Enhanced Disease Susceptibility1 Regulates Immune Response in Lotus japonicus
Abstract
1. Introduction
2. Results
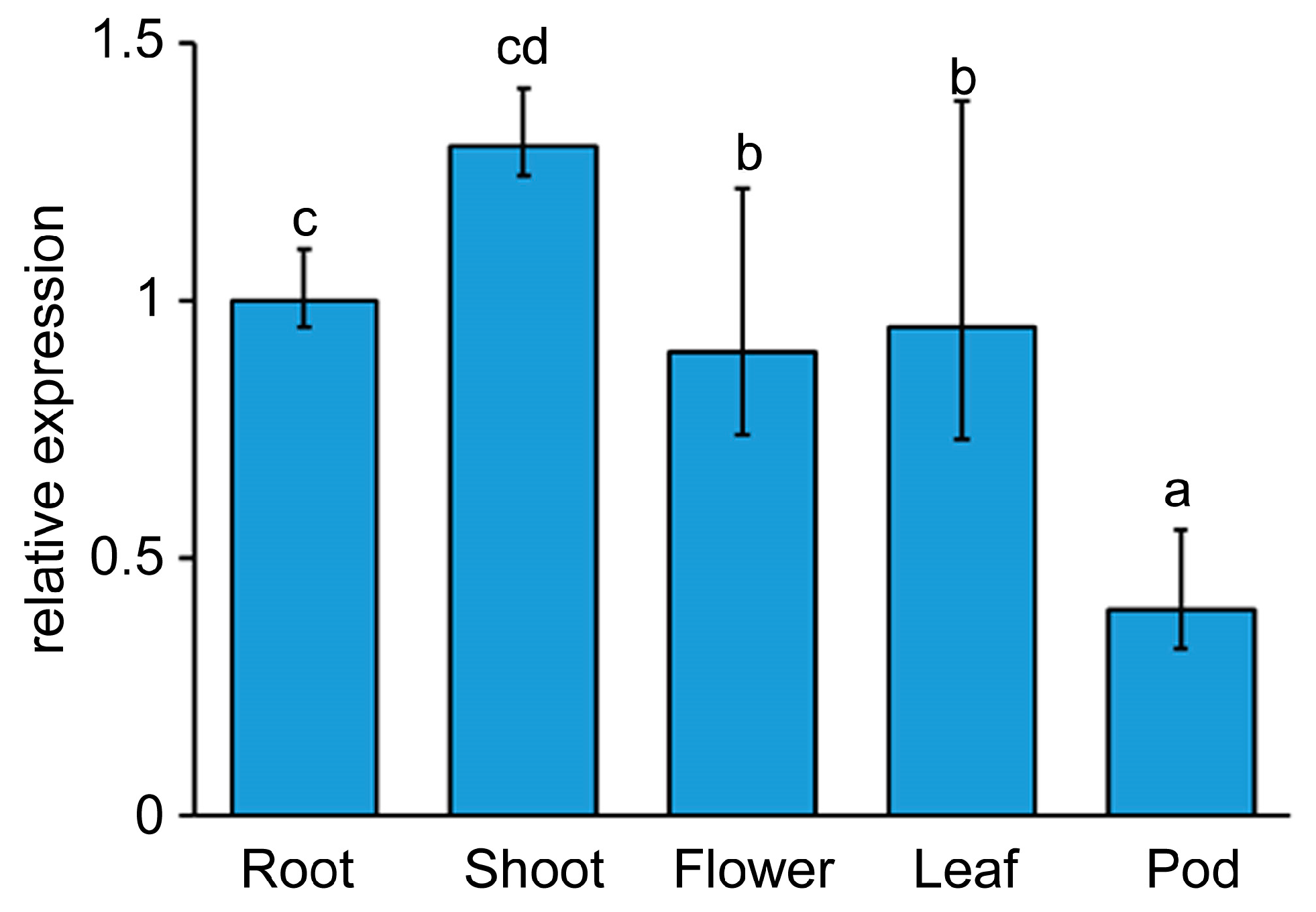
2.1. LjEDS1 Is an EDS1 Orthologs in L. japonicus
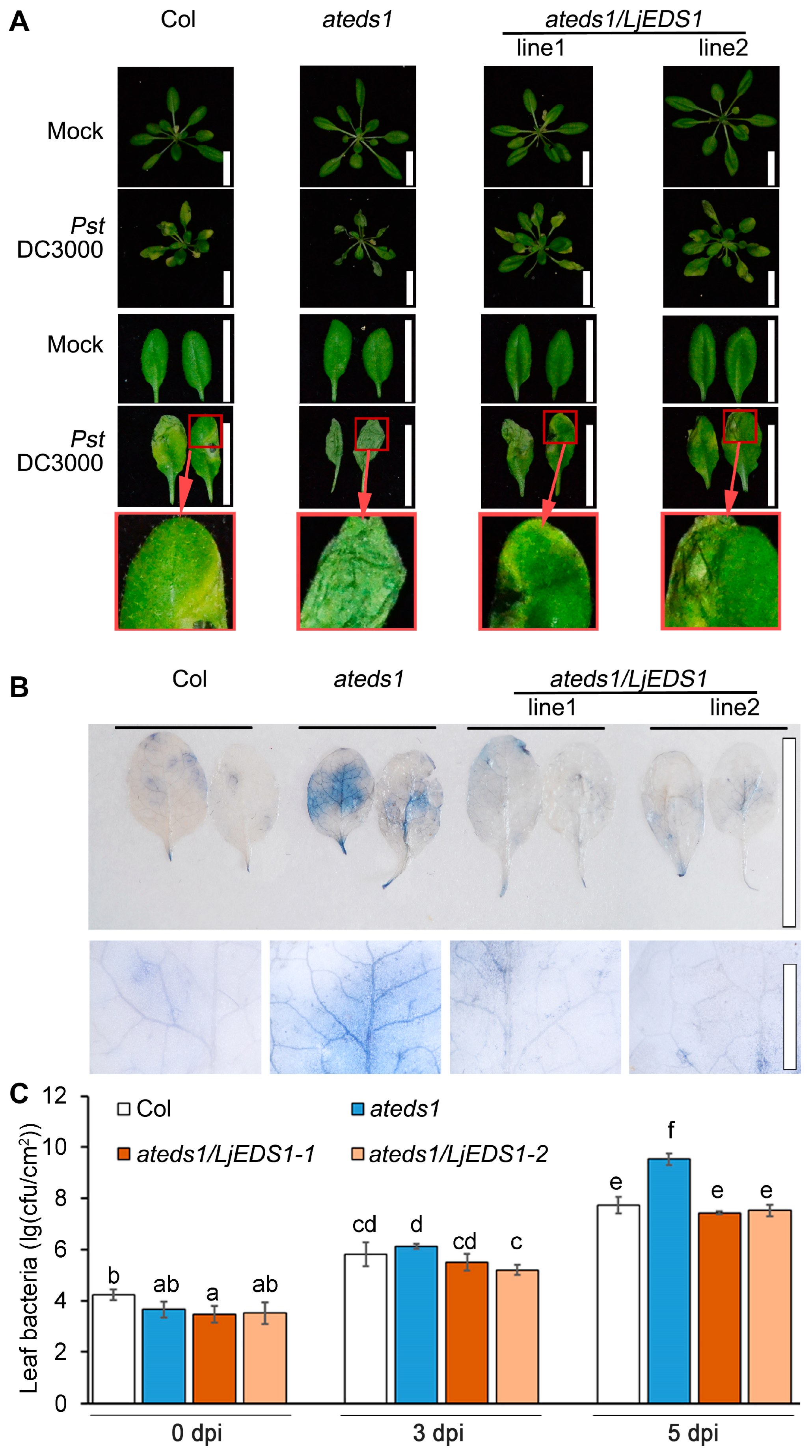
2.2. LjEDS1 Positively Regulates Disease Resistance to Pst DC3000 in Arabidopsis
