The Role of ESS2/DGCR14: Is It an Essential Factor in Splicing and Transcription?
Abstract
1. Cloning of ESS2 and Its Structure
1.1. Cloning of ESS2 (DGCR14)
1.2. ESS2 Interactants
1.3. Mutations of ESS2 and Disease
2. Function of ESS2
2.1. ESS2 Function in Yeast
2.2. ESS2-Dependent Splicing Regulation in Arabidopsis
2.3. ESS2-Dependent Mutated mRNA Splicing Regulation in C. elegans
2.4. ESS2 Function in Drosophila
2.5. ESS2 Function in Mammals
2.5.1. ESS2 Function in Neurons
2.5.2. ESS2 Function in T Cells
2.5.3. ESS2 Regulates the NF-κB/CHD1 Pathway in Prostate Cancer
3. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gong, W.; Emanuel, B.S.; Collins, J.; Kim, D.H.; Wang, Z.; Chen, F.; Zhang, G.; Roe, B.; Budarf, M.L. A transcription map of the DiGeorge and velo-cardio-facial syndrome minimal critical region on 22q11. Hum. Mol. Genet. 1996, 5, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, E.A.; Rizzu, P.; Antonacci, R.; Jurecic, V.; Delmas-Mata, J.; Lee, C.C.; Kim, U.J.; Scambler, P.J.; Baldini, A. A transcription map in the CATCH22 critical region: Identification, mapping, and ordering of four novel transcripts expressed in heart. Genomics 1996, 32, 104–112. [Google Scholar] [CrossRef]
- Scambler, P.J. The 22q11 deletion syndromes. Hum. Mol. Genet. 2000, 9, 2421–2426. [Google Scholar] [CrossRef]
- Robin, N.H.; Shprintzen, R.J. Defining the clinical spectrum of deletion 22q11.2. J. Pediatr. 2005, 147, 90–96. [Google Scholar] [CrossRef] [PubMed]
- McDonald-McGinn, D.M.; Sullivan, K.E. Chromosome 22q11.2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome). Medicine 2011, 90, 1–18. [Google Scholar] [CrossRef]
- Sullivan, K.E. Chromosome 22q11.2 deletion syndrome and DiGeorge syndrome. Immunol. Rev. 2019, 287, 186–201. [Google Scholar] [CrossRef]
- Lindsay, E.A.; Halford, S.; Wadey, R.; Scambler, P.J.; Baldini, A. Molecular cytogenetic characterization of the DiGeorge syndrome region using fluorescence in situ hybridization. Genomics 1993, 17, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Rizzu, P.; Lindsay, E.A.; Taylor, C.; O’Donnell, H.; Levy, A.; Scambler, P.; Baldini, A. Cloning and comparative mapping of a gene from the commonly deleted region of DiGeorge and Velocardiofacial syndromes conserved in C. elegans. Mamm. Genome 1996, 7, 639–643. [Google Scholar] [CrossRef]
