HIF-1α Pathway in COVID-19: A Scoping Review of Its Modulation and Related Treatments
Abstract
1. Introduction
2. Materials and Methods
3. Results
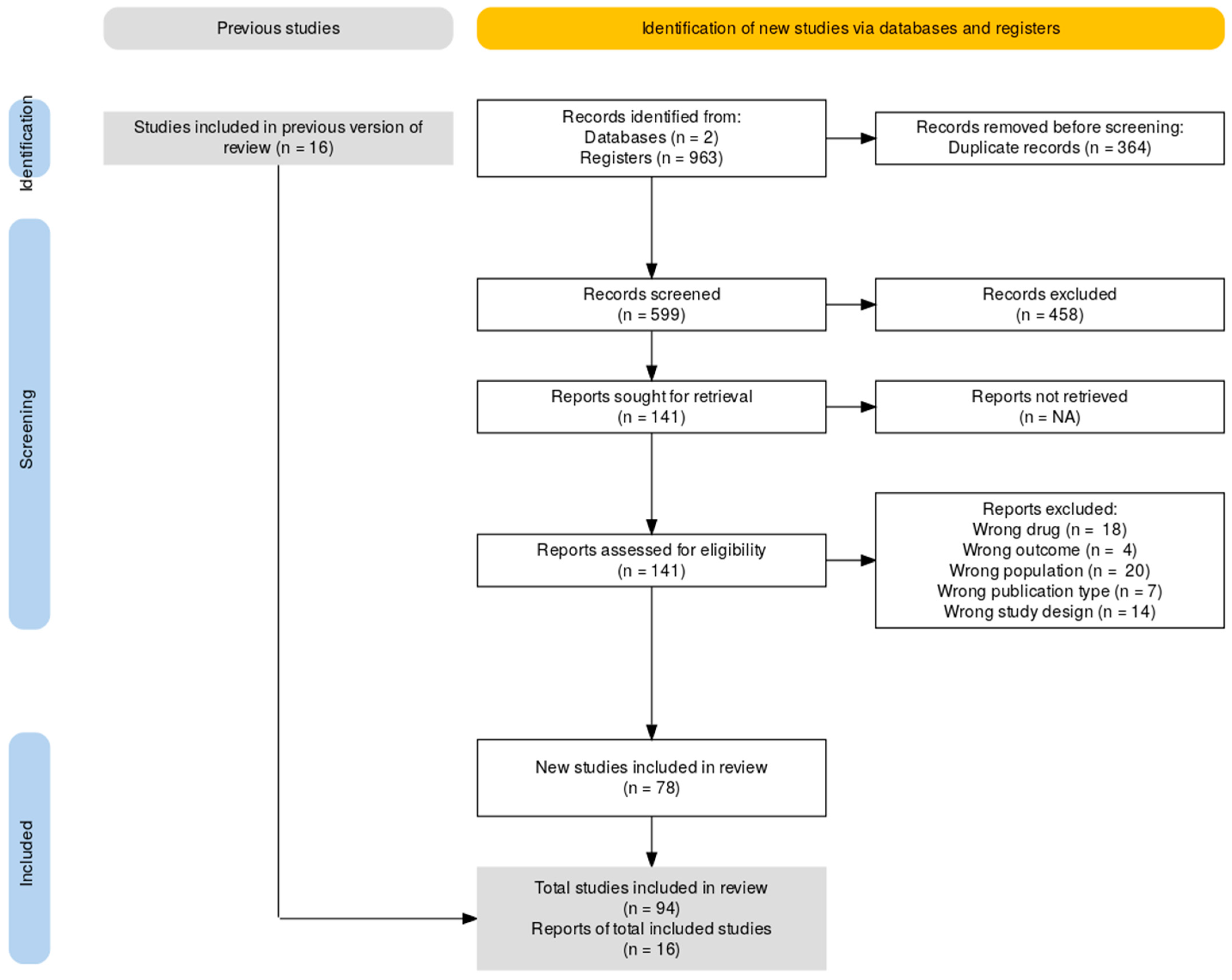
Search
4. Discussion
4.1. Hypoxia-Inducible Factor (HIF) Physiologic Pathway
4.1.1. Regulation of HIF Under Normoxic Conditions
4.1.2. Regulation of HIF Under Hypoxic Conditions
4.2. HIF Signaling in COVID-19
4.2.1. Silent Hypoxia in COVID-19
4.2.2. Post-Acute COVID-19 Syndrome: Does It Have Any Relation to the Role of HIF-1α?
4.3. The TNF-α/NF-κB/HIF-1α/VEGF Pathway Induced by SARS-CoV-2
4.4. Modulation of Viral Entry Through HIF-1α
4.4.1. Promotion of Viral Replication Through Metabolic Reprogramming
4.4.2. HIF Modulation of SARS-CoV-2 Viral Entry: Mechanisms Involving ACE2 and TMPRSS2
4.5. New Insights on COVID-19 Treatment: Innovative Drugs Targeting the HIF Pathway
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. COVID-19 Deaths Dashboard; World Health Organization: Geneva, Switzerland, 2025; Available online: https://data.who.int/dashboards/covid19/deaths?n=o (accessed on 3 March 2025).
