Molecular Hydrogen Ameliorates Anti-Desmoglein 1 Antibody-Induced Pemphigus-Associated Interstitial Lung Disease by Inhibiting Oxidative Stress
Abstract
1. Introduction
2. Results
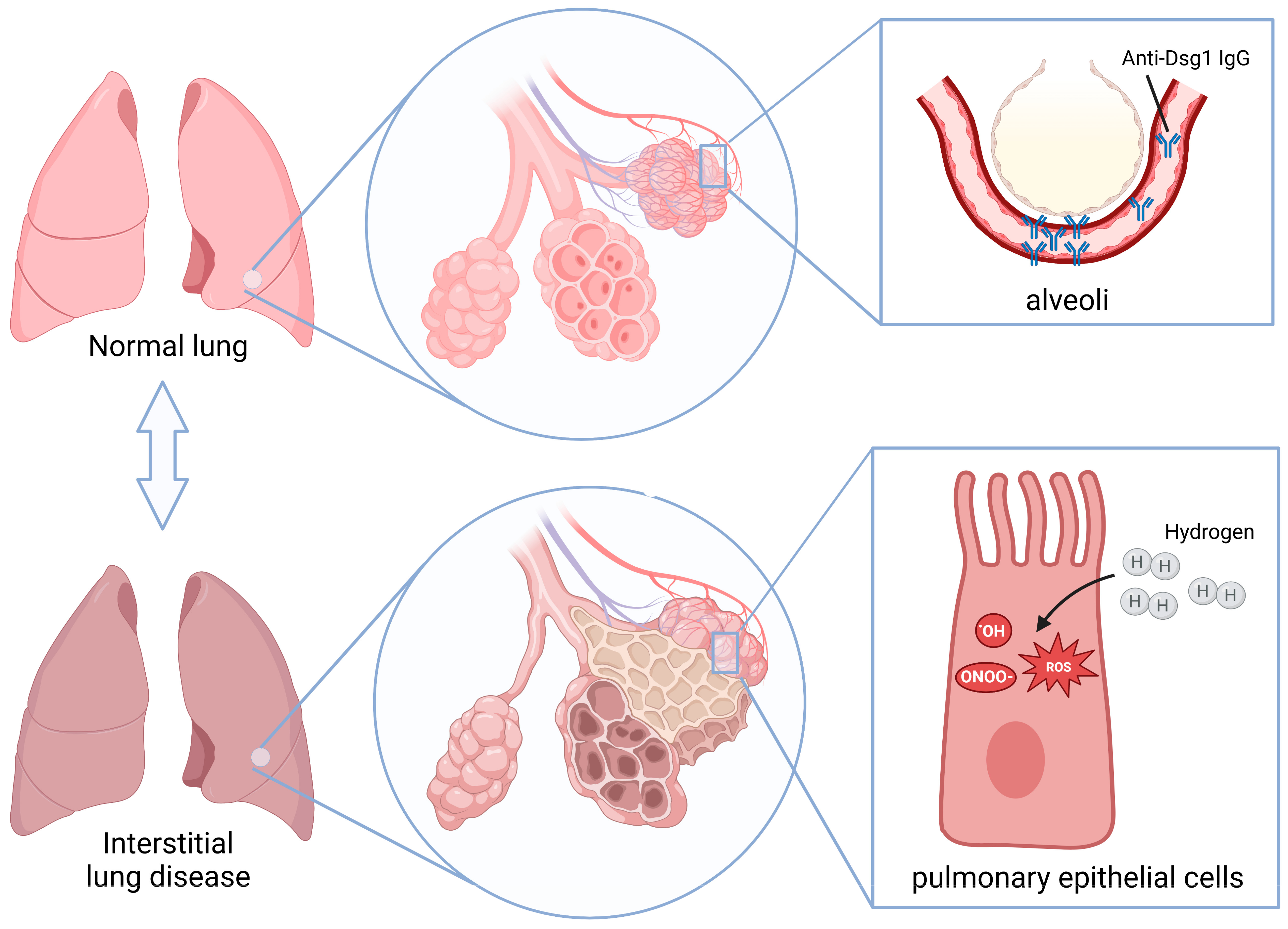
2.1. Anti-Dsg 1 Antibody-Induced Interstitial Lung Disease in the Pemphigus Mouse Model
2.2. Anti-Dsg 1 Antibody Induced Increased Oxidative Stress in the Lungs of Mice
2.3. H2 Improved Interstitial Lung Disease in P-ILD Mice
2.4. H2 Inhibited Oxidative Stress in the Lungs of P-ILD Mice
3. Discussion
4. Materials and Methods
4.1. Serum
4.2. Animals
4.3. Hydrogen-Rich Water (HW)
4.4. Serum IgG Extraction
4.5. Pemphigus Mouse Model
4.6. Histopathology
4.7. Ashcroft Scoring
4.8. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
4.9. Sircol Assay
4.10. ROS Staining
4.11. Intervention
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Cat | catalase |
| Col1a1 | collagen, type I, alpha 1 |
| Col1a2 | collagen, type I, alpha 2 |
| Col3a1 | collagen, type III, alpha 1 |
| DIF | direct immunofluorescence |
| Dsg | desmoglein |
| GPx | glutathione peroxidase |
| H2 | hydrogen molecules |
| HH | anti-Dsg1H/3H |
| HL | anti-Dsg1H/3L |
| HW | hydrogen-rich water |
| IL-13 | interleukin-13 |
| IL-1β | interleukin-1β |
| IL-6 | interleukin-6 |
| ILD | interstitial lung disease |
| LH | anti-Dsg1L/3H |
| LL | anti-Dsg1L/3L |
| N | normal healthy people |
| NAC | N-Acetylcysteine |
| NS | normal saline |
| P-ILD | pemphigus associated interstitial lung disease |
| ROS | reactive oxygen species |
| RT-qPCR | real-time quantitative polymerase chain reaction |
| S100A4 | S100 calcium binding protein A4 |
| Sod | superoxide |
| TGF-β | transforming growth factor-beta |
| TNF-α | tumor necrosis factor-alpha |
| α-SMA | α-smooth muscle actin |
