Abstract
Drought stress substantially impacts the development and viability of Populus spp., which are essential for forestry and bioenergy production. This review summarizes and describes the functions of phytohormones, such as abscisic acid, auxins, and ethylene, in modulating physiological and molecular responses to water scarcity. Drought-induced ABA-mediated stomatal closure and root extension are essential adaptation processes. Furthermore, auxin–ABA (abscisic acid) interactions augment root flexibility, whereas ethylene regulates antioxidant defenses to alleviate oxidative stress. The advantageous function of endophytic bacteria, specifically plant growth-promoting rhizobacteria (PGPR), can augment drought resistance in spruce trees by enhancing nutrient absorption and stimulating root development. Structural adaptations encompass modifications in root architecture, including enhanced root length and density, which augment water uptake efficiency. Similarly, Arbuscular Mycorrhizal Fungi (AMF) significantly enhance stress resilience in forest trees. AMF establishes symbiotic relationships with plant roots, improving water and nutrient uptake, particularly phosphorus, during drought conditions. Furthermore, morphological alterations at the root–soil interface enhance interaction with soil moisture reserves. This review examines the complex mechanisms by which these hormones influence plant responses to water shortage, aiming to offer insights into prospective techniques for improving drought tolerance in common tree species and highlights the importance of hormone control in influencing the adaptive responses of prominent trees to drought stress, providing significant implications for research and practical applications in sustainable forestry and agriculture. These findings lay the groundwork for improving drought tolerance in Populus spp. by biotechnological means and by illuminating the complex hormonal networks that confer drought resistance.
1. Introduction
Everywhere across the globe, drought is a significant abiotic stressor that has a detrimental impact on the development, productivity, and survival of plants [1]. The reduction in stomatal transpiration, the enhancement of the roots’ capacity to absorb water, the preservation of cellular water content, and the activation of the antioxidant system in order to maintain redox homeostasis are the mechanisms by which plants compensate for the effects of drought stress [2]. Cold, drought, extreme temperatures, salinity, and pathogen invasion increase reactive oxygen species (ROS), causing oxidative stress. Oxidative stresses damage proteins, lipids, and DNA, limiting respiration and photosynthesis [3]. Severe drought stress, the primary abiotic factor affecting Populus growth, can significantly impede its growth, development, and reproductive capacity [4,5]. The Populus forest covers about 8 million hectares (ha), which is more than 14% of China’s planted forest area, and has a total carbon store of around 261 metric tons (Tg), according to the country’s ninth national forest inventory [6]. In the genus Populus, approximately 30 different types of trees are collectively referred to as “poplars”. The trees are usually categorized into six groups: Populus, Tacamahaca, Leucoides, Turanga, Abaso, and Aigeiros [7]. The genus represents one of the most complex groups in plant taxonomy, partly because of the frequent occurrence of natural hybridization [8]. Populus species are most diverse in the US and China, where the former classifies 29 species and 27 hybrids [9]. Consequently, it is imperative to identify innovative drought-resistant genes, elucidate their regulatory processes, and develop new drought-resistant types of Populus spp. [10]. Gibberellin, auxin, cytokinin, ethylene, strigolactone, jasmonic acid, salicylic acid, and brassinosteroid are the eight known phytohormones that trigger a chain reaction of responses to stimuli in the environment [11]. Additionally, antioxidant enzyme overexpression during drought stress can reduce reactive oxygen species (ROS)-induced oxidative damage [12]. An essential intercellular signal molecule, hydrogen peroxide (H2O2), is a reactive oxygen species (ROS) generated by plant cells metabolic processes. H2O2 regulates a number of downstream gene expression changes through its interactions with a number of plant hormones and signaling pathways, including salicylic acid (SA), jasmonic acid (JA), auxin, ethylene (ETH), and abscisic acid (ABA) [13]. With their role as chemical messengers synthesized and transported throughout the plant, these hormones regulate a wide range of physiological and developmental processes [14]. Of particular note, the abundance of JA and SA hormones in higher plant species has focused research on plant response and defense mechanisms [15]. Phytohormones and reactive oxygen species (ROS) are intricately interdependent, with the former regulating the latter’s synthesis [16].
To better understand how various phytohormones, such as cytokinin, auxins, and abscisic acid, regulate the biochemical and physiological responses of poplar trees to drought-induced stress, this review will examine the literature on the topic. Molecular methods of interaction with stress-responsive genes and signaling cascades. The effects of soil moisture and microbial interactions on phytohormonal control are likewise unclear. To address these gaps, we analyze the functions of principal phytohormones in regulating biochemical and physiological responses of poplar trees during drought stress. We examined the molecular processes by which phytohormones modulate stress-responsive gene expression. We also examined the interplay of phytohormones, soil moisture, and microbial populations in improving drought resilience and obtained insights into the intricate hormonal signaling networks, establishing a basis for biotechnological approaches to enhance the drought resilience of Populus spp.
2. Harmful Effects of Drought Stress on Plants
Drought stress has a profound impact on agricultural productivity, particularly by reducing crop yield and compromising grain quality [17,18]. It is widely regarded as one of the most limiting factors in modern agriculture [18]. Drought conditions during key developmental stages such as flowering and grain filling can drastically reduce grain size and number, leading to significant yield losses in staple crops like wheat and maize [19,20]. Moreover, insufficient soil moisture affects nutrient uptake, further stunting plant growth and reducing overall crop performance. The combined effect of climate change and frequent drought events has already led to declining agricultural productivity, which is expected to worsen in the coming years [21,22,23]. This intensifies concerns about food insecurity, particularly in vulnerable regions affected by erratic rainfall patterns and extended dry seasons [24].
In addition to yield losses, drought also affects the nutritional and health value of crops. Water stress induces a shift in metabolic pathways and triggers complex defense mechanisms, altering the biosynthesis of key metabolites and nutritional compounds [25]. As a result, the quality of produce—such as protein content, oil composition, and micronutrient levels—may be negatively affected, further diminishing the value of agricultural outputs.
At the physiological level, drought leads to a reduction in soil water content (SWC), which in turn lowers hydraulic conductivity from the roots to the leaves. Plants respond by closing their stomata to conserve water, especially under conditions of high vapor pressure deficit (VPD). While this response limits water loss, it also restricts carbon dioxide intake, resulting in reduced CO2 fixation and photosynthesis [26]. Prolonged stomatal closure impairs carbohydrate production and causes a carbon imbalance, thereby affecting energy availability for growth and development.
Water deficit also causes osmotic stress due to increased solute concentration in cells, reducing turgor pressure and impairing cell expansion and organ development. The stalling of the photosynthetic electron transport chain results in the production of ROS which affects various macromolecules [20]. Consequently, drought stress often leads to the overproduction of reactive oxygen species (ROS), which damage proteins, lipids, and cellular membranes. Xylem cavitation and embolism become more likely under low soil water potential, which may result in partial or complete hydraulic failure and even plant death [27,28].
Reactive oxygen species (ROS) are generated through several metabolic pathways located in different cellular compartments. In the chloroplasts, ROS, such as singlet oxygen and superoxide, are produced during photosynthesis, particularly under high light intensity or drought conditions that limit CO2 availability. In the mitochondria, the electron transport chain leaks electrons during respiration, forming superoxide radicals, especially under stress conditions that disrupt ATP synthesis. In the peroxisomes, photorespiration leads to hydrogen peroxide (H2O2) accumulation, while in the apoplast, ROS are produced by NADPH oxidases during pathogen attack or abiotic stress [29]. To mitigate ROS-induced damage, plants activate a range of detoxification mechanisms. These include enzymatic antioxidants like superoxide dismutase (SOD), ascorbate peroxidase (APX), catalase (CAT), glutathione peroxidase (GPX), and peroxiredoxin (PRX), as well as non-enzymatic antioxidants like ascorbate and glutathione (GSH) [30]. Chloroplasts act as environmental sensors; their photosynthetic apparatus is known to be affected by the number of biotic (bacteria, viruses, etc.) and abiotic (heat, strong light, salt, hypoxia, etc.) stresses. Apart from assaying ROS production, another indicator of the damage to photosystems is decreased chlorophyll fluorescence [31]. The levels of antioxidant enzyme activity and chlorophyll fluorescence are impacted by drought [32].
These defense systems, although critical, are often overwhelmed under severe drought conditions, leading to oxidative stress and cellular injury. An overview of these harmful effects is illustrated in Figure 1.
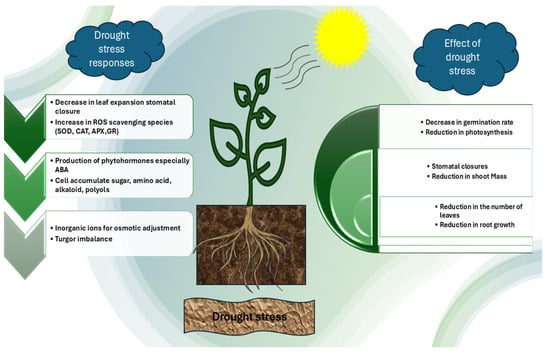
Figure 1.
In response to water scarcity, plants close their stomata, which reduces transpiration and photosynthesis. As a result, plants may experience stunted growth, withering, or even death in extreme circumstances due to their inability to absorb enough water.
3. Drought Stress and Hormonal Influences on Root–Soil Interface Adaptations
Hormones trigger these changes. Stomatal closure and root development depend on abscisic acid (ABA). Drought resilience depends on hormone balance, including cytokinins and auxins, which affect root structure [33]. The PtrABR1 gene in Populus trichocarpa is significantly upregulated by drought stress, facilitating lateral root development and improving drought resilience. This indicates that hormonal mechanisms, including abscisic acid (ABA) and other hormones, are essential in governing root responses during water deficiency circumstances [34]. Drought-tolerant Populus species have an increased fine root density and root architecture to facilitate water absorption. Water retention under low-moisture conditions is helped by specialized tissues such as thicker rhizodermis [35]. Fine roots are primarily responsible for completing nitrogen (N) and carbon (C) cycles, as well as absorbing large amounts of water and nutrients, because of their short lifespan and quick turnover rate [36,37,38]. This ensures that there will be enough water and nutrients for plants to develop and undergo photosynthesis [38]. A root’s delicate anatomical structure is a direct reflection of its growth and development, demonstrating the intimate relationship between anatomy and physiological function [39]. Environmental factors can affect the structure of fine roots [37]. In addition to directly influencing plant roots, temperature can indirectly affect fine roots by altering the soil’s nutritional conditions [40]. Spreading roots that are deeper assists plants absorb more water and nutrients in colder climates. Also, high temperatures accelerate fine root development [41,42]. The response of fine roots to drought is influenced by the intricate interplay between anatomical structure and function [43]. Without water, hydraulic conductivity, root length, rhizosphere wettability, soil structure, and porosity vary. In the rhizosphere, plants deposit organic substances. This influences chemical, biological, and physical rhizospheres. Mucilage, secretions, root exudates, sloughed-off cells, dead root cells, and root enzymes exhibit distinct properties when moisture levels are low in rhizodeposits [44]. Root exudates contain mostly proteins and mucilage, with low-molecular-weight carbohydrates, amino acids, flavonoids, coumarin, and carboxylates. Multiple-purpose root exudates alter stress tolerance [45]. Due to limited carbon transfer from plants to bacteria and non-mycorrhizal fungi in the rhizosphere, droughts impede plant–microorganism interactions [46,47,48]. Studies indicate that grapevines exhibiting elevated levels of suberin in their roots demonstrate enhanced resilience to drought conditions [49]. However, a root may become more susceptible to damage if suberin accumulates in its fine roots. Research has also shown that lignin deposition can enhance drought tolerance by forming an impermeable water-resistant barrier around xylem tissue [50]. The rhizosphere microbiome is the bacterial community inside a few millimeters of plant roots [51]. Comprehending the molecular mechanisms behind these changes is crucial for enhancing drought tolerance in Populus species. Research indicates that altering hormonal pathways may improve root resistance and overall plant performance during drought stress [52].
