In Vivo Insights into the Role of Astragaloside IV in Preventing and Treating Civilization Diseases: A Comprehensive Review
Abstract
1. Introduction
2. Physicochemical Properties
3. Bioavailability
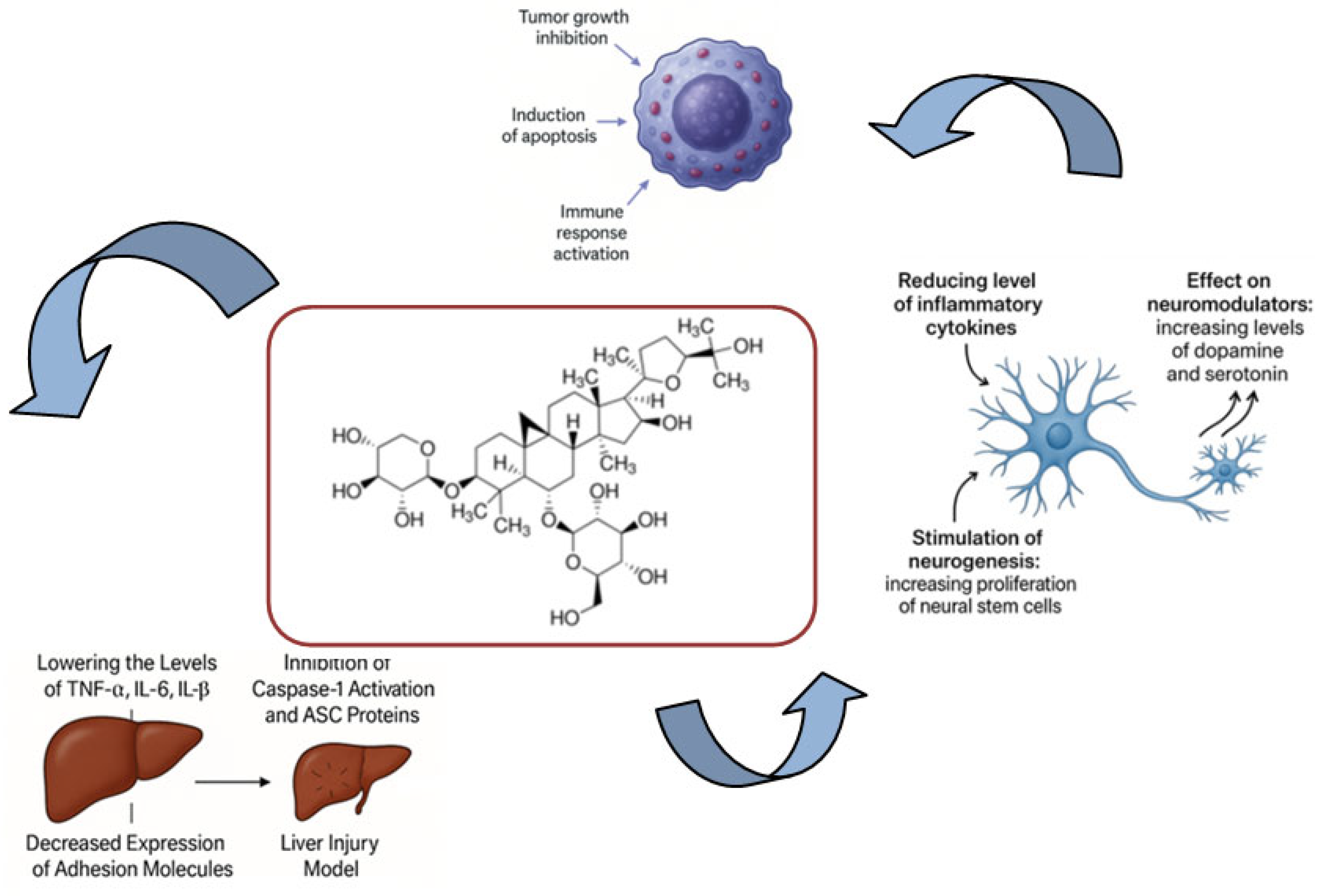
4. Anti-Inflammatory Potential
5. Neuroprotection and Cognition Enhancement
6. Anticarcinogenicity
| Type | Organism | Dose | Mechanism | References |
|---|---|---|---|---|
| Sanhuang decoction | Wistar male rats weighing 150–160 g, clean grade | 0.5 g/kg SASP enema (10 mL/kg b.w.) and 60 g/kg MSD enema (10 mL/kg b.w.) | The TNF-α, IL-1β and IL-6 levels in the MSD group significantly decreased | [101] |
| Sanhuang decoction | Female nude mice (6 weeks old, 18–22 g) | 4 g/kg | Positive role in inhibit ing the growth of MCF-7 cancer xenografts in vivo, the expression of IL-6 and TNF-α was observed to be obviously de- creased, the expression of VEGF, MMP-2, and MMP-9 was observed to be down-regulated in the Sanhuang decoction treatment group | [102] |
| ASIV | Male C57BL/6J mice (5 weeks old) | 40 mg/kg, 80 nM | Ameliorated cancer-associated inflammation, de- creased the expression of inflammatory factors such as TGF-β and IL-10, and suppressed M2 macro- phage polarization and de- creased the density of M2 macrophages | [95] |
| ASIV with saponins and cyclophosphamide | Male BALB/c nude mice (6weeks old, weighing 20 ± 2 g) | 20 mg/kg/day, 5 μM | AS and SRP improved the hematopoietic function and cured myelosuppression in CTX- induced myelosuppression mice | [96] |
| ASIV | Four-week-old BALB/c nude mice weighing 15–17 g | 25 mg/kg/d, 25 μM | Decreased growth of cervical cancer tumor | [97] |
7. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lacaille-Dubois, M.A.; Wagner, H. A review of the biological and pharmacological activities of saponins. Phytomedicine 1996, 2, 363–386. [Google Scholar] [CrossRef] [PubMed]
- Lasztity, R.; Hidvegi, M.; Bata, A. Saponins in food. Food Rev. Int. 1998, 14, 371–390. [Google Scholar] [CrossRef]
- Oleszek, W.A. Chromatographic determination of plant saponins. J. Chromatogr. A 2002, 967, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Hostettmann, K.; Marston, A. Saponins. Chemistry and Pharmacology of Natural Products; Cambridge University Press: Cambridge, UK, 2005; ISBN 10:0521020174. [Google Scholar]
- Vicken, J.-P.; Heng, L.; de Groot, A.; Gruppen, H. Saponins, classification and occurrence in the plant kingdom. Phytochemistry 2007, 68, 275–297. [Google Scholar] [CrossRef]
- Grenby, T.H. Intense sweeteners for the food industry: An overview. Trends Food Sci. Technol. 1991, 2, 2–6. [Google Scholar] [CrossRef]
- Kitagawa, I. Licorice root. A natural sweetener and an important ingredient in Chinese medicine. Pure Appl. Chem. 2002, 74, 1189–1198. [Google Scholar] [CrossRef]
- Heng, L.; Vincken, J.-P.; van Koningsveld, G.A.; Legger, A.; Gruppen, H.; van Boekel, T.; Roozen, J.; Voragen, F. Bitterness of saponins and their content in dry peas. J. Sci. Food Agric. 2006, 86, 1225–1231. [Google Scholar] [CrossRef]
- Price, K.R.; Johnson, I.T.; Fenwick, G.R. The chemistry and biological significance of saponins in foods and feedstuffs. Crit. Rev. Food Sci. Nutr. 1987, 26, 27–35. [Google Scholar] [CrossRef]
- Attele, A.S.; Wu, J.A.; Yuan, C.S. Ginseng pharmacology. Multiple constituents and multiple actions. Biochem. Pharmacol. 1999, 58, 1685–1693. [Google Scholar] [CrossRef]
- Oda, K.; Matsuda, H.; Murakami, T.; Katayama, S.; Ohgitani, T.; Yoshikawa, M. Adjuvant and haemolytic activities of 47 saponins derived from medicinal and food plants. Biol. Chem. 2000, 381, 67–74. [Google Scholar]
- Sparg, S.G.; Light, M.; Van Staden, J. Biological activities and distribution of plants saponins. J. Ethnopharmacol. 2004, 94, 219–243. [Google Scholar] [CrossRef] [PubMed]
- Abe, I.; Romer, M.; Prestwich, G.C. Enzymatic cyclization of squalene and oxidosqualene to sterols and triterpenes. Chem. Rev. 1993, 93, 2189–2206. [Google Scholar] [CrossRef]
- Dey, P.M.; Harborne, J.B. Methods in Plant Biochemistry; Academic Press: London, UK, 1997. [Google Scholar]
- European Pharmacopoeia (Ph. Eur.), 11.7th ed.; The Council of Europe: Strasbourg, France, 2023.
