Silver Nanoparticle-Induced Nephrotoxicity in Zebrafish (Danio rerio)
Abstract
1. Introduction
2. Results
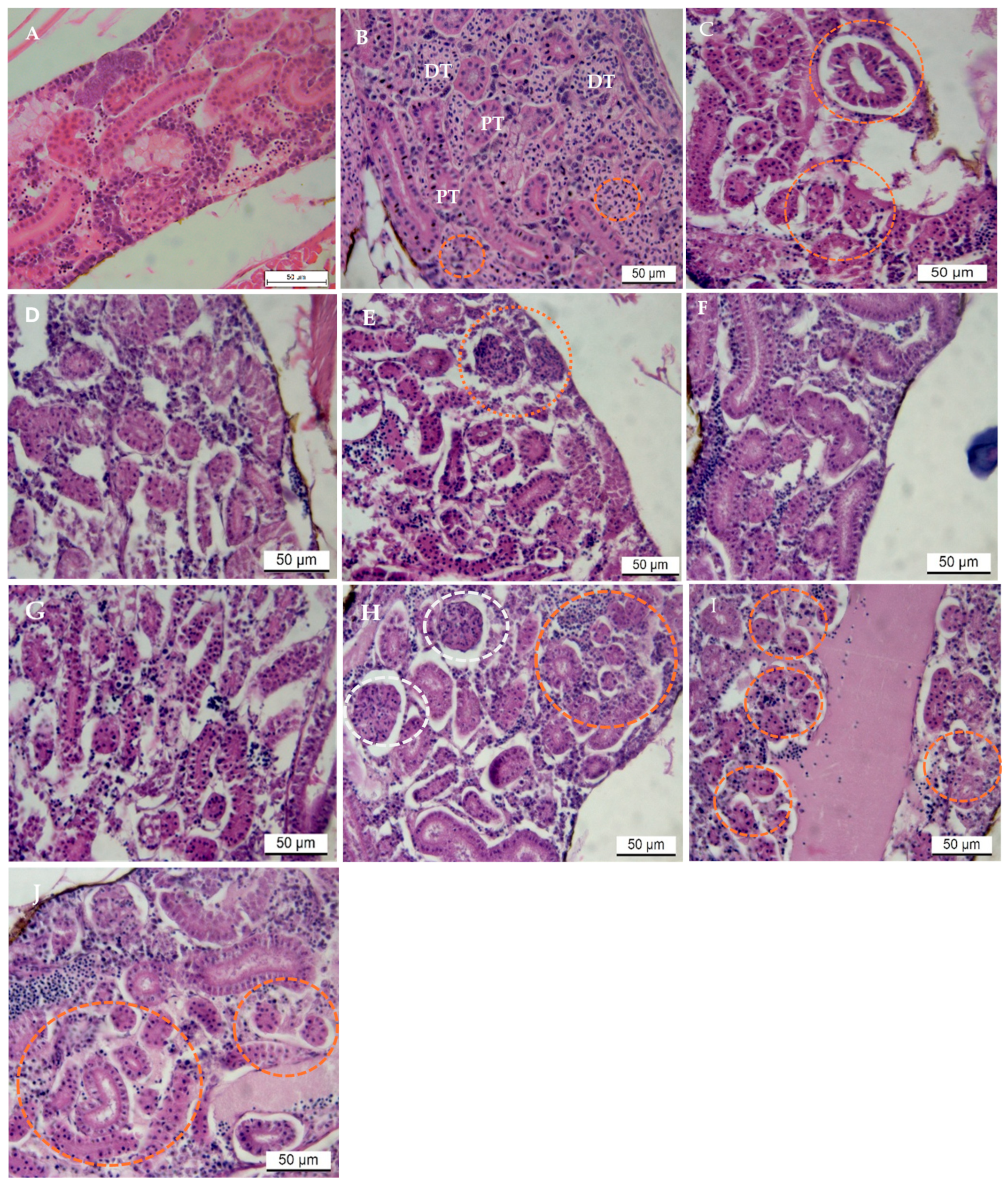
2.1. Pathological Observations in the Kidney: H&E Stain
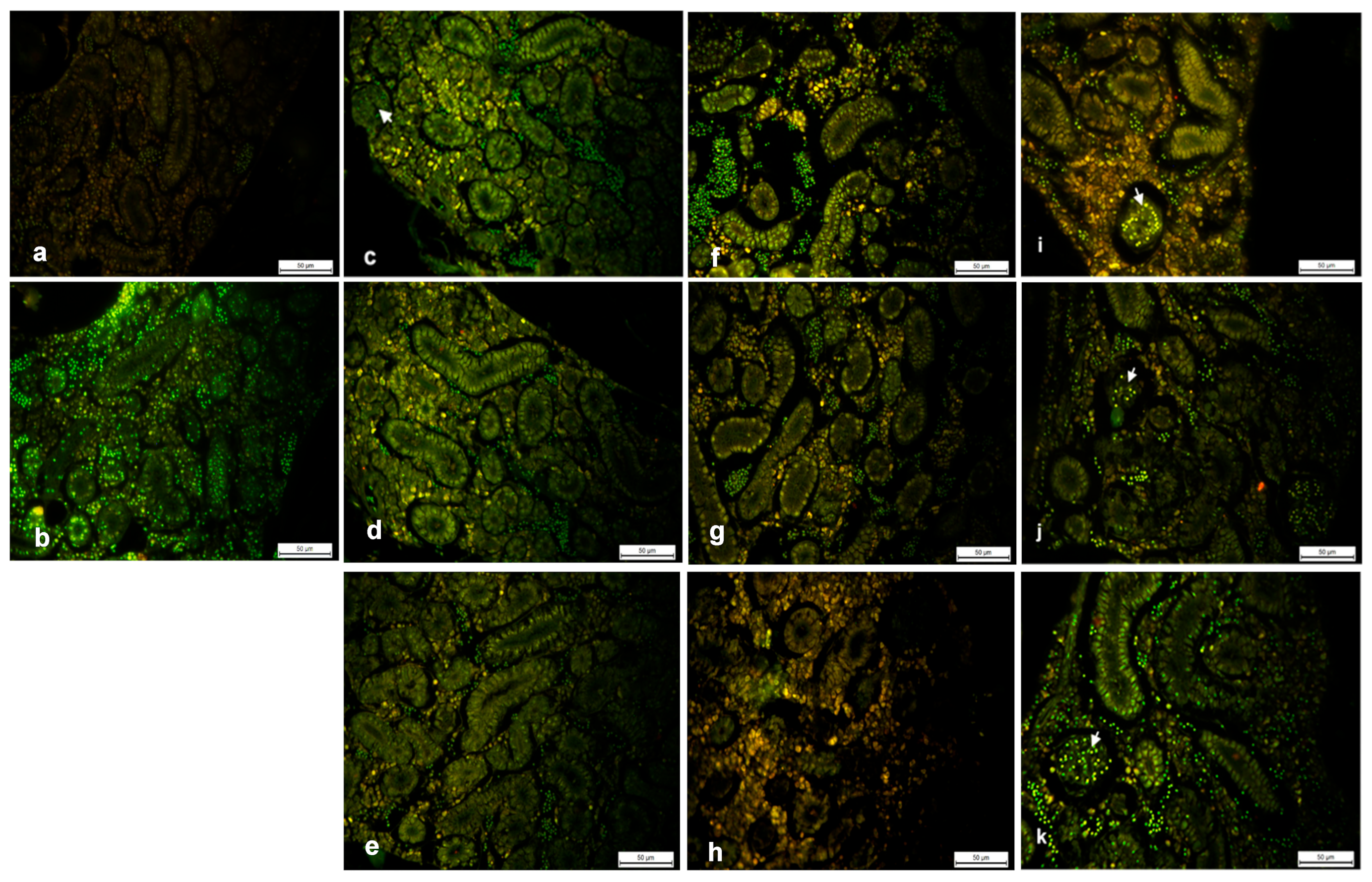
2.2. Fluorescent Staining: Acridine Orange (AO)
3. Discussion
3.1. The Emerging Threat of Silver Nanoparticles in Aquatic Ecosystems
3.2. Histopathological Alterations in Zebrafish Kidneys: Morphological Evidence of Nephrotoxicity
3.3. Alterations in Bowman’s Space and Implications for Glomerular Filtration
3.4. Renal Tubulogenesis in Response to Nanoparticle Exposure: A Regenerative Attempt?
3.5. Inflammatory Cell Infiltration and Haematological Response
3.6. Molecular Mechanisms Underlying Teleost Kidney Regeneration After Nanoparticle Damage
3.7. Synergistic Effects of Silver Nanoparticles and Other Environmental Stressors
3.8. Advanced Methodologies for Differentiating Apoptosis and Necrosis in Renal Damage
3.9. Long-Term Ecological Implications and Future Research Directions
4. Material and Methods
4.1. Zebrafish Maintenance
4.2. Chemicals
4.3. Toxicity Testing of AgNPs
4.4. Sample Fixation and Tissue Sectioning
4.5. Ethical Approval
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eker, F.; Duman, H.; Akdaşçi, E.; Witkowska, A.M.; Bechelany, M.; Karav, S. Silver nanoparticles in therapeutics and beyond: A review of mechanism insights and applications. Nanomaterials 2024, 14, 1618. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M.; Ashengroph, M. Mycosynthesis of AgNPs: Mechanisms of nanoparticle formation and antimicrobial activities. Expert Rev. Anti-Infect. Ther. 2023, 21, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Frippiat, T.; Art, T.; Delguste, C. Silver Nanoparticles as Antimicrobial Agents in Veterinary Medicine: Current Applications and Future Perspectives. Nanomaterials 2025, 15, 202. [Google Scholar] [CrossRef] [PubMed]
- Gungordu Er, S.; Edirisinghe, M.; Tabish, T.A. Graphene-based nanocomposites as antibacterial, antiviral and antifungal agents. Adv. Healthc. Mater. 2023, 12, 2201523. [Google Scholar] [CrossRef]
- Harine, A.; Ranjani, S.; Hemalatha, S. Antifungal efficacy of Citrusfusion mediated silver nanoparticles in Candida species. BMC Biotechnol. 2025, 25, 18. [Google Scholar] [CrossRef]
- Hashem, A.H.; Saied, E.; Amin, B.H.; Alotibi, F.O.; Al-Askar, A.A.; Arishi, A.A.; Elkady, F.M.; Elbahnasawy, M.A. Antifungal activity of biosynthesized silver nanoparticles (AgNPs) against aspergilli causing aspergillosis: Ultrastructure Study. J. Funct. Biomater. 2022, 13, 242. [Google Scholar] [CrossRef]
