Unlocking the Pharmacological Potential of Myricetin Against Various Pathogenesis
Abstract
1. Introduction
2. Methodology
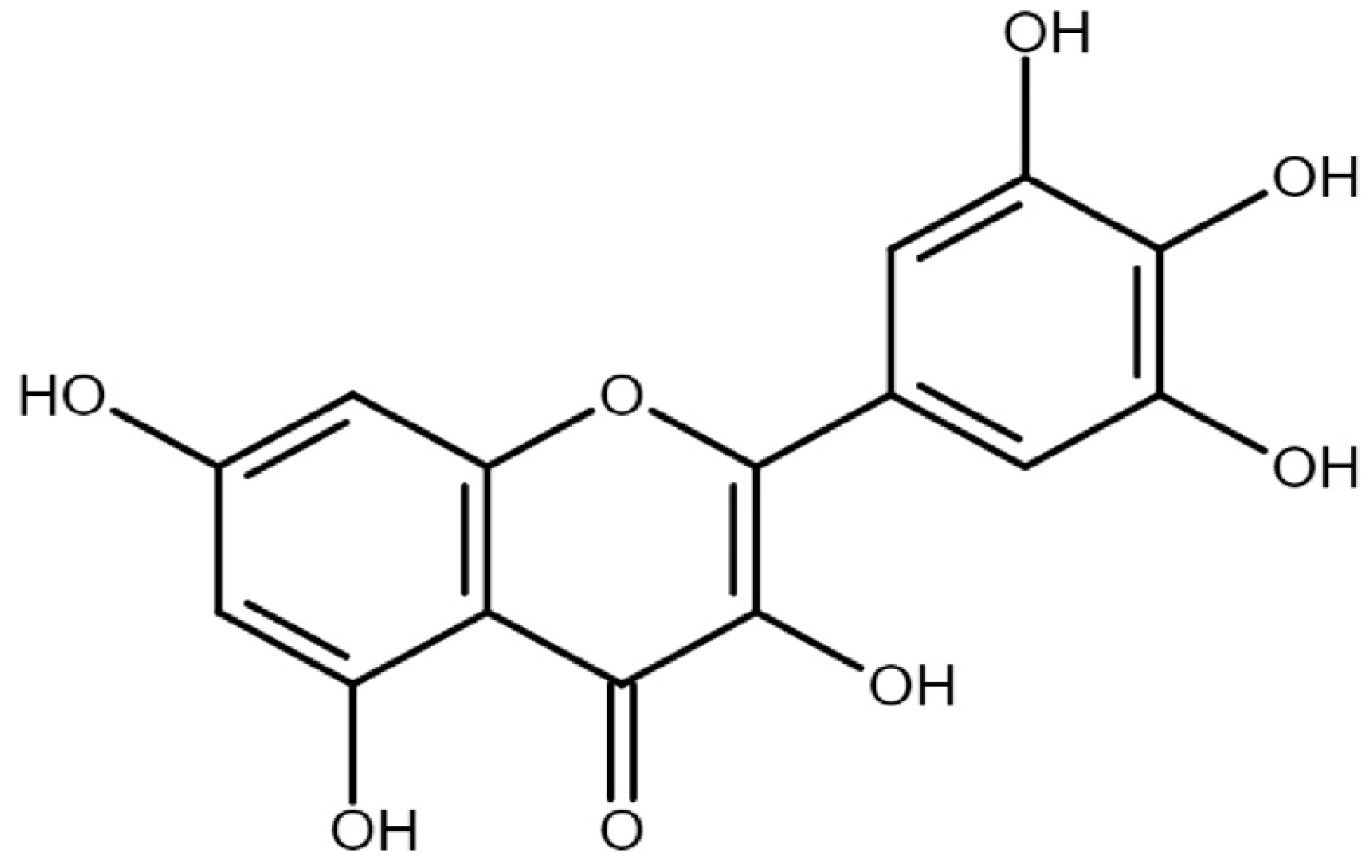
3. Structure, Sources, Daily Intake, and Pharmacokinetics of Myricetin
4. Effects of Myricetin on Human Health
4.1. Antioxidant Potential
4.2. Anti-Inflammatory Effects
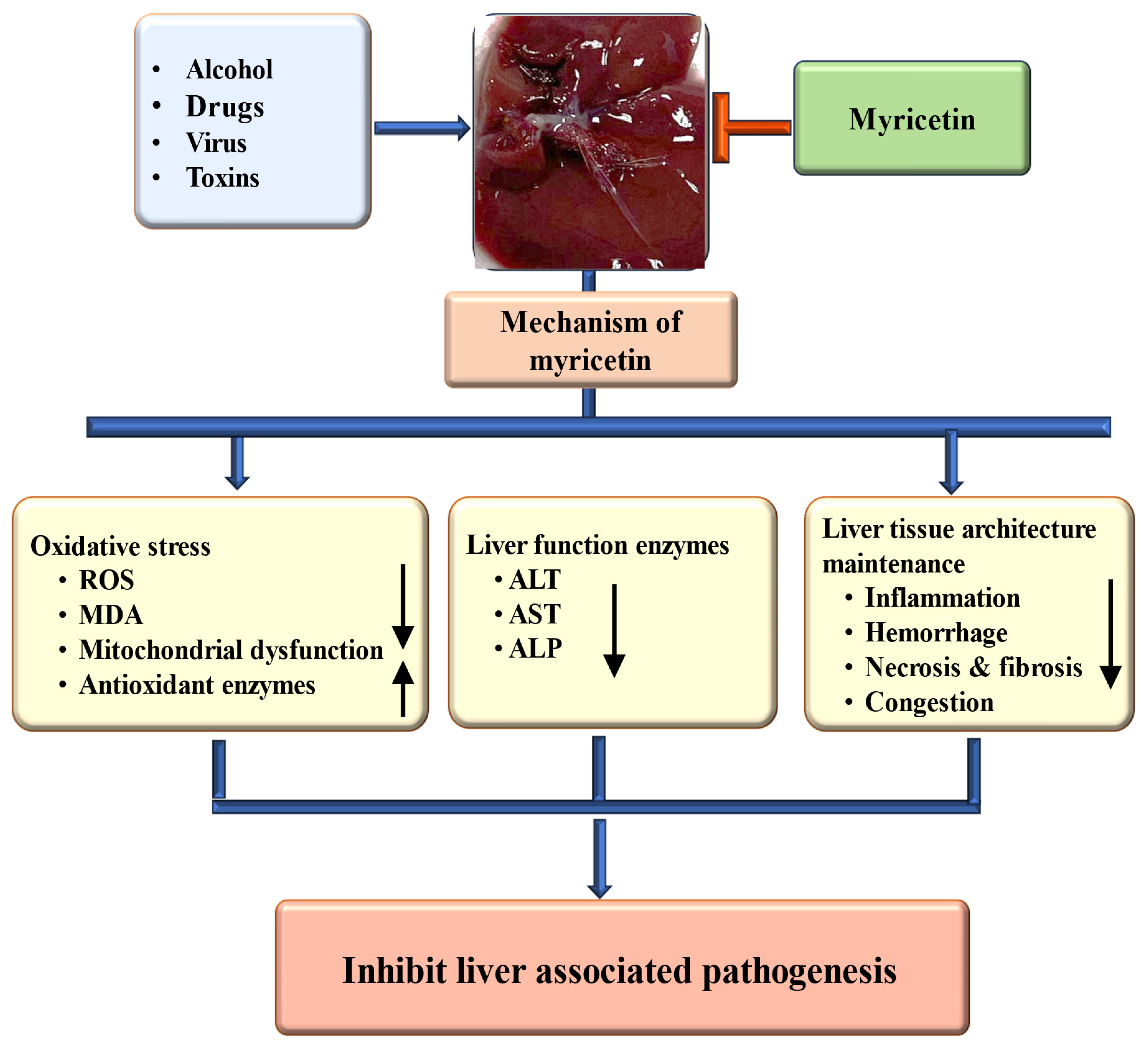
4.3. Hepatoprotective Effects
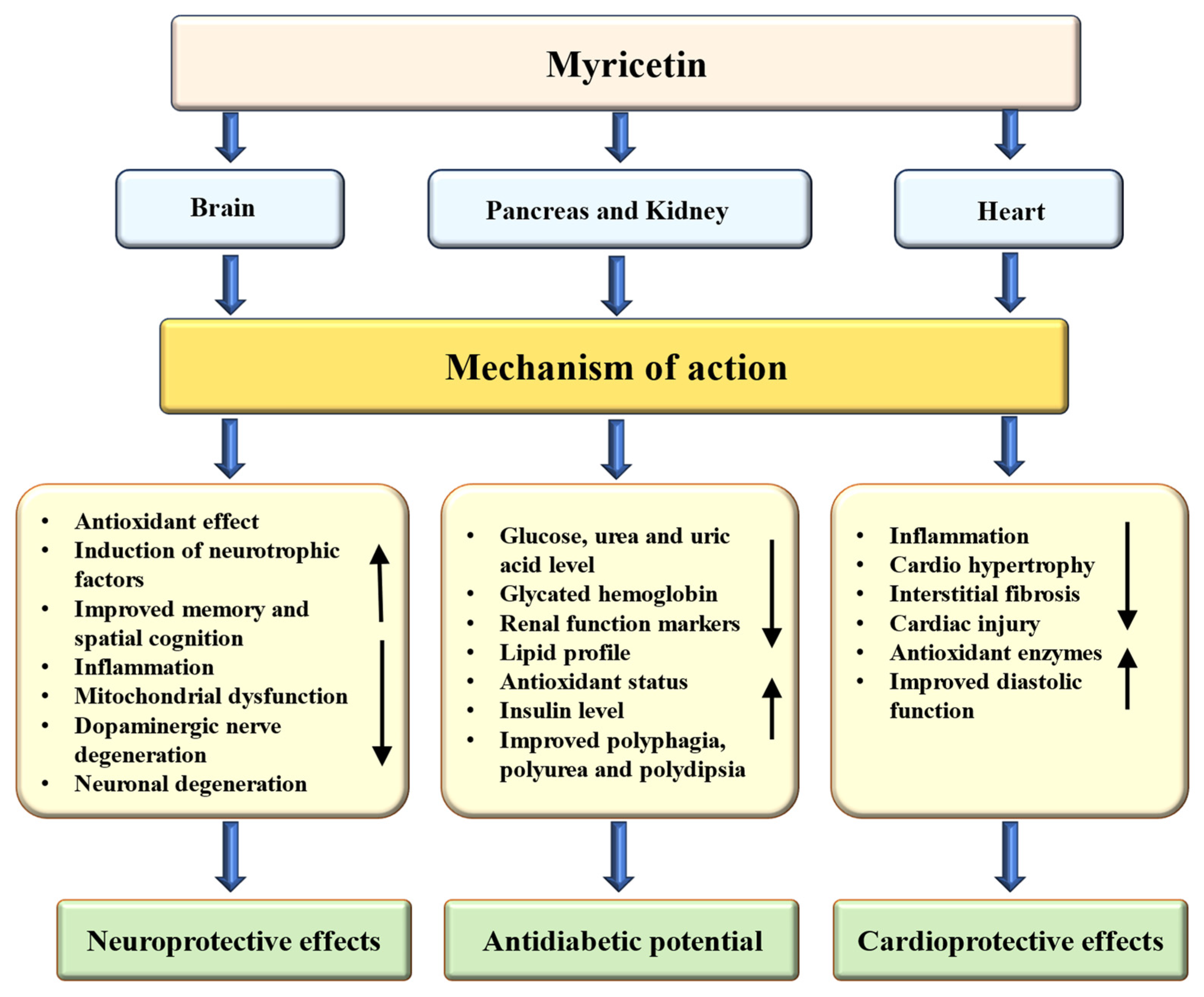
4.4. Anti-Diabetic Potential
| Activity | Types of Study | Doses | Findings | Ref. |
|---|---|---|---|---|
| Anti-diabetic potential | Male albino Wistar rats, in vivo | 1.0 mg/kg bw |
| [90] |
| Male albino Wistar rats, in vivo | 1.0 mg/kg bw |
| [91] | |
| Male mice, in vivo | 75, 150 and 300 mg/kg |
| [92] | |
| Mice model, in vivo | 200 mg/kg/day |
| [93] | |
| Mice model, in vivo | 100 mg/kg/day |
| [94] | |
| Rat model, in vivo | 50 and 200 mg/kg body weight |
| [96] | |
| Rats model, in vivo | 1 mg/kg per injection |
| [97] | |
| Rats model, in vivo | 0.5, 1.0 and 1.5 mg/kg bw |
| [98] | |
| Rats model, in vivo | 6 mg/day |
| [99] | |
| Rats model, in vivo | 1.0 mg/kg |
| [100] |
4.5. Cardioprotective Effects
| Activity | Model | Dose | Outcome | Ref. |
|---|---|---|---|---|
| Mice, in vivo | 100 mg/kg |
| [62] | |
| Wistar rats, in vivo | 100 and 300 mg/kg, p.o |
| [105] | |
| H9c2 cardiomyocyte cell line, in vitro | 5, 10, 20, 40 Μm |
| [106] | |
| Rat model, in vivo | 5 μM |
| [107] | |
| Mice model, in vivo | 200 mg/kg/d |
| [93] | |
| H9c2 cells, in vitro | 25 μg/mL |
| [108] | |
| Mice model, in vivo | 300 mg/kg/day |
| [108] | |
| Male rats, in vivo | 2.5 mg/kg and 5 mg/kg |
| [109] | |
| Rats model, in vivo | 25 and 50 mg/kg |
| [111] | |
| H9c2 cells, in vitro | 1, 5, 10, & 15 μM |
| [60] |
4.6. Neuroprotective Effects
| Disease | Types of Study | Model | Doses | Outcome | Ref. |
|---|---|---|---|---|---|
| Alzheimer’s disease | In vivo | Rat models | 5 or 10 mg/kg |
| [114] |
| In vivo | Mice model | 20 mg/kg |
| [115] | |
| In vitro | SH-SY5Y cells | 5–20 µM |
| [115] | |
| In vitro | SH-SY5Y | 4, 1, 0.25, 0.063, 0.016 µM |
| [116] | |
| In vivo | Mice model | 25 or 50 mg/kg |
| [116] | |
| Parkinson’s disease | In vitro | SH-SY5Y cells | 50 µM |
| [117] |
| In vivo | Rat model | 25 g/kg |
| [117] | |
| In vivo | Rat model | 2.5, 5, or 10 mg/kg |
| [118] | |
