Clinical Significance of Marginal Zinc Deficiency as a Predictor of Covert Hepatic Encephalopathy in Patients with Liver Cirrhosis
Abstract
1. Introduction
2. Results
2.1. Comparison of Clinical Characteristics Between the CHE and No-CHE Groups
2.2. Risk Factors for CHE in Patients with Cirrhosis
2.3. Diagnostic Accuracy of Zinc Level for CHE
2.4. Clinical Characteristics According to Zinc Level
3. Discussion
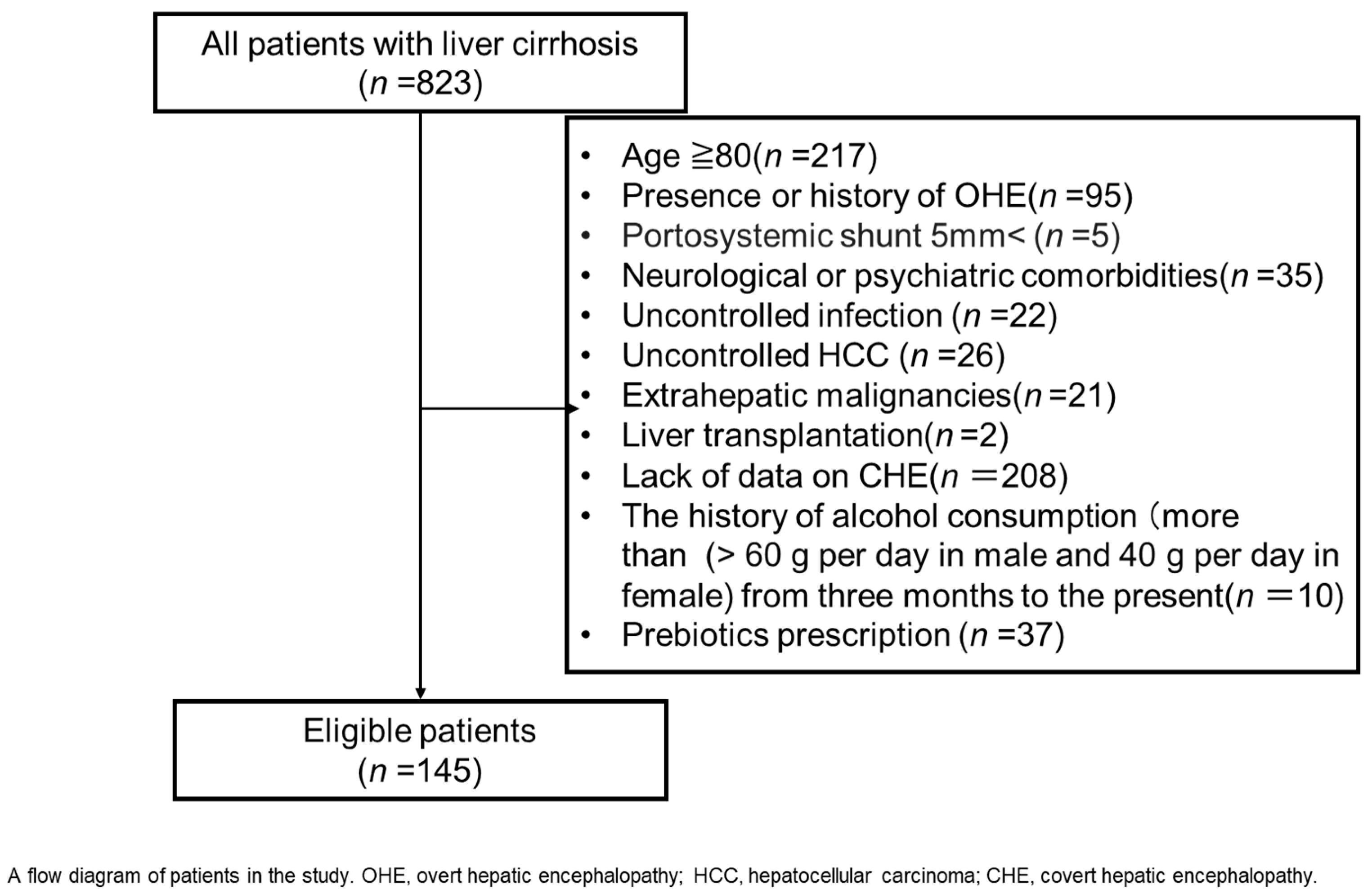
4. Patients and Methods
Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HE | Hepatic encephalopathy |
| CHEJ | Covert hepatic encephalopathy |
| HGS | Hand grip strength |
| QOL | Quality of life |
| NCT | Number connection tests |
| HCC | Hepatocellular carcinoma |
| BTR | Branched-chain amino acid to tyrosine ratio |
References
- Patidar, K.R.; Thacker, L.R.; Wade, J.B.; Sterling, R.K.; Sanyal, A.J.; Siddiqui, M.S.; Matherly, S.C.; Stravitz, T.R.; Puri, P.; Luketic, V.A. Covert hepatic encephalopathy is independently associated with poor survival and increased risk of hospitalization. Am. J. Gastroenterol. 2014, 109, 1757–1763. [Google Scholar] [CrossRef]
- Weissenborn, K. Hepatic encephalopathy: Definition, clinical grading and diagnostic principles. Drugs 2019, 79, 5–9. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Heuman, D.M.; Sterling, R.K.; Sanyal, A.J.; Siddiqui, M.; Matherly, S.; Luketic, V.; Stravitz, R.T.; Fuchs, M.; Thacker, L.R. Validation of encephalapp, smartphone-based stroop test, for the diagnosis of covert hepatic encephalopathy. Clin. Gastroenterol. Hepatol. 2015, 13, 1828–1835.e1. [Google Scholar] [CrossRef]
- Acharya, C.; Shaw, J.; Duong, N.; Fagan, A.; McGeorge, S.; Wade, J.B.; Thacker, L.R.; Bajaj, J.S. Quickstroop, a shortened version of encephalapp, detects covert hepatic encephalopathy with similar accuracy within one minute. Clin. Gastroenterol. Hepatol. 2023, 21, 136–142. [Google Scholar] [CrossRef]
- Matuszczak, M.; Kiljanczyk, A.; Marciniak, W.; Derkacz, R.; Stempa, K.; Baszuk, P.; Bryśkiewicz, M.; Sun, P.; Cheriyan, A.; Cybulski, C.; et al. Zinc and its antioxidant properties: The potential use of blood zinc levels as a marker of cancer risk in BRCA1 mutation carriers. Antioxidants 2024, 13, 609. [Google Scholar] [CrossRef]
