Integrated Multi-Omics Analysis Reveals Key Regulators of Bovine Oocyte Maturation
Abstract
1. Introduction
2. Results
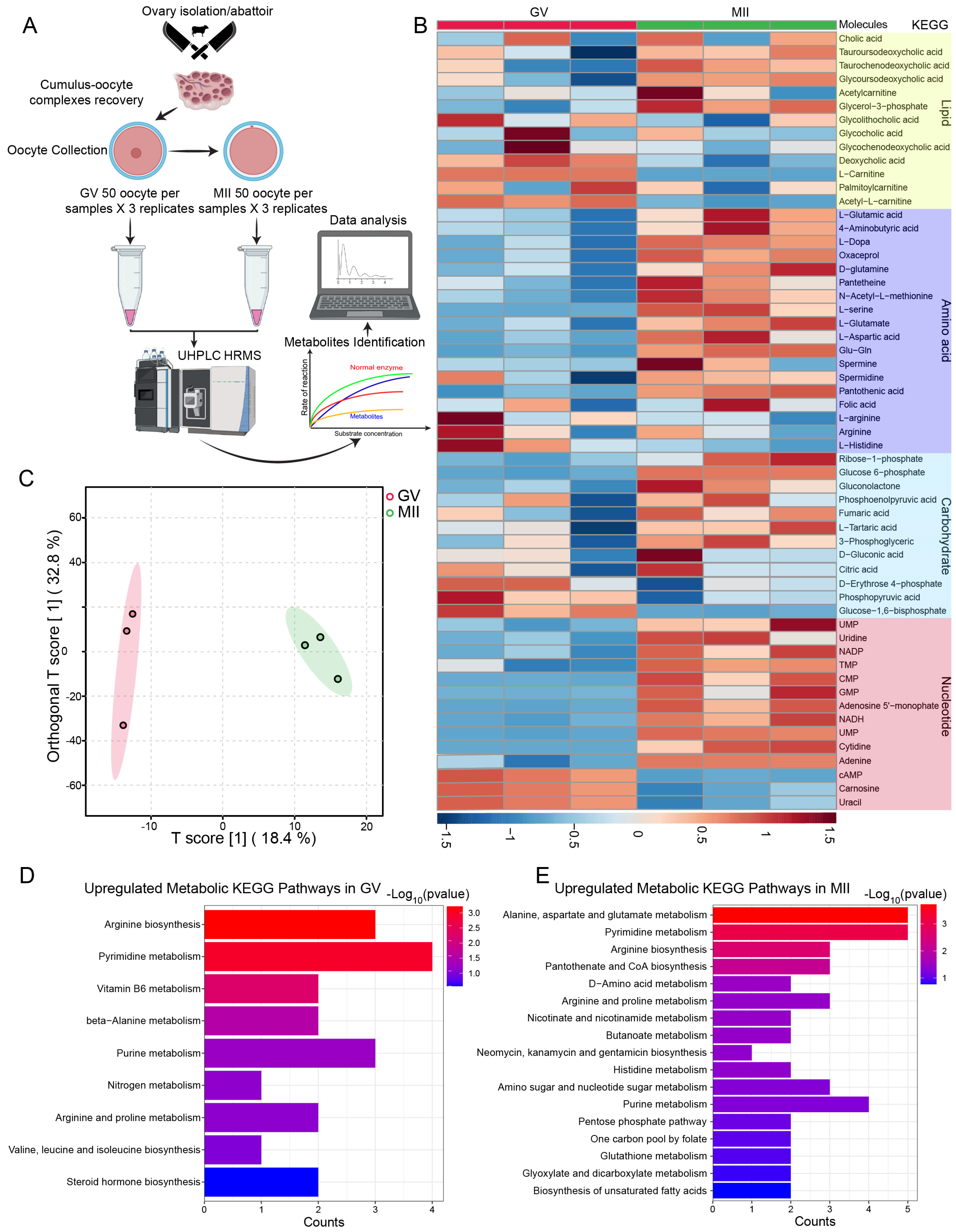
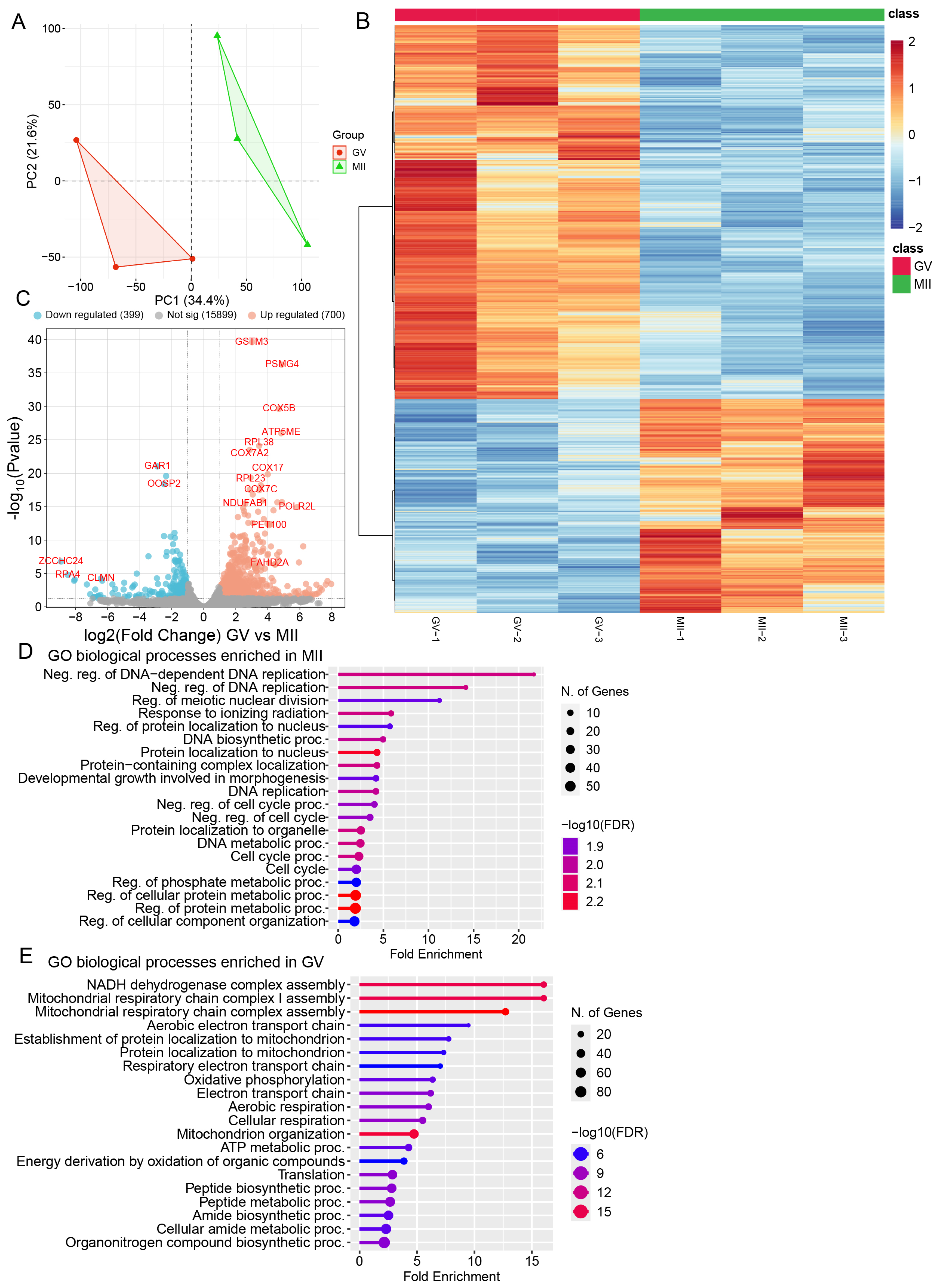
2.1. Metabolomic and Transcriptomic Profiling of Bovine Oocyte Maturation
2.2. Lipid Metabolism During Bovine Oocyte Maturation
2.2.1. Reduced Fatty Acid β-Oxidation and Accumulation of Long-Chain Fatty Acid During Oocyte Maturation
2.2.2. Enhanced Fatty Acid Biosynthesis and Their Metabolism During Bovine Oocyte Maturation
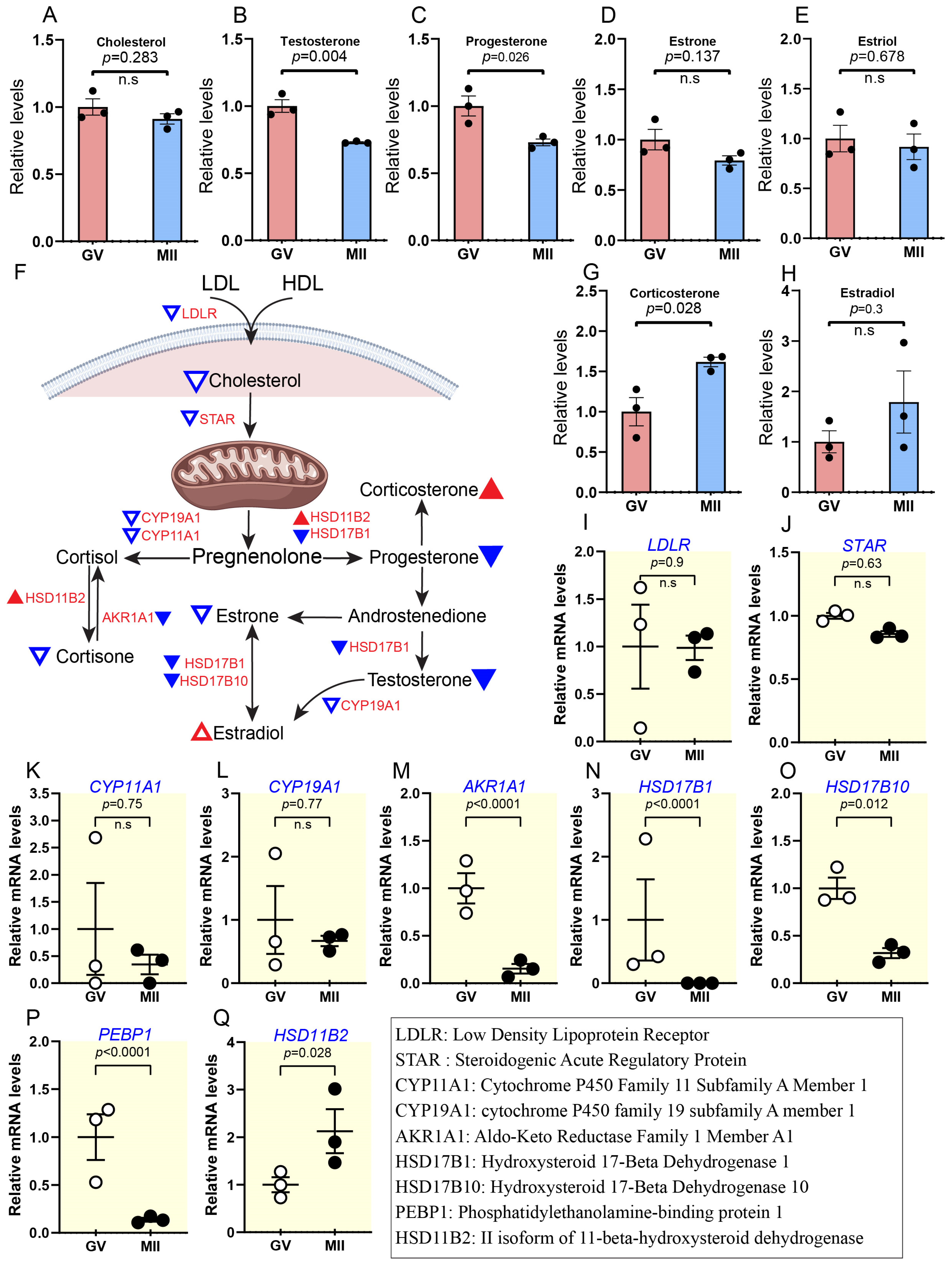
2.2.3. Reduced Cholesterol Levels Coincide with Decreased Steroid Hormone Activity During Maturation
2.2.4. Elevated Bile Acids and Their Derivatives During Oocyte Maturation
2.3. Metabolic Characteristics of Carbohydrate Metabolism Activity During Bovine Oocyte Maturation
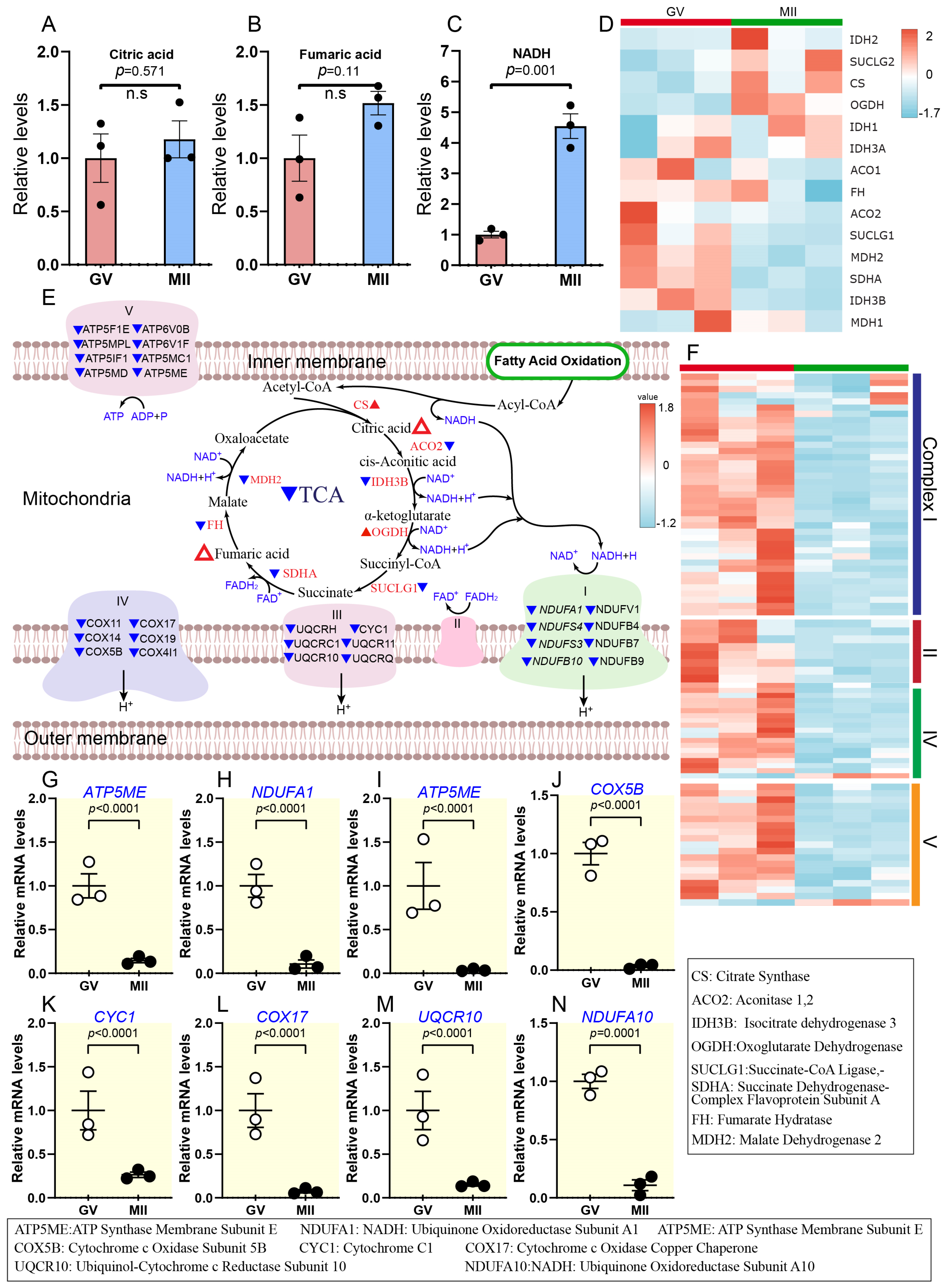
2.3.1. Activity of Citric Acid Cycle and Inhibition of Oxidative Phosphorylation During Oocyte Maturation
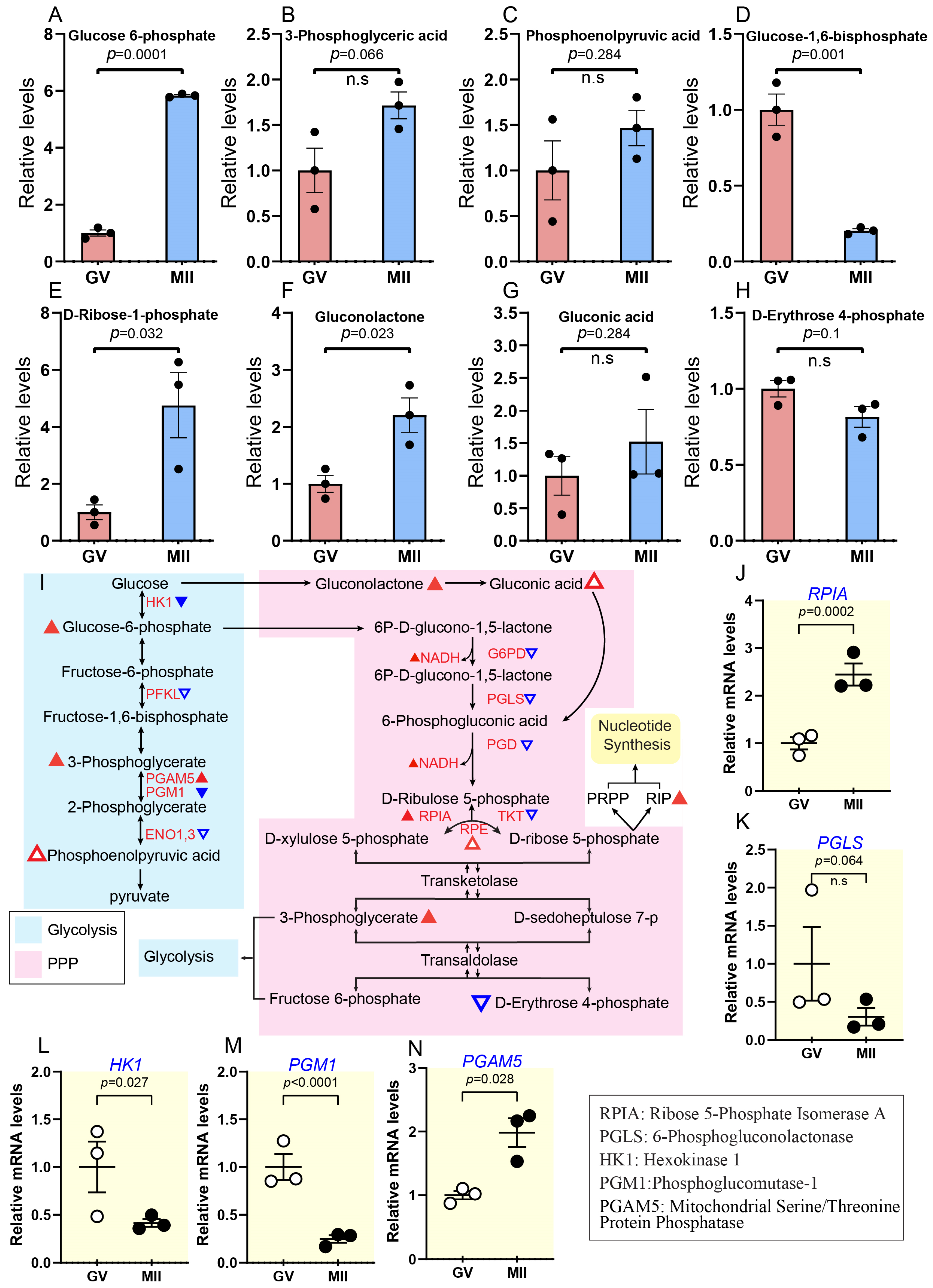
2.3.2. Persistent Increase in Glycolysis and Pentose Phosphate Pathway During Oocyte Maturation
2.3.3. Increased Hexosamine Biosynthesis Pathway During Oocyte Maturation
2.4. Metabolic Characteristics of Amino Acids During Bovine Oocyte Maturation
Increased Glutamic Acid Synthesis and Polyamine Metabolism Drive the Catabolism of Arginine and Proline
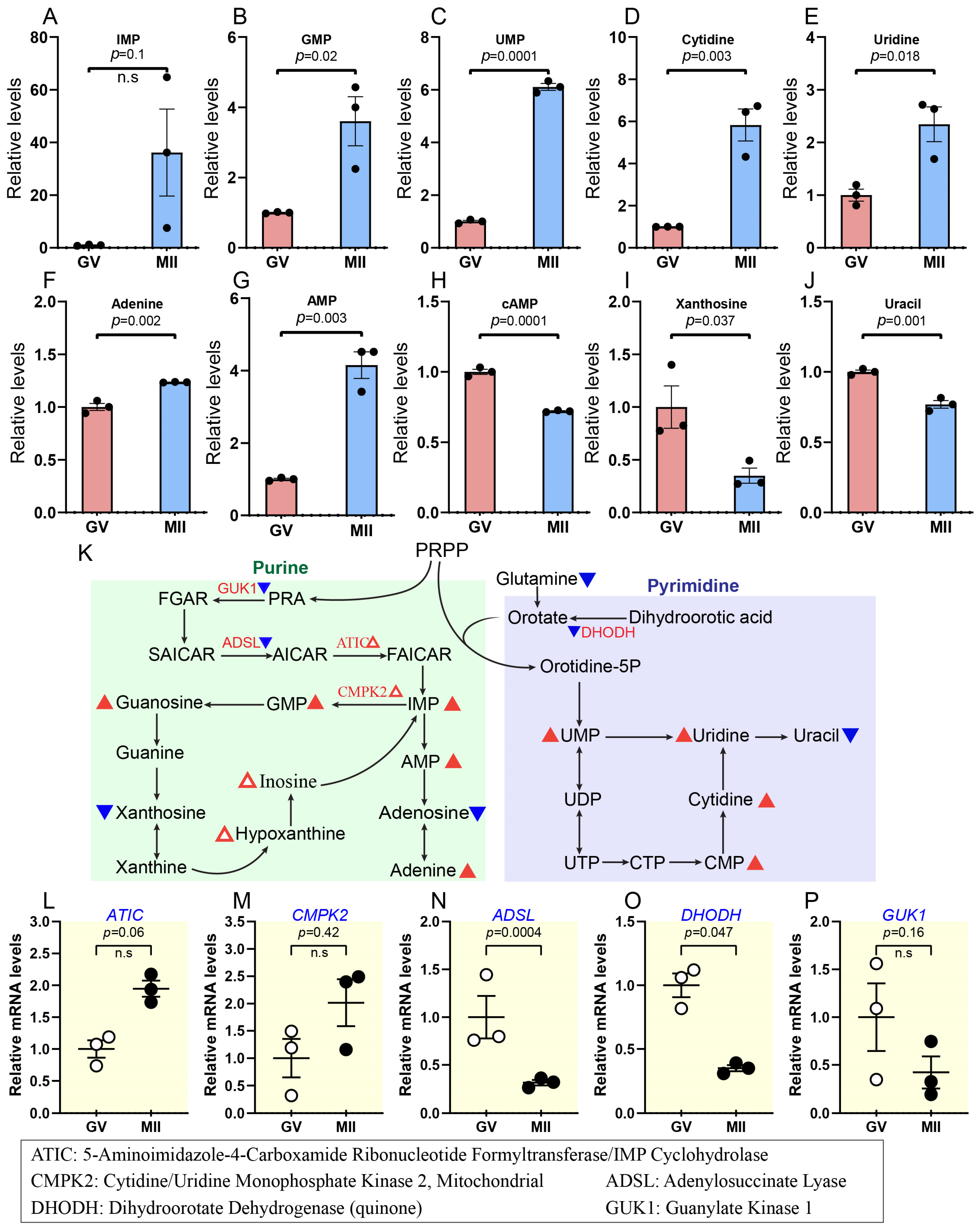
2.5. Progressive Increase in Nucleotide Synthesis During Oocyte Maturation
3. Discussion
4. Materials and Methods
4.1. Bovine Oocyte Maturation and Sample Collection
4.2. Metabolomic Analysis
4.2.1. Metabolomic Sample Collection
4.2.2. UHPLC-MS/MS Analyses
4.2.3. Metabolomic Data Analysis
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GV | Germinal Vesicle |
| MII | Metaphase II |
| IVM | In Vitro Maturation |
| COCs | Cumulus–Oocyte Complexes |
| LCFAs | Long-Chain Fatty Acids |
| TCA Cycle | Citric Acid Cycle (Tricarboxylic Acid Cycle) |
| OXPHOS | Oxidative Phosphorylation |
| PPP | Pentose Phosphate Pathway |
| SGOC | Serine–Glycine–One-Carbon |
| PUFAs | Polyunsaturated Fatty Acids |
| ART | Assisted Reproductive Technologies |
| UHPLC-HRMS | Ultra-High-Performance Liquid Chromatography–High-Resolution Mass Spectrometry |
| OPLS-DA | Orthogonal Partial Least-Squares Discriminant Analysis |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| GO | Gene Ontology |
| DEGs | Differentially Expressed Genes |
| FAO | Fatty Acid β-Oxidation |
| CPT1 | Carnitine Palmitoyltransferase I |
| CACT | Carnitine-Acylcarnitine Translocase |
| CPT2 | Carnitine Palmitoyltransferase II |
| ARA | Arachidonic Acid |
| EPA | Eicosapentaenoic Acid |
| PEP | Phosphoenolpyruvic acid |
| PFK | Phosphofructokinase |
| DPA | Docosapentaenoic Acid |
| G3P | Glycerol-3-Phosphate |
| LPE 20:4 | Lysophosphatidylethanolamine |
| LPC 18:1 | Lysophosphatidylcholine |
| PC 40:4 | Phosphatidylcholine |
| DPPC | DL-Dipalmitoylphosphatidylcholine |