| In vitro | SH-SY5Y cells | 12.5, 25, 50 µM |
| [118] | |
| In vivo | Drosophila model | 250, 500, 750, 1000 µM |
| [119] | |
| In vivo | Drosophila Model | 10, 20 and 40 μM |
| [120] | |
| Epilepsy | In vivo | Mice model | 50, 100 mg/kg |
| [122] |
| In vivo | Mice model | 200 mg/kg |
| [123] |
4.7. Anti-Cancer Potential
4.8. Role in Respiratory Disease
4.9. Effects of Myricetin on Digestive System/Inflammatory Bowel Disease
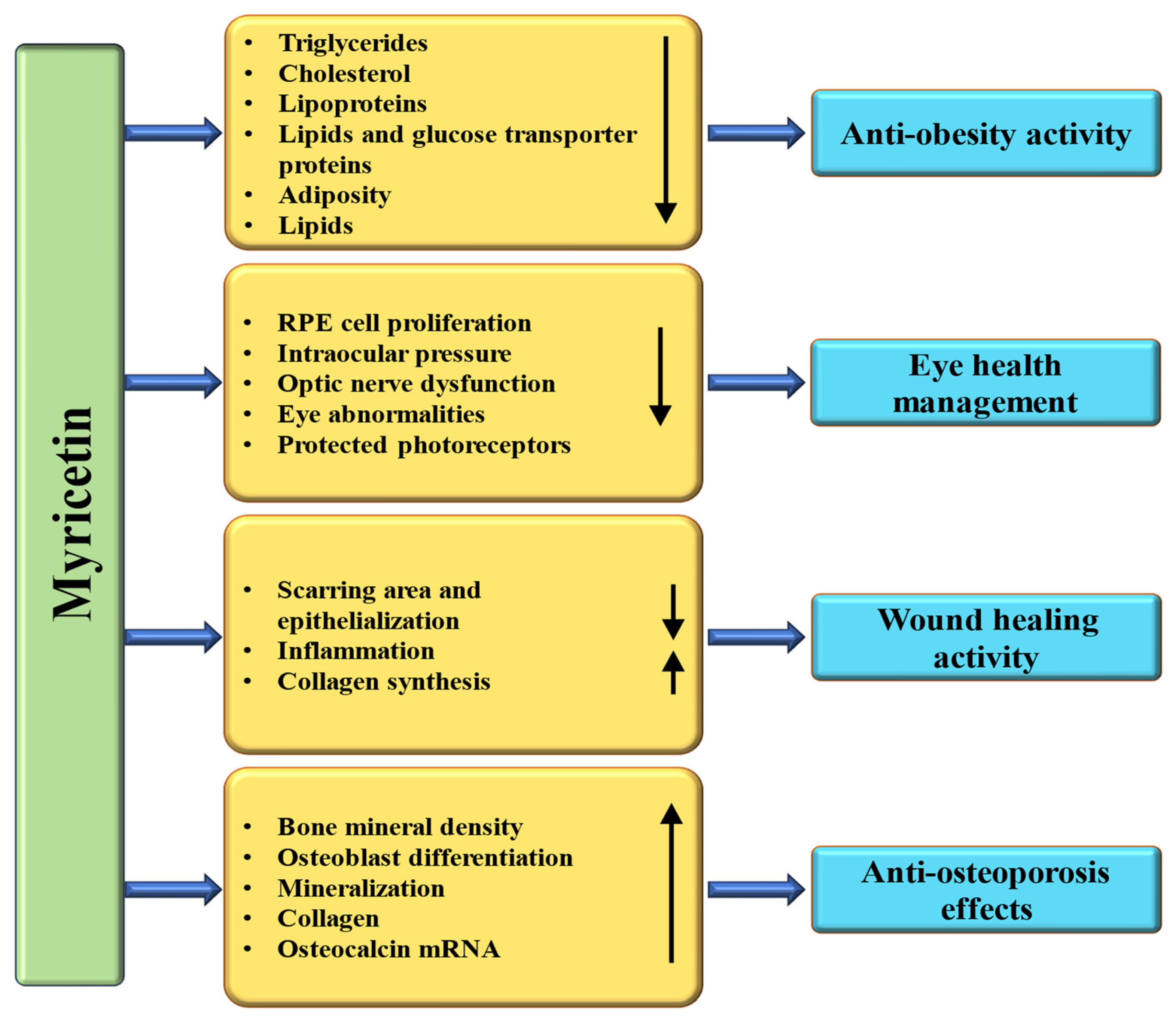
4.10. Anti-Obesity Properties
4.11. Wound Healing Effects
4.12. Anti-Analgesic Activity
4.13. Anti-Platelet Aggregation Potential
4.14. Effect on Bone Disease
4.15. Effect on Eye Disease
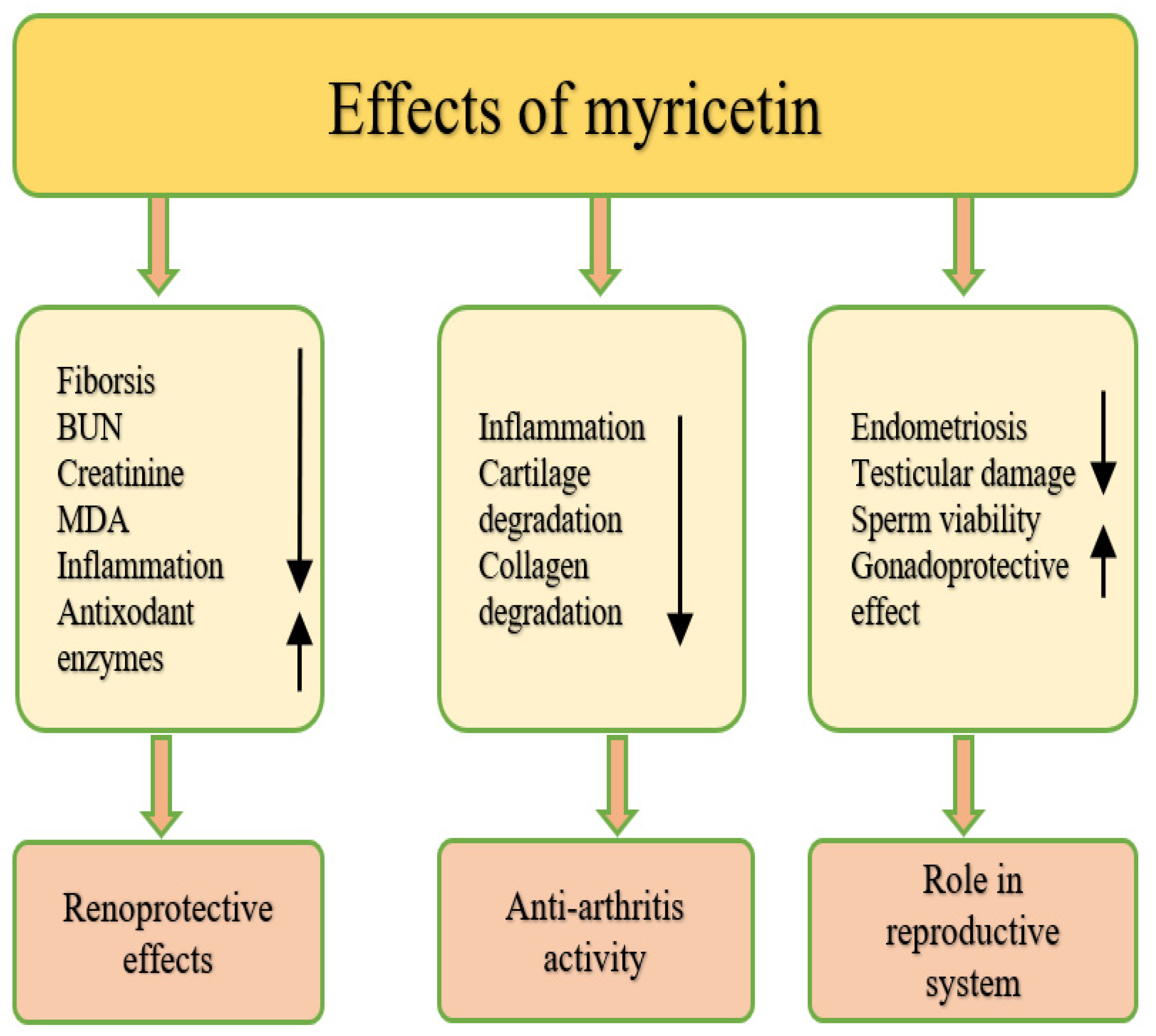
4.16. Effect on Osteoarthritis
4.17. Renoprotective Effects
| Activity | Study Types | Model | Dose | Outcomes | Ref. |
|---|---|---|---|---|---|
| Lung injury protective effects | In vivo | Mice model/murine sepsis model | 100 mg/kg |
| [146] |
| In vivo | Rat model | 10, 20 and 40 mg/kg |
| [67] | |
| In vivo | Mice model | 50 mg/kg |
| [147] | |
| Anti-ulcerative colitis effects | In vivo | Mice model | 200, 100 or 50 mg/kg |
| [153] |
| In vivo | Mice model | 80 mg/kg |
| [155] | |
| Anti-obesity potential | In vivo | Mice model | 150 mg/kg |
| [49] |
| In vivo | Mice model | 400 mg/kg |
| [161] | |
| Wound healing effects | In vivo | Rat model | 10 and 20% myricetin |
| [164] |
| Anti-allodynic effect | In vivo | Rat model | 0.1–10 mg/kg |
| [168] |
| Anti-osteoporosis effects | In vivo | Rat model | 1 or 2.5 mg/kg |
| [176] |
| In vitro | MC3T3-E1 cells | 20 μM |
| [176] | |
| In vivo | Rat model | 50 mg/kg |
| [177] | |
| Role in periodontitis and osteoporosis | In vivo | Mice model | 2 or 5 mg/kg |
| [180] |
| Intraocular pressure-lowering activity | In vivo | Rabbit model | 1 mg |
| [183] |
| Role in glaucoma | In vivo | Rat model | 25, 50 or 100 mg/kg |
| [185] |
| Anti-osteoarthritis effects | In vitro | Mouse chondrocyte | 12.5, 25 and 50 μM |
| [190] |
| In vivo | Mouse model | 10 mg/kg |
| [190] | |
| Renoprotective effects | In vivo | Mice model | 3 mg/kg |
| [66] |
| In vivo | Mice model | 50 and 200 mg/kg |
| [193] | |
| Role in the reproductive system | In vitro | VK2 and End1 | 5, 10, 20, 50 and 100 μM |
| [194] |
4.18. Role in the Reproductive System
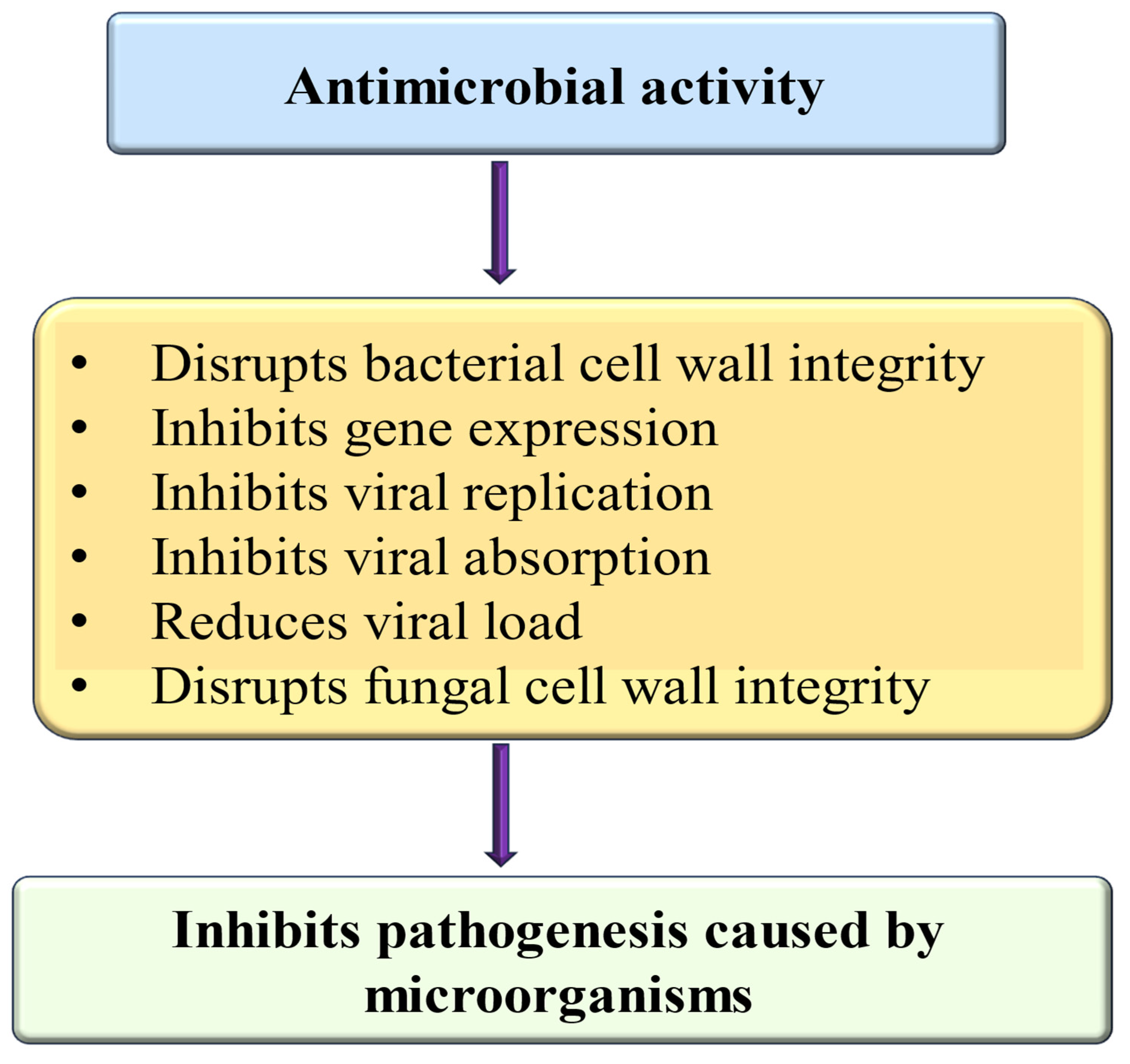
4.19. Anti-Microbial Effects
- I.