References
- Schmidt, E.; Kasperkiewicz, M.; Joly, P. Pemphigus. Lancet 2019, 394, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Kasperkiewicz, M.; Ellebrecht, C.T.; Takahashi, H.; Yamagami, J.; Zillikens, D.; Payne, A.S.; Amagai, M. Pemphigus. Nat. Rev. Dis. Primers 2017, 3. [Google Scholar] [CrossRef] [PubMed]
- Chams-Davatchi, C.; Valikhani, M.; Daneshpazhooh, M.; Esmaili, N.; Balighi, K.; Hallaji, Z.; Barzegari, M.; Akhiani, M.; Ghodsi, Z.; Mortazavi, H.; et al. Pemphigus: Analysis of 1209 cases. Int. J. Dermatol. 2005, 44, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, L.; Ma, L.; Yang, F.; Chen, S.; Yang, J.; Gao, H.; Tang, C.; Zhao, Y.; Zhang, Z.; et al. Epidemiological Insights into Autoimmune Bullous Diseases in China: A Comprehensive Analysis. J. Epidemiol. Glob. Health 2024. [Google Scholar] [CrossRef]
- Tang, C.; Wang, L.; Liu, Q.; Chen, Z.; Yang, J.; Gao, H.; Guan, C.; He, S.; Zhang, L.; Zheng, S.; et al. Pulmonary interstitial lesions in pemphigus mouse model: Verifying pemphigus may not be only limited to skin and mucosa. Exp. Dermatol. 2024, 33, e15136. [Google Scholar] [CrossRef]
- Hata, T.; Nishimoto, S.; Nagao, K.; Takahashi, H.; Yoshida, K.; Ohyama, M.; Yamada, T.; Asano, K.; Amagai, M. Ectopic expression of epidermal antigens renders the lung a target organ in paraneoplastic pemphigus. J. Immunol. 2013, 191, 83–90. [Google Scholar] [CrossRef]
- Horimasu, Y.; Ishikawa, N.; Taniwaki, M.; Yamaguchi, K.; Hamai, K.; Iwamoto, H.; Ohshimo, S.; Hamada, H.; Hattori, N.; Okada, M.; et al. Gene expression profiling of idiopathic interstitial pneumonias (IIPs): Identification of potential diagnostic markers and therapeutic targets. BMC Med. Genet. 2017, 18, 88. [Google Scholar] [CrossRef]