Drought stress regulates root architecture, water uptake, and stress resilience through complex hormonal signaling at the root–soil interface. Auxins, cytokinins, and ethylene regulate root elongation and branching, while ABA (abscisic acid) stimulates deep root growth and stomatal closure. Figure 2 shows how hormones improve water uptake and drought adaptation.
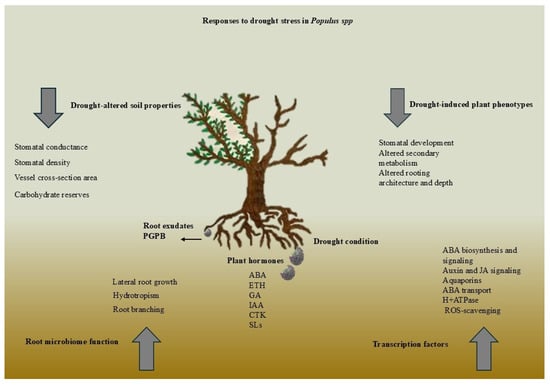
Figure 2.
Hormonal networks significantly influence the adaptations of plants at the root–soil interface under drought conditions by modulating root architecture, regulating water uptake mechanisms, and integrating complex signaling pathways that enhance resilience against water stress.
4. Role of Endophytes in Drought Tolerance
Piriformospora indica colonization primarily transpires in the cortex and root epidermis of many host plants, including poplar, rice, barley, tobacco, Arabidopsis, and maize [53]. Plants experiencing abiotic stresses, including salinity, hydric stress, drought, low temperatures, and heavy metal toxicity [54] derive advantages from the support of P. indica [55]. Plants may provide microbes with 15–20% of their photosynthetic output in return for beneficial services; however, this is generally surpassed by the advantages conferred by bacteria [56]. The fungus is probably the best-recognized endophyte [57]. Plants that colonize Piriformospora indica symbiotically grow more quickly and are less affected by biotic stress [58]. Trichoderma spp. has been widely used in agricultural applications due to its well-known biological control mechanism [59]. Root rot disease and other prevalent plant diseases can be effectively treated by Trichoderma spp., according to recent research [60], via which the root architecture is well controlled and the length is increased [61]. Plant growth is influenced by a multitude of factors, including temperature, light intensity, nutrient availability, and microbial ecology. The roots release a great deal of photosynthetic byproducts, which enrich the soil in the area immediately around the roots, creating a nutrient-rich environment known as the rhizosphere [62]. Furthermore, it was identified that T. virens and T. atroviride produce compounds associated with auxin and indole acetic acid (IAA). IAA is a plant hormone of the auxin class [63].
Recent research has revealed multiple strains of drought-resistant endophytic fungus from various plant species, as illustrated in Figure 3.
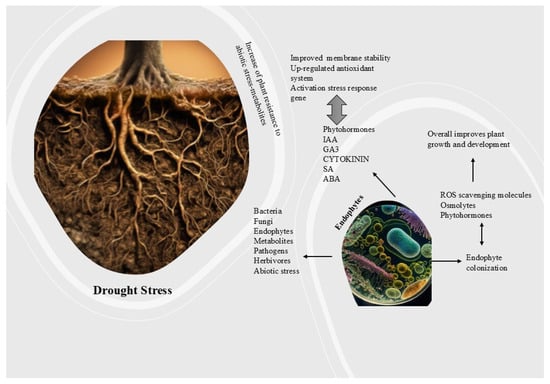
Figure 3.
Drought-resistant endophytes offer a promising approach to improving plant resistance against climate-related water stress. These microorganisms can substantially aid sustainable agricultural practices and forest management strategies by enhancing nutrient absorption, facilitating advantageous hormonal alterations, and improving physiological responses to drought, thereby mitigating its effects on plant health and productivity.
5. Phytohormone Regulation of Root Development Under Drought
Plants produce a diverse array of phytohormones, such as auxin, cytokinin (CK), gibberellic acid (GA), ethylene, salicylic acid (SA), and jasmonic acid (JA), to facilitate plant development. These phytohormones facilitate a diverse array of dynamic yet precisely calibrated molecular responses throughout the life cycle of a plant [64]. Some of the drought-related activities that phytohormones including ethylene, salicylic acid, jasmonic acid, and abscisic acid regulate include root architectural adjustments and root cell osmotic potential preservation. Root development and growth depend on phytohormonal regulation and signaling in both ideal and poor conditions. When plants experience drought stress, they change the structure and function of their roots, and ABA (abscisic acid) is a key component in this process [65,66]. A lot of studies have focused on ABA (abscisic acid), a well-known plant hormone that suppresses development in response to stress, as a potential way to increase drought tolerance in plants [67]. Many methods have been used to isolate phytohormones to learn more about their effects. Chromatography is one of the two most used techniques for separating plant growth hormones [68,69]. Calcium enhances seedling growth with ABA (abscisic acid), IAA, and MeJA, but inhibits growth with GA3 or SA [70]. Increased respiration by the shoots is another mechanism by which drought stress keeps metabolic activity constant. Then, the glucose levels in the citrus plants’ storage organs drop [27]. Additionally, to counteract these adverse conditions, plant growth regulators are sprayed externally [71]. See Table 1 for a summary of how phytohormones improve plant growth, development, and productivity while simultaneously increasing their drought resistance.

Table 1.
Phytohormones function to protect plants from drought stress.
6. Role of Hormones
6.1. Auxin
Auxin is crucial in drought conditions as it facilitates the formation of a robust root system. Indole-acetic acid (IAA), a naturally occurring auxin, is biosynthesized in plants in a tryptophan-dependent yet independent manner [81]. PeFUS3 regulated lateral root growth during drought stress through auxin signaling. Furthermore, PeFUS3 directly enhanced the expression of PePYL3, and poplar lines with overexpressed PePYL3 demonstrated markedly improved drought resistance [82]. PtHB180-OE plants exhibited pleiotropic phenotypes, characterized by enhanced plant height and reduced leaf area due to auxin modulation. PtHB180 may serve as a viable candidate gene for enhancing drought resistance through a genetic transformation in poplar [83]. The IAA17.1/HSFA5a module collectively regulates flavonol production and controls ROS buildup, consequently altering the root system of poplar to respond to salinity stress [84]. Auxin’s negative control of DRO1 during drought, which in turn changes the root growth symmetry [85]. Contrarily, research into auxin’s role in drought stress has made use of TLD1/OsGH3.13, the gene encoding indole-3-acetic acid (IAA)–amido synthase; this, in turn, enhances plant resistance to drought stress by increasing the expression of genes involved in late embryogenesis abundant (LEA). The bulk of the Aux/IAA genes found in rice were also found to be expressed when the plant was under drought stress. The research found that potatoes and poplar trees with overexpressed YUC6 genes have improved drought tolerance and auxin overproduction characteristics [86]. Moreover, under both normal and dry conditions, the interaction between ABA (abscisic acid) and auxin signaling is crucial for regulating root growth and development. The phytohormone abscisic acid (ABA), referred to as the “stress hormone”, alters several morphological, physiological, biochemical, and molecular processes in plant root tissues to regulate a poplar’s drought resistance [87].
6.2. Cytokinin
Cytokinins are crucial for the maturation of plant regulatory mechanisms and adaptations to drought stress [88,89]. These hormones play a central role in cell division (cytokinesis) in both roots and shoots. Under drought stress, cytokinins negatively regulate root system acidosis by modifying root structure and metabolism [90]. In Populus species, drought stress has been shown to reduce endogenous cytokinin levels, contributing to increased root-to-shoot ratio, which is a typical adaptive response to water limitation [91]. Moreover, cytokinin signaling interacts with other hormonal pathways to influence root architecture in Populus, helping in the formation of deeper and more efficient root systems for water uptake. Transgenic studies in other species (e.g., tobacco and rice) that overexpress cytokinin oxidase (CKX) exhibit enhanced root elongation and lateral root formation, a trait also observed in drought-resistant Populus genotypes [92,93]. In Populus tremula × alba, drought has been found to downregulate genes related to cytokinin biosynthesis and signaling, correlating with observed reductions in shoot growth and delayed leaf senescence [10]. This indicates a key regulatory role of cytokinin in drought adaptation of Populus.
6.3. Gibberellinses
Gibberellins (GAs) is the name given to the tetracyclic diterpenoids of carboxylic acids. The primary roles of GAs in plants are as growth regulators and as defense mechanisms against abiotic stressors such as drought. GAs maintain their functions in plants throughout their entire lifespan. In both the immature and mature stages of plant development, gibberellins are utilized to enhance tissue growth by increasing the length of cells and the rate of cell division. Furthermore, they enhance the reproductive and vegetative stages of plant life [94]. Since the recognition and verification of several genes and genetic variations involved in crop drought responses, valuable resources have been made available for the breeding of drought-resistant cultivars using both conventional and cutting-edge gene editing and transgenic technologies [95]. PtoMYB142 regulates gibberellin catabolism in reaction to drought stress by directly connecting to the promoter of PtoGA2ox4, a GA2-oxidase gene activated during drought conditions. The CRISPR/Cas9-mediated deletion of PtoMYB142 diminished drought resistance [96]. Two modules within the photoperiod pathway explain how poplar (Populus spp.) buds go into dormancy: GIGANTEA-like genes (GIs) and the circadian oscillator LATE ELONGATED HYPOCOTYL 2 (LHY2) both control the main target for winter dormancy induction, FLOWERING LOCUS T2 (FT2). But under short-day (SD) conditions, changing LHY2 and GIs does not stop growth and bud development entirely; therefore, other regulatory modules must be involved. Poplar has PtoHY5a, which is an ortholog of the photomorphogenesis regulatory factor ELONGATED HYPOCOTYL 5 (HY5) has the dual effect of boosting PtoFT2 expression directly and suppressing LHY2’s circadian oscillation indirectly. So, PtoHY5a prevents SD-induced bud setting and halts development. Therefore, PtoHY5a knockdown promotes dormancy induction. Furthermore, PtoHY5a controls gibberellic acid (GA) concentrations in apical buds, which inhibits poplar bud-break. Also, following phytochrome PHYB2, PtoHY5a regulates photoperiodic growth. So, PtoHY5a regulates the seasonal growth of poplars by controlling GA levels to regulate bud-break and the PtoPHYB2-PtoHY5a-PtoFT2 module to establish the start of winter dormancy [97].
