Long-Term Real-World Effectiveness of Dupilumab vs. Upadacitinib in Early Treatment Responders with Atopic Dermatitis: Results from Central European Health Fund Registry
Abstract
1. Introduction
2. Results
2.1. Demographics and Patient Baseline Characteristics
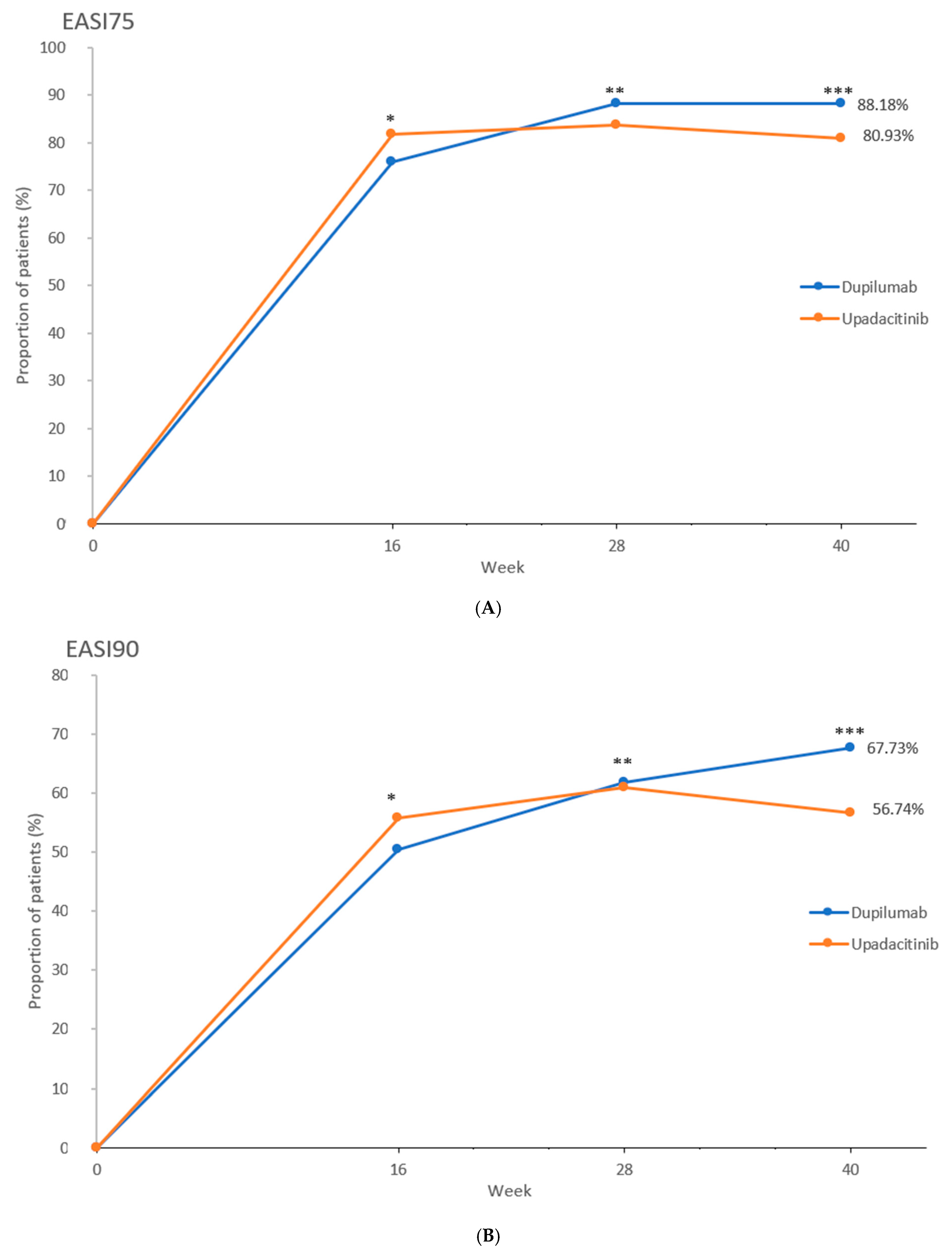
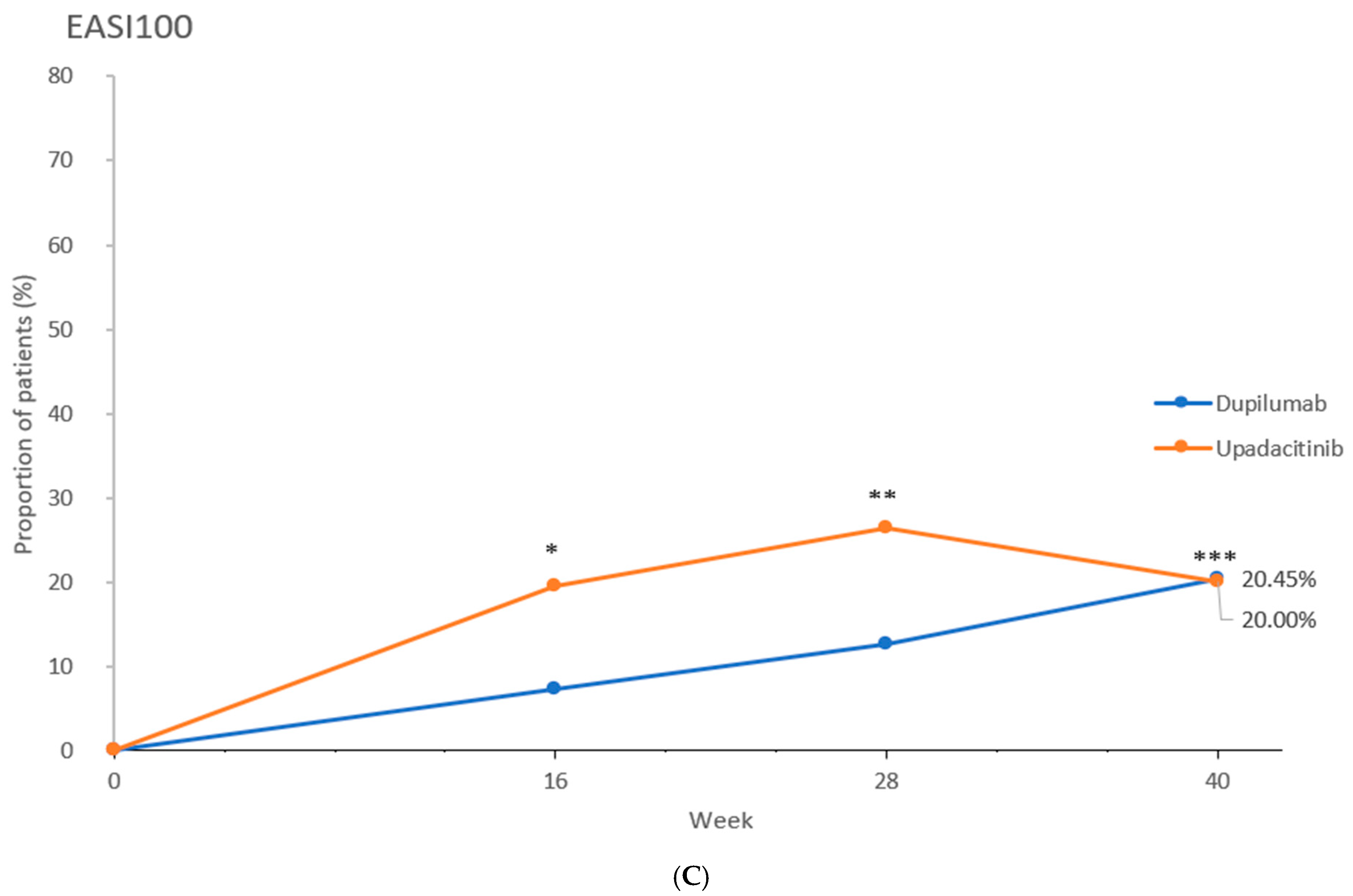
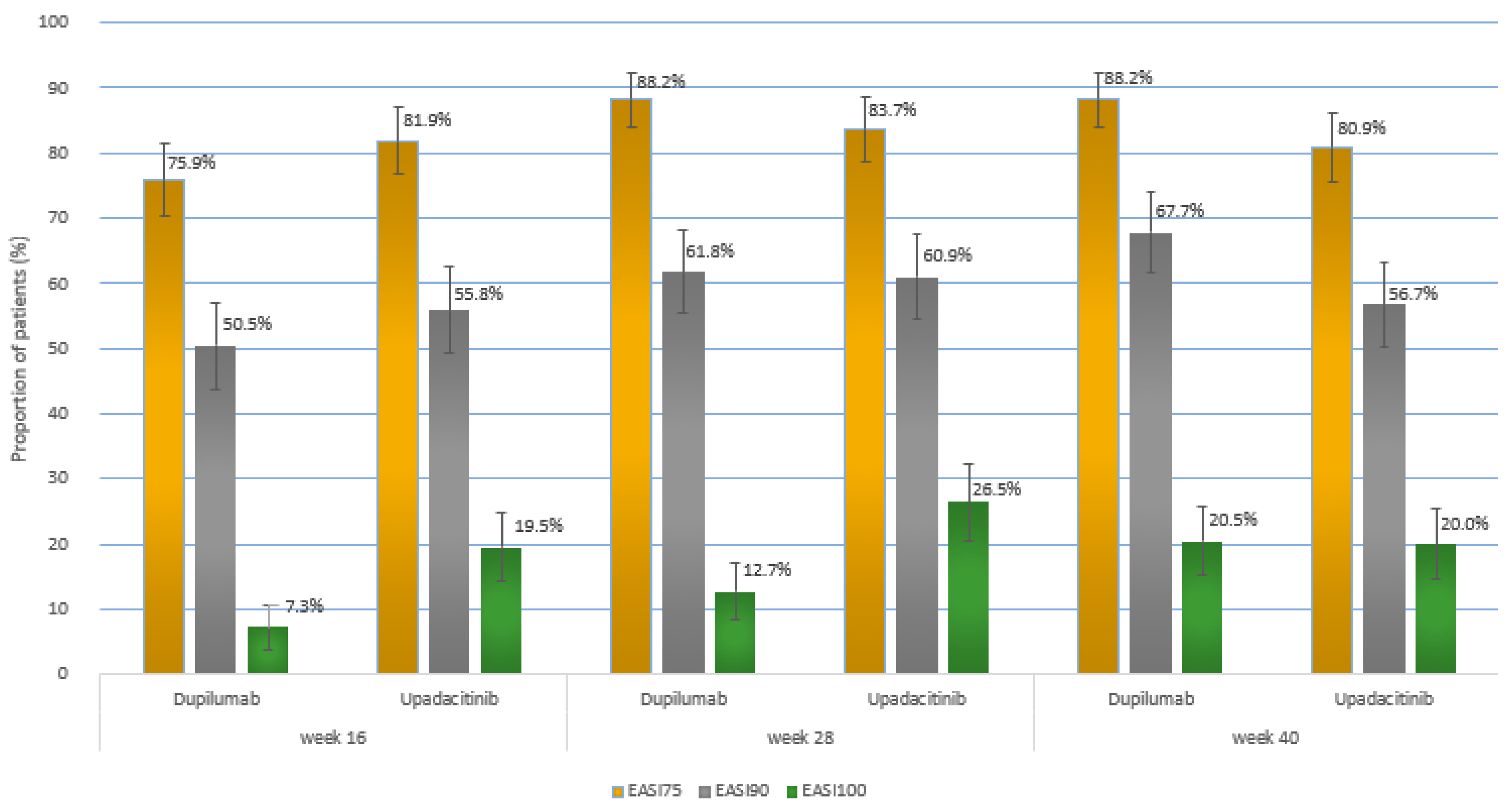
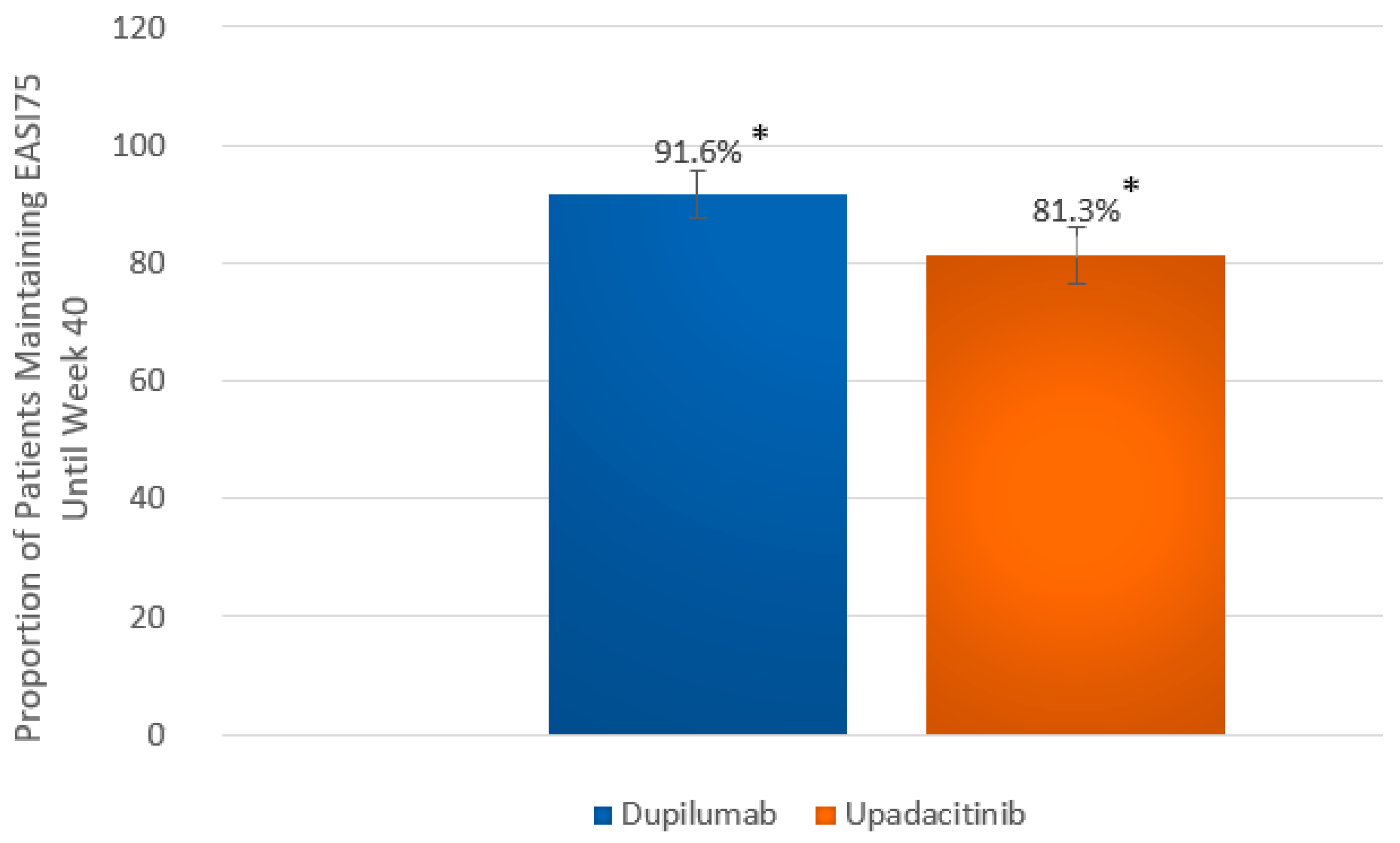
2.2. Efficacy Outcomes
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Patients
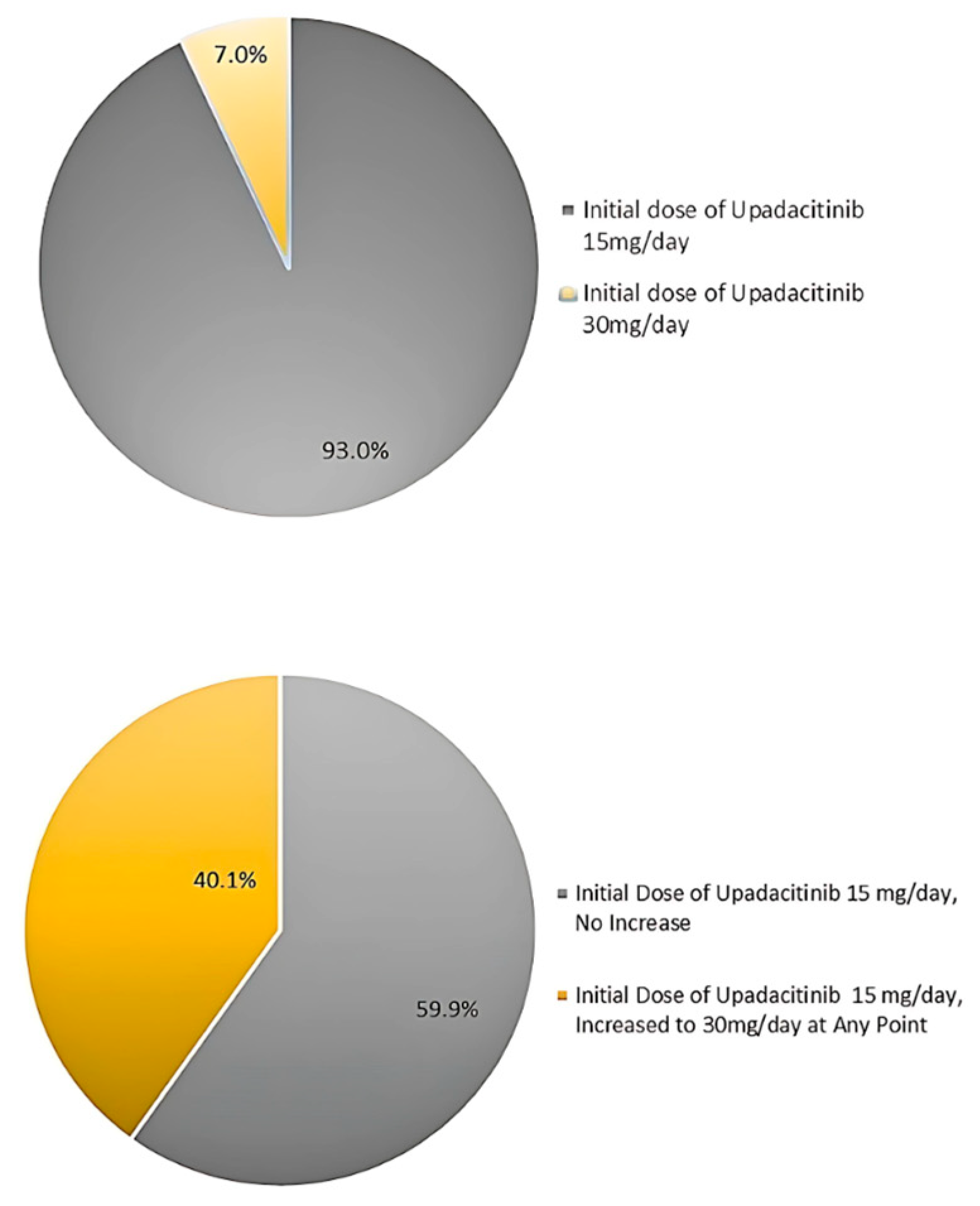
4.3. Treatment Dosage and Concomitant Medications
4.4. Efficacy Parameters
4.5. Statistical Analysis
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Atopic Dermatitis |
| CDLQI | Children’s Dermatology Life Quality Index |
| CI | confidence interval |
| DLQI | Dermatology Life Quality Index |
| DUPI | dupilumab |
| EASI | Eczema Area and Severity Index |
| EASI75 | at least 75% improvement in the Eczema Area and Severity Index |
| EASI90 | at least 90% improvement in the Eczema Area and Severity Index |
| EASI100 | 100% improvement in the Eczema Area and Severity Index |
| IFN-γ | interferon gamma |
| IL-4 | interleukin 4 |
| IL-13 | interleukin 13 |
| IL-22 | interleukin 22 |
| IL-31 | interleukin 31 |
| JAK-STAT | Janus kinase–signal transducer and activator of transcription signaling pathway |
| q2w | every two weeks |
| SD | standard deviation |
| SmPC | Summary of Product Characteristics |
| TSLP | thymic stromal lymphopoietin |
| UPA | upadacitinib |
| wk | week |
| wks | weeks |
References
