Fecal Arachidonic Acid: A Potential Biomarker for Inflammatory Bowel Disease Severity
Abstract
1. Introduction
2. Results
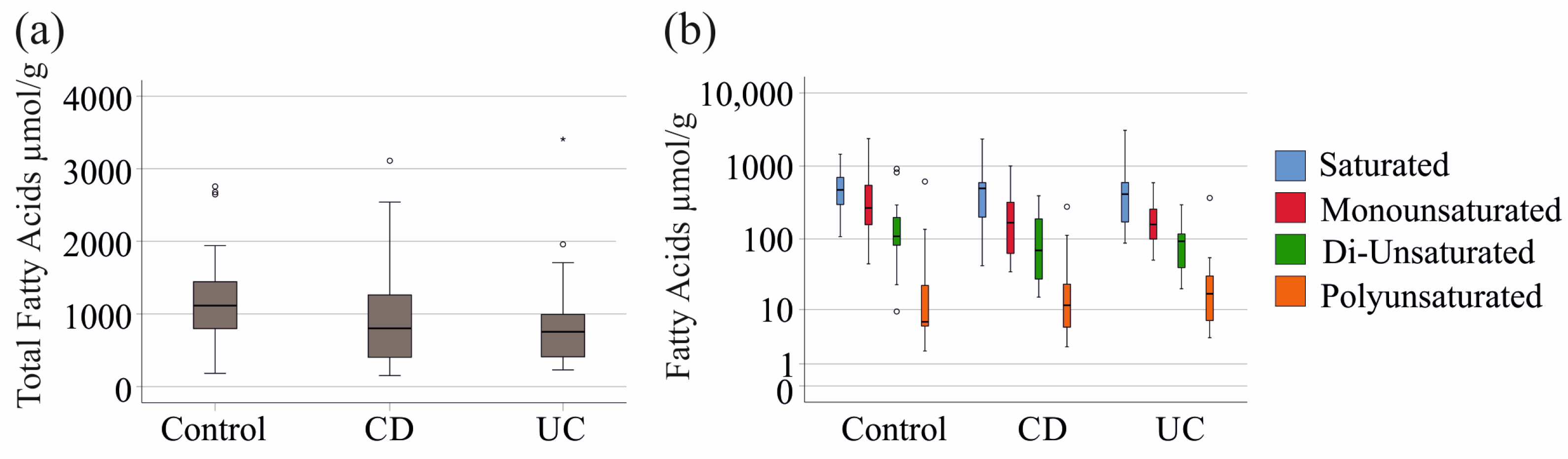
2.1. Fecal Fatty Acid Levels in Patients with IBD and Controls
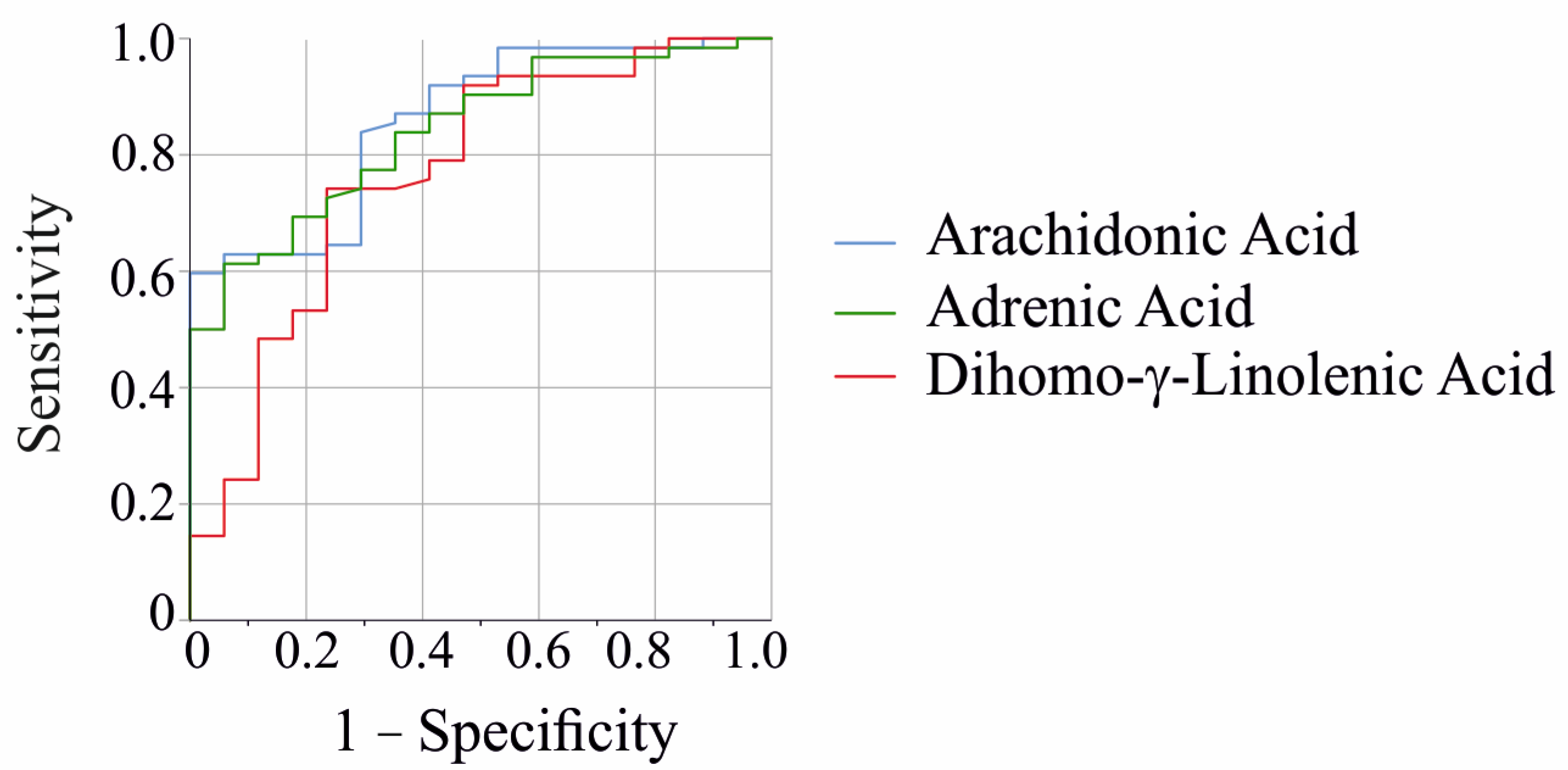
