Interaction Between Glucagon-like Peptide 1 and Its Analogs with Amyloid-β Peptide Affects Its Fibrillation and Cytotoxicity
Abstract
1. Introduction
2. Results
2.1. Interaction Between GLP-1RAs and Monomeric Aβ
2.2. Concentration-Dependent Changes in Quaternary Structure of GLP-1RAs
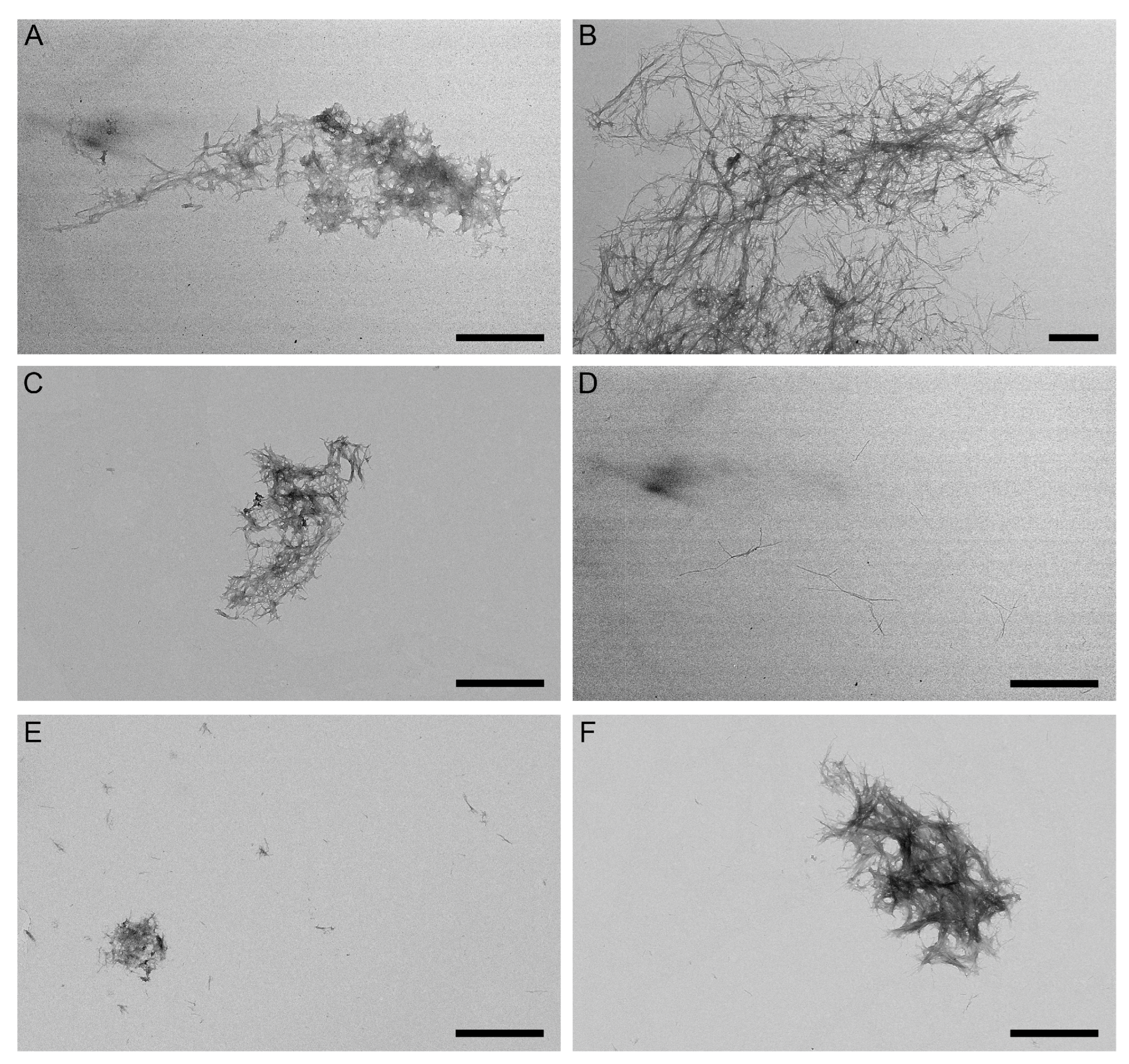
2.3. Effect of GLP-1RAs on Aβ Fibrillation
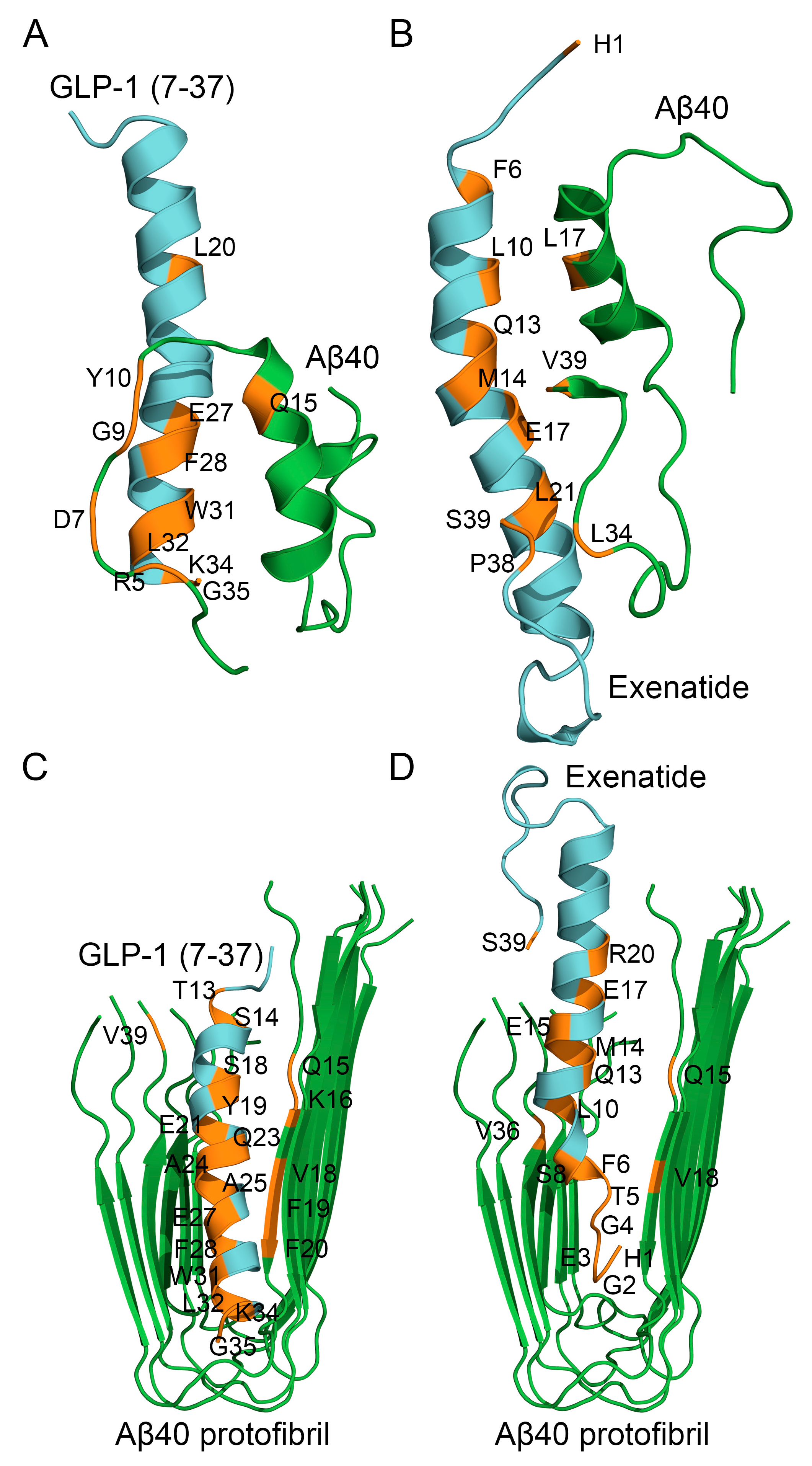
2.4. Structural Modeling of Complexes Between Aβ40 or Its Protofibril and GLP-1(7-37)/Exen
2.5. Effect of GLP-1RAs on Aβ Cytotoxicity Toward Human Neuroblastoma Cells
3. Materials and Methods
3.1. Materials
3.2. BLI Measurements
3.3. SPR Measurements
3.4. Dynamic Light Scattering Measurements
3.5. Structural Modeling
3.6. ThT Fluorescence Assay
3.7. Transmission Electron Microscopy
3.8. Cell Viability Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-ME | 2-mercaptoethanol |
| Aβ | amyloid-β peptide |
| Aβ40/Aβ42 | amyloid-β peptide, residues 1-40/42 |
| AD | Alzheimer’s disease |
| APP | amyloid precursor protein |
| BBB | blood–brain barrier |
| CNS | central nervous system |
| DM | diabetes mellitus |
| DM2 | type 2 diabetes mellitus |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DMSO | dimethyl sulfoxide |
| DPP-4 | dipeptidyl peptidase-4 |
| EDAC/sulfo-NHS | 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride/N-hydroxysulfosuccinimide |
| EDTA | ethylenediaminetetraacetic acid |
| EM | electron microscope |
| Exen | exendin-4/exenatide |
| GLP-1 | glucagon-like peptide 1 |
| GLP-1(7-36), GLP-1(7-37) | N-terminally truncated forms of glucagon-like peptide 1, residues 7-36 or 7-37 |
| GLP-1R | glucagon-like peptide 1 receptor |
| GLP-1RA | glucagon-like peptide 1 receptor agonist |
| HSA | human serum albumin |
| Lira | liraglutide |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| MWm | molecular mass calculated from the amino acid sequence |
| MWRh | molecular mass calculated from the hydrodynamic radius |
| NMR | nuclear magnetic resonance |
| PA | palmitic acid |
| PDB | Protein Data Bank |
| Sema | semaglutide |
| SDS | sodium dodecyl sulfate |
| TEM | transmission electron microscopy |
| TFA | trifluoroacetic acid |
| ThT | thioflavin T |
| Tris | tris(hydroxymethyl)aminomethane |
| TWEEN | polyethylene glycol sorbitan monolaurate |
References