- Deckers, I.A.G.; McLean, S.; Linssen, S.; Mommers, M.; van Schayck, C.P.; Sheikh, A. Investigating International Time Trends in the Incidence and Prevalence of Atopic Eczema 1990–2010: A Systematic Review of Epidemiological Studies. PLoS ONE 2012, 7, e39803. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zhang, D.; Yang, Y.; Huang, Y.; Wang, L.; Yao, X.; Lu, Q. Global Epidemiology of Atopic Dermatitis: A Comprehensive Systematic Analysis and Modelling Study. Br. J. Dermatol. 2023, 190, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Paternoster, L.; Standl, M.; Waage, J.; Baurecht, H.; Hotze, M.; Strachan, D.P.; Curtin, J.A.; Bønnelykke, K.; Tian, C.; Takahashi, A.; et al. Multi-Ancestry Genome-Wide Association Study of 21,000 Cases and 95,000 Controls Identifies New Risk Loci for Atopic Dermatitis. Nat. Genet. 2015, 47, 1449–1456. [Google Scholar] [CrossRef] [PubMed]
- Pinart, M.; Benet, M.; Annesi-Maesano, I.; von Berg, A.; Berdel, D.; Carlsen, K.C.L.; Carlsen, K.-H.; Bindslev-Jensen, C.; Eller, E.; Fantini, M.P.; et al. Comorbidity of Eczema, Rhinitis, and Asthma in IgE-Sensitised and Non-IgE-Sensitised Children in MeDALL: A Population-Based Cohort Study. Lancet Respir. Med. 2014, 2, 131–140. [Google Scholar] [CrossRef]
- Drislane, C.; Irvine, A.D. The Role of Filaggrin in Atopic Dermatitis and Allergic Disease. Ann. Allergy Asthma Immunol. 2020, 124, 36–43. [Google Scholar] [CrossRef]
- Weidinger, S.; Beck, L.A.; Bieber, T.; Kabashima, K.; Irvine, A.D. Atopic Dermatitis. Nat. Rev. Dis. Primers 2018, 4, 1. [Google Scholar] [CrossRef]
- Steinhoff, M.; Ahmad, F.; Pandey, A.; Datsi, A.; AlHammadi, A.; Al-Khawaga, S.; Al-Malki, A.; Meng, J.; Alam, M.; Buddenkotte, J. Neuroimmune Communication Regulating Pruritus in Atopic Dermatitis. J. Allergy Clin. Immunol. 2022, 149, 1875–1898. [Google Scholar] [CrossRef]