- Namba, C.; Tohyama, M.; Hanakawa, Y.; Murakami, M.; Shirakata, Y.; Matsumoto, T.; Suemori, K.; Ishii, N.; Hashimoto, T.; Sayama, K. Paraneoplastic pemphigus associated with fatal bronchiolitis obliterans and intractable mucosal erosions: Treatment with cyclosporin in addition to steroid, rituximab and intravenous immunoglobulin. J. Dermatol. 2016, 43, 419–422. [Google Scholar] [CrossRef]
- Fullerton, S.H.; Woodley, D.T.; Smoller, B.R.; Anhalt, G.J. Paraneoplastic pemphigus with autoantibody deposition in bronchial epithelium after autologous bone marrow transplantation. JAMA 1992, 267, 1500–1502. [Google Scholar] [CrossRef]
- Bast, A.; Weseler, A.R.; Haenen, G.R.; den Hartog, G.J. Oxidative stress and antioxidants in interstitial lung disease. Curr. Opin. Pulm. Med. 2010, 16, 516–520. [Google Scholar] [CrossRef]
- Raghu, G.; Rochwerg, B.; Zhang, Y.; Garcia, C.A.; Azuma, A.; Behr, J.; Brozek, J.L.; Collard, H.R.; Cunningham, W.; Homma, S.; et al. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2015, 192, e3–e19. [Google Scholar] [CrossRef] [PubMed]
- Behr, J.; Maier, K.; Degenkolb, B.; Krombach, F.; Vogelmeier, C. Antioxidative and clinical effects of high-dose N-acetylcysteine in fibrosing alveolitis. Adjunctive therapy to maintenance immunosuppression. Am. J. Respir. Crit. Care Med. 1997, 156, 1897–1901. [Google Scholar] [CrossRef]
- Muramatsu, Y.; Sugino, K.; Ishida, F.; Tatebe, J.; Morita, T.; Homma, S. Effect of inhaled N-acetylcysteine monotherapy on lung function and redox balance in idiopathic pulmonary fibrosis. Respir. Investig. 2016, 54, 170–178. [Google Scholar] [CrossRef]