- Astragalus membranaceus. Monograph. Altern. Med. Rev. 2003, 8, 72–77. [Google Scholar]
- Kwon, H.J.; Hwang, J.; Lee, S.K.; Park, Y.D. Astragaloside content in the periderm, cortex, and xylem of Astragalus membranaceus root. J. Nat. Med. 2013, 67, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Kemper, K.J.; Small, R. Astragalus (Astragalus membranaceous) Longwood Herbal Task Force and The Center for Holistic Pediatric Education and Research. 1999, pp. 1–18. Available online: https://magistralbr.caldic.com/storage/product-files/527279452.PDF?utm_source=chatgpt.com (accessed on 29 April 2024).
- Yuan, F.; Yang, Y.; Liu, L.; Zhou, P.; Zhu, Y.; Chai, Y.; Chen, K.; Tang, W.; Huang, Q.; Zhang, C. Research progress on the mechanism of astragaloside IV in the treatment of asthma. Heliyon 2023, 9, 22149. [Google Scholar] [CrossRef]
- Chen, T.Q.; Yang, P.Y.; Jia, Y.J. Molecular mechanisms of astragaloside-IV in cancer therapy (Review). Int. J. Mol. Med. 2021, 47, 13. [Google Scholar] [CrossRef]
- Ren, S.; Zhang, H.; Mu, Y.; Sun, M.; Liu, P. Pharmacological effects of Astragaloside IV: A literature review. J. Tradit. Chin. Med. 2013, 33, 413–416. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, L.; Yang, Y.; Liu, Y. Cycloastragenol: An exciting novel candidate for 566 age-associated diseases. Exp. Ther. Med. 2018, 16, 2175–2182. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, C.; Gao, L.; Du, G.; Qin, X. Astragaloside IV derived from Astragalus membranaceus: A research review on the pharmacological effects. Adv. Pharm. 2020, 87, 89–112. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, H.; Zhang, Y.; Cheng, Z.; Wan, M.; Qin, W.; Li, P.; Feng, J.; Shao, S.; Xue, W.; et al. Recent pharmacological advances in the treatment of cardiovascular events with Astragaloside IV. Biomed. Pharmacother. 2023, 168, 115752. [Google Scholar] [CrossRef]
- Stępnik, K.; Kukula-Koch, W. In Silico Studies on Triterpenoid Saponins Permeation through the Blood-Brain Barrier Combined with Postmortem Research on the Brain Tissues of Mice Affected by Astragaloside IV Administration. Int. J. Mol. Sci. 2020, 21, 2534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhu, L.L.; Chen, G.G.; Du, Y. Pharmacokinetics of astragaloside iv in beagle dogs. Eur. J. Drug Metab. Pharmacokinet. 2007, 32, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.R.; Wang, G.J.; Wu, X.L.; Li, H.; Xie, H.T.; Lv, H.; Sun, J.G. Absorption enhancement study of astragaloside IV based on its transport mechanism in caco-2 cells. Eur. J. Drug Metab. Pharmacokinet. 2006, 31, 5–10. [Google Scholar] [CrossRef]
- Chang, Y.X.; Sun, Y.G.; Li, J.; Zhang, Q.-H.; Guo, X.-R.; Zhang, B.-L.; Jin, H.; Gao, X.-M. The experimental study of Astragalus membranaceus on meridian tropsim: The distribution study of astragaloside IV in rat tissues. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 911, 71–75. [Google Scholar] [CrossRef]
- Yu, S.; Peng, W.; Qiu, F.; Zhang, G. Research progress of astragaloside IV in the treatment of atopic diseases. Biomed. Pharmacother. 2022, 156, 113989. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, G.; Fawcett, J.P. Determination of Astragaloside IV in rat plasma by liquid chromatography electrospray ionization mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 801, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Yin, J.; Xie, L.; Zhang, J.; Zou, C.; Zou, J.; Liu, F.; Ju, W.; Li, P. Pharmacokinetics and tolerance of toalastragalosides after intravenous infusion of astragalosides injection in healthy Chinese volunteers. Phytomedicine 2013, 20, 1105–1111. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, C.; Liu, R.; Li, H.; Zhang, J.; Mao, C.; Chen, C. Quantitative determination of Astragaloside IV, a natural product with cardioprotective activity, in plasma, urine and other biological samples by HPLC coupled with tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2005, 822, 170–177. [Google Scholar] [CrossRef]
- Huang, C.; Wang, G.; Li, H.; Xie, H.; Sun, J.; Lv, H.; Lv, T. Sensitive and selective liquid chromatography-electrospray ionisation-mass spectrometry analysis of astragaloside-IV in rat plasma. J. Pharm. Biomed. Anal. 2006, 40, 788–793. [Google Scholar] [CrossRef]