| NPPC | Natriuretic Peptide C |
| GVBD | Germinal Vesicle Breakdown |
| cAMP | Cyclic Adenosine Monophosphate |
| BAs | Bile Acids |
| FF | Follicular Fluid |
| IVF | In Vitro Fertilization |
| HBP | Hexosamine Biosynthesis Pathway |
| UDP-GlcNAc | Uridine Diphosphate N-Acetylglucosamine |
| GlcNAc-1P | N-Acetyl-α-D-Glucosamine 1-Phosphate |
| GlcNAc | N-Acetyl-D-Glucosamine |
| HAS2 | Hyaluronic Acid Synthase 2 |
| ROS | Reactive Oxygen Species |
| ETC | Electron Transport Chain |
| R5P | Ribose-5-Phosphate |
| R1P | D-Ribose-1-Phosphate |
| RPIA | Ribose-5-Phosphate Isomerase A |
| RPE | Ribulose-5-Phosphate 3-Epimerase |
| G6P | Glucose 6-phosphate |
| G1,6 BP | Glucose-1,6-bisphosphate |
| N-Acetyl-GA | N-Acetyl-Glutamic Acid |
| IMP | Inosine Monophosphate |
| AMP | Adenosine Monophosphate |
| GMP | Guanosine Monophosphate |
| UMP | Uridine Monophosphate |
| DTDP | Deoxythymidine Diphosphate |
| ADP | Adenosine Diphosphate |
References
- Hoshino, Y. Updating the markers for oocyte quality evaluation: Intracellular temperature as a new index. Reprod. Med. Biol. 2018, 17, 434–441. [Google Scholar] [CrossRef] [PubMed]
- van der Reest, J.; Nardini Cecchino, G.; Haigis, M.C.; Kordowitzki, P. Mitochondria: Their relevance during oocyte ageing. Ageing Res. Rev. 2021, 70, 101378. [Google Scholar] [CrossRef] [PubMed]
- Sha, Q.-Q.; Zheng, W.; Wu, Y.-W.; Li, S.; Guo, L.; Zhang, S.; Lin, G.; Ou, X.-H.; Fan, H.-Y. Dynamics and clinical relevance of maternal mRNA clearance during the oocyte-to-embryo transition in humans. Nat. Commun. 2020, 11, 4917. [Google Scholar] [CrossRef] [PubMed]
- Baldini, G.M.; Lot, D.; Malvasi, A.; Laganà, A.S.; Vimercati, A.; Dellino, M.; Cicinelli, E.; Baldini, D.; Trojano, G. Abnormalities of Oocyte Maturation: Mechanisms and Implications. Int. J. Mol. Sci. 2024, 25, 12197. [Google Scholar] [CrossRef]
- Conti, M.; Franciosi, F. Acquisition of oocyte competence to develop as an embryo: Integrated nuclear and cytoplasmic events. Hum. Reprod. Update 2018, 24, 245–266. [Google Scholar] [CrossRef]
- Duarte-da-Fonseca Dias, S.; Palmeira-de-Oliveira, A.; Rolo, J.; Gomes-Ruivo, P.; Hélio Oliani, A.; Palmeira-de-Oliveira, R.; Martinez-de-Oliveira, J.; Pinto-de-Andrade, L. Parameters influencing the maturation of bovine oocyte: A review. Anim. Prod. Sci. 2022, 62, 751–764. [Google Scholar] [CrossRef]
- Hashimoto, S.; Gamage, U.; Inoue, Y.; Iwata, H.; Morimoto, Y. Nicotinamide mononucleotide boosts the development of bovine oocyte by enhancing mitochondrial function and reducing chromosome lagging. Sci. Rep. 2025, 15, 310. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Wen, R.; Yang, N.; Zhang, T.-N.; Liu, C.-F. Roles of protein post-translational modifications in glucose and lipid metabolism: Mechanisms and perspectives. Mol. Med. 2023, 29, 93. [Google Scholar] [CrossRef]