- Gustavsson, A.; Norton, N.; Fast, T.; Frölich, L.; Georges, J.; Holzapfel, D.; Kirabali, T.; Krolak-Salmon, P.; Rossini, P.M.; Ferretti, M.T.; et al. Global estimates on the number of persons across the Alzheimer’s disease continuum. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2023, 19, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Harris, E. FDA Green-Lights Second Alzheimer Drug, Donanemab. JAMA 2024, 332, 524. [Google Scholar] [CrossRef]
- 2024 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2024, 20, 3708–3821. [CrossRef] [PubMed]
- Ameen, T.B.; Kashif, S.N.; Abbas, S.M.I.; Babar, K.; Ali, S.M.S.; Raheem, A. Unraveling Alzheimer’s: The promise of aducanumab, lecanemab, and donanemab. Egypt. J. Neurol. Psychiatry Neurosurg. 2024, 60, 72. [Google Scholar] [CrossRef]
- Sadigh-Eteghad, S.; Sabermarouf, B.; Majdi, A.; Talebi, M.; Farhoudi, M.; Mahmoudi, J. Amyloid-beta: A crucial factor in Alzheimer’s disease. Med. Princ. Pract. 2015, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, R.J.; Wong, P.C. Amyloid precursor protein processing and Alzheimer’s disease. Annu. Rev. Neurosci. 2011, 34, 185–204. [Google Scholar] [CrossRef]
- Glenner, G.G.; Wong, C.W. Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 1984, 120, 885–890. [Google Scholar] [CrossRef]
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef]
- Vander Zanden, C.M.; Wampler, L.; Bowers, I.; Watkins, E.B.; Majewski, J.; Chi, E.Y. Fibrillar and Nonfibrillar Amyloid Beta Structures Drive Two Modes of Membrane-Mediated Toxicity. Langmuir 2019, 35, 16024–16036. [Google Scholar] [CrossRef]
- Arvanitakis, Z.; Wilson, R.S.; Bienias, J.L.; Evans, D.A.; Bennett, D.A. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch. Neurol. 2004, 61, 661–666. [Google Scholar] [CrossRef]
- Ott, A.; Stolk, R.P.; Hofman, A.; van Harskamp, F.; Grobbee, D.E.; Breteler, M.M. Association of diabetes mellitus and dementia: The Rotterdam Study. Diabetologia 1996, 39, 1392–1397. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Han, W.N.; Chai, S.F.; Li, Y.; Fu, C.J.; Wang, C.F.; Cai, H.Y.; Li, X.Y.; Wang, X.; Hölscher, C.; et al. Semaglutide promotes the transition of microglia from M1 to M2 type to reduce brain inflammation in APP/PS1/tau mice. Neuroscience 2024, 563, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Overview: Type 2 diabetes. In InformedHealth.org [Internet]; Institute for Quality and Efficiency in Health Care (IQWiG): Cologne, Germany, 2006.