- Boopathi, V.; Nahar, J.; Murugesan, M.; Subramaniyam, S.; Kong, B.M.; Choi, S.-K.; Lee, C.-S.; Ling, L.; Yang, D.U.; Yang, D.C.; et al. In silico and in vitro inhibition of host-based viral entry targets and cytokine storm in COVID-19 by ginsenoside compound K. Heliyon 2023, 9, e19341. [Google Scholar] [CrossRef] [PubMed]
- Roshan, V.D.; Ahmadian, M.; Nasiri, K.; Akbari, A.; Ghasemi, M.; Borujeni, N.N.; Zahedmanesh, F.; Chashmi, S.M.N.; Imani, F. Exercise-induced expression of SARS-CoV-2 entry receptors: Impact of mask modality, sex, and exercise intensity. J. Sports Med. Phys. Fit. 2023, 63, 319–328. [Google Scholar] [CrossRef]
- Ardanuy, J.; Johnson, R.; Dillen, C.; Taylor, L.; Hammond, H.; Weston, S.; Frieman, M. Pyronaridine tetraphosphate is an efficacious antiviral and anti-inflammatory active against multiple highly pathogenic coronaviruses. mBio 2023, 14, e0158723. [Google Scholar] [CrossRef]
- Liu, A.; Zhang, X.; Li, R.; Zheng, M.; Yang, S.; Dai, L.; Wu, A.; Hu, C.; Huang, Y.; Xie, M.; et al. Overexpression of the SARS-CoV-2 receptor ACE2 is induced by cigarette smoke in bronchial and alveolar epithelia. J. Pathol. 2021, 253, 17–30. [Google Scholar] [CrossRef]
- Bank, S.; De, S.K.; Bankura, B.; Maiti, S.; Das, M.; A Khan, G. ACE/ACE2 balance might be instrumental to explain the certain comorbidities leading to severe COVID-19 cases. Biosci. Rep. 2021, 41, BSR20202014. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.-R.; Geng, R.; Li, Q.; Chen, Y.; Li, S.-F.; Wang, Q.; Min, J.; Yang, Y.; Li, B.; Jiang, R.-D.; et al. ACE2-independent infection of T lymphocytes by SARS-CoV-2. Signal Transduct. Target. Ther. 2022, 7, 83. [Google Scholar] [CrossRef]
- Wicik, Z.; Eyileten, C.; Nowak, A.; Keshwani, D.; Simões, S.N.; Martins, D.C.; Klos, K.; Wlodarczyk, W.; Assinger, A.; Soldacki, D.; et al. Alteration of circulating ACE2-network related microRNAs in patients with COVID-19. Sci. Rep. 2024, 14, 13573. [Google Scholar] [CrossRef]
- Beyerstedt, S.; Casaro, E.B.; Rangel, É.B. COVID-19: Angiotensin-Converting Enzyme 2 (ACE2) Expression and Tissue Susceptibility to SARS-CoV-2 Infection. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 905–919. [Google Scholar] [CrossRef]
- Zhang, H.; Penninger, J.M.; Li, Y.; Zhong, N.; Slutsky, A.S. Angiotensin-Converting Enzyme 2 (ACE2) as a SARS-CoV-2 Receptor: Molecular Mechanisms and Potential Therapeutic Target. Intensive Care Med. 2020, 46, 586–590. [Google Scholar] [CrossRef]
- Ballering, A.V.; van Zon, S.K.R.; Hartman, T.C.O.; Rosmalen, J.G.M. Persistence of somatic symptoms after COVID-19 in the Netherlands: An observational cohort study. Lancet 2022, 400, 452–461. [Google Scholar] [CrossRef]
- Zha, S.; Liu, X.; Yao, Y.; He, Y.; Wang, Y.; Zhang, Q.; Zhang, J.; Yi, Y.; Xiao, R.; Hu, K. Short-term intermittent hypoxia exposure for dyspnea and fatigue in post-acute sequelae of COVID-19: A randomized controlled study. Respir. Med. 2024, 232, 107763. [Google Scholar] [CrossRef] [PubMed]
- Romão, P.R.; Teixeira, P.C.; Schipper, L.; da Silva, I.; Filho, P.S.; Júnior, L.C.R.; Peres, A.; da Fonseca, S.G.; Monteiro, M.C.; Lira, F.S.; et al. Viral load is associated with mitochondrial dysfunction and altered monocyte phenotype in acute severe SARS-CoV-2 infection. Int. Immunopharmacol. 2022, 108, 108697. [Google Scholar] [CrossRef] [PubMed]
- Miggiolaro, A.F.R.S.; da Silva, F.P.G.; Wiedmer, D.B.; Godoy, T.M.; Borges, N.H.; Piper, G.W.; Oricil, A.G.G.; Klein, C.K.; Hlatchuk, E.C.; Dagostini, J.C.H.; et al. COVID-19 and Pulmonary Angiogenesis: The Possible Role of Hypoxia and Hyperinflammation in the Overexpression of Proteins Involved in Alveolar Vascular Dysfunction. Viruses 2023, 15, 706. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.J.; Vadakke-Madathil, S.; Croft, L.B.; Brody, R.I.; Chaudhry, H.W. HIF-1α Cardioprotection in COVID-19 Patients. JACC Basic Transl. Sci. 2022, 7, 67–69. [Google Scholar] [CrossRef]
- Zalpoor, H.; Rezaei, M.; Yahyazadeh, S.; Ganjalikhani-Hakemi, M. Flt3-ITD mutated acute myeloid leukemia patients and COVID-19: Potential roles of autophagy and HIF-1α in leukemia progression and mortality. Hum. Cell 2022, 35, 1304–1305. [Google Scholar] [CrossRef]
- Ivan, M.; Kondo, K.; Yang, H.; Kim, W.; Valiando, J.; Ohh, M.; Salic, A.; Asara, J.M.; Lane, W.S.; Kaelin, W.G., Jr. HIFalpha Targeted for VHL-Mediated Destruction by Proline Hydroxylation. Science 2001, 292, 464–468. [Google Scholar] [CrossRef]
- Kaelin, W.G., Jr.; Ratcliffe, P.J. Oxygen Sensing by Metazoans: The Central Role of the HIF Hydroxylase Pathway. Mol. Cell 2008, 30, 393–402. [Google Scholar] [CrossRef]
- Zhang, H.; Bosch-Marce, M.; Shimoda, L.A.; Tan, Y.S.; Baek, J.H.; Wesley, J.B.; Gonzalez, F.J.; Semenza, G.L. Mitochondrial Autophagy Is an HIF-1-dependent Adaptive Metabolic Response to Hypoxia. J. Biol. Chem. 2008, 283, 10892–10903. [Google Scholar] [CrossRef]
- Manalo, D.J.; Rowan, A.; Lavoie, T.; Natarajan, L.; Kelly, B.D.; Ye, S.Q.; Garcia, J.G.N.; Semenza, G.L. Transcriptional Regulation of Vascular Endothelial Cell Responses to Hypoxia by HIF-1. Blood 2005, 105, 659–669. [Google Scholar] [CrossRef]
- Codo, A.C.; Davanzo, G.G.; de Brito Monteiro, L.; de Souza, G.F.; Muraro, S.P.; Virgilio-Da-Silva, J.V.; Prodonoff, J.S.; Carregari, V.C.; de Biagi Junior, C.A.O.; Crunfli, F.; et al. Elevated Glucose Levels Favor SARS-CoV-2 Infection and Monocyte Response through a HIF-1α/Glycolysis-Dependent Axis. Cell Metab. 2020, 32, 437–446.e5. [Google Scholar] [CrossRef]
- Palazon, A.; Goldrath, A.W.; Nizet, V.; Johnson, R.S. HIF Transcription Factors, Inflammation, and Immunity. Immunity 2014, 41, 518–528. [Google Scholar] [CrossRef] [PubMed]
- McGettrick, A.F.; O’neill, L.A.J. The Role of HIF in Immunity and Inflammation. Cell Metab. 2020, 32, 524–536. [Google Scholar] [CrossRef]