6.4. Abscisic Acid
Abscisic acid is an essential phytohormone for signaling during drought stress [98]. Our current understanding of the role of expansins in woody plants is limited. The Populus and expansin gene family, which is 36 members strong and is subdivided into 4 subfamilies, was the focus of a recent study. They also investigated Populus tremula L. expansin genes (PtEXs), along with their molecular composition. Additionally, they investigated how phytohormones and abiotic stresses affected the expression of these genes [99]. Additional research indicates that when PdNF-YB21 is overexpressed in poplar trees, it enhances drought resistance through the root xylem channel development which is both bigger and highly lignified. Conversely, nf-yb21, a poplar mutant generated by CRISPR/Cas9, exhibited stunted root development and reduced drought resilience. PdNF-YB21 interacted with PdFUSCA3, also known as PdFUS3, a transcription factor with a B3 domain. An essential gene involved in abscisic acid (ABA) synthesis, PdNCED3, was directly induced by PdFUS3. A dramatic increase in the concentration of ABA (abscisic acid) in the dry roots of poplar trees was a result of this. The co-expression of poplar NF-YB21 and FUS3 significantly increased the expression of PdNCED3. Additionally, ABA (abscisic acid) improved root development and drought resistance by increasing indoleacetic acid transport to root tips [100].
6.5. Salicylic Acid
One of the most important hormones that plants have for dealing with biotic and abiotic stresses and for balancing development and immunity is salicylic acid (SA), which is also called the sixth phytohormone [101]. Methodical identification and exploration of SA biosynthesis are thus crucial to plant science and the development of novel medicines. The model plant Arabidopsis thaliana has been the subject of research in terms of SA biosynthesis [102]. In their study, Xiao et al. utilized a combination of genome-wide association studies (GWASs), metabolite and expression profiling methods, and Populus to investigate the genetic architecture of SA biosynthesis. A total of 300 unique Populus tomentosa Carr. individuals had nine different SA biosynthesis levels, and initial intermediates were measured [103]. Drought, according to previous studies, increased endogenous JA and ABA (abscisic acid) levels in Brassica napus, which in turn increased ABA/SA and (ABA + JA)/SA [104]. There was a drop in reducing potential [NAD(P)H/NAD(P) + and GSH/GSSG] and an increase in reactive oxygen species (ROS) and proline alongside the changes in endogenous hormonal balance. The SA pretreatment effectively removed the excess O2 that had built up due to the drought [104]. Furthermore, salicylic acid levels were five times greater in drought-stricken plants than in normally occurring evergreen Phillyrea augustifolia shrubby plants [105].
6.6. Ethylene
Researchers discovered that ethylene enhances plants’ resilience to drought conditions [106]. Several developmental processes are regulated by the multifunctional phytohormone ethylene when environmental conditions are unfavorable [107]. Ethylene alters RSA by enhancing auxin production and the machinery that transports it. Multiple investigations have shown that ethylene causes an increase in the expression of auxin efflux genes (PIN 1, 2, and 4), as well as the inflow gene (AUX1) [108]. Ethylene positively regulates poplar’s responses to canker caused by the hemibiotrophic fungus Dothiorella gregaria. Applying the biosynthetic precursor of ethylene, 1-aminocyclopropane-1-carboxylic acid (ACC), to Populus tomentosa significantly enhanced the plant’s disease resistance, along with enhancing H2O2 accumulation and the expression of the pathogen-related protein (PR) gene. An inhibitor of ethylene production, aminoethoxyvinyl glycine (AVG), was used to reduce disease resistance. Populus tomentosa showed improved defenses and disease resistance after overexpressing the PtoACO7 gene, which is involved in ethylene production. In addition, they demonstrated that the ethylene-induced defensive response, which does not rely on the salicylic acid pathway, requires ROS signaling. In poplars, elevated H2O2 levels and the production of the NADPH oxidases PtoRbohD/RbohF were seen as a result of the overexpression of ACC or PtoACO7. Inhibiting NADPH oxidase decreased PR gene expressions and ethylene-induced disease resistance while treating H2O2 completely restored AVG-induced disease hypersensitivity. It follows that ethylene contributes to disease resistance through ROS formation and PR gene expression activation. Changing ethylene production or its signaling route could significantly improve disease resistance in woody plants [109].
6.7. Brassinosteroids
Plant growth hormones are considered an effective means of mitigating the adverse effects of salt. Brassinosteroids (BRs) are phytohormones that exhibit significant benefits against various abiotic stressors. BRs can alleviate the detrimental effects of salt stress by enhancing water and nutrient absorption, membrane integrity, antioxidant functions, and osmolyte production while preserving hormonal equilibrium [110]. Previous research has shown that brassinosteroids improve drought stress responses in Arabidopsis, wheat, and Brassica species [111]. In Arabidopsis, a bifunctional cytochrome P450 monooxygenase known as AtCYP90D1 has been studied for its role in the production of brassinolides, namely in the C-23 hydroxylation phase. In this study, the functional characteristics of PtoCYP90D1, one of the AtCYP90D1 homologous genes from Populus tomentosa, are revealed. According to the qRT-PCR analysis, PtoCYP90D1 was found to be highly expressed in both roots and old leaves. In poplar, PtoCYP90D1-OE overexpression improved cell layers, xylem area, growth, and biomass yield. Transgenic plants displayed significantly taller and wider stems compared to their wild counterparts. In contrast, CRISPR/Cas9 transgenic plants expressing the PtoCYP90D1-KO mutant had much lower biomass production. Following this, studies demonstrated that PtoCYP90D1-KO lines did not exhibit a significant increase in cell wall components compared to wild-type plants, while PtoCYP90D1-OE lines did. In sum, the data demonstrate that PtoCYP90D1 has a positive impact on the growth rate and biomass output of poplar trees, which is beneficial for all the agricultural and industrial applications of this versatile plant [112].
6.8. Jasmonic Acid
Jasmonic acid (JA) is a lipid-derived phytohormone known for regulating defense mechanisms and improving plant tolerance to abiotic stresses such as drought [113]. JA and its derivatives, collectively called jasmonates (JAs), modulate resource allocation and inhibit root growth under stress conditions [114,115]. In Populus species, drought stress induces the upregulation of jasmonic acid biosynthesis genes such as LOX, AOS, and OPR3, which are key components of the JA signaling pathway [77]. This hormonal shift is associated with enhanced expression of stress-related genes and changes in root architecture. Specifically, in Populus euphratica, JA signaling has been implicated in root hydrotropism and lateral root development under osmotic stress [116]. The MYC2 transcription factor, central to JA signaling, is also responsive in Populus trichocarpa under drought, indicating a conserved role in stress adaptation. Moreover, comparative transcriptomic studies have revealed that JA-responsive genes are differentially expressed in drought-tolerant vs. susceptible Populus genotypes, underscoring the hormone’s role in species-specific drought resilience [117].
6.9. Peptides
It is evident that safer and more eco-friendly growth regulators are essential for sustainable agro-forestry output. A peptide is a small biological molecule that makes up the plant proteome. Since the initial revelation of signal peptides in plants, a plethora of short peptides (sPEPs, 2–100 amino acid residues) encoded by tiny open reading frames (sORFs) have been found. Plant development, biotic response, signal transduction, and growth are all regulated by these peptides [118]. However, whether or not these peptides facilitate drought-related long-distance signaling remains unclear. An important peptide for shoot apical meristem growth is CLAVATA3 (CLV3), which has been the subject of much research in plants. In land plants, the phytohormone abscisic acid controls stomatal motility to prevent water loss. However, no signaling molecules that can facilitate the accumulation of abscisic acid in leaves have been identified thus far [119]. Researchers have found that the CLE25 peptide sends signals through vascular tissues to control transpiration in Arabidopsis, which in turn affects abscisic acid generation and stomatal regulation. The root’s resistance to drought stress was enhanced by the expression of the peptide-related gene in the vascular tissues. The peptides travel up the plant’s stems to its leaves, where they regulate the abscisic acid accumulation and trigger stomatal closure, making the plant more resistant to drought stress [120]. Additional peptides include phytosulfokine (PSK), linked to cell proliferation; LUREs, which direct pollen tube growth; RALF, which modulates root development; STOMAGEN, associated with stomatal development; and CIF, related to the formation of the Casparian strip diffusion barrier. A significant peptide associated with salinity and drought stress has recently been identified by researchers—AtPep3 [120]. To improve poplar tree management and breeding programs to boost their drought resilience in forestry and agriculture, it is vital to understand these hormonal changes, as will be shown in Figure 4.
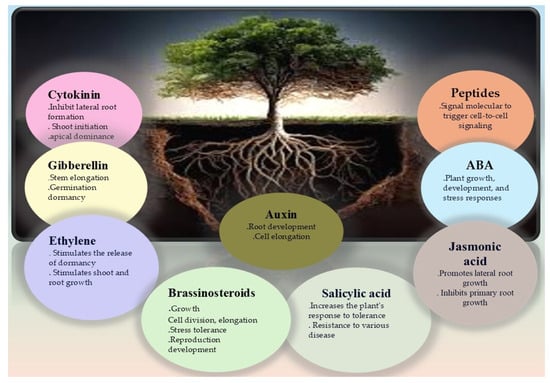
Figure 4.
When plants are drought-stricken, phytohormones mediate a cascade of physiological reactions that control root development. Root growth and stomatal closure are both aided by auxins and cytokinin, which in turn promote water uptake, and abscisic acid (ABA) is essential for both processes. Hormones such as ethylene and jasmonic acid have a role in stress signaling and adaptive responses, which allow plants to improve their drought resilience by optimizing their root architecture.
7. Plant Growth-Promoting Rhizobacteria Improve Plant Health
To reduce stress, Rhizobacteria and mycorrhizae stimulate phytohormone, siderophore, antioxidants, phosphate solubilization, and ethylene reduction. They are used in solid, liquid, metabolite, or polymeric forms depending on crop needs [121]. Drought stress affects plant morphology, energy metabolism, signaling pathways, reactive oxygen species generation, and hormone synthesis. However, several attempts to overcome this impediment were unsuccessful and only temporarily effective. Plant growth-promoting rhizobacteria (PGPR) generates indole acetic acid and gibberellins. PGPR produced active enzymes under drought and waterlogged conditions. This technology enhances plant growth and crop yield while being ecologically friendly [122]. Various tissue types have been identified as colonizing trees [123] and nodes, foliage, and stalks [124]. Invasive fungi can be found in wood, bark, and other organic materials [125]. Many distinct dark septate endophytes have been identified in plant roots from different parts of the globe (DSE) [126]. Each isolate was tested for nitrogen fixation, phosphate solubilization/mineralization, IAA, and siderophore synthesis [127].