- Francés-Herrero, E.; Lorenzo-Rebenaque, L.; Casto-Rebollo, C.; Vicente, J.S.; Sebastian-Leon, P.; Bueno-Fernandez, C.; Rodríguez-Eguren, A.; Gómez-Álvarez, M.; Faus, A.; Diaz-Gimeno, P.; et al. Oviductal extracellular matrix hydrogels enhance in vitro culture of rabbit embryos and reduce deficiencies during assisted reproductive technologies. Sci. Rep. 2024, 14, 27579. [Google Scholar] [CrossRef]
- Gualtieri, R.; De Gregorio, V.; Candela, A.; Travaglione, A.; Genovese, V.; Barbato, V.; Talevi, R. In Vitro Culture of Mammalian Embryos: Is There Room for Improvement? Cells 2024, 13, 996. [Google Scholar] [CrossRef]
- Xiong, Y.-Y.; Zhu, H.-Y.; Shi, R.-J.; Wu, Y.-F.; Fan, Y.; Jin, L. Regulation of glucose metabolism: Effects on oocyte, preimplantation embryo, assisted reproductive technology and embryonic stem cell. Heliyon 2024, 10, e38551. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Qin, J.; Du, K.; Zhang, L.; Jia, G.; Ye, J.; Liang, Q.; Yang, Q.; Zhou, G. Integrated ultrasensitive metabolomics and single-cell transcriptomics identify crucial regulators of sheep oocyte maturation and early embryo development in vitro. J. Adv. Res. 2024, in press. [CrossRef] [PubMed]
- Huang, J.; Chen, P.; Jia, L.; Li, T.; Yang, X.; Liang, Q.; Zeng, Y.; Liu, J.; Wu, T.; Hu, W.; et al. Multi-Omics Analysis Reveals Translational Landscapes and Regulations in Mouse and Human Oocyte Aging. Adv. Sci. 2023, 10, 2301538. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhu, S.; Shu, W.; Guo, Y.; Guan, Y.; Zeng, J.; Wang, H.; Han, L.; Zhang, J.; Liu, X.; et al. Characterization of Metabolic Patterns in Mouse Oocytes during Meiotic Maturation. Mol. Cell 2020, 80, 525–540.e9. [Google Scholar] [CrossRef]
- Gao, M.; Chen, M.; Chen, Q.; Zhu, S.; Wang, H.; Yang, W.; Wang, X.; Wang, Q.; Gu, L. Integration of parallel metabolomics and transcriptomics reveals metabolic patterns in porcine oocytes during maturation. Front. Endocrinol. 2023, 14, 1131256. [Google Scholar] [CrossRef]
- Zhang, X.; Ge, J.; Wang, Y.; Chen, M.; Guo, X.; Zhu, S.; Wang, H.; Wang, Q. Integrative Omics Reveals the Metabolic Patterns During Oocyte Growth. Mol. Cell. Proteom. 2024, 23, 100862. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, W.; Gao, M.; Li, J.; Wang, Q.; Chen, M.; Gu, L. Combined analysis of temporal metabolomics and transcriptomics reveals the metabolic patterns in goat oocytes during maturation. Theriogenology 2024, 218, 69–78. [Google Scholar] [CrossRef]
- Zhao, J.; Yao, K.; Yu, H.; Zhang, L.; Xu, Y.; Chen, L.; Sun, Z.; Zhu, Y.; Zhang, C.; Qian, Y.; et al. Metabolic remodelling during early mouse embryo development. Nat. Metab. 2021, 3, 1372–1384. [Google Scholar] [CrossRef]
- Graf, A.; Krebs, S.; Zakhartchenko, V.; Schwalb, B.; Blum, H.; Wolf, E. Fine mapping of genome activation in bovine embryos by RNA sequencing. Proc. Natl. Acad. Sci. USA 2014, 111, 4139–4144. [Google Scholar] [CrossRef]
- Liu, T.; Qu, J.; Tian, M.; Yang, R.; Song, X.; Li, R.; Yan, J.; Qiao, J. Lipid Metabolic Process Involved in Oocyte Maturation During Folliculogenesis. Front. Cell Dev. Biol. 2022, 10, 806890. [Google Scholar] [CrossRef]