- Yang, M.; Qi, M.; Xu, L.; Huang, P.; Wang, X.; Sun, J.; Shi, J.; Hu, Y. Differential host circRNA expression profiles in human lung epithelial cells infected with SARS-CoV-2. Infect. Genet. Evol. 2021, 93, 104923. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Chu, S.; Shapiro, S.; Young, L.; Russo, M.; De Paepe, M.E. Placental SARS-CoV-2 distribution correlates with level of tissue oxygenation in COVID-19-associated necrotizing histiocytic intervillositis/perivillous fibrin deposition. Placenta 2021, 117, 187–193. [Google Scholar] [CrossRef]
- Ackermann, M.; Ackermann, M.; Kamp, J.C.; Kamp, J.C.; Werlein, C.; Werlein, C.; Walsh, C.L.; Walsh, C.L.; Stark, H.; Stark, H.; et al. The fatal trajectory of pulmonary COVID-19 is driven by lobular ischemia and fibrotic remodelling. eBioMedicine 2022, 85, 104296. [Google Scholar] [CrossRef] [PubMed]
- Krenytska, D.; Strubchevska, K.; Kozyk, M.; Vovk, T.; Halenova, T.; Kot, L.; Raksha, N.; Savchuk, O.; Falalyeyeva, T.; Tsyryuk, O.; et al. Circulating levels of inflammatory cytokines and angiogenesis-related growth factors in patients with osteoarthritis after COVID-19. Front. Med. 2023, 10, 1168487. [Google Scholar] [CrossRef]
- Wulandari, E.; Hapsari, R.; Tjakradidjaja, F.; Alfiah; Suri, A. The Cytoglobin Expression Under Hypoxic Conditions in COVID-19 Cases. Open Biomark J. 2023, 13, e187531832304120. [Google Scholar] [CrossRef]
- Abdolahi, S.; Hosseini, M.; Rezaei, R.; Mohebbi, S.R.; Rostami-Nejad, M.; Mojarad, E.N.; Mirjalali, H.; Yadegar, A.; Aghdaei, H.A.; Zali, M.R.; et al. Evaluation of miR-200c-3p and miR-421-5p levels during immune responses in the admitted and recovered COVID-19 subjects. Infect. Genet. Evol. 2022, 98, 105207. [Google Scholar] [CrossRef]
- Alghanem, B.; Mansour, F.A.; Shaibah, H.; Almuhalhil, K.; Almourfi, F.; Alamri, H.S.; Alajmi, H.; Rashid, M.; Alroqi, F.; Jalouli, M.; et al. Quantitative proteomics analysis of COVID-19 patients: Fetuin-A and tetranectin as potential modulators of innate immune responses. Heliyon 2023, 9, e15224. [Google Scholar] [CrossRef]
- Manivannan, J.; Sundaresan, L. Systems level insights into the impact of airborne exposure on SARS-CoV-2 pathogenesis and COVID-19 outcome—A multi-omics big data study. Gene Rep. 2021, 25, 101312. [Google Scholar] [CrossRef]
- Wang, K.; Khoramjoo, M.; Srinivasan, K.; Gordon, P.M.; Mandal, R.; Jackson, D.; Sligl, W.; Grant, M.B.; Penninger, J.M.; Borchers, C.H.; et al. Sequential multi-omics analysis identifies clinical phenotypes and predictive biomarkers for long COVID. Cell Rep. Med. 2023, 4, 101254. [Google Scholar] [CrossRef]
- Barman, R.K.; Mukhopadhyay, A.; Maulik, U.; Das, S. A network biology approach to identify crucial host targets for COVID-19. Methods 2022, 203, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, N.; Saha, I.; Sharma, N. Interactome of human and SARS-CoV-2 proteins to identify human hub proteins associated with comorbidities. Comput. Biol. Med. 2021, 138, 104889. [Google Scholar] [CrossRef] [PubMed]
- Rahman, H.; Rana, H.K.; Peng, S.; Kibria, G.; Islam, Z.; Mahmud, S.M.H.; Moni, M.A. Bioinformatics and system biology approaches to identify pathophysiological impact of COVID-19 to the progression and severity of neurological diseases. Comput. Biol. Med. 2021, 138, 104859. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Sharma, B.; Mazid, I.; Akhand, R.N.; Das, M.; Marufatuzzahan, M.; Chowdhury, T.A.; Azim, K.F.; Hasan, M. Identification and host response interaction study of SARS-CoV-2 encoded miRNA-like sequences: An in silico approach. Comput. Biol. Med. 2021, 134, 104451. [Google Scholar] [CrossRef]
- Saleki, K.; Aram, C.; Alijanizadeh, P.; Khanmirzaei, M.H.; Vaziri, Z.; Ramzankhah, M.; Azadmehr, A. Matrix metalloproteinase/Fas ligand (MMP/FasL) interaction dynamics in COVID-19: An in silico study and neuroimmune perspective. Heliyon 2024, 10, e30898. [Google Scholar] [CrossRef]
- Sheerin, D.; Abhimanyu; Peton, N.; Vo, W.; Allison, C.C.; Wang, X.; Johnson, W.E.; Coussens, A.K. Immunopathogenic overlap between COVID-19 and tuberculosis identified from transcriptomic meta-analysis and human macrophage infection. iScience 2022, 25, 104464. [Google Scholar] [CrossRef]
- Wu, Q.; Coumoul, X.; Grandjean, P.; Barouki, R.; Audouze, K. Endocrine disrupting chemicals and COVID-19 relationships: A computational systems biology approach. Environ. Int. 2021, 157, 106232. [Google Scholar] [CrossRef]
- Yuan, J.; Chen, F.; Fan, D.; Jiang, Q.; Xue, Z.; Zhang, J.; Yu, X.; Li, K.; Qu, J.; Su, J. EyeDiseases: An integrated resource for dedicating to genetic variants, gene expression and epigenetic factors of human eye diseases. NAR Genom. Bioinform. 2021, 3, lqab050. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, Y.; Li, C.; Stine, L.D.; Wang, P.-H.; Turnbull, M.W.; Wu, H.; Liu, Q. Ectopic expression of SARS-CoV-2 S and ORF-9B proteins alters metabolic profiles and impairs contractile function in cardiomyocytes. Front. Cell Dev. Biol. 2023, 11, 1110271. [Google Scholar] [CrossRef]
- Caldwell, B.A.; Wu, Y.; Wang, J.; Li, L. Altered DNA methylation underlies monocyte dysregulation and immune exhaustion memory in sepsis. Cell Rep. 2024, 43, 113894. [Google Scholar] [CrossRef]
- Du, T.; Gao, C.; Lu, S.; Liu, Q.; Yu, W.; Li, W.; Sun, Y.Q.; Tang, C.; Wang, J.; Gao, J.; et al. Differential Transcriptomic Landscapes of SARS-CoV-2 Variants in Multiple Organs from Infected Rhesus Macaques. Genom. Proteom. Bioinform. 2023, 21, 1014–1029. [Google Scholar] [CrossRef] [PubMed]
- Lei, H. Hypoxia and Activation of Neutrophil Degranulation-Related Genes in the Peripheral Blood of COVID-19 Patients. Viruses 2024, 16, 201. [Google Scholar] [CrossRef]
- Maria, N.I.; Rapicavoli, R.V.; Alaimo, S.; Bischof, E.; Stasuzzo, A.; Broek, J.A.C.; Pulvirenti, A.; Mishra, B.; Duits, A.J.; Ferro, A. Application of the PHENotype SIMulator for rapid identification of potential candidates in effective COVID-19 drug repurposing. Heliyon 2023, 9, e14115. [Google Scholar] [CrossRef] [PubMed]