Whether useful or harmful, microbes can infect plants and have the rhizosphere competence to enter and multiply in plants and be transferred to other hosts. Endophyte colonization depends on plant tissue type, genotype, microbial taxon and strain, and biotic and abiotic environmental situations [128]. The inoculum density, age, and species of the plant, growing medium, fungal genus, and rate of conidia application considerations affect endophytic colonization [129]. JA controls root endophyte density [130]. Many investigations have shown that Enterobacter sp. does not encode cellulose-degrading proteins, consistent with its nonpathogenic behavior during endophyte–poplar tree contact [131]. Research indicates that the influence of rhizobacteria strains JS and charcoal mineral fertilizer on biomass yield and physiological characteristics is beneficial for cultivating poplar trees in marginal soils, such as reclaimed land, and promotes wood pellet utilization by enhancing soil quality [132]. Endophytic colonization is a vital component of plant–microbe interactions that improve plant health, especially under challenging conditions such as drought, as shown in Figure 5.
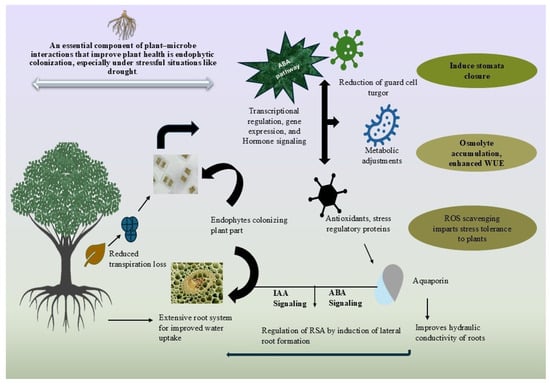
Figure 5.
Endophytic colonization involves beneficial microbes that reside inside plant tissues, promoting growth and stress tolerance. These microbes can help plants overcome drought by enhancing nutrient uptake, inducing stress resistance pathways, and improving water use efficiency. Ultimately, endophytic colonization contributes to improved plant health and survival in challenging environments like drought.
8. Transgenic Approaches
Improving plant productivity, quality, and tolerance to abiotic and biotic stress can be achieved by plant genetic transformation [133]. Various established genetic transformation techniques can reliably incorporate additional genes into the nuclear genomes of diverse plant species. Despite decades of technical progress, effective plant transformation and regeneration continue to pose challenges for numerous species [134]. Transgenic plant regeneration and biomolecule delivery are the two primary processes in plant genetic engineering. The process of biomolecules penetrating plant cells and then regenerating transgenic plants from in vitro grown explants, whether by de novo organogenesis or somatic embryogenesis, is the primary obstacle to successful plant genetic transformation [135]. In addition, from the uses already mentioned, there is a lot of room for improvement when it comes to figuring Populus species with new and improved traits [136]. Multiple Populus species have been genetically modified by Agrobacterium tumefaciens [137]. The study illustrates the durable genetic change in two poplar species, Populus angustifolia and Populus balsamifera, mediated by Agrobacterium tumefaciens. The binary vector pCAMBIA-Npro-long-Luc contains the luciferase reporter gene [138]. Genes linked to drought response, including the AREB1 gene, which controls ABA (abscisic acid) signaling, can be precisely edited thanks to this technology. Research has indicated that when drought circumstances are present, CRISPR-modified plants have enhanced physiological characteristics [139]. Several research has shown how CRISPR-based genetic alteration can improve the quality of wood [140,141]. In recent years, CRISPR/Cas9 genome editing technologies have been utilized in popular plants [142]. The efficacy of Cas12a in woody tree species remains unknown. The hybrid poplar (Populus alba × Populus glandulosa) clone 84 K is utilized to evaluate specific alterations through the CRISPR/Cas12a system [143,144].
9. Discussion
The present analysis highlights the central role of hormonal regulation in enabling Populus species to cope with drought stress. Populus, as a model genus for woody plants and a key component of many forest ecosystems, exhibits complex and dynamic responses to water deficiency. These responses are primarily driven by intricate hormonal signaling pathways that modulate root architecture, stomatal regulation, and metabolic adaptation under drought conditions.
Multiple phytohormones, including auxin, cytokinin, gibberellins (GAs), abscisic acid (ABA), salicylic acid (SA), ethylene, brassinosteroids (BRs), jasmonic acid (JA), and peptide hormones, play significant roles in drought adaptation in Populus. Among them, ABA is the most extensively studied, known for inducing stomatal closure to minimize transpirational water loss and maintain internal water balance. This regulation is particularly critical for Populus, as it often grows in environments where rapid adjustments to water availability are essential for survival.
Root system plasticity is another hallmark of drought adaptation in Populus. Under drought conditions, Populus species alter their root morphology by extending deeper taproots and enhancing lateral root development to access deeper soil moisture. Auxins promote these changes by stimulating lateral root formation and elongation, thus increasing the root system’s capacity to absorb water even in arid soils. This adaptive strategy often compensates for reduced aboveground growth due to water limitation.
Cytokinins, known for regulating shoot and root development, help delay leaf senescence and promote root initiation under drought stress conditions in Populus. GAs, although generally growth-promoting, are often downregulated during drought to reduce elongation and conserve energy. SA and BRs contribute to stress mitigation by enhancing antioxidant enzyme activities, improving photosynthetic performance, and regulating stress-responsive gene expression. JA, while more traditionally associated with defense mechanisms, also influences root system architecture and drought-responsive signaling. Recent findings indicate that peptide hormones serve as intercellular messengers, enhancing the coordination of stress responses across various tissues in Populus.
In addition to hormonal modulation, Populus undergoes several physiological and structural changes to withstand drought. Metabolically, drought stress induces the accumulation of osmoprotectants like proline and soluble sugars, which help stabilize cellular structures and mitigate oxidative damage caused by reactive oxygen species (ROS). These ROS, primarily produced in organelles such as chloroplasts, mitochondria, and peroxisomes, can damage membranes and proteins if not regulated. The hormone-mediated upregulation of antioxidant enzymes—such as superoxide dismutase (SOD), catalase (CAT), and peroxidases—plays a vital role in Populus’ ability to counteract oxidative stress.
Structural adjustments at the root–soil interface are equally crucial. Populus species frequently modify their root anatomy, enhancing hydraulic conductivity while minimizing vulnerability to cavitation. Symbiotic relationships with mycorrhizal fungi further improve drought resistance by increasing water and nutrient uptake. Such associations are particularly beneficial to Populus, given its fast-growing nature and high water demand.
Collectively, these hormonal and structural responses underscore the adaptive resilience of Populus under drought stress. The integration of signaling pathways, root system plasticity, and protective metabolic processes allows Populus to maintain functional integrity and resource acquisition during water deficit conditions.
10. Conclusions
Understanding the hormonal regulation of drought stress responses is essential for enhancing the resilience of Populus species, which are particularly vulnerable to water limitations. As drought becomes increasingly frequent and severe due to climate change, improving the drought tolerance of Populus is crucial for sustainable forestry, biomass production, and ecosystem health.
Future research should focus on unraveling the molecular mechanisms through which phytohormones influence drought adaptation specifically in Populus. Special attention must be given to the genetic networks that mediate hormone signaling in response to environmental stimuli. The use of advanced omics technologies such as genomics, transcriptomics, proteomics, and metabolomics will be instrumental in identifying key genes and pathways involved in hormonal regulation during drought stress in Populus.
Breeding and biotechnological strategies for drought-resilient Populus varieties are another vital avenue. Approaches such as marker-assisted selection, genetic transformation, and genome editing tools like CRISPR-Cas9 can be employed to improve root traits, enhance hormone signaling pathways, and boost overall stress tolerance. Identifying genetic markers linked to favorable drought response traits in Populus will accelerate the selection and development of robust genotypes.
It is also essential to validate laboratory findings under field conditions to assess the performance of hormone-regulated responses in real-world environments. Long-term trials will help determine the practical utility of these traits in various Populus genotypes and guide future forest management practices.
Given the escalating global issue of water scarcity, advancing our understanding of how Populus species respond to drought at the physiological, molecular, and ecological levels is critical. An interdisciplinary approach involving ecologists, plant physiologists, geneticists, and forestry experts will be key to developing holistic strategies that enhance Populus resilience while maintaining ecosystem sustainability. Strengthening these adaptive mechanisms will not only secure Populus productivity but also contribute to broader efforts in climate change mitigation and sustainable land use.
Author Contributions
We acknowledge the contributions of S.A., S.T. and S.S.H. in the development and execution of this study. We extend our appreciation to M.L., X.W. and L.T.Q.Q. for their valuable contributions and insights. Special thanks to S.C. and W.Z., the corresponding authors, for their guidance and support, which were crucial in shaping our research direction. All authors have read and agreed to the published version of the manuscript.
Funding
National Key Research and Development Program of China (2021YFD2200800) and the Fundamental Research Funds for the Central Universities (2572022CG06).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Correction Statement
This article has been republished with a minor correction to the Funding statement. This change does not affect the scientific content of the article.