- Matuszczak, M.; Kiljanczyk, A.; Marciniak, W.; Derkacz, R.; Stempa, K.; Baszuk, P.; Bryśkiewicz, M.; Cybulski, C.; Dębniak, T.; Gronwald, J.; et al. Antioxidant properties of zinc and copper-blood zinc-to copper-ratio as a marker of cancer risk BRCA1 mutation carriers. Antioxidants 2024, 13, 841. [Google Scholar] [CrossRef]
- Qi, Z.; Liu, K.J. The Interaction of zinc and the blood-brain barrier under physiological and ischemic conditions. Toxicol. Appl. Pharmacol. 2019, 364, 114–119. [Google Scholar] [CrossRef]
- Miwa, T.; Hanai, T.; Toshihide, M.; Ogiso, Y.; Imai, K.; Suetsugu, A.; Takai, K.; Shiraki, M.; Katsumura, N.; Shimizu, M. Zinc Deficiency predicts overt hepatic encephalopathy and mortality in liver cirrhosis patients with minimal hepatic encephalopathy. Hepatol. Res. 2021, 51, 662–673. [Google Scholar] [CrossRef]
- Bañares, J.; Aceituno, L.; Ruiz-Ortega, L.; Pons, M.; Abraldes, J.G.; Genescà, J. Zinc supplementation to improve prognosis in patients with compensated advanced chronic liver disease: A Multicenter, randomized, double-blind, placebo-controlled clinical trial. Hepatol. Commun. 2024, 8, e0524. [Google Scholar] [CrossRef] [PubMed]
- Semeya, A.A.; Elgamal, R.; Othman, A.A.A. Correlation of serum zinc levels with hepatic encephalopathy severity in patients with decompensated liver cirrhosis: A prospective observational study from Egypt. Biol. Trace Elem. Res. 2025, 1–13. [Google Scholar] [CrossRef]
- Hanai, T.; Shiraki, M.; Watanabe, S.; Imai, K.; Suetsugu, A.; Takai, K.; Moriwaki, H.; Shimizu, M. Prognostic significance of minimal hepatic encephalopathy in patients with liver cirrhosis in Japan: A propensity score-matching analysis. J. Gastroenterol. Hepatol. 2019, 34, 1809–1816. [Google Scholar] [CrossRef]
- Hanai, T.; Shiraki, M.; Nishimura, K.; Miwa, T.; Maeda, T.; Ogiso, Y.; Imai, K.; Suetsugu, A.; Takai, K.; Shimizu, M. Usefulness of the stroop test in diagnosing minimal hepatic encephalopathy and predicting overt hepatic encephalopathy. Hepatol. Commun. 2021, 5, 1518–1526. [Google Scholar] [CrossRef]
- Soma, N.; Uchida, Y.; Kouyama, J.-I.; Naiki, K.; Usui, N.; Sato, A.; Yamada, S.; Tsuji, S.; Ando, S.; Sugawara, K. Serum Zinc Levels as Predictors of Covert Hepatic Encephalopathy in Patients with Liver Cirrhosis. J. Gastroenterol. 2025, 60, 96–106. [Google Scholar] [CrossRef]