- Saccon, E.; Chen, X.; Mikaeloff, F.; Rodriguez, J.E.; Szekely, L.; Vinhas, B.S.; Krishnan, S.; Byrareddy, S.N.; Frisan, T.; Végvári, Á.; et al. Cell-type-resolved quantitative proteomics map of interferon response against SARS-CoV-2. iScience 2021, 24, 102420. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, Z.; Ran, P.; Xiang, H.; Xu, Z.; Xu, N.; Deng, M.; Zhu, L.; Yin, Y.; Feng, J.; et al. Serum proteome reveals distinctive molecular features of H7N9- and SARS-CoV-2-infected patients. Cell Rep. 2024, 43, 114900. [Google Scholar] [CrossRef]
- Pooladanda, V.; Thatikonda, S.; Sunnapu, O.; Tiwary, S.; Vemula, P.K.; Talluri, M.V.N.K.; Godugu, C. iRGD conjugated nimbolide liposomes protect against endotoxin induced acute respiratory distress syndrome. Nanomed. Nanotechnol. Biol. Med. 2021, 33, 102351. [Google Scholar] [CrossRef]
- Shrimali, N.M.; Agarwal, S.; Kaur, S.; Bhattacharya, S.; Bhattacharyya, S.; Prchal, J.T.; Guchhait, P. α-Ketoglutarate Inhibits Thrombosis and Inflammation by Prolyl Hydroxylase-2 Mediated Inactivation of Phospho-Akt. eBioMedicine 2021, 73, 103672. [Google Scholar] [CrossRef]
- Keller, G.A.; Colaianni, I.; Coria, J.; Di Girolamo, G.; Miranda, S. Clinical and biochemical short-term effects of hyperbaric oxygen therapy on SARS-Cov-2+ hospitalized patients with hypoxemic respiratory failure. Respir. Med. 2023, 209, 107155. [Google Scholar] [CrossRef]
- Kotagiri, P.; Mescia, F.; Hanson, A.L.; Turner, L.; Bergamaschi, L.; Peñalver, A.; Richoz, N.; Moore, S.D.; Ortmann, B.M.; Dunmore, B.J.; et al. The impact of hypoxia on B cells in COVID-19. eBioMedicine 2022, 77, 103878. [Google Scholar] [CrossRef]
- Whiley, L.; Lawler, N.G.; Zeng, A.X.; Lee, A.; Chin, S.-T.; Bizkarguenaga, M.; Bruzzone, C.; Embade, N.; Wist, J.; Holmes, E.; et al. Cross-Validation of Metabolic Phenotypes in SARS-CoV-2 Infected Subpopulations Using Targeted Liquid Chromatography–Mass Spectrometry (LC-MS). J. Proteome Res. 2024, 23, 1313–1327. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Hypoxia-Inducible Factors in Physiology and Medicine. Cell 2012, 148, 399–408. [Google Scholar] [CrossRef]
- Terraneo, L.; Samaja, M. Comparative Response of Brain to Chronic Hypoxia and Hyperoxia. Int. J. Mol. Sci. 2017, 18, 1914. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Jiang, B.-H.; Rue, E.A.; Semenza, G.L. Hypoxia-Inducible Factor 1 Is a BASIC-HELIX-LOOP-HELIX-PAS HETERODIMER REGULATED by Cellular O2 Tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510–5514. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, J.A.; Jiang, B.-H.; Iyer, N.V.; Agani, F.; Leung, S.W.; Koos, R.D.; Semenza, G.L. Activation of Vascular Endothelial Growth Factor Gene Transcription by Hypoxia-Inducible Factor 1. Mol. Cell. Biol. 1996, 16, 4604–4613. [Google Scholar] [CrossRef]
- Semenza, G.L. Pharmacologic Targeting of Hypoxia-Inducible Factors. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 379–403. [Google Scholar] [CrossRef]
- Kyoto Encyclopedia of Genes and Genomes (KEGG). KEGG Pathway Database: HIF-1 Signaling Pathway. Available online: https://www.kegg.jp/pathway/hsa04066 (accessed on 16 January 2025).
- Chen, C.; Pore, N.; Behrooz, A.; Ismail-Beigi, F.; Maity, A. Regulation of Glut1 mRNA by Hypoxia-Inducible Factor-1: Interaction Between H-Ras and Hypoxia. J. Biol. Chem. 2001, 276, 9519–9525. [Google Scholar] [CrossRef]
- Jaśkiewicz, M.; Moszyńska, A.; Króliczewski, J.; Cabaj, A.; Bartoszewska, S.; Charzyńska, A.; Gebert, M.; Dąbrowski, M.; Collawn, J.F.; Bartoszewski, R. The transition from HIF-1 to HIF-2 during prolonged hypoxia results from reactivation of PHDs and HIF1A mRNA instability. Cell. Mol. Biol. Lett. 2022, 27, 109. [Google Scholar] [CrossRef]
- Brahimi-Horn, M.C.; Pouysségur, J. Oxygen, a Source of Life and Stress. FEBS Lett. 2007, 581, 3582–3591. [Google Scholar] [CrossRef]
- Fritch, E.J.; Mordant, A.L.; Gilbert, T.S.; Wells, C.I.; Yang, X.; Barker, N.K.; Madden, E.A.; Dinnon, K.H.; Hou, Y.J.; Tse, L.V.; et al. Investigation of the Host Kinome Response to Coronavirus Infection Reveals PI3K/mTOR Inhibitors as Betacoronavirus Antivirals. J. Proteome Res. 2023, 22, 3159–3177. [Google Scholar] [CrossRef]
- Li, Y.; Chu, F.; Li, P.; Johnson, N.; Li, T.; Wang, Y.; An, R.; Wu, D.; Chen, J.; Su, Z.; et al. Potential effect of Maxing Shigan decoction against coronavirus disease 2019 (COVID-19) revealed by network pharmacology and experimental verification. J. Ethnopharmacol. 2021, 271, 113854. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, S.; Zhang, Z.; Lee, X.; Wu, W.; Huang, Z.; Lei, Z.; Xu, W.; Chen, D.; Wu, X.; et al. Association between the nasopharyngeal microbiome and metabolome in patients with COVID-19. Synth. Syst. Biotechnol. 2021, 6, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Manohar, K.; Gupta, R.K.; Gupta, P.; Saha, D.; Gare, S.; Sarkar, R.; Misra, A.; Giri, L. FDA approved L-type channel blocker Nifedipine reduces cell death in hypoxic A549 cells through modulation of mitochondrial calcium and superoxide generation. Free. Radic. Biol. Med. 2021, 177, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Tabassum, T.; Araf, Y.; Al Nahid, A.; Ullah, M.A.; Hosen, M.J. Silent hypoxia in COVID-19: Pathomechanism and possible management strategy. Mol. Biol. Rep. 2021, 48, 3863–3869. [Google Scholar] [CrossRef]
- Devaux, C.A.; Lagier, J.-C. Unraveling the Underlying Molecular Mechanism of ‘Silent Hypoxia’ in COVID-19 Patients Suggests a Central Role for Angiotensin II Modulation of the AT1R-Hypoxia-Inducible Factor Signaling Pathway. J. Clin. Med. 2023, 12, 2445. [Google Scholar] [CrossRef]
- Chapola, H.; de Bastiani, M.A.; Duarte, M.M.; Freitas, M.B.; Schuster, J.S.; de Vargas, D.M.; Klamt, F. A comparative study of COVID-19 transcriptional signatures between clinical samples and preclinical cell models in the search for disease master regulators and drug repositioning candidates. Virus Res. 2023, 326, 199053. [Google Scholar] [CrossRef]
