MicroRNA-29c-3p and -126a Contribute to the Decreased Angiogenic Potential of Aging Endothelial Progenitor Cells
Abstract
1. Introduction
2. Results
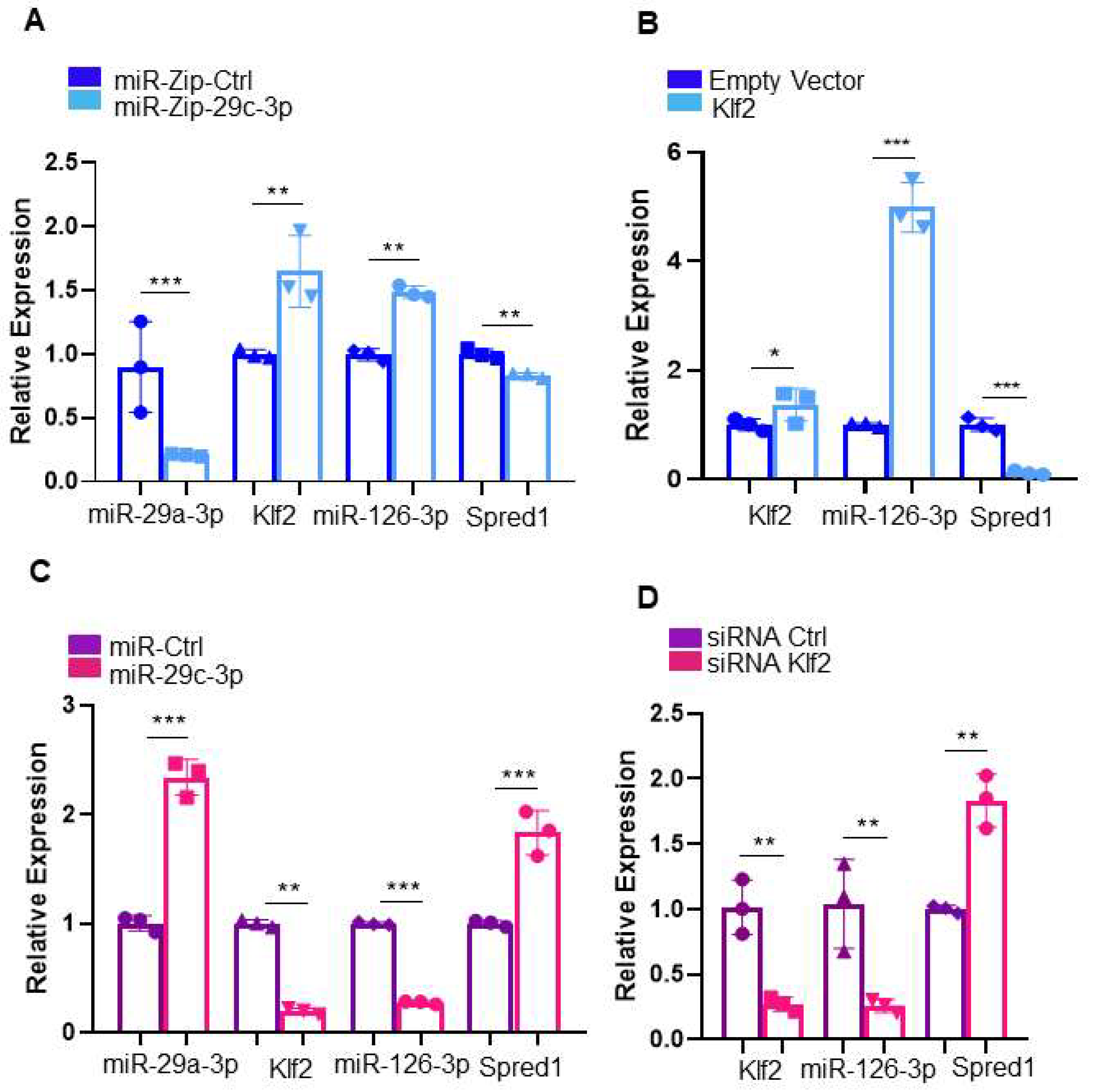
2.1. MiR-29c-3p-Klf2 Is a miRNA-mRNA Pair Dysregulated During EPC Aging
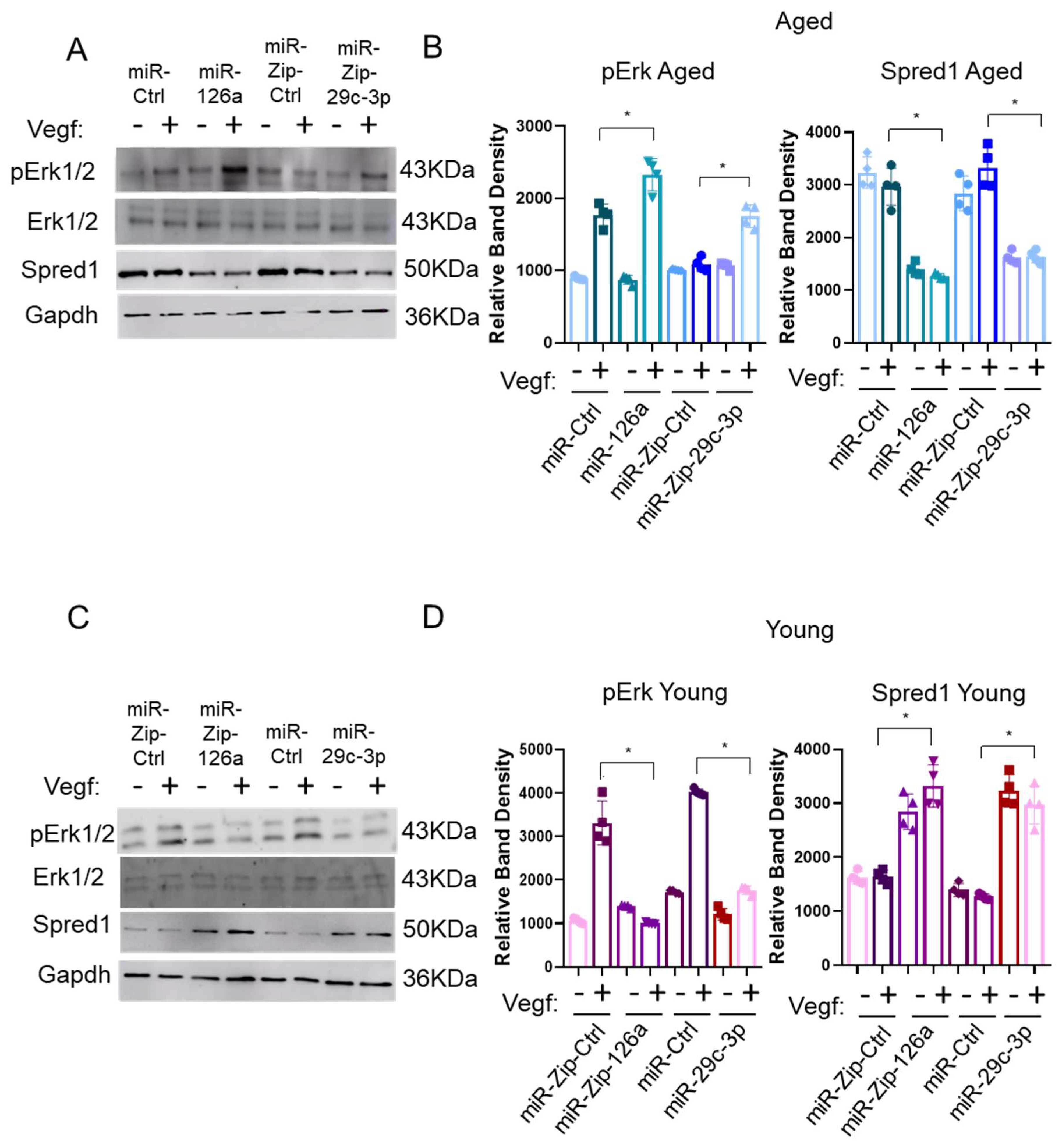
2.2. Establishing the miR-29c-3p–Klf2–miR-126a–Spred1-Vegf Axis
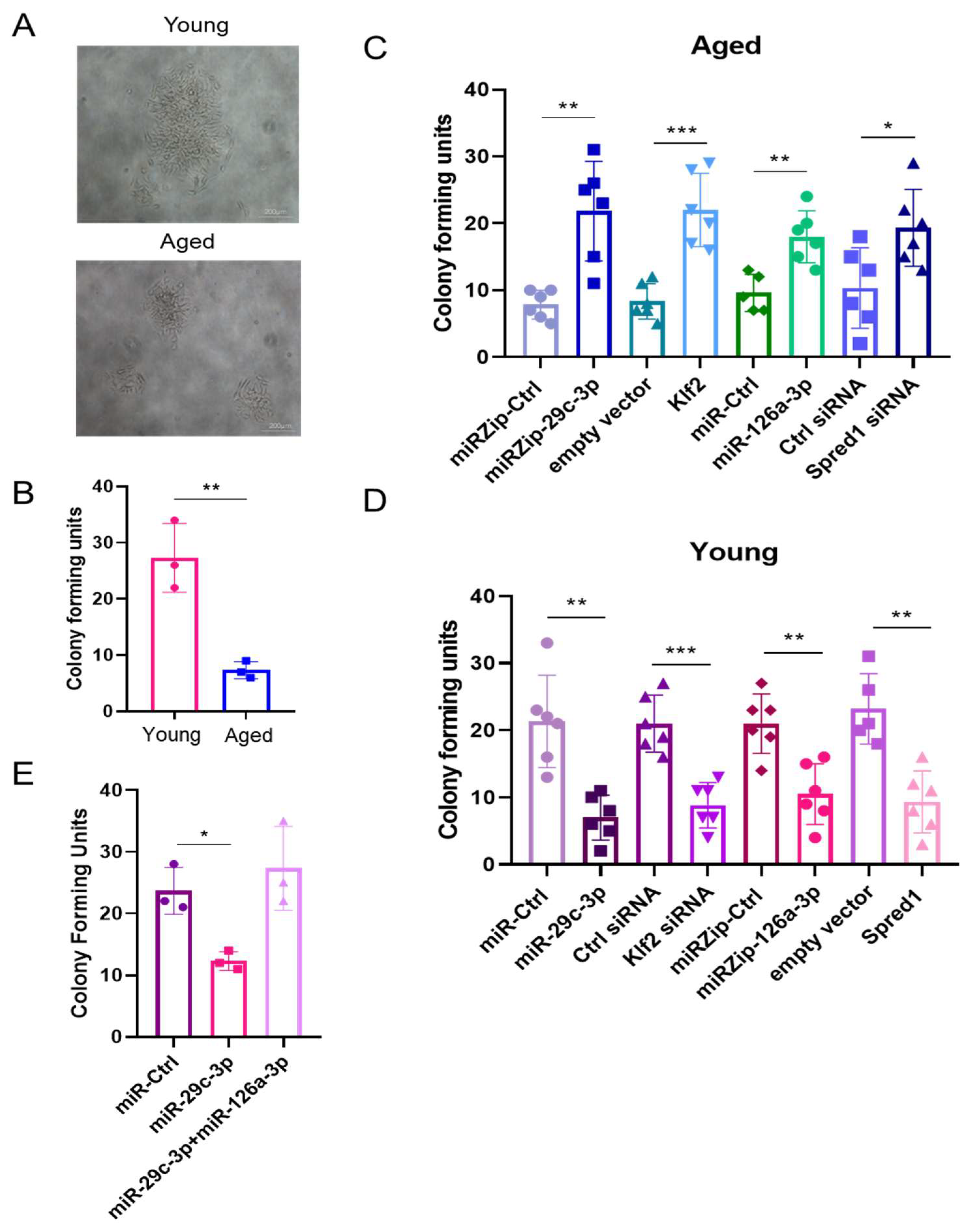
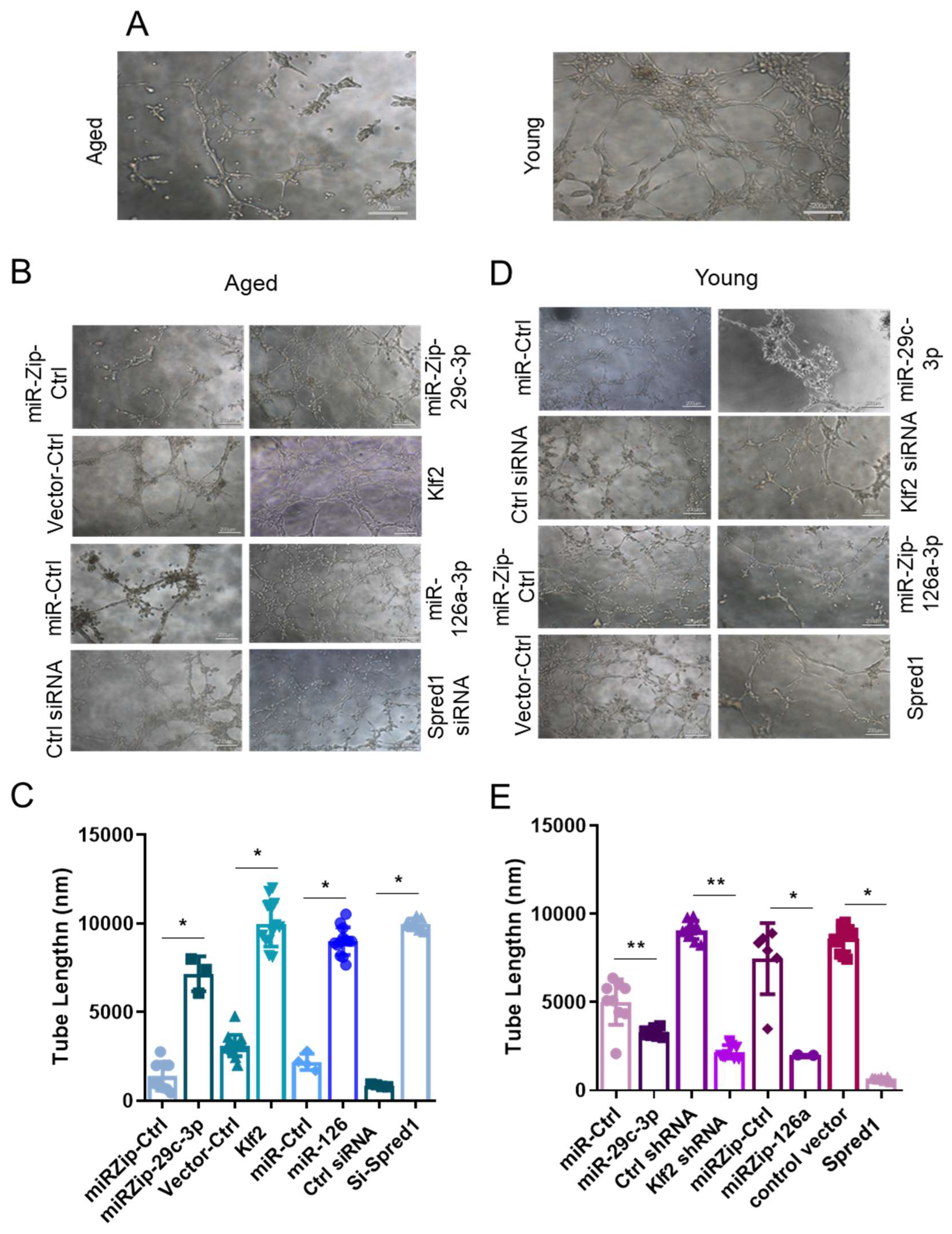
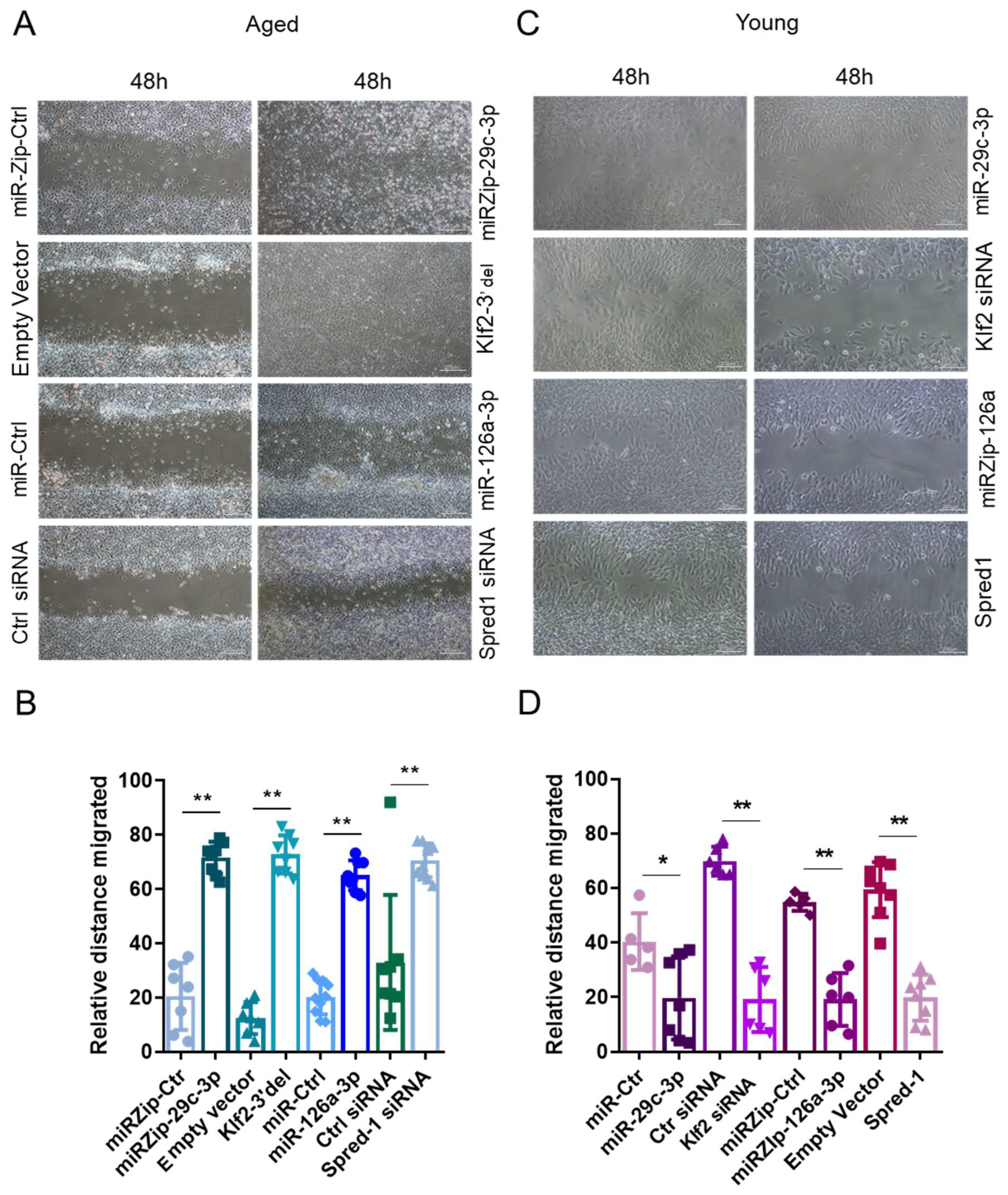
2.3. The miR-29c-3p → ↓Klf2 → ↓miR-126a → ↑Spred1 → ↓VEGF Signaling Pathway Regulates the Self-Renewal Potential, Vascular Tube Formation, and Migration of EPCs
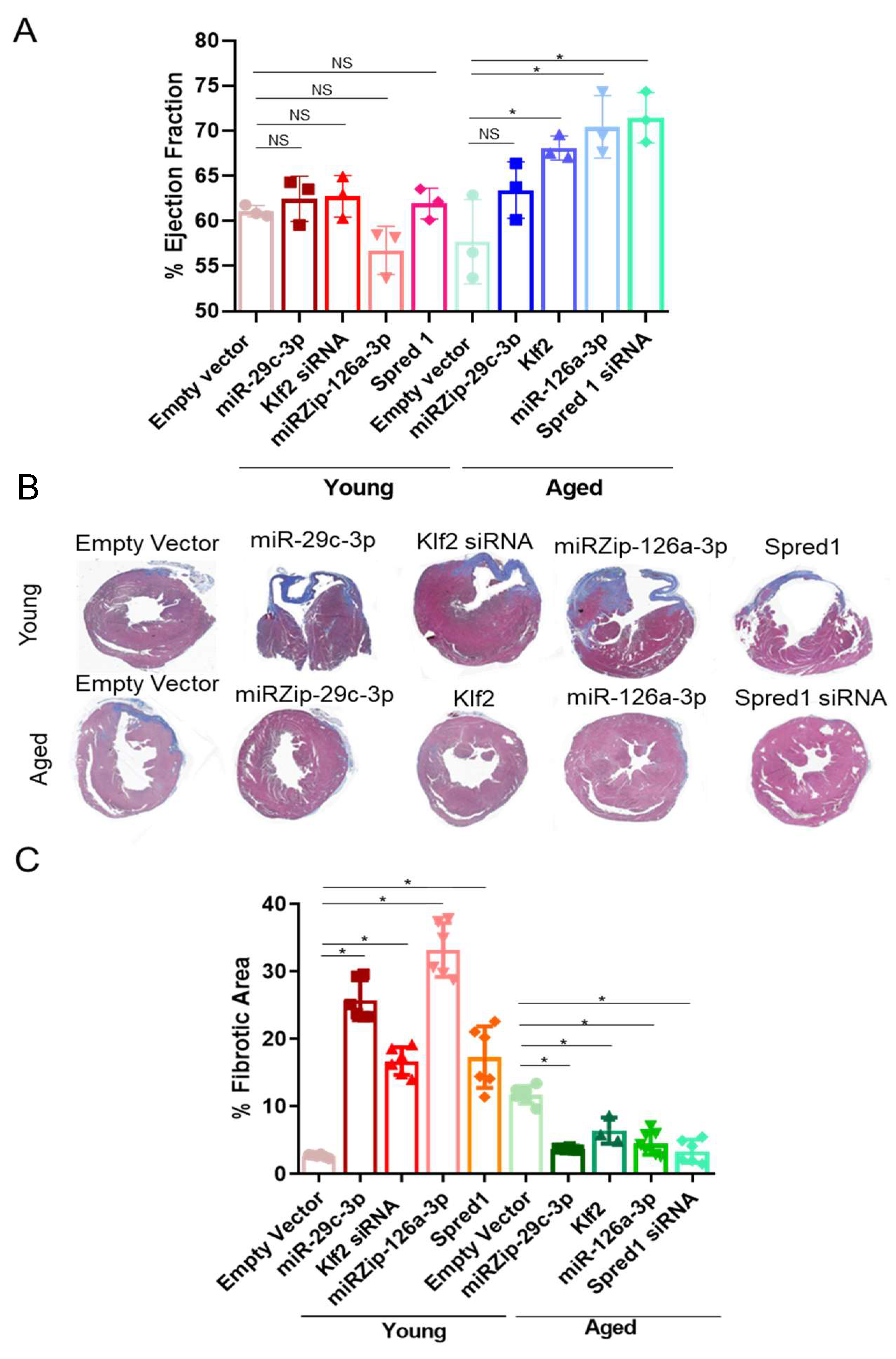
2.4. Modulation of the ↑miR-29c-3p → ↓Klf2 → ↓miR-126a → ↑Spred1 → ↓VEGF Signaling Pathway Impacts Cardiac Repair In Vivo
3. Discussion
4. Methods
4.1. Animals
4.2. Quantitative RT-PCR
4.3. MiRNA and mRNA Profiling Analysis
4.4. Validating Potential miRNA-mRNA Regulatory Relationships in 293T Cell Lines
4.5. Isolation and Culture of EPCs
4.6. MiRNA and shRNA Modulation in EPCs
4.7. Self-Renewal Assay
4.8. In Vitro Angiogenesis Assay
4.9. Western Blotting
4.10. Myocardial Ischemia (MI) Model and Cell Therapy
4.11. Histological Examination
4.12. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMI | Acute Myocardial Infarction |
| CFU | Colony-forming unit |
| COPD | Chronic Obstructive Pulmonary Disease |
| CVD | Cardiovascular disease |
| ECG | Electrocardiogram |
| ECs | Endothelial cells |
| EPCs | Endothelial Progenitor Cells |
| Gluc | Gaussia Luciferase |
| Hmga2 | High-mobility group AT-hook 2 |
