Biotechnological Applications of Biogenic Nanomaterials from Red Seaweed: A Systematic Review (2014–2024)
Abstract
1. Introduction
2. Results
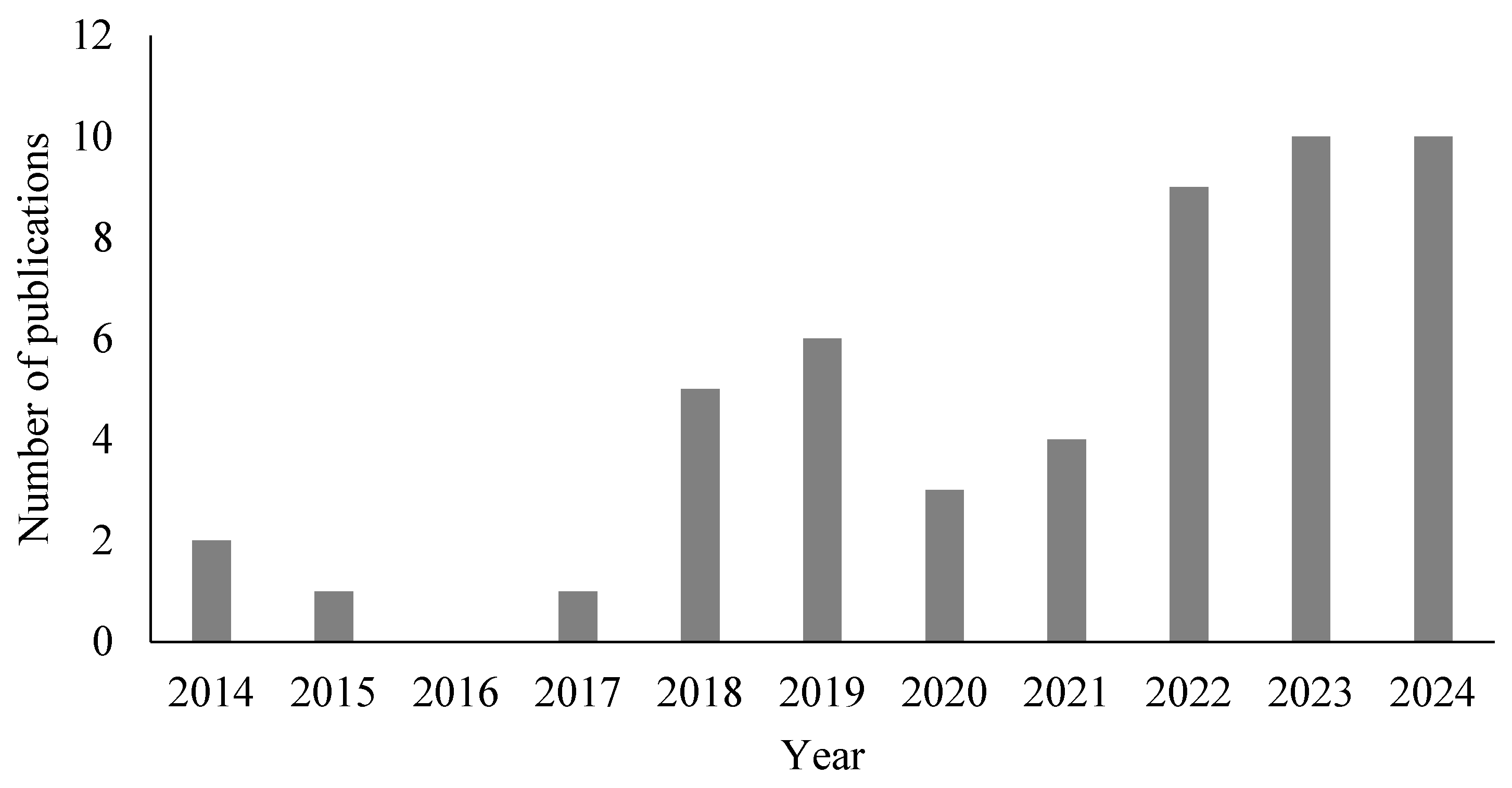
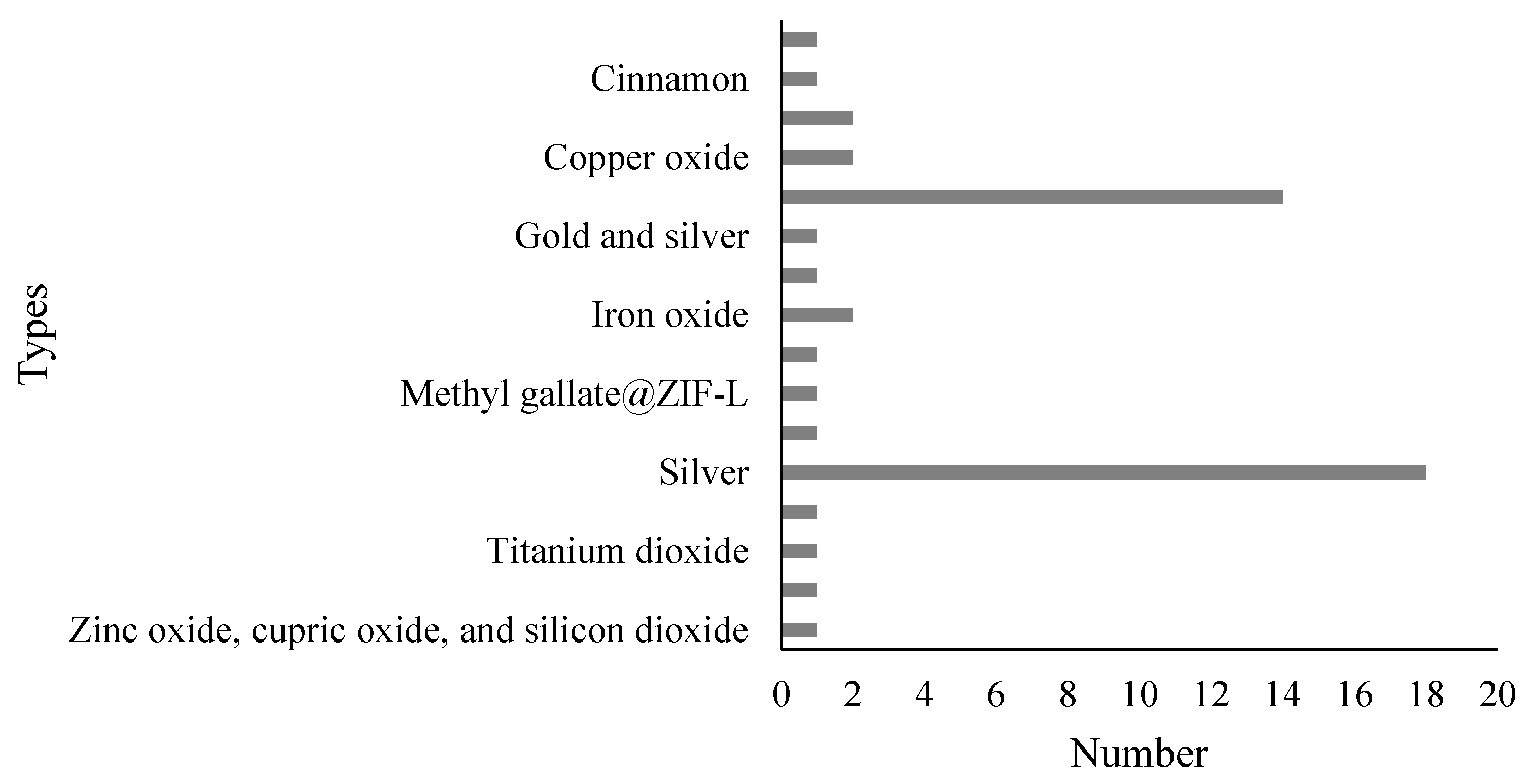
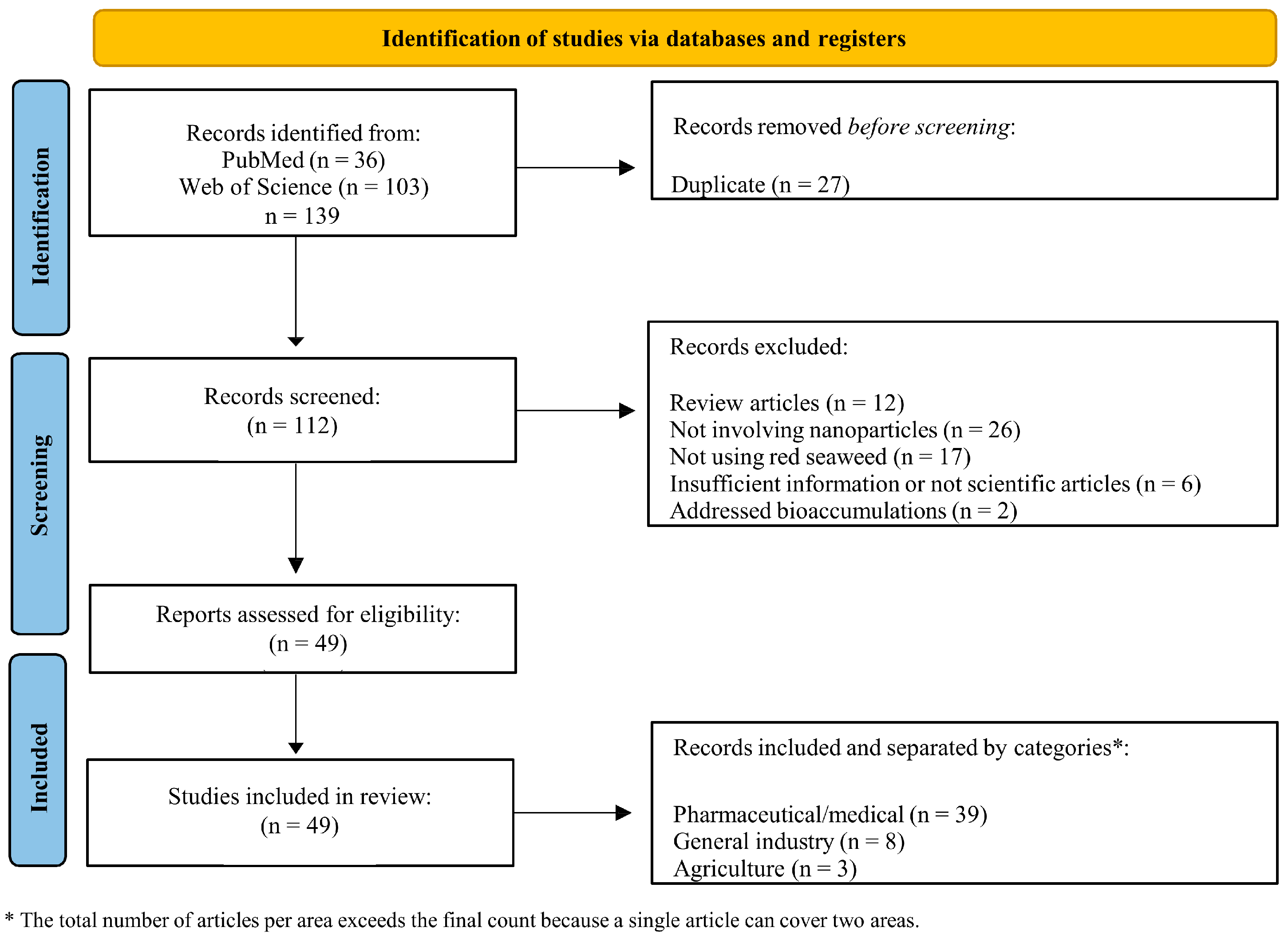
2.1. Paper Selection and Characteristics
2.2. Pharmaceutical/Medical Field
| Nº | Target Effect | Species Used | Core Nanoparticle | Results/Potential | References |
|---|---|---|---|---|---|
| 1 | Cytotoxic activity in cancer cell lines | Corallina officinalis | Gold | Strong NP cytotoxic activity was observed against breast cancer cells, indicating its potential for anticancer therapy | [26] |
| 2 | Bactericidal application and cytotoxic activity | Pterocladiella capillacea | Silver | Strong anticancer activity and high bactericidal efficacy of NPs exhibited against Gram-positive strains, highlighting their potential in cancer treatment and bacterial infections | [27] |
| 3 | Cytotoxic activities | Laurencia aldingensis and Laurenciella sp. | Silver | The NPs demonstrated low toxicity to healthy human cells and high cytotoxicity against uterine sarcoma cells, highlighting their potential as therapeutic agents for tumor treatment | [59] |
| 4 | Vector control | Gracilaria firma | Silver | Significant larvicidal activity of NPs was observed, proving their application as a potential eco-friendly larvicidal agent against A. aegypti | [51] |
| 5 | Antitumor activity | In-house purified carrageenan | Gold | NPs exhibited significant cytotoxic activity against cancer cells, highlighting their potential for the formulation of cancer treatments | [63] |
| 6 | Methodology optimization | Palmaria decipiens | Gold and silver | The green synthesis led to NPs with potential therapeutic and medical applications due to the active components involved in the reduction and stabilization processes | [62] |
| 7 | Monitoring 5-fluorouracile in pharmaceutical formulations | Porphyra genus (commercial form—nori) | Gold | The proposed device demonstrated high sensitivity to 5-fluorouracile and was successfully applied for the analysis of injectable pharmaceutical samples, as a viable alternative for drug monitoring in pharmaceutical formulations | [66] |
| 8 | Antimicrobial activity | Gelidium amansii | Silver | Strong antimicrobial activity was observed, indicating the potential of AgNPs as a coating material for biofouling control | [46] |
| 9 | Antibacterial activity | Gracilaria birdiae | Silver | Antibacterial activity of AgNPs against E. coli and S. aureus was observed, suggesting that these NPs can be used as a model for future nanomedicine projects or drug delivery systems | [48] |
| 10 | Biocompatibility against different cell lines | Gracilaria verrucosa | Gold | These AuNPs demonstrated high biocompatibility on normal cells, showing no significant cytotoxic effects, and suitable are for future applications as drug nanovehicles | [39] |
| 11 | Anticancer drug release | In-house purified Carrageenan | Gold | These AuNPs were developed as a drug delivery system showing higher biocompatibility on normal cells than soluble drug (epirubicin) and stronger dose-dependent cytotoxicity against HepG2 cells, suggesting their potential as targeted drug delivery agent | [54] |
| 12 | Anticancer activities | Gracilaria debilis | Methyl gallate encapsulated on ZIF-L | Significant cytotoxic effects were observed against lung cancer cell lines, suggesting its application as a promising agent for cancer treatment | [50] |
| 13 | Antimicrobial activity | Pterocladiella capillacea | Iron oxide | These IONPs exhibited a strong antibacterial activity and a low antifungal activity, suggesting their use for pharmaceutical and biomedical applications, particularly as antibacterial agent | [41] |
| 14 | Antioxidant, antibacterial, and anticancer activities | Acanthophora spicifera | Gold | Significant antioxidant, antibacterial, and anticancer activities of these AuNPs were observed, highlighting their potential for the development of new drugs | [43] |
| 15 | Antibacterial activity e detection of 5-fluorouracil (5-FU) | Porphyra genus (commercial form—nori) | Silver | AgNPs exhibited antibacterial activity against S. aureus and E. coli and were applied for the electrochemical detection of 5-FU, demonstrating the potential as a promising analytical tool for quality control | [67] |
| 16 | Antibacterial and anticancer activity | Spyridia filamentosa | Silver | Dose-dependent antibacterial activity and cytotoxic effects of AgNPs on breast cancer cells were obtained, highlighting their potential for applications in food, textile industries, and medicine | [61] |
| 17 | Antioxidant activity | Crassiphycus birdiae | Silver | The AgNPs exhibited enhanced antioxidant activity, suggesting their use in various applications | [45] |
| 18 | Antioxidant, hypoglycemic, and cytotoxic activities | Gracilaria lemaneiformis | Selenium | The nanoconjugates exhibited significant antioxidant, hypoglycemic, and cytotoxic activities, along with low hemolytic properties, demonstrating potential as an antioxidant supplement and a possible medication in diabetes | [54] |
