The Potential Role of Exosomes in Communication Between Astrocytes and Endothelial Cells
Abstract
1. Introduction
1.1. The Blood–Brain Barrier Role in CNS
1.2. Importance of Endothelial Cells in the BBB
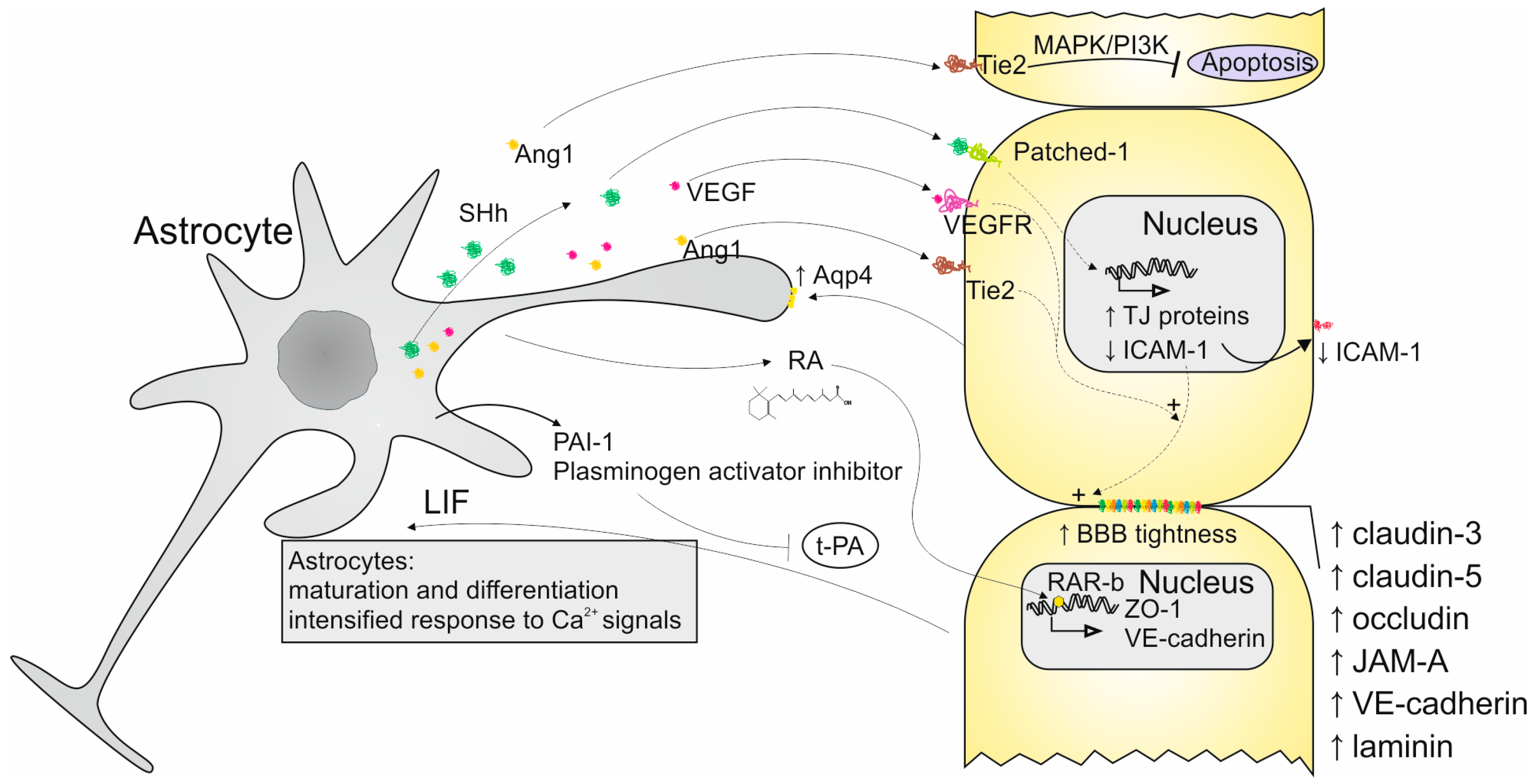
1.3. Importance of Astrocytes in the BBB
2. Interactions Between ECs and Astrocytes
3. Exosomes as the Strategy of Communication Between Astrocytes and ECs
3.1. Characteristics of Exosomes Secreted by Astrocytes
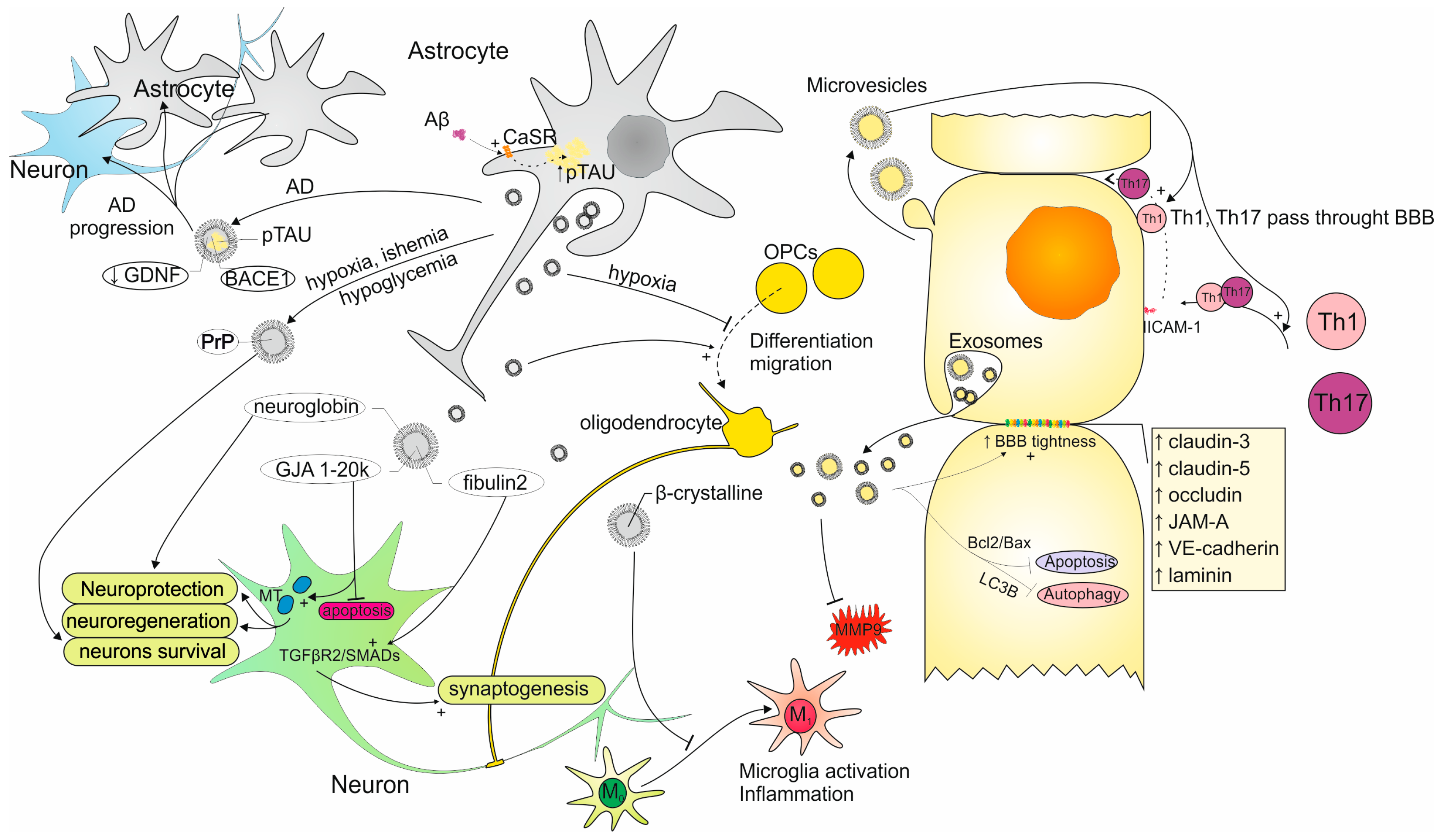
3.1.1. Astrocyte-Derived Exosomes Impact on CNS Cells
3.1.2. AS-Exos Role in Neuronal Protection and Regeneration
3.1.3. AS-Exos Importance in Neurodegenerative and Neuroinflammatory Diseases
3.1.4. Functions of miRNAs as the AS-Exos Cargo
3.2. Characteristics of Exosomes Secreted by ECs
3.2.1. Involvement of Exosomes Secreted by ECs in the BBB Regulation
3.2.2. EC-Exos Role in Neurodegenerative and Neuroinflammatory Conditions
3.2.3. Functions of miRNAs as the EC-Exos Cargo
4. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Kadry, H.; Noorani, B.; Cucullo, L. A Blood–Brain Barrier Overview on Structure, Function, Impairment, and Biomarkers of Integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.J.; Asselin, M.-C.; Hinz, R.; Parkes, L.M.; Allan, S.; Schiessl, I.; Boutin, H.; Dickie, B.R. In Vivo Methods for Imaging Blood–Brain Barrier Function and Dysfunction. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1051–1083. [Google Scholar] [CrossRef] [PubMed]
- Cash, A.; Theus, M.H. Mechanisms of Blood–Brain Barrier Dysfunction in Traumatic Brain Injury. Int. J. Mol. Sci. 2020, 21, 3344. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Blood-Brain Barrier Biology and Methodology. J. Neurovirol. 1999, 5, 556–569. [Google Scholar] [CrossRef]
- Yu, X.; Ji, C.; Shao, A. Neurovascular Unit Dysfunction and Neurodegenerative Disorders. Front. Neurosci. 2020, 14, 334. [Google Scholar] [CrossRef]
- Hladky, S.B.; Barrand, M.A. Fluid and Ion Transfer across the Blood–Brain and Blood–Cerebrospinal Fluid Barriers; a Comparative Account of Mechanisms and Roles. Fluids Barriers CNS 2016, 13, 19. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The Blood–Brain Barrier: Structure, Regulation and Drug Delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef]
- Gastfriend, B.D.; Palecek, S.P.; Shusta, E.V. Modeling the Blood–Brain Barrier: Beyond the Endothelial Cells. Curr. Opin. Biomed. Eng. 2018, 5, 6–12. [Google Scholar] [CrossRef]
- Langen, U.H.; Ayloo, S.; Gu, C. Development and Cell Biology of the Blood-Brain Barrier. Annu. Rev. Cell Dev. Biol. 2019, 35, 591–613. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Yang, J.; Ronaldson, P.T.; Davis, T.P. Structure, Function, and Regulation of the Blood-Brain Barrier Tight Junction in Central Nervous System Disorders. Front. Physiol. 2020, 11, 914. [Google Scholar] [CrossRef]
- Wibbe, N.; Ebnet, K. Cell Adhesion at the Tight Junctions: New Aspects and New Functions. Cells 2023, 12, 2701. [Google Scholar] [CrossRef]