| Klf2 | Kruppel-like Factor 2 |
| KMT5 | Histone lysine methyl transferase 5 |
| LAD | Left anterior descending branch of the coronary artery |
| Lin- BMCs | Lineage negative bone marrow cells |
| LVEDd | Left Ventricular End-Diastolic Diameter |
| MEEBO | Mouse Exonic-Evidence Based-Oligonucleotide |
| MI | Myocardial infarction |
| Plk2 | Polo-like Kinase 2 |
| PVDF | Polyvinylidene fluoride |
| SCF | Stem cell factor |
| SEAP | Alkaline Phosphatase |
| TUNEL | Terminal deoxynucleotidyl transferase dUTP nick end labeling |
References
- Tyrrell, D.J.; Goldstein, D.R. Ageing and atherosclerosis: Vascular intrinsic and extrinsic factors and potential role of IL-6. Nat. Rev. Cardiol. 2021, 18, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Venkatesulu, B.P.; Mahadevan, L.S.; Aliru, M.L.; Yang, X.; Bodd, M.H.; Singh, P.K.; Yusuf, S.W.; Abe, J.-I.; Krishnan, S. Radiation-induced endothelial vascular injury: A review of possible mechanisms. JACC Basic Transl. Sci. 2018, 3, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Mak, A.; Chan, J.K.Y. Endothelial function and endothelial progenitor cells in systemic lupus erythematosus. Nat. Rev. Rheumatol. 2022, 18, 286–300. [Google Scholar] [CrossRef]
- Deng, S.; Wang, H.; Jia, C.; Zhu, S.; Chu, X.; Ma, Q.; Wei, J.; Chen, E.; Zhu, W.; Macon, C.J.; et al. MicroRNA-146a induces lineage-negative bone marrow cell apoptosis and senescence by targeting Polo-like kinase 2 expression. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wei, J.; Da Fonseca Ferreira, A.; Wang, H.; Zhang, L.; Zhang, Q.; Bellio, M.A.; Chu, X.-M.; Khan, A.; Jayaweera, D.; et al. Rejuvenation of senescent endothelial progenitor cells by extracellular vesicles derived from mesenchymal stromal cells. JACC Basic Transl. Sci. 2020, 5, 1127–1141. [Google Scholar] [CrossRef]