- Homma, S.; Azuma, A.; Taniguchi, H.; Ogura, T.; Mochiduki, Y.; Sugiyama, Y.; Nakata, K.; Yoshimura, K.; Takeuchi, M.; Kudoh, S. Efficacy of inhaled N-acetylcysteine monotherapy in patients with early stage idiopathic pulmonary fibrosis. Respirology 2012, 17, 467–477. [Google Scholar] [CrossRef]
- Demedts, M.; Behr, J.; Buhl, R.; Costabel, U.; Dekhuijzen, R.; Jansen, H.M.; MacNee, W.; Thomeer, M.; Wallaert, B.; Laurent, F.; et al. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2005, 353, 2229–2242. [Google Scholar] [CrossRef]
- Bridgeman, M.M.; Marsden, M.; Selby, C.; Morrison, D.; MacNee, W. Effect of N-acetyl cysteine on the concentrations of thiols in plasma, bronchoalveolar lavage fluid, and lung tissue. Thorax 1994, 49, 670. [Google Scholar] [CrossRef]
- Cotgreave, I.A.; Eklund, A.; Larsson, K.; Moldéus, P.W. No penetration of orally administered N-acetylcysteine into bronchoalveolar lavage fluid. Eur. J. Respir. Dis. 1987, 70, 73–77. [Google Scholar]
- Hamzeh, N.; Li, L.; Barkes, B.; Huang, J.; Canono, B.; Gillespie, M.; Maier, L.; Day, B. The effect of an oral anti-oxidant, N-Acetyl-cysteine, on inflammatory and oxidative markers in pulmonary sarcoidosis. Respir. Med. 2016, 112, 106–111. [Google Scholar] [CrossRef]
- Sakamoto, S.; Kataoka, K.; Kondoh, Y.; Kato, M.; Okamoto, M.; Mukae, H.; Bando, M.; Suda, T.; Yatera, K.; Tanino, Y.; et al. Pirfenidone plus inhaled N-acetylcysteine for idiopathic pulmonary fibrosis: A randomised trial. Eur. Respir. J. 2021, 57, 2000348. [Google Scholar] [CrossRef]
- Yang, M.; Dong, Y.; He, Q.; Zhu, P.; Zhuang, Q.; Shen, J.; Zhang, X.; Zhao, M. Hydrogen: A Novel Option in Human Disease Treatment. Oxid. Med. Cell Longev. 2020, 2020, 8384742. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Kawai, D.; Takaki, A.; Nakatsuka, A.; Wada, J.; Tamaki, N.; Yasunaka, T.; Koike, K.; Tsuzaki, R.; Matsumoto, K.; Miyake, Y.; et al. Hydrogen-rich water prevents progression of nonalcoholic steatohepatitis and accompanying hepatocarcinogenesis in mice. Hepatology 2012, 56, 912–921. [Google Scholar] [CrossRef]