| 19 | Anticancer and antimicrobial activity | Gracilaria foliifera | Gold | The AuNPs exhibited anticancer effects and antimicrobial activity, suggesting their application as promising agent for these purposes | [53] |
| 20 | Immunomodulatory, antioxidant, and antitumoral activity | Chondrus crispus, Gelidium corneum, and Porphyra linearis | Gold | Antioxidant and immunomodulatory properties of AuNPs were observed, where P. linearis-derived AuNPs exhibited the best antioxidant capacity, suggesting their potential applications in immunotherapy | [44] |
| 21 | Antibacterial activity | Kappaphycus alvarezii | Silver | A good antibacterial activity of AgNPs against various E. coli strains was observed, indicating their potential as safe and effective alternative to antibiotics for preventing bacterial infections | [34] |
| 22 | Antibacterial activity | Halymenia porphyriformis and Solieria robusta | Silver | These AgNPs exhibited moderate antibacterial activity and may serve as potential agents for the treatment of oral bacterial pathogens | [56] |
| 23 | Antioxidant activity | Jania rubens | Chitosan-tripolyphosphate | These NPs demonstrated strong antioxidant activity and effective radical elimination | [58] |
| 24 | Antibacterial, antioxidant and anticancer activities | Hypnea valentiae | Silver | Activity of the AgNPs was observed on all the evaluated biological tests, demonstrating a strong capacity to eliminate free radicals, action against four pathogens, and effectiveness against human colon and lung cancer cells | [31] |
| 25 | Antioxidant, antimicrobial, and anticarcinogenic activity | Halymenia venusta | Gold | Antioxidant, antimicrobial, and anticancer activities of the AuNPs were obtained, highlighting the inhibition of free radicals and lung cancer cells, demonstrating significant potential for this purpose | [57] |
| 26 | Anticancer activity | Kappaphycus alvarezii | Graphene oxide | A new multifunctional nanohybrid system for drug delivery was developed, demonstrating significant cytotoxic activity against cancer cells, suggesting a potential as biocompatible nanomaterial and a model for anticancer drugs | [36] |
| 27 | Antibacterial activity | Gelidiella acerosa | Gold | Tests demonstrated antibacterial activity of these AuNPs against S. aureus, indicating potential for biomedical applications | [47] |
| 28 | Treatment of risk factors associated with kidney injury | Gracilaria oblongata | Titanium dioxide | Extracts of G. oblongata protect against acute kidney injury by TiO2 NPs by reducing oxidative stress and inflammation | [55] |
| 29 | Antioxidant and anticancer activity | Hypnea valentiae | Gold | These AuNPs exhibited strong antioxidant activity and potential anticancer effects, proving capability in eliminating free radicals and inhibiting the growth of lung cancer cells | [32] |
| 30 | Antioxidant and anticarcinogenic activity | Champia parvula | Gold | Significant antioxidant activity and anticancer effects of these AuNPs against lung cancer cells were observed, indicating potential for the development of new anticancer drugs | [37] |
| 31 | Antioxidant and antitumor potential | Hypnea valentiae | Iron oxide | These IONPs demonstrated significant antioxidant activity and anticancer efficacy, particularly against breast cancer cells, suggesting their potential for future targeted treatments for this cancer | [43] |
| 32 | Anticarcinogenic activity | Porphyridium purpureum | Silver and zinc oxide | The nanocomposite exhibited strong cytotoxicity against breast cancer cells, highlighting its significant potential for anticancer therapies | [60] |
| 33 | Antimicrobial activity | Kappaphycus alvarezii | Silver | Effective antimicrobial action of these AgNPs was observed, indicating their potential for applications for food preservation and biomedical products | [35] |
| 34 | Antibacterial activity | Gracilaria fisheri | Gold and silver | Significant antibacterial activity against shrimp pathogens of NPs was observed. Additionally, these NPs improved the survival rate of infected shrimps, suggesting their potential as a promising agent to fight against Vibrio sp. Infections | [52] |
| 35 | Anticoagulant properties | Acanthophora sp. | Copper | These CuNPs exhibited a strong anticoagulant effect, suggesting their potential as anticoagulant | [42] |
| 36 | Antimicrobial activity | Gracilaria verrucosa | Copper oxide | A limited antimicrobial activity of these CuONPs was observed, inhibiting only E. coli at the highest concentrations tested, indicating the need for further research on antimicrobial potential of these NPs | [40] |
| 37 | Larvicidal and neurotoxicity activity | Gracilaria corticata | Silver | These AgNPs showed efficacy in killing the larvae of A. aegypti, A. stephensi, and C. quinquefasciatus, indicating their potential to be used as nano larvicidal agent | [49] |
| 38 | Drug deliver for cancer therapy | κ-carrageenan (commercial) | Copper oxide | The CuO based NPs exhibited strong cytotoxicity and effective paclitaxel release, with metabolic alterations, and inhibited cell proliferation linked to apoptosis from reactive oxygen species. It also reduced mitochondrial membrane potential, showcasing a novel targeted drug delivery approach | [65] |