- Sun, S.; Meng, Y.; Li, M.; Tang, X.; Hu, W.; Wu, W.; Li, G.; Pang, Q.; Wang, W.; Liu, B. CD133+ endothelial-like stem cells restore neovascularization and promote longevity in progeroid and naturally aged mice. Nat. Aging 2023, 3, 1401–1414. [Google Scholar] [CrossRef]
- Shang, R.; Lee, S.; Senavirathne, G.; Lai, E.C. microRNAs in action: Biogenesis, function and regulation. Nat. Rev. Genet. 2023, 24, 816–833. [Google Scholar] [CrossRef]
- Zhu, S.; Deng, S.; Ma, Q.; Zhang, T.; Jia, C.; Zhuo, D.; Yang, F.; Wei, J.; Wang, L.; Dykxhoorn, D.M.; et al. MicroRNA-10A* and MicroRNA-21 modulate endothelial progenitor cell senescence via suppressing high-mobility group A2. Circ. Res. 2013, 112, 152–164. [Google Scholar] [CrossRef]
- Lee, J.S.; Yu, Q.; Shin, J.T.; Sebzda, E.; Bertozzi, C.; Chen, M.; Mericko, P.; Stadtfeld, M.; Zhou, D.; Cheng, L.; et al. Klf2 is an essential regulator of vascular hemodynamic forces in vivo. Dev. Cell 2006, 11, 845–857. [Google Scholar] [CrossRef]
- Ji, J.S.; Xu, M.; Song, J.J.; Zhao, Z.-W.; Chen, M.-J.; Chen, W.-Q.; Tu, J.-F.; Ji, J.-S. Inhibition of microRNA-126 promotes the expression of Spred1 to inhibit angiogenesis in hepatocellular carcinoma after transcatheter arterial chemoembolization: In vivo study. OncoTargets Ther. 2016, 9, 4357–4367. [Google Scholar]