Abbreviations
The following abbreviations are used in this manuscript:
| ABA | Abscisic acid |
| IAA | Indole-3-acetic acid (a form of auxin) |
| CKX | Cytokinin oxidase |
| GA or GAs | Gibberellins |
| SA | Salicylic acid |
| JA | Jasmonic acid |
| JAs | Jasmonates (includes JA and derivatives) |
| LOX | Lipoxygenase |
| AOS | Allene oxide synthase |
| OPR3 | 12-oxophytodienoate reductase 3 |
| MYC2 | (Not an acronym; it is a transcription factor involved in JA signaling) |
| CK | Cytokinin |
| ROS | Reactive oxygen species |
| CAT | Catalase |
| SOD | Superoxide dismutase |
| APX | Ascorbate peroxidase |
| GPX | Glutathione peroxidase |
| PRX | Peroxiredoxin |
| GSH | Glutathione |
| AMF | Arbuscular Mycorrhizal Fungi |
| PGPR | Plant growth-promoting rhizobacteria |
| H2O2 | Hydrogen peroxide |
| RSL4 | Root hair defective six-like 4 (a transcription factor involved in root hair development) |
| ARR1/ARR12 | Arabidopsis response regulators (involved in cytokinin signaling) |
| FT2 | FLOWERING LOCUS T2 |
| HY5 | ELONGATED HYPOCOTYL 5 (photomorphogenesis regulatory factor) |
| CRISPR/Cas9 | Clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9 |
| LEA | Late embryogenesis abundant (genes associated with stress tolerance) |
| JAZ | Jasmonate ZIM-domain (repressor proteins in JA signaling) |
| COI1 | Coronatine-insensitive 1 (receptor in JA pathway) |
| JAUP1 | Jasmonic acid upregulated protein 1 |
| DSE | Dark septate endophytes |
| BRs | Brassinosteroids |
| EPF | Epidermal patterning factor |
| CK | Cytokinin |
| GA2ox4 | Gibberellin 2-oxidase 4 (enzyme involved in GA catabolism) |
References
- Zhang, H.; Ye, S.; Wang, N.; Xu, Z.; Gong, S. Analyses of the bHLH gene family in Populus trichocarpa reveal roles of four PtbHLHs in regulating the drought stress response. Environ. Exp. Bot. 2024, 228, 106046. [Google Scholar] [CrossRef]
- Nyaupane, S.; Poudel, M.R.; Panthi, B.; Dhakal, A.; Paudel, H.; Bhandari, R. Drought stress effect, tolerance, and management in wheat–A review. Cogent Food Agric. 2024, 10, 2296094. [Google Scholar] [CrossRef]
- Farheen, J.; Mansoor, S.; Abideen, Z. Exogenously applied salicylic acid improved growth, photosynthetic pigments and oxidative stability in mungbean seedlings (Vigna radiata) at salt stress. Pak. J. Bot. 2018, 50, 901–912. [Google Scholar]
- Ahmed, U.; Rao, M.J.; Qi, C.; Xie, Q.; Noushahi, H.A.; Yaseen, M.; Shi, X.; Zheng, B. Expression profiling of flavonoid biosynthesis genes and secondary metabolites accumulation in Populus under drought stress. Molecules 2021, 26, 5546. [Google Scholar] [CrossRef]
- Wu, J.; Liu, S.; Feng, Q.; Cao, X.; Xing, H.; Shi, Z. Response of Leaf Non-Structural Carbohydrates to Elevation in Dioecious Plants, Populus cathayana and Hippophae rhamnoides. Forests 2025, 16, 246. [Google Scholar] [CrossRef]
- Poudel, D.R.; Chen, H.Y.; KC, M.; Ge, Z.; Bown, H.E.; Ruan, H. Understory vegetation dynamics across a poplar plantation chronosequence in reclaimed coastal saline soil. Forests 2019, 10, 764. [Google Scholar] [CrossRef]
- Biselli, C.; Vietto, L.; Rosso, L.; Cattivelli, L.; Nervo, G.; Fricano, A. Advanced breeding for biotic stress resistance in poplar. Plants 2022, 11, 2032. [Google Scholar] [CrossRef]
- Tahiri, A.; Ait Aabd, N.; Qessaoui, R.; Mimouni, A.; Bouharroud, R. Genetic Diversity and Breeding of Cactus (Opuntia spp.). In Breeding of Ornamental Crops: Potted Plants and Shrubs; Springer: Cham, Switzerland, 2025; pp. 153–193. [Google Scholar]
- Shi, Y.-J.; Mi, J.-X.; Huang, J.-L.; Tian, F.-F.; He, F.; Zhong, Y.; Yang, H.-B.; Wang, F.; Xiao, Y.; Yang, L.-K. A new species of Populus and the extensive hybrid speciation arising from it on the Qinghai-Tibet Plateau. Mol. Phylogenet. Evol. 2024, 196, 108072. [Google Scholar] [CrossRef]
- Kim, T.-L.; Lim, H.; Denison, M.I.J.; Oh, C. Transcriptomic and physiological analysis reveals genes associated with drought stress responses in Populus alba × Populus glandulosa. Plants 2023, 12, 3238. [Google Scholar] [CrossRef]
- Nahakpam, S.; Shah, K.; Kundu, M.; Heikham, R.S. Role of phytohormones as master regulators during the abiotic stress. In Stress Tolerance in Horticultural Crops; Elsevier: Amsterdam, Netherlands, 2021; pp. 347–369. [Google Scholar]
- Wang, B.; Zhang, J.; Pei, D.; Yu, L. Combined effects of water stress and salinity on growth, physiological, and biochemical traits in two walnut genotypes. Physiol. Plant. 2021, 172, 176–187. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, J.; Fu, X.; Zhao, C.; Zhang, W.; Gao, H.; Zhu, C.; Song, X.; Zhao, Y.; An, Y. PagPXYs improve drought tolerance by regulating reactive oxygen species homeostasis in the cambium of Populus alba × P. glandulosa. Plant Sci. 2024, 344, 112106. [Google Scholar] [CrossRef] [PubMed]
- Reichheld, J.-P.; Dard, A.; Belin, C. Redox Regulation of Plant Development. In Redox Regulation of Differentiation and De-Differentiation; CRC Press: Boca Raton, FL, USA, 2021; pp. 15–36. [Google Scholar]
- Wang, Y.; Mostafa, S.; Zeng, W.; Jin, B. Function and mechanism of jasmonic acid in plant responses to abiotic and biotic stresses. Int. J. Mol. Sci. 2021, 22, 8568. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Gupta, R.; Shokat, S.; Iqbal, N.; Kocsy, G.; Pérez-Pérez, J.M.; Riyazuddin, R. Ascorbate, plant hormones and their interactions during plant responses to biotic stress. Physiol. Plant. 2024, 176, e14388. [Google Scholar] [CrossRef] [PubMed]
- Begna, T. Effects of drought stress on crop production and productivity. Int. J. Res. Stud. Agric. Sci. 2020, 6, 34–43. [Google Scholar]
- Al Hinai, M.S.; Rehman, A.; Siddique, K.H.; Farooq, M. The Role of Trehalose in Improving Drought Tolerance in Wheat. J. Agron. Crop Sci. 2025, 211, e70053. [Google Scholar] [CrossRef]
- Dietz, K.J.; Zörb, C.; Geilfus, C.M. Drought and crop yield. Plant Biol. 2021, 23, 881–893. [Google Scholar] [CrossRef]
- Buckley, T.N.; Magney, T.S.; Berny Mier y Teran, J.C.; Mills, C.; Palkovic, A.; Parker, T.A.; Pierce, M.A.; Wadhwani, Y.; Wong, C.Y.; Gepts, P. Diversity in stomatal and hydraulic responses to post-flowering drought in common (Phaseolus vulgaris) and tepary (P. acutifolius) beans. Plant Cell Environ. 2025, 48, 51–64. [Google Scholar] [CrossRef]
- Elemike, E.E.; Uzoh, I.M.; Onwudiwe, D.C.; Babalola, O.O. The role of nanotechnology in the fortification of plant nutrients and improvement of crop production. Appl. Sci. 2019, 9, 499. [Google Scholar] [CrossRef]
- Bal, S.K.; Minhas, P.S. Atmospheric stressors: Challenges and coping strategies. In Abiotic Stress Management for Resilient Agriculture; Springer: Singapore, 2017; pp. 9–50. [Google Scholar]
- Hafez, E.; Seleiman, M. Response of barley quality traits, yield and antioxidant enzymes to water-stress and chemical inducers. Int. J. Plant Prod. 2017, 11, 477–490. [Google Scholar]
- Raza, M.A.S.; Muhammad, F.; Farooq, M.; Aslam, M.U.; Akhter, N.; Toleikienė, M.; Binobead, M.A.; Ali, M.A.; Rizwan, M.; Iqbal, R. ZnO-nanoparticles and stage-based drought tolerance in wheat (Triticum aestivum L.): Effect on morpho-physiology, nutrients uptake, grain yield and quality. Sci. Rep. 2025, 15, 5309. [Google Scholar] [CrossRef]
- Naeem, M.Y. The Impact of Drought Stress on the Nutritional Quality of Vegetables. In Drought Stress: Review and Recommendations; Springer: Cham, Switzerland 2025; pp. 143–158. [Google Scholar]
- Fu, Z.; Ciais, P.; Prentice, I.C.; Gentine, P.; Makowski, D.; Bastos, A.; Luo, X.; Green, J.K.; Stoy, P.C.; Yang, H. Atmospheric dryness reduces photosynthesis along a large range of soil water deficits. Nat. Commun. 2022, 13, 989. [Google Scholar] [CrossRef] [PubMed]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, V.; Rezaeizadeh, A.; Mondak, B.; Rasoulnia, A.; Domínguez-Figueroa, J.; Carrillo, L.; Romero-Hernandez, G.; Medina, J. Unraveling the role of autophagy and antioxidants in anther and pistil responses to heat stress in rapeseed (Brassica napus L.). Plant Cell Rep. 2025, 44, 51. [Google Scholar] [CrossRef]
- Hendrix, S.; Vanbuel, I.; Colemont, J.; Bos Calderó, L.; Hamzaoui, M.A.; Kunnen, K.; Huybrechts, M.; Cuypers, A. Jack of all trades: Reactive oxygen species in plant responses to stress combinations and priming-induced stress tolerance. J. Exp. Bot. 2025, eraf065. [Google Scholar] [CrossRef]
- Jha, Y. Regulation of photosynthesis under stress. In Improving Stress Resilience in Plants; Elsevier: Amsterdam, Netherlands, 2024; pp. 35–48. [Google Scholar]
- Abid, G.; M’hamdi, M.; Mingeot, D.; Aouida, M.; Aroua, I.; Muhovski, Y.; Sassi, K.; Souissi, F.; Mannai, K.; Jebara, M. Effect of drought stress on chlorophyll fluorescence, antioxidant enzyme activities and gene expression patterns in faba bean (Vicia faba L.). Arch. Agron. Soil. Sci. 2017, 63, 536–552. [Google Scholar] [CrossRef]
- Yan, S.; Weng, B.; Jing, L.; Bi, W.; Yan, D. Adaptive pathway of summer maize under drought stress: Transformation of root morphology and water absorption law. Front. Earth Sci. 2022, 10, 1020553. [Google Scholar] [CrossRef]