- Anti-bacterial potential
- II.
- Anti-viral effects
- III.
- Anti-fungal effects
| Activities | Species | Key Findings | Ref. |
|---|---|---|---|
| Anti-bacterial | Staphylococcus aureus |
| [198] |
| Escherichia coli |
| [199] | |
| S. aureus |
| [201] | |
| S. aureus |
| [200] | |
| SARS-CoV-2 |
| [205] | |
| Anti-viral | Pseudorabies virus |
| [206] |
| Transmissible gastroenteritis virus |
| [208] | |
| Herpes simplex virus |
| [209] | |
| Candida albicans |
| [210] | |
| Anti-fungal | Candida albicans |
| [211] |
5. Synergetic Effects of Myricetin with Other Drugs
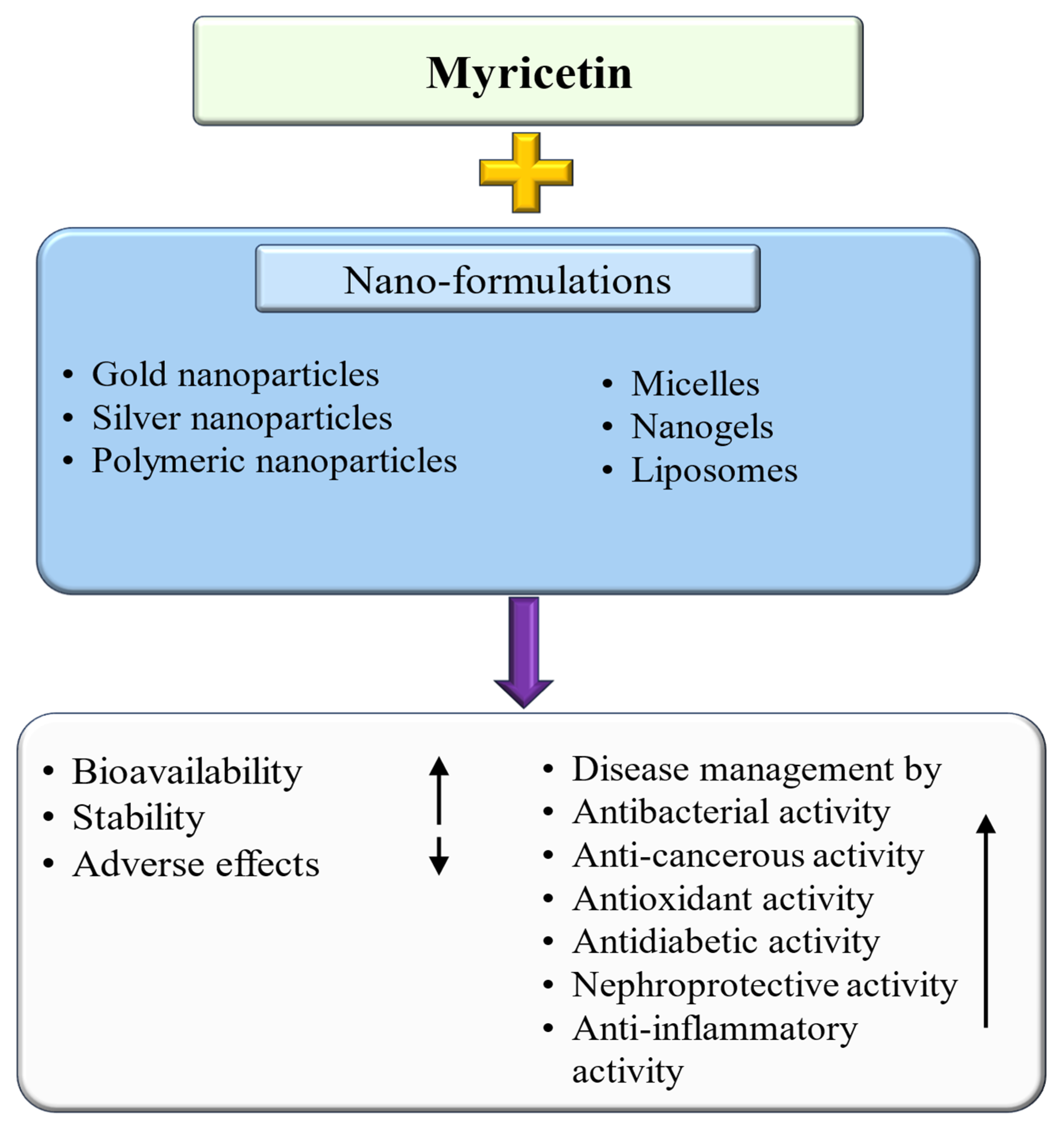
6. Myricetin-Based Nanoformulation and Its Role in Disease Management
| Nanoformulations | Activity | Key Findings | Ref. |
|---|---|---|---|
| Myricetin-mediated silver nanoparticles | Antibacterial |
| [225] |
| Myricetin-gold nanoparticles | Anti-cancerous |
| [226] |
| Myricetin liposomal nanoformulation | Antioxidant |
| [227] |
| TPGS modified pro-liposome of myricetin | Hepatoprotective |
| [228] |
| MYR pH-sensitive liposomes | Anti-hyperuricemic |
| [229] |
| Myricetin-loaded solid lipid nanoparticles | Anti-cancerous |
| [231] |
| Myricetin nanofibers | Photoprotective |
| [232] |
| Myricetin encapsulated chitosan nanoformulation | Diabetes management |
| [234] |
| Myricetin-loaded nanomicelles | Nephroprotective |
| [235] |
7. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
List of Abbreviations
| GSH | Glutathione |
| T-AOC | Total antoxidant capacity |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| MDA | Malondialdehyde |
| NAFLD | Nonalcoholic fatty liver disease |
| ROS | Reactive oxygen species |
| AGEs | Advanced glycation end-products |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 |
| iNOS | Inducible nitric oxide synthase |
| ALP | Alkaline phosphatase |
| ALT | Alanine transaminase |
| AST | Aspartate aminotransferase |
| COX-2 | Cyclooxygenase-2 |
| TNF-α | Tumor necrosis factor-α |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| IL | Interleukin |
| STAT | Signal transducer and activator of transcription |
| MMP | Matrix metalloproteinase |
| Cmax | Maximum concentration |
| IBD | Inflammatory bowel diseases |
| 5-FU | 5-Fluorouracil |
| CCl4 | Carbon tetrachloride |
| LPS | Lipopolysaccharide |
| BALF | Bronchoalveolar Lavage Fluid |
| STZ | Streptozotocin |
| HFD | High-fat diet |
| MAPK | Mitogen-activated protein kinase |
| TLR | Toll-like receptor |
| OA | Osteoarthritis |
| BUN | Blood Urea Nitrogen |
| VEGF | Vascular endothelil growth factor |
| PTZ | Pentylenetetrazole |
| PCO | Polycystic Ovary Syndrome |
| HIF-1α | Hypoxia-inducible factor-1α |
| IOP | Intraocular pressure |
| MPO | Myeloperoxidase |
| POAG | Primary open-angle glaucoma |
| MIC | Minimum inhibitory concentration |
| AuNP | Gold nanoparticle |
| AgNP | Silver nanoparticle |
| SLN | Solid nanoparticle |
| CDAHFD | Choline-deficient, L-amino acid-defined, high-fat diet |
| HSC | Hepatic stellate cells |
| NASH | Nonalcoholic steatohepatitis |
| TREM-1 | Triggering receptor expressed on myeloid cells-1 |
| Pi3k/Akt/mTOR | Phosphoinositide 3 kinase/Akt/mTOR |
| CDK | Cyclin-dependent kinase |
| Fe-NTA | Ferric iron nitrilotriacetate |
| GSH-Px | Glutathione peroxidase |
| TBARS | Thiobarbituric acid reactive substance |
| PGN | Peptidoglycan |
| mTOR | Mammalian target of rapamycin |
References
- van Wyk, A.; Prinsloo, G. Medicinal plant harvesting, sustainability and cultivation in South Africa. Biol. Conserv. 2018, 227, 335–342. [Google Scholar] [CrossRef]
- Torres-León, C.; Ramírez, F.R.; Aguirre-Joya, J.A.; Ramírez-Moreno, A.; Chávez-González, M.L.; Aguillón-Gutierrez, D.R.; Camacho-Guerra, L.; Ramírez-Guzmán, N.; Vélez, S.H.; Aguilar, C.N. Medicinal plants used by rural communities in the arid zone of Viesca and Parras Coahuila in northeast Mexico. Saudi Pharm. J. 2023, 31, 21–28. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). World Health Organization Traditional Medicine Strategy 2014–2023; WHO: Geneva, Switzerland, 2013; pp. 1–78. [Google Scholar]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef]
- Anwar, S.; Raut, R.; Alsahli, M.A.; Almatroudi, A.; Alfheeaid, H.; Alzahrani, F.M.; Khan, A.A.; Allemailem, K.S.; Almatroodi, S.A.; Rahmani, A.H. Role of Ajwa Date Fruit Pulp and Seed in the Management of Diseases through In Vitro and In Silico Analysis. Biology 2022, 11, 78. [Google Scholar] [CrossRef]
- Anwar, S.; Almatroodi, S.A.; Almatroudi, A.; Allemailem, K.S.; Joseph, R.J.; Khan, A.A.; Alrumaihi, F.; Alsahli MAHusain Rahmani, A. Biosynthesis of silver nanoparticles using Tamarix articulata leaf extract: An effective approach for attenuation of oxidative stress mediated diseases. Int. J. Food Prop. 2021, 24, 677–701. [Google Scholar] [CrossRef]
- Barakat, H.; Aljutaily, T.; Almujaydil, M.S.; Algheshairy, R.M.; Alhomaid, R.M.; Almutairi, A.S.; Alshimali, S.I.; Abdellatif, A.A. Amygdalin: A review on its characteristics, antioxidant potential, gastrointestinal microbiota intervention, anticancer therapeutic and mechanisms, toxicity, and encapsulation. Biomolecules 2022, 12, 1514. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Khan, A.A.; Aloliqi, A.A.; Syed, M.A.; Rahmani, A.H. Therapeutic potential of Tamarix aphylla in the prevention of lung injury through the regulation of inflammation, oxidative stress and cell-signaling molecules. Appl. Sci. 2022, 12, 9925. [Google Scholar] [CrossRef]
- Mani, V.; Arfeen, M.; Dhaked, D.K.; Mohammed, H.A.; Amirthalingam, P.; Elsisi, H.A. Neuroprotective effect of methanolic Ajwa seed extract on lipopolysaccharide-induced memory dysfunction and neuroinflammation: In vivo, molecular docking and dynamics studies. Plants 2023, 12, 934. [Google Scholar] [CrossRef]
- Santos, E.L.; Maia, B.; Ferriani, A.P.; Teixeira, S.D. Flavonoids: Classification, biosynthesis and chemical ecology. In Flavonoids: From Biosynthesis to Human Health; IntechOpen: London, UK, 2017; Volume 13, pp. 78–94. [Google Scholar]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Ferraz, C.R.; Carvalho, T.T.; Manchope, M.F.; Artero, N.A.; Rasquel-Oliveira, F.S.; Fattori, V.; Casagrande, R.; Verri, W.A. Therapeutic Potential of Flavonoids in Pain and Inflammation: Mechanisms of Action, Pre-Clinical and Clinical Data, and Pharmaceutical Development. Molecules 2020, 25, 762. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhang, X.; Zeng, F. Biological Functions and Health Benefits of Flavonoids in Fruits and Vegetables: A Contemporary Review. Foods 2025, 14, 155. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Siddiqui, M.A.; Khan, M.M.; Ajmal, M.; Ahsan, R.; Rahaman, A.; Ahmad, A.; Arshad; Khushtar, M. Current Pharmacological Trends on Myricetin. Drug Res. 2020, 70, 448–454. [Google Scholar] [CrossRef]
- Rehman, M.U.; Rather, I.A. Myricetin abrogates cisplatin-induced oxidative stress, inflammatory response, and goblet cell disintegration in colon of wistar rats. Plants 2019, 9, 28. [Google Scholar] [CrossRef]
- Yao, Q.; Li, S.; Li, X.; Wang, F.; Tu, C. Myricetin modulates macrophage polarization and mitigates liver inflammation and fibrosis in a murine model of nonalco-holic steatohepatitis. Front. Med. 2020, 7, 71. [Google Scholar] [CrossRef]
- Lu, Y.; Sun, J.; Yang, M.; Xing, Y.; Zhu, W.; Zhu, J.; Ma, X.; Wang, Y.; Wang, L.; Jia, Y. Myricetin Induces Ferroptosis and Inhibits Gastric Cancer Progression by Targeting NOX4. J. Agric. Food Chem. 2024, 72, 6178–6188. [Google Scholar] [CrossRef]
- Rashid, S.M.; Wali, A.F.; Ali, S.; Rehman, M.U.; Maqbool, M.T.; Nadeem, A.; Ahmad, S.F.; Siddiqui, N. Myricetin (3,3′,4′,5,5′,7-hexahydroxyflavone) prevents ethanol-induced biochemical and inflammatory damage in the liver of Wistar rats. Hum. Exp. Toxicol. 2022, 41, 09603271211066843. [Google Scholar] [CrossRef]
- Ong, K.C.; Khoo, H.E. Biological effects of myricetin. Gen Pharmacol. 1997, 29, 121–126. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F. Flavonols (kaempeferol, quercetin, myricetin) contents of selected fruits, vegetables and medicinal plants. Food Chem. 2008, 108, 879–884. [Google Scholar] [CrossRef]
- ahan, N.; Khalil-Ur-Rahman, A.S.; Asi, M.R. Phenolic acid and flavonol contents of gemmo-modified and native extracts of some indigenous medicinal plants. Pak. J. Bot. 2013, 45, 1515–1519. [Google Scholar]
- Mustafa, R.; Hamid, A.A.; Mohamed, S.; Abu Bakar, F. Total phenolic compounds, flavonoids, and radical scavenging activity of 21 selected tropical plants. J. Food Sci. 2010, 75, C28–C35. [Google Scholar] [CrossRef]
- Häkkinen, S.H.; Kärenlampi, S.O.; Heinonen, I.M.; Mykkänen, H.M.; Törrönen, A.R. Content of the flavonols quercetin, myricetin, and kaempferol in 25 edible berries. J. Agric. Food Chem. 1999, 47, 2274–2279. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, C.; Yaman, M. Determination of Myricetin in medicinal plants by high-performance liquid chromatography. Instrum. Sci. Technol. 2015, 43, 44–52. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Antioxidant constituents of three selected red and green color Amaranthus leafy vegetable. Sci. Rep. 2019, 9, 18233. [Google Scholar] [CrossRef] [PubMed]
- Nardini, M.; Garaguso, I. Characterization of bioactive compounds and antioxidant activity of fruit beers. Food Chem. 2020, 305, 125437. [Google Scholar] [CrossRef]
- Martos, I.; Ferreres, F.; Yao, L.; D’Arcy, B.; Caffin, N.; Tomás-Barberán, F.A. Flavonoids in monospecific eucalyptus honeys from Australia. J. Agric. Food Chem. 2000, 48, 4744–4748. [Google Scholar] [CrossRef]
- Ali, M.C.; Chen, J.; Zhang, H.; Li, Z.; Zhao, L.; Qiu, H. Effective extraction of flavonoids from Lycium barbarum L. fruits by deep eutectic solvents-based ultrasound-assisted extraction. Talanta 2019, 203, 16–22. [Google Scholar] [CrossRef]
- Huamán-Castilla, N.L.; Mariotti-Celis, M.S.; Pérez-Correa, J.R. Polyphenols of Carménère grapes. Mini-Rev. Org. Chem. 2017, 14, 176–186. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, S.M.; Wu, K.; Willett, W.C.; Fuchs, C.S.; Giovannucci, E. Flavonoid intake and colorectal cancer risk in men and women. Am. J. Epidemiology 2006, 164, 644–651. [Google Scholar] [CrossRef]
- Mullie, P.; Clarys, P.; Deriemaeker, P.; Hebbelinck, M. Estimation of daily human intake of food flavonoids. Plant Foods Hu-Man Nutr. 2007, 62, 93–98. [Google Scholar] [CrossRef]