- Luceri, A.; Francese, R.; Lembo, D.; Ferraris, M.; Balagna, C. Silver nanoparticles: Review of antiviral properties, mechanism of action and applications. Microorganisms 2023, 11, 629. [Google Scholar] [CrossRef]
- Parihar, M.; Chauhan, R.; Singh, R. Silver nanoparticles: A review of their therapeutic potential and biocompatibility. IJBS 2025, 7, 10–12. [Google Scholar] [CrossRef]
- Li, X.; Shi, L.; Song, Z.; Geng, Z.; Yan, Y. The antibacterial activity and formation mechanism of quercetin-coated silver nanoparticles and protein complex. J. Mol. Struct. 2025, 1334, 141878. [Google Scholar] [CrossRef]
- Rima, M.; Villeneuve-Faure, C.; Pilloux, L.; Roques, C.; El Garah, F.; Makasheva, K. From Adhesion to Biofilms Formation and Resilience: Exploring the Impact of Silver Nanoparticles-Based Biomaterials on Pseudomonas aeruginosa. Biofilm 2025, 9, 100267. [Google Scholar] [CrossRef]
- Darwesh, O.M.; Marzoog, A.; Matter, I.A.; Okla, M.K.; El-Tayeb, M.A.; Aufy, M.; Dawoud, T.M.; Abdel-Maksoud, M.A. Natural dyes developed by microbial-nanosilver to produce antimicrobial and anticancer textiles. Microb. Cell Factories 2024, 23, 189. [Google Scholar] [CrossRef] [PubMed]
- Foteva, T. Nanotechnology in the cosmetic industry. J. Chem. Technol. Metall. 2024, 59, 3–14. [Google Scholar] [CrossRef]
- George, N.; Devi, D.G. Phytonano silver for cosmetic formulation-synthesis, characterization, and assessment of antimicrobial and antityrosinase potential. Discov. Nano 2024, 19, 65. [Google Scholar] [CrossRef] [PubMed]
- Jatoi, A.W.; Channa, N.; Abro, M.I.; Aftab, U.; Agheem, M.H.; Hussain, F. Colored antimicrobial protective clothing produced by dopamine-based AgNPs generation method. Biomed. Mater. Devices 2024, 2, 1049–1064. [Google Scholar] [CrossRef]
- Ong, W.T.J.; Yeap, S.P.; Haque, J.; Nyam, K.L. Green synthesis of silver nanoparticles using Kenaf leaves extract and their antibacterial potential in acne management. J. Nanoparticle Res. 2024, preprint. [Google Scholar] [CrossRef]
- Saha, S.K.; Alam, M.R.; Repon, M.R.; Siddique, A.B.; Ali, A.; Chowdhury, T.A. Development of antimicrobial textile using in-situ AgNPs and evaluation of comfort performance for biomedical applications. SPE Polym. 2025, 6, e70001. [Google Scholar] [CrossRef]
- Elhenawy, S.; Khraisheh, M.; AlMomani, F.; Al-Ghouti, M.; Selvaraj, R.; Al-Muhtaseb, A.a. Emerging nanomaterials for drinking water purification: A new era of water treatment technology. Nanomaterials 2024, 14, 1707. [Google Scholar] [CrossRef]