- Nicoli, S.; Standley, C.; Walker, P.; Hurlstone, A.; Fogarty, K.E.; Lawson, N.D. MicroRNA-mediated integration of haemodynamics and Vegf signalling during angiogenesis. Nature 2010, 464, 1196–1200. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.; Qin, S.; Wu, R.; Zhou, X.; Tang, X.; Zhang, S.B.; Zhao, Q.; Wang, H.; Liu, Y.; Han, X.; et al. Role of MiR-126a-3p in endothelial injury in endotoxic mice. Crit. Care Med. 2016, 44, e639–e650. [Google Scholar] [CrossRef] [PubMed]
- Fish, J.E.; Santoro, M.M.; Morton, S.U.; Yu, S.; Yeh, R.-F.; Wythe, J.D.; Ivey, K.N.; Bruneau, B.G.; Stainier, D.Y.R.; Srivastava, D. miR-126 regulates angiogenic signaling and vascular integrity. Dev. Cell 2008, 15, 272–284. [Google Scholar] [CrossRef]
- Wang, S.; Aurora, A.B.; Johnson, B.A.; Qi, X.; McAnally, J.; Hill, J.A.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev. Cell 2008, 15, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and therapeutic aspects of angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [CrossRef]
- Sharpless, N.E.; DePinho, R.A. How stem cells age and why this makes us grow old. Nat. Rev. Mol. Cell Biol. 2007, 8, 703–713. [Google Scholar] [CrossRef]
- Inomata, K.; Aoto, T.; Binh, N.T.; Okamoto, N.; Tanimura, S.; Wakayama, T.; Iseki, S.; Hara, E.; Masunaga, T.; Shimizu, H.; et al. Genotoxic stress abrogates renewal of melanocyte stem cells by triggering their differentiation. Cell 2009, 137, 1088–1099. [Google Scholar] [CrossRef]
- Conboy, I.M.; Conboy, M.J.; Wagers, A.J.; Girma, E.R.; Weissman, I.L.; Rando, T.A. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 2005, 433, 760–764. [Google Scholar] [CrossRef]