- Fernández, R.; González, S.; Rey, S.; Cortés, P.P. Carotid Body Chemoreceptors: Physiology, Pathology, and Implications for Health and Disease. Physiol. Rev. 2021, 101, 1177–1235. [Google Scholar] [CrossRef]
- Kjellberg, A.; Zhao, A.; Lussier, A.; Hassler, A.; Al-Ezerjawi, S.; Boström, E.; Catrina, S.-B.; Bergman, P.; Rodriguez-Wallberg, K.A.; Lindholm, P. Hyperbaric oxygen therapy as an immunomodulatory intervention in COVID-19-induced ARDS: Exploring clinical outcomes and transcriptomic signatures in a randomised controlled trial. Pulm. Pharmacol. Ther. 2024, 87, 102330. [Google Scholar] [CrossRef]
- Yesilkaya, N.; Tellioglu, T.M.; Unay, F.C.; İner, H.; Besir, Y.; Gokalp, O.; Yılık, L.; Gurbuz, A. Histopathologic Evaluation of COVID-19 Patients with Peripheral Arterial Thromboembolism: Does Clot Composition Make Any Sense? Ann. Vasc. Surg. 2021, 74, 80–87. [Google Scholar] [CrossRef]
- Vaz de Paula, C.B.; Nagashima, S.; Liberalesso, V.; Collete, M.; da Silva, F.P.G.; Oricil, A.G.G.; Barbosa, G.S.; da Silva, G.V.C.; Wiedmer, D.B.; Dezidério, F.d.S.; et al. COVID-19: Análise imuno-histoquímica das vias de sinalização do TGF-β na fibrose pulmonar. Rev. Int. Ciênc. Mol. 2022, 23, 168. [Google Scholar] [CrossRef]
- Ackermann, M.; Mentzer, S.J.; Kolb, M.; Jonigk, D. Inflammation and Intussusceptive Angiogenesis in COVID-19: Everything in and Out of Flow. Eur. Respir. J. 2020, 56, 2003147. [Google Scholar] [CrossRef] [PubMed]
- Baskol, G.; Özel, M.; Saracoglu, H.; Ulger, B.; Unuvar, G.K.; Onuk, S.; Bayram, A.; Akin, A.K.; Muhtaroglu, S.; Sagiroglu, P.; et al. New Avenues to Explore in SARS-CoV-2 Infection: Both TRIM25 and TRIM56 Positively Correlate with VEGF, GAS6, and sAXL in COVID-19 Patients. Viral Immunol. 2022, 35, 690–699. [Google Scholar] [CrossRef]
- Weenink, R.P.; de Jonge, S.W.; van Hulst, R.A.; Wingelaar, T.T.; van Ooij, P.-J.A.M.; Immink, R.V.; Preckel, B.; Hollmann, M.W. Perioperative Hyperoxyphobia: Justified or Not? Benefits and Harms of Hyperoxia during Surgery. J. Clin. Med. 2020, 9, 642. [Google Scholar] [CrossRef] [PubMed]
- Iosef, C.; Knauer, M.J.; Nicholson, M.; Van Nynatten, L.R.; Cepinskas, G.; Draghici, S.; Han, V.K.M.; Fraser, D.D. Plasma proteome of Long-COVID patients indicates HIF-mediated vasculo-proliferative disease with impact on brain and heart function. J. Transl. Med. 2023, 21, 377. [Google Scholar] [CrossRef] [PubMed]
- Naidu, A.S.; Wang, C.-K.; Rao, P.; Mancini, F.; Clemens, R.A.; Wirakartakusumah, A.; Chiu, H.-F.; Yen, C.-H.; Porretta, S.; Mathai, I.; et al. Precision nutrition to reset virus-induced human metabolic reprogramming and dysregulation (HMRD) in long-COVID. NPJ Sci. Food 2024, 8, 19. [Google Scholar] [CrossRef]
- Pawlik, M.; Rinneberg, G.; Koch, A.; Meyringer, H.; Loew, T.; Kjellberg, A. Is there a rationale for hyperbaric oxygen therapy in the patients with Post COVID syndrome? Eur. Arch. Psychiatry Clin. Neurosci. 2024, 274, 1797–1817. [Google Scholar] [CrossRef]
- Ketenci, S.; Kalaycı, M.U.; Dündar, B.; Duranay, R.; Aynacıoğlu, A.Ş. Elevated serum midkine levels in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infected patients. Int. Immunopharmacol. 2022, 110, 108939. [Google Scholar] [CrossRef]
- Choi, H.S.; Choi, A.Y.; Kopp, J.B.; Winkler, C.A.; Cho, S.K. Review of COVID-19 Therapeutics by Mechanism: From Discovery to Approval. J. Korean Med. Sci. 2024, 39, e134. [Google Scholar] [CrossRef]
- Ambikan, A.T.; Yang, H.; Krishnan, S.; Akusjärvi, S.S.; Gupta, S.; Lourda, M.; Sperk, M.; Arif, M.; Zhang, C.; Nordqvist, H.; et al. Multi-omics personalized network analyses highlight progressive disruption of central metabolism associated with COVID-19 severity. Cell Syst. 2022, 13, 665–681.e4. [Google Scholar] [CrossRef]
- Kim, J.-W.; Tchernyshyov, I.; Semenza, G.L.; Dang, C.V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006, 3, 177–185. [Google Scholar] [CrossRef]
- Tannahill, G.M.; Curtis, A.M.; Adamik, J.; Palsson-McDermott, E.M.; McGettrick, A.F.; Goel, G.; Frezza, C.; Bernard, N.J.; Kelly, B.; Foley, N.H.; et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 2013, 496, 238–242. [Google Scholar] [CrossRef]
- Zhu, B.; Wu, Y.; Huang, S.; Zhang, R.; Son, Y.M.; Li, C.; Cheon, I.S.; Gao, X.; Wang, M.; Chen, Y.; et al. Uncoupling of macrophage inflammation from self-renewal modulates host recovery from respiratory viral infection. Immunity 2021, 54, 1200–1218.e9. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, J.W.; Dybas, J.M.; Fazelinia, H.; Kim, M.S.; Frere, J.; Zhang, Y.; Albrecht, Y.S.; Murdock, D.G.; Angelin, A.; Singh, L.N.; et al. Core mitochondrial genes are down-regulated during SARS-CoV-2 infection of rodent and human hosts. Sci. Transl. Med. 2023, 15, eabq1533. [Google Scholar] [CrossRef]
- de Andrade, L.G.M.; de Sandes-Freitas, T.V.; Requiao-Mourã, L.R.; Viana, L.A.; Cristelli, M.P.; Garcia, V.D.; Alcântara, A.L.C.; Esmeraldo, R.d.M.; Filho, M.A.; Pacheco-Silva, A.; et al. Development and validation of a simple web-based tool for early prediction of COVID-19-associated death in kidney transplant recipients. Am. J. Transplant. 2021, 22, 610–625. [Google Scholar] [CrossRef]
- Knudson, C.J.; Férez, M.; Alves-Peixoto, P.; Erkes, D.A.; Melo-Silva, C.R.; Tang, L.; Snyder, C.M.; Sigal, L.J. Mechanisms of Antiviral Cytotoxic CD4 T Cell Differentiation. J. Virol. 2021, 95, JVI0056621. [Google Scholar] [CrossRef] [PubMed]
- Akebia Therapeutics. Akebia Therapeutics Announces Initial Findings from Investigator-Sponsored Clinical Study Evaluating Vadadustat for the Prevention and Treatment of Acute Respiratory Distress Syndrome (ARDS) in Subjects with COVID-19 and Hypoxemia. PR Newswire, 25 July 2022. Available online: https://www.prnewswire.com/news-releases/akebia-therapeutics-announces-initial-findings-from-investigator-sponsored-clinical-study-evaluating-vadadustat-for-the-prevention-and-treatment-of-acute-respiratory-distress-syndrome-ards-in-subjects-with-covid-19-and-hypoxemia-301600306.html (accessed on 10 January 2025).