- Kalakonda, P.; Thodeti, M.; Ganneboina, C.; Ankathi, K.; Kathri, S.; Begari, K.; Kante, H.S.; Jupalli, V.; Khaderabad, Y.; Vijaylaxmi, S. Eco-Friendly fabrication of silver nanoparticles for sustainable water purification and antibacterial synergy. Plasmonics 2024, 19, 2857–2869. [Google Scholar] [CrossRef]
- Mayakrishnan, V.; Venkatesan, R.; Madhavan, A.A. Development and Characterization of Antimicrobial Nisin/MMT K10/AgNPs Nanocomposite Coatings on Oxygen Plasma Surface-Modified Polypropylene for Food Packaging Applications. Food Bioprocess Technol. 2024, 18, 3553–3565. [Google Scholar] [CrossRef]
- Trotta, F.; Da Silva, S.; Massironi, A.; Mirpoor, S.F.; Lignou, S.; Ghawi, S.K.; Charalampopoulos, D. Advancing food preservation: Sustainable green-AgNPs bionanocomposites in paper-starch flexible packaging for prolonged shelf life. Polymers 2024, 16, 941. [Google Scholar] [CrossRef]
- Wang, X.; Xuan, S.; Ding, K.; Jin, P.; Zheng, Y.; Wu, Z. Photothermal controlled antibacterial Ta4C3Tx-AgNPs/nanocellulose bioplastic food packaging. Food Chem. 2024, 448, 139126. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Cui, R.; Zhou, C.; Yang, S. Fabrication and performance evaluation of a Janus solar-driven steam generator using AgNPs@ C composite for sustainable water purification. Desalination 2024, 573, 117204. [Google Scholar] [CrossRef]
- Aminzai, M.T.; Yildirim, M.; Yabalak, E. Metallic nanoparticles unveiled: Synthesis, characterization, and their environmental, medicinal, and agricultural applications. Talanta 2024, 280, 126790. [Google Scholar] [CrossRef]
- Bamigbade, A.A.; Dare, E.O.; Badmus, B.S.; Bamgbose, T. Direct Electron Transfer and Electro-Catalytic Activity of Non-Enzymatic Glucose Biosensor Based on Silver Nanoparticle (AgNPs) Stabilized with Sodium Tripolyphosphate (NaTPP) Cross-Linked Chitosan. Eng. Chem. 2025, 9, 33–51. [Google Scholar] [CrossRef]
- Kishnani, V.; Makadia, R.A.; Natarajan, S.; Joseph, J.; Gupta, A. Development of a silver–polyaniline functionalized biosensor for non-enzymatic lactic acid detection. Mater. Adv. 2025, 6, 766–776. [Google Scholar] [CrossRef]
- Rupanshi; Kumar, V.; Yadav, N.; Singh, D.; Beniwal, V.; Chhabra, J.; Singh, B. Biogenic Silver Nanoparticles as Next-Generation Green Catalysts for Multifaceted Applications. Trans. Tianjin Univ. 2025, 1–34. [Google Scholar] [CrossRef]