- Zhang, W.D.; Zhang, C.; Liu, R.H.; Li, H.L.; Zhang, J.T.; Mao, C.; Moran, S.; Chen, C.L. Preclinical pharmacokinetics and tissue distribution of a natural cardioprotective agent astragaloside IV in rats and dogs. Life Sci. 2006, 79, 808–815. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, Q.; Chen, G.G.; Wei, P.; Tu, C.Y. Pharmacokinetics of Astragaloside IV in rats by liquid chromatography coupled with tandem mass spectrometry. Eur. J. Drug Metab. Pharm. 2005, 30, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.N.; Song, Y.L.; Ruan, J.Q.; Wang, Y.T.; Yan, R. Pharmacokinetic evidence on the contribution of intestinal bacterial conversion to beneficial effects of astragaloside IV, a marker compound of astragali radix, in traditional oral use of the herb. Drug Metab. Pharmacokinet. 2012, 27, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Guo, X.; Yuan, B.; Yu, W.; Suo, H.; Li, Z.; Xu, H. Disposition of Astragaloside IV via enterohepatic circulation is affected by the activity of the intestinal microbiome. J. Agric. Food Chem. 2015, 63, 6084–6093. [Google Scholar] [CrossRef]
- Qing, L.S.; Peng, S.L.; Liang, J.; Ding, L.S. Astragalosidic acid: A new water-soluble derivative of Astragaloside IV prepared using remarkably simple TEMPOmediated oxidation. Molecules 2017, 22, 1275. [Google Scholar] [CrossRef]
- Sun, W.X.; Zhang, Z.F.; Xie, J.; He, Y.; Cheng, Y.; Ding, L.S.; Luo, P.; Qing, L.S. Determination of a astragaloside IV derivative LS-102 in plasma by ultraperformance liquid chromatography-tandem mass spectrometry in dog plasma and its application in a pharmacokinetic study. Phytomedicine 2019, 53, 243–251. [Google Scholar] [CrossRef]
- Qing, L.-S.; Chen, T.-B.; Sun, W.-X.; Chen, L.; Luo, P.; Zhang, Z.-F.; Ding, L.-S. Ding, Pharmacokinetics comparison, intestinal absorption and acute toxicity assessment of a novel water-soluble Astragaloside IV derivative (Astragalosidic Acid, LS-102). Eur. J. Drug Metab. Pharmcokinet. 2019, 44, 251–259. [Google Scholar] [CrossRef]
- Gui, D.; Guo, Y.; Wang, F.; Liu, W.; Chen, J.; Chen, Y.; Huang, J.; Wang, N. Astragaloside IV, a novel antioxidant, prevents glucose-induced podocyte apoptosis in vitro and in vivo. PLoS ONE 2012, 7, 39824. [Google Scholar] [CrossRef]
- Yu, S.-Y.; Ouyang, H.T.; Yang, J.Y.; Huang, X.L.; Yang, T.; Duan, J.P.; Cheng, J.P.; Chen, Y.X.; Yang, Y.J.; Qiong, P. Subchronic toxicity studies of Radix Astragali extract in rats and dogs. J. Ethnopharmacol. 2007, 110, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Xuying, W.; Jiangbo, Z.; Yuping, Z.; Xili, M.; Yiwen, Z.; Tianbao, Z.; Weidong, Z. Effect of astragaloside IV on the general and peripartum reproductive toxicity in Sprague-Dawley rats. Int. J. Toxicol. 2010, 29, 505–516. [Google Scholar] [CrossRef]
- Jiangbo, Z.; Xuying, W.; Yuping, Z.; Xili, M.; Yiwen, Z.; Tianbao, Z. Effect of astragaloside IV on the embryo-fetal development of Sprague-Dawley rats and New Zealand White rabbits. J. Appl. Toxicol. 2009, 29, 381–385. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef]
- Stępnik, K.; Kukula-Koch, W.; Plazinski, W.; Gawel, K.; Gaweł-Bęben, K.; Khurelbat, D.; Boguszewska-Czubara, A. Significance of Astragaloside IV from the Roots of Astragalus mongholicus as an Acetylcholinesterase Inhibitor-From the Computational and Biomimetic Analyses to the In Vitro and In Vivo Studies of Safety. Int. J. Mol. Sci. 2023, 24, 9152. [Google Scholar] [CrossRef] [PubMed]
- Pehourcq, F.; Jarry, C.; Bannwarth, B. Potential of immobilized artificial membrane chromatography for lipophilicity determination of arylpropionic acid non-steroidal anti-inflammatory drugs. J. Pharm. Biomed. Anal. 2003, 33, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Barbato, F.; La Rotonda, M.I.; Quaglia, F. Interactions of Nonsteroidal Antiinflammatory Drugs with Phospholipids: Comparison between Octanol/Buffer Partition Coefficients and Chromatographic Indexes on Immobilized Artificial Membranes. J. Pharm. Sci. 1997, 86, 225–229. [Google Scholar] [CrossRef]