- Choudhery, M.S.; Khan, M.; Mahmood, R.; Mehmood, A.; Khan, S.N.; Riazuddin, S. Bone marrow derived mesenchymal stem cells from aged mice have reduced wound healing, angiogenesis, proliferation and anti-apoptosis capabilities. Cell Biol. Int. 2012, 36, 747–753. [Google Scholar] [CrossRef]
- Vasa, M.; Fichtlscherer, S.; Aicher, A.; Adler, K.; Urbich, C.; Martin, H.; Zeiher, A.M.; Dimmeler, S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ. Res. 2001, 89, E1–E7. [Google Scholar] [CrossRef]
- Laufs, U.; Werner, N.; Link, A.; Endres, M.; Wassmann, S.; Jurgens, K.; Miche, E.; Böhm, M.; Nickenig, G. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation 2004, 109, 220–226. [Google Scholar] [PubMed]
- Kuehbacher, A.; Urbich, C.; Zeiher, A.M.; Dimmeler, S. Role of dicer and drosha for endothelial microRNA expression and angiogenesis. Circ. Res. 2007, 101, 59–68. [Google Scholar] [CrossRef]
- Roger, V.L. Epidemiology of heart failure. Circ. Res. 2013, 113, 646–659. [Google Scholar]
- Pei, C.Z.; Liu, B.; Li, Y.T.; Fang, L.; Zhang, Y.; Li, Y.G.; Meng, S. MicroRNA-126 protects against vascular injury by promoting homing and maintaining stemness of late outgrowth endothelial progenitor cells. Stem Cell Res. Ther. 2020, 11, 28. [Google Scholar] [PubMed]
- Chu, M.; Zhao, Y.; Feng, Y.; Zhang, H.; Liu, J.; Cheng, M.; Li, L.; Shen, W.; Cao, H.; Li, Q.; et al. MicroRNA-126 participates in lipid metabolism in mammary epithelial cells. Mol. Cell. Endocrinol. 2017, 454, 77–86. [Google Scholar] [CrossRef]