| 39 | Antioxidant, antimicrobial e anticancer activity | Champia parvula | Silver | Significant antioxidant, antimicrobial and anticancer activities of these AgNPs were observed, with high efficacy against lung cancer cells and the control of bacterial and fungal pathogens. Therefore, they show promise for the development of new nanomedicines | [38] |
2.3. General Industry
| Nº | Target Effect | Species Used | Core Nanoparticle | Results/Potential | References |
|---|---|---|---|---|---|
| 1 | Detection and rapid removal of Hg2+ ions | Gracilaria canaliculata | Gold | Newly synthesized Gold NPs were applied in a glass probe chemiosensor, demonstrating the effective detection and removal of mercury ions from water, with high sensitivity and reusability | [71] |
| 2 | Potential to absorb Ismate violet 2R ions | Pterocladia capillacea | Zinc oxide | NPs were developed as highly effective for removing Ismate Violet 2R dye from aqueous solutions, showing a potential for water treatment applications | [72] |
| 3 | Methodology optimization | Palmaria palmata | Silver | The algae demonstrated significant capacity to accumulate silver NPs, indicating its potential for removing environmentally dangerous nanomaterials | [68] |
| 4 | Multiple industrial applications | Kappaphycus alvarezii | Zinc oxide, cupric oxide, and silicon dioxide | Bio-nanocomposite films with enhanced antibacterial properties and modified physical characteristics were developed, suggesting applications in food industry | [69] |
| 5 | Methodology optimization | κ-Carrageenan (commercial) | Magnetite | NPs with high catalytic activity for synthesizing dihydropyrano[2,3-c]pyrazoles were proposed, suggesting applications as a reusable and cost-effective biobased catalyst for acid-catalyzed organic reactions | [73] |
| 6 | Packaging | Kappaphycus alvarezii | Cinnamon | Improved mechanical, thermal, and antibacterial properties of novel nanofilms were demonstrated for food packaging applications | [70] |
| 7 | Photocatalytic degradation | Gelidiella acerosa | Gold | Degradation of commercially important dyes was obtained, suggesting environmental applications | [47] |
| 8 | Packaging | κ-Carrageenan (commercial) | Copper | Nanocomposite films exhibiting enhanced thermal stability, elasticity, and antimicrobial properties were proposed as promising, and environment-friendly alternative for food packaging | [74] |
2.4. Agriculture
3. Discussion
Gaps and Prospects
4. Material and Methods
4.1. Study Design
4.2. Quality Assessment e Data Extraction
4.3. Inclusion/Exclusion Criteria
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Martínez-Ballesta, M.; Gil-Izquierdo, Á.; García-Viguera, C.; Domínguez-Perles, R. Nanoparticles and Controlled Delivery for Bioactive Compounds: Outlining Challenges for New “Smart-Foods” for Health. Foods 2018, 7, 72. [Google Scholar] [CrossRef]
- Tiwari, P.; Adil, M.; Bose, S.K.; Chen, J.-T.; Dufosse, L. The Exploitation of Marine Seaweeds for Nanoparticle Biosynthesis and Applications. In Advanced Nanotechnology in Plants; CRC Press: Boca Raton, FL, USA, 2024; pp. 226–237. [Google Scholar] [CrossRef]
- Gupta, D.; Boora, A.; Thakur, A.; Gupta, T.K. Green and Sustainable Synthesis of Nanomaterials: Recent Advancements and Limitations. Environ. Res. 2023, 231, 116316. [Google Scholar] [CrossRef]
- Malik, A.Q.; Mir, T.U.G.; Kumar, D.; Mir, I.A.; Rashid, A.; Ayoub, M.; Shukla, S. A Review on the Green Synthesis of Nanoparticles, Their Biological Applications, and Photocatalytic Efficiency against Environmental Toxins. Environ. Sci. Pollut. Res. Int. 2023, 30, 69796–69823. [Google Scholar] [CrossRef]
- Osman, A.I.; Zhang, Y.; Farghali, M.; Rashwan, A.K.; Eltaweil, A.S.; Abd El-Monaem, E.M.; Mohamed, I.M.A.; Badr, M.M.; Ihara, I.; Rooney, D.W.; et al. Synthesis of Green Nanoparticles for Energy, Biomedical, Environmental, Agricultural, and Food Applications: A Review. Environ. Chem. Lett. 2024, 22, 841–887. [Google Scholar] [CrossRef]
- Kumar, Y.; Tarafdar, A.; Badgujar, P.C. Seaweed as a Source of Natural Antioxidants: Therapeutic Activity and Food Applications. J. Food Qual. 2021, 2021, 5753391. [Google Scholar] [CrossRef]
- Park, E.; Yu, H.; Lim, J.-H.; Hee Choi, J.; Park, K.-J.; Lee, J. Seaweed Metabolomics: A Review on Its Nutrients, Bioactive Compounds and Changes in Climate Change. Food Res. Int. 2023, 163, 112221. [Google Scholar] [CrossRef]
- Polat, S.; Trif, M.; Rusu, A.; Šimat, V.; Čagalj, M.; Alak, G.; Meral, R.; Özogul, Y.; Polat, A.; Özogul, F. Recent Advances in Industrial Applications of Seaweeds. Crit. Rev. Food Sci. Nutr. 2023, 63, 4979–5008. [Google Scholar] [CrossRef] [PubMed]
- Cotas, J.; Leandro, A.; Pacheco, D.; Gonçalves, A.M.M.; Pereira, L. A Comprehensive Review of the Nutraceutical and Therapeutic Applications of Red Seaweeds (Rhodophyta). Life 2020, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.; Cotas, J.; Pacheco, D.; Ihle, K.; Hillinger, A.; Cascais, M.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Red Seaweed (Rhodophyta) Phycocolloids: A Road from the Species to the Industry Application. Mar. Drugs 2024, 22, 432. [Google Scholar] [CrossRef]