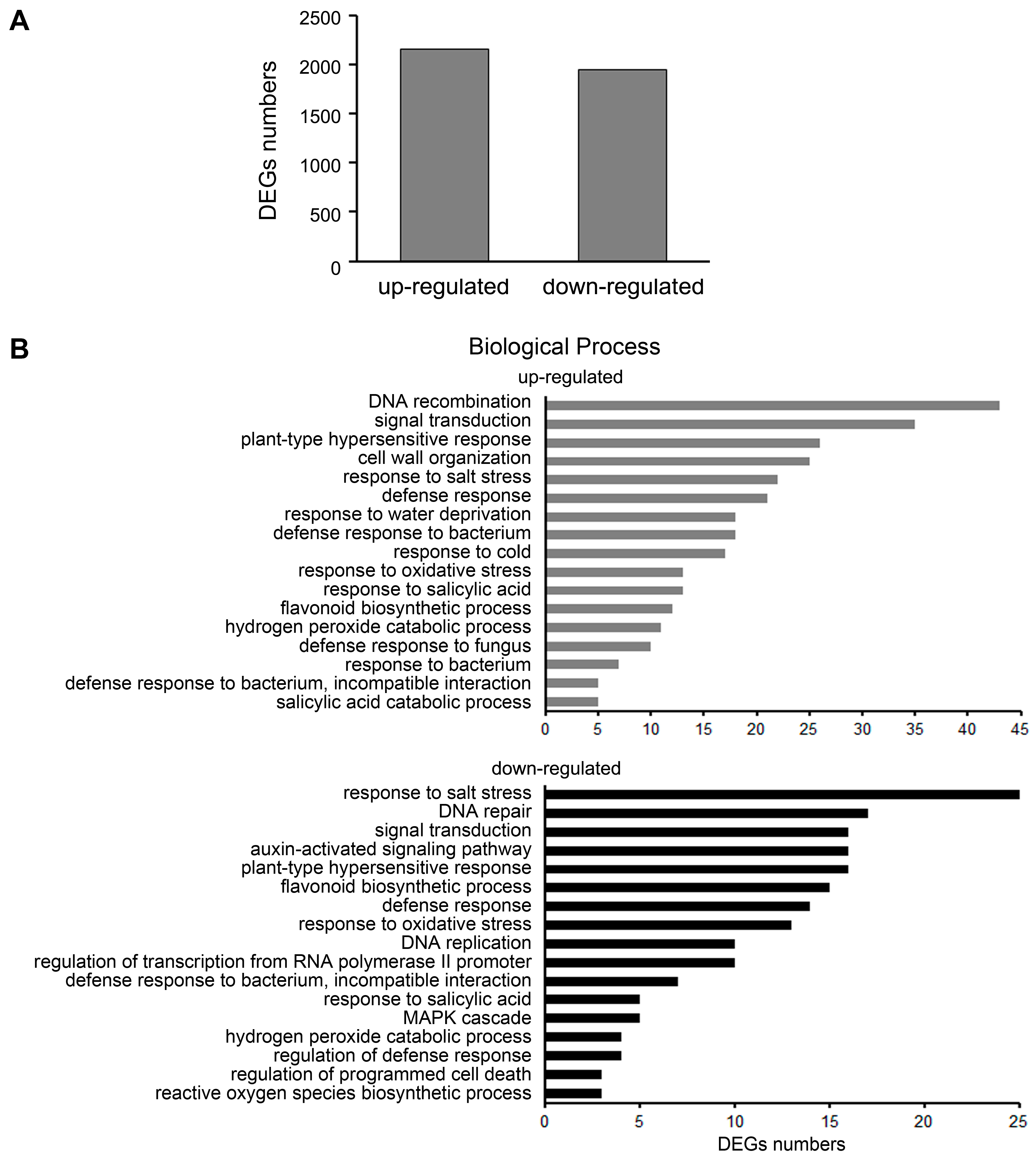
2.3. Differential Gene Expression Analysis in ljeds1 Roots
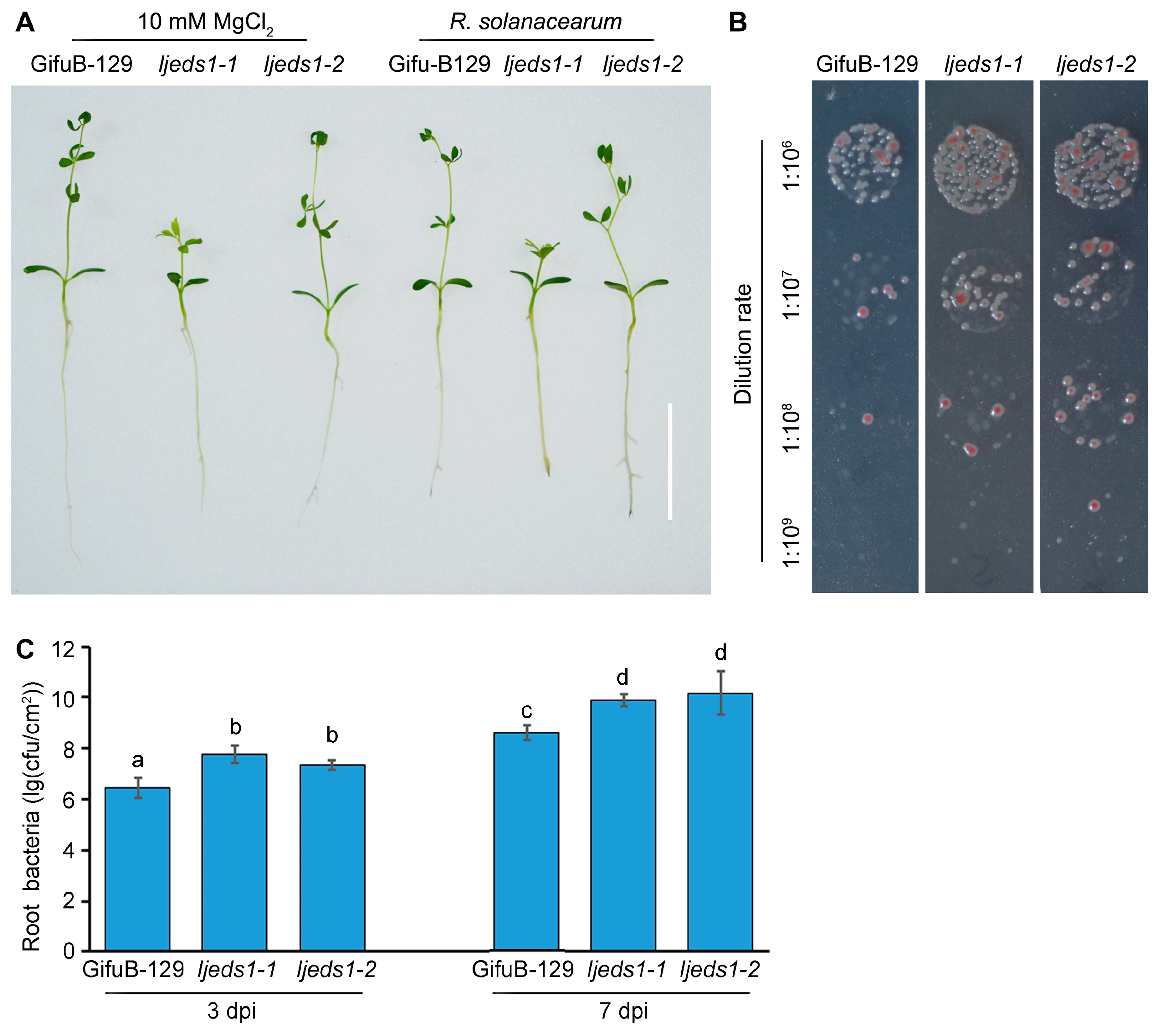
2.4. ljeds1 Mutants Are Susceptible to R. solanacearum
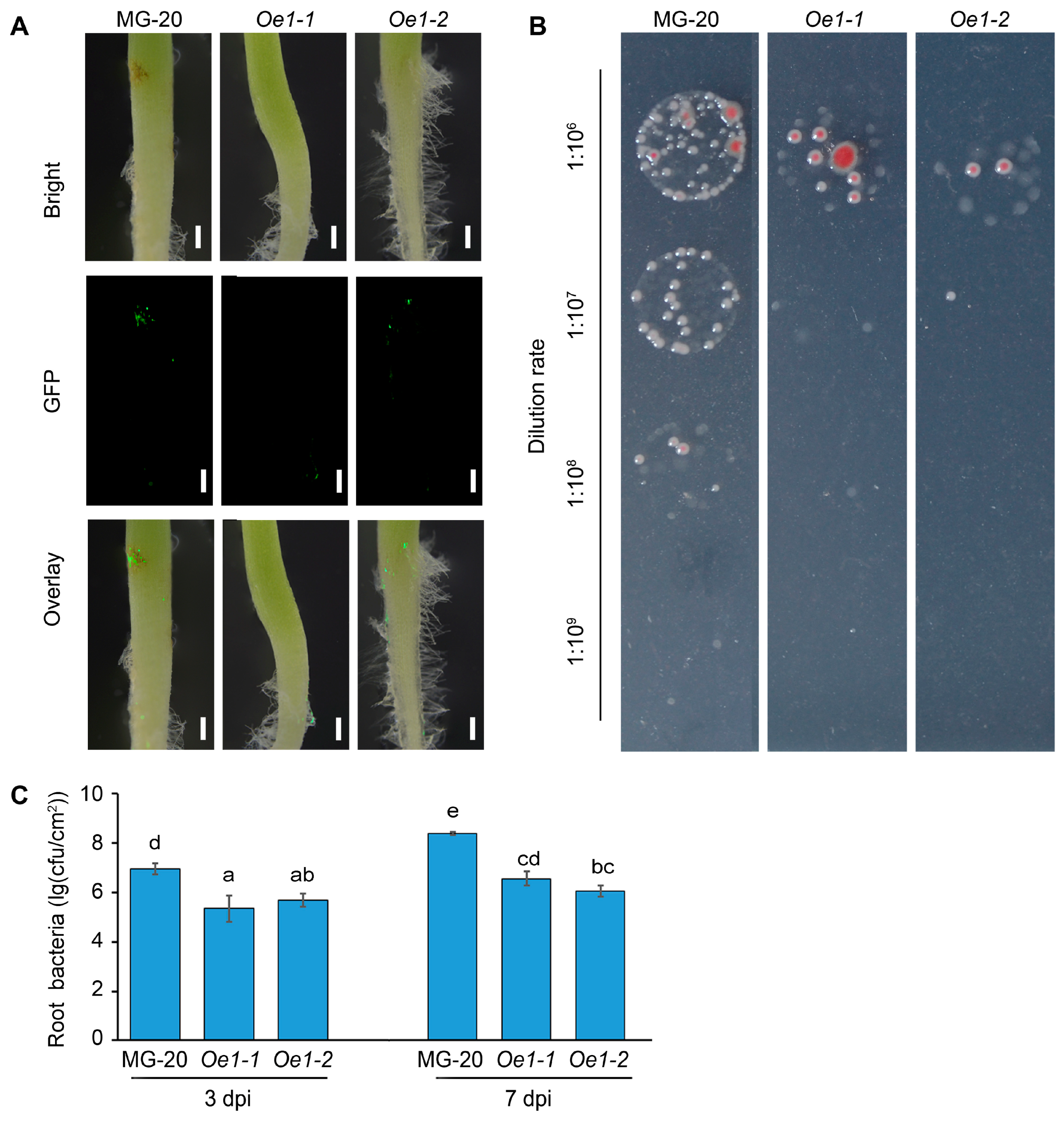
2.5. LjEDS1 Overexpression Enhances Resistance to R. solanacearum