- Luissint, A.-C.; Artus, C.; Glacial, F.; Ganeshamoorthy, K.; Couraud, P.-O. Tight Junctions at the Blood Brain Barrier: Physiological Architecture and Disease-Associated Dysregulation. Fluids Barriers CNS 2012, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- González-Mariscal, L.; Betanzos, A.; Ávila-Flores, A. MAGUK Proteins: Structure and Role in the Tight Junction. Semin. Cell Dev. Biol. 2000, 11, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Nilles, K.L.; Williams, E.I.; Betterton, R.D.; Davis, T.P.; Ronaldson, P.T. Blood–Brain Barrier Transporters: Opportunities for Therapeutic Development in Ischemic Stroke. Int. J. Mol. Sci. 2022, 23, 1898. [Google Scholar] [CrossRef]
- Williams, E.I.; Betterton, R.D.; Davis, T.P.; Ronaldson, P.T. Transporter-Mediated Delivery of Small Molecule Drugs to the Brain: A Critical Mechanism That Can Advance Therapeutic Development for Ischemic Stroke. Pharmaceutics 2020, 12, 154. [Google Scholar] [CrossRef]
- Chaves, J.C.S.; Dando, S.J.; White, A.R.; Oikari, L.E. Blood-Brain Barrier Transporters: An Overview of Function, Dysfunction in Alzheimer’s Disease and Strategies for Treatment. Biochim. et Biophys. Acta (BBA)-Mol. Basis Dis. 2024, 1870, 166967. [Google Scholar] [CrossRef]
- Brzica, H.; Abdullahi, W.; Ibbotson, K.; Ronaldson, P.T. Role of Transporters in Central Nervous System Drug Delivery and Blood-Brain Barrier Protection: Relevance to Treatment of Stroke. J. Cent. Nerv. Syst. Dis. 2017, 9, 117957351769380. [Google Scholar] [CrossRef]
- Aller, S.G.; Yu, J.; Ward, A.; Weng, Y.; Chittaboina, S.; Zhuo, R.; Harrell, P.M.; Trinh, Y.T.; Zhang, Q.; Urbatsch, I.L.; et al. Structure of P-Glycoprotein Reveals a Molecular Basis for Poly-Specific Drug Binding. Science 2009, 323, 1718–1722. [Google Scholar] [CrossRef]
- Mao, Q.; Unadkat, J.D. Role of the Breast Cancer Resistance Protein (BCRP/ABCG2) in Drug Transport—An Update. AAPS J. 2015, 17, 65–82. [Google Scholar] [CrossRef]
- Bakos, É.; Evers, R.; Szakács, G.; Tusnády, G.E.; Welker, E.; Szabó, K.; de Haas, M.; van Deemter, L.; Borst, P.; Váradi, A.; et al. Functional Multidrug Resistance Protein (MRP1) Lacking the N-Terminal Transmembrane Domain. J. Biol. Chem. 1998, 273, 32167–32175. [Google Scholar] [CrossRef]
- Wang, N.; Yvan-Charvet, L.; Lütjohann, D.; Mulder, M.; Vanmierlo, T.; Kim, T.; Tall, A.R. ATP-binding Cassette Transporters G1 and G4 Mediate Cholesterol and Desmosterol Efflux to HDL and Regulate Sterol Accumulation in the Brain. FASEB J. 2008, 22, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Galochkina, T.; Chong, M.N.F.; Challali, L.; Abbar, S.; Etchebest, C. New Insights into GluT1 Mechanics during Glucose Transfer. Sci. Rep. 2019, 9, 998. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.; Obaidat, A.; Hagenbuch, B. OATPs, OATs and OCTs: The Organic Anion and Cation Transporters of the SLCO and SLC22A Gene Superfamilies. Br. J. Pharmacol. 2012, 165, 1260–1287. [Google Scholar] [CrossRef] [PubMed]