- Kaliszan, R.; Kaliszan, A.; Wainer, I.W. Deactivated hydrocarbonaceous silica and immobilized artificial membrane stationary phases in high-performance liquid chromatographic determination of hydrophobicities of organic bases: Relationship to log P and CLOGP. J. Pharm. Biomed. Anal. 1993, 11, 505–511. [Google Scholar] [CrossRef]
- Flieger, J.; Pizon, M.; Plech, T. Chromatographic behavior of new antiepileptic active compounds on different reversed-phase materials. J. Chromatogr. A 2014, 1338, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Sztanke, M.; Rzymowska, J.; Janicka, M.; Sztanke, K. Synthesis, structure elucidation, determination of antiproliferative activities, lipophilicity indices and pharmacokinetic properties of novel fused azaisocytosine-like congeners. Arab. J. Chem. 2019, 12, 4044–4064. [Google Scholar] [CrossRef]
- Janicka, M.; Sztanke, M.; Sztanke, K. Predicting the Blood-Brain Barrier Permeability of New Drug-like Compounds via HPLC with Various Stationary Phases. Molecules 2020, 25, 487. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Feng, X.; Xu, Y.; Zhou, L.; Wang, C.; Wang, M. Astragaloside IV Attenuates Fatty Acid-Induced Renal Tubular Injury in Diabetic Kidney Disease by Inhibiting Fatty Acid Transport Protein-2. Phytomedicine 2024, 134, 155991. [Google Scholar] [CrossRef]
- Zha, C.; Qi, Y.; Xing, F.; Li, J. Astragaloside IV Inhibits the Pyroptosis in the Acute Kidney Injury through Targeting the SIRT1/FOXO3a Axis. Chem. Pharm. Bull. 2024, 72, 923–931. [Google Scholar] [CrossRef]
- Guo, J.; Le, Y.; Yuan, A.; Liu, J.; Chen, H.; Qiu, J.; Wang, C.; Dou, X.; Yuan, X.; Lu, D. Astragaloside IV Ameliorates Cisplatin-Induced Liver Injury by Modulating Ferroptosis-Dependent Pathways. J. Ethnopharmacol. 2024, 328, 118080. [Google Scholar] [CrossRef]
- Tian, L.; Zhao, J.L.; Kang, J.Q.; Guo, S.B.; Zhang, N.; Shang, L.; Zhang, Y.L.; Zhang, J.; Jiang, X.; Lin, Y. Astragaloside IV Alleviates the Experimental DSS-Induced Colitis by Remodeling Macrophage Polarization Through STAT Signaling. Front. Immunol. 2021, 12, 740565. [Google Scholar] [CrossRef]
- Fu, X.; Sun, Z.; Long, Q.; Tan, W.; Ding, H.; Liu, X.; Wu, L.; Wang, Y.; Zhang, W. Glycosides from BuyangHuanwu Decoction Inhibit Atherosclerotic Inflammation via JAK/STAT Signaling Pathway. Phytomedicine 2022, 105, 154385. [Google Scholar] [CrossRef]
- Leng, B.; Li, C.; Sun, Y.; Zhao, K.; Zhang, L.; Lu, M.-L.; Wang, H.-X. Protective Effect of Astragaloside IV on High Glucose-Induced Endothelial Dysfunction via Inhibition of P2X7R Dependent P38 MAPK Signaling Pathway. Oxid. Med. Cell Longev. 2020, 2020, 5070415. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, O.; Zhang, W.; Liu, L.; Xu, C. Astragaloside IV Exerts Anti-Inflammatory Role in Endometriosis by Downregulating TLR4/NF-κB Pathway. Trop. J. Pharm. Res. 2019, 18, 539–545. [Google Scholar] [CrossRef]
- Leng, B.; Zhang, Y.; Liu, X.; Zhang, Z.; Liu, Y.; Wang, H.; Lu, M. Astragaloside IV Suppresses High Glucose-Induced NLRP3 Inflammasome Activation by Inhibiting TLR4/NF-κB and CaSR. Mediat. Inflamm. 2019, 2019, 1082497. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xiao, W.; Pei, C.; Wang, M.; Wang, X.; Huang, D.; Wang, F.; Wang, Z. Astragaloside IV Alleviates PM2.5-Induced Lung Injury in Rats by Modulating TLR4/MyD88/NF-κB Signalling Pathway. Int. Immunopharmacol. 2021, 91, 107290. [Google Scholar] [CrossRef]
- Liu, T.; Ai, L.; Jiang, A.; Wang, Y.; Jiang, R.; Liu, L. Astragaloside IV Suppresses the Proliferation and Inflammatory Response of Human Epidermal Keratinocytes and Ameliorates Imiquimod-Induced Psoriasis-like Skin Damage in Mice. Allergol. Immunopathol. 2024, 52, 44–50. [Google Scholar] [CrossRef]
- Zhai, P.; Chen, Q.; Wang, X.; Ouyang, X.; Yang, M.; Dong, Y.; Li, J.; Li, Y.; Luo, S.; Liu, Y.; et al. The Combination of Tanshinone IIA and Astragaloside IV Attenuates Myocardial Ischemia-Reperfusion Injury by Inhibiting the STING Pathway. Chin. Med. 2024, 19, 34. [Google Scholar] [CrossRef]
- Yue, Y.; Liu, X.; Pang, L.; Liu, Y.; Lin, Y.; Xiang, T.; Li, J.; Liao, S.; Jiang, Y. Astragalus Polysaccharides/PVA Nanofiber Membranes Containing Astragaloside IV-Loaded Liposomes and Their Potential Use for Wound Healing. Evid. Based Complement. Altern. Med. 2022, 2022, 9716271. [Google Scholar] [CrossRef]