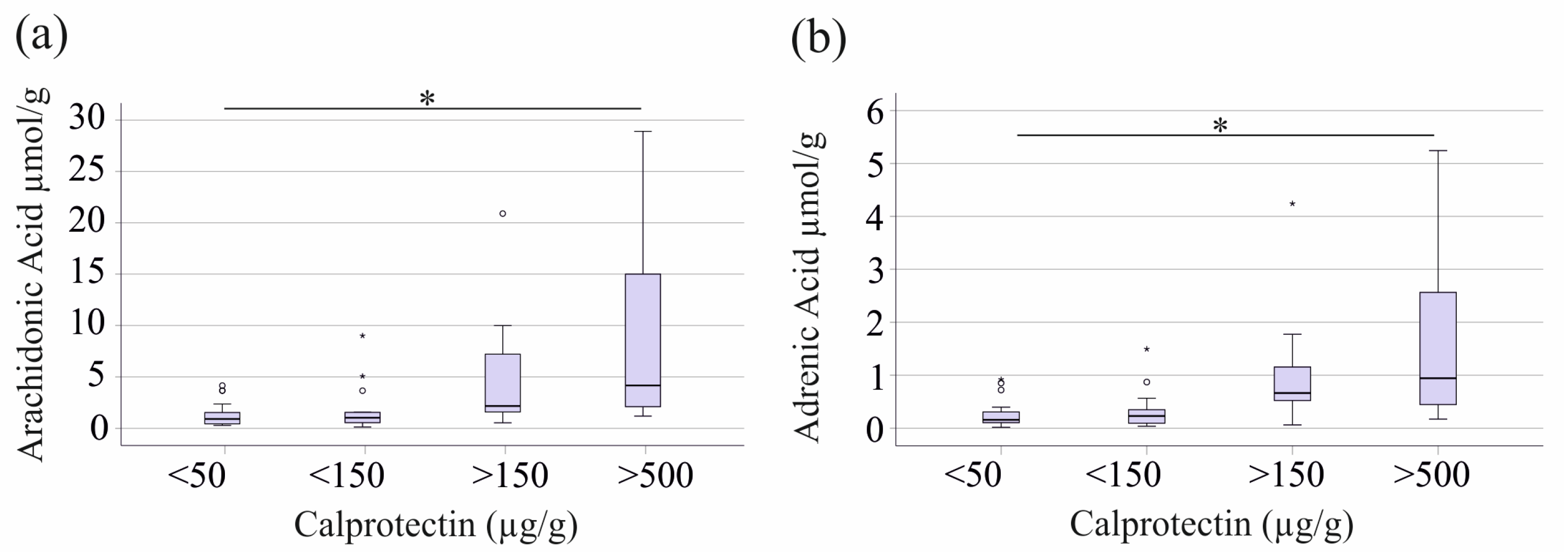
2.2. Fecal Levels of Arachidonic Acid and Adrenic Acid Increase with Fecal Calprotectin, but Show No Correlation with CRP or Markers of Renal Function
2.3. Correlations of Fecal Fatty Acids with Serum Cholesterol and Triglyceride Levels
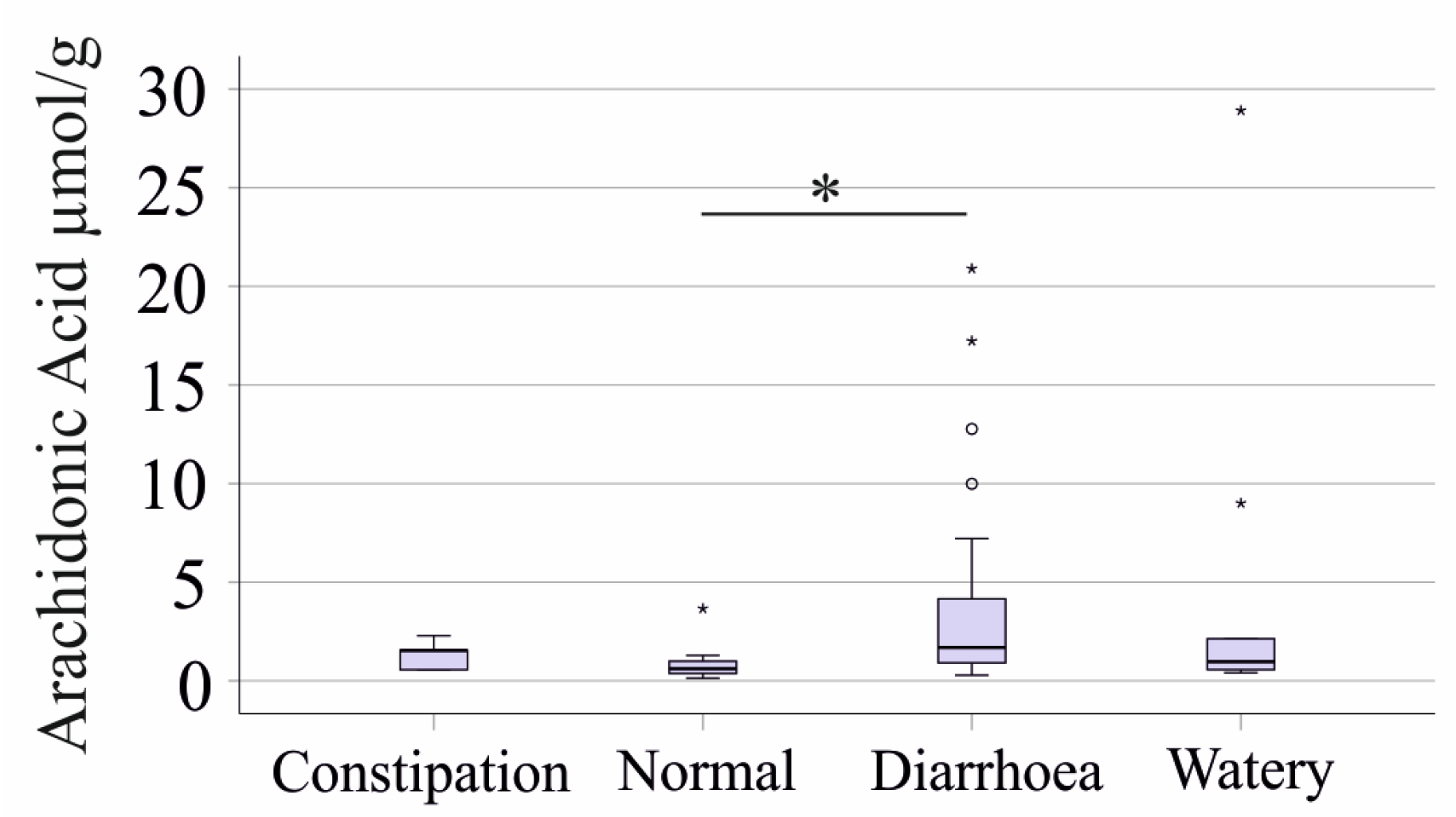
2.4. Associations of Fecal Fatty Acids with the Bristol Stool Chart and Gastrointestinal Symptom Rating Scale
- Constipation (Types 1 and 2): 5 patients.
- Normal (Types 3 and 4): 15 patients.
- Diarrhea (Types 5 and 6): 33 patients.
- Watery Stool (Type 7): 9 patients.