- Fact.MR. Red Seaweed Extract Market. Available online: https://www.factmr.com/report/3810/red-seaweed-extract-market (accessed on 10 December 2024).
- Yang, J.-I.; Yeh, C.-C.; Lee, J.-C.; Yi, S.-C.; Huang, H.-W.; Tseng, C.-N.; Chang, H.-W. Aqueous Extracts of the Edible Gracilaria Tenuistipitata Are Protective against H2O2-Induced DNA Damage, Growth Inhibition, and Cell Cycle Arrest. Molecules 2012, 17, 7241–7254. [Google Scholar] [CrossRef]
- Torres, M.D.; Flórez-Fernández, N.; Domínguez, H. Integral Utilization of Red Seaweed for Bioactive Production. Mar. Drugs 2019, 17, 314. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.V.; Pacheco, D.; Cotas, J.; Mouga, T.; Afonso, C.; Pereira, L. Red Seaweed Pigments from a Biotechnological Perspective. Phycology 2021, 2, 1–29. [Google Scholar] [CrossRef]
- Yun, E.J.; Yu, S.; Kim, Y.-A.; Liu, J.-J.; Kang, N.J.; Jin, Y.-S.; Kim, K.H. In Vitro Prebiotic and Anti-Colon Cancer Activities of Agar-Derived Sugars from Red Seaweeds. Mar. Drugs 2021, 19, 213. [Google Scholar] [CrossRef]
- Aluta, U.P.; Aderolu, A.Z.; Ishola, I.O.; Alyassin, M.; Morris, G.A.; Olajide, O.A. Chemical Characterisation of Sulfated Polysaccharides from the Red Seaweed Centroceras clavulatum and Their in Vitro Immunostimulatory and Antioxidant Properties. Food Hydrocoll. Health 2023, 3, 100135. [Google Scholar] [CrossRef]
- Sharan, L.V.; Vennila, J.J. Phyto-Pharmacological Investigation of Marine Red Algae Kappaphycus alvarezii (Doty) Doty Ex Silva for Oral Diseases. Int. J. Algae 2021, 23, 183–209. [Google Scholar] [CrossRef]
- Sudhakar, M.P.; Dharani, G.; Paramasivam, A. Evaluation of Antimicrobial, Antioxidant and Cytotoxicity Potential of R-Phycoerythrin Extracted from Gracilaria corticata Seaweed. Curr. Res. Green Sustain. Chem. 2023, 6, 100352. [Google Scholar] [CrossRef]
- Vasudevan, M.T.; Rangaraj, K.; Ramesh, R.; Muthusami, S.; Govindasamy, C.; Khan, M.I.; Arulselvan, P.; Muruganantham, B. Inhibitory Effects of Gracilaria edulis and Gracilaria salicornia against the MGMT and VEGFA Biomarkers Involved in the Onset and Advancement of Glioblastoma Using in Silico and in Vitro Approaches. Biotechnol. Appl. Biochem. 2025, 72, 207–224. [Google Scholar] [CrossRef]
- Govindaraju, K.; Kiruthiga, V.; Kumar, V.G.; Singaravelu, G. Extracellular Synthesis of Silver Nanoparticles by a Marine Alga, Sargassum Wightii Grevilli and Their Antibacterial Effects. J. Nanosci. Nanotechnol. 2009, 9, 5497–5501. [Google Scholar] [CrossRef]
- Vivek, M.; Kumar, P.S.; Steffi, S.; Sudha, S. Biogenic Silver Nanoparticles by Gelidiella acerosa Extract and Their Antifungal Effects. Avicenna J. Med. Biotechnol. 2011, 3, 143–148. [Google Scholar]
- Castro, L.; Blázquez, M.L.; Muñoz, J.A.; González, F.; Ballester, A. Biological Synthesis of Metallic Nanoparticles Using Algae. IET Nanobiotechnol. 2013, 7, 109–116. [Google Scholar] [CrossRef]
- Kumar, P.; Senthamil Selvi, S.; Govindaraju, M. Seaweed-mediated biosynthesis of silver nanoparticles using Gracilaria corticata for its antifungal activity against Candida spp. Appl. Nanosci. 2013, 3, 495–500. [Google Scholar] [CrossRef]
- Teodor, E.D.; Ungureanu, O.; Gatea, F.; Radu, G.L. The Potential of Flavonoids and Tannins from Medicinal Plants as Anticancer Agents. Anticancer Agents Med. Chem. 2020, 20, 2216–2227. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Andrikopoulos, N.; Velonia, K.; Tang, H.; Cai, R.; Ding, F.; Ke, P.C.; Chen, C. Chemical and Biophysical Signatures of the Protein Corona in Nanomedicine. J. Am. Chem. Soc. 2022, 144, 9184–9205. [Google Scholar] [CrossRef]
- El-Kassas, H.Y.; El-Sheekh, M.M. Cytotoxic Activity of Biosynthesized Gold Nanoparticles with an Extract of the Red Seaweed Corallina officinalis on the MCF-7 Human Breast Cancer Cell Line. Asian Pac. J. Cancer Prev. 2014, 15, 4311–4317. [Google Scholar] [CrossRef]
- El Kassas, H.Y.; Attia, A.A. Bactericidal Application and Cytotoxic Activity of Biosynthesized Silver Nanoparticles with an Extract of the Red Seaweed Pterocladiella capillacea on the HepG2 Cell Line. Asian Pac. J. Cancer Prev. 2014, 15, 1299–1306. [Google Scholar] [CrossRef]
- Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as Drug Delivery Systems: A Review of the Implication of Nanoparticles’ Physicochemical Properties on Responses in Biological Systems. Polymers 2023, 15, 1596. [Google Scholar] [CrossRef]
- Kannt, A.; Wieland, T. Managing Risks in Drug Discovery: Reproducibility of Published Findings. Naunyn Schmiedeberg’s. Arch. Pharmacol. 2016, 389, 353–360. [Google Scholar] [CrossRef]
- Rónavári, A.; Igaz, N.; Adamecz, D.I.; Szerencsés, B.; Molnar, C.; Kónya, Z.; Pfeiffer, I.; Kiricsi, M. Green Silver and Gold Nanoparticles: Biological Synthesis Approaches and Potentials for Biomedical Applications. Molecules 2021, 26, 844. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, S.; Palaniyandi, T.; Shanmugam, R.; Tharani; Rajendran, B.K.; Sivaji, A. Biomedical Potential of Silver Nanoparticles Capped with Active Ingredients of Hypnea valentiae, Red Algae Species. Part. Sci. Technol. 2022, 40, 686–696. [Google Scholar] [CrossRef]