- Khanal, P.; Patil, V.S.; Bhandare, V.V.; Dwivedi, P.S.; Shastry, C.S.; Patil, B.M.; Gurav, S.S.; Harish, D.R.; Roy, S. Computational investigation of benzalacetophenone derivatives against SARS-CoV-2 as potential multi-target bioactive compounds. Comput. Biol. Med. 2022, 146, 105668. [Google Scholar] [CrossRef]
- Lewis, S.A.; Cinco, I.R.; Doratt, B.M.; Blanton, M.B.; Hoagland, C.; Newman, N.; Davies, M.; Grant, K.A.; Messaoudi, I. Chronic alcohol consumption dysregulates innate immune response to SARS-CoV-2 in the lung. eBioMedicine 2023, 97, 104812. [Google Scholar] [CrossRef]
- Pang, J.; Xu, F.; Aondio, G.; Li, Y.; Fumagalli, A.; Lu, M.; Valmadre, G.; Wei, J.; Bian, Y.; Canesi, M.; et al. Efficacy and tolerability of bevacizumab in patients with severe COVID-19. Nat. Commun. 2021, 12, 814. [Google Scholar] [CrossRef]
- Das, J.K.; Chakraborty, S.; Roy, S. A scheme for inferring viral-host associations based on codon usage patterns identifies the most affected signaling pathways during COVID-19. J. Biomed. Inform. 2021, 118, 103801. [Google Scholar] [CrossRef]
- Devaux, C.A.; Raoult, D. The impact of COVID-19 on populations living at high altitude: Role of hypoxia-inducible factors (HIFs) signaling pathway in SARS-CoV-2 infection and replication. Front. Physiol. 2022, 13, 960308. [Google Scholar] [CrossRef]
- Dong, Q.; Tan, Y.; Tang, G.; Wu, Z.; Li, A.; Qin, X.; Li, S.; Liao, H.; Xiao, J.; Huang, Q.; et al. Neuroprotective potentials of ferulic acid against intracerebral hemorrhage COVID-19 through using network pharmacology approach and molecular docking analysis. Curr. Res. Toxicol. 2023, 5, 100123. [Google Scholar] [CrossRef] [PubMed]



| Methods | Relationship with HIF | Reference |
|---|---|---|
| Immunohistochemistry | HIF-1α upregulation confers cytoprotective responses in endothelial cells in the hearts of COVID-19 patients. Human heart tissue samples | Wang et al., 2022 [15] |
| HIF-1α was equally expressed in COVID-19 patients and the control group. The overexpression of HIF-1α was associated with greater transcription of VEGF. Human lung tissue samples | Miggiolaro et al., 2023 [14] | |
| Carbonic anhydrase IX (CAIX) expression represents an indirect indicator of the upregulation of HIF-1 in human villous trophoblastic and stromal cells. Human placenta samples | Mao et al., 2022 [25] | |
| COVID-19 patients exhibited spatial heterogeneity of Angiopoietin-2, HIF-1α, and TGF-β as indicators of microischemia in affected lung tissue samples compared to healthy controls. Human lung tissue samples | Ackermann et al., 2022 [26] | |
| HIF-1α transcriptionally upregulates ACE2 expression. Mouse and human bronchioles samples | Liu et al., 2021 [5] | |
| ELISA | The paper’s analysis did not reveal any significant difference in the plasma level of transcription factor HIF-1α. Human plasma samples | Krenytska et al., 2023 [27] |
| HIF-1α upregulation in hypoxia promotes the transcription of Cygb, which is produced to supply oxygen in tissues during hypoxic conditions like SARS-CoV-2 infection. Human nose–throat swab samples | Wulandari et al., 2023 [28] | |
| PCR | ACE-2 is regulated by miR-421-5p, leading to the development of immunothrombosis; miR-421-5p also acts on hypoxia response repressor elements (HRR), resulting in an inflammatory imbalance mediated by the overexpression of HIF and its genes, which results in increased intensity and lung damage. Human serum samples | Abdolari et al., 2022 [29] |
| Virus-induced HIF-1α activation leads to increased expression of Fetuin-A, which has anti-inflammatory properties and can modulate the immune response. Human serum samples | Alghanem et al., 2023 [30] | |
| Multi-omics | SARS-CoV-2 modulated the AKT/mTOR/HIF-1α pathway, which regulates glycolysis and glutamatelysis, consequently inhibiting SARS-CoV-2 replication. Mouse lung tissue samples | Ardanuy et al., 2023 [4] |
| HIF-1α pathway is upregulated in particulate matter exposed endothelial cells. Human endothelial cell samples | Manivannan et al., 2021 [31] | |
| Targeting HIF-1α could help mitigate hypoxia-related damage or inflammatory effects in conditions like PACS. Human serum samples | Wang et al., 2023 [32] | |
| In Silico | PKA-inducible HIF-1α was shown to increase coagulation factors and thrombus formation. Therefore, targeting PKA modulation, promoting HIF-1α downregulating, should be considered as a COVID-19 therapeutic. Protein–Protein Interactions (PPIs): human samples | Barman et al., 2022 [33] |
| Cytokine storm in SARS-CoV-2 infected lung tissue may be due to the HIF-1α-regulated overexpression of ACE2 and TMPRSS2. Human samples: lung cancer cell line, breast cancer cell line, colorectal adenocarcinoma cell line | Boopathi et al., 2023 [2] | |
| The HIF-1α signaling pathway is significantly affected by SARS-CoV-2 infection, as indicated by the enrichment of human hub proteins in this pathway. PPIs: human samples | Ghosh et al., 2021 [34] | |
| The glycolysis/gluconeogenesis and HIF-1α signaling pathways are shown to be associated with COVID-19 and neurological diseases. PPIs: human samples | Rahman et al., 2021 [35] | |
| HIF-1α plays a role as a transcriptional regulator of the adaptive response to hypoxia, tumorigenesis and metastasis, based on human genes targeted by SARS-CoV-2 encoded miRNAs. Human lung epithelium samples | Roy et al., 2021 [36] | |
| In COVID-19, the angiogenesis process, stimulated by HIF-1α, is accelerated by MMP (Matrix Metalloproteinases) and NRP (Neuropilins) cooperation, leading to significant tissue damage. Human samples | Saleki et al., 2024 [37] | |
| SLC2A3 encodes the glucose uptake transporter GLUT3 and LCP1 (L-Plastin). They are induced during hypoxia by STAT-3-HIF-1α signaling and regulate macrophage infiltration. Human samples | Sheerin et al., 2022 [38] | |