- Hayashida, K.; Sano, M.; Ohsawa, I.; Shinmura, K.; Tamaki, K.; Kimura, K.; Endo, J.; Katayama, T.; Kawamura, A.; Kohsaka, S.; et al. Inhalation of hydrogen gas reduces infarct size in the rat model of myocardial ischemia-reperfusion injury. Biochem. Biophys. Res. Commun. 2008, 373, 30–35. [Google Scholar] [CrossRef]
- Wynn, T.A. Integrating mechanisms of pulmonary fibrosis. J. Exp. Med. 2011, 208, 1339–1350. [Google Scholar] [CrossRef]
- Kolb, M.; Margetts, P.J.; Anthony, D.C.; Pitossi, F.; Gauldie, J. Transient expression of IL-1beta induces acute lung injury and chronic repair leading to pulmonary fibrosis. J. Clin. Investig. 2001, 107, 1529–1536. [Google Scholar] [CrossRef]
- Zhu, Z.; Homer, R.J.; Wang, Z.; Chen, Q.; Geba, G.P.; Wang, J.; Zhang, Y.; Elias, J.A. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J. Clin. Investig. 1999, 103, 779–788. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Terasaki, Y.; Terasaki, M.; Kanazawa, S.; Kokuho, N.; Urushiyama, H.; Kajimoto, Y.; Kunugi, S.; Maruyama, M.; Akimoto, T.; Miura, Y.; et al. Effect of H2 treatment in a mouse model of rheumatoid arthritis-associated interstitial lung disease. J. Cell Mol. Med. 2019, 23, 7043–7053. [Google Scholar] [CrossRef]
- Yang, L.; Lin, Z.; Wang, Y.; Li, C.; Xu, W.; Li, Q.; Yao, W.; Song, Z.; Liu, G. Nickle(II) ions exacerbate bleomycin-induced pulmonary inflammation and fibrosis by activating the ROS/Akt signaling pathway. Environ. Sci. Pollut. Res. Int. 2018, 25, 4406–4418. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Kato, T.; Ito, M.; Azuma, Y.; Fukasawa, Y.; Ohno, K.; Kojima, S. Hydrogen ameliorates pulmonary hypertension in rats by anti-inflammatory and antioxidant effects. J. Thorac. Cardiovasc. Surg. 2015, 150, 645–654.e3. [Google Scholar] [CrossRef]