- Koch, W. Dietary Polyphenols—Important Non-Nutrients in the Prevention of Chronic Noncommunicable Diseases. A Systematic Review. Nutrients 2019, 11, 1039. [Google Scholar] [CrossRef]
- Yao, M.; Zhang, L.; Wang, L. Astragaloside IV: A promising natural neuroprotective agent for neurological disorders. Biomed. Pharmacother. 2023, 159, 114229. [Google Scholar] [CrossRef] [PubMed]
- Costa, I.M.; Lima, F.O.V.; Fernandes, L.C.B.; Norrara, B.; Neta, F.I.; Alves, R.D.; Cavalcanti, J.R.; Lucena, E.E.; Cavalcante, J.S.; Rego, A.C.; et al. Astragaloside IV Supplementation Promotes A Neuroprotective Effect in Experimental Models of Neurological Disorders: A Systematic Review. Curr. Neuropharmacol. 2019, 17, 648–665. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chen, T.; Wang, R. Astragaloside IV ameliorates cognitive impairment and protects oligodendrocytes from antioxidative stress via regulation of the SIRT1/Nrf2 signaling pathway. Neurochem. Int. 2023, 167, 105535. [Google Scholar] [CrossRef]
- Kalaria, R.N.; Maestre, G.E.; Arizaga, R.; Friedland, R.P.; Galasko, D.; Hall, K.; A Luchsinger, J.; Ogunniyi, A.; Perry, E.K.; Potocnik, F.; et al. Alzheimer’s disease and vascular dementia in developing countries: Prevalence, management, and risk factors. Lancet Neurol. 2008, 7, 812–826. [Google Scholar] [CrossRef]
- Roh, J.H.; Lee, J.H. Recent updates on subcortical ischemic vascular dementia. J. Stroke 2014, 16, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Nogueiras, R.; Habegger, K.M.; Chaudhary, N.; Finan, B.; Banks, A.S.; Dietrich, M.O.; Horvath, T.L.; Sinclair, D.A.; Pfluger, P.T.; Tschöop, M.H. Sirtuin 1 and sirtuin 3: Physiological modulators of metabolism. Physiol. Rev. 2012, 92, 1479–1514. [Google Scholar] [CrossRef]
- Hwang, J.W.; Yao, H.; Caito, S.; Sundar, I.K.; Rahman, I. Redox regulation of SIRT1 in inflammation and cellular senescence. Free Radic. Biol. Med. 2013, 61, 95–110. [Google Scholar] [CrossRef]
- Chang, H.C.; Guarente, L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol. Metabol. 2014, 25, 138–145. [Google Scholar] [CrossRef]
- Peng, D.; Qiao, H.Z.; Tan, H.Y.; Wang, Y.-X.; Luo, D.; Qiao, L.-J.; Cai, Y.-F.; Zhang, S.-J.; Wang, Q.; Guan, L. Ligustilide ameliorates cognitive impairment via AMPK/SIRT1 pathway in vascular dementia rat. Metab. Brain Dis. 2022, 37, 1401–1414. [Google Scholar] [CrossRef]
- Peng, D.; Wang, Y.X.; Huang, T.H.; Luo, D.; Qiao, L.-J.; Wang, Q.; Guan, L.; Cai, Y.-F.; Zhang, S.-J. Ligustilide improves cognitive impairment via regulating the SIRT1/IRE1alpha/XBP1s/CHOP pathway in vascular dementia rats. Oxid. Med. Cell Longev. 2022, 2022, 6664990. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Deng, C.; Lv, J.; Fan, C.; Hu, W.; Di, S.; Yan, X.; Ma, Z.; Liang, Z.; Yang, Y. Nrf2 weaves an elaborate network of neuroprotection against stroke. Mol. Neurobiol. 2017, 54, 1440–1455. [Google Scholar] [CrossRef]
- Liao, S.; Wu, J.; Liu, R.; Wang, S.; Luo, J.; Yang, Y.; Qin, Y.; Li, T.; Zheng, X.; Song, J.; et al. A novel compound DBZ ameliorates neuroinflammation in LPS-stimulated microglia and ischemic stroke rats: Role of Akt (Ser473)/GSK3beta(Ser9)-mediated Nrf2 activation. Redox Biol. 2020, 36, 101644. [Google Scholar] [CrossRef]
- Arioz, B.I.; Tastan, B.; Tarakcioglu, E.; Tufekci, K.U.; Olcum, M.; Ersoy, N.; Bagriyanik, A.; Genc, K.; Genc, S. Melatonin attenuates LPS-induced acute depressive-like behaviors and microglial NLRP3 inflammasome activation through the SIRT1/Nrf2 pathway. Front. Immunol. 2019, 10, 1511. [Google Scholar] [CrossRef]
- He, L.L.; Wang, Y.C.; Ai, Y.T.; Wang, L.; Gu, S.M.; Wang, P.; Long, Q.H.; Hu, H. Qiangji decoction alleviates neurodegenerative changes and hippocampal neuron apoptosis induced by Dgalactose via regulating AMPK/SIRT1/NF-kappaB signaling pathway. Front. Pharmacol. 2021, 12, 735812. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Chen, X.; Zaeem, M.; Zhang, W.; Song, L.; Chen, L.; Mubwandarikwa, J.; Chen, X.; Xiao, J.; Xie, L.; et al. Sesamol attenuates neuroinflammation by regulating the AMPK/SIRT1/NF-kappaB signaling pathway after spinal cord injury in mice. Oxid. Med. Cell Longev. 2022, 2022, 8010670. [Google Scholar] [CrossRef]