- Michailidis, M.; Moraitou, D.; Tata, D.A.; Kalinderi, K.; Papamitsou, T.; Papaliagkas, V. Alzheimer’s Disease as Type 3 Diabetes: Common Pathophysiological Mechanisms between Alzheimer’s Disease and Type 2 Diabetes. Int. J. Mol. Sci. 2022, 23, 2687. [Google Scholar] [CrossRef] [PubMed]
- Kandimalla, R.; Thirumala, V.; Reddy, P.H. Is Alzheimer’s disease a Type 3 Diabetes? A critical appraisal. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1078–1089. [Google Scholar] [CrossRef]
- Michailidis, M.; Tata, D.A.; Moraitou, D.; Kavvadas, D.; Karachrysafi, S.; Papamitsou, T.; Vareltzis, P.; Papaliagkas, V. Antidiabetic Drugs in the Treatment of Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 4641. [Google Scholar] [CrossRef]
- Mudaliar, S.; Henry, R.R. The incretin hormones: From scientific discovery to practical therapeutics. Diabetologia 2012, 55, 1865–1868. [Google Scholar] [CrossRef]
- Cobble, M. Differentiating among incretin-based therapies in the management of patients with type 2 diabetes mellitus. Diabetol. Metab. Syndr. 2012, 4, 8. [Google Scholar] [CrossRef]
- Meier, J. The role of incretin-based therapies in the management of type 2 diabetes mellitus: Perspectives on the past, present and future. Diabetes Mellit. 2019, 22, 461–466. [Google Scholar] [CrossRef]
- Lim, G.E.; Brubaker, P.L. Glucagon-like Peptide 1 Secretion by the L-Cell: The View From Within. Diabetes 2006, 55, S70–S77. [Google Scholar] [CrossRef]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef]
- Müller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef]
- Holt, M.K.; Richards, J.E.; Cook, D.R.; Brierley, D.I.; Williams, D.L.; Reimann, F.; Gribble, F.M.; Trapp, S. Preproglucagon Neurons in the Nucleus of the Solitary Tract Are the Main Source of Brain GLP-1, Mediate Stress-Induced Hypophagia, and Limit Unusually Large Intakes of Food. Diabetes 2019, 68, 21–33. [Google Scholar] [CrossRef]
- Kastin, A.J.; Akerstrom, V.; Pan, W. Interactions of glucagon-like peptide-1 (GLP-1) with the blood-brain barrier. J. Mol. Neurosci. 2002, 18, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; DeFronzo, R.A.; Gastaldelli, A.; Holst, J.J. Glucagon-like Peptide-1 and the Central/Peripheral Nervous System: Crosstalk in Diabetes. Trends Endocrinol. Metab. 2017, 28, 88–103. [Google Scholar] [CrossRef]
- Monney, M.; Jornayvaz, F.R.; Gariani, K. GLP-1 receptor agonists effect on cognitive function in patients with and without type 2 diabetes. Diabetes Metab. 2023, 49, 101470. [Google Scholar] [CrossRef] [PubMed]
- Holscher, C. Novel dual GLP-1/GIP receptor agonists show neuroprotective effects in Alzheimer’s and Parkinson’s disease models. Neuropharmacology 2018, 136, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Abbas, T.; Faivre, E.; Holscher, C. Impairment of synaptic plasticity and memory formation in GLP-1 receptor KO mice: Interaction between type 2 diabetes and Alzheimer’s disease. Behav. Brain Res. 2009, 205, 265–271. [Google Scholar] [CrossRef]
- During, M.J.; Cao, L.; Zuzga, D.S.; Francis, J.S.; Fitzsimons, H.L.; Jiao, X.; Bland, R.J.; Klugmann, M.; Banks, W.A.; Drucker, D.J.; et al. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat. Med. 2003, 9, 1173–1179. [Google Scholar] [CrossRef]
- Perry, T.; Lahiri, D.K.; Sambamurti, K.; Chen, D.; Mattson, M.P.; Egan, J.M.; Greig, N.H. Glucagon-like peptide-1 decreases endogenous amyloid-beta peptide (Abeta) levels and protects hippocampal neurons from death induced by Abeta and iron. J. Neurosci. Res. 2003, 72, 603–612. [Google Scholar] [CrossRef]
- Qin, Z.; Sun, Z.; Huang, J.; Hu, Y.; Wu, Z.; Mei, B. Mutated recombinant human glucagon-like peptide-1 protects SH-SY5Y cells from apoptosis induced by amyloid-beta peptide (1-42). Neurosci. Lett. 2008, 444, 217–221. [Google Scholar] [CrossRef]
- Ussher, J.R.; Drucker, D.J. Cardiovascular biology of the incretin system. Endocr. Rev. 2012, 33, 187–215. [Google Scholar] [CrossRef]
- Hui, H.; Farilla, L.; Merkel, P.; Perfetti, R. The short half-life of glucagon-like peptide-1 in plasma does not reflect its long-lasting beneficial effects. Eur. J. Endocrinol. 2002, 146, 863–869. [Google Scholar] [CrossRef]
- Plamboeck, A.; Holst, J.J.; Carr, R.D.; Deacon, C.F. Neutral Endopeptidase 24.11 and Dipeptidyl Peptidase IV are Both Involved in Regulating the Metabolic Stability of Glucagon-like Peptide-1 in vivo. In Dipeptidyl Aminopeptidases in Health and Disease; Back, N., Cohen, I.R., Kritchevsky, D., Lajtha, A., Paoletti, R., Eds.; Springer: Boston, MA, USA, 2003; pp. 303–312. [Google Scholar]
- Collins, L.; Costello, R.A. Glucagon-like Peptide-1 Receptor Agonists. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Gupta, V. Glucagon-like peptide-1 analogues: An overview. Indian J. Endocrinol. Metab. 2013, 17, 413–421. [Google Scholar] [CrossRef]
- Li, Y.; Duffy, K.B.; Ottinger, M.A.; Ray, B.; Bailey, J.A.; Holloway, H.W.; Tweedie, D.; Perry, T.; Mattson, M.P.; Kapogiannis, D.; et al. GLP-1 receptor stimulation reduces amyloid-beta peptide accumulation and cytotoxicity in cellular and animal models of Alzheimer’s disease. J. Alzheimer’s Dis. JAD 2010, 19, 1205–1219. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Jiang, R.; Xu, Y.; Zhao, X.; Li, Y. Exendin-4 antagonizes Aβ1-42-induced attenuation of spatial learning and memory ability. Exp. Ther. Med. 2016, 12, 2885–2892. [Google Scholar] [CrossRef]