- Li, Q.; Hu, L.; Li, J.; Yu, P.; Hu, F.; Wan, B.; Xu, M.; Cheng, H.; Yu, W.; Jiang, L.; et al. Hydrogen Attenuates Endotoxin-Induced Lung Injury by Activating Thioredoxin 1 and Decreasing Tissue Factor Expression. Front. Immunol. 2021, 12, 625957. [Google Scholar] [CrossRef]
- Wang, S.T.; Bao, C.; He, Y.; Tian, X.; Yang, Y.; Zhang, T.; Xu, K.F. Hydrogen gas (XEN) inhalation ameliorates airway inflammation in asthma and COPD patients. QJM Int. J. Med. 2020, 113, 870–875. [Google Scholar] [CrossRef]
- Meng, J.; Liu, L.; Wang, D.; Yan, Z.; Chen, G. Hydrogen gas represses the progression of lung cancer via down-regulating CD47. Biosci. Rep. 2020, 40. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.G.; Sun, W.Z.; Hu, J.Y.; Jie, Z.J.; Xu, J.F.; Cao, J.; Song, Y.L.; Wang, C.H.; Wang, J.; Zhao, H.; et al. Hydrogen/oxygen therapy for the treatment of an acute exacerbation of chronic obstructive pulmonary disease: Results of a multicenter, randomized, double-blind, parallel-group controlled trial. Respir. Res. 2021, 22, 149. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Jiang, D.; Geng, J.; Dong, R.; Dai, H. Hydrogen inhalation attenuated bleomycin-induced pulmonary fibrosis by inhibiting transforming growth factor-β1 and relevant oxidative stress and epithelial-to-mesenchymal transition. Exp. Physiol. 2019, 104, 1942–1951. [Google Scholar] [CrossRef]
- Ashcroft, T.; Simpson, J.M.; Timbrell, V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J. Clin. Pathol. 1988, 41, 467–470. [Google Scholar] [CrossRef]