- Song, B.; Zhou, W. Amarogentin has protective effects against sepsis-induced brain injury via modulating the AMPK/SIRT1/NF-kappaB pathway. Brain Res. Bull. 2022, 189, 44–56. [Google Scholar] [CrossRef]
- Liu, G.; Song, J.; Guo, Y.; Wang, T.; Zhou, Z. Astragalus injection protects cerebral ischemic injury by inhibiting neuronal apoptosis and the expression of JNK3 after cerebral ischemia reperfusion in rats. Behav. Brain Funct. 2013, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Wan-Yu, L.; Chung-Hsiang, L.; Chao-Hsiang, C.; Ching-Liang, H. Proteomics analysis of protein biomarkers in Astragalus membranaceus- and Astragaloside IV-treated brain tissues in ischemia-reperfusion injured rats. J. Tradit. Complement. Med. 2021, 11, 369–374. [Google Scholar]
- Liu, X.; Ding, Y.; Jiang, C.; Xin, Y.; Ma, X.; Xu, M.; Wang, Q.; Hou, B.; Li, Y.; Zhang, S.; et al. Astragaloside IV Mediates Radiation-Induced Neuronal Damage through Activation of BDNF-TrkB Signaling. Phytomedicine 2024, 132, 155803. [Google Scholar] [CrossRef]
- Chen, X.; Wu, H.; Chen, H.; Wang, Q.; Xie, X.-J.; Shen, J. Astragaloside VI Promotes Neural Stem Cell Proliferation and Enhances Neurological Function Recovery in Transient Cerebral Ischemic Injury via Activating EGFR/MAPK Signaling Cascades. Mol. Neurobiol. 2019, 56, 3053–3067. [Google Scholar] [CrossRef]
- Wang, L.; Liu, C.; Wang, L.; Tang, B. Astragaloside IV Mitigates Cerebral Ischaemia-Reperfusion Injury via Inhibition of P62/Keap1/Nrf2 Pathway-Mediated Ferroptosis. Eur. J. Pharmacol. 2023, 944, 175516. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Li, J.; Qiao, R.; Luo, J.; Liu, Y. Tetramethylpyrazine and Astragaloside IV Have Synergistic Effects against Spinal Cord Injury-Induced Neuropathic Pain via the OIP5-AS1/miR-34a/Sirt1/NF-κB Axis. Int. Immunopharmacol. 2023, 115, 109546. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Yang, D.; Cheng, X.-Y.; Yang, H.; Yang, X.-H.; Liu, H.-T.; Wang, R.; Zheng, P.; Yao, Y.; Li, J. Astragaloside IV Ameliorates Cognitive Impairment and Neuroinflammation in an Oligomeric Aβ Induced Alzheimer’s Disease Mouse Model via Inhibition of Microglial Activation and NADPH Oxidase Expression. Biol. Pharm. Bull. 2021, 44, 1688–1696. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.-L.; Xie, X.-H.; Ding, J.-H.; Du, R.-H.; Hu, G. Astragaloside IV Inhibits Astrocyte Senescence: Implication in Parkinson’s Disease. J. Neuroinflamm. 2020, 17, 105. [Google Scholar] [CrossRef]
- Tohda, C.; Tamura, T.; Matsuyama, S.; Komatsu, K. Promotion of axonal maturation and prevention of memory loss in mice by extracts of Astragalus mongholicus. Br. J. Pharmacol. 2006, 149, 532–541. [Google Scholar] [CrossRef]
- Stępnik, K.; Kukula-Koch, W.; Boguszewska-Czubara, A.; Gawel, K. Astragaloside IV as a Memory-Enhancing Agent: In Silico Studies with In Vivo Analysis and Post Mortem ADME-Tox Profiling in Mice. Int. J. Mol. Sci. 2024, 25, 4021. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, L.; Wang, J.; Xu, X.; Ni, S.; Liu, M.; Hu, K. Nose to Brain Delivery of Astragaloside IV by β-Asarone Modified Chitosan Nanoparticles for Multiple Sclerosis Therapy. Int. J. Pharm. 2023, 644, 123351. [Google Scholar] [CrossRef]
- Chen, X.; Shen, J.; Zhou, Q.; Jin, X.; Liu, H.; Gao, R. Astragaloside VI Ameliorates Post-Stroke Depression via Upregulating the NRG-1-Mediated MEK/ERK Pathway. Pharmaceuticals 2022, 15, 1551. [Google Scholar] [CrossRef]
- Li, F.; Cao, K.; Wang, M.; Liu, Y.; Zhang, Y. Astragaloside IV exhibits anti-tumor function in gastric cancer via targeting circRNA dihydrolipoamide S-succinyltransferase (circDLST)/miR-489-3p/eukaryotic translation initiation factor 4A1(EIF4A1) pathway. Bioengineered 2022, 13, 10111–10122. [Google Scholar] [CrossRef]
- Xu, F.; Cui, W.Q.; Wei, Y.; Cui, J.; Qiu, J.; Hu, L.L.; Gong, W.Y.; Dong, J.C.; Liu, B.J. Astragaloside IV inhibits lung cancer progression and metastasis by modulating macrophage polarization through AMPK signaling. J. Exp. Clin. Cancer Res. 2018, 37, 207. [Google Scholar] [CrossRef]