- Hu, Y.; Deng, F.; Song, J.; Lin, J.; Li, X.; Tang, Y.; Zhou, J.; Tang, T.; Zheng, L. Evaluation of miR-29c inhibits endotheliocyte migration and angiogenesis of human endothelial cells by suppressing the insulin like growth factor 1. Am. J. Transl. Res. 2015, 7, 866–877. [Google Scholar] [PubMed]
- Su, M.; Niu, Y.; Dang, Q.; Qu, J.; Zhu, D.; Tang, Z.; Gou, D. Circulating microRNA profiles based on direct S-Poly(T)Plus assay for detection of coronary heart disease. J. Cell. Mol. Med. 2020, 24, 5984–5997. [Google Scholar]
- Tran, K.-V. Circulating extracellular RNAs, myocardial remodeling, and heart failure in patients with acute coronary syndrome. J. Clin. Transl. Res. 2019, 5, 33–43. [Google Scholar]
- Williams, A.L.; Walton, C.B.; MacCannell, K.A.; Avelar, A.; Shohet, R.V. HIF-1 regulation of miR-29c impairs SERCA2 expression and cardiac contractility. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H554–H565. [Google Scholar]
- Lyu, G.; Guan, Y.; Zhang, C.; Zong, L.; Sun, L.; Huang, X.; Huang, L.; Zhang, L.; Tian, X.-L.; Zhou, Z.; et al. TGF-β signaling alters H4K20me3 status via miR-29 and contributes to cellular senescence and cardiac aging. Nat. Commun. 2018, 9, 2560. [Google Scholar] [CrossRef]
- Brillante, S.; Volpe, M.; Indrieri, A. Advances in MicroRNA Therapeutics: From Preclinical to Clinical Studies. Hum. Gene Ther. 2024, 35, 628–648. [Google Scholar] [CrossRef]
- Rupaimoole, R.; Slack, F.J. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017, 16, 203–222. [Google Scholar] [CrossRef] [PubMed]
- What will it take to get miRNA therapies to market. Nat. Biotechnol. 2024, 42, 1623–1624. [CrossRef]