- Khan, R.; Jiang, X.; Hameed, U.; Shi, Q. Role of Lipid Metabolism and Signaling in Mammalian Oocyte Maturation, Quality, and Acquisition of Competence. Front. Cell Dev. Biol. 2021, 9, 639704. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, C.; Caramujo, M.J. The Various Roles of Fatty Acids. Molecules 2018, 23, 2583. [Google Scholar] [CrossRef] [PubMed]
- Houten, S.M.; Wanders, R.J.A. A general introduction to the biochemistry of mitochondrial fatty acid β-oxidation. J. Inherit. Metab. Dis. 2010, 33, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Koves, T.R.; Ussher, J.R.; Noland, R.C.; Slentz, D.; Mosedale, M.; Ilkayeva, O.; Bain, J.; Stevens, R.; Dyck, J.R.B.; Newgard, C.B.; et al. Mitochondrial Overload and Incomplete Fatty Acid Oxidation Contribute to Skeletal Muscle Insulin Resistance. Cell Metab. 2008, 7, 45–56. [Google Scholar] [CrossRef]
- Vanella, A.; Russo, A.; Acquaviva, R.; Campisi, A.; Di Giacomo, C.; Sorrenti, V.; Barcellona, M.L. L-Propionyl-carnitine as superoxide scavenger, antioxidant, and DNA cleavage protector. Cell Biol. Toxicol. 2000, 16, 99–104. [Google Scholar] [CrossRef]
- Shen, Z.; Ma, Y.; Gao, M.; Gu, L. Temporal metabolomics analysis reveals the metabolic patterns in goat cumulus cells during oocyte maturation. Gene 2024, 928, 148772. [Google Scholar] [CrossRef]
- Pawlak, P.; Malyszka, N.; Szczerbal, I.; Kolodziejski, P. Fatty acid induced lipolysis influences embryo development, gene expression and lipid droplet formation in the porcine cumulus cells. Biol. Reprod. 2020, 103, 36–48. [Google Scholar] [CrossRef]
- Gaspar, M.L.; Hofbauer, H.F.; Kohlwein, S.D.; Henry, S.A. Coordination of storage lipid synthesis and membrane biogenesis: Evidence for cross-talk between triacylglycerol metabolism and phosphatidylinositol synthesis. J. Biol. Chem. 2011, 286, 1696–1708. [Google Scholar] [CrossRef]
- Khajeh, M.; Rahbarghazi, R.; Nouri, M.; Darabi, M. Potential role of polyunsaturated fatty acids, with particular regard to the signaling pathways of arachidonic acid and its derivatives in the process of maturation of the oocytes: Contemporary review. Biomed. Pharmacother. 2017, 94, 458–467. [Google Scholar] [CrossRef]
- Soares, A.C.S.; Lodde, V.; Barros, R.G.; Price, C.A.; Luciano, A.M.; Buratini, J. Steroid hormones interact with natriuretic peptide C to delay nuclear maturation, to maintain oocyte–cumulus communication and to improve the quality of in vitro-produced embryos in cattle. Reprod. Fertil. Dev. 2017, 29, 2217–2224. [Google Scholar] [CrossRef]
- Vítek, L.; Haluzík, M. The role of bile acids in metabolic regulation. J. Endocrinol. 2016, 228, R85–R96. [Google Scholar] [CrossRef] [PubMed]
- Ticho, A.L.; Malhotra, P.; Dudeja, P.K.; Gill, R.K.; Alrefai, W.A. Intestinal Absorption of Bile Acids in Health and Disease. Compr. Physiol. 2019, 10, 21–56. [Google Scholar] [CrossRef] [PubMed]