- Lv, X.-H.; Lu, Q.; Deng, K.; Yang, J.-L.; Yang, L. Prevalence and characteristics of covert/minimal hepatic encephalopathy in patients with liver cirrhosis: A systematic review and meta-analysis. Am. J. Gastroenterol. 2024, 119, 690–699. [Google Scholar] [CrossRef]
- Gairing, S.J.; Mangini, C.; Zarantonello, L.; Gioia, S.; Nielsen, E.J.; Danneberg, S.; Gabriel, M.; Ehrenbauer, A.F.; Bloom, P.P.; Ripoll, C. Prevalence of minimal hepatic encephalopathy in patients with liver cirrhosis: A multicenter study. Am. J. Gastroenterol. 2023, 118, 2191–2200. [Google Scholar] [CrossRef]
- Kaji, K.; Okita, K.; Suzuki, K.; Sato, I.; Fujisawa, M.; Yoshiji, H. Association between serum albumin and cognitive dysfunction in hepatic encephalopathy: An exploratory data analysis. JGH Open 2021, 5, 207–212. [Google Scholar] [CrossRef]
- Kondo, Y.; Iwasa, M.; Kawaratani, H.; Miyaaki, H.; Hanai, T.; Kon, K.; Hirano, H.; Shimizu, M.; Yoshiji, H.; Okita, K. Proposal of stroop test cut-off values as screening for neuropsychological impairments in cirrhosis: A Japanese multicenter study. Hepatol. Res. 2021, 51, 674–681. [Google Scholar] [CrossRef]
- Kiouri, D.P.; Tsoupra, E.; Peana, M.; Perlepes, S.P.; Stefanidou, M.E.; Chasapis, C.T. Multifunctional role of zinc in human health: An update. EXCLI J. 2023, 22, 809–827. [Google Scholar] [CrossRef]
- Brugger, D.; Windisch, W.M. Adaption of body zinc pools in weaned piglets challenged with subclinical zinc deficiency. Br. J. Nutr. 2019, 121, 849–858. [Google Scholar] [CrossRef]
- Katayama, K.; Kawaguchi, T.; Shiraishi, K.; Ito, T.; Suzuki, K.; Koreeda, C.; Ohtake, T.; Iwasa, M.; Tokumoto, Y.; Endo, R. The prevalence and implication of zinc deficiency in patients with chronic liver disease. J. Clin. Med. Res. 2018, 10, 437–444. [Google Scholar] [CrossRef]
- Katayama, K. Zinc and protein metabolism in chronic liver diseases. Nutr. Res. 2020, 74, 1–9. [Google Scholar] [CrossRef]
- Murata, K.; Namisaki, T.; Fujimoto, Y.; Takeda, S.; Enomoto, M.; Takaya, H.; Tsuji, Y.; Shibamoto, A.; Suzuki, J.; Kubo, T.; et al. Clinical significance of serum zinc levels on the development of sarcopenia in cirrhotic patients. Cancer Diagn. Progn. 2022, 2, 184–193. [Google Scholar] [CrossRef]
- Kodama, H.; Tanaka, M.; Naito, Y.; Katayama, K.; Moriyama, M. Japan’s practical guidelines for zinc deficiency with a particular focus on taste disorders, inflammatory bowel disease, and liver cirrhosis. Int. J. Mol. Sci. 2020, 21, 2941. [Google Scholar] [CrossRef]