- Bonneau, E.; Neveu, B.; Kostantin, E.; Tsongalis, G.J.; De Guire, V. How close are miRNAs from clinical practice? A perspective on the diagnostic and therapeutic market. EJIFCC 2019, 30, 114–127. [Google Scholar]
- Agarwal, V.; Bell, G.W.; Nam, J.W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. eLife 2015, 4, e05005. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, X. miRDB: An online database for prediction of functional microRNA targets. Nucleic Acids Res. 2020, 48, D127–D131. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Stewart, S.A.; Dykxhoorn, D.M.; Palliser, D.; Mizuno, H.; Yu, E.Y.; An, D.S.; Sabatini, D.M.; Chen, I.S.; Hahn, W.C.; Sharp, P.A.; et al. Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA 2003, 9, 493–501. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Gao, W.; Li, F.; Bo, Y.; Zhu, M.; Fu, R.; Liu, Q.; Wen, S.; Wang, B. Identification and characterization of CD133+CD44+ cancer stem cells from human laryngeal squamous cell carcinoma cell lines. J. Cancer 2017, 8, 497–506. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, X.; Li, Y.; Goldschmidt-Clermont, P.J.; Dong, C. Aging in the atherosclerosis milieu may accelerate the consumption of bone marrow endothelial progenitor cells. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 113–119. [Google Scholar] [CrossRef]
- De Villiers, C.; Riley, P.R. Mouse models of myocardial infarction: Comparing permanent ligation and ischemia-reperfusion. Dis. Model. Mech. 2020, 13, dmm046565. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Yang, X.P.; Nass, O.; Sabbah, H.N.; Peterson, E.; Carretero, O.A. Chronic heart failure induced by coronary artery ligation in Lewis inbred rats. Am. J. Physiol. 