- Nagy, R.A.; Hollema, H.; Andrei, D.; Jurdzinski, A.; Kuipers, F.; Hoek, A.; Tietge, U.J.F. The Origin of Follicular Bile Acids in the Human Ovary. Am. J. Pathol. 2019, 189, 2036–2045. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Duron, H.E.; Chaudhuri, D. Beyond the TCA cycle: New insights into mitochondrial calcium regulation of oxidative phosphorylation. Biochem. Soc. Trans. 2023, 51, 1661–1673. [Google Scholar] [CrossRef]
- Kierans, S.J.; Taylor, C.T. Glycolysis: A multifaceted metabolic pathway and signalling hub. J. Biol. Chem. 2024, 300, 107906. [Google Scholar] [CrossRef]
- Qiao, J.; Yu, Z.; Zhou, H.; Wang, W.; Wu, H.; Ye, J. The Pentose Phosphate Pathway: From Mechanisms to Implications for Gastrointestinal Cancers. Int. J. Mol. Sci. 2025, 26, 610. [Google Scholar] [CrossRef]
- Arnold, P.K.; Finley, L.W.S. Regulation and function of the mammalian tricarboxylic acid cycle. J. Biol. Chem. 2023, 299, 102838. [Google Scholar] [CrossRef]
- Stincone, A.; Prigione, A.; Cramer, T.; Wamelink, M.M.; Campbell, K.; Cheung, E.; Olin-Sandoval, V.; Grüning, N.M.; Krüger, A.; Tauqeer Alam, M.; et al. The return of metabolism: Biochemistry and physiology of the pentose phosphate pathway. Biol. Rev. Camb. Philos. Soc. 2015, 90, 927–963. [Google Scholar] [CrossRef]
- Neumann, N.; Friz, S.; Forchhammer, K. Glucose-1, 6-bisphosphate, a key metabolic regulator, is synthesized by a distinct family of α-phosphohexomutases widely distributed in prokaryotes. mBio 2022, 13, e01469-22. [Google Scholar] [CrossRef]
- Varizhuk, I.V.; Oslovsky, V.E.; Solyev, P.N.; Drenichev, M.S.; Mikhailov, S.N. Synthesis of α-d-Ribose 1-Phosphate and 2-Deoxy-α-d-Ribose 1-Phosphate Via Enzymatic Phosphorolysis of 7-Methylguanosine and 7-Methyldeoxyguanosine. Curr. Protoc. 2022, 2, e347. [Google Scholar] [CrossRef]
- Gao, N.; Shang, J.; Huynh, D.; Manthati, V.L.; Arias, C.; Harding, H.P.; Kaufman, R.J.; Mohr, I.; Ron, D.; Falck, J.R.; et al. Mannose-6-phosphate regulates destruction of lipid-linked oligosaccharides. Mol. Biol. Cell 2011, 22, 2994–3009. [Google Scholar] [CrossRef] [PubMed]
- Lay, K.M.; Ashizawa, K.; Nakada, T.; Tatemoto, H. N-glycosylation of zona glycoproteins during meiotic maturation is involved in sperm-zona pellucida interactions of porcine oocytes. Theriogenology 2011, 75, 1146–1152. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, Y.; Uzuhashi, R.; Iwata, H.; Monji, Y.; Kuwayama, T. Changes in the sperm-zona pellucida binding properties during porcine oocyte maturation. J. Mamm. Ova Res. 2010, 27, 130–135. [Google Scholar] [CrossRef]
- Sutton-McDowall, M.L.; Gilchrist, R.B.; Thompson, J.G. The pivotal role of glucose metabolism in determining oocyte developmental competence. Reproduction 2010, 139, 685. [Google Scholar] [CrossRef]
- Chen, M.; Yang, W.; Guo, Y.; Hou, X.; Zhu, S.; Sun, H.; Guo, X.; Chen, M.; Wang, Q. Multi-omics reveal the metabolic patterns in mouse cumulus cells during oocyte maturation. J. Ovarian Res. 2023, 16, 156. [Google Scholar] [CrossRef]