- Viswanathan, S.; Palaniyandi, T.; Chellam, D.C.; Ahmed, M.F.; Shoban, N.; Pushpakumar, M.; Abdul Wahab, M.R.; Baskar, G.; Ravi, M.; Sivaji, A.; et al. Anti-Cancer Activity of Hypnea valentiae Seaweed Loaded Gold Nanoparticles through EMT Signaling Pathway in A549 Cells. Biochem. Syst. Ecol. 2023, 107, 104606. [Google Scholar] [CrossRef]
- Baskar, G.; Palaniyandi, T.; Ravi, M.; Viswanathan, S.; Wahab, M.R.A.; Surendran, H.; Govindaraj, M.; Sugumaran, A.; Almutairi, M.H.; Almutairi, B.O. Biosynthesis of Iron Oxide Nanoparticles from Red Seaweed Hypnea valentiae and Evaluation of Their Antioxidant and Antitumor Potential via the AKT/PI3K Pathway. Process Biochem. 2024, 141, 155–169. [Google Scholar] [CrossRef]
- Khan, M.S.; Ranjani, S.; Hemalatha, S. Synthesis and Characterization of Kappaphycus alvarezii Derived Silver Nanoparticles and Determination of Antibacterial Activity. Mater. Chem. Phys. 2022, 282, 125985. [Google Scholar] [CrossRef]
- Jaffar, S.S.; Saallah, S.; Misson, M.; Siddique, S.; Roslan, J.; Lenggoro, W. Green Synthesis, Characterization and Antimicrobial Efficacy of Silver Nanoparticles from Kappaphycus alvarezii Extract. Res. Chem. Intermed. 2024, 50, 3435–3452. [Google Scholar] [CrossRef]
- Kesevan, S.; Rajesh, D.; Shanmugam, J.; Aruna, S.; Gopal, M.; Vijayakumar, S. Biocompatible Polysaccharide Fabricated Graphene Oxide Nanoparticles: A Versatile Nanodrug Carrier to Deliver κ- Carrageenan against Cancer Cells. Int. J. Biol. Macromol. 2023, 244, 125322. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, S.; Palaniyandi, T.; Kannaki, P.; Shanmugam, R.; Baskar, G.; Rahaman, A.M.; Paul, L.T.D.; Rajendran, B.K.; Sivaji, A. Biogenic Synthesis of Gold Nanoparticles Using Red Seaweed Champia parvula and Its Anti-Oxidant and Anticarcinogenic Activity on Lung Cancer. Part. Sci. Technol. 2023, 41, 241–249. [Google Scholar] [CrossRef]
- Viswanathan, S.; Palaniyandi, T.; Shanmugam, R.; Karunakaran, S.; Pandi, M.; Wahab, M.R.A.; Baskar, G.; Rajendran, B.K.; Sivaji, A.; Moovendhan, M. Synthesis, Characterization, Cytotoxicity, and Antimicrobial Studies of Green Synthesized Silver Nanoparticles Using Red Seaweed Champia parvula. Biomass Convers. Biorefin. 2024, 14, 7387–7400. [Google Scholar] [CrossRef]
- Chellapandian, C.; Ramkumar, B.; Puja, P.; Shanmuganathan, R.; Pugazhendhi, A.; Kumar, P. Gold Nanoparticles Using Red Seaweed Gracilaria verrucosa: Green Synthesis, Characterization and Biocompatibility Studies. Process Biochem. 2019, 80, 58–63. [Google Scholar] [CrossRef]
- Marmiroli, M.; Villani, M.; Scarponi, P.; Carlo, S.; Pagano, L.; Sinisi, V.; Lazzarini, L.; Pavlicevic, M.; Marmiroli, N. Green Synthesis of CuO Nanoparticles from Macroalgae Ulva lactuca and Gracilaria verrucosa. Nanomaterials 2024, 14, 1157. [Google Scholar] [CrossRef]
- Salem, D.M.S.A.; Ismail, M.M.; Aly-Eldeen, M.A. Biogenic Synthesis and Antimicrobial Potency of Iron Oxide (Fe3O4) Nanoparticles Using Algae Harvested from the Mediterranean Sea, Egypt. Egypt. J. Aquat. Res. 2019, 45, 197–204. [Google Scholar] [CrossRef]
- Krishnaswamy, J.; Christupaul Roseline, P.; Kannan, K.; Dhanraj, G.; Sivaperumal, P. Biosynthesis, Characterization, and Anticoagulant Properties of Copper Nanoparticles from Red Seaweed of Acanthophora sp. Phytochem. Anal. 2024. [Google Scholar] [CrossRef]
- Babu, B.; Palanisamy, S.; Vinosha, M.; Anjali, R.; Kumar, P.; Pandi, B.; Tabarsa, M.; You, S.; Prabhu, N.M. Bioengineered Gold Nanoparticles from Marine Seaweed Acanthophora spicifera for Pharmaceutical Uses: Antioxidant, Antibacterial, and Anticancer Activities. Bioprocess Biosyst. Eng. 2020, 43, 2231–2242. [Google Scholar] [CrossRef] [PubMed]
- González-Ballesteros, N.; Diego-González, L.; Lastra-Valdor, M.; Grimaldi, M.; Cavazza, A.; Bigi, F.; Rodríguez-Argüelles, M.C.; Simón-Vázquez, R. Immunomodulatory and Antitumoral Activity of Gold Nanoparticles Synthesized by Red Algae Aqueous Extracts. Mar. Drugs 2022, 20, 182. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, C.G.; Viana, R.L.S.; Teixeira, D.I.A.; Rocha, H.A.O. Síntese verde de nanopartículas antioxidantes feitas com prata e polissacarídeos sulfatados da alga Gracilaria birdiae. HOLOS 2021, 5, 1–19. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Prabakar, D.; Jacob, J.M.; Karuppusamy, I.; Saratale, R.G. Synthesis and Characterization of Silver Nanoparticles Using Gelidium amansii and Its Antimicrobial Property against Various Pathogenic Bacteria. Microb. Pathog. 2018, 114, 41–45. [Google Scholar] [CrossRef]
- Subbulakshmi, A.; Durgadevi, S.; Anitha, S.; Govarthanan, M.; Biruntha, M.; Rameshthangam, P.; Kumar, P. Biogenic Gold Nanoparticles from Gelidiella acerosa: Bactericidal and Photocatalytic Degradation of Two Commercial Dyes. Appl. Nanosci. 2023, 13, 4033–4042. [Google Scholar] [CrossRef]