1997, 272, H722–H727. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dykxhoorn, D.M.; Da Fonseca Ferreira, A.; Gomez, K.; Shi, J.; Zhu, S.; Zhang, L.; Wang, H.; Wei, J.; Zhang, Q.; Macon, C.J.; et al. MicroRNA-29c-3p and -126a Contribute to the Decreased Angiogenic Potential of Aging Endothelial Progenitor Cells. Int. J. Mol. Sci. 2025, 26, 4259. https://doi.org/10.3390/ijms26094259
Dykxhoorn DM, Da Fonseca Ferreira A, Gomez K, Shi J, Zhu S, Zhang L, Wang H, Wei J, Zhang Q, Macon CJ, et al. MicroRNA-29c-3p and -126a Contribute to the Decreased Angiogenic Potential of Aging Endothelial Progenitor Cells. International Journal of Molecular Sciences. 2025; 26(9):4259. https://doi.org/10.3390/ijms26094259
Chicago/Turabian StyleDykxhoorn, Derek M., Andrea Da Fonseca Ferreira, Karenn Gomez, Jianjun Shi, Shoukang Zhu, Lukun Zhang, Huilan Wang, Jianqin Wei, Qianhuan Zhang, Conrad J. Macon, and et al. 2025. "MicroRNA-29c-3p and -126a Contribute to the Decreased Angiogenic Potential of Aging Endothelial Progenitor Cells" International Journal of Molecular Sciences 26, no. 9: 4259. https://doi.org/10.3390/ijms26094259
APA StyleDykxhoorn, D. M., Da Fonseca Ferreira, A., Gomez, K., Shi, J., Zhu, S., Zhang, L., Wang, H., Wei, J., Zhang, Q., Macon, C. J., Hare, J. M., Marzouka, G. R., Wang, L., & Dong, C. (2025). MicroRNA-29c-3p and -126a Contribute to the Decreased Angiogenic Potential of Aging Endothelial Progenitor Cells. International Journal of Molecular Sciences, 26(9), 4259. https://doi.org/10.3390/ijms26094259