- Garabadu, D.; Verma, J. Exendin-4 attenuates brain mitochondrial toxicity through PI3K/Akt-dependent pathway in amyloid beta (1–42)-induced cognitive deficit rats. Neurochem. Int. 2019, 128, 39–49. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Xu, Z.; Chen, S.; Yao, W.; Gao, X. GLP-1 receptor agonists downregulate aberrant GnT-III expression in Alzheimer’s disease models through the Akt/GSK-3β/β-catenin signaling. Neuropharmacology 2018, 131, 190–199. [Google Scholar] [CrossRef]
- Song, X.; Sun, Y.; Wang, Z.; Su, Y.; Wang, Y.; Wang, X. Exendin-4 alleviates β-Amyloid peptide toxicity via DAF-16 in a Caenorhabditis elegans model of Alzheimer’s disease. Front. Aging Neurosci. 2022, 14, 955113. [Google Scholar] [CrossRef]
- Aviles-Olmos, I.; Dickson, J.; Kefalopoulou, Z.; Djamshidian, A.; Kahan, J.; Ell, P.; Whitton, P.; Wyse, R.; Isaacs, T.; Lees, A.; et al. Motor and cognitive advantages persist 12 months after exenatide exposure in Parkinson’s disease. J. Parkinsons Dis. 2014, 4, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Mullins, R.J.; Mustapic, M.; Chia, C.W.; Carlson, O.; Gulyani, S.; Tran, J.; Li, Y.; Mattson, M.P.; Resnick, S.; Egan, J.M.; et al. A Pilot Study of Exenatide Actions in Alzheimer’s Disease. Curr. Alzheimer Res. 2019, 16, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Xin, X.; Ma, J.; Tan, C.; Osna, N.; Mahato, R.I. Therapeutic targets, novel drugs, and delivery systems for diabetes associated NAFLD and liver fibrosis. Adv. Drug Deliv. Rev. 2021, 176, 113888. [Google Scholar] [CrossRef]
- Meece, J. Pharmacokinetics and Pharmacodynamics of Liraglutide, a Long-Acting, Potent Glucagon-like Peptide-1 Analog. Pharmacotherapy 2009, 29, 33S–42S. [Google Scholar] [CrossRef]
- Jantrapirom, S.; Nimlamool, W.; Chattipakorn, N.; Chattipakorn, S.; Temviriyanukul, P.; Inthachat, W.; Govitrapong, P.; Potikanond, S. Liraglutide Suppresses Tau Hyperphosphorylation, Amyloid Beta Accumulation through Regulating Neuronal Insulin Signaling and BACE-1 Activity. Int. J. Mol. Sci. 2020, 21, 1725. [Google Scholar] [CrossRef]
- McClean, P.L.; Parthsarathy, V.; Faivre, E.; Hölscher, C. The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer’s disease. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 6587–6594. [Google Scholar] [CrossRef]
- McClean, P.L.; Hölscher, C. Liraglutide can reverse memory impairment, synaptic loss and reduce plaque load in aged APP/PS1 mice, a model of Alzheimer’s disease. Neuropharmacology 2014, 76, 57–67. [Google Scholar] [CrossRef]
- McClean, P.L.; Jalewa, J.; Hölscher, C. Prophylactic liraglutide treatment prevents amyloid plaque deposition, chronic inflammation and memory impairment in APP/PS1 mice. Behav. Brain Res. 2015, 293, 96–106. [Google Scholar] [CrossRef]
- Gejl, M.; Gjedde, A.; Egefjord, L.; Moller, A.; Hansen, S.B.; Vang, K.; Rodell, A.; Braendgaard, H.; Gottrup, H.; Schacht, A.; et al. In Alzheimer’s Disease, 6-Month Treatment with GLP-1 Analog Prevents Decline of Brain Glucose Metabolism: Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Front. Aging Neurosci. 2016, 8, 108. [Google Scholar] [CrossRef]
- Watson, K.T.; Wroolie, T.E.; Tong, G.; Foland-Ross, L.C.; Frangou, S.; Singh, M.; McIntyre, R.S.; Roat-Shumway, S.; Myoraku, A.; Reiss, A.L.; et al. Neural correlates of liraglutide effects in persons at risk for Alzheimer’s disease. Behav. Brain Res. 2019, 356, 271–278. [Google Scholar] [CrossRef]
- Lau, J.; Bloch, P.; Schaffer, L.; Pettersson, I.; Spetzler, J.; Kofoed, J.; Madsen, K.; Knudsen, L.B.; McGuire, J.; Steensgaard, D.B.; et al. Discovery of the Once-Weekly Glucagon-like Peptide-1 (GLP-1) Analogue Semaglutide. J. Med. Chem. 2015, 58, 7370–7380. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.F.; Zhang, D.; Hu, W.M.; Liu, D.X.; Li, L. Semaglutide-mediated protection against Abeta correlated with enhancement of autophagy and inhibition of apotosis. J. Clin. Neurosci. 2020, 81, 234–239. [Google Scholar] [CrossRef]
- Yang, X.; Feng, P.; Zhang, X.; Li, D.; Wang, R.; Ji, C.; Li, G.; Holscher, C. The diabetes drug semaglutide reduces infarct size, inflammation, and apoptosis, and normalizes neurogenesis in a rat model of stroke. Neuropharmacology 2019, 158, 107748. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, C.; He, Y.; Zhang, Y.; Li, Q.; Zhang, T.; Zhao, B.; Tong, A.; Zhong, Q.; Zhong, Z. Semaglutide ameliorates Alzheimer’s disease and restores oxytocin in APP/PS1 mice and human brain organoid models. Biomed. Pharmacother. Biomed. Pharmacother. 2024, 180, 117540. [Google Scholar] [CrossRef]
- A Research Study Investigating Semaglutide in People with Early Alzheimer’s Disease (EVOKE Plus). Available online: https://ctv.veeva.com/study/a-research-study-investigating-semaglutide-in-people-with-early-alzheimers-disease-evoke-plus (accessed on 24 April 2005).