- Gong, W.; Emanuel, B.S.; Galili, N.; Kim, D.H.; Roe, B.; Driscoll, D.A.; Budarf, M.L. Structural and mutational analysis of a conserved gene (DGSI) from the minimal DiGeorge syndrome critical region. Hum. Mol. Genet. 1997, 6, 267–276. [Google Scholar] [CrossRef]
- Lindsay, E.A.; Harvey, E.L.; Scambler, P.J.; Baldini, A. ES2, a gene deleted in DiGeorge syndrome, encodes a nuclear protein and is expressed during early mouse development, where it shares an expression domain with a Goosecoid-like gene. Hum. Mol. Genet. 1998, 7, 629–635. [Google Scholar] [CrossRef]
- Guna, A.; Butcher, N.J.; Bassett, A.S. Comparative mapping of the 22q11.2 deletion region and the potential of simple model organisms. J. Neurodev. Disord. 2015, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Herold, N.; Will, C.L.; Wolf, E.; Kastner, B.; Urlaub, H.; Luhrmann, R. Conservation of the protein composition and electron microscopy structure of Drosophila melanogaster and human spliceosomal complexes. Mol. Cell Biol. 2009, 29, 281–301. [Google Scholar] [CrossRef]
- Bessonov, S.; Anokhina, M.; Will, C.L.; Urlaub, H.; Luhrmann, R. Isolation of an active step I spliceosome and composition of its RNP core. Nature 2008, 452, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Hegele, A.; Kamburov, A.; Grossmann, A.; Sourlis, C.; Wowro, S.; Weimann, M.; Will, C.L.; Pena, V.; Luhrmann, R.; Stelzl, U. Dynamic protein-protein interaction wiring of the human spliceosome. Mol. Cell 2012, 45, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Kastner, B.; Will, C.L.; Stark, H.; Luhrmann, R. Structural Insights into Nuclear pre-mRNA Splicing in Higher Eukaryotes. Cold Spring Harb. Perspect. Biol. 2019, 11, a032417. [Google Scholar] [CrossRef]
- Dybkov, O.; Preussner, M.; El Ayoubi, L.; Feng, V.Y.; Harnisch, C.; Merz, K.; Leupold, P.; Yudichev, P.; Agafonov, D.E.; Will, C.L.; et al. Regulation of 3′ splice site selection after step 1 of splicing by spliceosomal C* proteins. Sci. Adv. 2023, 9, eadf1785. [Google Scholar] [CrossRef]
- Zhan, X.; Lu, Y.; Zhang, X.; Yan, C.; Shi, Y. Mechanism of exon ligation by human spliceosome. Mol. Cell 2022, 82, 2769–2778.e2764. [Google Scholar] [CrossRef]
- Fica, S.M.; Oubridge, C.; Wilkinson, M.E.; Newman, A.J.; Nagai, K. A human postcatalytic spliceosome structure reveals essential roles of metazoan factors for exon ligation. Science 2019, 363, 710–714. [Google Scholar] [CrossRef]