- Vogiatzoglou, A.; Mulligan, A.A.; Lentjes, M.A.H.; Luben, R.N.; Spencer, J.P.E.; Schroeter, H.; Khaw, K.-T.; Kuhnle, G.G.C. Flavonoid intake in European adults (18 to 64 years). PLoS ONE 2015, 10, e0128132. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.-H.; Li, C.; Choi, J.-S. Effects of myricetin, an antioxidant, on the pharmacokinetics of losartan and its active metabo-lite, EXP-3174, in rats: Possible role of cytochrome P450 3A4, cytochrome P450 2C9 and P-glycoprotein inhibition by myricetin. J. Pharm. Pharmacol. 2010, 62, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Lin, G.; Xie, Y.; Duan, J.; Ma, P.; Li, G.; Ji, G. Quantitative Determination of Myricetin in Rat Plasma by Ultra Performance Liquid Chromatography Tandem Mass Spectrometry and Its Absolute Bioavailability. Drug Res. 2014, 64, 516–522. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Alzohairy, M.A.; Khan, A.A.; Alsahli, M.A.; Almatroodi, S.A.; Rahmani, A.H. Protective effects of thymoquinone, an active compound of nigella sativa, on rats with Benzo (a) pyrene-Induced Lung injury through regulation of oxidative stress and inflammation. Molecules 2021, 26, 3218. [Google Scholar] [CrossRef]
- Alharbi, H.O.A.; Alshebremi, M.; Babiker, A.Y.; Rahmani, A.H. The Role of Quercetin, a Flavonoid in the Management of Pathogenesis Through Regulation of Oxidative Stress, Inflammation, and Biological Activities. Biomolecules 2025, 15, 151. [Google Scholar] [CrossRef]
- Rahmani, A.H.; Almatroudi, A.; Allemailem, K.S.; Alharbi, H.O.A.; Babiker, A.Y.; Althwab, S.A.; Alsuhaymi, N.; Alsugoor, M.H.; Khan, A.A.; Al-Megrin, W.A.I. Oleuropein, a phenolic component of Olea europaea L. ameliorates CCl 4-induced liver injury in rats through the regulation of oxidative stress and inflammation. Eur. Rev. Med. Pharmacol. Sci. 2024, 28, 1259–1271. [Google Scholar]
- Morel, I.; Abaléa, V.; Sergent, O.; Cillard, P.; Cillard, J. Involvement of phenoxyl radical intermediates in lipid antioxidant action of myricetin in iron-treated rat hepatocyte culture. Biochem. Pharmacol. 1998, 55, 1399–1404. [Google Scholar] [CrossRef]
- Hassan, F.A.; Al-Shawi, N.N. Antioxidant and Anti-Inflammatory Effects of Myricetin on 5-Fluorouracil-Induced Hepatotoxicity in Male Rats. Afr. J. Biomed. Res. 2024, 27, 3705–3713. [Google Scholar]
- Abalea, V.; Cillard, J.; Dubos, M.-P.; Sergent, O.; Cillard, P.; Morel, I. Repair of iron-induced DNA oxidation by the flavonoid myricetin in primary rat hepatocyte cultures. Free. Radic. Biol. Med. 1999, 26, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, Y.; Li, P.; Wu, Z.; Chen, Y.; Fu, Y.; Wu, H.; Ye, Y.; Wang, J.; Yang, Z.; et al. The protective effects of myricetin against acute liver failure via inhibiting inflammation and regulating oxidative stress via Nrf2 signaling. Nat. Prod. Res. 2023, 37, 798–802. [Google Scholar] [CrossRef]
- Barzegar, A. Antioxidant activity of polyphenolic myricetin in vitro cell- free and cell-based systems. Mol. Biol. Res. Commun. 2016, 5, 87–95. [Google Scholar] [PubMed]
- Salimi, A.; Jamali, Z.; Shabani, M. Antioxidant potential and inhibition of mitochondrial permeability transition pore by myricetin reduces aluminium phosphide-induced cytotoxicity and mitochondrial impairments. Front. Pharmacol. 2021, 12, 719081. [Google Scholar] [CrossRef]
- Pandey, K.B.; Mishra, N.; Rizvi, S.I. Protective role of myricetin on oxidative stress markers in human erythrocytes subjected to oxidative stress. Nat. Prod. Commun. 2009, 4, 1934578X0900400211. [Google Scholar] [CrossRef]
- Xia, S.-F.; Le, G.-W.; Wang, P.; Qiu, Y.-Y.; Jiang, Y.-Y.; Tang, X. Regressive effect of myricetin on hepatic steatosis in mice fed a high-fat diet. Nutrients 2016, 8, 799. [Google Scholar] [CrossRef]
- Su, H.-M.; Feng, L.-N.; Zheng, X.-D.; Chen, W. Myricetin protects against diet-induced obesity and ameliorates oxidative stress in C57BL/6 mice. J. Zhejiang Univ. B 2016, 17, 437–446. [Google Scholar] [CrossRef]
- Choi, H.-N.; Kang, M.-J.; Lee, S.-J.; Kim, J.-I. Ameliorative effect of myricetin on insulin resistance in mice fed a high-fat, high-sucrose diet. Nutr. Res. Pr. 2014, 8, 544–549. [Google Scholar] [CrossRef]
- Ma, J.; Liu, J.; Chen, Y.; Yu, H.; Xiang, L. Myricetin Improves Impaired Nerve Functions in Experimental Diabetic Rats. Front. Endocrinol. 2022, 13, 915603. [Google Scholar] [CrossRef]
- Rahmani, A.H.; A Alharbi, H.O.; Khan, A.A.; Babiker, A.Y.; Alam Rizvi, M.M. Therapeutic potential of resveratrol, a polyphenol in the prevention of liver injury induced by diethylnitrosamine (DEN) through the regulation of inflammation and oxidative stress. Funct. Foods Health Dis. 2024, 14, 898–920. [Google Scholar] [CrossRef]
- Rahmani, A.H.; Babiker, A.Y. Review on role of honey in disease prevention and treatment through modulation of biological activities. Open Life Sci. 2025, 20, 20251069. [Google Scholar] [CrossRef] [PubMed]
- Alzohairy, M.A.; Khan, A.A.; Ansari, M.A.; Babiker, A.Y.; Alsahli, M.A.; Almatroodi, S.A.; Rahmani, A.H. Protective effect of quercetin, a Flavonol against benzo (a) pyrene-induced lung injury via inflammation, oxidative stress, angiogenesis and Cyclooxygenase-2 Signalling molecule. Appl. Sci. 2021, 11, 8675. [Google Scholar] [CrossRef]
- Serafini, M.; Peluso, I.; Raguzzini, A. Flavonoids as Anti-Inflammatory Agents. Proc. Nutr. Soc. 2010, 69, 273–278. [Google Scholar] [CrossRef]
- García-Lafuente, A.; Guillamón, E.; Villares, A.; Rostagno, M.A.; Martínez, J.A. Flavonoids as Anti-Inflammatory Agents: Implications in Cancer and Cardiovascular Disease. Inflamm. Res. 2009, 58, 537–552. [Google Scholar] [CrossRef]
- Read, M.A. Flavonoids: Naturally occurring anti-inflammatory agents. Am. J. Pathol. 1995, 147, 235–237. [Google Scholar]
- Ferrali, M.; Signorini, C.; Caciotti, B.; Sugherini, L.; Ciccoli, L.; Giachetti, D.; Comporti, M. Protection against oxidative damage of erythrocyte membrane by the flavonoid quercetin and its relation to iron chelating activity. FEBS Lett. 1997, 416, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.O.; Yin, H.H.; Park, S.H.; Byun, E.B.; Ha, H.Y.; Jang, S.I. Anti-inflammatory activity of myricetin from Diospyros lotus through suppression of NF-κB and STAT1 activation and Nrf2-mediated HO-1 induction in lipopolysaccharide-stimulated RAW264.7 macrophages. Biosci. Biotechnol. Biochem. 2016, 80, 1520–1530. [Google Scholar] [CrossRef]
- Rosas-Martínez, M.; Gutiérrez-Venegas, G. Myricetin Inhibition of Peptidoglycan-Induced COX-2 Expression in H9c2 Cardiomyocytes. Prev. Nutr. Food Sci. 2019, 24, 202–209. [Google Scholar] [CrossRef]
- Fu, R.H.; Liu, S.P.; Chu, C.L.; Lin, Y.H.; Ho, Y.C.; Chiu, S.C.; Lin, W.Y.; Shyu, W.C.; Lin, S.Z. Myricetin attenuates lipopolysaccharide-stimulated activation of mouse bone marrow-derived dendritic cells through suppression of IKK/NF-κB and MAPK sig-nalling pathways. J. Sci. Food Agric. 2013, 93, 76–84. [Google Scholar] [CrossRef]
- Zhang, N.; Feng, H.; Liao, H.; Chen, S.; Yang, Z.; Deng, W.; Tang, Q. Myricetin attenuated LPS induced cardiac injury in vivo and in vitro. Phytotherapy Res. 2018, 32, 459–470. [Google Scholar] [CrossRef]
- Lee, C.S. Flavonoid myricetin inhibits TNF-stimulated production of inflammatory mediators by suppressing the Akt, mTOR and NF-κB pathways in human keratinocytes. Eur. J. Pharmacol. 2016, 784, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zheng, Y. Myricetin protects keratinocyte damage induced by UV through IκB/NFκb signaling pathway. J. Cosmet. Dermatol. 2017, 16, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Choi, E.M. Myricetin inhibits IL-1β-induced inflammatory mediators in SW982 human synovial sarcoma cells. Int. Immunopharmacol. 2010, 10, 812–814. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.M.; Khalaf, M.M.; Sadek, S.A.; Abo-Youssef, A.M. Protective effects of apigenin and myricetin against cisplatin-induced nephrotoxicity in mice. Pharm. Biol. 2017, 55, 766–774. [Google Scholar] [CrossRef]
- Mao, M.; Huang, M. Myricetin attenuates lung inflammation and provides protection against lipopolysaccharide-induced acute lung injury by inhibition of NF-κB pathway in rats. Trop. J. Pharm. Res. 2017, 16, 2585. [Google Scholar] [CrossRef]
- Chen, K.; Yang, C.; Li, T.; Zouboulis, C.C.; Huang, Y. Suppression of Propionibacterium acnes-stimulated proinflammatory cytokines by Chinese bayberry extracts and its active constituent myricetin in human sebocytes in vitro. Phytotherapy Res. 2019, 33, 1104–1113. [Google Scholar] [CrossRef]
- Kuo, P.L. Myricetin inhibits the induction of anti-Fas IgM-, tumor necrosis factor-alpha- and interleukin-1beta-mediated apoptosis by Fas pathway inhibition in human osteoblastic cell line MG-63. Life Sci. 2005, 77, 2964–2976. [Google Scholar] [CrossRef]
- Jang, J.-H.; Lee, S.H.; Jung, K.; Yoo, H.; Park, G. Inhibitory Effects of Myricetin on Lipopolysaccharide-Induced Neuroinflammation. Brain Sci. 2020, 10, 32. [Google Scholar] [CrossRef]
- Kan, X.; Liu, B.; Guo, W.; Wei, L.; Lin, Y.; Guo, Y.; Gong, Q.; Li, Y.; Xu, D.; Cao, Y.; et al. Myricetin relieves LPS-induced mastitis by inhibiting inflammatory response and repairing the blood–milk barrier. J. Cell. Physiol. 2019, 234, 16252–16262. [Google Scholar] [CrossRef]
- Rehm, J.; Samokhvalov, A.V.; Shield, K.D. Global burden of alcoholic liver diseases. J. Hepatol. 2013, 59, 160–168. [Google Scholar] [CrossRef]
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global burden of liver disease: 2023 update. J. Hepatol. 2023, 79, 516–537. [Google Scholar] [CrossRef] [PubMed]
- Adewusi, E.; Afolayan, A. A review of natural products with hepatoprotective activity. J. Med. Plants Res. 2010, 4, 1318–1334. [Google Scholar]
- Almatroodi, S.A.; Alsahli, M.A.; Alharbi, H.M.; Khan, A.A.; Husain Rahmani, A. Epigallocatechin-3-gallate (EGCG), an active con-stituent of green tea: Implications in the prevention of liver injury induced by diethylnitrosamine (DEN) in rats. Appl. Sci. 2019, 9, 4821. [Google Scholar] [CrossRef]
- Abushady, E.A.; Elagaty, S.M.; Nassef, N.A.; Abdelhamid, G.S. The potential hepatoprotective effect of quercetin on cholestatic liver injury in rats. Qjm Int. J. Med. 2020, 113 (Suppl. S1), hcaa065-002. [Google Scholar] [CrossRef]
- Oke, G.O.; Abiodun, A.A.; Imafidon, C.E.; Monsi, B.F. Zingiber officinale (Roscoe) mitigates CCl4-induced liver histopathology and biochemical derangements through antioxidant, membrane-stabilizing and tissue-regenerating potentials. Toxicol. Rep. 2019, 6, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Sun, Q.; Li, W.; Lu, Z.; Xu, H.; Shi, J.; Xu, Z. The common dietary flavonoid myricetin attenuates liver fibrosis in carbon tetrachloride treated mice. Mol. Nutr. Food Res. 2017, 61, 1600392. [Google Scholar] [CrossRef]