- Sun, L.; Dong, X.; Song, X. PtrABR1 increases tolerance to drought stress by enhancing lateral root formation in Populus trichocarpa. Int. J. Mol. Sci. 2023, 24, 13748. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Karimi, M.; Venditti, A.; Zahra, N.; Siddique, K.H.; Farooq, M. Plant Adaptation to Drought Stress: The Role of Anatomical and Morphological Characteristics in Maintaining the Water Status. J. Soil. Sci. Plant Nutr. 2024, 25, 409–427. [Google Scholar] [CrossRef]
- Hu, M.; Zou, B.; Huang, Z.; Wang, S.; Su, X.; Ding, X.; Zheng, G.; Chen, H.Y. Fine root biomass and necromass dynamics of Chinese fir plantations and natural secondary forests in subtropical China. For. Ecol. Manag. 2021, 496, 119413. [Google Scholar] [CrossRef]
- Huaraca Huasco, W.; Riutta, T.; Girardin, C.A.; Hancco Pacha, F.; Puma Vilca, B.L.; Moore, S.; Rifai, S.W.; del Aguila-Pasquel, J.; Araujo Murakami, A.; Freitag, R. Fine root dynamics across pantropical rainforest ecosystems. Glob. Change Biol. 2021, 27, 3657–3680. [Google Scholar] [CrossRef] [PubMed]
- Endo, I.; Kobatake, M.; Tanikawa, N.; Nakaji, T.; Ohashi, M.; Makita, N. Anatomical patterns of condensed tannin in fine roots of tree species from a cool-temperate forest. Ann. Bot. 2021, 128, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Guo, Y.; Sheng, J.; Yuan, Y.; Zhang, W.H.; Bai, W. Using anatomical traits to understand root functions across root orders of herbaceous species in a temperate steppe. New Phytol. 2022, 234, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, P.L.; Clemmensen, K.E.; Michelsen, A.; Jonasson, S.; Ström, L. Plant and microbial uptake and allocation of organic and inorganic nitrogen related to plant growth forms and soil conditions at two subarctic tundra sites in Sweden. Arct. Antarct. Alp. Res. 2008, 40, 171–180. [Google Scholar] [CrossRef]
- Zadworny, M.; McCormack, M.L.; Mucha, J.; Reich, P.B.; Oleksyn, J. Scots pine fine roots adjust along a 2000-km latitudinal climatic gradient. New Phytol. 2016, 212, 389–399. [Google Scholar] [CrossRef]
- Malhotra, A.; Brice, D.J.; Childs, J.; Graham, J.D.; Hobbie, E.A.; Vander Stel, H.; Feron, S.C.; Hanson, P.J.; Iversen, C.M. Peatland warming strongly increases fine-root growth. Proc. Natl. Acad. Sci. USA 2020, 117, 17627–17634. [Google Scholar] [CrossRef]
- Reingwirtz, I.; Uretsky, J.; Cuneo, I.F.; Knipfer, T.; Reyes, C.; Walker, M.A.; McElrone, A.J. Inherent and stress-induced responses of fine root morphology and anatomy in commercial grapevine rootstocks with contrasting drought resistance. Plants 2021, 10, 1121. [Google Scholar] [CrossRef]
- George, T.S.; Bulgarelli, D.; Carminati, A.; Chen, Y.; Jones, D.; Kuzyakov, Y.; Schnepf, A.; Wissuwa, M.; Roose, T. Bottom-up perspective–The role of roots and rhizosphere in climate change adaptation and mitigation in agroecosystems. Plant Soil. 2024, 500, 297–323. [Google Scholar] [CrossRef]
- Chai, Y.N.; Schachtman, D.P. Root exudates impact plant performance under abiotic stress. Trends Plant Sci. 2022, 27, 80–91. [Google Scholar] [CrossRef]
- Tak, Y.; Yadav, V.K.; Gautam, C.; Kumar, R.; Kaur, M. Drought stress alleviation in plants by soil microbial interactions. In Microbiological Activity for Soil and Plant Health Management; Springer: Singapore, 2021; pp. 133–159. [Google Scholar]
- Gamalero, E.; Glick, B.R. Recent advances in bacterial amelioration of plant drought and salt stress. Biology 2022, 11, 437. [Google Scholar] [CrossRef]
- Rahim, R.; Jahromi, O.E.; Amelung, W.; Kroener, E. Rhizosheath formation depends on mucilage concentration and water content. Plant Soil. 2024, 495, 649–661. [Google Scholar] [CrossRef]
- Yıldırım, K.; Yağcı, A.; Sucu, S.; Tunç, S. Responses of grapevine rootstocks to drought through altered root system architecture and root transcriptomic regulations. Plant Physiol. Biochem. 2018, 127, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.K.; Gupta, S.K.; Dwivedi, V.; Chattopadhyay, D. Lignin deposition in chickpea root xylem under drought. Plant Signal. Behav. 2020, 15, 1754621. [Google Scholar] [CrossRef] [PubMed]
- White III, R.A.; Rivas-Ubach, A.; Borkum, M.I.; Köberl, M.; Bilbao, A.; Colby, S.M.; Hoyt, D.W.; Bingol, K.; Kim, Y.-M.; Wendler, J.P. The state of rhizospheric science in the era of multi-omics: A practical guide to omics technologies. Rhizosphere 2017, 3, 212–221. [Google Scholar] [CrossRef]
- Kang, J.; Peng, Y.; Xu, W. Crop root responses to drought stress: Molecular mechanisms, nutrient regulations, and interactions with microorganisms in the rhizosphere. Int. J. Mol. Sci. 2022, 23, 9310. [Google Scholar] [CrossRef]
- Kundu, A.; Vadassery, J. Molecular mechanisms of Piriformospora indica mediated growth promotion in plants. Plant Signal. Behav. 2022, 17, 2096785. [Google Scholar] [CrossRef]
- Hassan, S.S.; Zhao, J.; Tahir, S.; Khan, I.; Yang, G.; Zhao, B. Optimizing Ge Enrichment in Lyophyllum decastes Fermentation for Enhanced Biological Activity. Fermentation 2024, 10, 641. [Google Scholar] [CrossRef]
- Sun, C.; Johnson, J.M.; Cai, D.; Sherameti, I.; Oelmüller, R.; Lou, B. Piriformospora indica confers drought tolerance in Chinese cabbage leaves by stimulating antioxidant enzymes, the expression of drought-related genes and the plastid-localized CAS protein. J. Plant Physiol. 2010, 167, 1009–1017. [Google Scholar] [CrossRef]
- Badar, A.; Aqueel, R.; Nawaz, A.; Ijaz, U.Z.; Malik, K.A. Microbiota transplantation for cotton leaf curl disease suppression—Core microbiome and transcriptome dynamics. Commun. Biol. 2025, 8, 380. [Google Scholar] [CrossRef]
- Hilszczańska, D. Endophytes–characteristics and possibilities of application in forest management. Leśne Pr. Badaw. 2016, 77, 276–282. [Google Scholar] [CrossRef][Green Version]
- Hassani, D.; Khalid, M.; Huang, D.; Zhang, Y.-D. Morphophysiological and molecular evidence supporting the augmentative role of Piriformospora indica in mitigation of salinity in Cucumis melo L. Acta Biochim. Biophys. Sin. 2019, 51, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Zin, N.A.; Badaluddin, N.A. Biological functions of Trichoderma spp. for agriculture applications. Ann. Agric. Sci. 2020, 65, 168–178. [Google Scholar] [CrossRef]
- El_Komy, M.H.; Saleh, A.A.; Eranthodi, A.; Molan, Y.Y. Characterization of novel Trichoderma asperellum isolates to select effective biocontrol agents against tomato Fusarium wilt. Plant Pathol. J. 2015, 31, 50. [Google Scholar] [CrossRef]
- Cai, F.; Yu, G.; Wang, P.; Wei, Z.; Fu, L.; Shen, Q.; Chen, W. Harzianolide, a novel plant growth regulator and systemic resistance elicitor from Trichoderma harzianum. Plant Physiol. Biochem. 2013, 73, 106–113. [Google Scholar] [CrossRef]
- Yuan, H.; Zhu, Z.; Liu, S.; Ge, T.; Jing, H.; Li, B.; Liu, Q.; Lynn, T.M.; Wu, J.; Kuzyakov, Y. Microbial utilization of rice root exudates: 13 C labeling and PLFA composition. Biol. Fertil. Soils 2016, 52, 615–627. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Alfaro-Cuevas, R.; López-Bucio, J. Trichoderma spp. improve growth of Arabidopsis seedlings under salt stress through enhanced root development, osmolite production, and Na+ elimination through root exudates. Mol. Plant-Microbe Interact. 2014, 27, 503–514. [Google Scholar] [CrossRef]
- Salvi, P.; Manna, M.; Kaur, H.; Thakur, T.; Gandass, N.; Bhatt, D.; Muthamilarasan, M. Phytohormone signaling and crosstalk in regulating drought stress response in plants. Plant Cell Rep. 2021, 40, 1305–1329. [Google Scholar] [CrossRef]
- Swain, R.; Sahoo, S.; Behera, M.; Rout, G.R. Instigating prevalent abiotic stress resilience in crop by exogenous application of phytohormones and nutrient. Front. Plant Sci. 2023, 14, 1104874. [Google Scholar] [CrossRef]
- Ranjan, A.; Sinha, R.; Lal, S.K.; Bishi, S.K.; Singh, A.K. Phytohormone signalling and cross-talk to alleviate aluminium toxicity in plants. Plant Cell Rep. 2021, 40, 1331–1343. [Google Scholar] [CrossRef]
- Li, S.; Yuan, J.; Zhou, F.; Liu, Y.; Xie, H.; Jia, W.; Chao, Y.; Han, L. Modulating ABA-dependent growth and development by overexpressing cytochrome P450 ABA 8′-hydroxylase in Medicago truncatula. Environ. Exp. Bot. 2025, 229, 106060. [Google Scholar] [CrossRef]
- Stec, N.; Banasiak, J.; Jasiński, M. Abscisic acid-an overlooked player in plant-microbe symbioses formation? Acta Biochim. Pol. 2016, 63, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Floková, K.; Tarkowská, D.; Miersch, O.; Strnad, M.; Wasternack, C.; Novák, O. UHPLC–MS/MS based target profiling of stress-induced phytohormones. Phytochemistry 2014, 105, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Xu, J.; Qi, J.; Lu, X.; Liu, Y.; Xiong, J.; Yu, W.; Li, C. Genome-wide identification of SlIQMs and the regulatory effect of calcium on tomato seedlings under drought stress and phytohormone treatment. Plant Cell Rep. 2025, 44, 70. [Google Scholar] [CrossRef]
- Kamran, M.; Danish, M.; Saleem, M.H.; Malik, Z.; Parveen, A.; Abbasi, G.H.; Jamil, M.; Ali, S.; Afzal, S.; Riaz, M. Application of abscisic acid and 6-benzylaminopurine modulated morpho-physiological and antioxidative defense responses of tomato (Solanum lycopersicum L.) by minimizing cobalt uptake. Chemosphere 2021, 263, 128169. [Google Scholar] [CrossRef]