- Chapman, H.A. Epithelial-mesenchymal interactions in pulmonary fibrosis. Annu. Rev. Physiol. 2011, 73, 413–435. [Google Scholar] [CrossRef]



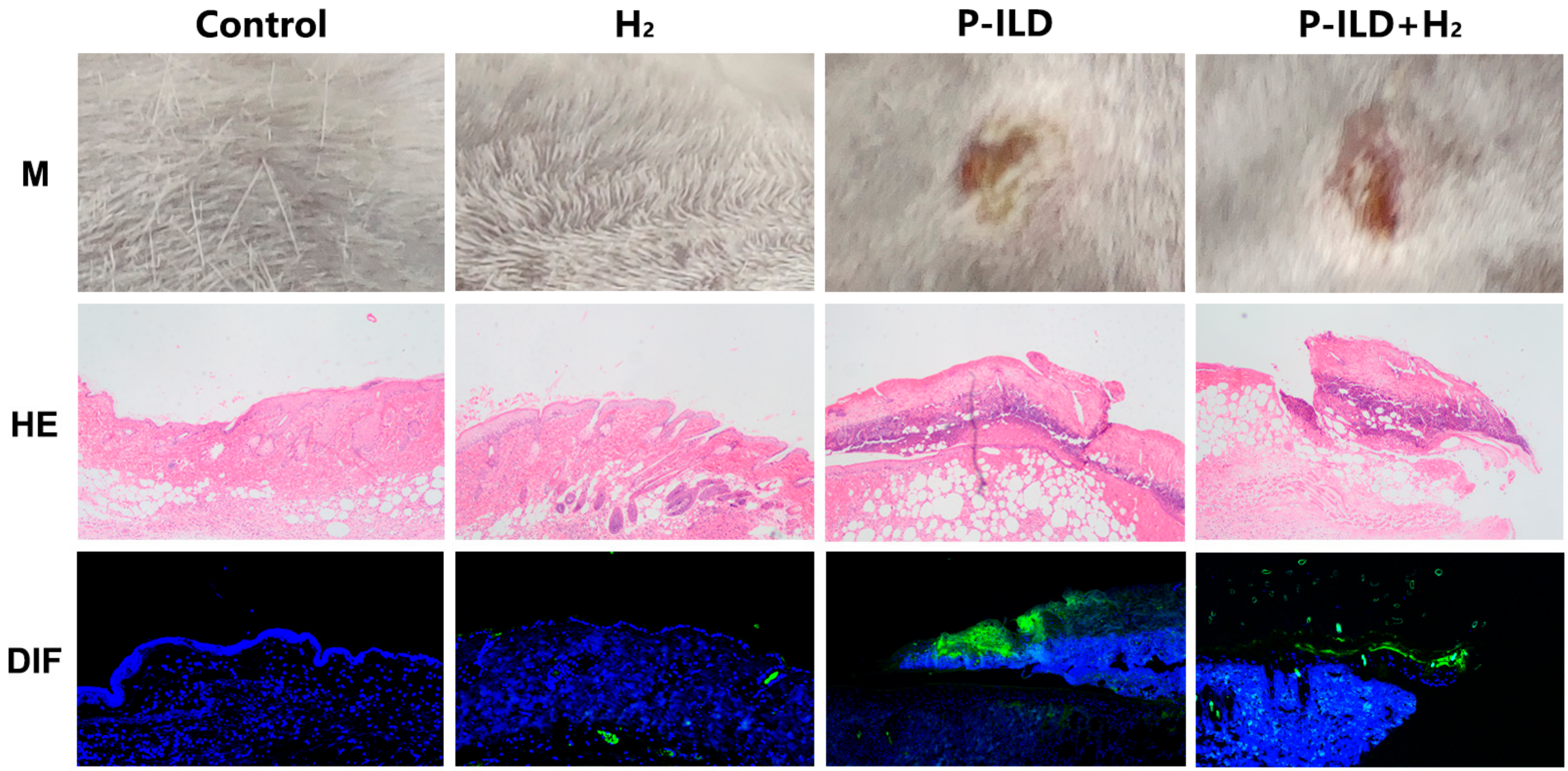
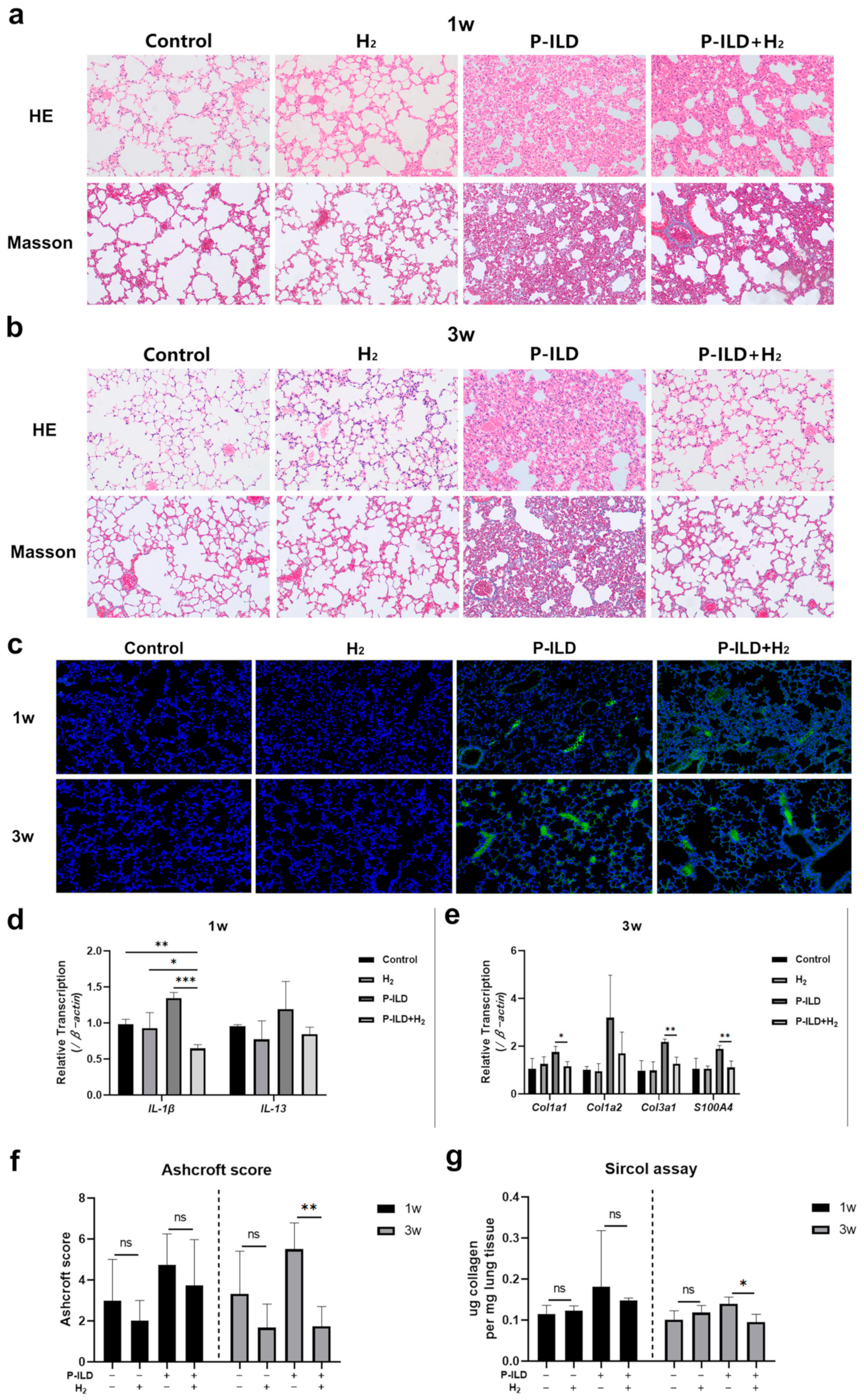


| Groups | Anti-Dsg 1 Antibody Titers (0.1–20 U/mL) | Anti-Dsg 3 Antibody Titers (0.1–20 U/mL) |
|---|---|---|
| Anti-Dsg 1H/3L (HL) | ≥100 | ≤10 |
| Anti-Dsg 1L/3H (LH) | ≤10 | ≥100 |
| Anti-Dsg 1H/3H (HH) | ≥100 | ≥100 |
| Anti-Dsg 1L/3L (LL) | ≤10 | ≤10 |
| Normal healthy (N) | ≤10 | ≤10 |
| Name | Species | Sequence (5′ to 3′) |
|---|---|---|
| α-SMA-F | Mouse | GAGCGTGGCTATTCCTTCGT |
| α-SMA-R | Mouse | GCCCATCAGGCAACTCGTAA |
| β-actin-F | Mouse | GGCTGTATTCCCCTCCATCG |
| β-actin-R | Mouse | CCAGTTGGTAACAATGCCATGT |
| Col1a1-F | Mouse | GGTCCACAAGGTTTCCAAGG |
| Col1a1-R | Mouse | GCTGTTCCAGGCAATCCAC |
| Col1a2-F | Mouse | GGACCCGTTGGCAAAGATG |
| Col1a2-R | Mouse | CACCAGGAGGACCAGGAG |
| Col3a1-F | Mouse | GAGGAAACAGAGGTGAAAGAGG |
| Col3a1-R | Mouse | CAGCAATGGCAGCAGCAC |
| IL-1β-F | Mouse | AAGGAGAACCAAGCAACGACAAAA |
| IL-1β-R | Mouse | TGGGGAACTCTGCAGACTCAAACT |
| IL-13-F | Mouse | TGAGCAACATCACACAAGACC |
| IL-13-R | Mouse | GGCCTTGCGGTTACAGAGG |
| S100A4-F | Mouse | TGAGCAACTTGGACAGCAACA |
| S100A4-R | Mouse | CTTCTTCCGGGGCTCCTTATC |
| TGF-β-F | Mouse | ATTCCTGGCGTTACCTTGG |
| TGF-β-R | Mouse | CCTGTATTCCGTCTCCTTGG |
| Cat-F | Mouse | AGCGACCAGATGAAGCAGTG |
| Cat-R | Mouse | TCCGCTCTCTGTCAAAGTGTG |
| GPx-1-F | Mouse | AGTCCACCGTGTATGCCTTCT |
| GPx-1-R | Mouse | GAGACGCGACATTCTCAATGA |
| GPx-2-F | Mouse | GAGCTGCAATGTCGCTTTCC |
| GPx-2-R | Mouse | TGGGTAAGACTAAAGGTGGGC |
| GPx-3-F | Mouse | CCTTTTAAGCAGTATGCAGGCA |
| GPx-3-R | Mouse | CAAGCCAAATGGCCCAAGTT |
| Sod1-F | Mouse | AACCAGTTGTGTTGTCAGGAC |