- Duelli, R.; Enerson, B.E.; Gerhart, D.Z.; Drewes, L.R. Expression of Large Amino Acid Transporter LAT1 in Rat Brain Endothelium. J. Cereb. Blood Flow Metab. 2000, 20, 1557–1562. [Google Scholar] [CrossRef]
- Gasser, P.J.; Hurley, M.M.; Chan, J.; Pickel, V.M. Organic Cation Transporter 3 (OCT3) Is Localized to Intracellular and Surface Membranes in Select Glial and Neuronal Cells within the Basolateral Amygdaloid Complex of Both Rats and Mice. Brain Struct. Funct. 2017, 222, 1913–1928. [Google Scholar] [CrossRef]
- Zidarič, T.; Gradišnik, L.; Velnar, T. Astrocytes and Human Artificial Blood-Brain Barrier Models. Bosn. J. Basic. Med. Sci. 2022, 22, 651–672. [Google Scholar] [CrossRef]
- Cruz, J.V.R.; Batista, C.; Diniz, L.P.; Mendes, F.A. The Role of Astrocytes and Blood–Brain Barrier Disruption in Alzheimer’s Disease. Neuroglia 2023, 4, 209–221. [Google Scholar] [CrossRef]
- Cabezas, R.; Ávila, M.; Gonzalez, J.; El-Bachá, R.S.; Báez, E.; García-Segura, L.M.; Coronel, J.C.J.; Capani, F.; Cardona-Gomez, G.P.; Barreto, G.E. Astrocytic Modulation of Blood Brain Barrier: Perspectives on Parkinson’s Disease. Front. Cell Neurosci. 2014, 8, 211. [Google Scholar] [CrossRef]
- Attwell, D.; Buchan, A.M.; Charpak, S.; Lauritzen, M.; MacVicar, B.A.; Newman, E.A. Glial and Neuronal Control of Brain Blood Flow. Nature 2010, 468, 232–243. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The Blood–Brain Barrier. Cold Spring Harb Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Pannasch, U.; Derangeon, M.; Chever, O.; Rouach, N. Astroglial Gap Junctions Shape Neuronal Network Activity. Commun. Integr. Biol. 2012, 5, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Nakamura, Y.; Lo, E.H.; Hayakawa, K. Astrocyte Signaling in the Neurovascular Unit After Central Nervous System Injury. Int. J. Mol. Sci. 2019, 20, 282. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, S.F.; Bortolotto, V.; Canonico, P.L.; Sortino, M.A.; Grilli, M. Astrocyte-Derived Paracrine Signals: Relevance for Neurogenic Niche Regulation and Blood–Brain Barrier Integrity. Front. Pharmacol. 2019, 10, 1346. [Google Scholar] [CrossRef] [PubMed]
- Heithoff, B.P.; George, K.K.; Phares, A.N.; Zuidhoek, I.A.; Munoz-Ballester, C.; Robel, S. Astrocytes Are Necessary for Blood–Brain Barrier Maintenance in the Adult Mouse Brain. Glia 2021, 69, 436–472. [Google Scholar] [CrossRef]
- Jackson, R.J.; Meltzer, J.C.; Nguyen, H.; Commins, C.; Bennett, R.E.; Hudry, E.; Hyman, B.T. APOE4 Derived from Astrocytes Leads to Blood–Brain Barrier Impairment. Brain 2022, 145, 3582–3593. [Google Scholar] [CrossRef]
- Manu, D.R.; Slevin, M.; Barcutean, L.; Forro, T.; Boghitoiu, T.; Balasa, R. Astrocyte Involvement in Blood–Brain Barrier Function: A Critical Update Highlighting Novel, Complex, Neurovascular Interactions. Int. J. Mol. Sci. 2023, 24, 17146. [Google Scholar] [CrossRef]
- Pivoriūnas, A.; Verkhratsky, A. Astrocyte–Endotheliocyte Axis in the Regulation of the Blood–Brain Barrier. Neurochem. Res. 2021, 46, 2538–2550. [Google Scholar] [CrossRef]