- Gu, X.; Zhu, L.Y.; Xu, Z.Y.; Shen, K.P. Astragaloside IV and Saponins of RhizomaPolygonati Cure Cyclophosphamide-Induced Myelosuppression in Lung Adenocarcinoma via Down-Regulating miR-142-3p. Front. Oncol. 2021, 11, 630921. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; He, Z.; Cai, Y. Quantitative proteomics analysis of differentially expressed proteins induced by astragaloside IV in cervical cancer cell invasion. Cell Mol. Biol. Lett. 2020, 25, 25. [Google Scholar] [CrossRef] [PubMed]
- Qiu, T.; Li, D.; Liu, Y.; Ren, H.; Yang, X.; Luo, W. Astragaloside IV Inhibits the Proliferation of Human Uterine Leiomyomas by Targeting IDO1. Cancers 2022, 14, 4424. [Google Scholar] [CrossRef]
- Liu, F.; Ran, F.; He, H.; Chen, L. Astragaloside IV Exerts Anti-tumor Effect on Murine Colorectal Cancer by Re-educating Tumor-Associated Macrophage. Arch. Immunol. Ther. Exp. 2020, 68, 33. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, Y.; Wu, T.; Li, Y.; Wang, Q. Astragaloside IV Attenuates Programmed Death-Ligand 1-Mediated Immunosuppression during Liver Cancer Development via the miR-135b-5p/CNDP1 Axis. Cancers 2023, 15, 5048. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, T.; Zhai, J.P.; Wang, L.H.; Chen, J. Effects of modified Sanhuang decoction enema on serum tumor necrosis factor-α and colonic mucosa interleukin-1β, interleukin-6 levels in ulcerative colitis rats. Chin. J. Integr. Med. 2014, 20, 865–869. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Yao, C.; Bian, W.H.; Chen, X.; Xue, J.X.; Zhu, Z.Y.; Ying, Y.; Xu, Y.L.; Wang, C. Effects of Astragaloside IV on treatment of breast cancer cells execute possibly through regulation of Nrf2 via PI3K/AKT/mTOR signaling pathway. Food Sci. Nutr. 2019, 7, 3403–3413. [Google Scholar] [CrossRef]

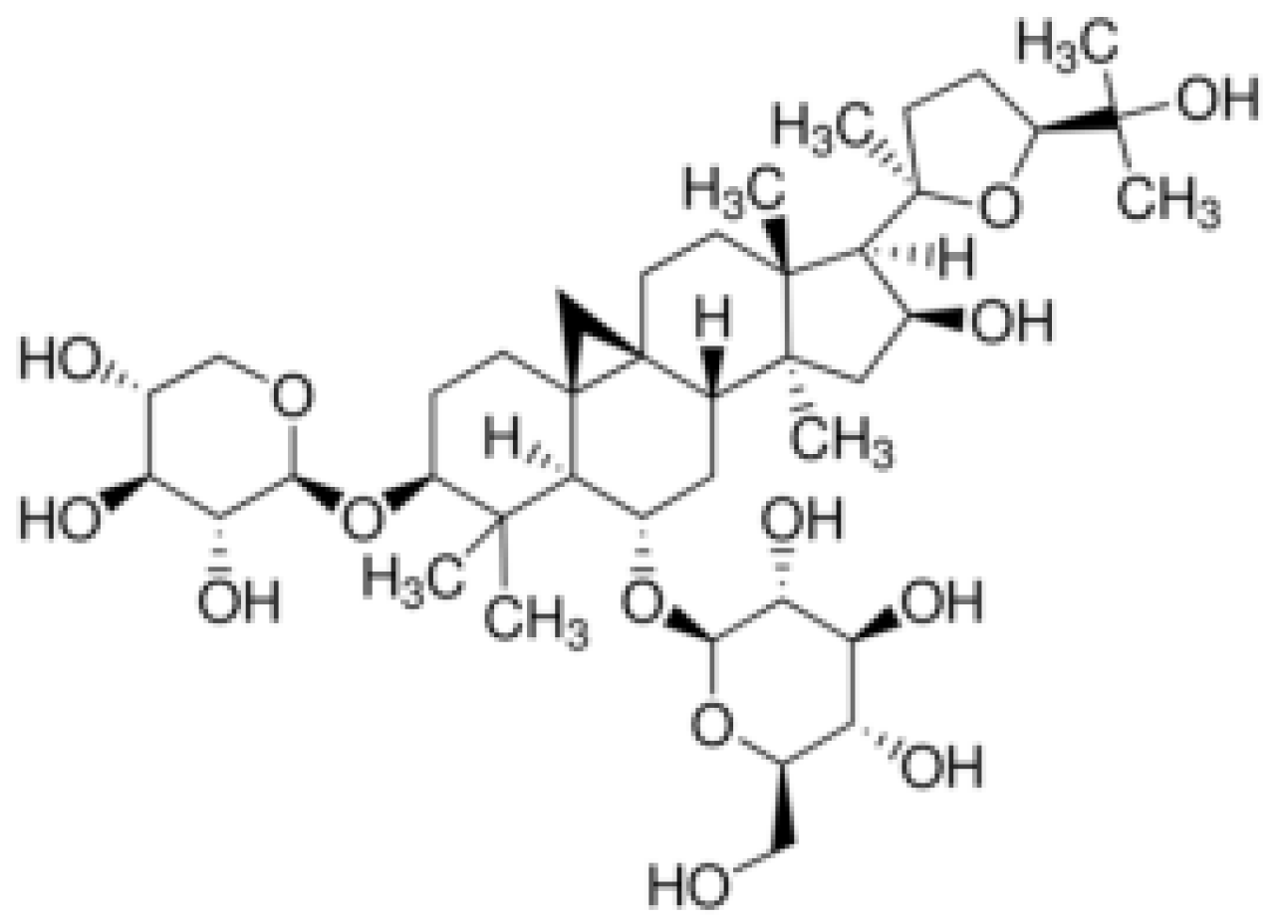
| Name of Property | Description/Value |
|---|---|
| Molecular weight | 784.87 g/mol |
| TPSA | 228.22 Å2 |
| LogPow | 3.757 |
| LogPcw | −9.015 |
| LogPhw | −9.3 |
| Physical Description | A crystalline solid |
| Color | White (yellow) crystallinepowder |
| Flash Point | 495.5 °C |
| Melting Point | 295–296 °C |
| Boiling Point | 895.7 °C |
| Density | 1.39 g/cm3 |
| Solubility | DMF: 20 mg/mL DMSO: 30 mg/mL DMSO:PBS (pH = 7.2) (1:1 v/v): 0.5 mg/mL |
| Organ | Mean Concentration [ng/mL] | Standard Deviation [ng/mL] |
|---|---|---|
| Liver | 916 | 506 |
| Kidneys | 587 | 301 |
| Lungs | 463 | 494 |
| Spleen | 216 | 114 |
| Heart | 90.9 | 45.7 |
| Dose [mL] | T1/2 [h] | AUC [μgh/mL] | Cmax [g/mL] |
|---|---|---|---|
| 200 | 2.14 | 4.38 | 2.12 |
| 300 | 2.59 | 9.75 | 3.59 |
| 400 | 2.62 | 13.59 | 3.71 |
| 500 | 2.69 | 18.22 | 5.17 |
| Animals | Cl [mg/kg/min] | Fl [%] | AUC [mgh/mL] | Cmax [mg/mL] | EBA [%] |
|---|---|---|---|---|---|
| Rats | 3.00 | 5.43 | 289.16 | 3.78 | 2.20 |
| Dogs | 4.00 | 12.90 | 156.04 | 4.39 | 7.40 |
| Drug | Model | Animals | Dose | Effect | References |
|---|---|---|---|---|---|
| ASIV | Diabetic kidney disease | 6-week-old rats | 10 or 20 mg/kg b.w./day for 8 weeks | ↓ blood glucose level ↓ ratio of urinary albumin to creatinine ↓ disorder of lipid metabolism ↓ injury in diabetic kidneys ↓ proteinuria | [53] |
| ASIV | Acute kidney injury | 4-week-old SpragueDawley (SD) male rats | 5 or 10 mg/kg b.w.p.o. ASIV | no inflammatory infiltration ↓ necrosis of epithelial cells ↓ BUN, SCR levels ↓ IL-18, IL-1beta, GSDMD-N and cleaved-caspase-1 levels | [54] |
| ASIV | Cisplatin-induced liver injury | Mice | 40 or 80 mg/kg b.w. | Significant improvement in inflammatory and oxidative stress conditions; inhibition of ferroptosis | [55] |
| ASIV | Psoriasis model of skin lesions and inflammation | ↓ IL-6 ↓ TNF-alpha | [62] | ||
| Glucosides (ASIV, paeoniflorin, amygdalin) and BuyangHuanwu Decoction | Atherosclerotic inflammation | 6–8-week-old ApoE−/− and C57BL/6J mice | 4 weeks, 2.772 g/kg/day of BYHWD, 0.167 or 0.084 g/kg/day of glucosides | ↓ inflammatory response ↓ protein expression of JAK2, STAT1, STAT3, VCAM-1, ICMA-1proteins, IL-6, and TNF-alpha in aorta wall ↓ TC, TG, LCLC-c ↑ HDL | [57] |
| ASIV | Lung injury model | Male SpragueDawley rats | 50 or 100 mg/kg b.w. ASIV | ↓ lung injury ↓ lung dry–wet ratio ↓ IL-6, TNF-alpha, CRP ↓ oxidative response in lung tissue Impact on TLR4/MyD88/NF-κB pathway | [61] |