- Xia, S.F.; Qiu, Y.Y.; Chen, L.M.; Jiang, Y.Y.; Huang, W.; Xie, Z.X.; Tang, X.; Sun, J. Myricetin alleviated hepatic steatosis by acting on microRNA-146b/thyroid hormone receptor b pathway in high-fat diet fed C57BL/6J mice. Food Funct. 2019, 10, 1465–1477. [Google Scholar] [CrossRef]
- Lv, H.; An, B.; Yu, Q.; Cao, Y.; Liu, Y.; Li, S. The hepatoprotective effect of myricetin against lipopolysaccharide and D-galactosamine-induced fulminant hepatitis. Int. J. Biol. Macromol. 2020, 155, 1092–1104. [Google Scholar] [CrossRef]
- Rostami, A.; Baluchnejadmojarad, T.; Roghani, M. Hepatoprotective Effect of Myricetin following Lipopolysaccharide/DGalactosamine: Involvement of Autophagy and Sirtuin 1. Curr. Mol. Pharmacol. 2023, 16, 419–433. [Google Scholar]
- Sun, W.-L.; Li, X.-Y.; Dou, H.-Y.; Wang, X.-D.; Li, J.-D.; Shen, L.; Ji, H.-F. Myricetin supplementation decreases hepatic lipid synthesis and inflammation by modulating gut microbiota. Cell Rep. 2021, 36, 109641. [Google Scholar] [CrossRef]
- Berköz, M.; Ünal, S.; Karayakar, F.; Yunusoğlu, O.; Özkan-Yılmaz, F.; Özlüer-Hunt, A.; Aslan, A. Prophylactic effect of myricetin and apigenin against lipopolysaccharide-induced acute liver injury. Mol. Biol. Rep. 2021, 48, 6363–6373. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-N.; Shin, J.-Y.; Kim, J.-I. Ameliorative Effect of Myricetin on Nonalcoholic Fatty Liver Disease in ob/ob Mice. J. Med. Food 2021, 24, 1092–1099. [Google Scholar] [CrossRef]
- Zamljen, T.; Medič, A.; Veberič, R.; Hudina, M.; Štampar, F.; Slatnar, A. Apple Fruit (Malus domestica Borkh.) Metabolic Re-sponse to Infestation by Invasive Brown Marmorated Stink Bug (Halyomorpha halys Stal.). Horticulturae 2021, 7, 212. [Google Scholar] [CrossRef]
- Emon, N.U.; Alam, S.; Rudra, S.; Al Haidar, I.K.; Farhad, M.; Rana, M.E.H.; Ganguly, A. Antipyretic activity of the leaves ex-tract of Caesalpinia digyna Rottl along with phytoconstituent’s binding affinity to COX-1, COX-2 and mPGES-1 receptors: An in vivo and in silico approaches (Antipyretic activity of Caesalpinia digyna Rottl). Saudi J. Biol. Sci. 2021, 28, 5302–5309. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.H.; Alsahli, M.A.; Khan, A.A.; Almatroodi, S.A. Quercetin, a plant flavonol attenuates diabetic complications, renal tissue damage, renal oxidative stress and inflammation in streptozotocin-induced diabetic rats. Metabolites 2023, 13, 130. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Alnuqaydan, A.M.; Babiker, A.Y.; Almogbel, M.A.; Khan, A.A.; Husain Rahmani, A. 6-Gingerol, a bioactive compound of ginger attenuates renal damage in streptozotocin-induced diabetic rats by regulating the oxidative stress and in-flammation. Pharmaceutics 2021, 13, 317. [Google Scholar] [CrossRef]
- Alsulaim, A.K.; Almutaz, T.H.; Albati, A.A.; Rahmani, A.H. Therapeutic Potential of Curcumin, a Bioactive Compound of Turmeric, in Prevention of Streptozotocin-Induced Diabetes through the Modulation of Oxidative Stress and Inflammation. Molecules 2023, 29, 128. [Google Scholar] [CrossRef]
- Kandasamy, N.; Ashokkumar, N. Protective effect of bioflavonoid myricetin enhances carbohydrate metabolic enzymes and insulin signaling molecules in streptozotocin–cadmium induced diabetic nephrotoxic rats. Toxicol. Appl. Pharmacol. 2014, 279, 173–185. [Google Scholar] [CrossRef]
- Kandasamy, N.; Ashokkumar, N. Renoprotective effect of myricetin restrains dyslipidemia and renal mesangial cell proliferation by the suppression of sterol regulatory element binding proteins in an experimental model of diabetic nephropathy. Eur. J. Pharmacol. 2014, 743, 53–62. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, Y.; Li, X.; Zhu, L.; Wang, X.; Li, L.; Sun, H.; Han, X.; Li, J. Myricetin relieves the symptoms of type 2 diabetes mice and regulates intestinal microflora. Biomed. Pharmacother. 2022, 153, 113530. [Google Scholar] [CrossRef]
- Liao, H.H.; Zhu, J.X.; Feng, H.; Ni, J.; Zhang, N.; Chen, S.; Liu, H.J.; Yang, Z.; Deng, W.; Tang, Q.Z. Myricetin possesses potential protective effects on diabetic cardiomyopathy through inhibiting IκBα/NFκB and enhancing Nrf2/HO-1. Oxidative Med. Cell. Longev. 2017, 2017, 8370593. [Google Scholar] [CrossRef] [PubMed]
- yang, Z.J.; Wang, H.R.; Wang, Y.I.; Zhai, Z.H.; Wang, L.W.; Li, L.; Zhang, C.; Tang, L. Myricetin Attenuated Diabetes-Associated Kidney Injuries and Dysfunction via Regulating Nuclear Factor (Erythroid Derived 2)-Like 2 and Nuclear Factor-κB Signaling. Front. Pharmacol. 2019, 10, 647. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Bao, Z.; Hu, Z.; Wu, S.; Tian, C.; Zhou, Y.; Ding, Z.; Tan, X. Myricetin alleviates diabetic cardiomyopathy by regulating gut microbiota and their metabolites. Nutr. Diabetes 2024, 14, 10. [Google Scholar] [CrossRef]
- Qian, J.; Zhang, J.; Chen, Y.; Dai, C.; Fan, J.; Guo, H. Hypoglycemic activity and mechanisms of myricetin. Nat. Prod. Res. 2022, 36, 6177–6180. [Google Scholar] [CrossRef]
- Liu, I.-M.; Tzeng, T.-F.; Liou, S.-S.; Lan, T.-W. Myricetin, a naturally occurring flavonol, ameliorates insulin resistance induced by a high-fructose diet in rats. Life Sci. 2007, 81, 1479–1488. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, N.; Ashokkumar, N. Myricetin, a natural flavonoid, normalizes hyperglycemia in streptozotocin-cadmium-induced experimental diabetic nephrotoxic rats. Biomed. Prev. Nutr. 2012, 2, 246–251. [Google Scholar] [CrossRef]
- Ozcan, F.; Ozmen, A.; Akkaya, B.; Aliciguzel, Y.; Aslan, M. Beneficial effect of myricetin on renal functions in streptozotocin-induced diabetes. Clin. Exp. Med. 2012, 12, 265–272. [Google Scholar] [CrossRef]
- Liu, I.-M.; Liou, S.-S.; Lan, T.-W.; Hsu, F.-L.; Cheng, J.-T. Myricetin as the active principle of Abelmoschus moschatus to lower plasma glucose in streptozotocin-induced diabetic rats. Planta Medica 2005, 71, 617–621. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the gbd 2019 study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Andersson, C.; Johnson, A.D.; Benjamin, E.J.; Levy, D.; Vasan, R.S. 70-year legacy of the framingham heart study. Nat. Rev. Cardiol. 2019, 16, 687–698. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Wu, J.H.Y. Flavonoids, dairy foods, and cardiovascular and metabolic health: A review of emerging biologic pathways. Circ. Res. 2018, 122, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Deb, P.K.; Priya, S.; Medina, K.D.; Devi, R.; Walode, S.G.; Rudrapal, M. Dietary Flavonoids: Cardioprotective Potential with Antioxidant Effects and Their Pharmacokinetic, Toxicological and Therapeutic Concerns. Molecules 2021, 26, 4021. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.; Mohan, M.; Kasture, S.; Maxia, A.; Ballero, M. Cardioprotective potential of myricetin in isoproterenol-induced myocardial infarction in Wistar rats. Phytother Res. 2009, 23, 1361–1366. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Fan, B. Myricetin protects cardiomyocytes from LPS-induced injury. Herz 2018, 43, 265–274. [Google Scholar] [CrossRef]
- Qiu, Y.; Cong, N.; Liang, M.; Wang, Y.; Wang, J. Systems Pharmacology Dissection of the Protective Effect of Myricetin Against Acute Ischemia/Reperfusion-Induced Myocardial Injury in Isolated Rat Heart. Cardiovasc. Toxicol. 2017, 17, 277–286. [Google Scholar] [CrossRef]
- Zhang, B.; Shen, Q.; Chen, Y.; Pan, R.; Kuang, S.; Liu, G.; Sun, G.; Sun, X. Myricitrin Alleviates Oxidative Stress-induced Inflammation and Apoptosis and Protects Mice against Diabetic Cardiomyopathy. Sci. Rep. 2017, 7, srep44239. [Google Scholar] [CrossRef] [PubMed Central]
- Li, J.; Luo, T.; Zhao, Y.; Wang, D.; Jin, Y.; Wu, Z.; Yang, G.; Qi, X. Cardioprotective potentials of myricetin on doxorubicin-induced cardiotoxicity based on biochemical and transcriptomic analysis. Biomed. Pharmacother. 2024, 175, 116748. [Google Scholar] [CrossRef]
- Nie, N.; Li, Z.; Li, W.; Huang, X.; Jiang, Z.; Shen, Y. Myricetin ameliorates experimental autoimmune myocarditis in mice by modulating immune response and inhibiting MCP-1 expression. Eur. J. Pharmacol. 2023, 942, 175549. [Google Scholar] [CrossRef]
- Arafah, A.; Rehman, M.U.; Ahmad, A.; AlKharfy, K.M.; Alqahtani, S.; Jan, B.L.; Almatroudi, N.M. Myricetin (3,3′,4′,5,5′,7-Hexahydroxyflavone) Prevents 5-Fluorouracil-Induced Cardiotoxicity. ACS Omega 2022, 7, 4514–4524. [Google Scholar] [CrossRef]
- Feigin, V.L.; Abajobir, A.A.; Abate, K.H.; Abd-Allah, F.; Abdulle, A.M.; Abera, S.F.; Abyu, G.Y.; Ahmed, M.B.; Aichour, A.N.; Aichour, I.; et al. Global, Regional, and National Burden of Neurological Disorders During 1990–2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017, 16, 877–897. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Ramezani, M.; Darbandi, N.; Khodagholi, F.; Hashemi, A. Myricetin protects hippocampal CA3 pyramidal neurons and improves learning and memory impairments in rats with Alzheimer’s disease. Neural Regen. Res. 2016, 11, 1976–1980. [Google Scholar] [CrossRef]
- Wang, L.; Tang, Z.; Li, B.; Peng, Y.; Yang, X.; Xiao, Y.; Ni, R.; Qi, X.-L. Myricetin ameliorates cognitive impairment in 3×Tg Alzheimer’s disease mice by regulating oxidative stress and tau hyperphosphorylation. Biomed. Pharmacother. 2024, 177, 116963. [Google Scholar] [CrossRef]
- Wang, B.; Zhong, Y.; Gao, C.; Li, J. Myricetin ameliorates scopolamine-induced memory impairment in mice via inhibiting acetylcholinesterase and down-regulating brain iron. Biochem. Biophys. Res. Commun. 2017, 490, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.-C.; Xie, Z.-G.; Gu, M.-J.; Wang, C.-D.; Xu, L.-M.; Gao, C.; Yuan, X.-L.; Wu, Y.; Hu, Y.-Q.; Cao, Y.; et al. Myricetin mitigates motor disturbance and decreases neuronal ferroptosis in a rat model of Parkinson’s disease. Sci. Rep. 2024, 14, 15107. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Liu, J.; Ma, D.; Chen, G.; Wang, W.; Fu, S. Myricetin prevents dopaminergic neurons from undergoing neuroinflammation-mediated degeneration in a lipopolysaccharide-induced Parkinson’s disease model. J. Funct. Foods 2018, 45, 452–461. [Google Scholar] [CrossRef]
- Karuppaiah, J.; Dhanraj, V.; Balakrishnan, R.; Elangovan, N. Myricetin attenuates neurodegeneration and cognitive impairment in Parkinsonism. Front. Biosci. 2018, 10, 481–494. [Google Scholar] [CrossRef]
- Ara, G.; Afzal, M.; Jyoti, S.; Naz, F.; Rahul; Siddique, Y.H. Effect of Myricetin on the loss of dopaminergic neurons in the transgenic Drosophila model of Parkinson’s disease. Curr. Drug Ther. 2019, 14, 58–64. [Google Scholar] [CrossRef]
- Ma, Z.-G.; Wang, J.; Jiang, H.; Liu, T.-W.; Xie, J.-X. Myricetin reduces 6-hydroxydopamine-induced dopamine neuron degeneration in rats. NeuroReport 2007, 18, 1181–1185. [Google Scholar] [CrossRef]