- Zeng, D.; Dai, L.-J.; Li, X.; Li, W.; Qu, G.-Z.; Li, S. Genome-Wide Identification of the ERF Transcription Factor Family for Structure Analysis, Expression Pattern, and Response to Drought Stress in Populus alba × Populus glandulosa. Int. J. Mol. Sci. 2023, 24, 3697. [Google Scholar] [CrossRef]
- Wang, S.; Fan, Y.; Du, S.; Zhao, K.; Liu, Q.; Yao, W.; Zheng, T.; Han, Y. PtaERF194 inhibits plant growth and enhances drought tolerance in poplar. Tree Physiol. 2022, 42, 1678–1692. [Google Scholar] [CrossRef]
- Pan, H.; He, Z.; Liu, L.; Cai, R.; Huang, H.; Xie, X.; Cao, X.; Li, Y.; Qiu, W.; Lu, Z. A Genome-Wide Characterization of Receptor-like Cytoplasmic Kinase IV Subfamily Members in Populus deltoides Identifies the Potential Role of PdeCRCK6 in Plant Osmotic Stress Responses. Plants 2024, 13, 3371. [Google Scholar] [CrossRef]
- Zhou, M.; Cheng, H.; Chiang, V.L.; Li, W.; Yang, C.; Wang, C. PtrbZIP3 transcription factor regulates drought tolerance of Populus trichocarpa. Environ. Exp. Bot. 2023, 208, 105231. [Google Scholar] [CrossRef]
- Xu, R.; Liu, W.-G.; Huang, T.-W.; Li, B.-R.; Dai, H.-X.; Yang, X.-D. Drought stress-induced the formation of heteromorphic leaves of Populus euphratica Oliv: Evidence from gene transcriptome. Front. Plant Sci. 2023, 14, 1194169. [Google Scholar] [CrossRef]
- Tikhomirova, T.S.; Krutovsky, K.V.; Shestibratov, K.A. Molecular traits for adaptation to drought and salt stress in birch, oak and poplar species. Forests 2022, 14, 7. [Google Scholar] [CrossRef]
- Han, Y.; Lou, X.; Zhang, W.; Xu, T.; Tang, M. Arbuscular mycorrhizal fungi enhanced drought resistance of Populus cathayana by regulating the 14-3-3 family protein genes. Microbiol. Spectr. 2022, 10, e0245621. [Google Scholar] [CrossRef] [PubMed]
- Gan, H.; Chu, J.; Shi, W.; Luo, Z.-B. Physiological and transcriptomic regulation of Populus simonii fine roots exposed to a heterogeneous phosphorus environment in soil. Environ. Exp. Bot. 2024, 219, 105646. [Google Scholar] [CrossRef]
- Jia, M.; Wang, Y.; Jin, H.; Li, J.; Song, T.; Chen, Y.; Yuan, Y.; Hu, H.; Li, R.; Wu, Z. Comparative Genomics Analysis of the Populus Epidermal Pattern Factor (EPF) Family Revealed Their Regulatory Effects in Populus euphratica Stomatal Development. Int. J. Mol. Sci. 2024, 25, 10052. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, M.R.; Bicalho, E.M.; Pereira, E.G.; Guilherme, L.R.G.; Marchiori, P.E.R. Drought tolerance: A perspective about leaf venation and the role of auxin. Theor. Exp. Plant Physiol. 2025, 37, 11. [Google Scholar] [CrossRef]
- Liu, S.J.; Zhang, H.; Jin, X.T.; Niu, M.X.; Feng, C.H.; Liu, X.; Liu, C.; Wang, H.L.; Yin, W.; Xia, X. PeFUS3 Drives Lateral Root Growth Via Auxin and ABA Signalling Under Drought Stress in Populus. Plant Cell Environ. 2025, 48, 664–681. [Google Scholar] [CrossRef]
- Xin, H.; Li, L.; Chen, Z.; Hong, X.; Wang, S.; Wang, J. Genome-wide analysis of the poplar homeobox gene family and the identification of PtHB180 as an important regulator of plant developmental processes and drought stress response. Ind. Crops Prod. 2024, 215, 118713. [Google Scholar] [CrossRef]
- Song, Q.; He, F.; Kong, L.; Yang, J.; Wang, X.; Zhao, Z.; Zhang, Y.; Xu, C.; Fan, C.; Luo, K. The IAA17. 1/HSFA5a module enhances salt tolerance in Populus tomentosa by regulating flavonol biosynthesis and ROS levels in lateral roots. New Phytol. 2024, 241, 592–606. [Google Scholar] [CrossRef]
- Uga, Y.; Sugimoto, K.; Ogawa, S.; Rane, J.; Ishitani, M.; Hara, N.; Kitomi, Y.; Inukai, Y.; Ono, K.; Kanno, N. Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat. Genet. 2013, 45, 1097–1102. [Google Scholar] [CrossRef]
- Colebrook, E.H.; Thomas, S.G.; Phillips, A.L.; Hedden, P. The role of gibberellin signalling in plant responses to abiotic stress. J. Exp. Biol. 2014, 217, 67–75. [Google Scholar] [CrossRef]
- Ndayambaza, B.; Si, J.; Zhao, X.; Zhao, Y.; Zhou, D.; Jia, B.; Zhu, X.; Liu, Z.; Bai, X.; Wang, B. Comprehensive Genomic Analysis of Trihelix Transcription Factor Genes and Their Expression Underlying Abiotic Stress in Euphrates Poplar (Populus euphratica). Plants 2025, 14, 662. [Google Scholar] [CrossRef]
- Li, W.; Herrera-Estrella, L.; Tran, L.-S.P. The Yin–Yang of cytokinin homeostasis and drought acclimation/adaptation. Trends Plant Sci. 2016, 21, 548–550. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.; Vankova, R.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.-S.P. Cytokinins: Metabolism and function in plant adaptation to environmental stresses. Trends Plant Sci. 2012, 17, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Pospíšilová, H.; Jiskrova, E.; Vojta, P.; Mrizova, K.; Kokáš, F.; Čudejková, M.M.; Bergougnoux, V.; Plíhal, O.; Klimešová, J.; Novák, O. Transgenic barley overexpressing a cytokinin dehydrogenase gene shows greater tolerance to drought stress. New Biotechnol. 2016, 33, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Kurepa, J.; Smalle, J.A. Auxin/cytokinin antagonistic control of the shoot/root growth ratio and its relevance for adaptation to drought and nutrient deficiency stresses. Int. J. Mol. Sci. 2022, 23, 1933. [Google Scholar] [CrossRef]
- Gao, S.; Xiao, Y.; Xu, F.; Gao, X.; Cao, S.; Zhang, F.; Wang, G.; Sanders, D.; Chu, C. Cytokinin-dependent regulatory module underlies the maintenance of zinc nutrition in rice. New Phytol. 2019, 224, 202–215. [Google Scholar] [CrossRef]
- Ramireddy, E.; Hosseini, S.A.; Eggert, K.; Gillandt, S.; Gnad, H.; von Wirén, N.; Schmülling, T. Root engineering in barley: Increasing cytokinin degradation produces a larger root system, mineral enrichment in the shoot and improved drought tolerance. Plant Physiol. 2018, 177, 1078–1095. [Google Scholar] [CrossRef]
- Kang, S.-M.; Hamayun, M.; Khan, M.A.; Iqbal, A.; Lee, I.-J. Bacillus subtilis JW1 enhances plant growth and nutrient uptake of Chinese cabbage through gibberellins secretion. J. Appl. Bot. Food Qual. 2019, 92, 172–178. [Google Scholar]
- He, Z.; Zhang, P.; Jia, H.; Zhang, S.; Nishawy, E.; Sun, X.; Dai, M. Regulatory mechanisms and breeding strategies for crop drought resistance. New Crops 2024, 1, 100029. [Google Scholar] [CrossRef]
- Song, Q.; Kong, L.; Yang, J.; Lin, M.; Zhang, Y.; Yang, X.; Wang, X.; Zhao, Z.; Zhang, M.; Pan, J. The transcription factor PtoMYB142 enhances drought tolerance in Populus tomentosa by regulating gibberellin catabolism. Plant J. 2024, 118, 42–57. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, Z.; Feng, Q.; Long, T.; Ding, J.; Shu, P.; Deng, H.; Yu, P.; Tan, W.; Liu, S. Elongated Hypocotyl 5a modulates Flowering Locus T2 and gibberellin levels to control dormancy and bud break in poplar. Plant Cell 2024, 36, 1963–1984. [Google Scholar] [CrossRef]
- Lamlom, S.F.; Abdelghany, A.M.; Farouk, A.; Alwakel, E.S.; Makled, K.M.; Bukhari, N.A.; Hatamleh, A.A.; Ren, H.; El-Sorady, G.A.; Shehab, A. Biochemical and yield response of spring wheat to drought stress through gibberellic and abscisic acids. BMC Plant Biol. 2025, 25, 5. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Zhou, F.; Chen, Y.; Wu, H.; Yin, T. Genome-wide analysis of the expansin gene family in Populus and characterization of expression changes in response to phytohormone (abscisic acid) and abiotic (low-temperature) stresses. Int. J. Mol. Sci. 2023, 24, 7759. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, Y.; Wang, X.; Han, X.; An, Y.; Lin, S.; Shen, C.; Wen, J.; Liu, C.; Yin, W. Root-specific NF-Y family transcription factor, PdNF-YB21, positively regulates root growth and drought resistance by abscisic acid-mediated indoylacetic acid transport in Populus. New Phytol. 2020, 227, 407–426. [Google Scholar] [CrossRef]
- Rodriguez-Lopez, J.; Arrojo, M.; Paz, E.; Paramo, M.; Costas, J. Identification of relevant hub genes for early intervention at gene coexpression modules with altered predicted expression in schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 98, 109815. [Google Scholar] [CrossRef]
- Lefevere, H.; Bauters, L.; Gheysen, G. Salicylic acid biosynthesis in plants. Front. Plant Sci. 2020, 11, 338. [Google Scholar] [CrossRef]
- Xiao, L.; Du, Q.; Fang, Y.; Quan, M.; Lu, W.; Wang, D.; Si, J.; El-Kassaby, Y.A.; Zhang, D. Genetic architecture of the metabolic pathway of salicylic acid biosynthesis in Populus. Tree Physiol. 2021, 41, 2198–2215. [Google Scholar] [CrossRef]
- La, V.H.; Lee, B.-R.; Zhang, Q.; Park, S.-H.; Islam, M.T.; Kim, T.-H. Salicylic acid improves drought-stress tolerance by regulating the redox status and proline metabolism in Brassica rapa. Hortic. Environ. Biotechnol. 2019, 60, 31–40. [Google Scholar] [CrossRef]
- Hamayun, M.; Hussain, A.; Iqbal, A.; Khan, S.A.; Khan, M.A.; Lee, I.-J. An endophytic fungus Gliocladium cibotii regulates metabolic and antioxidant system of Glycine max and Helianthus annuus under heat stress. Pol. J. Environ. Stud. 2021, 30, 1631–1640. [Google Scholar]
- Ji, Y.; Lang, D.; Xu, Z.; Ma, X.; Bai, Q.; Zhang, W.; Zhang, X.; Zhao, Q. Bacillus pumilus G5 combined with silicon enhanced flavonoid biosynthesis in drought-stressed Glycyrrhiza uralensis Fisch. by regulating jasmonate, gibberellin and ethylene crosstalk. Plant Physiol. Biochem. 2025, 220, 109560. [Google Scholar] [CrossRef]