- Fetouh, H.; Abd_Ellah, S.; Fadhil, F.M.; Ahmed, A.G.; Sallam, E.; Alazmi, M.; Samy, Ů.A.M.; Taha, A.; Hattawi, S.N.; El Desouky, J. Potential health risk effects of silver nanoparticles on aquatic ecosystem: Regulations and guidelines. Alex. J. Sci. Technol. 2024, 2, 99–113. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Zhang, Y.; Ruan, X.; Cheng, H.; Ge, Q.; Zhang, L. Efficient V-Shaped Substrate for Surface and Volume Enhanced Raman Spectroscopic Analysis of Bioaerosol: Prevention from Potential Health Risk. Environ. Health 2024, 2, 212–220. [Google Scholar] [CrossRef]
- Dube, E.; Okuthe, G.E. Engineered nanoparticles in aquatic systems: Toxicity and mechanism of toxicity in fish. Emerg. Contam. 2023, 9, 100212. [Google Scholar] [CrossRef]
- Li, C.; Liu, Z.; Xu, Y.; Chen, X.; Zhang, Q.; Hu, L.; Lv, Z.; Liu, X.; Xiao, T.; Li, D. AgNPs-induced oxidative stress and inflammation confer an increased susceptibility to aquatic reovirus infection. Aquaculture 2024, 586, 740748. [Google Scholar] [CrossRef]
- Wang, F.; Zhou, L.; Mu, D.; Zhang, H.; Zhang, G.; Huang, X.; Xiong, P. Current research on ecotoxicity of metal-based nanoparticles: From exposure pathways, ecotoxicological effects to toxicity mechanisms. Front. Public Health 2024, 12, 1390099. [Google Scholar] [CrossRef] [PubMed]
- Kong, I.C.; Ko, K.-S.; Koh, D.-C. Evaluation of the effects of particle sizes of silver nanoparticles on various biological systems. Int. J. Mol. Sci. 2020, 21, 8465. [Google Scholar] [CrossRef] [PubMed]
- Dang, F.; Huang, Y.; Wang, Y.; Zhou, D.; Xing, B. Transfer and toxicity of silver nanoparticles in the food chain. Environ. Sci. Nano 2021, 8, 1519–1535. [Google Scholar] [CrossRef]
- Yoo-Iam, M.; Chaichana, R.; Satapanajaru, T. Toxicity, bioaccumulation and biomagnification of silver nanoparticles in green algae (Chlorella sp.), water flea (Moina macrocopa), blood worm (Chironomus spp.) and silver barb (Barbonymus gonionotus). Chem. Speciat. Bioavailab. 2014, 26, 257–265. [Google Scholar] [CrossRef]
- Zhou, K.; Hu, Y.; Zhang, L.; Yang, K.; Lin, D. The role of exopolymeric substances in the bioaccumulation and toxicity of Ag nanoparticles to algae. Sci. Rep. 2016, 6, 32998. [Google Scholar] [CrossRef]
- Asharani, P.; Lianwu, Y.; Gong, Z.; Valiyaveettil, S. Comparison of the toxicity of silver, gold and platinum nanoparticles in developing zebrafish embryos. Nanotoxicology 2011, 5, 43–54. [Google Scholar] [CrossRef]
- Massarsky, A.; Dupuis, L.; Taylor, J.; Eisa-Beygi, S.; Strek, L.; Trudeau, V.L.; Moon, T.W. Assessment of nanosilver toxicity during zebrafish (Danio rerio) development. Chemosphere 2013, 92, 59–66. [Google Scholar] [CrossRef]