2.5. Fecal Fatty Acids in Relation to Time Since First Diagnosis and Disease Localization
2.6. Effects of Medication on Fecal Fatty Acid Composition
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Stool Collection, Fecal Fatty Acid, and Calprotectin Analysis
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vavricka, S.R.; Rogler, G. New insights into the pathogenesis of Crohn’s disease: Are they relevant for therapeutic options? Swiss Med. Wkly. 2009, 139, 527–534. [Google Scholar] [CrossRef]
- Seyedian, S.S.; Nokhostin, F.; Malamir, M.D. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J. Med. Life 2019, 12, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Scholmerich, J. Inflammatory bowel disease: Pandora’s box, present and future. Ann. N. Y. Acad. Sci. 2006, 1072, 365–378. [Google Scholar] [CrossRef]
- Gajendran, M.; Loganathan, P.; Catinella, A.P.; Hashash, J.G. A comprehensive review and update on Crohn’s disease. Dis. Mon. 2018, 64, 20–57. [Google Scholar] [CrossRef]
- Brown, S.J.; Mayer, L. The immune response in inflammatory bowel disease. Am. J. Gastroenterol. 2007, 102, 2058–2069. [Google Scholar] [CrossRef] [PubMed]
- Sergeant, S.; Rahbar, E.; Chilton, F.H. Gamma-linolenic acid, Dihommo-gamma linolenic, Eicosanoids and Inflammatory Processes. Eur. J. Pharmacol. 2016, 785, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Turini, M.E.; DuBois, R.N. Cyclooxygenase-2: A therapeutic target. Annu. Rev. Med. 2002, 53, 35–57. [Google Scholar] [CrossRef]
- Kefalakes, H.; Stylianides, T.J.; Amanakis, G.; Kolios, G. Exacerbation of inflammatory bowel diseases associated with the use of nonsteroidal anti-inflammatory drugs: Myth or reality? Eur. J. Clin. Pharmacol. 2009, 65, 963–970. [Google Scholar] [CrossRef]
- Pearl, D.S.; Masoodi, M.; Eiden, M.; Brummer, J.; Gullick, D.; McKeever, T.M.; Whittaker, M.A.; Nitch-Smith, H.; Brown, J.F.; Shute, J.K.; et al. Altered colonic mucosal availability of n-3 and n-6 polyunsaturated fatty acids in ulcerative colitis and the relationship to disease activity. J. Crohns Colitis 2014, 8, 70–79. [Google Scholar] [CrossRef]
- Maimo-Barcelo, A.; Martin-Saiz, L.; Barcelo-Nicolau, M.; Salivo, S.; Perez-Romero, K.; Rodriguez, R.M.; Martin, J.; Martinez, M.A.; Garcia, M.; Amengual, I.; et al. Lipid signature associated with chronic colon inflammation reveals a dysregulation in colonocyte differentiation process. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2024, 1869, 159528. [Google Scholar] [CrossRef]
- Harris, D.W.; Smith, P.R.; Swan, C.H. Determination of prostaglandin synthetase activity in rectal biopsy material and its significance in colonic disease. Gut 1978, 19, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Boughton-Smith, N.K.; Hawkey, C.J.; Whittle, B.J. Biosynthesis of lipoxygenase and cyclo-oxygenase products from [14C]-arachidonic acid by human colonic mucosa. Gut 1983, 24, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Sommersberger, S.; Gunawan, S.; Elger, T.; Fererberger, T.; Loibl, J.; Huss, M.; Kandulski, A.; Krautbauer, S.; Muller, M.; Liebisch, G.; et al. Altered fecal bile acid composition in active ulcerative colitis. Lipids Health Dis. 2023, 22, 199. [Google Scholar] [CrossRef]
- Tews, H.C.; Elger, T.; Gunawan, S.; Fererberger, T.; Sommersberger, S.; Loibl, J.; Huss, M.; Liebisch, G.; Muller, M.; Kandulski, A.; et al. Fecal short chain fatty acids and urinary 3-indoxyl sulfate do not discriminate between patients with Crohn s disease and ulcerative colitis and are not of diagnostic utility for predicting disease severity. Lipids Health Dis. 2023, 22, 164. [Google Scholar] [CrossRef]
- Tiratterra, E.; Franco, P.; Porru, E.; Katsanos, K.H.; Christodoulou, D.K.; Roda, G. Role of bile acids in inflammatory bowel disease. Ann. Gastroenterol. 2018, 31, 266–272. [Google Scholar] [CrossRef]
- Zhuang, X.; Li, T.; Li, M.; Huang, S.; Qiu, Y.; Feng, R.; Zhang, S.; Chen, M.; Xiong, L.; Zeng, Z. Systematic Review and Meta-analysis: Short-Chain Fatty Acid Characterization in Patients With Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 1751–1763. [Google Scholar] [CrossRef]