- Michinaga, S.; Hishinuma, S.; Koyama, Y. Roles of Astrocytic Sonic Hedgehog Production and Its Signal for Regulation of the Blood-Brain Barrier Permeability. Vitam. Horm. 2024, 126, 97–111. [Google Scholar]
- Michinaga, S.; Koyama, Y. Dual Roles of Astrocyte-Derived Factors in Regulation of Blood-Brain Barrier Function after Brain Damage. Int. J. Mol. Sci. 2019, 20, 571. [Google Scholar] [CrossRef]
- Hultman, K.; Björklund, U.; Hansson, E.; Jern, C. Potentiating Effect of Endothelial Cells on Astrocytic Plasminogen Activator Inhibitor Type-1 Gene Expression in an in Vitro Model of the Blood–Brain Barrier. Neuroscience 2010, 166, 408–415. [Google Scholar] [CrossRef]
- Abbott, N.J. Astrocyte–Endothelial Interactions and Blood–Brain Barrier Permeability. J. Anat. 2002, 200, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Taylor, X.; Cisternas, P.; Jury, N.; Martinez, P.; Huang, X.; You, Y.; Redding-Ochoa, J.; Vidal, R.; Zhang, J.; Troncoso, J.; et al. Activated Endothelial Cells Induce a Distinct Type of Astrocytic Reactivity. Commun. Biol. 2022, 5, 282. [Google Scholar] [CrossRef] [PubMed]
- Schiera, G.; Di Liegro, C.M.; Schirò, G.; Sorbello, G.; Di Liegro, I. Involvement of Astrocytes in the Formation, Maintenance, and Function of the Blood–Brain Barrier. Cells 2024, 13, 150. [Google Scholar] [CrossRef] [PubMed]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The Exosome Journey: From Biogenesis to Uptake and Intracellular Signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef]
- Xu, M.; Ji, J.; Jin, D.; Wu, Y.; Wu, T.; Lin, R.; Zhu, S.; Jiang, F.; Ji, Y.; Bao, B.; et al. The Biogenesis and Secretion of Exosomes and Multivesicular Bodies (MVBs): Intercellular Shuttles and Implications in Human Diseases. Genes Dis. 2023, 10, 1894–1907. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Schmidt, O.; Teis, D. The ESCRT Machinery. Curr. Biol. 2012, 22, R116–R120. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.-H.; Kim, J.-H. A Comprehensive Review on Factors Influences Biogenesis, Functions, Therapeutic and Clinical Implications of Exosomes. Int. J. Nanomed. 2021, 16, 1281–1312. [Google Scholar] [CrossRef]
- Anthony, D.F.; Shiels, P.G. Exploiting Paracrine Mechanisms of Tissue Regeneration to Repair Damaged Organs. Transplant. Res. 2013, 2, 10. [Google Scholar] [CrossRef]
- Ai, Y.; Guo, C.; Garcia-Contreras, M.; Sánchez , B.L.S.; Saftics, A.; Shodubi, O.; Raghunandan, S.; Xu, J.; Tsai, S.J.; Dong, Y.; et al. Endocytosis Blocks the Vesicular Secretion of Exosome Marker Proteins. Sci. Adv. 2024, 10, eadi9156. [Google Scholar] [CrossRef] [PubMed]
- Sędzik, M.; Rakoczy, K.; Sleziak, J.; Kisiel, M.; Kraska, K.; Rubin, J.; Łuniewska, W.; Choromańska, A. Comparative Analysis of Exosomes and Extracellular Microvesicles in Healing Pathways: Insights for Advancing Regenerative Therapies. Molecules 2024, 29, 3681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, R.; Chen, Y.; Wang, M.; Du, J. Crosstalk between Oxidative Stress and Exosomes. Oxidative Med. Cell Longev. 2022, 2022, 3553617. [Google Scholar] [CrossRef]