- Sun, Z.Q.; Meng, F.H.; Tu, L.X.; Sun, L. Myricetin attenuates the severity of seizures and neuroapoptosis in pentylenetetrazole kindled mice by regulating the of BDNF-TrkB signaling pathway and modulating matrix metalloproteinase-9 and GABAA. Exp. Ther. Med. 2019, 17, 3083–3091. [Google Scholar] [CrossRef]
- Demyashkin, G.; Blinova, E.; Grigoryan, M.; Parshenkov, M.; Skovorodko, P.; Ius, V.; Lebed, A.; Shegay, P.; Kaprin, A. Neuroprotective Effects of Myricetin on PTZ-Induced Seizures in Mice: Evaluation of Oxidation, Neuroinflammation and Metabolism, and Apoptosis in the Hippocampus. Curr. Issues Mol. Biol. 2024, 46, 8914–8944. [Google Scholar] [CrossRef]
- Bhatt, A.P.; Redinbo, M.R.; Bultman, S.J. The role of the microbiome in cancer development and therapy. CA A Cancer J. Clin. 2017, 67, 326–344. [Google Scholar] [CrossRef] [PubMed]
- Cronin, K.A.; Lake, A.J.; Scott, S.; Sherman, R.L.; Noone, A.M.; Howlader, N.; Henley, S.J.; Anderson, R.N.; Firth, A.U.; Ma, J.; et al. Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer 2018, 124, 2785–2800. [Google Scholar] [CrossRef] [PubMed]
- Madhuri, S.; Pandey, G. Some anticancer medicinal plants of foreign origin. Curr. Sci. 2009, 25, 779–783. [Google Scholar]
- Cragg, G.M.; Pezzuto, J.M. Natural Products as a Vital Source for the Discovery of Cancer Chemotherapeutic and Chemopreventive Agents. Med. Princ. Pract. 2016, 25 (Suppl. S2), 41–59. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Alsahli, M.A.; Rahmani, A.H. Berberine: Berberine: An important emphasis on its anticancer effects through modulation of various cell signaling pathways. Molecules 2022, 27, 5889. [Google Scholar] [CrossRef]
- Almajali, B.; Al-Jamal, H.A.; Taib, W.R.; Ismail, I.; Johan, M.F.; Doolaanea, A.A.; Ibrahim, W.N. Thymoquinone, as a novel therapeutic candidate of cancers. Pharmaceuticals 2021, 14, 369. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Syed, M.A.; Rahmani, A.H. Potential therapeutic targets of curcumin, most abundant active compound of turmeric spice: Role in the management of various types of cancer. Recent Pat. Anti-Cancer Drug Discov. 2021, 16, 3–29. [Google Scholar] [CrossRef]
- Huang, H.; Chen, A.Y.; Rojanasakul, Y.; Ye, X.; Rankin, G.O.; Chen, Y.C. Dietary compounds galangin and myricetin suppress ovarian cancer cell angiogenesis. J. Funct. Foods 2015, 15, 464–475. [Google Scholar] [CrossRef]
- Zhou, Z.; Mao, W.; Li, Y.; Qi, C.; He, Y. Myricetin inhibits breast tumor growth and angiogenesis by regulating VEGF/VEGFR2 and p38MAPK signaling pathways. Anat. Rec. 2019, 302, 2186–2192. [Google Scholar] [CrossRef]
- Niu, X.; Ding, X.; Tong, Q.; Huang, X.; Ma, X.; Li, Z.; Wang, Q.; Wang, Y. Myricetin inhibits 4 T1 breast tumor growth in mice via induc-tion of Nrf-2/GPX4 pathway-mediated Ferroptosis. Toxicol. Appl. Pharmacol. 2024, 3, 116990. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-H.; Lee, J.-H.; Woo, J.-S.; Jung, G.-H.; Jung, S.-H.; Han, E.-J.; Kim, B.; Cho, S.D.; Nam, J.S.; Che, J.H.; et al. Myricetin induces apoptosis and autophagy in human gastric cancer cells through inhibition of the PI3K/Akt/mTOR pathway. Heliyon 2022, 8, e09309. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Chen, X.; Wang, Y.; Du, Y.; Sun, Q.; Zang, W.; Zhao, G. Myricetin inhibits proliferation and induces apoptosis and cell cycle arrest in gastric cancer cells. Mol. Cell. Biochem. 2015, 408, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Phillips, P.; Sangwan, V.; Borja-Cacho, D.; Dudeja, V.; Vickers, S.; Saluja, A. Myricetin induces pancreatic cancer cell death via the induction of apoptosis and inhibition of the phosphatidylinositol 3-kinase (PI3K) signaling pathway. Cancer Lett. 2011, 308, 181–188. [Google Scholar] [CrossRef]
- Li, H.G.; Chen, J.X.; Xiong, J.H.; Zhu, J.W. Myricetin exhibits anti-glioma potential by inducing mitochondrial-mediated apoptosis, cell cycle arrest, inhibition of cell migration and ROS generation. J BUON 2016, 21, 182–190. [Google Scholar]
- Yang, W.; Su, J.; Li, M.; Li, T.; Wang, X.; Zhao, M.; Hu, X. Myricetin induces autophagy and cell cycle arrest of HCC by inhibiting MARCH1-regulated Stat3 and p38 MAPK signaling pathways. Front. Pharmacol. 2021, 12, 709526. [Google Scholar] [CrossRef]
- Zhang, X.H.; Zou, Z.Q.; Xu, C.W.; Shen, Y.Z.; Li, D. Myricetin induces G2/M phase arrest in HepG2 cells by inhibiting the activity of the cyclin B/Cdc2 complex. Mol. Med. Rep. 2011, 4, 273–277. [Google Scholar] [CrossRef]
- Ji, A.; Hu, L.; Ma, D.; Qiang, G.; Yan, D.; Zhang, G.; Jiang, C. Myricetin Induces Apoptosis and Protective Autophagy through Endoplasmic Reticulum Stress in Hepatocellular Carcinoma. Evid. Based Complement. Altern. Med. 2022, 2022, 3115312. [Google Scholar] [CrossRef]
- Yang, H.-W.; Lan, Y.; Li, A.; Wu, H.; Song, Z.-W.; Wan, A.-L.; Wang, Y.; Li, S.-B.; Ji, S.; Wang, Z.-C.; et al. Myricetin suppresses TGF-β-induced epithelial-to-mesenchymal transition in ovarian cancer. Front. Pharmacol. 2023, 14, 1288883. [Google Scholar] [CrossRef]
- Senggunprai, L.; Tuponchai, P.; Kukongviriyapan, V.; Prawan, A.; Kongpetch, S. Myricetin ameliorates cytokine-induced migration and invasion of cholangiocarcinoma cells via suppression of STAT3 pathway. J. Cancer Res. Ther. 2019, 15, 157–163. [Google Scholar] [CrossRef]
- Xie, M.; Liu, X.; Cao, X.; Guo, M.; Li, X. Trends in prevalence and incidence of chronic respiratory diseases from 1990 to 2017. Respir. Res. 2020, 21, 49. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Bibi, S.; Rahaman, S.; Rahman, F.; Islam, F.; Khan, M.S.; Hasan, M.M.; Parvez, A.; Hossain, A.; Maeesa, S.K.; et al. Natural therapeutics and nutraceuticals for lung diseases: Traditional significance, phytochemistry, and pharmacology. Biomed. Pharmacother. 2022, 150, 113041. [Google Scholar] [CrossRef]
- Abdulaal, W.H.; Omar, U.M.; Zeyadi, M.; El-Agamy, D.S.; Alhakamy, N.A.; Almalki, N.A.; Asfour, H.Z.; Al-Rabia, M.W.; Alzain, A.A.; Mohamed, G.A.; et al. Protective effect of kaempferol glucoside against lipopolysaccharide-caused acute lung injury via targeting Nrf2/NF-κB/NLRP3/GSDMD: Integrating experimental and computational studies. Saudi Pharm. J. 2024, 32, 102073. [Google Scholar] [CrossRef]
- Xu, H.; Qi, Q.; Yan, X. Myricetin ameliorates sepsis-associated acute lung injury in a murine sepsis model. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, B.; Yang, G. The protective effects and mechanisms of myricetin on LPS-induced acute lung injury of BALB/c mice. Int. J. Tradit. Chin. Med. 2019, 6, 154–159. [Google Scholar]
- Huang, W.-C.; Wu, S.-J.; Yeh, K.-W.; Huang, T.; Liou, C.-J. Protective effects of myricetin on airway inflammation and oxidative stress in ovalbumin-induced asthma mice. J. Nutr. Biochem. 2024, 123, 109485. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, H.; Liang, L.; Bi, Z.; Wang, Y.; Gao, S.; Wang, M.; Li, H.; Miao, Y.; Deng, R.; et al. Myricetin ameliorates bleomycin-induced pulmonary fibrosis in mice by inhibiting TGF-β signaling via targeting HSP90β. Biochem. Pharmacol. 2020, 178, 114097. [Google Scholar] [CrossRef] [PubMed]
- Dmochowska, N.; Wardill, H.R.; Hughes, P.A. Advances in Imaging Specific Mediators of Inflammatory Bowel Disease. Int. J. Mol. Sci. 2018, 19, 2471. [Google Scholar] [CrossRef]
- Gubatan, J.; Kulkarni, C.V.; Talamantes, S.M.; Temby, M.; Fardeen, T.; Sinha, S.R. Dietary Exposures and Interventions in Inflammatory Bowel Disease: Current Evidence and Emerging Concepts. Nutrients 2023, 15, 579. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Zhao, J.; Hong, T.; Dong, M.; Meng, Y.; Mu, J. Protective effect of myricetin in dextran sulphate sodium-induced murine ulcerative colitis. Mol. Med. Rep. 2013, 7, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-L.; Yang, J.; Qu, X.-J.; Meng, J.; Miao, R.-R.; Cui, S.-X. M10, a Myricetin-3-O-b-D-Lactose Sodium Salt, Prevents Ulcerative Colitis Through Inhibiting Necroptosis in Mice. Front. Pharmacol. 2020, 11, 557312. [Google Scholar] [CrossRef]
- Qu, X.; Li, Q.; Song, Y.; Xue, A.; Liu, Y.; Qi, D.; Dong, H. Potential of myricetin to restore the immune balance in dextran sulfate sodium-induced acute murine ulcerative colitis. J. Pharm. Pharmacol. 2020, 72, 92–100. [Google Scholar] [CrossRef]
- Wang, F.; Song, Z.-Y.; Qu, X.-J.; Li, F.; Zhang, L.; Li, W.-B.; Cui, S.-X. M10, a novel derivative of Myricetin, prevents ulcerative colitis and colorectal tumor through attenuating robust endoplasmic reticulum stress. Carcinogenesis 2018, 39, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Li, H. Obesity: Epidemiology, pathophysiology, and therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jin, Y.; Choi, Y.; Park, T. Resveratrol exerts anti-obesity effects via mechanisms involving down-regulation of adipogenic and inflammatory processes in mice. Biochem. Pharmacol. 2011, 81, 1343–1351. [Google Scholar] [CrossRef]
- Aguirre, L.; Milton-Laskibar, I.; Hijona, E.; Bujanda, L.; Rimando, A.M.; Portillo, M.P. Effects of pterostilbene in brown adipose tissue from obese rats. J. Physiol. Biochem. 2016, 73, 457–464. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, S.-T.; Yang, X.; You, P.-P.; Zhang, W. Myricetin suppresses differentiation of 3 T3-L1 preadipocytes and enhances lipolysis in adipocytes. Nutr. Res. 2015, 35, 317–327. [Google Scholar] [CrossRef]
- Hu, T.; Yuan, X.; Wei, G.; Luo, H.; Lee, H.J.; Jin, W. Myricetin-induced brown adipose tissue activation prevents obesity and insulin resistance in db/db mice. Eur. J. Nutr. 2018, 57, 391–403. [Google Scholar] [CrossRef]
- Chang, C.J.; Tzeng, T.-F.; Liou, S.-S.; Chang, Y.-S.; Liu, I.-M. Myricetin increases hepatic peroxisome proliferator-activated receptor protein expression and decreases plasma lipids and adiposity in rats. Evid. Based Complement. Altern. Med. 2012, 2012, 787152. [Google Scholar] [CrossRef]
- Trinh, X.-T.; Long, N.-V.; Van Anh, L.T.; Nga, P.T.; Giang, N.N.; Chien, P.N.; Nam, S.-Y.; Heo, C.-Y. A Comprehensive Review of Natural Compounds for Wound Healing: Targeting Bioactivity Perspective. Int. J. Mol. Sci. 2022, 23, 9573. [Google Scholar] [CrossRef]
- Elshamy, A.I.; Ammar, N.M.; Hassan, H.A.; El-Kashak, W.A.; Al-Rejaie, S.S.; Abd-ElGawad, A.M.; Farrag, A.H. Topical Wound Healing Activity of Myricetin Isolated from Tecomaria capensis v. aurea. Molecules 2020, 25, 4870. [Google Scholar] [CrossRef]
- Elloumi, W.; Mahmoudi, A.; Ortiz, S.; Boutefnouchet, S.; Chamkha, M.; Sayadi, S. Wound healing potential of quercetin-3-O-rhamnoside and myricetin-3-O-rhamnoside isolated from Pistacia lentiscus distilled leaves in rats model. Biomed. Pharmacother. 2022, 146, 112574. [Google Scholar] [CrossRef]