| HIF-1α upregulation activates both SARS-CoV-2 infection and inflammatory response and plays a role in aggravation of COVID-19. Human blood cells: g T (CD4 + helper T and CD8 + cytotoxic T), B, and natural killer (NK) | Shen et al., 2022 [7] | |
| HIF-1α pathway enriches a set of proteins that are linked to both the predisposing diseases and to the endocrine-disrupting chemical. Human samples | Wu et al., 2020 [39] | |
| In the retina, upregulation of the HIF-1α pathway in SARS-CoV-2 infection promotes the expression of VEGF, which stimulates angiogenesis. Human eye tissue samples | Yuan et al., 2021 [40] | |
| Upregulation of HIF-1α in SARS-CoV-2 infection may lead to cytokine storm. Human cardiomyocyte samples | Zhang et al., 2023 [41] | |
| Transcriptomic Analysis | Hypoxic activation of HIF-1α is related to MAPK, NF-kB and IL-6 signaling, which shows its role in cytokine production. Mice monocyte cell samples | Caldwell et al., 2024 [42] |
| SARS-CoV-2 infection leads to hypoxic lung tissue conditions, which triggers the HIF-1α pathway. Rhesus macaques solid organs samples | Du et al., 2023 [43] | |
| There is a molecular link between HIF-1α and neutrophil degranulation in blood. The correlation was more consistent in altitude-related hypoxia than that in COVID-19 or other respiratory infections. Human neutrophils samples | Lei et al., 2024 [44] | |
| HIF-1α pathway modulates genes related to early inflammatory response, immune response, and cell signal transduction. It acts as a parental gene of circRNAs and plays biological functions in SARS-CoV-2 infection. Human bronchial epithelial cells | Yang et al., 2021 [24] | |
| Proteomic Analysis | Proteomic pathway analysis in SARS-CoV-2 infected human host cells revealed an upregulation of HIF-1α. Human lung and airway cell samples | Maria et al., 2023 [45] |
| Proteomics-based studies have observed that SARS-CoV-2 causes global proteomic changes after 48 h SARS-CoV-2 post-infection, specifically in pathways related to HIF-1α. Human lung tissue samples | Sacoon et al., 2021 [46] | |
| Upregulation of the HIF signaling pathway and ROS production were gradually enhanced during the disease progression in SARS-CoV-2-infected patients. Human serum samples | Wang et al., 2021 [47] | |
| HIF-1α pathway modulates genes related to early inflammatory response, immune response, and cell signal transduction. It acts as a parental gene of circRNAs and plays biological functions in SARS-CoV-2 infection. Human lung epithelial cell samples | Wang et al., 2021 [24] | |
| Western blotting | HIF-1α transcriptionally upregulates ACE2 expression. Mouse and human bronchioles samples | Liu et al., 2021 [5] |
| HIF-1α enhances the production of pro-inflammatory cytokines, especially IL-6 and TNF-α. Human bronchial epithelial cell samples | Pooladanda et al., 2021 [48] | |
| In normoxia, PHD2 degrades HIF-1α and HIF-2α; in hypoxia induced by SARS-CoV-2 infection, HIF level is increased in activated platelets, promoting platelet activation, aggregation, and inflammatory signaling. Human platelet and monocyte cell samples | Shrimali et al., 2021 [49] | |
| Multiplex assay | It was postulated that the return to normoxia after a mild hyperoxia stimulus is sensed as a hypoxic trigger, which induces HIF-1α activation and then VEGF synthesis. Human monocyte cell samples | Keller et al., 2023 [50] |
| Cytometry Immunophenotyping | B cells seem particularly sensitive to perturbations in oxygenation and HIF activity. Mice B cell samples | Kotagiri et al., 2022 [51] |
| Flow Cytometry | Ethanol consumption resulted in transcriptional shifts in the immune landscape of the lung. Infiltrating monocytes associated with migration were decreased while inflammatory HIF-1α signaling increased. Mice lung tissue samples | Ardanuy et al., 2023 [4] |
| TUNEL Assay | Upregulation of HIF-1α transcription factor at 48 h SARS-CoV-2 post-infection compared to 24 h. Mice lung tissue samples | Ardanuy et al., 2023 [4] |
| Mass Spectrometry | Hydroxyglutaric Acid increases concentrations during times of tissue hypoxia via a HIF-dependent pathway; Hydroxyglutaric Acid upregulation may influence the adaptive immune response to SARS-CoV-2, with reports of accumulation, activation and differentiation of CD8+ T-cells. Human serum samples | Whiley et al., 2024 [52] |
| Gene ID | Gene Name | Function |
|---|---|---|
| VEGF | Vascular Endothelial Growth Factor | Promotes angiogenesis by stimulating new blood vessel formation to enhance oxygen delivery to tissues. |
| EPO | Erythropoietin | Stimulates erythropoiesis, increasing oxygen-carrying capacity in the blood. |
| SLC2A1 | Solute Carrier Family 2 Member 1 (GLUT1) | Facilitates glucose transport across the plasma membrane, critical for cellular metabolism, particularly under hypoxia. |
| LDHA | Lactate Dehydrogenase A | Catalyzes the conversion of pyruvate to lactate, allowing continuous ATP production via anaerobic glycolysis. |
| PGK1 | Phosphoglycerate Kinase 1 | A key enzyme in glycolysis that catalyzes ATP generation through substrate-level phosphorylation. |
| NOS2 | Nitric Oxide Synthase 2 (Inducible) | Produces nitric oxide, a signaling molecule involved in vasodilation and cellular responses to hypoxia. |
| NOS3 | Nitric Oxide Synthase 3 (Endothelial) | Regulates nitric oxide production in endothelial cells, promoting vasodilation and blood flow. |
| HMOX1 | Heme Oxygenase 1 | Degrades heme into biliverdin, iron, and carbon monoxide, providing cytoprotective effects against oxidative stress. |
| HK1/HK2 | Hexokinase 1/2 | Catalyzes the phosphorylation of glucose to glucose-6-phosphate, the first step of glycolysis. |