- Sakurai, K.; Furukawa, S.; Katsurada, T.; Otagiri, S.; Yamanashi, K.; Nagashima, K.; Onishi, R.; Yagisawa, K.; Nishimura, H.; Ito, T. Effectiveness of administering zinc acetate hydrate to patients with inflammatory bowel disease and zinc deficiency: A retrospective observational two-center study. Intest. Res. 2021, 20, 78–89. [Google Scholar] [CrossRef]
- Yokokawa, H.; Fukuda, H.; Saita, M.; Miyagami, T.; Takahashi, Y.; Hisaoka, T.; Naito, T. Serum zinc concentrations and characteristics of zinc deficiency/marginal deficiency among Japanese subjects. J. Gen. Fam. Med. 2020, 21, 248–255. [Google Scholar] [CrossRef]
- Fukushima, M.; Miyaaki, H.; Sasaki, R.; Nakao, Y.; Haraguchi, M.; Takahashi, K.; Ozawa, E.; Miuma, S.; Nakao, K. Benefits of liver volume and serum zinc level assessment for the screening of covert hepatic encephalopathy in patients with child–pugh class A cirrhosis. Diagnostics 2024, 15, 23. [Google Scholar] [CrossRef]
- Shen, Y.C.; Chang, Y.H.; Fang, C.J.; Lin, Y.S. Zinc supplementation in patients with cirrhosis and hepatic encephalopathy: A systematic review and meta-analysis. Nutr. J. 2019, 18, 34. [Google Scholar] [CrossRef]
- Chen, L.; Shen, Q.; Liu, Y.; Zhang, Y.; Sun, L.; Ma, X.; Song, N.; Xie, J. Homeostasis and metabolism of iron and other metal ions in neurodegenerative diseases. Signal Transduct. Target. Ther. 2025, 10, 31. [Google Scholar] [CrossRef]
- Muro, P.; Zhang, L.; Li, S.; Zhao, Z.; Jin, T.; Mao, F.; Mao, Z. The emerging role of oxidative stress in inflammatory bowel disease. Front. Endocrinol. 2024, 15, 1390351. [Google Scholar] [CrossRef]
- Simicic, D.; Cudalbu, C.; Pierzchala, K. Overview of oxidative stress findings in hepatic encephalopathy: From cellular and ammonium-based animal models to human data. Anal. Biochem. 2022, 654, 114795. [Google Scholar] [CrossRef]
- Katayama, K.; Kakita, N. Possible pathogenetic role of ammonia in liver cirrhosis without hyperammonemia of venous blood: The so-called latency period of abnormal ammonia metabolism. Hepatol. Res. 2024, 54, 235–243. [Google Scholar] [CrossRef]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef]