- Kelly-Welch, A.E.; Hanson, E.M.; Boothby, M.R.; Keegan, A.D. Interleukin-4 and Interleukin-13 Signaling Connections Maps. Science 2003, 300, 1527–1528. [Google Scholar] [CrossRef]
- Chiricozzi, A.; Maurelli, M.; Peris, K.; Girolomoni, G. Targeting IL-4 for the Treatment of Atopic Dermatitis. Immunotargets Ther. 2020, 9, 151–156. [Google Scholar] [CrossRef]
- Howell, M.D.; Kuo, F.I.; Smith, P.A. Targeting the Janus Kinase Family in Autoimmune Skin Diseases. Front. Immunol. 2019, 10, 2342. [Google Scholar] [CrossRef]
- He, H.; Guttman-Yassky, E. JAK Inhibitors for Atopic Dermatitis: An Update. Am. J. Clin. Dermatol. 2019, 20, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Harb, H.; Chatila, T.A. Mechanisms of Dupilumab. Clin. Exp. Allergy 2020, 50, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Thaçi, D.; Simpson, E.L.; Deleuran, M.; Kataoka, Y.; Chen, Z.; Gadkari, A.; Eckert, L.; Akinlade, B.; Graham, N.M.H.; Pirozzi, G.; et al. Efficacy and Safety of Dupilumab Monotherapy in Adults with Moderate-to-Severe Atopic Dermatitis: A Pooled Analysis of Two Phase 3 Randomized Trials (LIBERTY AD SOLO 1 and LIBERTY AD SOLO 2). J. Dermatol. Sci. 2019, 94, 266–275. [Google Scholar] [CrossRef]
- Blauvelt, A.; de Bruin-Weller, M.; Gooderham, M.; Cather, J.C.; Weisman, J.; Pariser, D.; Simpson, E.L.; Papp, K.A.; Hong, H.C.-H.; Rubel, D.; et al. Long-Term Management of Moderate-to-Severe Atopic Dermatitis with Dupilumab and Concomitant Topical Corticosteroids (LIBERTY AD CHRONOS): A 1-Year, Randomised, Double-Blinded, Placebo-Controlled, Phase 3 Trial. Lancet 2017, 389, 2287–2303. [Google Scholar] [CrossRef]