| ALDOA | Aldolase, Fructose-Bisphosphate A | A glycolytic enzyme that cleaves fructose-1,6-bisphosphate into glyceraldehyde-3-phosphate and dihydroxyacetone phosphate. |
| ENO1 | Enolase 1 | Catalyzes the conversion of 2-phosphoglycerate to phosphoenolpyruvate in glycolysis. |
| PFKFB3/PFKL | 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase3/Phosphofructokinase, Liver Type | Regulates glycolysis through control of fructose-2,6-bisphosphate levels, an allosteric activator of phosphofructokinase-1. |
| PDK1 | Pyruvate Dehydrogenase Kinase 1 | Inhibits pyruvate dehydrogenase, reducing oxidative metabolism and favoring anaerobic glycolysis. |
| TIME1 | TIMP Metallopeptidase Inhibitor 1 | Inhibits matrix metalloproteinases, regulating tissue remodeling and angiogenesis. |
| ITGB2 | Integrin Subunit Beta 2 (CD18) | Component of β2 integrins, involved in cell adhesion and immune responses. |
| CD142 | Coagulation Factor III (Tissue Factor) | Regulates blood coagulation and inflammation in hypoxic conditions. |
| TFRC | Transferrin Receptor | Mediates iron uptake, which is essential for cellular respiration and hemoglobin synthesis. |
| FLT1 | Fms Related Receptor Tyrosine Kinase 1 (VEGFR-1) | VEGF receptor is involved in angiogenesis regulation and vascular permeability. |
| EGF | Epidermal Growth Factor | Stimulates cell proliferation and tissue regeneration. |
| SERPINE1 | Serpin Family E Member 1 (PAI-1) | Regulates fibrinolysis and contributes to thrombosis under hypoxia. |
| ANGPT1 | Angiopoietin 1 | Modulates vascular stability and remodeling. |
| SINGLE | TEK Receptor Tyrosine Kinase (Tie-2) | A receptor for angiopoietins, crucial for vascular integrity maintenance. |
| EDN1 | Endothelin 1 | Potent vasoconstrictor regulated by HIF-1α, involved in blood pressure control. |
| NPPA | Natriuretic Peptide A (ANP) | Regulates fluid-electrolyte balance and blood pressure. |
| BCL2 | BCL2 Apoptosis Regulator | Anti-apoptotic protein that promotes cell survival under hypoxic stress. |
| CDKN1A/CDKN1B | Cyclin Dependent Kinase Inhibitor 1A/1B (p21/p27) | Inhibitors of cyclin/CDK complexes, involved in cell cycle control and stress response. |
| Study | Drug | Population | Methods | HIF Related Results |
|---|---|---|---|---|
| Lewis, S. A. et al. (2023) [90] | Alcohol (ethanol) | Vero E6 Cells (to obtain SARS-CoV-2 virus) Samples of Bronchoalveolar Lavage (BAL): Monkeys (n = 11) and Humans (n = 6). | Flow Cytometry Luminex scRNA-Seq. Gene Set Enrichment analysis | Higher HIF-1α levels were found in the BAL of rhesus monkeys and humans after six months of chronic alcohol consumption. The DEGs in myeloid cells, such as alveolar macrophages and monocytes, indicate HIF-1α pathway activation. |
| VSTAT Trial (2022) [88] | Vadadustat (AKB-6548) | 449 adult subjects in five hospitals who were randomized 1:1 to vadadustat 900 mg or placebo once daily orally for up to 14 days while hospitalized | Phase 2, randomized, double-blind, placebo-controlled trial. | Vadadusta is an oral hypoxia-inducible factor prolyl hydroxylase inhibitor (HIF-PHI). The VSTAT study assessed vadadustat’s efficacy against a placebo in severe COVID-19 patients using the NIAID Ordinal Scale (NIAID-OS). The results suggest a therapeutic potential but are insufficient for a definitive conclusion under the pre-established parameters. The safety of the drug was comparable to placebo, with no signs of additional toxicity. |
| Liu et al. (2020) [91] | Bevacizumab (Avastin) | Adult patients with severe COVID-19, characterized by hypoxemia and radiological evidence of pneumonia (n = 26). | Open-label, single-arm clinical trial (single-arm). | Bevacizumab (Avastin) is a monoclonal antibody that inhibits VEGF, which is regulated by HIF-1α. In severe COVID-19 patients, hypoxemia and inflammatory stress stabilize HIF-1α, leading to increased VEGF transcription, which contributes to angiogenesis, vascular permeability, and pulmonary edema. Specifically, the administration of bevacizumab to patients with severe COVID-19 led to a significant improvement in oxygenation parameters and the resolution of pulmonary infiltrates, suggesting that the inhibition of VEGF—a transcriptional target of HIF1α—reduces vascular permeability inflammation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, F.P.G.; Matte, R.; Wiedmer, D.B.; da Silva, A.P.G.; Menin, R.M.; Barbosa, F.B.; Meneguzzi, T.A.M.; Pereira, S.B.; Fausto, A.T.; Klug, L.; et al. HIF-1α Pathway in COVID-19: A Scoping Review of Its Modulation and Related Treatments. Int. J. Mol. Sci. 2025, 26, 4202. https://doi.org/10.3390/ijms26094202
da Silva FPG, Matte R, Wiedmer DB, da Silva APG, Menin RM, Barbosa FB, Meneguzzi TAM, Pereira SB, Fausto AT, Klug L, et al. HIF-1α Pathway in COVID-19: A Scoping Review of Its Modulation and Related Treatments. International Journal of Molecular Sciences. 2025; 26(9):4202. https://doi.org/10.3390/ijms26094202
Chicago/Turabian Styleda Silva, Felipe Paes Gomes, Rafael Matte, David Batista Wiedmer, Arthur Paes Gomes da Silva, Rafaela Makiak Menin, Fernanda Bressianini Barbosa, Thainá Aymê Mocelin Meneguzzi, Sabrina Barancelli Pereira, Amanda Terres Fausto, Larissa Klug, and et al. 2025. "HIF-1α Pathway in COVID-19: A Scoping Review of Its Modulation and Related Treatments" International Journal of Molecular Sciences 26, no. 9: 4202. https://doi.org/10.3390/ijms26094202
APA Styleda Silva, F. P. G., Matte, R., Wiedmer, D. B., da Silva, A. P. G., Menin, R. M., Barbosa, F. B., Meneguzzi, T. A. M., Pereira, S. B., Fausto, A. T., Klug, L., Melim, B. P., & Beltrão, C. J. (2025). HIF-1α Pathway in COVID-19: A Scoping Review of Its Modulation and Related Treatments. International Journal of Molecular Sciences, 26(9), 4202. https://doi.org/10.3390/ijms26094202