- Campagna, F.; Montagnese, S.; Ridola, L.; Senzolo, M.; Schiff, S.; De Rui, M.; Pasquale, C.; Nardelli, S.; Pentassuglio, I.; Merkel, C. The animal naming test: An easy tool for the assessment of hepatic encephalopathy. Hepatology 2017, 66, 198–208. [Google Scholar] [CrossRef]
- NeSmith, M.; Ahn, J.; Flamm, S.L. Contemporary understanding and management of overt and covert hepatic encephalopathy. Gastroenterol. Hepatol. 2016, 12, 91–100. [Google Scholar]
- Crawford, A.C.; Lehtovirta-Morley, L.E.; Alamir, O.; Niemiec, M.J.; Alawfi, B.; Alsarraf, M.; Skrahina, V.; Costa, A.C.B.P.; Anderson, A.; Yellagunda, S. Biphasic zinc compartmentalisation in a human fungal pathogen. PLoS Pathog. 2018, 14, e1007013. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Konishi, M.; Kato, A.; Kato, M.; Kooka, Y.; Sawara, K.; Endo, R.; Torimura, T.; Suzuki, K.; Takikawa, Y. Updating the neuropsychological test system in Japan for the elderly and in a modern touch screen tablet society by resetting the cut-off values. Hepatol. Res. 2017, 47, 1335–1339. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]

| Variables | All Patients (n = 145) | No-CHE (n = 54) | CHE (n = 91) | p a |
|---|---|---|---|---|
| Age, years | 66.7 ± 11.5 | 69.2 ± 8.8 | 65.2 ± 12.6 | 0.052 |
| Male, n (%) | 86 (59.3) | 30 (55.6) | 56 (61.5) | 0.49 |
| BMI, kg/m2 | 25.0 ± 4.7 | 23.6 ± 4.0 | 25.9 ± 4.9 | 0.0045 |
| Etiology HCV/HBV/ALD/NASH/other b | 32/18/32/29/34 | 8/8/12/11/15 | 24/10/20/18/19 | 0.53 |
| HCC, n (%) | 18 (12.4) | 7 (13.0) | 11 (12.1) | 1 |
| Esophagogastric varices, n (%) | 83 (57.2) | 33 (61.1) | 50 (54.9) | 0.49 |
| Ascites, n (%) | 38 (26.2) | 13 (24.1) | 25 (27.5) | 0.70 |
| Child-Pugh score | 6 (5–8) | 6 (5–7) | 6 (5–9) | 0.051 |
| Child-Pugh class (A/B/C) | 86/42/17 | 36/16/2 | 50/26/15 | 0.060 |
| mALBI score | −2.27 ± 0.75 | −2.45 ± 0.50 | −2.17 ± 0.85 | 0.032 |
| HGS, kg | ||||
| Male | 31.8 ± 10.1 | 30.8 ± 9.7 | 32.4 ± 10.3 | 0.52 |
| Female | 17.5 ± 6.1 | 19.5 ± 5.6 | 15.9 ± 6.0 | 0.030 |
| Reduced HGS, % | 41.1 | 36.7 | 44.0 | 0.46 |
| PT, % | 83.9 ± 22.5 | 88.4 ± 19.3 | 81.2 ± 23.9 | 0.065 |
| Platelet, ×104/μL | 11.6 ± 6.0 | 11.7 ± 6.1 | 11.5 ± 5.9 | 0.89 |
| BUN, mg/dL | 18.2 ± 11.0 | 16.7 ± 9.0 | 19.0 ± 11.9 | 0.21 |
| Creatinine, mg/dL | 0.80 (0.65–0.98) | 0.76 (0.64–0.91) | 0.83 (0.66–1.06) | 0.17 |
| AST, U/L | 35 (26–45) | 36 (25–42) | 35 (26–49) | 0.40 |