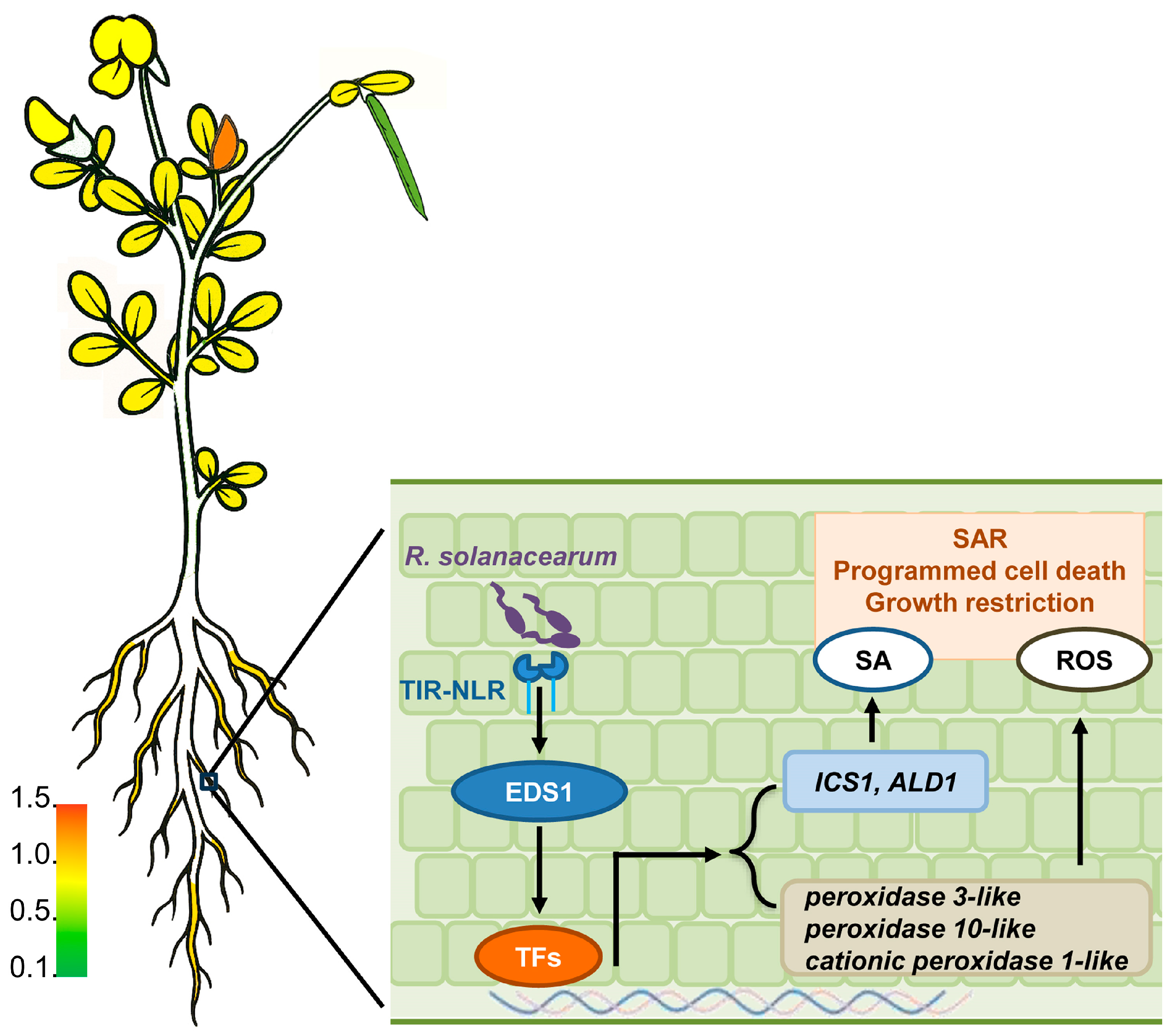
3. Discussion
4. Materials and Methods
4.1. Growth Conditions and Treatments of L. japonicus
4.2. Growth Conditions and Treatments of Arabidopsis
4.3. RNA-Seq and Transcriptome Data Analysis
4.4. RNA Isolation and Expression Analysis