- Pecoraro, R.; Salvaggio, A.; Scalisi, E.M.; Iaria, C.; Lanteri, G.; Copat, C.; Ferrante, M.; Fragalà, G.; Zimbone, M.; Impellizzeri, G. Evaluation of the effects of silver nanoparticles on Danio rerio cornea: Morphological and ultrastructural analysis. Microsc. Res. Tech. 2019, 82, 1297–1301. [Google Scholar] [CrossRef]
- Abramenko, N.; Semenova, M.; Khina, A.; Zherebin, P.; Krutyakov, Y.; Krysanov, E.; Kustov, L. The toxicity of coated silver nanoparticles and their stabilizers towards Paracentrotus lividus sea urchin embryos. Nanomaterials 2022, 12, 4003. [Google Scholar] [CrossRef]
- Elmonem, M.A.; Berlingerio, S.P.; Van den Heuvel, L.P.; De Witte, P.A.; Lowe, M.; Levtchenko, E.N. Genetic renal diseases: The emerging role of zebrafish models. Cells 2018, 7, 130. [Google Scholar] [CrossRef]
- Tingaud-Sequeira, A.; Calusinska, M.; Finn, R.N.; Chauvigné, F.; Lozano, J.; Cerdà, J. The zebrafish genome encodes the largest vertebrate repertoire of functional aquaporins with dual paralogy and substrate specificities similar to mammals. BMC Evol. Biol. 2010, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Okuthe, G.E. DNA and RNA pattern of staining during oogenesis in zebrafish (Danio rerio): A confocal microscopy study. Acta Histochem. 2013, 115, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Okuthe, G.E.; Hanrahan, S.; Fabian, B.C. Early gonad development in zebrafish (Danio rerio). Afr. J. Biotechnol. 2014, 13, 3433–3442. [Google Scholar] [CrossRef]
- McKee, R.A.; Wingert, R.A. Zebrafish renal pathology: Emerging models of acute kidney injury. Curr. Pathobiol. Rep. 2015, 3, 171–181. [Google Scholar] [CrossRef]
- Queiroz, L.G.; Faustino, L.A.; de Oliveira, P.F.; Pompêo, M.; de Torresi, S.I.C. Transformative nanobioplasmonic effects: Toxicological implications of plasmonic silver nanoparticles in aquatic biological models. Sci. Total Environ. 2024, 954, 176592. [Google Scholar] [CrossRef]
- Kim, W.-I.; Pak, S.-W.; Lee, S.-J.; Park, S.-H.; Shin, I.-S.; Moon, C.; Yu, W.-J.; Kim, S.-H.; Kim, J.-C. In vitro study of silver nanoparticles-induced embryotoxicity using a rat whole embryo culture model. Toxicol. Res. 2024, 41, 189–197. [Google Scholar] [CrossRef]
- Mortazavi Moghadam, F.S.; Rasouli, S.; Mortazavi Moghadam, F.A. In Vivo Study and Cytotoxicity of Migrated Silver Nanoparticles (AgNPs) from Cellulose/LDPE/AgNP Nanocomposite in Highly Perishable Food (Fish Fillet) Packaging. ACS Food Sci. Technol. 2025, 3, 1024–1041. [Google Scholar] [CrossRef]