| ALT, U/L | 23 (17–31) | 23 (16–30) | 23 (18–35) | 0.17 |
| γ-GTP, mg/dL | 49 (23–98) | 50 (21–117) | 48 (24–87) | 0.79 |
| ALP, U/L | 117 (85–230) | 112 (86–195) | 117 (85–252) | 0.61 |
| Albumin, g/dL | 3.76 ± 0.73 | 3.93 ± 0.54 | 3.67 ± 0.80 | 0.033 |
| Total Bilirubin, mg/dL | 1.3 (1.0–2.1) | 1.2 (1.0–1.9) | 1.4 (1.0–2.3) | 0.43 |
| ChE, U/L | 209 ± 97 | 219 ± 90 | 204 ± 100 | 0.34 |
| NH3, μg/dL | 52.5 ± 42.5 | 38.6 ± 21.5 | 60.7 ± 49.3 | 0.0025 |
| BTR | 4.76 ± 2.00 | 5.12 ± 1.95 | 4.50 ± 2.01 | 0.079 |
| Zinc, μg/dL | 65.6 ± 18.5 | 73.0 ± 13.9 | 60.7 ± 19.7 | <0.001 |
| EAA | 0.292 ± 0.121 | 0.305 ± 0.117 | 0.285 ± 0.124 | 0.37 |
| AFP, ng/mL | 3.5 (2.3–5.9) | 3.9 (2.5–6.2) | 3.4 (2.2–5.1) | 0.33 |
| 25-hydroxyvitamin D, ng/mL | 15.1 ± 6.3 | 15.7 ± 6.1 | 14.7 ± 6.4 | 0.39 |
| Type IV collagen 7S, ng/mL | 8.88 ± 7.54 | 6.55 ± 2.89 | 10.4 ± 9.12 | 0.0036 |
| Type III procollagen-N-peptide, U/m | 0.914 ± 0.543 | 0.823 ± 0.329 | 1.01 ± 0.64 | 0.045 |
| M2BPGi, C.O.I | 2.55 (1.12–4.84) | 2.41 (1.06–4.54) | 2.80 (1.23–5.45) | 0.29 |
| FIB-4 index | 4.87 (2.83–7.14) | 4.82 (3.33–6.62) | 4.91 (2.74–7.29) | 0.99 |
| APRI | 1.10 (0.61–1.96) | 1.00 (0.61–1.59) | 1.18 (0.61–2.03) | 0.39 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Age ≤ 63, Years | 2.09 (0.97–4.51) | 0.061 | ||
| BMI ≥ 25.5, kg/m2 | 1.86 (0.91–3.81) | 0.089 | ||
| Child-Pugh score ≥ 8 | 2.22 (1.01–4.89) | 0.047 | ||
| mALBI score ≥ −1.8 | 2.98 (1.20–7.42) | 0.019 | ||
| HGS, kg | ||||
| Male ≤ 28 | 0.70 (0.25–1.94) | 0.49 | ||
| Female ≤ 18 | 2.83 (0.93–8.62) | 0.068 | ||
| PT < 65, % | 3.11 (1.18–8.18) | 0.022 | 1.56 (0.43–5.62) | 0.50 |
| Platelet < 4.1, ×104/μL | 3.74 (0.44–31.9) | 0.23 | ||
| BUN ≥ 14.0, mg/dL | 1.26 (0.62–2.55) | 0.53 | ||
| Creatinine ≥ 1.04, mg/dL | 2.27 (0.90–5.72) | 0.082 | ||
| AST ≥ 37, U/L | 0.74 (0.32–1.71) | 0.48 | ||
| ALT ≥ 25, U/L | 1.18 (0.59–2.34) | 0.64 | ||
| γ-GTP ≥ 74, mg/dL | 0.50 (0.25–1.01) | 0.054 | ||
| ALP ≥ 92, U/L | 1.55 (0.740–3.23) | 0.25 | ||
| Albumin < 3.1, g/dL | 4.73 (1.55–14.5) | <0.001 | 2.16 (0.49–9.55) | 0.31 |
| Total bilirubin ≥ 2.2, mg/dL | 1.89 (0.81–4.44) | 0.141 | ||
| ChE < 179, U/L | 2.13 (1.04–4.35) | 0.038 | ||
| NH3 ≥ 77.1, μg/dL | 4.52 (1.47–13.9) | 0.0084 | 1.62 (0.45–5.86) | 0.46 |
| BTR < 3.8 | 2.5 (1.17–5.34) | 0.018 | ||