- Cummings, J.L.; Atri, A.; Feldman, H.H.; Hansson, O.; Sano, M.; Knop, F.K.; Johannsen, P.; León, T.; Scheltens, P. evoke and evoke+: Design of two large-scale, double-blind, placebo-controlled, phase 3 studies evaluating efficacy, safety, and tolerability of semaglutide in early-stage symptomatic Alzheimer’s disease. Alzheimer’s Res. Ther. 2025, 17, 14. [Google Scholar] [CrossRef] [PubMed]
- Deryusheva, E.I.; Shevelyova, M.P.; Rastrygina, V.A.; Nemashkalova, E.L.; Vologzhannikova, A.A.; Machulin, A.V.; Nazipova, A.A.; Permyakova, M.E.; Permyakov, S.E.; Litus, E.A. In Search for Low-Molecular-Weight Ligands of Human Serum Albumin That Affect Its Affinity for Monomeric Amyloid beta Peptide. Int. J. Mol. Sci. 2024, 25, 4975. [Google Scholar] [CrossRef] [PubMed]
- Kamynina, A.V.; Esteras, N.; Koroev, D.O.; Bobkova, N.V.; Balasanyants, S.M.; Simonyan, R.A.; Avetisyan, A.V.; Abramov, A.Y.; Volpina, O.M. Synthetic Fragments of Receptor for Advanced Glycation End Products Bind Beta-Amyloid 1–40 and Protect Primary Brain Cells From Beta-Amyloid Toxicity. Front. Neurosci. 2018, 12, 681. [Google Scholar] [CrossRef]
- Lv, J.; Pan, Y.; Li, X.; Cheng, D.; Liu, S.; Shi, H.; Zhang, Y. The Imaging of Insulinomas Using a Radionuclide-Labelled Molecule of the GLP-1 Analogue Liraglutide: A New Application of Liraglutide. PLoS ONE 2014, 9, e96833. [Google Scholar] [CrossRef]
- Jacobsen, L.V.; Flint, A.; Olsen, A.K.; Ingwersen, S.H. Liraglutide in Type 2 Diabetes Mellitus: Clinical Pharmacokinetics and Pharmacodynamics. Clin. Pharmacokinet. 2016, 55, 657–672. [Google Scholar] [CrossRef]
- Yang, X.D.; Yang, Y.Y. Clinical Pharmacokinetics of Semaglutide: A Systematic Review. Drug Des. Dev. Ther. 2024, 18, 2555–2570. [Google Scholar] [CrossRef]
- Mehta, P.D.; Pirttila, T.; Mehta, S.P.; Sersen, E.A.; Aisen, P.S.; Wisniewski, H.M. Plasma and cerebrospinal fluid levels of amyloid beta proteins 1-40 and 1-42 in Alzheimer disease. Arch. Neurol. 2000, 57, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Zapadka, K.L.; Becher, F.J.; Uddin, S.; Varley, P.G.; Bishop, S.; Gomes Dos Santos, A.L.; Jackson, S.E. A pH-Induced Switch in Human Glucagon-like Peptide-1 Aggregation Kinetics. J. Am. Chem. Soc. 2016, 138, 16259–16265. [Google Scholar] [CrossRef]
- Prada Brichtova, E.; Krupova, M.; Bour, P.; Lindo, V.; Gomes Dos Santos, A.; Jackson, S.E. Glucagon-like peptide 1 aggregates into low-molecular-weight oligomers off-pathway to fibrillation. Biophys. J. 2023, 122, 2475–2488. [Google Scholar] [CrossRef]
- Wang, K.; Chen, K. Direct Assessment of Oligomerization of Chemically Modified Peptides and Proteins in Formulations using DLS and DOSY-NMR. Pharm. Res. 2023, 40, 1329–1339. [Google Scholar] [CrossRef]
- Venanzi, M.; Savioli, M.; Cimino, R.; Gatto, E.; Palleschi, A.; Ripani, G.; Cicero, D.; Placidi, E.; Orvieto, F.; Bianchi, E. A spectroscopic and molecular dynamics study on the aggregation process of a long-acting lipidated therapeutic peptide: The case of semaglutide. Soft Matter 2020, 16, 10122–10131. [Google Scholar] [CrossRef]
- Wang, Y.; Lomakin, A.; Kanai, S.; Alex, R.; Benedek, G.B. Transformation of oligomers of lipidated peptide induced by change in pH. Mol. Pharm. 2015, 12, 411–419. [Google Scholar] [CrossRef]