- Jansson, J.; Willing, B.; Lucio, M.; Fekete, A.; Dicksved, J.; Halfvarson, J.; Tysk, C.; Schmitt-Kopplin, P. Metabolomics reveals metabolic biomarkers of Crohn’s disease. PLoS ONE 2009, 4, e6386. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lin, Y.; Sheng, X.; Xu, J.; Hou, X.; Li, Y.; Zhang, H.; Guo, H.; Yu, Z.; Ren, F. Arachidonic Acid Promotes Intestinal Regeneration by Activating WNT Signaling. Stem Cell Rep. 2020, 15, 374–388. [Google Scholar] [CrossRef]
- Wang, X.; Lin, H.; Gu, Y. Multiple roles of dihomo-gamma-linolenic acid against proliferation diseases. Lipids Health Dis. 2012, 11, 25. [Google Scholar] [CrossRef]
- Singh, N.; Barnych, B.; Wagner, K.M.; Wan, D.; Morisseau, C.; Hammock, B.D. Adrenic Acid-Derived Epoxy Fatty Acids Are Naturally Occurring Lipids and Their Methyl Ester Prodrug Reduces Endoplasmic Reticulum Stress and Inflammatory Pain. ACS Omega 2021, 6, 7165–7174. [Google Scholar] [CrossRef]
- Kwon, S.J.; Khan, M.S.; Kim, S.G. Intestinal Inflammation and Regeneration—Interdigitating Processes Controlled by Dietary Lipids in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2024, 25, 1311. [Google Scholar] [CrossRef]
- Zhao, M.; Feng, R.; Ben-Horin, S.; Zhuang, X.; Tian, Z.; Li, X.; Ma, R.; Mao, R.; Qiu, Y.; Chen, M. Systematic review with meta-analysis: Environmental and dietary differences of inflammatory bowel disease in Eastern and Western populations. Aliment. Pharmacol. Ther. 2022, 55, 266–276. [Google Scholar] [CrossRef]
- Siddiqui, I.; Majid, H.; Abid, S. Update on clinical and research application of fecal biomarkers for gastrointestinal diseases. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Sands, B.E. Biomarkers of Inflammation in Inflammatory Bowel Disease. Gastroenterology 2015, 149, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- Freeman, K.; Taylor-Phillips, S.; Willis, B.H.; Ryan, R.; Clarke, A. Test accuracy of faecal calprotectin for inflammatory bowel disease in UK primary care: A retrospective cohort study of the IMRD-UK data. BMJ Open 2021, 11, e044177. [Google Scholar] [CrossRef]
- Blad, N.; Palmqvist, R.; Karling, P. Pre-diagnostic faecal calprotectin levels in patients with colorectal cancer: A retrospective study. BMC Cancer 2022, 22, 315. [Google Scholar] [CrossRef] [PubMed]
- Turvill, J.; Aghahoseini, A.; Sivarajasingham, N.; Abbas, K.; Choudhry, M.; Polyzois, K.; Lasithiotakis, K.; Volanaki, D.; Kim, B.; Langlands, F.; et al. Faecal calprotectin in patients with suspected colorectal cancer: A diagnostic accuracy study. Br. J. Gen. Pract. 2016, 66, e499–e506. [Google Scholar] [CrossRef]
- Song, E.M.; Byeon, J.S.; Lee, S.M.; Yoo, H.J.; Kim, S.J.; Lee, S.H.; Chang, K.; Hwang, S.W.; Yang, D.H.; Jeong, J.Y. Fecal Fatty Acid Profiling as a Potential New Screening Biomarker in Patients with Colorectal Cancer. Dig. Dis. Sci. 2018, 63, 1229–1236. [Google Scholar] [CrossRef]
- Ben-Mustapha, Y.; Ben-Fradj, M.K.; Hadj-Taieb, S.; Serghini, M.; Ben Ahmed, M.; Boubaker, J.; Feki, M. Altered mucosal and plasma polyunsaturated fatty acids, oxylipins, and endocannabinoids profiles in Crohn’s disease. Prostaglandins Other Lipid Mediat. 2023, 168, 106741. [Google Scholar] [CrossRef]
- Manfredi, M.; Conte, E.; Barberis, E.; Buzzi, A.; Robotti, E.; Caneparo, V.; Cecconi, D.; Brandi, J.; Vanni, E.; Finocchiaro, M.; et al. Integrated serum proteins and fatty acids analysis for putative biomarker discovery in inflammatory bowel disease. J. Proteom. 2019, 195, 138–149. [Google Scholar] [CrossRef]
- Lai, Y.; Xue, J.; Liu, C.W.; Gao, B.; Chi, L.; Tu, P.; Lu, K.; Ru, H. Serum Metabolomics Identifies Altered Bioenergetics, Signaling Cascades in Parallel with Exposome Markers in Crohn’s Disease. Molecules 2019, 24, 449. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cao, W.; Zhang, S.; Chen, D.; Liu, L. The Role of Calprotectin in the Diagnosis and Treatment of Inflammatory Bowel Disease. Int. J. Mol. Sci. 2025, 26, 1996. [Google Scholar] [CrossRef]