- Noma, K.; Goncharov, A.; Jin, Y. Systematic analyses of rpm-1 suppressors reveal roles for ESS-2 in mRNA splicing in Caenorhabditis elegans. Genetics 2014, 198, 1101–1115. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, Z.; Iomini, C.; Dutcher, S.K. Identifying RNA splicing factors using IFT genes in Chlamydomonas reinhardtii. Open Biol. 2018, 8, 170211. [Google Scholar] [CrossRef]
- Kanno, T.; Venhuizen, P.; Wu, M.T.; Chiou, P.; Chang, C.L.; Kalyna, M.; Matzke, A.J.M.; Matzke, M. A Collection of Pre-mRNA Splicing Mutants in Arabidopsis thaliana. G3 Genes Genomes Genet. 2020, 10, 1983–1996. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Tadesse, D.; Zhang, J.; Yao, T.; Zhang, L.; Jawdy, S.S.; Devireddy, A.; Zheng, K.; Smith, E.B.; Morrell-Falvey, J.; et al. AtDGCR14L contributes to salt-stress tolerance via regulating pre-mRNA splicing in Arabidopsis. Plant J. 2024, 120, 2668–2682. [Google Scholar] [CrossRef] [PubMed]
- Kalkat, M.; Resetca, D.; Lourenco, C.; Chan, P.K.; Wei, Y.; Shiah, Y.J.; Vitkin, N.; Tong, Y.; Sunnerhagen, M.; Done, S.J.; et al. MYC Protein Interactome Profiling Reveals Functionally Distinct Regions that Cooperate to Drive Tumorigenesis. Mol. Cell 2018, 72, 836–848.e837. [Google Scholar] [CrossRef] [PubMed]
- Goos, H.; Kinnunen, M.; Salokas, K.; Tan, Z.; Liu, X.; Yadav, L.; Zhang, Q.; Wei, G.H.; Varjosalo, M. Human transcription factor protein interaction networks. Nat. Commun. 2022, 13, 766. [Google Scholar] [CrossRef]
- Takada, I. DGCR14 induces Il17a gene expression through the RORgamma/BAZ1B/RSKS2 complex. Mol. Cell Biol. 2015, 35, 344–355. [Google Scholar] [CrossRef]
- Yang, P.B.; Hou, P.P.; Liu, F.Y.; Hong, W.B.; Chen, H.Z.; Sun, X.Y.; Li, P.; Zhang, Y.; Ju, C.Y.; Luo, L.J.; et al. Blocking PPARgamma interaction facilitates Nur77 interdiction of fatty acid uptake and suppresses breast cancer progression. Proc. Natl. Acad. Sci. USA 2020, 117, 27412–27422. [Google Scholar] [CrossRef]
- Evans, R.M.; Mangelsdorf, D.J. Nuclear Receptors, RXR, and the Big Bang. Cell 2014, 157, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Burris, T.P.; de Vera, I.M.S.; Cote, I.; Flaveny, C.A.; Wanninayake, U.S.; Chatterjee, A.; Walker, J.K.; Steinauer, N.; Zhang, J.; Coons, L.A.; et al. International Union of Basic and Clinical Pharmacology CXIII: Nuclear Receptor Superfamily-Update 2023. Pharmacol. Rev. 2023, 75, 1233–1318. [Google Scholar] [CrossRef]