| ASIV and Astragalus polysaccharides-loaded nanofibers | Diabetic rat wound inflammation model | Four groups of rats weighing 180–220 g | PVA nanofibers with ASIV and polysaccharides from Astragalus for 15 days | ↓ wound area ↑ tissue proliferation No infection ↑ cell adhesion ↑ cell migration ↓ neutrophils ↓ inflammation ↑ collagen fibers | [64] |
| ASIV | High glucose-induced endothelial dysfunction model | Sprague Dawley rats | 40 or 80 mg/kg/day of ASIV for 8 weeks | ↑ endothelial relaxation ↑ eNOS ↑ NO ↓ inflammation and oxidative stress in diabetic model | [58] |
| ASIV | Endometriosis | 6-week-old female mice | 0,5, 10 or 30 mg/kg b.w./day for 5 weeks | ↓ inflammation ↓ TLR4/NF-ĸBsignaling ↓ expression ofTNF-alpha, Ccl-2, IL-1beta and IL-6 | [59] |
| ASIV with tanshinone IIA | Myocardial ischemia (30 min) and infarction | 8–9-week-old male C57BL/6 mice | i.p. injections of 15 mg/kg/day ASIV, 10 mg/kg/day Ta-IIA, or in combination: ASIV (15 mg/kg) + Ta-IIA (10 mg/kg) or ASIV (10 mg/kg) + Ta-IIA (5 mg/kg) or Ta-IIA (15 mg/kg) + ASIV (20 mg/kg) | ↓ mRNA expression of IL-6, IL-1beta, iNOS, TNF-alpha ↑ SOD and GSH levels | [63] |
| Drug | Model | Animals | Dose | Effect | References |
|---|---|---|---|---|---|
| ASIV | Radiation-induced neuronal damage | Mice Thy1-YFP line H | 40 mg/kg b.w./day ASIV, i.p. for 4 weeks | ↑ BDNF-TrkB signaling ↑ (Ngf, Bdnf, Gap-43, Ras, Psd-95, Arc, Creb, c-Fos) genes, PSD-95 and F-actin | [84] |
| Memory impairment model with scopolamine | Swiss-type mice | 25 mg/kg b.w.i.p. ASIV | ↑ memory impairments ↑ phosphatidylcholine level | [91] | |
| Cerebral ischemic injury model | SpragueDawley rats 260–280 g | 2 µg/kg/day ASIV i.v. for 7 days | ↑ EGRF/MAPK cascades ↑ astrogenic and neuronal formation | [85] | |
| Post-stroke depression model | Male Sprague Dawley rats 200–240 g | 2 μg/kg ASIV i.v. for 7 days | ↑ NRG-1-Mediated MEK/ERK Pathway ↑ dopamine ↑ serotonin | [93] | |
| Cerebral-ischaemia reperfusion injury model | Male Sprague Dawley rats 5–7 week-old 200–220 g | 28 mg/kg ASIV i.g. | ↑ P62/Keap1/Nrf2 pathway ↓ ferroptosis, cerebral reperfusion injury ↓ MCAO/R-induced brain damage ↑ Nrf2 protein, p62 ↓Keap1 | [86] | |
| Spinal cord injury model | Sprague Dawley rats 5–8 weeks old, 18–220 g | 20 mg/kg b.w./day, i.p. | ↑ OIP5-AS1 ↑ Sirt1 ↓ neuropathic pain ↓ miR-34a | [87] | |
| Oligomeric Aβ-induced Alzheimer’s Disease mouse model | Male ICR mice 22–25 g | 20, 40, 80 mg/kg b.w./day i.g. ASIV | ↓ IL-1beta, IL-6, TNF-alpha, ROS ↓ microglial activation, ↓ NADPHoxidase protein expression ↓ neuronal damage | [88] | |
| Model of Parkinson’s disease | Mice | 20 mg/kg ASIV | ↓ astrocytes senescence ↑ dopamine neurons ↓ ROS, damaged mitochondria in substantia nigra | [89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stępnik, K.; Jarząb, A.; Niedźwiadek, R.; Głowniak-Lipa, A.; Głowniak, K.; Kukula-Koch, W. In Vivo Insights into the Role of Astragaloside IV in Preventing and Treating Civilization Diseases: A Comprehensive Review. Int. J. Mol. Sci. 2025, 26, 4250. https://doi.org/10.3390/ijms26094250
Stępnik K, Jarząb A, Niedźwiadek R, Głowniak-Lipa A, Głowniak K, Kukula-Koch W. In Vivo Insights into the Role of Astragaloside IV in Preventing and Treating Civilization Diseases: A Comprehensive Review. International Journal of Molecular Sciences. 2025; 26(9):4250. https://doi.org/10.3390/ijms26094250
Chicago/Turabian StyleStępnik, Katarzyna, Agata Jarząb, Rafał Niedźwiadek, Anna Głowniak-Lipa, Kazimierz Głowniak, and Wirginia Kukula-Koch. 2025. "In Vivo Insights into the Role of Astragaloside IV in Preventing and Treating Civilization Diseases: A Comprehensive Review" International Journal of Molecular Sciences 26, no. 9: 4250. https://doi.org/10.3390/ijms26094250
APA StyleStępnik, K., Jarząb, A., Niedźwiadek, R., Głowniak-Lipa, A., Głowniak, K., & Kukula-Koch, W. (2025). In Vivo Insights into the Role of Astragaloside IV in Preventing and Treating Civilization Diseases: A Comprehensive Review. International Journal of Molecular Sciences, 26(9), 4250. https://doi.org/10.3390/ijms26094250


_Kim.png)