- Alghoul, Z.; Yang, C.; Merlin, D. The Current Status of Molecular Biomarkers for Inflammatory Bowel Disease. Biomedicines 2022, 10, 1492. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Qian, Y.; Li, H.; Deng, J. Combination of serum lipids and cancer antigens as a novel marker for colon cancer diagnosis. Lipids Health Dis. 2018, 17, 261. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, N.; Kakutani, S.; Kawashima, H.; Shibata, H.; Morita, I. Dietary supplementation of arachidonic acid increases arachidonic acid and lipoxin A4 contents in colon, but does not affect severity or prostaglandin E2 content in murine colitis model. Lipids Health Dis. 2014, 13, 30. [Google Scholar] [CrossRef]
- van Rheenen, P.F.; Van de Vijver, E.; Fidler, V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: Diagnostic meta-analysis. BMJ 2010, 341, c3369. [Google Scholar] [CrossRef]
- Jukic, A.; Bakiri, L.; Wagner, E.F.; Tilg, H.; Adolph, T.E. Calprotectin: From biomarker to biological function. Gut 2021, 70, 1978–1988. [Google Scholar] [CrossRef]
- Hrabovsky, V.; Zadak, Z.; Blaha, V.; Hyspler, R.; Karlik, T.; Martinek, A.; Mendlova, A. Cholesterol metabolism in active Crohn’s disease. Wien. Klin. Wochenschr. 2009, 121, 270–275. [Google Scholar] [CrossRef]
- Feingold, K.R.; Grunfeld, C. The Effect of Inflammation and Infection on Lipids and Lipoproteins. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Chen, H.; Li, W.; Hu, J.; Xu, F.; Lu, Y.; Zhu, L.; Shen, H. Association of serum lipids with inflammatory bowel disease: A systematic review and meta-analysis. Front. Med. 2023, 10, 1198988. [Google Scholar] [CrossRef]
- Hettenbach, A.; Elger, T.; Huss, M.; Liebisch, G.; Höring, M.; Loibl, J.; Kandulski, A.; Müller, M.; Tews, H.; Buechler, C. Cholesteryl ester species but not serum proprotein convertase subtilisin/kexin type 9 levels decline in male patients with active inflammatory bowel disease. Pathophysiology 2025, 32, 13. [Google Scholar] [CrossRef]
- Kwon, R.S.; Carey, M.C. Do steroids ameliorate bile acid malabsorption in Crohn’s disease? Gut 2004, 53, 10–11. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, L.R.; Jenabzadeh, P. IBD and Bile Acid Absorption: Focus on Pre-clinical and Clinical Observations. Front. Physiol. 2020, 11, 564. [Google Scholar] [CrossRef]
- Scoville, E.A.; Allaman, M.M.; Adams, D.W.; Motley, A.K.; Peyton, S.C.; Ferguson, S.L.; Horst, S.N.; Williams, C.S.; Beaulieu, D.B.; Schwartz, D.A.; et al. Serum Polyunsaturated Fatty Acids Correlate with Serum Cytokines and Clinical Disease Activity in Crohn’s Disease. Sci. Rep. 2019, 9, 2882. [Google Scholar] [CrossRef]
- Nielsen, O.H.; Ahnfelt-Ronne, I.; Elmgreen, J. Abnormal metabolism of arachidonic acid in chronic inflammatory bowel disease: Enhanced release of leucotriene B4 from activated neutrophils. Gut 1987, 28, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, S.; Hillier, K.; Smith, C. Increased arachidonic acid levels in phospholipids of human colonic mucosa in inflammatory bowel disease. Clin. Sci. 1987, 73, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.Y.; Zhang, Y.H.; Xiao, A.F.; Zhang, A.H.; Fang, B.S. Key Enzymes in Fatty Acid Synthesis Pathway for Bioactive Lipids Biosynthesis. Front. Nutr. 2022, 9, 851402. [Google Scholar] [CrossRef]
- Buechler, C.; Pohl, R.; Aslanidis, C. Pro-Resolving Molecules-New Approaches to Treat Sepsis? Int. J. Mol. Sci. 2017, 18, 476. [Google Scholar] [CrossRef]
- Masoodi, M.; Pearl, D.S.; Eiden, M.; Shute, J.K.; Brown, J.F.; Calder, P.C.; Trebble, T.M. Altered colonic mucosal Polyunsaturated Fatty Acid (PUFA) derived lipid mediators in ulcerative colitis: New insight into relationship with disease activity and pathophysiology. PLoS ONE 2013, 8, e76532. [Google Scholar] [CrossRef]
- Kikut, J.; Mokrzycka, M.; Drozd, A.; Grzybowska-Chlebowczyk, U.; Zietek, M.; Szczuko, M. Involvement of Proinflammatory Arachidonic Acid (ARA) Derivatives in Crohn’s Disease (CD) and Ulcerative Colitis (UC). J. Clin. Med. 2022, 11, 1861. [Google Scholar] [CrossRef]