- Yu, L.; Jearawiriyapaisarn, N.; Lee, M.P.; Hosoya, T.; Wu, Q.; Myers, G.; Lim, K.C.; Kurita, R.; Nakamura, Y.; Vojtek, A.B.; et al. BAP1 regulation of the key adaptor protein NCoR1 is critical for gamma-globin gene repression. Genes. Dev. 2018, 32, 1537–1549. [Google Scholar] [CrossRef]
- DiBlasi, E.; Shabalin, A.A.; Monson, E.T.; Keeshin, B.R.; Bakian, A.V.; Kirby, A.V.; Ferris, E.; Chen, D.; William, N.; Gaj, E.; et al. Rare protein-coding variants implicate genes involved in risk of suicide death. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2021, 186, 508–520. [Google Scholar] [CrossRef]
- Hoogendoorn, B.; Coleman, S.L.; Guy, C.A.; Smith, S.K.; O’Donovan, M.C.; Buckland, P.R. Functional analysis of polymorphisms in the promoter regions of genes on 22q11. Hum. Mutat. 2004, 24, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Duan, S.; Du, J.; Li, X.; Xu, Y.; Zhang, Z.; Wang, Y.; Huang, G.; Feng, G.; He, L. Transmission disequilibrium test provides evidence of association between promoter polymorphisms in 22q11 gene DGCR14 and schizophrenia. J. Neural Transm. 2006, 113, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.H.; Yoon, J.S.; Kwon, J.B.; Kim, H.W.; Wang, Y.P. Identification of genomic aberrations by array comparative genomic hybridization in patients with aortic dissections. Korean J. Thorac. Cardiovasc. Surg. 2011, 44, 123–130. [Google Scholar] [CrossRef]
- Lindsay, E.A.; Botta, A.; Jurecic, V.; Carattini-Rivera, S.; Cheah, Y.C.; Rosenblatt, H.M.; Bradley, A.; Baldini, A. Congenital heart disease in mice deficient for the DiGeorge syndrome region. Nature 1999, 401, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Taricani, L.; Tejada, M.L.; Young, P.G. The fission yeast ES2 homologue, Bis1, interacts with the Ish1 stress-responsive nuclear envelope protein. J. Biol. Chem. 2002, 277, 10562–10572. [Google Scholar] [CrossRef]
- Sengupta, S.; Kennemer, A.; Patrick, K.; Tsichlis, P.; Guerau-de-Arellano, M. Protein Arginine Methyltransferase 5 in T Lymphocyte Biology. Trends Immunol. 2020, 41, 918–931. [Google Scholar] [CrossRef]
- Zheng, J.; Li, B.; Wu, Y.; Wu, X.; Wang, Y. Targeting Arginine Methyltransferase PRMT5 for Cancer Therapy: Updated Progress and Novel Strategies. J. Med. Chem. 2023, 66, 8407–8427. [Google Scholar] [CrossRef]