- Caponnetto, F.; Manini, I.; Skrap, M.; Palmai-Pallag, T.; Di Loreto, C.; Beltrami, A.P.; Cesselli, D.; Ferrari, E. Size-Dependent Cellular Uptake of Exosomes. Nanomedicine 2017, 13, 1011–1020. [Google Scholar] [CrossRef]
- Wen, J.; Creaven, D.; Luan, X.; Wang, J. Comparison of Immunotherapy Mediated by Apoptotic Bodies, Microvesicles and Exosomes: Apoptotic Bodies’ Unique Anti-Inflammatory Potential. J. Transl. Med. 2023, 21, 478. [Google Scholar] [CrossRef]
- Rezaie, J.; Feghhi, M.; Etemadi, T. A Review on Exosomes Application in Clinical Trials: Perspective, Questions, and Challenges. Cell Commun. Signal. 2022, 20, 145. [Google Scholar] [CrossRef]
- Tan, F.; Li, X.; Wang, Z.; Li, J.; Shahzad, K.; Zheng, J. Clinical Applications of Stem Cell-Derived Exosomes. Signal Transduct. Target. Ther. 2024, 9, 17. [Google Scholar] [CrossRef]
- Chivet, M.; Hemming, F.; Pernet-Gallay, K.; Fraboulet, S.; Sadoul, R. Emerging Role of Neuronal Exosomes in the Central Nervous System. Front. Physiol. 2012, 3, 145. [Google Scholar] [CrossRef]
- Venturini, A.; Passalacqua, M.; Pelassa, S.; Pastorino, F.; Tedesco, M.; Cortese, K.; Gagliani, M.C.; Leo, G.; Maura, G.; Guidolin, D.; et al. Exosomes From Astrocyte Processes: Signaling to Neurons. Front. Pharmacol. 2019, 10, 1452. [Google Scholar] [CrossRef]
- Patel, M.R.; Weaver, A.M. Astrocyte-Derived Small Extracellular Vesicles Promote Synapse Formation via Fibulin-2-Mediated TGF-β Signaling. Cell Rep. 2021, 34, 108829. [Google Scholar] [CrossRef]
- Xu, Y.; Tian, Y.; Wang, Y.; Xu, L.; Song, G.; Wu, Q.; Wang, W.; Xie, M. Exosomes Derived from Astrocytes after Oxygen-Glucose Deprivation Promote Differentiation and Migration of Oligodendrocyte Precursor Cells in Vitro. Mol. Biol. Rep. 2021, 48, 5473–5484. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, H.; Zhang, P.; Gao, Y.; Ma, H.; Gao, T.; Liu, H.; Hua, W.; Zhang, L.; Zhang, X.; et al. The Functions of Exosomes Targeting Astrocytes and Astrocyte-Derived Exosomes Targeting Other Cell Types. Neural Regen. Res. 2024, 19, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zheng, P.; Hong, T.; Wang, Y.; Liu, N.; He, B.; Zou, S.; Ren, D.; Duan, J.; Zhao, L.; et al. Astrocytes-derived Exosomes Induce Neuronal Recovery after Traumatic Brain Injury via Delivering Gap Junction Alpha 1-20 k. J. Tissue Eng. Regen. Med. 2020, 14, 412–423. [Google Scholar] [CrossRef]
- Pei, X.; Li, Y.; Zhu, L.; Zhou, Z. Astrocyte-Derived Exosomes Suppress Autophagy and Ameliorate Neuronal Damage in Experimental Ischemic Stroke. Exp. Cell Res. 2019, 382, 111474. [Google Scholar] [CrossRef]
- Guitart, K.; Loers, G.; Buck, F.; Bork, U.; Schachner, M.; Kleene, R. Improvement of Neuronal Cell Survival by Astrocyte-derived Exosomes under Hypoxic and Ischemic Conditions Depends on Prion Protein. Glia 2016, 64, 896–910. [Google Scholar] [CrossRef]