- Barbarot, S.; Wollenberg, A.; Silverberg, J.I.; Deleuran, M.; Pellacani, G.; Armario-Hita, J.C.; Chen, Z.; Shumel, B.; Eckert, L.; Gadkari, A.; et al. Dupilumab Provides Rapid and Sustained Improvement in SCORAD Outcomes in Adults with Moderate-to-Severe Atopic Dermatitis: Combined Results of Four Randomized Phase 3 Trials. J. Dermatol. Treat. 2022, 33, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.L.; Papp, K.A.; Blauvelt, A.; Chu, C.-Y.; Hong, H.C.; Katoh, N.; Calimlim, B.M.; Thyssen, J.P.; Chiou, A.S.; Bissonnette, R.; et al. Efficacy and Safety of Upadacitinib in Patients With Moderate to Severe Atopic Dermatitis. JAMA Dermatol. 2022, 158, 404. [Google Scholar] [CrossRef]
- Reich, K.; Teixeira, H.D.; de Bruin-Weller, M.; Bieber, T.; Soong, W.; Kabashima, K.; Werfel, T.; Zeng, J.; Huang, X.; Hu, X.; et al. Safety and Efficacy of Upadacitinib in Combination with Topical Corticosteroids in Adolescents and Adults with Moderate-to-Severe Atopic Dermatitis (AD Up): Results from a Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet 2021, 397, 2169–2181. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Teixeira, H.D.; Simpson, E.L.; Papp, K.A.; Pangan, A.L.; Blauvelt, A.; Thaçi, D.; Chu, C.-Y.; Hong, H.C.; Katoh, N.; et al. Once-Daily Upadacitinib versus Placebo in Adolescents and Adults with Moderate-to-Severe Atopic Dermatitis (Measure Up 1 and Measure Up 2): Results from Two Replicate Double-Blind, Randomised Controlled Phase 3 Trials. Lancet 2021, 397, 2151–2168. [Google Scholar] [CrossRef]