- Genau, A.C.; Li, Z.; Renzaglia, K.S.; Fernandez Pozo, N.; Nogue, F.; Haas, F.B.; Wilhelmsson, P.K.I.; Ullrich, K.K.; Schreiber, M.; Meyberg, R.; et al. HAG1 and SWI3A/B control of male germ line development in P. patens suggests conservation of epigenetic reproductive control across land plants. Plant Reprod. 2021, 34, 149–173. [Google Scholar] [CrossRef]
- Sheta, M.; Yoshida, K.; Kanemoto, H.; Calderwood, S.K.; Eguchi, T. Stress-Inducible SCAND Factors Suppress the Stress Response and Are Biomarkers for Enhanced Prognosis in Cancers. Int. J. Mol. Sci. 2023, 24, 5168. [Google Scholar] [CrossRef]
- Huang, W.P.; Ellis, B.C.S.; Hodgson, R.E.; Sanchez Avila, A.; Kumar, V.; Rayment, J.; Moll, T.; Shelkovnikova, T.A. Stress-induced TDP-43 nuclear condensation causes splicing loss of function and STMN2 depletion. Cell Rep. 2024, 43, 114421. [Google Scholar] [CrossRef]
- Takada, I.; Tsuchiya, M.; Yanaka, K.; Hidano, S.; Takahashi, S.; Kobayashi, T.; Ogawa, H.; Nakagawa, S.; Makishima, M. Ess2 bridges transcriptional regulators and spliceosomal complexes via distinct interacting domains. Biochem. Biophys. Res. Commun. 2018, 497, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Nakata, K.; Abrams, B.; Grill, B.; Goncharov, A.; Huang, X.; Chisholm, A.D.; Jin, Y. Regulation of a DLK-1 and p38 MAP kinase pathway by the ubiquitin ligase RPM-1 is required for presynaptic development. Cell 2005, 120, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Baumbach, J.; Hummel, P.; Bickmeyer, I.; Kowalczyk, K.M.; Frank, M.; Knorr, K.; Hildebrandt, A.; Riedel, D.; Jackle, H.; Kuhnlein, R.P. A Drosophila in vivo screen identifies store-operated calcium entry as a key regulator of adiposity. Cell Metab. 2014, 19, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Toprak, U.; Hegedus, D.; Dogan, C.; Guney, G. A journey into the world of insect lipid metabolism. Arch. Insect Biochem. Physiol. 2020, 104, e21682. [Google Scholar] [CrossRef]
- Arioka, Y.; Shishido, E.; Kushima, I.; Suzuki, T.; Saito, R.; Aiba, A.; Mori, D.; Ozaki, N. Chromosome 22q11.2 deletion causes PERK-dependent vulnerability in dopaminergic neurons. EBioMedicine 2021, 63, 103138. [Google Scholar] [CrossRef]
- Santinha, A.J.; Klingler, E.; Kuhn, M.; Farouni, R.; Lagler, S.; Kalamakis, G.; Lischetti, U.; Jabaudon, D.; Platt, R.J. Transcriptional linkage analysis with in vivo AAV-Perturb-seq. Nature 2023, 622, 367–375. [Google Scholar] [CrossRef]