| Sod1-R | Mouse | CCACCATGTTTCTTAGAGTGAGG |
| Sod2-F | Mouse | CAGACCTGCCTTACGACTATGG |
| Sod2-R | Mouse | CTCGGTGGCGTTGAGATTGTT |
| Sod3-F | Mouse | CCTTCTTGTTCTACGGCTTGC |
| Sod3-R | Mouse | GCGTGTCGCCTATCTTCTCAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, C.; Wang, L.; Chen, Z.; Shi, X.; Chen, Y.; Yang, J.; Gao, H.; Guan, C.; He, S.; Zhang, L.; et al. Molecular Hydrogen Ameliorates Anti-Desmoglein 1 Antibody-Induced Pemphigus-Associated Interstitial Lung Disease by Inhibiting Oxidative Stress. Int. J. Mol. Sci. 2025, 26, 4203. https://doi.org/10.3390/ijms26094203
Tang C, Wang L, Chen Z, Shi X, Chen Y, Yang J, Gao H, Guan C, He S, Zhang L, et al. Molecular Hydrogen Ameliorates Anti-Desmoglein 1 Antibody-Induced Pemphigus-Associated Interstitial Lung Disease by Inhibiting Oxidative Stress. International Journal of Molecular Sciences. 2025; 26(9):4203. https://doi.org/10.3390/ijms26094203
Chicago/Turabian StyleTang, Chang, Lanting Wang, Zihua Chen, Xiangguang Shi, Yahui Chen, Jin Yang, Haiqing Gao, Chenggong Guan, Shan He, Luyao Zhang, and et al. 2025. "Molecular Hydrogen Ameliorates Anti-Desmoglein 1 Antibody-Induced Pemphigus-Associated Interstitial Lung Disease by Inhibiting Oxidative Stress" International Journal of Molecular Sciences 26, no. 9: 4203. https://doi.org/10.3390/ijms26094203
APA StyleTang, C., Wang, L., Chen, Z., Shi, X., Chen, Y., Yang, J., Gao, H., Guan, C., He, S., Zhang, L., Zheng, S., Yang, F., Chen, S.-A., Ma, L., Zhang, Z., Zhao, Y., Liu, Q., Wang, J., & Luo, X. (2025). Molecular Hydrogen Ameliorates Anti-Desmoglein 1 Antibody-Induced Pemphigus-Associated Interstitial Lung Disease by Inhibiting Oxidative Stress. International Journal of Molecular Sciences, 26(9), 4203. https://doi.org/10.3390/ijms26094203