- Blauvelt, A.; Teixeira, H.D.; Simpson, E.L.; Costanzo, A.; De Bruin-Weller, M.; Barbarot, S.; Prajapati, V.H.; Lio, P.; Hu, X.; Wu, T.; et al. Efficacy and Safety of Upadacitinib vs Dupilumab in Adults With Moderate-to-Severe Atopic Dermatitis. JAMA Dermatol. 2021, 157, 1047. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Bunick, C.G.; Hong, H.C.; Mendes-Bastos, P.; Stein Gold, L.; Costanzo, A.; Ibrahim, N.; Sancho, C.; Wu, X.; Han, Y.; et al. Efficacy and Safety of Upadacitinib versus Dupilumab in Adults and Adolescents with Moderate-to-Severe Atopic Dermatitis: Week 16 Results of an Open-Label Randomized Efficacy Assessor-Blinded Head-to-Head Phase IIIb/IV Study (Level Up). Br. J. Dermatol. 2024, 192, 36–45. [Google Scholar] [CrossRef]
- Huang, D.; Lu, J.; Tan, F. Improvement of Pruritus and Efficacy in the Early Stage of Therapy with Upadacitinib, Abrocitinib, or Dupilumab in Chinese Patients with Moderate-to-Severe Atopic Dermatitis: Prospective, Cohort, Observational Study. Dermatitis 2024, 35, 77–83. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, J.; Xiong, S.; Zhang, J.; Ye, Q.; Xue, R.; Tian, X.; Zhong, J.; Zhu, H.; Gao, A.; et al. Comparative Effectiveness of Upadacitinib versus Dupilumab for Moderate-to-Severe Atopic Dermatitis: A Retrospective Cohort Study. Int. Immunopharmacol. 2024, 143, 113383. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, S.; König, A.; Gerger, V.; Rummenigge, C.; Müller, A.C.; Jung, T.; Frank, A.; Tassopoulos, G.; Laurent, E.; Kaufmann, R.; et al. Efficacy and Treatment Satisfaction of Different Systemic Therapies in Children and Adolescents with Moderate-to-Severe Atopic Dermatitis: A Real-World Study. J. Clin. Med. 2023, 12, 1175. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, J.I.; Lei, D.; Yousaf, M.; Janmohamed, S.R.; Vakharia, P.P.; Chopra, R.; Chavda, R.; Gabriel, S.; Patel, K.R.; Singam, V.; et al. What Are the Best Endpoints for Eczema Area and Severity Index and Scoring Atopic Dermatitis in Clinical Practice? A Prospective Observational Study*. Br. J. Dermatol. 2021, 184, 888–895. [Google Scholar] [CrossRef]