- Yokoo, M.; Kimura, N.; Abe, H.; Sato, E. Influence of hyaluronan accumulation during cumulus expansion on in vitro porcine oocyte maturation. Zygote 2008, 16, 309–314. [Google Scholar] [CrossRef]
- Bresnahan, D.R.; Catandi, G.D.; Peters, S.O.; Maclellan, L.J.; Broeckling, C.D.; Carnevale, E.M. Maturation and culture affect the metabolomic profile of oocytes and follicular cells in young and old mares. Front. Cell Dev. Biol. 2024, 11, 1280998. [Google Scholar] [CrossRef]
- Lieu, E.L.; Nguyen, T.; Rhyne, S.; Kim, J. Amino acids in cancer. Exp. Mol. Med. 2020, 52, 15–30. [Google Scholar] [CrossRef]
- Egbujor, M.C.; Olaniyan, O.T.; Emeruwa, C.N.; Saha, S.; Saso, L.; Tucci, P. An insight into role of amino acids as antioxidants via NRF2 activation. Amino Acids 2024, 56, 23. [Google Scholar] [CrossRef]
- Walker, M.C.; van der Donk, W.A. The many roles of glutamate in metabolism. J. Ind. Microbiol. Biotechnol. 2016, 43, 419–430. [Google Scholar] [CrossRef]
- Mullen, N.J.; Singh, P.K. Nucleotide metabolism: A pan-cancer metabolic dependency. Nat. Rev. Cancer 2023, 23, 275–294. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Wang, X.; Li, J.; Sun, H.; Zhu, S.; Ge, J.; Han, L.; Shen, B.; Wang, Q. KAS-seq profiling captures transcription dynamics during oocyte maturation. J. Ovarian Res. 2024, 17, 23. [Google Scholar] [CrossRef] [PubMed]
- Obaseki, E.; Adebayo, D.; Bandyopadhyay, S.; Hariri, H. Lipid droplets and fatty acid-induced lipotoxicity: In a nutshell. FEBS Lett. 2024, 598, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.; Pereira-Dutra, F.S.; Reis, P.A.; Cunha-Fernandes, T.; Yoshinaga, M.Y.; Souza-Moreira, L.; Souza, E.K.; Barreto, E.A.; Silva, T.P.; Espinheira-Silva, H.; et al. Prevention of lipid droplet accumulation by DGAT1 inhibition ameliorates sepsis-induced liver injury and inflammation. JHEP Rep. 2024, 6, 100984. [Google Scholar] [CrossRef]
- Ducharme, N.A.; Bickel, P.E. Minireview: Lipid Droplets in Lipogenesis and Lipolysis. Endocrinology 2008, 149, 942–949. [Google Scholar] [CrossRef]
- Shi, M.; Sirard, M.A. Metabolism of fatty acids in follicular cells, oocytes, and blastocysts. Reprod. Fertil. 2022, 3, R96–R108. [Google Scholar] [CrossRef]
- Ishizuka, Y.; Nakao, S.; Kamisako, T.; Yamaga, K.; Nakagata, N.; Ishizaki, H.; Takeo, T. In vivo fertilization improved the cryotolerance and developmental ability of vitrified-warmed rat fertilized oocytes. Sci. Rep. 2024, 14, 24198. [Google Scholar] [CrossRef]
- Pei, Z.; Deng, K.; Xu, C.; Zhang, S. The molecular regulatory mechanisms of meiotic arrest and resumption in Oocyte development and maturation. Reprod. Biol. Endocrinol. 2023, 21, 90. [Google Scholar] [CrossRef]
- Li, R.; Albertini, D.F. The road to maturation: Somatic cell interaction and self-organization of the mammalian oocyte. Nat. Rev. Mol. Cell Biol. 2013, 14, 141–152. [Google Scholar] [CrossRef]
- Tadros, W.; Lipshitz, H.D. The maternal-to-zygotic transition: A play in two acts. Development 2009, 136, 3033–3042. [Google Scholar] [CrossRef]