- Bessonov, S.; Anokhina, M.; Krasauskas, A.; Golas, M.M.; Sander, B.; Will, C.L.; Urlaub, H.; Stark, H.; Luhrmann, R. Characterization of purified human Bact spliceosomal complexes reveals compositional and morphological changes during spliceosome activation and first step catalysis. RNA 2010, 16, 2384–2403. [Google Scholar] [CrossRef]
- von Elsner, L.; Chai, G.; Schneeberger, P.E.; Harms, F.L.; Casar, C.; Qi, M.; Alawi, M.; Abdel-Salam, G.M.H.; Zaki, M.S.; Arndt, F.; et al. Biallelic FRA10AC1 variants cause a neurodevelopmental disorder with growth retardation. Brain 2022, 145, 1551–1563. [Google Scholar] [CrossRef]
- Sarafidou, T.; Galliopoulou, E.; Apostolopoulou, D.; Fragkiadakis, G.A.; Moschonas, N.K. Reconstruction of a Comprehensive Interactome and Experimental Data Analysis of FRA10AC1 May Provide Insights into Its Biological Role in Health and Disease. Genes 2023, 14, 568. [Google Scholar] [CrossRef]
- Gupta, M.; Pazour, G.J. Intraflagellar transport: A critical player in photoreceptor development and the pathogenesis of retinal degenerative diseases. Cytoskeleton 2024, 81, 556–568. [Google Scholar] [CrossRef]
- Raje, N.R.; Noel-MacDonnell, J.R.; Shortt, K.A.; Gigliotti, N.M.; Chan, M.A.; Heruth, D.P. T Cell Transcriptome in Chromosome 22q11.2 Deletion Syndrome. J. Immunol. 2022, 209, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Capone, A.; Volpe, E. Transcriptional Regulators of T Helper 17 Cell Differentiation in Health and Autoimmune Diseases. Front. Immunol. 2020, 11, 348. [Google Scholar] [CrossRef] [PubMed]
- Jetten, A.M. Retinoid-related orphan receptors (RORs): Critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl. Recept. Signal 2009, 7, e003. [Google Scholar] [CrossRef]
- Huang, W.; Littman, D.R. Regulation of RORgammat in Inflammatory Lymphoid Cell Differentiation. Cold Spring Harb. Symp. Quant. Biol. 2015, 80, 257–263. [Google Scholar] [CrossRef]
- Spirrison, A.N.; Lannigan, D.A. RSK1 and RSK2 as therapeutic targets: An up-to-date snapshot of emerging data. Expert. Opin. Ther. Targets 2024, 28, 1047–1059. [Google Scholar] [CrossRef]
- Takada, I.; Yogiashi, Y.; Makishima, M. The ribosomal S6 kinase inhibitor BI-D1870 ameliorated experimental autoimmune encephalomyelitis in mice. Immunobiology 2016, 221, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Takada, I.; Hidano, S.; Takahashi, S.; Yanaka, K.; Ogawa, H.; Tsuchiya, M.; Yokoyama, A.; Sato, S.; Ochi, H.; Nakagawa, T.; et al. Transcriptional coregulator Ess2 controls survival of post-thymic CD4(+) T cells through the Myc and IL-7 signaling pathways. J. Biol. Chem. 2022, 298, 102342. [Google Scholar] [CrossRef]