- Wolff, M.; Gast, K.; Evers, A.; Kurz, M.; Pfeiffer-Marek, S.; Schuler, A.; Seckler, R.; Thalhammer, A. A Conserved Hydrophobic Moiety and Helix-Helix Interactions Drive the Self-Assembly of the Incretin Analog Exendin-4. Biomolecules 2021, 11, 1305. [Google Scholar] [CrossRef]
- Calanna, S.; Christensen, M.; Holst, J.; Laferrère, B.; Gluud, L.; Vilsbøll, T.; Knop, F. Secretion of Glucagon-like Peptide-1 in Patients With Type 2 Diabetes Mellitus—Systematic Review and Meta-Analysis of Clinical Studies. Diabetologia 2013, 56, 965–972. [Google Scholar] [CrossRef]
- Balks, H.J.; Holst, J.J.; von zur Mühlen, A.; Brabant, G. Rapid Oscillations in Plasma Glucagon-like Peptide-1 (GLP-1) in Humans: Cholinergic Control of GLP-1 Secretion via Muscarinic Receptors1. J. Clin. Endocrinol. Metab. 1997, 82, 786–790. [Google Scholar] [CrossRef][Green Version]
- Fineman, M.; Flanagan, S.; Taylor, K.; Aisporna, M.; Shen, L.Z.; Mace, K.F.; Walsh, B.; Diamant, M.; Cirincione, B.; Kothare, P.; et al. Pharmacokinetics and Pharmacodynamics of Exenatide Extended-Release After Single and Multiple Dosing. Clin. Pharmacokinet. 2011, 50, 65–74. [Google Scholar] [CrossRef]
- Kastin, A.J.; Akerstrom, V. Entry of exendin-4 into brain is rapid but may be limited at high doses. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2003, 27, 313–318. [Google Scholar] [CrossRef]
- Gabery, S.; Salinas, C.G.; Paulsen, S.J.; Ahnfelt-Rønne, J.; Alanentalo, T.; Baquero, A.F.; Buckley, S.T.; Farkas, E.; Fekete, C.; Frederiksen, K.S.; et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight 2020, 5, e133429. [Google Scholar] [CrossRef]
- Lovshin, J.A.; Drucker, D.J. Incretin-based therapies for type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2009, 5, 262–269. [Google Scholar] [CrossRef]
- Dejgaard, T.; Frandsen, C.; Kielgast, U.; Størling, J.; Overgaard, A.; Svane, M.; Olsen, M.H.; Thorsteinsson, B.; Andersen, H.; Krarup, T.; et al. Liraglutide enhances insulin secretion and prolongs the remission period in adults with newly diagnosed type 1 diabetes (the NewLira study): A randomized, double-blind, placebo-controlled trial. Diabetes Obes. Metab. 2024, 26, 4905–4915. [Google Scholar] [CrossRef]
- Zheng, Z.; Zong, Y.; Ma, Y.; Tian, Y.; Pang, Y.; Zhang, C.; Gao, J. Glucagon-like peptide-1 receptor: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 234. [Google Scholar] [CrossRef]
- Desta, I.T.; Porter, K.A.; Xia, B.; Kozakov, D.; Vajda, S. Performance and Its Limits in Rigid Body Protein-Protein Docking. Structure 2020, 28, 1071–1081.e3. [Google Scholar] [CrossRef]
- Jarero-Basulto, J.J.; Gasca-Martínez, Y.; Rivera-Cervantes, M.C.; Gasca-Martínez, D.; Carrillo-González, N.J.; Beas-Zárate, C.; Gudiño-Cabrera, G. Cytotoxic Effect of Amyloid-β1-42 Oligomers on Endoplasmic Reticulum and Golgi Apparatus Arrangement in SH-SY5Y Neuroblastoma Cells. NeuroSci 2024, 5, 141–157. [Google Scholar] [CrossRef]
- Vander Zanden, C.M.; Chi, E.Y. Passive Immunotherapies Targeting Amyloid Beta and Tau Oligomers in Alzheimer’s Disease. J. Pharm. Sci. 2020, 109, 68–73. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wang, L.X.; Chen, Z.; Liu, L.B. Liraglutide prevents beta-amyloid-induced neurotoxicity in SH-SY5Y cells via a PI3K-dependent signaling pathway. Neurol. Res. 2016, 38, 313–319. [Google Scholar] [CrossRef]