- Clarke, H.J. Post-transcriptional control of gene expression during mouse oogenesis. Results Probl. Cell Differ. 2012, 55, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Conti, M.; Kunitomi, C. A genome-wide perspective of the maternal mRNA translation program during oocyte development. Semin. Cell Dev. Biol. 2024, 154, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.K.; Han, S.J. Post-transcriptional and post-translational regulation during mouse oocyte maturation. BMB Rep. 2011, 44, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.S.; Roundtree, I.A.; He, C. Post-transcriptional gene regulation by mRNA modifications. Nat. Rev. Mol. Cell Biol. 2017, 18, 31–42. [Google Scholar] [CrossRef]
- Anderson, P.; Kedersha, N. RNA granules: Post-transcriptional and epigenetic modulators of gene expression. Nat. Rev. Mol. Cell Biol. 2009, 10, 430–436. [Google Scholar] [CrossRef]
- Sugimura, S.; Matoba, S.; Hashiyada, Y.; Aikawa, Y.; Ohtake, M.; Matsuda, H.; Kobayashi, S.; Konishi, K.; Imai, K. Oxidative phosphorylation-linked respiration in individual bovine oocytes. J. Reprod. Dev. 2012, 58, 636–641. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive Oxygen Species Signaling and Oxidative Stress: Transcriptional Regulation and Evolution. Antioxidants 2024, 13, 312. [Google Scholar] [CrossRef]
- Khan, A.U.H.; Allende-Vega, N.; Gitenay, D.; Garaude, J.; Vo, D.-N.; Belkhala, S.; Gerbal-Chaloin, S.; Gondeau, C.; Daujat-Chavanieu, M.; Delettre, C.; et al. Mitochondrial Complex I activity signals antioxidant response through ERK5. Sci. Rep. 2018, 8, 7420. [Google Scholar] [CrossRef]
- Campbell, K.; Swann, K. Ca2+ oscillations stimulate an ATP increase during fertilization of mouse eggs. Dev. Biol. 2006, 298, 225–233. [Google Scholar] [CrossRef]
- Dalton, C.M.; Szabadkai, G.; Carroll, J. Measurement of ATP in single oocytes: Impact of maturation and cumulus cells on levels and consumption. J. Cell. Physiol. 2014, 229, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Brazill, J.M.; Liu, S.; Bello, C.; Zhu, Y.; Morimoto, M.; Cascio, L.; Pauly, R.; Diaz-Perez, Z.; Malicdan, M.C.V.; et al. Spermine synthase deficiency causes lysosomal dysfunction and oxidative stress in models of Snyder-Robinson syndrome. Nat. Commun. 2017, 8, 1257. [Google Scholar] [CrossRef] [PubMed]
- Satarker, S.; Wilson, J.; Kolathur, K.K.; Mudgal, J.; Lewis, S.A.; Arora, D.; Nampoothiri, M. Spermidine as an epigenetic regulator of autophagy in neurodegenerative disorders. Eur. J. Pharmacol. 2024, 979, 176823. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bai, J.; Cui, Z.; Li, Y.; Gao, Q.; Miao, Y.; Xiong, B. Polyamine metabolite spermidine rejuvenates oocyte quality by enhancing mitophagy during female reproductive aging. Nat. Aging 2023, 3, 1372–1386. [Google Scholar] [CrossRef]
- Tang, H.; Pang, S. Proline Catabolism Modulates Innate Immunity in Caenorhabditis elegans. Cell Rep. 2016, 17, 2837–2844. [Google Scholar] [CrossRef]