| Zinc < 74, μg/dL | 5.10 (2.35–11.1) | <0.001 | 3.22 (1.33–7.76) | 0.0093 |
| EAA ≥ 0.21 | 0.56 (0.23–1.32) | 0.18 | ||
| AFP ≥ 3.8, ng/mL | 0.51 (0.25–1.04) | 0.065 | ||
| 25-hydroxyvitamin D < 16.5, ng/mL | 2.52 (1.11–5.69) | 0.027 | ||
| Type IV collagen 7S ≥ 8.8, ng/mL | 3.24 (1.52–6.90) | 0.0023 | 1.59 (0.59–4.30) | 0.36 |
| P-III-P ≥ 1.0, U/m | 1.77 (0.80–3.92) | 0.157 | ||
| M2BPGi ≥ 6.5, C.O.I | 3.65 (0.98 –13.7) | 0.054 | ||
| FIB4 index ≥ 6.1 | 1.38 (0.67–2.84) | 0.38 | ||
| APRI ≥ 1.7 | 1.86 (0.85–4.07) | 0.12 | ||
| Variables | All Patients (n = 145) | Zn ≥ 74 (n = 43) | Zn < 74 (n = 102) | p a |
|---|---|---|---|---|
| Age, years | 66.7 ± 11.5 | 68.8 ± 8.8 | 65.8 ± 12.4 | 0.15 |
| Male, n (%) | 86 (59.3) | 23 (53.4) | 63 (61.8) | 0.36 |
| BMI, kg/m2 | 25.0 ± 4.7 | 23.9 ± 4.0 | 25.5 ± 4.9 | 0.073 |
| Etiology HCV/HBV/ALD/NASH/other b | 32/18/32/29/34 | 12/10/6/7/8 | 20/8/26/22/26 | 0.058 |
| HCC, n (%) | 18 (12.4) | 4 (9.3) | 14 (13.7) | 0.59 |
| Esophagogastric varices, n (%) | 83 (57.2) | 24 (55.8) | 59 (57.8) | 0.71 |
| Ascites, n (%) | 38 (26.2) | 7 (16.3) | 31 (30.4) | 0.099 |
| Child-Pugh score | 6 (5–8) | 6 (5–7) | 6 (5–9) | 0.051 |
| Child-Pugh class (A/B/C) | 86/42/17 | 34/9/0 | 52/33/17 | <0.001 |
| mALBI score | −2.27 ± 0.75 | −2.74 ± 0.43 | −2.08 ± 0.77 | <0.001 |
| HGS, kg | ||||
| Male | 31.8 ± 10.1 | 33.4 ± 9.2 | 31.1 ± 10.5 | 0.38 |
| Female | 17.5 ± 6.1 | 18.7 ± 6.2 | 16.8 ± 6.0 | 0.28 |
| Reduced HGS, % | 41.1 | 55.4 | 65.9 | 0.33 |
| CHE, n (%) | 91 (62.8) | 14 (32.6) | 77 (75.5) | <0.001 |
| PT, % | 83.9 ± 22.5 | 94.2 ± 22.5 | 79.5 ± 22.9 | <0.001 |
| Platelet, ×104/μL | 11.6 ± 6.0 | 12.9 ± 6.0 | 11.0 ± 5.9 | 0.078 |
| BUN, mg/dL | 18.2 ± 11.0 | 17.3 ± 5.3 | 18.5 ± 12.6 | 0.54 |
| Creatinine, mg/dL | 0.80 (0.65–0.98) | 0.76 (0.64–0.91) | 0.83 (0.66–1.06) | 0.17 |
| AST, U/L | 35 (26–45) | 36 (25–42) | 35 (26–49) | 0.40 |
| ALT, U/L | 23 (17–31) | 23 (16–30) | 23 (18–35) | 0.17 |
| γ-GTP, mg/dL | 49 (23–98) | 50 (21–117) | 48 (24–87) | 0.79 |
| ALP, U/L | 117 (85–230) | 112 (86–195) | 117 (85–252) | 0.61 |
| Albumin, g/dL | 3.76 ± 0.73 | 4.23 ± 0.48 | 3.57 ± 0.72 | <0.001 |
| Total bilirubin, mg/dL | 1.3 (1.0–2.1) | 1.2 (1.0–1.9) | 1.4 (1.0–2.3) | 0.43 |
| ChE, U/L | 209 ± 97 | 259 ± 88 | 189 ± 93 | <0.001 |
| NH3, μg/dL | 52.5 ± 42.5 | 33.9 ± 23.1 | 60.3 ± 46.2 | <0.001 |