- Vitek, L. Bile acid malabsorption in inflammatory bowel disease. Inflamm. Bowel Dis. 2015, 21, 476–483. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, F.; Yuan, J.; Li, J.; Jiang, D.; Zhang, J.; Li, H.; Wang, R.; Tang, J.; Huang, T.; et al. Effects of dietary fat on gut microbiota and faecal metabolites, and their relationship with cardiometabolic risk factors: A 6-month randomised controlled-feeding trial. Gut 2019, 68, 1417–1429. [Google Scholar] [CrossRef]
- Janczy, A.; Szymanski, M.; Stankiewicz, M.; Kaska, L.; Waleron, K.; Stelmanska, E.; Sledzinski, T.; Mika, A. Increased Amount of Polyunsaturated Fatty Acids in the Intestinal Contents of Patients with Morbid Obesity. Obes. Surg. 2023, 33, 1228–1236. [Google Scholar] [CrossRef]
- Dincer, B.T.; Usta, A.M.; Kural, A.; Helvaci, N.; Ucar, A.; Urganci, N. Can fecal calprotectin be used as a biomarker of non-alcoholic fatty liver disease in obese adolescents? BMC Pediatr. 2024, 24, 834. [Google Scholar] [CrossRef]
- Yarur, A.J.; Long, M.D.; Torres, J.; Nandi, N.; Cross, R.K.; Abbatemarco, A.M.; Blanco, D.; Niezychowski, W.; Crosby, C.; Wu, J.; et al. Body Mass Index Did Not Affect Efficacy and Safety of Etrasimod: A Post Hoc Analysis of the ELEVATE Ulcerative Colitis Clinical Program. Am. J. Gastroenterol. 2025; online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Sturm, A.; Maaser, C.; Calabrese, E.; Annese, V.; Fiorino, G.; Kucharzik, T.; Vavricka, S.R.; Verstockt, B.; van Rheenen, P.; Tolan, D.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 2: IBD scores and general principles and technical aspects. J. Crohns Colitis 2019, 13, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Ecker, J.; Scherer, M.; Schmitz, G.; Liebisch, G. A rapid GC-MS method for quantification of positional and geometric isomers of fatty acid methyl esters. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 897, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Horing, M.; Ejsing, C.S.; Hermansson, M.; Liebisch, G. Quantification of Cholesterol and Cholesteryl Ester by Direct Flow Injection High-Resolution Fourier Transform Mass Spectrometry Utilizing Species-Specific Response Factors. Anal. Chem. 2019, 91, 3459–3466. [Google Scholar] [CrossRef]
- Horing, M.; Ejsing, C.S.; Krautbauer, S.; Ertl, V.M.; Burkhardt, R.; Liebisch, G. Accurate quantification of lipid species affected by isobaric overlap in Fourier-Transform mass spectrometry. J. Lipid Res. 2021, 62, 100050. [Google Scholar] [CrossRef]
| Characteristics | IBD | Controls |
|---|---|---|
| Number (female/male) | 62 (28/34) | 17 (10/7) |
| Age (years) | 42 (19–78) | 48 (23–78) |
| Body Mass Index (kg/m2) | 24 (16–44) | not determined |
| C-reactive protein (mg/L) | 3 (0–144) | not determined |
| Creatinine (mg/dL) | 0.85 (0.51–1.25) | not determined |
| Glomerular filtration rate (mL/min) | 99 (61–136) | not determined |
| Fecal calprotectin (µg/g) | 62 (17–1616) | not determined |
| Fatty Acid (µmol/g Dry Weight) | Controls | IBD | |||||
|---|---|---|---|---|---|---|---|
| Median | Minimum | Maximum | Median | Minimum | Maximum | p-Value | |
| FA8:0 (Caprylic Acid) | 0.27 | 0.05 | 3.09 | 0.33 | 0.06 | 3.02 | ns |
| FA10:0 (Capric Acid) | 0.47 | 0.20 | 3.29 | 0.52 | 0.09 | 4.70 | ns |
| FA11:0 (Undecyclic Acid) | 0.10 | 0.04 | 0.39 | 0.10 | 0.00 | 0.83 | ns |
| FA12:0 (Lauric Acid) | 2.34 | 0.88 | 16.58 | 3.01 | 0.45 | 801.77 | ns |
| FA14:0 (Myristic Acid) | 10.63 | 4.69 | 31.39 | 14.84 | 1.60 | 348.33 | ns |
| FA14:1 c9 | 0.21 | 0.11 | 1.34 | 0.31 | 0.00 | 5.88 | ns |
| FA15:0 (Pentadecyclic Acid) | 6.89 | 2.33 | 18.69 | 6.37 | 0.38 | 21.22 | ns |
| FA16:0 (Palmitic Acid) | 200.46 | 54.80 | 474.02 | 237.45 | 24.56 | 950.08 | ns |
| FA16:1:c9 (Palmitoleic Acid) | 3.46 | 1.06 | 10.08 | 3.68 | 0.98 | 23.73 | ns |
| FA17:0 (Margaric Acid) | 3.95 | 1.49 | 17.61 | 3.94 | 0.38 | 23.81 | ns |