- ALAtawi, M.K.; AlAsmari, A.A.; AlAliany, A.D.; Almajed, M.M.; Sakran, M.I. Silver nanoparticles forensic uses and toxicity on vital organs and different body systems. Adv. Toxicol. Toxic Eff. 2024, 8, 015–029. [Google Scholar]
- Xie, Q.; Li, Z.; Chen, Y.; Zhao, Y.; Xu, Y.; Hong, Z.; Chen, Z.; Zhang, Z.; Xu, H.; Yin, Z. Mass Spectrometry Imaging Reveals the Morphology-Dependent Toxicological Effects of Nanosilvers on Multiple Organs of Adult Zebrafish (Danio rerio). Environ. Sci. Technol. 2024, 58, 10015–10027. [Google Scholar] [CrossRef]
- Sati, A.; Ranade, T.N.; Mali, S.N.; Ahmad Yasin, H.K.; Pratap, A. Silver Nanoparticles (AgNPs): Comprehensive Insights into Bio/Synthesis, Key Influencing Factors, Multifaceted Applications, and Toxicity—A 2024 Update. ACS Omega 2025, 10, 7549–7582. [Google Scholar] [CrossRef]
- Chernick, M.; Kennedy, A.J.; Thomas, T.; Scott, K.C.; Sipe, J.M.; Hendren, C.O.; Wiesner, M.R.; Hinton, D.E. Morphologic alterations across three levels of biological organization following oral exposure to silver-polymer nanocomposites in Japanese medaka (Oryzias latipes). Environ. Sci. Nano 2024, 11, 3317–3334. [Google Scholar] [CrossRef]
- Qiang, L.; Arabeyyat, Z.H.; Xin, Q.; Paunov, V.N.; Dale, I.J.; Lloyd Mills, R.I.; Rotchell, J.M.; Cheng, J. Silver nanoparticles in Zebrafish (Danio rerio) embryos: Uptake, growth and molecular responses. Int. J. Mol. Sci. 2020, 21, 1876. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Chen, Y.; Yang, Y.; Zhou, Y.; Su, P.; Yuan, S. Alleviation of bovine serum albumin on the neurotoxicity of silver nanoparticles in zebrafish (Danio rerio) larvae. Front. Mar. Sci. 2024, 11, 1473054. [Google Scholar] [CrossRef]
- Lee, Y.-L.; Shih, Y.-S.; Chen, Z.-Y.; Cheng, F.-Y.; Lu, J.-Y.; Wu, Y.-H.; Wang, Y.-J. Toxic effects and mechanisms of silver and zinc oxide nanoparticles on zebrafish embryos in aquatic ecosystems. Nanomaterials 2022, 12, 717. [Google Scholar] [CrossRef]
- Quevedo, A.C.; Lynch, I.; Valsami-Jones, E. Silver nanoparticle induced toxicity and cell death mechanisms in embryonic zebrafish cells. Nanoscale 2021, 13, 6142–6161. [Google Scholar] [CrossRef]
- Yan, N.; Wang, W.-X. Maternal transfer and biodistribution of citrate and luminogens coated silver nanoparticles in medaka fish. J. Hazard. Mater. 2022, 433, 128862. [Google Scholar] [CrossRef]
- Szudrowicz, H.; Kamaszewski, M.; Adamski, A.; Skrobisz, M.; Frankowska-ukawska, J.; Wójcik, M.; Bochenek, J.; Kawalski, K.; Martynow, J.; Bujarski, P. The Effects of Seven-Day Exposure to Silver Nanoparticles on Fertility and Homeostasis of Zebrafish (Danio rerio). Int. J. Mol. Sci. 2022, 23, 11239. [Google Scholar] [CrossRef]