- Kowsar, R.; Iranshahi, V.N.; Sadeghi, N.; Riasi, A.; Miyamoto, A. Urea influences amino acid turnover in bovine cumulus-oocyte complexes, cumulus cells and denuded oocytes, and affects in vitro fertilization outcome. Sci. Rep. 2018, 8, 12191. [Google Scholar] [CrossRef]
- Santos, R.R.; Schoevers, E.J.; Roelen, B.A.J. Usefulness of bovine and porcine IVM/IVF models for reproductive toxicology. Reprod. Biol. Endocrinol. 2014, 12, 117. [Google Scholar] [CrossRef]
- Luciano, A.M.; Sirard, M.-A. Successful in vitro maturation of oocytes: A matter of follicular differentiation. Biol. Reprod. 2017, 98, 162–169. [Google Scholar] [CrossRef]
- Martinez, C.A.; Rizos, D.; Rodriguez-Martinez, H.; Funahashi, H. Oocyte-cumulus cells crosstalk: New comparative insights. Theriogenology 2023, 205, 87–93. [Google Scholar] [CrossRef]
- Zhou, C.J.; Wu, S.N.; Shen, J.P.; Wang, D.H.; Kong, X.W.; Lu, A.; Li, Y.J.; Zhou, H.X.; Zhao, Y.F.; Liang, C.G. The beneficial effects of cumulus cells and oocyte-cumulus cell gap junctions depends on oocyte maturation and fertilization methods in mice. PeerJ 2016, 4, e1761. [Google Scholar] [CrossRef]
- Yu, H.; Shi, Y.; Wu, X.; Hu, B.; Jin, H.; Kassim, Y.; Iqbal, T.; Kandil, O.M.; Ismail, E.A.; Wang, H.; et al. TEAD3 and TEAD4 play overlapping role in bovine preimplantation development. Reproduction 2025, 169, e240307. [Google Scholar] [CrossRef] [PubMed]
- Want, E.J.; O’Maille, G.; Smith, C.A.; Brandon, T.R.; Uritboonthai, W.; Qin, C.; Trauger, S.A.; Siuzdak, G. Solvent-dependent metabolite distribution, clustering, and protein extraction for serum profiling with mass spectrometry. Anal. Chem. 2006, 78, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Barri, T.; Dragsted, L.O. UPLC-ESI-QTOF/MS and multivariate data analysis for blood plasma and serum metabolomics: Effect of experimental artefacts and anticoagulant. Anal. Chim. Acta 2013, 768, 118–128. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kassim, Y.; Sheng, H.; Xu, G.; Jin, H.; Iqbal, T.; Elashry, M.; Zhang, K. Integrated Multi-Omics Analysis Reveals Key Regulators of Bovine Oocyte Maturation. Int. J. Mol. Sci. 2025, 26, 3973. https://doi.org/10.3390/ijms26093973
Kassim Y, Sheng H, Xu G, Jin H, Iqbal T, Elashry M, Zhang K. Integrated Multi-Omics Analysis Reveals Key Regulators of Bovine Oocyte Maturation. International Journal of Molecular Sciences. 2025; 26(9):3973. https://doi.org/10.3390/ijms26093973
Chicago/Turabian StyleKassim, Yassin, Hao Sheng, Guangjun Xu, Hao Jin, Tariq Iqbal, Mostafa Elashry, and Kun Zhang. 2025. "Integrated Multi-Omics Analysis Reveals Key Regulators of Bovine Oocyte Maturation" International Journal of Molecular Sciences 26, no. 9: 3973. https://doi.org/10.3390/ijms26093973
APA StyleKassim, Y., Sheng, H., Xu, G., Jin, H., Iqbal, T., Elashry, M., & Zhang, K. (2025). Integrated Multi-Omics Analysis Reveals Key Regulators of Bovine Oocyte Maturation. International Journal of Molecular Sciences, 26(9), 3973. https://doi.org/10.3390/ijms26093973