| FA18:0 (Stearic Acid) | 167.70 | 35.14 | 1008.66 | 150.15 | 12.15 | 1600.20 | ns |
| FA18:1:c9:n-9 (Oleic Acid) | 254.40 | 24.93 | 2352.78 | 146.40 | 23.63 | 946.50 | ns |
| FA18:1 c11:n-7 (Cis-Vaccenic Acid) | 7.34 | 1.58 | 17.71 | 5.55 | 0.92 | 25.75 | ns |
| FA18:2:t9t12:n-6 (Linolelaidic Acid) | 0.76 | 0.05 | 5.02 | 0.27 | 0.00 | 4.57 | ns |
| FA18:2:c9c12:n-6 (Linoleic Acid) | 108.29 | 8.93 | 916.03 | 81.10 | 14.71 | 391.85 | ns |
| FA18:3:c6c9c12:n-6 (γ-Linolenic Acid) | 0.03 | 0.01 | 0.63 | 0.05 | 0.00 | 0.70 | ns |
| FA18:3 c9c12c15 n-3 (α-Linolenic acid) | 3.21 | 0.63 | 611.23 | 4.06 | 0.32 | 363.19 | ns |
| FA20:0 (Arachidic Acid) | 5.36 | 0.92 | 12.76 | 3.82 | 0.51 | 50.87 | ns |
| FA20:1 c11n-9 (Gondoic Acid) | 1.82 | 0.70 | 8.69 | 2.16 | 0.59 | 26.45 | ns |
| FA20:2:11c14c (Eicosadienoic Acid) | 0.23 | 0.10 | 0.51 | 0.42 | 0.07 | 2.78 | ns |
| FA21:0 (Heneicosylic Acid) | 0.21 | 0.08 | 0.34 | 0.23 | 0.06 | 0.99 | ns |
| FA20:3 c8c11c14 n-6 (Dihomo-γ-Linolenic Acid) | 0.15 | 0.00 | 3.27 | 0.81 | 0.06 | 10.50 | 0.022 |
| FA20:4 c5c8c11c14 n-6 (Arachidonic Acid) | 0.33 | 0.12 | 0.93 | 1.22 | 0.13 | 28.91 | <0.001 |
| FA20:3 c11c14c17 n-3 (Eicosatrienoic Acid) | 0.05 | 0.01 | 0.44 | 0.08 | 0.01 | 1.19 | ns |
| FA22:0 (Behenic Acid) | 3.88 | 0.60 | 18.01 | 2.52 | 0.85 | 28.08 | ns |
| FA20:4 c8c11c14c17 n-3 (Eicosatetraenoic Acid) | 0.05 | 0.00 | 0.49 | 0.04 | 0.01 | 3.15 | ns |
| FA22:1 c13 n-9 (Erucic Acid) | 0.54 | 0.21 | 3.10 | 1.18 | 0.26 | 7.70 | ns |
| FA20:5 c5c8c11c14c17 n-3 (Eicosapentaenoic Acid) | 0.12 | 0.04 | 0.67 | 0.12 | 0.03 | 10.01 | ns |
| FA22:2 c13c16 | 0.12 | 0.05 | 0.32 | 0.14 | 0.03 | 0.33 | ns |
| FA23:0 (Tricosylic Acid) | 0.59 | 0.17 | 1.20 | 0.57 | 0.15 | 2.55 | ns |
| FA22:4 7c10c13c16c (Adrenic Acid) | 0.07 | 0.02 | 0.30 | 0.28 | 0.02 | 5.24 | <0.001 |
| Statistical tests applied | Mann–Whitney U-test (two-group comparisons), Kruskal–Wallis test (multiple group comparisons), Spearman correlation (associations between variables), ROC analysis (diagnostic performance assessment), Youden’s statistic (optimal cut-off values) |
| Data are presented as | Boxplots (median, minimum, maximum). |
| Outlier identification | Boxplots highlight outliers with circles and asterisks |
| Normality testing | Kolmogorov–Smirnov and Shapiro–Wilk tests (p < 0.05 for all) indicated a non-normal distribution |
| Correction for multiple comparisons | p-values were adjusted by multiplying by 31 (total number of fatty acids analyzed). |
| Significance threshold | p < 0.05 |
| Table presentation | Data are reported as median, minimum, and maximum values |
| Software used | IBM SPSS Statistics 26.0 (IBM Corp., Armonk, NY, USA, released 2019) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huss, M.; Elger, T.; Kunst, C.; Loibl, J.; Krautbauer, S.; Liebisch, G.; Kandulski, A.; Müller, M.; Tews, H.C.; Buechler, C. Fecal Arachidonic Acid: A Potential Biomarker for Inflammatory Bowel Disease Severity. Int. J. Mol. Sci. 2025, 26, 4034. https://doi.org/10.3390/ijms26094034
Huss M, Elger T, Kunst C, Loibl J, Krautbauer S, Liebisch G, Kandulski A, Müller M, Tews HC, Buechler C. Fecal Arachidonic Acid: A Potential Biomarker for Inflammatory Bowel Disease Severity. International Journal of Molecular Sciences. 2025; 26(9):4034. https://doi.org/10.3390/ijms26094034
Chicago/Turabian StyleHuss, Muriel, Tanja Elger, Claudia Kunst, Johanna Loibl, Sabrina Krautbauer, Gerhard Liebisch, Arne Kandulski, Martina Müller, Hauke Christian Tews, and Christa Buechler. 2025. "Fecal Arachidonic Acid: A Potential Biomarker for Inflammatory Bowel Disease Severity" International Journal of Molecular Sciences 26, no. 9: 4034. https://doi.org/10.3390/ijms26094034
APA StyleHuss, M., Elger, T., Kunst, C., Loibl, J., Krautbauer, S., Liebisch, G., Kandulski, A., Müller, M., Tews, H. C., & Buechler, C. (2025). Fecal Arachidonic Acid: A Potential Biomarker for Inflammatory Bowel Disease Severity. International Journal of Molecular Sciences, 26(9), 4034. https://doi.org/10.3390/ijms26094034