- Kosai, P.; Jiraungkoorskul, W.; Thammasunthorn, T.; Jiraungkoorskul, K. Reduction of copper-induced histopathological alterations by calcium exposure in Nile tilapia (Oreochromis niloticus). Toxicol. Mech. Methods 2009, 19, 461–467. [Google Scholar] [CrossRef]
- Yazdanparast, T.; Sharifpour, I.; Soltani, M.; Esfahani, H.K. Evaluation of silver retention in different organs of zebrafish (Danio rerio) fed diet supplemented with silver nanoparticles. Int. J. Eng. Res. 2016, 5, 269–274. [Google Scholar]
- Huang, Y.; Wang, J.; Jiang, K.; Chung, E.J. Improving kidney targeting: The influence of nanoparticle physicochemical properties on kidney interactions. J. Control. Release 2021, 334, 127–137. [Google Scholar] [CrossRef]
- McCampbell, K.K.; Springer, K.N.; Wingert, R.A. Atlas of cellular dynamics during zebrafish adult kidney regeneration. Stem Cells Int. 2015, 2015, 547636. [Google Scholar] [CrossRef] [PubMed]
- Handy, R.D. Chronic effects of copper exposure versus endocrine toxicity: Two sides of the same toxicological process? Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2003, 135, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Noga, M.; Milan, J.; Frydrych, A.; Jurowski, K. Toxicological aspects, safety assessment, and green toxicology of silver nanoparticles (AgNPs)—Critical review: State of the art. Int. J. Mol. Sci. 2023, 24, 5133. [Google Scholar] [CrossRef] [PubMed]
- Diep, C.Q.; Ma, D.; Deo, R.C.; Holm, T.M.; Naylor, R.W.; Arora, N.; Wingert, R.A.; Bollig, F.; Djordjevic, G.; Lichman, B. Identification of adult nephron progenitors capable of kidney regeneration in zebrafish. Nature 2011, 470, 95–100. [Google Scholar] [CrossRef]
- Yan, Z.; Zhou, Y.; Zhu, P.; Bao, X.; Su, P. Polystyrene nanoplastics mediated the toxicity of silver nanoparticles in zebrafish embryos. Front. Mar. Sci. 2023, 10, 1195125. [Google Scholar] [CrossRef]
- Mansouri, B.; Rahmani, R.; Johari, S.; Azadi, N. Combined effects of silver nanoparticles and mercury on gill histopathology of zebrafih (Danio rerio). J. Coast. Life Med. 2016, 4, 421–425. [Google Scholar] [CrossRef]
- Bilberg, K.; Hovgaard, M.B.; Besenbacher, F.; Baatrup, E. In vivo toxicity of silver nanoparticles and silver ions in zebrafish (Danio rerio). J. Toxicol. 2012, 2012, 293784. [Google Scholar] [CrossRef]
- Uçak, R.; Topcu, Ş.O.; Sarı, İ. Analysis of apoptosis of kidney tissue by the tunel method and histomorphological changes in rabbit kidney model due to unilateral supravesical obstruction. J. Surg. Med. 2020, 4, 1057–1062. [Google Scholar] [CrossRef]
