O | √ | √ | −242 |
| Nitrofural (NF) | 198.136 | C6H6N4O4 | - | - | - | - | - |
| Nitrofural impurities | |||||||
| NF-Imp1-RED | 168.153 | C6H8N4O2 | pH 7.4, −1.0 V | +2H -2O | √ | √ | −399 |
| NF-Imp2-RED | 185.137 | C6H7N3O4 | pH 7.4, −0.7 V | +H -N | √ | X | −179 |
| Nitrofurantoin (NFT) | 238.157 | C8H6N4O5 | - | - | - | - | - |
| Nitrofurantoin impurities | |||||||
| NFT-Imp1-RED | 208.174 | C8H8N4O3 | pH 7.4, −1.0 V | +2H -2O | √ | √ | −400 |
| NFT-Imp2-RED | 206.158 | C8H6N4O3 | pH 7.4, −0.7 V | -2O | √ | X | −286 |
| NFT-Imp3-RED | 224.174 | C8H8N4O4 | pH 7.4, −1.0 V | +2H -O | √ | √ | −244 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Witkowska, A.B.; Stolarczyk, K.; Fusaro, M.; Leś, A.; Giebułtowicz, J.; Stolarczyk, E.U. Oxidation and Reduction of Hydrazones—Risk Factors Related to the Manufacture and Stability of the Drugs. Int. J. Mol. Sci. 2025, 26, 4295. https://doi.org/10.3390/ijms26094295
Witkowska AB, Stolarczyk K, Fusaro M, Leś A, Giebułtowicz J, Stolarczyk EU. Oxidation and Reduction of Hydrazones—Risk Factors Related to the Manufacture and Stability of the Drugs. International Journal of Molecular Sciences. 2025; 26(9):4295. https://doi.org/10.3390/ijms26094295
Chicago/Turabian StyleWitkowska, Anna B., Krzysztof Stolarczyk, Massimo Fusaro, Andrzej Leś, Joanna Giebułtowicz, and Elżbieta U. Stolarczyk. 2025. "Oxidation and Reduction of Hydrazones—Risk Factors Related to the Manufacture and Stability of the Drugs" International Journal of Molecular Sciences 26, no. 9: 4295. https://doi.org/10.3390/ijms26094295
APA StyleWitkowska, A. B., Stolarczyk, K., Fusaro, M., Leś, A., Giebułtowicz, J., & Stolarczyk, E. U. (2025). Oxidation and Reduction of Hydrazones—Risk Factors Related to the Manufacture and Stability of the Drugs. International Journal of Molecular Sciences, 26(9), 4295. https://doi.org/10.3390/ijms26094295