- Zhao, S.; Sheng, S.; Wang, Y.; Ding, L.; Xu, X.; Xia, X.; Zheng, J.C. Astrocyte-Derived Extracellular Vesicles: A Double-Edged Sword in Central Nervous System Disorders. Neurosci. Biobehav. Rev. 2021, 125, 148–159. [Google Scholar] [CrossRef]
- Willis, C.M.; Ménoret, A.; Jellison, E.R.; Nicaise, A.M.; Vella, A.T.; Crocker, S.J. A Refined Bead-Free Method to Identify Astrocytic Exosomes in Primary Glial Cultures and Blood Plasma. Front. Neurosci. 2017, 11, 335. [Google Scholar] [CrossRef]
- Szpakowski, P.; Ksiazek-Winiarek, D.; Czpakowska, J.; Kaluza, M.; Milewska-Jedrzejczak, M.; Glabinski, A. Astrocyte-Derived Exosomes Differentially Shape T Cells’ Immune Response in MS Patients. Int. J. Mol. Sci. 2023, 24, 7470. [Google Scholar] [CrossRef]
- Winston, C.N.; Goetzl, E.J.; Schwartz, J.B.; Elahi, F.M.; Rissman, R.A. Complement Protein Levels in Plasma Astrocyte-derived Exosomes Are Abnormal in Conversion from Mild Cognitive Impairment to Alzheimer’s Disease Dementia. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2019, 11, 61–66. [Google Scholar] [CrossRef]
- Chiarini, A.; Armato, U.; Gardenal, E.; Gui, L.; Dal Prà, I. Amyloid β-Exposed Human Astrocytes Overproduce Phospho-Tau and Overrelease It within Exosomes, Effects Suppressed by Calcilytic NPS 2143—Further Implications for Alzheimer’s Therapy. Front. Neurosci. 2017, 11, 217. [Google Scholar] [CrossRef]
- Goetzl, E.J.; Mustapic, M.; Kapogiannis, D.; Eitan, E.; Lobach, I.V.; Goetzl, L.; Schwartz, J.B.; Miller, B.L. Cargo Proteins of Plasma Astrocyte-derived Exosomes in Alzheimer’s Disease. FASEB J. 2016, 30, 3853–3859. [Google Scholar] [CrossRef] [PubMed]
- Gayen, M.; Bhomia, M.; Balakathiresan, N.; Knollmann-Ritschel, B. Exosomal MicroRNAs Released by Activated Astrocytes as Potential Neuroinflammatory Biomarkers. Int. J. Mol. Sci. 2020, 21, 2312. [Google Scholar] [CrossRef] [PubMed]
- Lafourcade, C.; Ramírez, J.P.; Luarte, A.; Fernández, A.; Wyneken, U. MIRNAS in Astrocyte-Derived Exosomes as Possible Mediators of Neuronal Plasticity. J. Exp. Neurosci. 2016, 10 (Suppl. S1), 1–9. [Google Scholar] [CrossRef]
- Sun, R.; Liao, W.; Lang, T.; Qin, K.; Jiao, K.; Shao, L.; Deng, C.; She, Y. Astrocyte-Derived Exosomal MiR-378a-5p Mitigates Cerebral Ischemic Neuroinflammation by Modulating NLRP3-Mediated Pyroptosis. Front. Immunol. 2024, 15, 1454116. [Google Scholar] [CrossRef]
- Wu, W.; Liu, J.; Yang, C.; Xu, Z.; Huang, J.; Lin, J. Astrocyte-Derived Exosome-Transported MicroRNA-34c Is Neuroprotective against Cerebral Ischemia/Reperfusion Injury via TLR7 and the NF-ΚB/MAPK Pathways. Brain Res. Bull. 2020, 163, 84–94. [Google Scholar] [CrossRef]
- Du, L.; Jiang, Y.; Sun, Y. Astrocyte-Derived Exosomes Carry MicroRNA-17-5p to Protect Neonatal Rats from Hypoxic-Ischemic Brain Damage via Inhibiting BNIP-2 Expression. Neurotoxicology 2021, 83, 28–39. [Google Scholar] [CrossRef]
- Ding, W.; Gu, Q.; Liu, M.; Zou, J.; Sun, J.; Zhu, J. Astrocytes-Derived Exosomes Pre-Treated by Berberine Inhibit Neuroinflammation after Stroke via MiR-182-5p/Rac1 Pathway. Int. Immunopharmacol. 2023, 118, 110047. [Google Scholar] [CrossRef]