- Popovic, B.; Nicolet, B.P.; Guislain, A.; Engels, S.; Jurgens, A.P.; Paravinja, N.; Freen-van Heeren, J.J.; van Alphen, F.P.J.; van den Biggelaar, M.; Salerno, F.; et al. Time-dependent regulation of cytokine production by RNA binding proteins defines T cell effector function. Cell Rep. 2023, 42, 112419. [Google Scholar] [CrossRef]
- Takahashi, S.; Takada, I.; Hashimoto, K.; Yokoyama, A.; Nakagawa, T.; Makishima, M.; Kume, H. ESS2 controls prostate cancer progression through recruitment of chromodomain helicase DNA binding protein 1. Sci. Rep. 2023, 13, 12355. [Google Scholar] [CrossRef]
- Takayama, K.I.; Fujimura, T.; Suzuki, Y.; Inoue, S. Identification of long non-coding RNAs in advanced prostate cancer associated with androgen receptor splicing factors. Commun. Biol. 2020, 3, 393. [Google Scholar] [CrossRef]
- Zhao, D.; Lu, X.; Wang, G.; Lan, Z.; Liao, W.; Li, J.; Liang, X.; Chen, J.R.; Shah, S.; Shang, X.; et al. Synthetic essentiality of chromatin remodelling factor CHD1 in PTEN-deficient cancer. Nature 2017, 542, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Holehouse, A.S.; Kragelund, B.B. The molecular basis for cellular function of intrinsically disordered protein regions. Nat. Rev. Mol. Cell Biol. 2024, 25, 187–211. [Google Scholar] [CrossRef] [PubMed]
- Meszaros, B.; Hatos, A.; Palopoli, N.; Quaglia, F.; Salladini, E.; Van Roey, K.; Arthanari, H.; Dosztanyi, Z.; Felli, I.C.; Fischer, P.D.; et al. Minimum information guidelines for experiments structurally characterizing intrinsically disordered protein regions. Nat. Methods 2023, 20, 1291–1303. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, K.; Hirose, T.; Inada, T.; Ito, T.; Kai, T.; Oyama, M.; Tomari, Y.; Yoda, T.; Nakagawa, S. Nondomain biopolymers: Flexible molecular strategies to acquire biological functions. Genes. Cells 2023, 28, 539–552. [Google Scholar] [CrossRef]
- So, B.R.; Di, C.; Cai, Z.; Venters, C.C.; Guo, J.; Oh, J.M.; Arai, C.; Dreyfuss, G. A Complex of U1 snRNP with Cleavage and Polyadenylation Factors Controls Telescripting, Regulating mRNA Transcription in Human Cells. Mol. Cell 2019, 76, 590–599.e594. [Google Scholar] [CrossRef]