- Aragão, A.P.; Oliveira, T.M.; Quelemes, P.V.; Perfeito, M.L.G.; Araújo, M.C.; Santiago, J.A.S.; Cardoso, V.S.; Quaresma, P.; Leite, J.R.S.A.; Silva, D.A. Green Synthesis of Silver Nanoparticles Using the Seaweed Gracilaria birdiae and Their Antibacterial Activity. Arab. J. Chem. 2019, 12, 4182–4188. [Google Scholar] [CrossRef]
- Naveenkumar, S.; Kamaraj, C.; Ragavendran, C.; Vaithiyalingam, M.; Sugumar, V.; Marimuthu, K. Gracilaria corticata Red Seaweed Mediate Biosynthesis of Silver Nanoparticles: Larvicidal, Neurotoxicity, Molecular Docking Analysis, and Ecofriendly Approach. Biomass Convers. Biorefin. 2024, 14, 20587–20609. [Google Scholar] [CrossRef]
- Prabhu, R.; Asik, M.R.; Anjali, R.; Archunan, G.; Prabhu, N.M.; Pugazhendhi, A.; Suganthy, N. Ecofriendly One Pot Fabrication of Methyl gallate@ZIF-L Nanoscale Hybrid as pH Responsive Drug Delivery System for Lung Cancer Therapy. Process Biochem. 2019, 84, 39–52. [Google Scholar] [CrossRef]
- Kalimuthu, K.; Panneerselvam, C.; Chou, C.; Lin, S.-M.; Tseng, L.-C.; Tsai, K.-H.; Murugan, K.; Hwang, J.-S. Predatory Efficiency of the Copepod Megacyclops formosanus and Toxic Effect of the Red Alga Gracilaria firma-Synthesized Silver Nanoparticles against the Dengue Vector Aedes Aegypti. Hydrobiologia 2017, 785, 359–372. [Google Scholar] [CrossRef]
- Kamble, M.T.; Soowannayan, C.; Chaicherd, S.; Medhe, S.V.; Rudtanatip, T.; Pissuwan, D.; Wongprasert, K. Bimetallic Nanoparticles with Sulfated Galactan Eliminate Vibrio parahaemolyticus in Shrimp Penaeus vannamei. Fish Shellfish Immunol. 2024, 151, 109753. [Google Scholar] [CrossRef]
- Algotiml, R.; Gab-alla, A.; Seoudi, R.; Abulreesh, H.H.; Ahmad, I.; Elbanna, K. Anticancer and antimicrobial activity of red sea seaweeds extracts-mediated gold nanoparticles. J. Pure Appl. Microbiol. 2022, 16, 207–225. [Google Scholar] [CrossRef]
- Tang, L.; Luo, X.; Wang, M.; Wang, Z.; Guo, J.; Kong, F.; Bi, Y. Synthesis, Characterization, in vitro Antioxidant and Hypoglycemic Activities of Selenium Nanoparticles Decorated with Polysaccharides of Gracilaria lemaneiformis. Int. J. Biol. Macromol. 2021, 193, 923–932. [Google Scholar] [CrossRef]
- Tu, J.; Hu, L.; Mohammed, K.J.; Le, B.N.; Chen, P.; Ali, E.; Ali, H.E.; Sun, L. Application of Logistic Regression, Support Vector Machine and Random Forest on the Effects of Titanium Dioxide Nanoparticles Using Macroalgae in Treatment of Certain Risk Factors Associated with Kidney Injuries. Environ. Res. 2023, 220, 115167. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.D.; Hanif, U.; Liaqat, I.; Shaheen, S.; Awan, U.F.; Ishtiaq, S.; Pereira, L.; Bahadur, S.; Khan, M.D. Application of Green Silver Nanoparticles Synthesized from the Red Seaweeds Halymenia porphyriformis and Solieria robusta against Oral Pathogenic Bacteria by Using Microscopic Technique. Front. Biosci. 2022, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Baskar, G.; Palaniyandi, T.; Viswanathan, S.; Rahaman Abdul Wahab, M.; Surendran, H.; Ravi, M.; Kumar Rajendran, B.; Govindasamy, G.; Sivaji, A.; Kaliamoorthy, S. Pharmacological Effect of Gold Nanoparticles from Red Algae Halymenia venusta on A549 Cell Line. Inorg. Chem. Commun. 2023, 155, 111005. [Google Scholar] [CrossRef]
- Maghraby, Y.R.; Farag, M.A.; G Kontominas, M.; Shakour, Z.T.; Ramadan, A.R. Nanoencapsulated Extract of a Red Seaweed (Rhodophyta) Species as a Promising Source of Natural Antioxidants. ACS Omega 2022, 7, 6539–6548. [Google Scholar] [CrossRef]
- Vieira, A.P.; Stein, E.M.; Andreguetti, D.X.; Colepicolo, P.; Ferreira, A.M.C. Preparation of Silver Nanoparticles Using Aqueous Extracts of the Red Algae Laurencia aldingensis and Laurenciella sp. and Their Cytotoxic Activities. J. Appl. Phycol. 2016, 28, 2615–2622. [Google Scholar] [CrossRef]
- Baskar, G.; Keerthana, K.; Supriya, A.; Pravin, R.; Abinesh, A.R.; Yuvaraaj, S.A. Synthesis of phycoerythrin-Ag-ZnO nanobiocomposite from marine red algae Porphyridium purpureum for anticancer applications against MCF-7 cell line. Indian J. Exp. Biol. 2024, 62, 393–399. [Google Scholar] [CrossRef]
- Valarmathi, N.; Ameen, F.; Almansob, A.; Kumar, P.; Arunprakash, S.; Govarthanan, M. Utilization of Marine Seaweed Spyridia filamentosa for Silver Nanoparticles Synthesis and Its Clinical Applications. Mater. Lett. 2020, 263, 127244. [Google Scholar] [CrossRef]
- González-Ballesteros, N.; González-Rodríguez, J.B.; Rodríguez-Argüelles, M.C.; Lastra, M. New Application of Two Antarctic Macroalgae Palmaria decipiens and Desmarestia menziesii in the Synthesis of Gold and Silver Nanoparticles. Polar Sci. 2018, 15, 49–54. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, X.; Gao, Y.; Yin, J.; Bai, M.; Wang, F. Green Synthesis of Gold Nanoparticles Using Carrageenan Oligosaccharide and Their in vitro Antitumor Activity. Mar. Drugs 2018, 16, 277. [Google Scholar] [CrossRef]
- Chen, X.; Han, W.; Zhao, X.; Tang, W.; Wang, F. Epirubicin-Loaded Marine Carrageenan Oligosaccharide Capped Gold Nanoparticle System for pH-Triggered Anticancer Drug Release. Sci. Rep. 2019, 9, 6754. [Google Scholar] [CrossRef]
- Singh, S.; Pal, K. Polyphenol Modified CuO Nanorods Capped by Kappa-Carrageenan for Controlled Paclitaxel Release in Furnishing Targeted Chemotherapy in Breast Carcinoma Cells. Int. J. Biol. Macromol. 2024, 255, 127893. [Google Scholar] [CrossRef] [PubMed]