| BTR | 4.76 ± 2.00 | 5.68 ± 1.71 | 4.32 ± 1.99 | <0.001 |
| EAA | 0.292 ± 0.121 | 0.271 ± 0.116 | 0.302 ± 0.123 | 0.19 |
| AFP, ng/mL | 3.5 (2.3–5.9) | 3.9 (2.5–6.2) | 3.4 (2.2–5.1) | 0.33 |
| 25-hydroxyvitamin D, ng/mL | 15.1 ± 6.3 | 17.8 ± 7.5 | 13.8 ± 5.1 | 0.0015 |
| Type IV collagen 7S, ng/mL | 8.88 ± 7.54 | 5.46 ± 2.34 | 10.50 ± 8.55 | <0.001 |
| P-III-P, U/m | 0.914 ± 0.543 | 0.650 ± 0.203 | 1.037 ± 0.606 | <0.001 |
| M2BPGi, C.O.I | 2.55 (1.12–4.84) | 2.41 (1.06–4.54) | 2.80 (1.23–5.45) | 0.29 |
| FIB4 index | 4.87 (2.83–7.14) | 4.82 (3.33–6.62) | 4.91 (2.74–7.29) | 1.0 |
| APRI | 1.10 (0.61–1.96) | 1.00 (0.61–1.59) | 1.18 (0.61–2.03) | 0.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuda, T.; Namisaki, T.; Shibamoto, A.; Asada, S.; Tomooka, F.; Kubo, T.; Koizumi, A.; Tanaka, M.; Iwai, S.; Inoue, T.; et al. Clinical Significance of Marginal Zinc Deficiency as a Predictor of Covert Hepatic Encephalopathy in Patients with Liver Cirrhosis. Int. J. Mol. Sci. 2025, 26, 4184. https://doi.org/10.3390/ijms26094184
Matsuda T, Namisaki T, Shibamoto A, Asada S, Tomooka F, Kubo T, Koizumi A, Tanaka M, Iwai S, Inoue T, et al. Clinical Significance of Marginal Zinc Deficiency as a Predictor of Covert Hepatic Encephalopathy in Patients with Liver Cirrhosis. International Journal of Molecular Sciences. 2025; 26(9):4184. https://doi.org/10.3390/ijms26094184
Chicago/Turabian StyleMatsuda, Takuya, Tadashi Namisaki, Akihiko Shibamoto, Shohei Asada, Fumimasa Tomooka, Takahiro Kubo, Aritoshi Koizumi, Misako Tanaka, Satoshi Iwai, Takashi Inoue, and et al. 2025. "Clinical Significance of Marginal Zinc Deficiency as a Predictor of Covert Hepatic Encephalopathy in Patients with Liver Cirrhosis" International Journal of Molecular Sciences 26, no. 9: 4184. https://doi.org/10.3390/ijms26094184
APA StyleMatsuda, T., Namisaki, T., Shibamoto, A., Asada, S., Tomooka, F., Kubo, T., Koizumi, A., Tanaka, M., Iwai, S., Inoue, T., Tsuji, Y., Fujinaga, Y., Nishimura, N., Sato, S., Kitagawa, K., Kaji, K., Mitoro, A., Asada, K., Takaya, H., ... Yoshiji, H. (2025). Clinical Significance of Marginal Zinc Deficiency as a Predictor of Covert Hepatic Encephalopathy in Patients with Liver Cirrhosis. International Journal of Molecular Sciences, 26(9), 4184. https://doi.org/10.3390/ijms26094184