- Sklenářová, R.; Svrčková, M.; Hodek, P.; Ulrichová, J.; Franková, J. Effect of the natural flavonoids myricetin and dihydromyricetin on the wound healing process in vitro. J. Appl. Biomed. 2021, 19, 149–158. [Google Scholar] [CrossRef]
- Tong, Y.; Zhou, X.-M.; Wang, S.-J.; Yang, Y.; Cao, Y.-L. Analgesic activity of myricetin isolated from Myrica rubra Sieb. et Zucc. leaves. Arch. Pharmacal Res. 2009, 32, 527–533. [Google Scholar] [CrossRef]
- Hagenacker, T.; Hillebrand, I.; Wissmann, A.; Büsselberg, D.; Schäfers, M. Anti-allodynic effect of the flavonoid myricetin in a rat model of neuropathic pain: Involvement of p38 and protein kinase C mediated modulation of Ca2+channels. Eur. J. Pain 2010, 14, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Córdova, M.M.; Werner, M.F.d.P.; da Silva, M.D.; Ruani, A.P.; Pizzolatti, M.G.; Santos, A.R. Further antinociceptive effects of myricitrin in chemical models of overt nociception in mice. Neurosci. Lett. 2011, 495, 173–177. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Shaheen, S.; El Haouari, M.; Azzini, E.; Butnariu, M.; Sarac, I.; Pentea, M.; Ramírez-Alarcón, K.; Martorell, M.; et al. Flavonoids as potential anti-platelet aggregation agents: From biochemistry to health promoting abilities. Crit. Rev. Food Sci. Nutr. 2022, 62, 8045–8058. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Zam, W.; Kumar, M.; Cardoso, S.M.; Pereira, O.R.; Ademiluyi, A.O.; Adeleke, O.; Moreira, A.C.; Živković, J.; et al. Phenolic bioactives as antiplatelet aggregation factors: The pivotal ingredients in maintaining cardiovascular health. Oxidative Med. Cell. Longev. 2021, 2021, 2195902. [Google Scholar] [CrossRef]
- Zang, B.-X.; Jin, M.; Wu, W.; Chen, W.-M.; Piao, Y.-Z.; Li, J.-R. Antagonistic effect of myricetin on platelet activing factor. Yao Xue Xue Bao Acta Pharm. Sin. 2003, 38, 831–833. [Google Scholar]
- Tzeng, S.-H.; Ko, W.-C.; Ko, F.-N.; Teng, C.-M. Inhibition of platelet aggregation by some flavonoids. Thromb. Res. 1991, 64, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Landolfi, R.; Mower, R.L.; Steiner, M. Modification of platelet function and arachidonic acid metabolism by bioflavonoids. Biochem. Pharmacol. 1984, 33, 1525–1530. [Google Scholar] [CrossRef]
- Gaspar, R.S.; da Silva, S.A.; Stapleton, J.; Fontelles, J.L.d.L.; Sousa, H.R.; Chagas, V.T.; Alsufyani, S.; Trostchansky, A.; Gibbins, J.M.; Paes, A.M.d.A. Myricetin, the Main Flavonoid in Syzygium cumini Leaf, Is a Novel Inhibitor of Platelet Thiol Isomerases PDI and ERp5. Front. Pharmacol. 2020, 10, 1678. [Google Scholar] [CrossRef]
- Fan, S.; Gao, X.; Chen, P.; Li, X. Myricetin ameliorates glucocorticoid-induced osteoporosis through the ERK signaling pathway. Life Sci. 2018, 207, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.; Chen, X.; Wang, T.; Zheng, W.; Chen, L.; Xu, Y. Possible osteoprotective effects of myricetin in STZ induced diabetic osteo-porosis in rats. Eur. J. Pharmacol. 2020, 866, 172805. [Google Scholar] [CrossRef]
- Ko, S.Y. Myricetin suppresses LPS-induced MMP expression in human gingival fibroblasts and inhibits osteoclastogenesis by downregulating NFATc1 in RANKL-induced RAW 264.7 cells. Arch. Oral Biol. 2012, 57, 1623–1632. [Google Scholar] [CrossRef]
- Ying, X.; Chen, X.; Feng, Y.; Xu, H.Z.; Chen, H.; Yu, K.; Cheng, S.; Peng, L. Myricetin enhances osteogenic differentiation through the activation of canonical Wnt/β-catenin signaling in human bone marrow stromal cells. Eur. J. Pharmacol. 2014, 738, 22–30. [Google Scholar] [CrossRef]
- Huang, J.; Wu, C.; Tian, B.; Zhou, X.; Ma, N.; Qian, Y. Myricetin prevents alveolar bone loss in an experimental ovariectomized mouse model of periodontitis. Int. J. Mol. Sci. 2016, 17, 422. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Hamamoto, R.; Uchiyama, S.; Ishiyama, K. Effects of flavonoid on calcium content in femoral tissue culture and parathyroid hormone-stimulated osteoclastogenesis in bone marrow culture in vitro. Mol. Cell. Biochem. 2007, 303, 83–88. [Google Scholar] [CrossRef]
- Chen, R.; Hollborn, M.; Grosche, A.; Reichenbach, A.; Wiedemann, P.; Bringmann, A.; Kohen, L. Effects of the vegetable polyphenols epigallocatechin-3-gallate, luteolin, apigenin, myricetin, quercetin, and cyanidin in primary cultures of human retinal pigment epithelial cells. Mol. Vis. 2014, 20, 242–258. [Google Scholar]
- Hodges, L.C.; Kearse, E.C.; Green, K. Intraocular pressure-lowering activity of phenolic antioxidants in normotensive rabbits. Curr. Eye Res. 1999, 19, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Laabich, A.; Manmoto, C.C.; Kuksa, V.; Leung, D.W.; Vissvesvaran, G.P.; Karliga, I.; Kamat, M.; Scott, I.L.; Fawzi, A.; Kubota, R. Protective effects of myricetin and related flavonols against A2E and light mediated-cell death in bovine retinal primary cell culture. Exp. Eye Res. 2007, 85, 154–165. [Google Scholar] [CrossRef]
- Qing, Y.; Li, Y.; Luo, L. Effect of myricetin on primary open-angle glaucoma. Transl. Neurosci. 2018, 9, 132–141. [Google Scholar]
- Nahar, N.; Mohamed, S.; Mustapha, N.M.; Fong, L.S.; Ishak, N.I.M. Gallic acid and myricetin-rich Labisia pumila extract mitigated multiple diabetic eye disorders in rats. J. Food Biochem. 2021, 45, e13948. [Google Scholar] [CrossRef]
- Bortoluzzi, A.; Furini, F.; Scirè, C.A. Osteoarthritis and its management—Epidemiology, nutritional aspects and environmental factors. Autoimmun. Rev. 2018, 17, 1097–1104. [Google Scholar] [CrossRef]
- Mabey, T.; Honsawek, S. Cytokines as biochemical markers for knee osteoarthritis. World J. Orthop. 2015, 6, 95–105. [Google Scholar] [CrossRef]
- Nelson, A. Osteoarthritis year in review 2017: Clinical. Osteoarthr. Cartil. 2018, 26, 319–325. [Google Scholar] [CrossRef]
- Yan, Z.; Lin, Z.; Wu, Y.; Zhan, J.; Qi, W.; Lin, J.; Shen, J.; Xue, X.; Pan, X. The protective effect of myricitrin in osteoarthritis: An in vitro and in vivo study. Int. Immunopharmacol. 2020, 84, 106511. [Google Scholar] [CrossRef]
- Liu, X.; Fang, H.; Zeng, C.; Cai, D. Myricetin attenuates osteoarthritis by blockade of the IL-1β/MAPK pathway. Osteoarthr. Cartil. 2018, 26, S84. [Google Scholar] [CrossRef]
- Pan, X.; Chen, T.; Zhang, Z.; Chen, X.; Chen, C.; Chen, L.; Wang, X.; Ying, X. Activation of Nrf2/HO-1 signal with Myricetin for attenuating ECM degradation in human chondrocytes and ameliorating the murine osteoarthritis. Int. Immunopharmacol. 2019, 75, 105742. [Google Scholar] [CrossRef]
- Sriset, Y.; Sukkasem, N.; Chatuphonprasert, W.; Jarukamjorn, K. Nephroprotective Effects of Hesperidin and Myricetin Against High-Fat Diet Plus Ethanol-Induced Renal Oxidative Damage in Mice. Rev. Bras. De Farm. 2022, 32, 555–562. [Google Scholar] [CrossRef]
- Park, S.; Song, G.; Lim, W. Myricetin inhibits endometriosis growth through cyclin E1 down-regulation in vitro and in vivo. J. Nutr. Biochem. 2020, 78, 108328. [Google Scholar] [CrossRef] [PubMed]
- Barlas, N.; Özer, S.; Karabulut, G. The estrogenic effects of apigenin, phloretin and myricetin based on uterotrophic assay in immature Wistar albino rats. Toxicol. Lett. 2014, 226, 35–42. [Google Scholar] [CrossRef]
- Zheng, N.; Wang, C.; Li, Y.; Fu, H.; Hu, T. Myricetin ameliorates polycystic ovary syndrome in mice by brown adipose tissue activation. Reproduction 2024, 167, e240034. [Google Scholar] [CrossRef]
- Golkar, T.; Bassenden, A.V.; Maiti, K.; Arya, D.P.; Schmeing, T.M.; Berghuis, A.M. Structural basis for plazomicin antibiotic action and resistance. Commun. Biol. 2021, 4, 729. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, M.; Pan, Y.; Cui, X.; Li, C. Enzymatic oxidation increases the antibacterial activity of myricetin against Staphylococcus aureus. Food Chem. 2025, 463, 141250. [Google Scholar] [CrossRef]
- Griep, M.A.; Blood, S.; Larson, M.A.; Koepsell, S.A.; Hinrichs, S.H. Myricetin inhibits Escherichia coli DnaB helicase but not primase. Bioorganic Med. Chem. 2007, 15, 7203–7208. [Google Scholar] [CrossRef]
- Zhou, J.-L.; Chen, H.-H.; Xu, J.; Huang, M.-Y.; Wang, J.-F.; Shen, H.-J.; Shen, S.-X.; Gao, C.-X.; Qian, C.-D. Myricetin acts as an inhibitor of type II NADH Dehydrogenase from Staphylococcus aureus. Molecules 2024, 29, 2354. [Google Scholar] [CrossRef]
- Kang, S.S.; Kim, J.G.; Lee, T.H.; Oh, K.B. Flavonols inhibit sortases and sortase-mediated Staphylococcus aureus clumping to fibrinogen. Biol. Pharm. Bull. 2006, 29, 1751–1755. [Google Scholar] [CrossRef]
- Jing, S.; Wang, L.; Wang, T.; Fan, L.; Chen, L.; Xiang, H.; Shi, Y.; Wang, D. Myricetin protects mice against MRSA-related lethal pneumonia by targeting ClpP. Biochem. Pharmacol. 2021, 192, 114753. [Google Scholar] [CrossRef]
- Silva, L.N.; Da Hora, G.C.A.; Soares, T.A.; Bojer, M.S.; Ingmer, H.; Macedo, A.J.; Trentin, D.S. Myricetin protects Galleria mellonella against Staphylococcus aureus infection and inhibits multiple virulence factors. Sci. Rep. 2017, 7, 2823. [Google Scholar] [CrossRef]
- Cetin-Karaca, H.; Newman, M.C. Antimicrobial efficacy of plant phenolic compounds against Salmonella and Escherichia Coli. Food Biosci. 2015, 11, 8–16. [Google Scholar] [CrossRef]
- Pan, H.; He, J.; Yang, Z.; Yao, X.; Zhang, H.; Li, R.; Xiao, Y.; Zhao, C.; Jiang, H.; Liu, Y.; et al. Myricetin possesses the potency against SARS-CoV-2 infection through blocking viral-entry facilitators and suppressing inflammation in rats and mice. Phytomedicine 2023, 116, 154858. [Google Scholar] [CrossRef]
- Hu, H.; Hu, Z.; Zhang, Y.; Wan, H.; Yin, Z.; Li, L.; Liang, X.; Zhao, X.; Yin, L.; Ye, G.; et al. Myricetin inhibits pseudorabies virus infection through direct inactivation and activating host antiviral defense. Front. Microbiol. 2022, 13, 985108. [Google Scholar] [CrossRef]
- Peng, S.; Fang, C.; He, H.; Song, X.; Zhao, X.; Zou, Y.; Li, L.; Jia, R.; Yin, Z. Myricetin exerts its antiviral activity against infectious bronchitis virus by inhibiting the deubiquitinating activity of papain-like protease. Poult. Sci. 2022, 101, 101626. [Google Scholar] [CrossRef]
- Fan, J.; Xi, P.; Liu, H.; Song, X.; Zhao, X.; Zhou, X.; Zou, Y.; Fu, Y.; Li, L.; Jia, R.; et al. Myricetin inhibits transmissible gastroenteritis virus replication by targeting papain-like protease deubiquitinating enzyme activity. Front. Microbiol. 2024, 15, 1433664. [Google Scholar] [CrossRef]
- Li, W.; Xu, C.; Hao, C.; Zhang, Y.; Wang, Z.; Wang, S.; Wang, W. Inhibition of herpes simplex virus by myricetin through targeting viral gD protein and cellular EGFR/PI3K/Akt pathway. Antivir. Res. 2020, 177, 104714. [Google Scholar] [CrossRef]