- Lima, D.; Calaça, G.N.; Viana, A.G.; Pessôa, C.A. Porphyran-Capped Gold Nanoparticles Modified Carbon Paste Electrode: A Simple and Efficient Electrochemical Sensor for the Sensitive Determination of 5-Fluorouracil. Appl. Surf. Sci. 2018, 427, 742–753. [Google Scholar] [CrossRef]
- Bojko, L.; Jonge, G.; Lima, D.; Lopes, L.C.; Viana, A.G.; Garcia, J.R.; Pessôa, C.A.; Wohnrath, K.; Inaba, J. Porphyran-Capped Silver Nanoparticles as a Promising Antibacterial Agent and Electrode Modifier for 5-Fluorouracil Electroanalysis. Carbohydr. Res. 2020, 498, 108193. [Google Scholar] [CrossRef] [PubMed]
- López-Mayán, J.J.; Álvarez-Fernández, B.; Peña-Vázquez, E.; Barciela-Alonso, M.C.; Moreda-Piñeiro, A.; Bermejo-Barrera, P. Ultrasonication Followed by Enzymatic Hydrolysis as a Sample Pre-Treatment for the Determination of Ag Nanoparticles in Edible Seaweed by SP-ICP-MS. Talanta 2022, 247, 123556. [Google Scholar] [CrossRef]
- Sudhakar, M.P.; Venkatnarayanan, S.; Dharani, G. Fabrication and Characterization of Bio-Nanocomposite Films Using κ-Carrageenan and Kappaphycus alvarezii Seaweed for Multiple Industrial Applications. Int. J. Biol. Macromol. 2022, 219, 138–149. [Google Scholar] [CrossRef]
- Rizal, S.; Abdul Khalil, H.P.S.; Abd Hamid, S.; Yahya, E.B.; Ikramullah, I.; Kurniawan, R.; Hazwan, C.M. Cinnamon-Nanoparticle-Loaded Macroalgal Nanocomposite Film for Antibacterial Food Packaging Applications. Nanomaterials 2023, 13, 560. [Google Scholar] [CrossRef] [PubMed]
- Parsaee, Z. Electrospun Nanofibers Decorated with Bio-Sonochemically Synthesized Gold Nanoparticles as an Ultrasensitive Probe in Amalgam-Based Mercury (II) Detection System. Ultrason. Sonochem. 2018, 44, 24–35. [Google Scholar] [CrossRef]
- Mansour, A.T.; Alprol, A.E.; Khedawy, M.; Abualnaja, K.M.; Shalaby, T.A.; Rayan, G.; Ramadan, K.M.A.; Ashour, M. Green Synthesis of Zinc Oxide Nanoparticles Using Red Seaweed for the Elimination of Organic Toxic Dye from an Aqueous Solution. Materials 2022, 15, 5169. [Google Scholar] [CrossRef]
- Heydari, F.; Bakhtiarian, M.; Khodaei, M.M. Preparation of Fe3O4@Carrageenan-Metformin Nanoparticles as a New Bio-Inspired Magnetic Nanocatalyst for the Synthesis of Dihydropyrano[2,3-c]Pyrazoles. Mater. Sci. Eng. B Solid State Mater. Adv. Technol. 2023, 296, 116686. [Google Scholar] [CrossRef]
- Kumari, S.; Kumari, A.; Ahmed, J.; Jasrotia, R.; Sillanpää, M.; Lakshmaiya, N.; Kondal, N.; Kandwal, A.; Sharma, R. Enhancing UV Protection and Antimicrobial Properties in Food Packaging through the Use of Copper Nanoparticles and κ-Carrageenan Based Nanocomposite Film. J. Inorg. Organomet. Polym. Mater. 2024, 34, 5538–5550. [Google Scholar] [CrossRef]
- Roseline, T.A.; Murugan, M.; Sudhakar, M.P.; Arunkumar, K. Nanopesticidal Potential of Silver Nanocomposites Synthesized from the Aqueous Extracts of Red Seaweeds. Environ. Technol. Innov. 2019, 13, 82–93. [Google Scholar] [CrossRef]
- Roseline, T.A.; Sudhakar, M.P.; Kulanthaiyesu, A. Synthesis of Silver Nanoparticle Composites Using Calliblepharis fimbriata Aqueous Extract, Phytochemical Stimulation, and Controlling Bacterial Blight Disease in Rice. ACS Agric. Sci. Technol. 2021, 1, 702–718. [Google Scholar] [CrossRef]
- Roseline, T.A.; Sudhakar, M.P.; Arunkumar, K. Aqueous Extraction of Red Seaweed Bioactive Compounds and Synthesis of Silver Nanoparticles for Agriculture Applications. J. Agric. Food Res. 2023, 14, 100769. [Google Scholar] [CrossRef]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New Approaches and Procedures for Cancer Treatment: Current Perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Sauter, E.R. Cancer Prevention and Treatment Using Combination Therapy with Natural Compounds. Expert Rev. Clin. Pharmacol. 2020, 13, 265–285. [Google Scholar] [CrossRef]
- Huang, M.; Lu, J.-J.; Ding, J. Natural Products in Cancer Therapy: Past, Present and Future. Nat. Prod. Bioprospect. 2021, 11, 5–13. [Google Scholar] [CrossRef]
- Alsaab, H.O.; Al-Hibs, A.S.; Alzhrani, R.; Alrabighi, K.K.; Alqathama, A.; Alwithenani, A.; Almalki, A.H.; Althobaiti, Y.S. Nanomaterials for Antiangiogenic Therapies for Cancer: A Promising Tool for Personalized Medicine. Int. J. Mol. Sci. 2021, 22, 1631. [Google Scholar] [CrossRef]
- Harun-Ur-Rashid, M.; Jahan, I.; Foyez, T.; Imran, A.B. Bio-Inspired Nanomaterials for Micro/Nanodevices: A New Era in Biomedical Applications. Micromachines 2023, 14, 1786. [Google Scholar] [CrossRef] [PubMed]
- Cotas, J.; Lomartire, S.; Gonçalves, A.M.M.; Pereira, L. From Ocean to Medicine: Harnessing Seaweed’s Potential for Drug Development. Int. J. Mol. Sci. 2024, 25, 797. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Sridhar, B.; Ram, S. Potential Seaweed-Derived Bioactive Compounds for Pharmaceutical Applications. In Multidisciplinary Applications of Marine Resources; Springer Nature Singapore: Singapore, 2024; pp. 211–242. ISBN 9789819750566. [Google Scholar]