- Pei, X.; Li, Y.; Zhu, L.; Zhou, Z. Astrocyte-Derived Exosomes Transfer MiR-190b to Inhibit Oxygen and Glucose Deprivation-Induced Autophagy and Neuronal Apoptosis. Cell Cycle 2020, 19, 906–917. [Google Scholar] [CrossRef]
- Long, X.; Yao, X.; Jiang, Q.; Yang, Y.; He, X.; Tian, W.; Zhao, K.; Zhang, H. Astrocyte-Derived Exosomes Enriched with MiR-873a-5p Inhibit Neuroinflammation via Microglia Phenotype Modulation after Traumatic Brain Injury. J. Neuroinflammation 2020, 17, 89. [Google Scholar] [CrossRef]
- Hosseinkhani, B.; Duran, G.; Hoeks, C.; Hermans, D.; Schepers, M.; Baeten, P.; Poelmans, J.; Coenen, B.; Bekar, K.; Pintelon, I.; et al. Cerebral Microvascular Endothelial Cell-Derived Extracellular Vesicles Regulate Blood–Brain Barrier Function. Fluids Barriers CNS 2023, 20, 95. [Google Scholar] [CrossRef]
- Dozio, V.; Sanchez, J. Characterisation of Extracellular Vesicle-subsets Derived from Brain Endothelial Cells and Analysis of Their Protein Cargo Modulation after TNF Exposure. J. Extracell. Vesicles 2017, 6, 1302705. [Google Scholar] [CrossRef]
- Huang, L.-Y.; Song, J.-X.; Cai, H.; Wang, P.-P.; Yin, Q.-L.; Zhang, Y.-D.; Chen, J.; Li, M.; Song, J.-J.; Wang, Y.-L.; et al. Healthy Serum-Derived Exosomes Improve Neurological Outcomes and Protect Blood–Brain Barrier by Inhibiting Endothelial Cell Apoptosis and Reversing Autophagy-Mediated Tight Junction Protein Reduction in Rat Stroke Model. Front. Cell Neurosci. 2022, 16, 841544. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qin, Y.; Chopp, M.; Li, C.; Kemper, A.; Liu, X.; Wang, X.; Zhang, L.; Zhang, Z.G. Ischemic Cerebral Endothelial Cell–Derived Exosomes Promote Axonal Growth. Stroke 2020, 51, 3701–3712. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Zhou, S.; Sun, C.; Cheng, D.; Zhang, Y.; Li, X.; Zhang, L.; Zhao, J.; Xu, D.; Bai, Y. Brain Endothelial Cell-Derived Exosomes Induce Neuroplasticity in Rats with Ischemia/Reperfusion Injury. ACS Chem. Neurosci. 2020, 11, 2201–2213. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Cheng, T.; Lai, X. Mechanism of Ischemic Brain Injury Repair by Endothelial Progenitor Cell-derived Exosomes. Mol. Med. Rep. 2022, 26, 269. [Google Scholar] [CrossRef]
- Pan, J.; He, R.; Huo, Q.; Shi, Y.; Zhao, L. Brain Microvascular Endothelial Cell Derived Exosomes Potently Ameliorate Cognitive Dysfunction by Enhancing the Clearance of Aβ Through Up-Regulation of P-Gp in Mouse Model of AD. Neurochem. Res. 2020, 45, 2161–2172. [Google Scholar] [CrossRef]
- Yang, D.; Li, Z.; Gao, G.; Li, X.; Liao, Z.; Wang, Y.; Li, W.; Zhang, Y.; Liu, W. Combined Analysis of Surface Protein Profile and MicroRNA Expression Profile of Exosomes Derived from Brain Microvascular Endothelial Cells in Early Cerebral Ischemia. ACS Omega 2021, 6, 22410–22421. [Google Scholar] [CrossRef]
- Lee, E.C.; Choi, D.; Lee, D.-H.; Oh, J.S. Engineering Exosomes for CNS Disorders: Advances, Challenges, and Therapeutic Potential. Int. J. Mol. Sci. 2025, 26, 3137. [Google Scholar] [CrossRef]