- Lee, H.-S.; Kim, Y. Myricetin Disturbs the Cell Wall Integrity and Increases the Membrane Permeability of Candida albicans. J. Microbiol. Biotechnol. 2022, 32, 37–45. [Google Scholar] [CrossRef]
- Mo, F.; Ma, J.; Yang, X.; Zhang, P.; Li, Q.; Zhang, J. In vitro and in vivo effects of the combination of myricetin and miconazole nitrate incorporated to thermosensitive hydrogels, on C. albicans biofilms. Phytomedicine 2020, 71, 153223. [Google Scholar] [CrossRef]
- Jacobs Jr, D.R.; Gross, M.D.; Tapsell, L.C. Food synergy: An operational concept for understanding nutrition. Am. J. Clin. Nutr. 2009, 89, 1543S–1548S. [Google Scholar] [CrossRef]
- Ganora, L. Herbal Constituents: Foundations of Phytochemistry, 1st ed.; Lisa Ganora: Louisville, KY, USA, 2009. [Google Scholar]
- Zhang, J.; Aray, B.; Zhang, Y.; Bai, Y.; Yuan, T.; Ding, S.; Xue, Y.; Huang, X.; Li, Z. Synergistic effect of cucurbitacin E and myricetin on Anti-Non-Small cell lung cancer: Molecular mechanism and therapeutic potential. Phytomedicine 2023, 111, 154619. [Google Scholar] [CrossRef] [PubMed]
- Pinto, H.B.; Brust, F.R.; Macedo, A.J.; Trentin, D.S. The antivirulence compound myricetin possesses remarkable synergistic effect with antibacterials upon multidrug resistant Staphylococcus aureus. Microb. Pathog. 2020, 149, 104571. [Google Scholar] [CrossRef] [PubMed]
- Hanci, H.; Igan, H. Antimicrobial synergistic effects of apigenin,(-)-epigallocatechin-3-gallate, myricetin and luteolin in combination with some antibiotics. Ann. Agric. Environ. Med. 2023, 30, 61–64. [Google Scholar] [CrossRef]
- Al-Abbasi, F.A.; Kazmi, I. Therapeutic role of kaempferol and myricetin in streptozotocin-induced diabetes synergistically via modulation in pancreatic amylase, glycogen storage and insulin secretion. Mol. Cell. Biochem. 2023, 478, 1927–1937. [Google Scholar] [CrossRef]
- Yi, J.-L.; Shi, S.; Shen, Y.-L.; Wang, L.; Chen, H.-Y.; Zhu, J.; Ding, Y. Myricetin and methyl eugenol combination enhances the anticancer activity, cell cycle arrest and apoptosis induction of cis-platin against HeLa cervical cancer cell lines. Int. J. Clin. Exp. Pathol. 2015, 8, 1116–1127. [Google Scholar]
- Yao, A.; Shen, Y.; Zhang, Z.; Zou, Z.; Wang, A.; Chen, S.; Zhang, H.; Chen, F.; Zhao, J.; Chen, Z.; et al. Sulforaphane and myricetin act synergistically to induce apoptosis in 3T3-L1 adipocytes. Mol. Med. Rep. 2018, 17, 2945–2951. [Google Scholar] [CrossRef]
- Chen, L.; Fan, T.; Wang, M.; Zhu, C.Y.; Feng, W.Y.; Li, Y.; Yang, H. Myricetin, a natural inhibitor of CD147, increases sensitivity of cis-platin in ovarian cancer. Expert Opin. Ther. Targets 2024, 28, 83–95. [Google Scholar] [CrossRef]
- Morales, P.; Haza, A.I. Selective apoptotic effects of piceatannol and myricetin in human cancer cells. J. Appl. Toxicol. 2012, 32, 986–993. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Chan, Y.S.; Danquah, M.K. Nano-formulations of drugs: Recent developments, impact and challenges. Biochimie 2016, 128, 99–112. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Anwer, S.T.; Mobashir, M.; Fantoukh, O.I.; Khan, B.; Imtiyaz, K.; Naqvi, I.H.; Alam Rizvi, M.M. Synthesis of Silver Nano Particles Using Myricetin and the In-Vitro Assessment of Anti-Colorectal Cancer Activity: In-Silico Integration. Int. J. Mol. Sci. 2022, 23, 11024. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ma, W.; Ali, I.; Zhao, H.; Wang, D.; Qiu, J. Green and facile synthesis and antioxidant and antibacterial evaluation of dietary myricetin-mediated silver nanoparticles. ACS Omega 2020, 5, 32632–32640. [Google Scholar] [CrossRef] [PubMed]
- Mohan, U.P.; Sriram, B.; Panneerselvam, T.; Devaraj, S.; MubarakAli, D.; Parasuraman, P.; Palanisamy, P.; Premanand, A.; Arunachalam, S.; Kunjiappan, S. Utilization of plant-derived Myricetin molecule coupled with ultrasound for the synthesis of gold nanoparticles against breast cancer. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 1963–1976. [Google Scholar] [CrossRef]
- Agraharam, G.; Girigoswami, A.; Girigoswami, K. Nanoencapsulated myricetin to improve antioxidant activity and bioavailabil-ity: A study on zebrafish embryos. Chemistry 2021, 4, 1–17. [Google Scholar] [CrossRef]
- Thant, Y.; Wang, Q.; Wei, C.; Liu, J.; Zhang, K.; Bao, R.; Zhu, Q.; Weng, W.; Yu, Q.; Zhu, Y.; et al. TPGS conjugated pro-liposomal nano-drug delivery system potentiate the antioxidant and hepatoprotective activity of Myricetin. J. Drug Deliv. Sci. Technol. 2021, 66, 102808. [Google Scholar] [CrossRef]
- Li, C.; Du, M.; Meng, L.; Adu-Frimpong, M.; Gong, C.; Zheng, S.; Shi, W.; Wang, Q.; Toreniyazov, E.; Ji, H.; et al. Preparation, characterisation, and pharmacodynamic study of myricetin pH-sensitive liposomes. J. Microencapsul. 2024, 41, 269–283. [Google Scholar] [CrossRef]
- Zhou, X.; Yi, L.; Lang, H.; Zhang, J.; Zhang, Q.; Yu, L.; Zhu, J.; Mi, M. Dihydromyricetin-encapsulated liposomes inhibit exhaustive exercise-induced liver inflammation by orchestrating M1/M2 macrophage polarization. Front. Pharmacol. 2022, 13, 887263. [Google Scholar] [CrossRef]
- Khatamian, N.; Motavalizadehkakhky, A.; Tabrizi, M.H.; Mehrzad, J.; Zhiani, R. Preparation and characterization of the myricetin-loaded solid lipid nanoparticles decorated with folic acid-bound chitosan and evaluation of its antitumor and anti-angiogenic activities in vitro and in vivo in mice bearing tumor models. Cancer Nanotechnol. 2023, 14, 9. [Google Scholar] [CrossRef]
- Lin, T.-C.; Yang, C.-Y.; Wu, T.-H.; Tseng, C.-H.; Yen, F.-L. Myricetin Nanofibers Enhanced Water Solubility and Skin Penetration for Increasing Antioxidant and Photoprotective Activities. Pharmaceutics 2023, 15, 906. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Chaturvedi, S.; Khan, M.A.; Rai, Y.; Bhatt, A.N.; Najmi, A.K.; Akhtar, M.; Mishra, A.K. Nanoemulsion potentiates the anti-cancer activity of Myricetin by effective inhibition of PI3K/AKT/mTOR pathway in triple-negative breast cancer cells. Med. Oncol. 2024, 41, 56. [Google Scholar] [CrossRef]
- Upadhyay, M.; Hosur, R.V.; Jha, A.; Bharti, K.; Mali, P.S.; Jha, A.K.; Mishra, B.; Kumar, A. Myricetin encapsulated chitosan nanoformulation for management of type 2 diabetes: Preparation, optimization, characterization and in vivo activity. Biomater. Adv. 2023, 153, 213542. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Wang, J.; Fei, F.; Gao, X.; Wu, X.; Shi, D.; Guo, C. Myricetin-loaded nanomicelles protect against cisplatin-induced acute kidney injury by inhibiting the DNA damage-cGAS–STING signaling pathway. Mol. Pharm. 2022, 20, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, N.H.; El-Aziz, N.K.A.; El-Naenaeey, E.-S.Y.; Abdelaziz, W.S.; Sewid, A.H. Antimicrobial potential of myricetin-coated zinc oxide nanocomposite against drug-resistant Clostridium perfringens. BMC Microbiol. 2023, 23, 79. [Google Scholar] [CrossRef]


| Activity | Study Types | Dose | Outcome | Ref. |
|---|---|---|---|---|
| Hepatoprotective effect | In vivo, mice model | 50 mg/kg |
| [78] |
| In vivo, mice model | 100 mg/kg |
| [79] | |
| In vivo, mice model | 25, 50, 100 mg/kg |
| [80] | |
| In vivo, mice model | 25 or 100 mg/kg |
| [81] | |
| In vivo, rat model | 25, 50 mg/kg |
| [19] | |
| In vivo, rat model | HFD containing 0.5% myricetin |
| [82] | |
| In vivo, mice model | 100, 200 mg/kg |
| [83] | |
| In vivo, mice model | 25, 50, 100 mg/kg |
| [44] | |
| In vivo, mice model | Diet containing 0.04% or 0.08% myricetin |
| [84] | |
| In vivo, mice model | 100 mg/kg |
| [17] | |
| In vitro, LPS-stimulated RAW264.7 macrophages | 50 μM |
| [17] | |
| In vivo, rats | 25, 50 mg/kg |
| [19] |
| Cancer | Modulation | Study Types | Model | Mechanism | Outcome | Refs. |
|---|---|---|---|---|---|---|
| Ovarian | Angiogenesis | In vitro | OVCAR-3 and A2780/CP70 | VEGF ↓ Angiogenesis ↓ |
| [131] |
| Breast | Angiogenesis | In vitro and in vivo | MDA-MB-231,4T1 & in vivo tumor xenograft model | VEGF ↓ VEGFR ↓ Angiogenesis ↓ |
| [132] |
| Breast | Nrf-2/GPX4 pathway | In vitro and in vivo | Breast tumors mice model 4 T1 | Nrf-2 ↓, GPX4 ↓ |
| [133] |
| Gastric | Apoptosis | In vitro and in vivo | AGS & Xenograft | Bcl-2/Bax ratio ↓ Bax ↑ Bcl2 ↓ |
| [134] |
| Gastric | PI3K/Akt/mtor | In vitro | AGS | p-PI3K, p-Akt and p-mTOR ↓ |
| [134] |
| Gastric | Apoptosis | In vitro | In vitro | Bcl-2 ↓ and pro-caspase-3 Bax and cleaved caspase-3 ↑ |
| [135] |
| Pancreatic cancer | Apoptosis | In vitro | MIA PaCa-2, Panc-1 and S2-013 | Caspase-3 and 9 ↑ Induction of apoptosis |
| [136] |
| Brain | Apoptosis | In vitro | U251 | Bax and Bad levels ↑ Bcl-2 and Bcl-xl ↓ |
| [137] |
| Liver | Cell cycle | In vitro | Hep3B and HepG2i | Blocking cell cycle at the G2/M phase cell number in the G0/G1 phase ↓ |
| [138] |
| Liver | Cell cycle | In vitro | HepG2 | Accumulation of cells in the G2/M phase ↑ Protein levels of the p53/p21 cascade ↑ |
| [139] |
| Liver | Autophagy | In vitro | SMMC-7721 and Hep3B | Ratio of LC3-II/LC3-I ↑ autophagic flux ↑ |
| [140] |
| Ovarian | PI3K/AKT | In vitro | A2780 and HO8910 | Phosphorylated ERK and PI3K/AKT ↓ |
| [141] |
| Bile duct | STAT3 pathway | In vitro | KKU-100 | STAT3 ↓ |
| [142] |
| Drugs/Compound | Activity | Key Finding | Ref. | |
|---|---|---|---|---|
| Myricetin | Cucurbitacin E | Anti-lung cancer |
| [214] |
| Oxacillin | Antibacterial |
| [215] | |
| Levofloxacin | Antibacterial |
| [216] | |
| Kaempferol | Anti-diabetic |
| [217] | |
| Methyl eugenol | Anti-cancerous |
| [218] | |
| Sulforaphane | Anti-obesity |
| [219] | |
| Cisplatin | Anti-cancerous |
| [220] | |
| Piceatannol | Anti-cancerous |
| [221] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almatroodi, S.A.; Rahmani, A.H. Unlocking the Pharmacological Potential of Myricetin Against Various Pathogenesis. Int. J. Mol. Sci. 2025, 26, 4188. https://doi.org/10.3390/ijms26094188
Almatroodi SA, Rahmani AH. Unlocking the Pharmacological Potential of Myricetin Against Various Pathogenesis. International Journal of Molecular Sciences. 2025; 26(9):4188. https://doi.org/10.3390/ijms26094188
Chicago/Turabian StyleAlmatroodi, Saleh A., and Arshad Husain Rahmani. 2025. "Unlocking the Pharmacological Potential of Myricetin Against Various Pathogenesis" International Journal of Molecular Sciences 26, no. 9: 4188. https://doi.org/10.3390/ijms26094188
APA StyleAlmatroodi, S. A., & Rahmani, A. H. (2025). Unlocking the Pharmacological Potential of Myricetin Against Various Pathogenesis. International Journal of Molecular Sciences, 26(9), 4188. https://doi.org/10.3390/ijms26094188