- Sorrells, S.; Toruno, C.; Stewart, R.A.; Jette, C. Analysis of apoptosis in zebrafish embryos by whole-mount immunofluorescence to detect activated Caspase 3. J. Vis. Exp. JoVE 2013, 51060. [Google Scholar]
- Morash, M.G.; Douglas, S.E.; Robotham, A.; Ridley, C.M.; Gallant, J.W.; Soanes, K.H. The zebrafish embryo as a tool for screening and characterizing pleurocidin host-defense peptides as anti-cancer agents. Dis. Models Mech. 2011, 4, 622–633. [Google Scholar] [CrossRef]
- Oluwafemi, O.S.; Vuyelwa, N.; Scriba, M.; Songca, S.P. Green controlled synthesis of monodispersed, stable and smaller sized starch-capped silver nanoparticles. Mater. Lett. 2013, 106, 332–336. [Google Scholar] [CrossRef]
- Chae, Y.J.; Pham, C.H.; Lee, J.; Bae, E.; Yi, J.; Gu, M.B. Evaluation of the toxic impact of silver nanoparticles on Japanese medaka (Oryzias latipes). Aquat. Toxicol. 2009, 94, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Griffitt, R.J.; Luo, J.; Gao, J.; Bonzongo, J.C.; Barber, D.S. Effects of particle composition and species on toxicity of metallic nanomaterials in aquatic organisms. Environ. Toxicol. Chem. 2008, 27, 1972–1978. [Google Scholar] [CrossRef] [PubMed]
- Laban, G.; Nies, L.F.; Turco, R.F.; Bickham, J.W.; Sepúlveda, M.S. The effects of silver nanoparticles on fathead minnow (Pimephales promelas) embryos. Ecotoxicology 2010, 19, 185–195. [Google Scholar] [CrossRef]
- Dunn, C.; Brettle, D.; Cockroft, M.; Keating, E.; Revie, C.; Treanor, D. Quantitative assessment of H&E staining for pathology: Development and clinical evaluation of a novel system. Diagn. Pathol. 2024, 19, 42. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okuthe, G.E.; Siguba, B. Silver Nanoparticle-Induced Nephrotoxicity in Zebrafish (Danio rerio). Int. J. Mol. Sci. 2025, 26, 4216. https://doi.org/10.3390/ijms26094216
Okuthe GE, Siguba B. Silver Nanoparticle-Induced Nephrotoxicity in Zebrafish (Danio rerio). International Journal of Molecular Sciences. 2025; 26(9):4216. https://doi.org/10.3390/ijms26094216
Chicago/Turabian StyleOkuthe, Grace Emily, and Busiswa Siguba. 2025. "Silver Nanoparticle-Induced Nephrotoxicity in Zebrafish (Danio rerio)" International Journal of Molecular Sciences 26, no. 9: 4216. https://doi.org/10.3390/ijms26094216
APA StyleOkuthe, G. E., & Siguba, B. (2025). Silver Nanoparticle-Induced Nephrotoxicity in Zebrafish (Danio rerio). International Journal of Molecular Sciences, 26(9), 4216. https://doi.org/10.3390/ijms26094216