- Malaguarnera, M.; Cabrera-Pastor, A. Emerging Role of Extracellular Vesicles as Biomarkers in Neurodegenerative Diseases and Their Clinical and Therapeutic Potential in Central Nervous System Pathologies. Int. J. Mol. Sci. 2024, 25, 10068. [Google Scholar] [CrossRef]

| miRNA | Potential Targets | Possible Effects | References |
|---|---|---|---|
| miR-520d-3p | BACE | β-amyloid protein regulation | [72] |
| miR-29a | APP | ||
| miR-let-7d miR-30d miR-31-3p miR-93-3p miR-145-5p | GFAP, aquaporin, vimentin, and amyloid precursor protein | Involvement in neurological disorders and brain trauma | [72] |
| miR-26a | CTDSP2 | Neurogenesis | |
| GSK-3β | Regeneration of axon | [73] | |
| PTEN | Intensification of neurite outgrowth | ||
| miR-378a-5p | NLRP3 | Reduction in pyroptosis and neuroinflammation | [74] |
| miR-34c | TLR7 | Reduced neuronal damage after ischemia/reperfusion | [75] |
| miRNA-17-5p | BNIP-2 | Protection of the brain from hypoxic–ischemic damage | [76] |
| miR-182-5p | Rac1 | Inhibition of neuroinflammation after ischemic stroke | [77] |
| miR-190b | Atg7 | Suppression of neuronal apoptosis and inhibition of autophagy caused by glucose deprivation | [78] |
| miR-873a-5p | ERK, NF-κB, and p65 | Enhancing microglia conversion into the M2 phenotype | [79] |
| miRNA | Potential Targets | Possible Effects | References |
|---|---|---|---|
| miR-27a miR-19a miR-195 miR-298 | NCAM1, SEMA6A, and SEMA7A PTEN RTN4 RHOG and RHOA | Inactivation of proteins suppressing axonal growth | [83] |
| miR-126-3p | PIK3R2 | Protection from the BBB damage | [84] |
| SDF-1 | Promotion of angiogenesis Enhancement of neurite outgrowth | ||
| miR-122-5p, miR-409-3p | Sbk1 and Syne2 | Cell proliferation and migration | [87] |
| miR-412-5p | Larp1 | Vasculature formation | |
| miR-379-5p | Krt26 | Regeneration of nerve fiber | |
| miR-494-3p | Hsf2 | Apoptosis | |
| miR-127-3p | Acat1 | Cellular adhesion |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czpakowska, J.; Głąbiński, A.; Szpakowski, P. The Potential Role of Exosomes in Communication Between Astrocytes and Endothelial Cells. Int. J. Mol. Sci. 2025, 26, 4676. https://doi.org/10.3390/ijms26104676
Czpakowska J, Głąbiński A, Szpakowski P. The Potential Role of Exosomes in Communication Between Astrocytes and Endothelial Cells. International Journal of Molecular Sciences. 2025; 26(10):4676. https://doi.org/10.3390/ijms26104676
Chicago/Turabian StyleCzpakowska, Joanna, Andrzej Głąbiński, and Piotr Szpakowski. 2025. "The Potential Role of Exosomes in Communication Between Astrocytes and Endothelial Cells" International Journal of Molecular Sciences 26, no. 10: 4676. https://doi.org/10.3390/ijms26104676
APA StyleCzpakowska, J., Głąbiński, A., & Szpakowski, P. (2025). The Potential Role of Exosomes in Communication Between Astrocytes and Endothelial Cells. International Journal of Molecular Sciences, 26(10), 4676. https://doi.org/10.3390/ijms26104676