- Litus, E.A.; Kazakov, A.S.; Deryusheva, E.I.; Nemashkalova, E.L.; Shevelyova, M.P.; Machulin, A.V.; Nazipova, A.A.; Permyakova, M.E.; Uversky, V.N.; Permyakov, S.E. Ibuprofen Favors Binding of Amyloid-beta Peptide to Its Depot, Serum Albumin. Int. J. Mol. Sci. 2022, 23, 6168. [Google Scholar] [CrossRef]
- Catanzariti, A.M.; Soboleva, T.A.; Jans, D.A.; Board, P.G.; Baker, R.T. An efficient system for high-level expression and easy purification of authentic recombinant proteins. Protein Sci. 2004, 13, 1331–1339. [Google Scholar] [CrossRef]
- Pace, C.N.; Vajdos, F.; Fee, L.; Grimsley, G.; Gray, T. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 1995, 4, 2411–2423. [Google Scholar] [CrossRef]
- De Ferrari, G.V.; Mallender, W.D.; Inestrosa, N.C.; Rosenberry, T.L. Thioflavin T is a fluorescent probe of the acetylcholinesterase peripheral site that reveals conformational interactions between the peripheral and acylation sites. J. Biol. Chem. 2001, 276, 23282–23287. [Google Scholar] [CrossRef]
- Uversky, V.N. Natively unfolded proteins: A point where biology waits for physics. Protein Sci. 2002, 11, 739–756. [Google Scholar] [CrossRef]
- Berman, H.M.; Burley, S.K. Protein Data Bank (PDB): Fifty-three years young and having a transformative impact on science and society. Q. Rev. Biophys. 2025, 58, e9. [Google Scholar] [CrossRef]
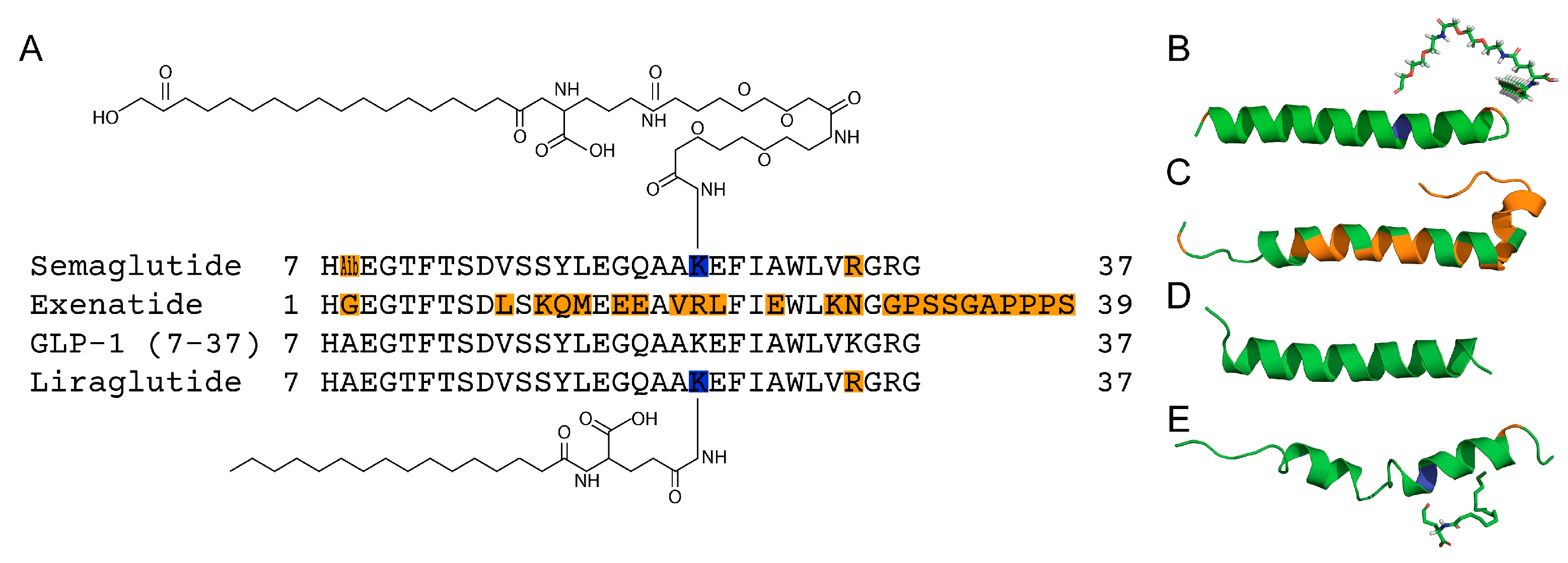
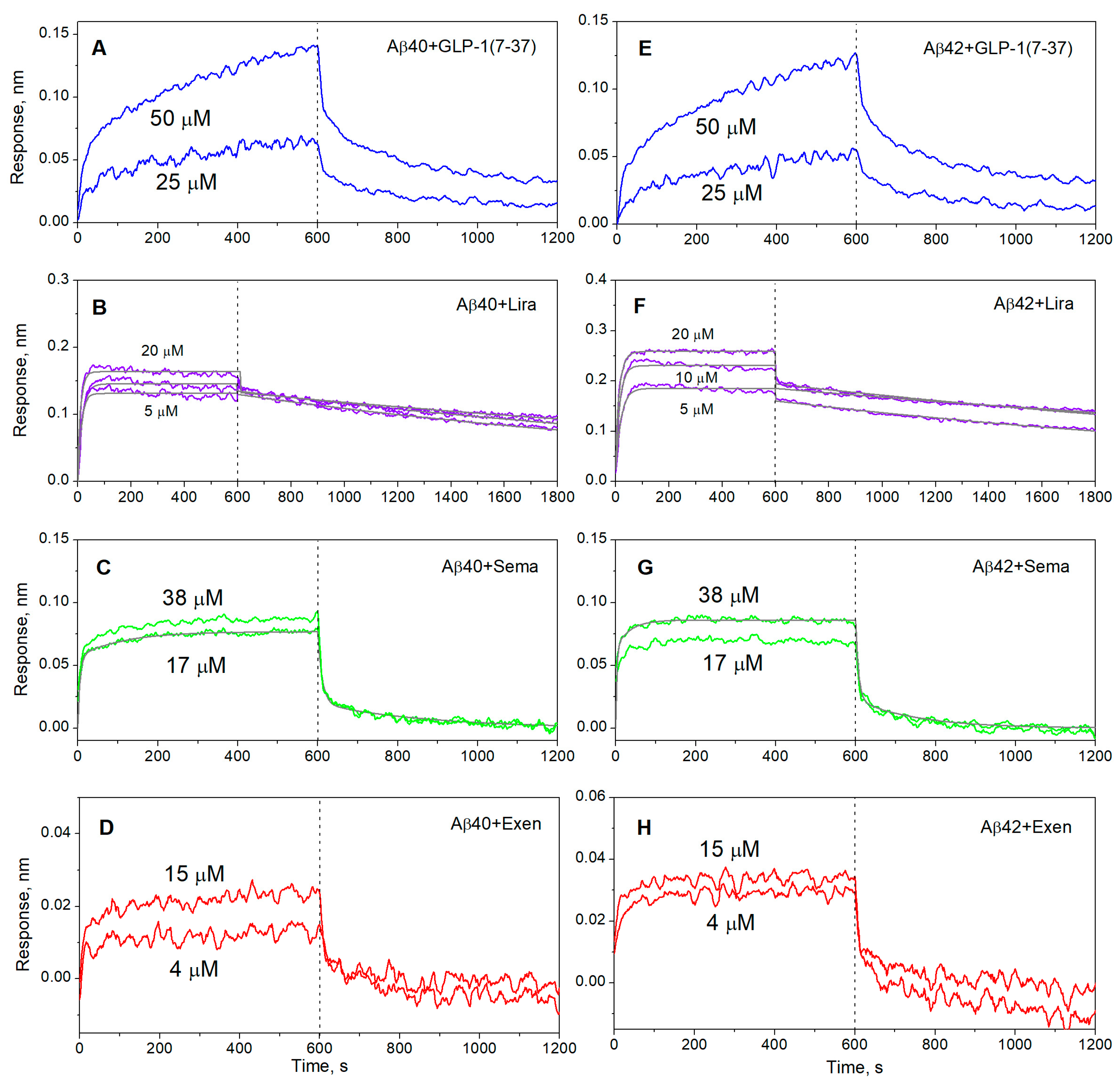
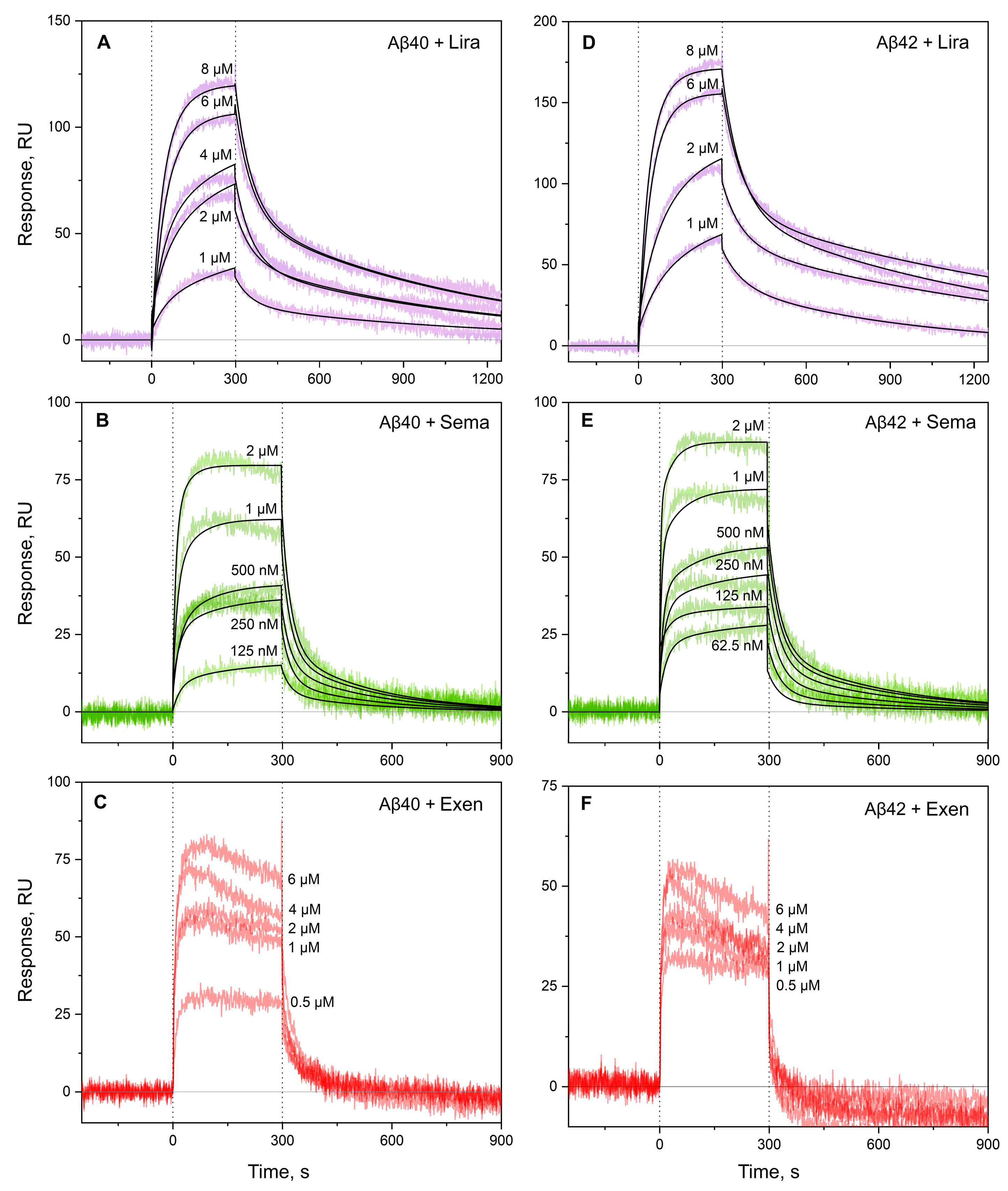


| [Lira], µM | ka, M−1s−1 | kd, s−1 | KD, M | ka, M−1s−1 | kd, s−1 | KD, M | |
| Lira | Aβ40 | Aβ42 | |||||
| 20 | (8.4 ± 2.8) × 103 | (9.0 ± 0.4) × 10−4 | (1.1 ± 0.4) × 10−7 | (5.7 ± 1.1) × 103 | (6.0 ± 0.2) × 10−4 | (1.1 ± 0.2) × 10−7 | |
| 10 | (7.3 ± 0.4) × 103 | (3.46 ± 0.07) × 10−4 | (4.8± 0.3) ×10−8 | (8.0 ± 0.7) × 103 | (4.82 ± 0.12) × 10−4 | (6.0 ± 0.2) × 10−8 | |
| 5 | (1.34 ± 0.09) × 104 | (5.56 ± 0.11) × 10−4 | (4.2 ± 0.3) × 10−8 | (1.21 ± 0.08) × 104 | (5.40 ± 0.12) × 10−4 | (4.5 ± 0.3) × 10−8 | |
| [Sema], µM | ka1, M−1s−1 | kd1, s−1 | KD1, M | ka2, M−1s−1 | kd2, s−1 | KD2, M | |
| Sema | Aβ40 | ||||||
| 17 | 310 ± 52 | (3.7 ± 0.2) × 10−3 | (1.2 ± 0.2) × 10−5 | (5.7 ± 1.1) × 103 | (9.1 ± 0.6) × 10−2 | (1.6 ± 0.3) × 10−5 | |
| Aβ42 | |||||||
| 38 | 582 ± 104 | (6.4 ± 0.6) × 10−3 | (1.1 ± 0.2) × 10−5 | (6.1 ± 2.7) × 103 | (1.34 ± 0.15) × 10−1 | (2.2 ± 1.0) × 10−5 | |
| [GLP-1RA], μM | ka1, M−1s−1 | kd1, s−1 | KD1, M | ka2, M−1s−1 | kd2, s−1 | KD2, M | |
| Aβ40 | |||||||
| Sema | 0.06–2 | (9.6 ± 2.4) × 103 | (3.2 ± 0.9) × 10−3 | (3.4 ± 0.5) × 10−7 | (3.90 ± 1.12) × 104 | (4.32 ± 0.12) × 10−2 | (1.2 ± 0.4) × 10−6 |
| Lira | 1–8 | (1.44 ± 0.05) × 103 | (1.19 ± 0.13) × 10−3 | (9.5 ± 0.7) × 10−7 | (1.9 ± 0.3) × 103 | (1.70 ± 0.10) × 10−2 | (9.1 ± 1.6) × 10−6 |
| Aβ42 | |||||||
| Sema | 0.06–2 | (1.26 ± 0.11) × 104 | (4.1 ± 1.4) × 10−3 | (3.4 ± 1.4) × 10−7 | (1.34 ± 0.18) × 105 | (3.8 ± 0.7) × 10−2 | (3.0 ± 0.9) × 10−7 |
| Lira | 1–8 | (2.24 ± 0.18) × 103 | (1.14 ± 0.10) × 10−3 | (5.2 ± 0.8) × 10−7 | (2.9 ± 0.04) × 103 | (1.64 ± 0.14) × 10−2 | (5.6 ± 0.3) × 10−6 |
| GLP-1RA | [GLP-1RA], µM | Rh, nm | MWRh, kDa | MWRh/MWm |