- Venters, C.C.; Oh, J.M.; Di, C.; So, B.R.; Dreyfuss, G. U1 snRNP Telescripting: Suppression of Premature Transcription Termination in Introns as a New Layer of Gene Regulation. Cold Spring Harb. Perspect. Biol. 2019, 11, a032235. [Google Scholar] [CrossRef]
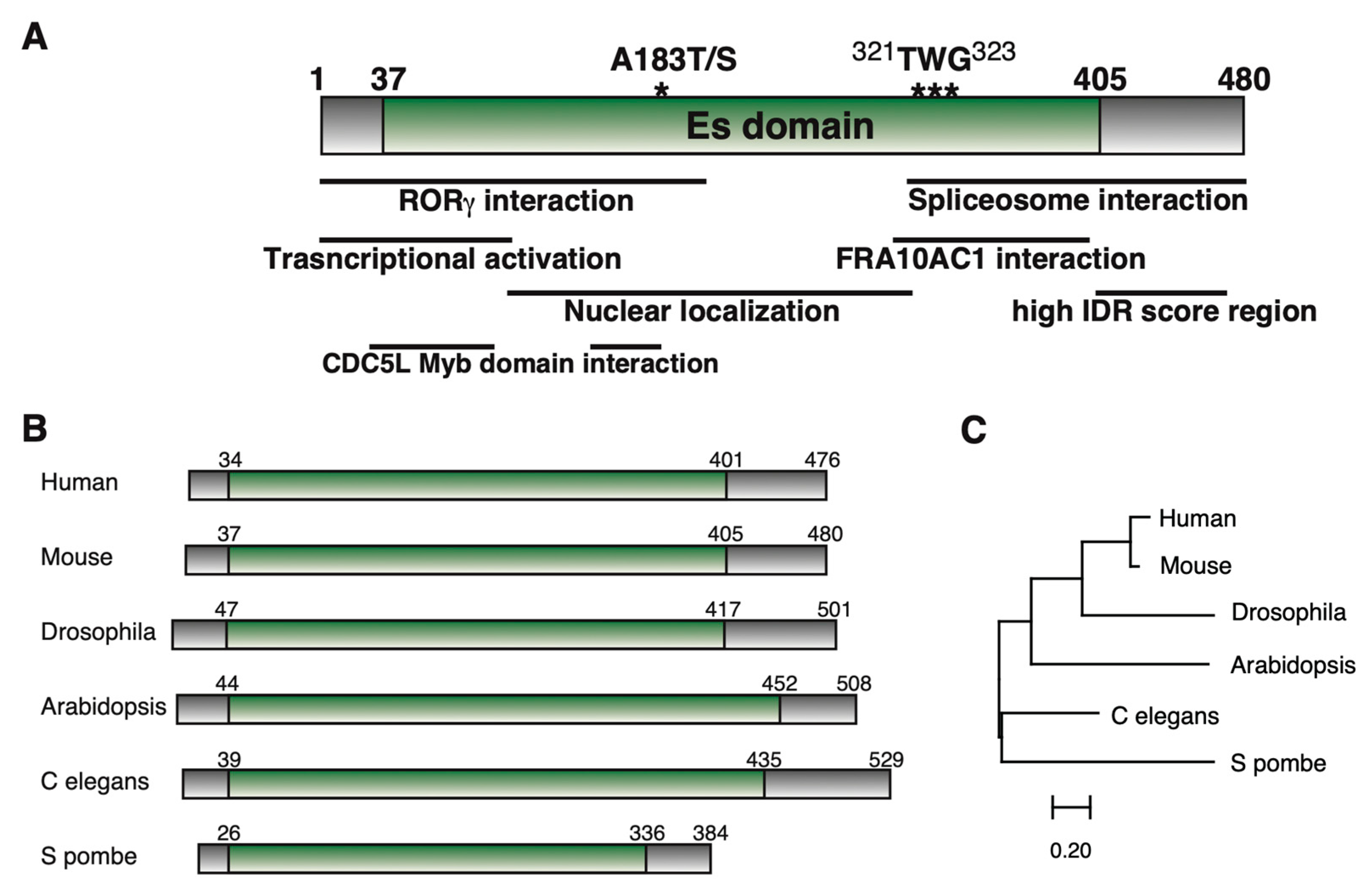
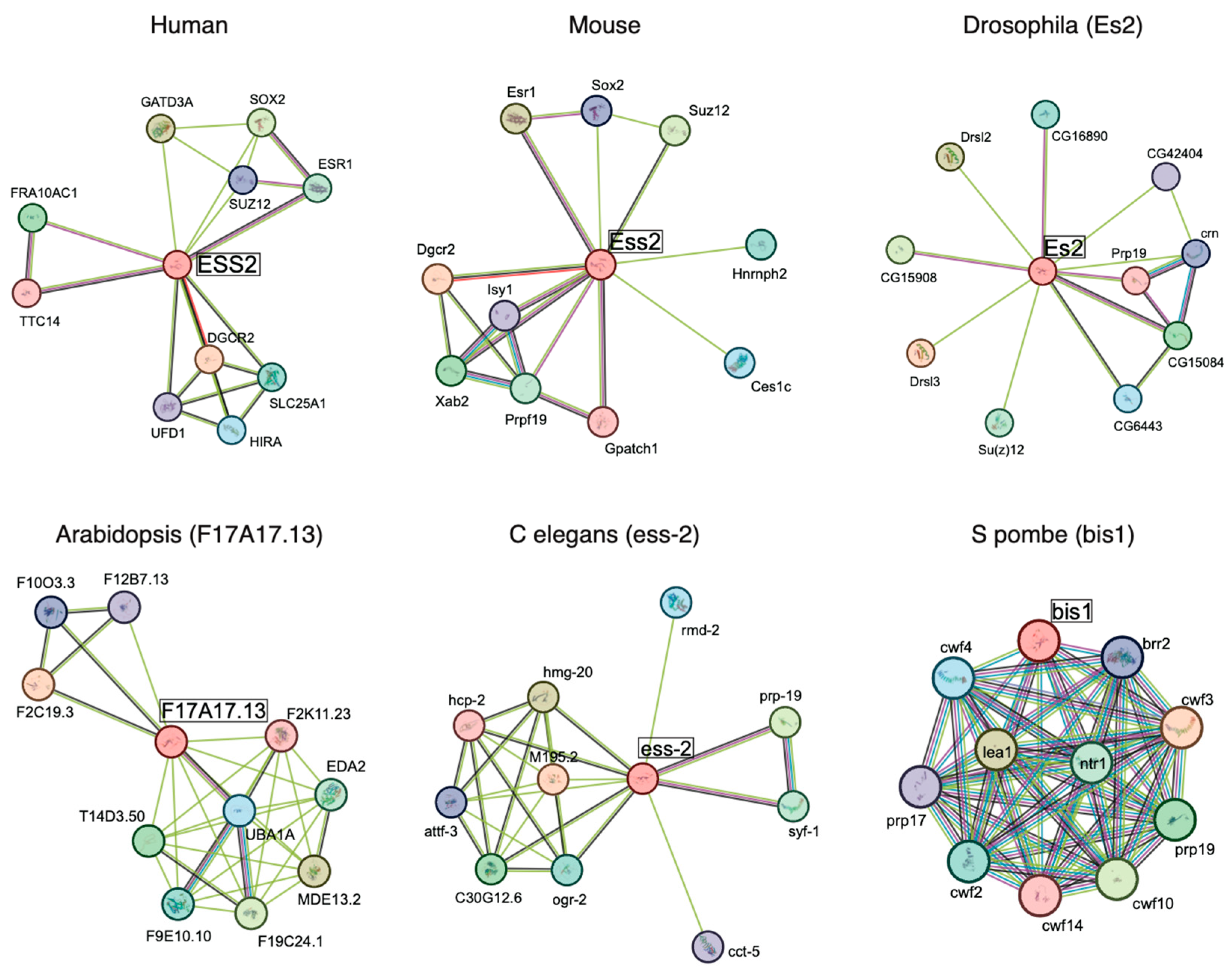
| Species | Functions | Interacting Proteins, Regulatory Factors | Ref. |
|---|---|---|---|
| Yeast | ESS2 overexpression; Cell elongation phenotype | Interact with ISH1, PRMT5 | [35] |
| ESS2 deficient; reduce viability | |||
| Green alga | IFT mutation splicing regulation | Regulate splicing with FRA10AC1 | [20] |
| Arabidopsis | Mutnants (Q80* and W365*) defect Pre-mRNA splicing | [21] | |
| Salt sress-dependent mRNA splicing | Regulate SWI3 mRNA expression | [22] | |
| C. elegans | Neuron development | [19] | |
| Drosophila | Fat cells regulation | [43] | |
| Mouse | CD4+ naive T cell maintenance, TH17 differentiation | Regulate mRNA expression and transcriptional activities of RORg/gt and Myc | [25,57] |
| Neuron | Regulate mRNA expression | [46] | |
| Human | Prostate cancer proliferation | Regulate such as NF-kB/CHD1-dependent mRNA expression | [59] |
| Neuron | Interact with FRA10AC1, mRNA and splicing regulation | [48,49] | |
| Dopamin neuron differentiation from iPS | PERK protein expression | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takada, I.; Hidano, S.; Nakagawa, T.; Nakagawa, S.; Makishima, M.; Takahashi, S. The Role of ESS2/DGCR14: Is It an Essential Factor in Splicing and Transcription? Int. J. Mol. Sci. 2025, 26, 4056. https://doi.org/10.3390/ijms26094056
Takada I, Hidano S, Nakagawa T, Nakagawa S, Makishima M, Takahashi S. The Role of ESS2/DGCR14: Is It an Essential Factor in Splicing and Transcription? International Journal of Molecular Sciences. 2025; 26(9):4056. https://doi.org/10.3390/ijms26094056
Chicago/Turabian StyleTakada, Ichiro, Shinya Hidano, Tohru Nakagawa, Shinichi Nakagawa, Makoto Makishima, and Sayuri Takahashi. 2025. "The Role of ESS2/DGCR14: Is It an Essential Factor in Splicing and Transcription?" International Journal of Molecular Sciences 26, no. 9: 4056. https://doi.org/10.3390/ijms26094056
APA StyleTakada, I., Hidano, S., Nakagawa, T., Nakagawa, S., Makishima, M., & Takahashi, S. (2025). The Role of ESS2/DGCR14: Is It an Essential Factor in Splicing and Transcription? International Journal of Molecular Sciences, 26(9), 4056. https://doi.org/10.3390/ijms26094056