- Dolgikh, V.A.; Pukhovaya, E.M.; Zemlyanskaya, E.V. Shaping ethylene response: The role of EIN3/EIL1 transcription factors. Front. Plant Sci. 2019, 10, 1030. [Google Scholar] [CrossRef]
- Pandey, B.K.; Bennett, M.J. Uncovering root compaction response mechanisms: New insights and opportunities. J. Exp. Bot. 2024, 75, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, H.; Yang, F.; Chai, S.; Wang, L.; de Dios, V.R.; Tan, W.; Yao, Y. Ethylene activates poplar defense against Dothiorella gregaria Sacc by regulating reactive oxygen species accumulation. Physiol. Plant. 2022, 174, e13726. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.U.; Guoqin, H.; Nawaz, M.; Shah, A.N.; Khan, T.A.; Haq, M.I.U.; Noor, M.A.; Ping, Z.; Qin, L.; Mostafa, Y.S. The role of brassinosteroids in plant physiological and molecular responses to counter salt stress and ensure food security: A review and future perspectives. Turk. J. Agric. For. 2025, 49, 1–23. [Google Scholar] [CrossRef]
- Sahni, S.; Prasad, B.D.; Liu, Q.; Grbic, V.; Sharpe, A.; Singh, S.P.; Krishna, P. Overexpression of the brassinosteroid biosynthetic gene DWF4 in Brassica napus simultaneously increases seed yield and stress tolerance. Sci. Rep. 2016, 6, 28298. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, S.; Tang, H.; Yao, X. Molecular cloning and characterization of a brassinosteriod biosynthesis-related gene PtoCYP90D1 from Populus tomentosa. BMC Genom. 2024, 25, 1047. [Google Scholar]
- Han, G.-Z. Evolution of jasmonate biosynthesis and signaling mechanisms. J. Exp. Bot. 2017, 68, 1323–1331. [Google Scholar] [CrossRef]
- Ruan, J.; Zhou, Y.; Zhou, M.; Yan, J.; Khurshid, M.; Weng, W.; Cheng, J.; Zhang, K. Jasmonic acid signaling pathway in plants. Int. J. Mol. Sci. 2019, 20, 2479. [Google Scholar] [CrossRef]
- Lu, C.; Ding, J.; Park, H.K.; Feng, H. High intensity ultrasound as a physical elicitor affects secondary metabolites and antioxidant capacity of tomato fruits. Food Control 2020, 113, 107176. [Google Scholar] [CrossRef]
- Rao, S.; Tian, Y.; Zhang, C.; Qin, Y.; Liu, M.; Niu, S.; Li, Y.; Chen, J. The JASMONATE ZIM-domain–OPEN STOMATA1 cascade integrates jasmonic acid and abscisic acid signaling to regulate drought tolerance by mediating stomatal closure in poplar. J. Exp. Bot. 2023, 74, 443–457. [Google Scholar] [CrossRef]
- Marqués-Gálvez, J.E.; Pandharikar, G.; Basso, V.; Kohler, A.; Lackus, N.D.; Barry, K.; Keymanesh, K.; Johnson, J.; Singan, V.; Grigoriev, I.V. Populus MYC2 orchestrates root transcriptional reprogramming of defence pathway to impair Laccaria bicolor ectomycorrhizal development. New Phytol. 2024, 242, 658–674. [Google Scholar] [CrossRef]
- Takahashi, F.; Hanada, K.; Kondo, T.; Shinozaki, K. Hormone-like peptides and small coding genes in plant stress signaling and development. Curr. Opin. Plant Biol. 2019, 51, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Nir, I.; Moshelion, M.; Weiss, D. The A rabidopsis GIBBERELLIN METHYL TRANSFERASE 1 suppresses gibberellin activity, reduces whole-plant transpiration and promotes drought tolerance in transgenic tomato. Plant Cell Environ. 2014, 37, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.H.; Wu, S.J.; Peng, Y.S.; Liu, R.N.; Chen, X.; Zhao, P.; Xu, P.; Zhu, J.B.; Jiao, G.L.; Pei, Y. Arabidopsis EDT 1/HDG 11 improves drought and salt tolerance in cotton and poplar and increases cotton yield in the field. Plant Biotechnol. J. 2016, 14, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Rawal, H.; Nautiyal, Y.; Sharma, B.; Tiwari, S. Microbial Inoculants and Their Role in Abiotic Stress Management. In Microbial Inoculants: Applications for Sustainable Agriculture; Springer: Singapore, 2024; pp. 163–201. [Google Scholar]
- Kumar, M.; Gupta, A.; Vandana, P.; Tiwari, L.D.; Patel, M.K.; Siddique, K.H. Recent advances of plant growth-promoting rhizobacteria (PGPR)-mediated drought and waterlogging stress tolerance in plants for sustainable agriculture. In Microbial Biostimulants for Plant Growth and Abiotic Stress Amelioration; Elsevier: Amsterdam, Netherlands, 2024; pp. 315–344. [Google Scholar]
- Milazzo, C.; Zulak, K.G.; Muria-Gonzalez, M.J.; Jones, D.; Power, M.; Bransgrove, K.; Bunce, M.; Lopez-Ruiz, F.J. High-throughput metabarcoding characterizes fungal endophyte diversity in the phyllosphere of a barley crop. Phytobiomes J. 2021, 5, 316–325. [Google Scholar] [CrossRef]
- Gazis, R.; Chaverri, P. Diversity of fungal endophytes in leaves and stems of wild rubber trees (Hevea brasiliensis) in Peru. Fungal Ecol. 2010, 3, 240–254. [Google Scholar] [CrossRef]
- Marčiulynienė, D.; Marčiulynas, A.; Lynikienė, J.; Vaičiukynė, M.; Gedminas, A.; Menkis, A. DNA-metabarcoding of belowground fungal communities in bare-root forest nurseries: Focus on different tree species. Microorganisms 2021, 9, 150. [Google Scholar] [CrossRef]
- Ruotsalainen, A.L.; Kauppinen, M.; Wäli, P.R.; Saikkonen, K.; Helander, M.; Tuomi, J. Dark septate endophytes: Mutualism from by-products? Trends Plant Sci. 2022, 27, 247–254. [Google Scholar] [CrossRef]
- Vaitiekūnaitė, D.; Kuusienė, S.; Beniušytė, E. Oak (Quercus robur) associated endophytic Paenibacillus sp. promotes poplar (Populus spp.) root growth in vitro. Microorganisms 2021, 9, 1151. [Google Scholar] [CrossRef]
- Hardoim, P.R.; Van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef]
- Bamisile, B.S.; Dash, C.K.; Akutse, K.S.; Keppanan, R.; Afolabi, O.G.; Hussain, M.; Qasim, M.; Wang, L. Prospects of endophytic fungal entomopathogens as biocontrol and plant growth promoting agents: An insight on how artificial inoculation methods affect endophytic colonization of host plants. Microbiol. Res. 2018, 217, 34–50. [Google Scholar] [CrossRef]
- Chen, X.; Marszałkowska, M.; Reinhold-Hurek, B. Jasmonic acid, not salicyclic acid restricts endophytic root colonization of rice. Front. Plant Sci. 2020, 10, 1758. [Google Scholar] [CrossRef] [PubMed]
- Taghavi, S.; van der Lelie, D.; Hoffman, A.; Zhang, Y.-B.; Walla, M.D.; Vangronsveld, J.; Newman, L.; Monchy, S. Genome sequence of the plant growth promoting endophytic bacterium Enterobacter sp. 638. PLoS Genet. 2010, 6, e1000943. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Woo, S.Y.; Kim, S.H.; Khaine, I.; Kwak, M.J.; Lee, H.K.; Lee, T.Y.; Lee, W.Y. Effects of increased soil fertility and plant growth-promoting rhizobacteria inoculation on biomass yield, energy value, and physiological response of poplar in short-rotation coppices in a reclaimed tideland: A case study in the Saemangeum area of Korea. Biomass Bioenergy 2017, 107, 29–38. [Google Scholar] [CrossRef]
- Yan, Y.; Zhu, X.; Yu, Y.; Li, C.; Zhang, Z.; Wang, F. Nanotechnology strategies for plant genetic engineering. Adv. Mater. 2022, 34, 2106945. [Google Scholar] [CrossRef]
- Ramkumar, T.; Lenka, S.; Arya, S.; Bansal, K. Biolistic DNA Delivery in Plants; Humana: New York, NY, USA, 2020. [Google Scholar]
- Xu, N.; Kang, M.; Zobrist, J.D.; Wang, K.; Fei, S.-z. Genetic transformation of recalcitrant upland switchgrass using morphogenic genes. Front. Plant Sci. 2022, 12, 781565. [Google Scholar] [CrossRef]
- Du, N.; Liu, X.; Li, Y.; Chen, S.; Zhang, J.; Ha, D.; Deng, W.; Sun, C.; Zhang, Y.; Pijut, P.M. Genetic transformation of Populus tomentosa to improve salt tolerance. Plant Cell Tissue Organ Cult. (PCTOC) 2012, 108, 181–189. [Google Scholar] [CrossRef]
- Pavlichenko, V.V.; Protopopova, M.V. Simplified Method for Agrobacterium-Mediated Genetic Transformation of Populus x berolinensis K. Koch. Methods Protoc. 2024, 7, 12. [Google Scholar] [CrossRef]
- Maheshwari, P.; Kovalchuk, I. Agrobacterium-mediated stable genetic transformation of Populus angustifolia and Populus balsamifera. Front. Plant Sci. 2016, 7, 296. [Google Scholar] [CrossRef]
- Roca Paixão, J.F.; Gillet, F.-X.; Ribeiro, T.P.; Bournaud, C.; Lourenço-Tessutti, I.T.; Noriega, D.D.; Melo, B.P.d.; de Almeida-Engler, J.; Grossi-de-Sa, M.F. Improved drought stress tolerance in Arabidopsis by CRISPR/dCas9 fusion with a Histone AcetylTransferase. Sci. Rep. 2019, 9, 8080. [Google Scholar] [CrossRef]
- Tahir, S.; Hassan, S.S.; Yang, L.; Ma, M.; Li, C. Detection Methods for Pine Wilt Disease: A Comprehensive Review. Plants 2024, 13, 2876. [Google Scholar] [CrossRef]
- Ali, A.; Zafar, M.M.; Farooq, Z.; Ahmed, S.R.; Ijaz, A.; Anwar, Z.; Abbas, H.; Tariq, M.S.; Tariq, H.; Mustafa, M. Breakthrough in CRISPR/Cas system: Current and future directions and challenges. Biotechnol. J. 2023, 18, 2200642. [Google Scholar] [CrossRef] [PubMed]
- Azeez, A.; Busov, V. CRISPR/Cas9-mediated single and biallelic knockout of poplar STERILE APETALA (PopSAP) leads to complete reproductive sterility. Plant Biotechnol. J. 2021, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Geng, Y.; Yao, J.; Fu, C.; Lu, M.; Wang, C.; Du, J. Efficient genome editing in Populus using CRISPR/Cas12a. Front. Plant Sci. 2020, 11, 593938. [Google Scholar] [CrossRef]
- Basu, U.; Riaz Ahmed, S.; Bhat, B.A.; Anwar, Z.; Ali, A.; Ijaz, A.; Gulzar, A.; Bibi, A.; Tyagi, A.; Nebapure, S.M. A CRISPR way for accelerating cereal crop improvement: Progress and challenges. Front. Genet. 2023, 13, 866976. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).