- Ross, F.W.R.; Boyd, P.W.; Filbee-Dexter, K.; Watanabe, K.; Ortega, A.; Krause-Jensen, D.; Lovelock, C.; Sondak, C.F.A.; Bach, L.T.; Duarte, C.M.; et al. Potential Role of Seaweeds in Climate Change Mitigation. Sci. Total Environ. 2023, 885, 163699. [Google Scholar] [CrossRef] [PubMed]
- Yong, W.T.L.; Thien, V.Y.; Misson, M.; Chin, G.J.W.L.; Said Hussin, S.N.I.; Chong, H.L.H.; Yusof, N.A.; Ma, N.L.; Rodrigues, K.F. Seaweed: A Bioindustrial Game-Changer for the Green Revolution. Biomass Bioenergy 2024, 183, 107122. [Google Scholar] [CrossRef]
- Pestovsky, Y.S.; Martínez-Antonio, A. The Use of Nanoparticles and Nanoformulations in Agriculture. J. Nanosci. Nanotechnol. 2017, 17, 8699–8730. [Google Scholar] [CrossRef]
- Singh, R.P.; Handa, R.; Manchanda, G. Nanoparticles in Sustainable Agriculture: An Emerging Opportunity. J. Control. Release 2021, 329, 1234–1248. [Google Scholar] [CrossRef]
- Li, Y.-J.; Harroun, S.G.; Su, Y.-C.; Huang, C.-F.; Unnikrishnan, B.; Lin, H.-J.; Lin, C.-H.; Huang, C.-C. Synthesis of Self-Assembled Spermidine-Carbon Quantum Dots Effective against Multidrug-Resistant Bacteria. Adv. Healthc. Mater. 2016, 5, 2545–2554. [Google Scholar] [CrossRef]
- Comparetti, E.J.; Romagnoli, G.G.; Gorgulho, C.M.; Pedrosa, V.d.A.; Kaneno, R. Anti-PSMA Monoclonal Antibody Increases the Toxicity of Paclitaxel Carried by Carbon Nanotubes. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 116, 111254. [Google Scholar] [CrossRef]
- Malina, T.; Poláková, K.; Hirsch, C.; Svoboda, L.; Zbořil, R. Toxicity of Carbon Nanomaterials-towards Reliable Viability Assessment via New Approach in Flow Cytometry. Int. J. Mol. Sci. 2021, 22, 7750. [Google Scholar] [CrossRef]
- Polash, S.A.; Pyreddy, S.; Abraham, A.N.; Mahasivam, S.; Bansal, V.; Varadi, L.; Bryant, G.; Shukla, R. Impact of Nucleic Acid Encapsulated MOF Crystal Phase on Protein Corona Formation. Mater. Adv. 2023, 4, 4761–4774. [Google Scholar] [CrossRef]
- Altammar, K.A. A Review on Nanoparticles: Characteristics, Synthesis, Applications, and Challenges. Front. Microbiol. 2023, 14, 1155622. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-Y.; Rheima, A.M.; Kadhim, M.M.; Ahmed, N.N.; Mohammed, S.H.; Abbas, F.H.; Abed, Z.T.; Mahdi, Z.M.; Abbas, Z.S.; Hachim, S.K.; et al. An Overview of Nanoparticles in Drug Delivery: Properties and Applications. S. Afr. J. Chem. Eng. 2023, 46, 233–270. [Google Scholar] [CrossRef]
- Damasco, J.A.; Ravi, S.; Perez, J.D.; Hagaman, D.E.; Melancon, M.P. Understanding Nanoparticle Toxicity to Direct a Safe-by-Design Approach in Cancer Nanomedicine. Nanomaterials 2020, 10, 2186. [Google Scholar] [CrossRef] [PubMed]
- Najahi-Missaoui, W.; Arnold, R.D.; Cummings, B.S. Safe Nanoparticles: Are We There Yet? Int. J. Mol. Sci. 2020, 22, 385. [Google Scholar] [CrossRef]
- Sengul, A.B.; Asmatulu, E. Toxicity of Metal and Metal Oxide Nanoparticles: A Review. Environ. Chem. Lett. 2020, 18, 1659–1683. [Google Scholar] [CrossRef]
- Foulkes, R.; Man, E.; Thind, J.; Yeung, S.; Joy, A.; Hoskins, C. The Regulation of Nanomaterials and Nanomedicines for Clinical Application: Current and Future Perspectives. Biomater. Sci. 2020, 8, 4653–4664. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
| Nº | Target Effect | Species Used | Core Nanoparticle | Results/Potential | References |
|---|---|---|---|---|---|
| 1 | Disease or pest control | Gracilaria corticata, Gracilaria edulis, Hypnea musciformis, and Spyridia hypnoides | Silver | Green synthesis of NPs with anti-phytopathogen activity as suitable resource for the formulation of nanopesticides | [75] |
| 2 | Control plant disease and support plant growth | Calliblepharis fimbriata | NP composites stimulate seed germination and are effective against agricultural bacteria, which can enhance the vegetative growth of rice | [76] | |
| 3 | Gracilaria crassa and Grateloupia lithophila | Green-synthesized NPs produced for controlling bacterial diseases in plants and for supporting plant growth | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, A.; Rilievo, G.; Magro, M.; Maraschin, M.; Vianello, F.; Lima, G.P.P. Biotechnological Applications of Biogenic Nanomaterials from Red Seaweed: A Systematic Review (2014–2024). Int. J. Mol. Sci. 2025, 26, 4275. https://doi.org/10.3390/ijms26094275
Nunes A, Rilievo G, Magro M, Maraschin M, Vianello F, Lima GPP. Biotechnological Applications of Biogenic Nanomaterials from Red Seaweed: A Systematic Review (2014–2024). International Journal of Molecular Sciences. 2025; 26(9):4275. https://doi.org/10.3390/ijms26094275
Chicago/Turabian StyleNunes, Aline, Graziano Rilievo, Massimiliano Magro, Marcelo Maraschin, Fabio Vianello, and Giuseppina Pace Pereira Lima. 2025. "Biotechnological Applications of Biogenic Nanomaterials from Red Seaweed: A Systematic Review (2014–2024)" International Journal of Molecular Sciences 26, no. 9: 4275. https://doi.org/10.3390/ijms26094275
APA StyleNunes, A., Rilievo, G., Magro, M., Maraschin, M., Vianello, F., & Lima, G. P. P. (2025). Biotechnological Applications of Biogenic Nanomaterials from Red Seaweed: A Systematic Review (2014–2024). International Journal of Molecular Sciences, 26(9), 4275. https://doi.org/10.3390/ijms26094275