- Schmitt, J.; Csötönyi, F.; Bauer, A.; Meurer, M. Determinants of Treatment Goals and Satisfaction of Patients with Atopic Eczema. JDDG J. Dtsch. Dermatol. Ges. 2008, 6, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Augustin, M.; Gottlieb, A.B.; Lebwohl, M.; Pinter, A.; Warren, R.B.; Puig, L.; Warham, R.; Lambert, J.; Wiegratz, S.; Szilagyi, B.; et al. Complete Skin Clearance Is Associated with the Greatest Benefits to Health-Related Quality of Life and Perceived Symptoms for Patients with Psoriasis. Dermatol. Ther. 2024, 14, 2841–2857. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Armstrong, A.; Blauvelt, A.; Reich, K. Assessment of Efficacy and Safety Outcomes Beyond Week 16 in Clinical Trials of Systemic Agents Used for the Treatment of Moderate to Severe Atopic Dermatitis in Combination with Topical Corticosteroids. Am. J. Clin. Dermatol. 2023, 24, 913–925. [Google Scholar] [CrossRef]
- Silverberg, J.I.; de Bruin-Weller, M.; Bieber, T.; Soong, W.; Kabashima, K.; Costanzo, A.; Rosmarin, D.; Lynde, C.; Liu, J.; Gamelli, A.; et al. Upadacitinib plus Topical Corticosteroids in Atopic Dermatitis: Week 52 AD Up Study Results. J. Allergy Clin. Immunol. 2022, 149, 977–987.e14. [Google Scholar] [CrossRef]
- Merola, J.F.; Sidbury, R.; Wollenberg, A.; Chen, Z.; Zhang, A.; Shumel, B.; Rossi, A.B. Dupilumab Prevents Flares in Adults with Moderate to Severe Atopic Dermatitis in a 52-Week Randomized Controlled Phase 3 Trial. J. Am. Acad. Dermatol. 2021, 84, 495–497. [Google Scholar] [CrossRef]