|---|---|---|---|---|
| GLP-1(7-37) | 5–83 | >92 | >7 × 105 | >210 |
| Lira | 105 | 3.08 ± 0.15 | 54.7 ± 7.8 | 15.6 ± 2.2 |
| 52 | 3.13 ± 0.05 | 57.1 ± 2.6 | 16.3 ± 0.7 | |
| 13 | 2.25 ± 0.12 | 22.7 ± 6.1 | 6.5 ± 1.7 | |
| 6 | 2.45 ± 0.16 | 28.8 ± 3.6 | 8.2 ± 1.0 | |
| Exen | 234 | 2.20 ± 0.01 | 24.58 ± 0.02 | 5.88 ± 0.04 |
| 115 | 2.27 ± 0.16 | 26.7 ± 5.7 | 6.4 ± 1.4 | |
| 29 | 1.53 ± 0.06 | 8.8 ± 0.9 | 2.1 ± 0.2 | |
| 15 | 1.39 ± 0.07 | 6.7 ± 0.9 | 1.6 ± 0.2 | |
| Sema | 47 | 1.22 ± 0.04 | 4.1 ± 0.4 | 1.2 ± 0.1 |
| 12 | 1.42 ± 0.15 | 6.2 ± 2.1 | 1.9 ± 0.6 |
| Minimal KD for Aβ Binding According to BLI | Effect on Aβ40 Fibrillation (Figure 4 and Figure 5) | Effect on Aβ Cytotoxicity to SH-SY5Y Cells (Figure 7) | Ability to Cross the BBB | AD Animal Data | Clinical Data, AD | |
|---|---|---|---|---|---|---|
| Lira | 4.2 × 10−8 M | No effect | Protection | + [47] | Prevents memory loss, reduces Aβ amyloid deposits [47,48,49] | No effect [50] |
| Sema | 1.1 × 10−5 M | Stimulation | Protection | − [74] | Positive effects on cognitive function, reduction of Aβ amyloid deposits [55] | Phase 3 clinical trials (NCT04777396 and NCT04777409) |
| Exen | ~(0.4–1.5) × 10−5 M | Inhibition | Protection | + [73] | Positive effects on learning and memory ability, reduces Aβ deposition [38,39,40,41] | No effect [43] |
| GLP-1(7-37) | ~(2.5–5.0) × 10−5 M | Inhibition | Increases Aβ40 cytotoxicity | + [24] | Positive effects on learning and memory [29] | No data |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Litus, E.A.; Shevelyova, M.P.; Vologzhannikova, A.A.; Deryusheva, E.I.; Chaplygina, A.V.; Rastrygina, V.A.; Machulin, A.V.; Alikova, V.D.; Nazipova, A.A.; Permyakova, M.E.; et al. Interaction Between Glucagon-like Peptide 1 and Its Analogs with Amyloid-β Peptide Affects Its Fibrillation and Cytotoxicity. Int. J. Mol. Sci. 2025, 26, 4095. https://doi.org/10.3390/ijms26094095
Litus EA, Shevelyova MP, Vologzhannikova AA, Deryusheva EI, Chaplygina AV, Rastrygina VA, Machulin AV, Alikova VD, Nazipova AA, Permyakova ME, et al. Interaction Between Glucagon-like Peptide 1 and Its Analogs with Amyloid-β Peptide Affects Its Fibrillation and Cytotoxicity. International Journal of Molecular Sciences. 2025; 26(9):4095. https://doi.org/10.3390/ijms26094095
Chicago/Turabian StyleLitus, Ekaterina A., Marina P. Shevelyova, Alisa A. Vologzhannikova, Evgenia I. Deryusheva, Alina V. Chaplygina, Victoria A. Rastrygina, Andrey V. Machulin, Valeria D. Alikova, Aliya A. Nazipova, Maria E. Permyakova, and et al. 2025. "Interaction Between Glucagon-like Peptide 1 and Its Analogs with Amyloid-β Peptide Affects Its Fibrillation and Cytotoxicity" International Journal of Molecular Sciences 26, no. 9: 4095. https://doi.org/10.3390/ijms26094095
APA StyleLitus, E. A., Shevelyova, M. P., Vologzhannikova, A. A., Deryusheva, E. I., Chaplygina, A. V., Rastrygina, V. A., Machulin, A. V., Alikova, V. D., Nazipova, A. A., Permyakova, M. E., Dotsenko, V. V., Permyakov, S. E., & Nemashkalova, E. L. (2025). Interaction Between Glucagon-like Peptide 1 and Its Analogs with Amyloid-β Peptide Affects Its Fibrillation and Cytotoxicity. International Journal of Molecular Sciences, 26(9), 4095. https://doi.org/10.3390/ijms26094095