| Characteristic | Patients, No. (%) Dupilumab, 300 mg q2w (Adults) Dupilumab 200/300 mg q2w (Adolescents; <60 kg or ≥60 kg, espectively) (n = 220) | Upadacitinib, 15 mg/30 mg Dose at Physician’s Discretion (n = 215) |
|---|---|---|
| Sex | ||
| Male | 118 (53.6) | 110 (51.2) |
| Female | 102 (46.4) | 105 (48.8) |
| Age, mean (SD) [range], yr | 29.3 (13.9) [12–75] | 28.2 (14.0) [12–75] |
| Age category | ||
| 12 to < 18 | 47 (21.4) | 59 (27.4) |
| 18 to < 40 | 119 (54.1) | 107 (49.8) |
| 40 to <65 | 50 (22.7) | 46 (21.4) |
| 65 to ≤75 | 4 (1.8) | 3 (1.4) |
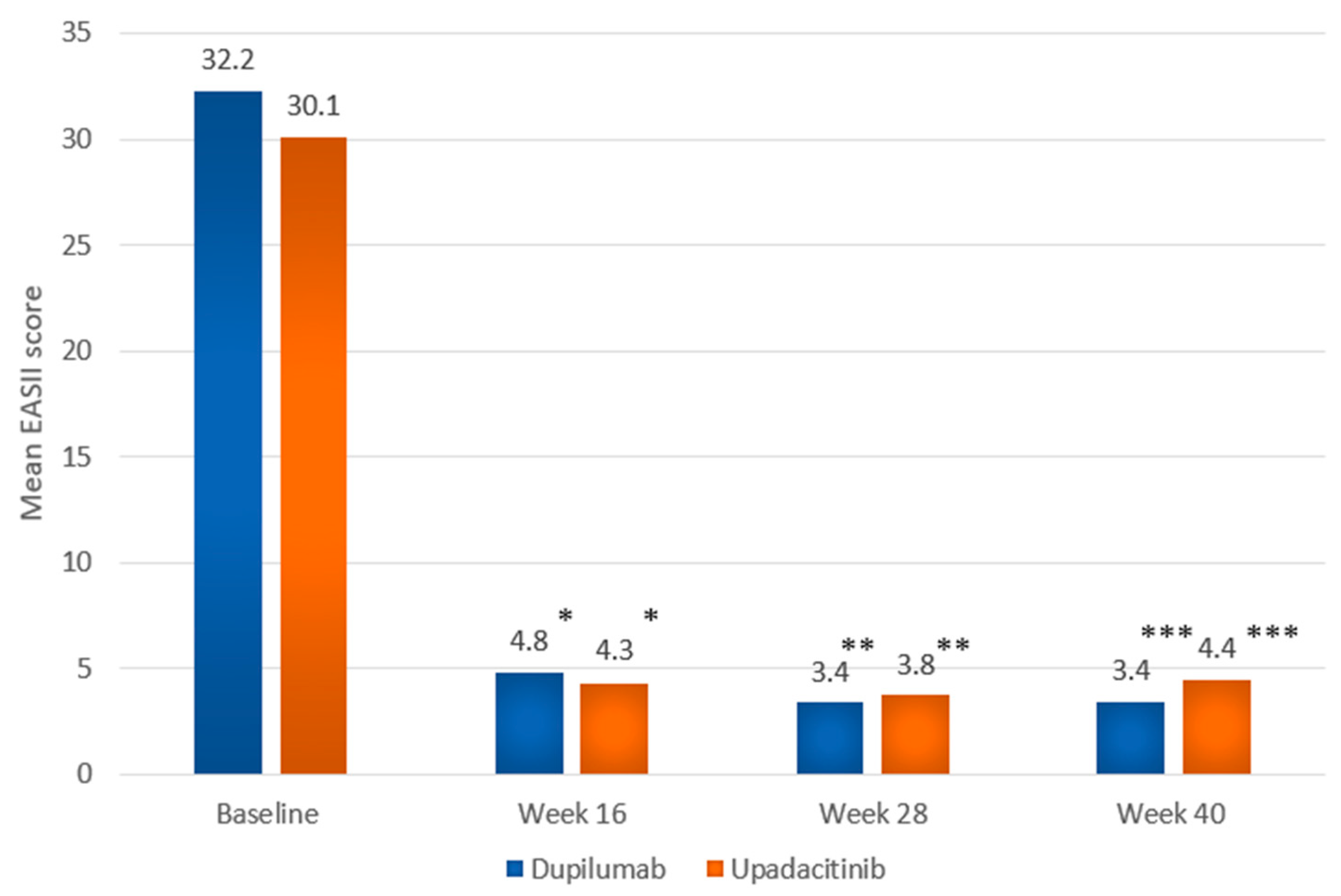
| EASI, mean (SD) [range] | 32.2 (11.1) [20.0–71.5] | 30.1 (10.3) [20.0–65.4] |
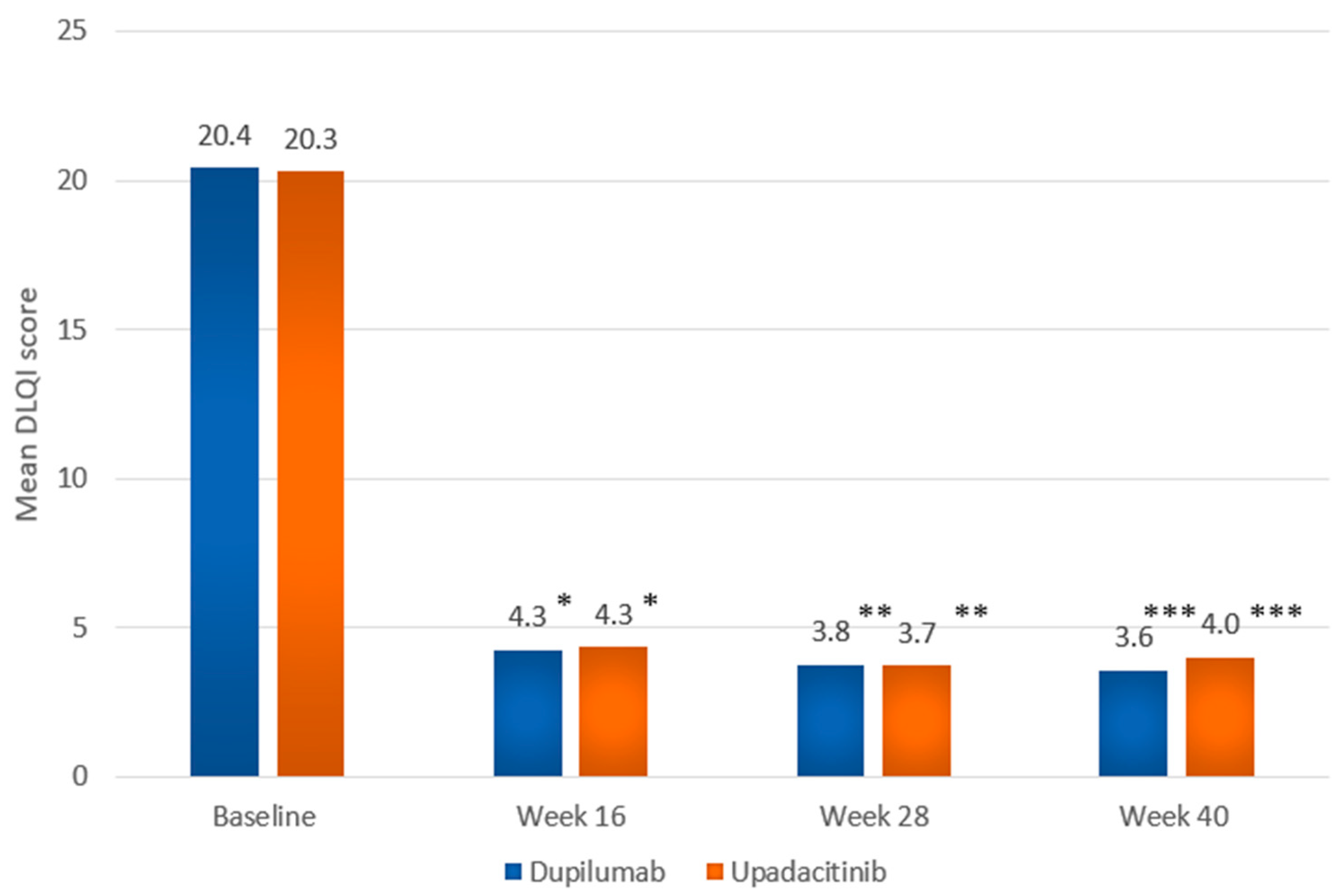
| DLQI, mean (SD) [range] | 20.4 (5.6) [8–30] | 20.3 (6.0) [5–30] |
| Previous treatment in the Polish National Health Fund Program | ||
| - with upadacitinib | 0 | 0 |
| - with baricitinib | 0 | <5 |
| - with abrocitinib | 0 | 0 |
| - with tralokinumab | 0 | 0 |
| - with dupilumab | 0 | <5 |
| Outcome | Dupilumab Group 300 mg q2w (Adults) 200/300 mg q2w (Adolescents; <60 kg or ≥60 kg, Respectively) (n = 220) | Upadacitinib Group 15 mg/30 mg Dose at Physician’s Discretion (n = 215) | p Value |
|---|---|---|---|
| Primary outcome | |||
| EASI75 at wk 40 a | 194 (88.2) [83.9–92.4] | 174 (80.9) [75.7–86.2] | p = 0.0498 |
| Key secondary outcomes | |||
| Proportion of patients maintaining EASI75 from wk 16 until wk 40 a | 153 (91.6) b [87.4–95.8] | 143 (81.3) c [75.5–87.0] | p = 0.008 |
| EASI90 at wk 40 a | 149 (67.7) [61.5–73.9] | 122 (56.7) [50.1–63.4] | p = 0.024 |
| EASI100 at wk 16 a | 16 (7.3) [3.8–10.7] | 42 (19.5) [14.2–24.8] | p < 0.001 |
| EASI100 at wk 28 a | 28 (12.7) [8.3–17.1] | 57 (26.5) [20.6–32.4] | p < 0.001 |
| EASI100 at wk 40 a | 45 (20.5) [15.1–25.8] | 43 (20.0) [14.7–25.3] | p = 1.000 |
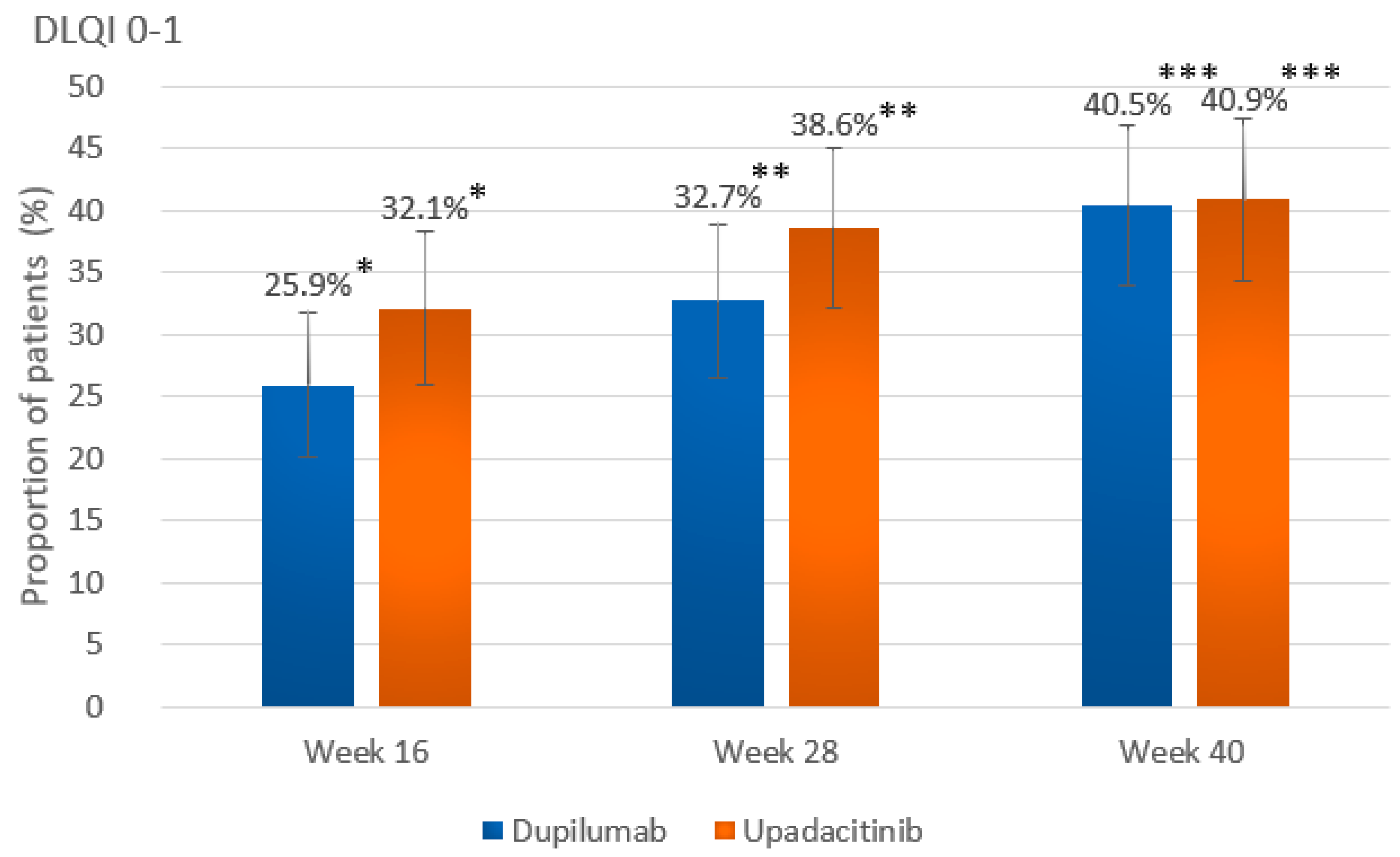
| DLQI 0-1 at wk 40 a | 89 (40.5) [34.0–46.9] | 88 (40.9) [34.4–47.5] | p = 0.997 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waligóra-Dziwak, K.; Dańczak-Pazdrowska, A.; Jenerowicz, D. Long-Term Real-World Effectiveness of Dupilumab vs. Upadacitinib in Early Treatment Responders with Atopic Dermatitis: Results from Central European Health Fund Registry. Int. J. Mol. Sci. 2025, 26, 4230. https://doi.org/10.3390/ijms26094230
Waligóra-Dziwak K, Dańczak-Pazdrowska A, Jenerowicz D. Long-Term Real-World Effectiveness of Dupilumab vs. Upadacitinib in Early Treatment Responders with Atopic Dermatitis: Results from Central European Health Fund Registry. International Journal of Molecular Sciences. 2025; 26(9):4230. https://doi.org/10.3390/ijms26094230
Chicago/Turabian StyleWaligóra-Dziwak, Katarzyna, Aleksandra Dańczak-Pazdrowska, and Dorota Jenerowicz. 2025. "Long-Term Real-World Effectiveness of Dupilumab vs. Upadacitinib in Early Treatment Responders with Atopic Dermatitis: Results from Central European Health Fund Registry" International Journal of Molecular Sciences 26, no. 9: 4230. https://doi.org/10.3390/ijms26094230
APA StyleWaligóra-Dziwak, K., Dańczak-Pazdrowska, A., & Jenerowicz, D. (2025). Long-Term Real-World Effectiveness of Dupilumab vs. Upadacitinib in Early Treatment Responders with Atopic Dermatitis: Results from Central European Health Fund Registry. International Journal of Molecular Sciences, 26(9), 4230. https://doi.org/10.3390/ijms26094230