4.5. Plasmid Construction and Plant Transformation
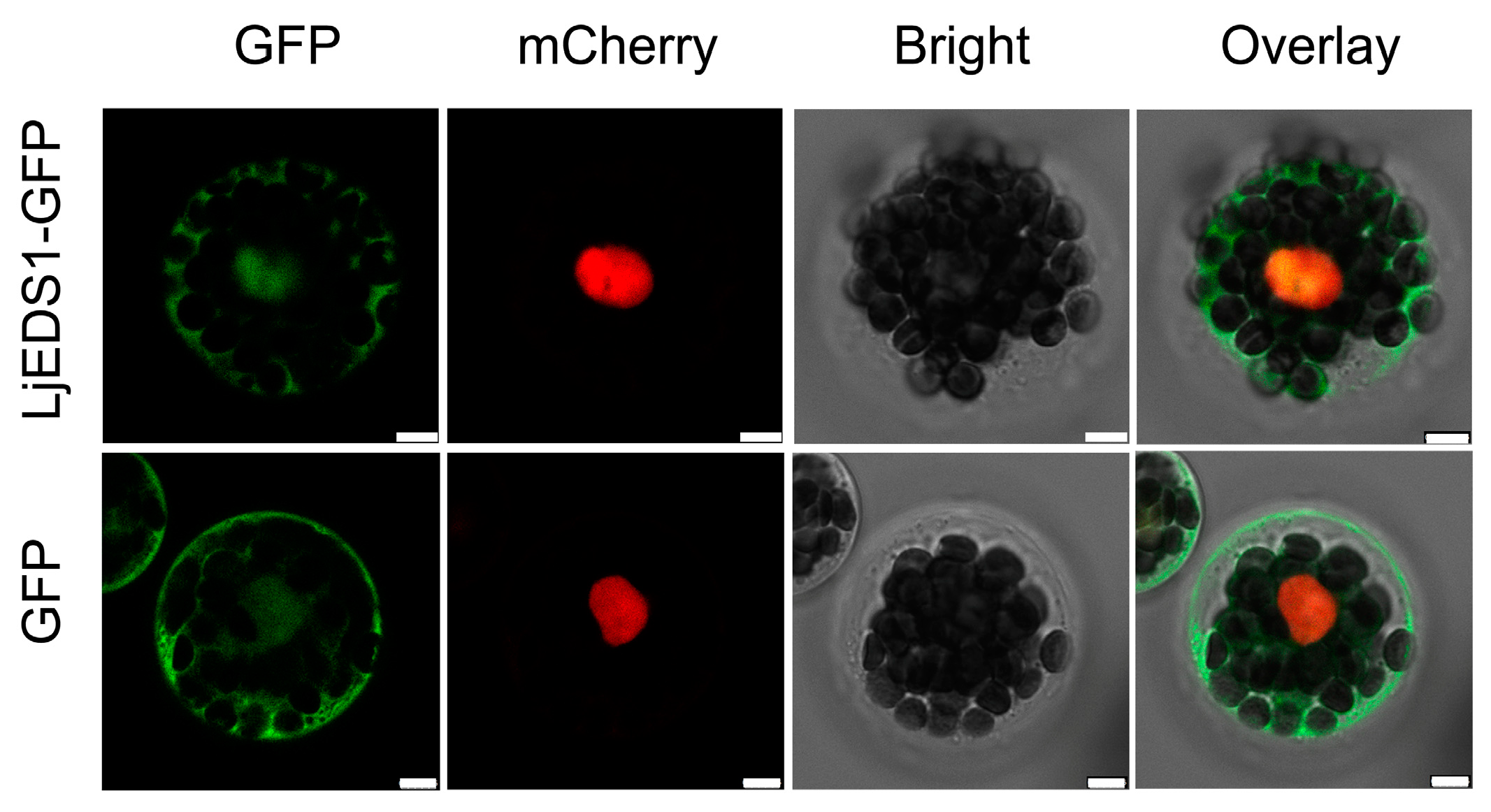
4.6. Subcellular Localization
4.7. Histochemistry Analysis
4.8. Sequence Analysis and Phylogenetic Tree Construction
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dou, D.; Zhou, J.M. Phytopathogen effectors subverting host immunity: Different foes, similar battleground. Cell Host Microbe. 2012, 12, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Dodds, P.N.; Rathjen, J.P. Plant immunity: Towards an integrated view of plant-pathogen interactions. Nat. Rev. Genet. 2010, 11, 539–548. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, J.; Kong, M.; Freeman, A.; Chen, H.; Liu, F. More stories to tell: NONEXPRESSOR OF PATHOGENESIS-RELATED GENES1, a salicylic acid receptor. Plant Cell Environ. 2021, 44, 1716–1727. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Zhang, L.; Xue, H.; Chen, Y.; Liu, X.; Del Pozo, J.C.; Zhao, C.; Lozano-Duran, R.; Macho, A.P. Cell wall-mediated root development is targeted by a soil-borne bacterial pathogen to promote infection. Cell Rep. 2024, 43, 114179. [Google Scholar]
- Pruitt, R.N.; Locci, F.; Wanke, F.; Zhang, L.; Saile, S.C.; Joe, A.; Karelina, D.; Hua, C.; Frohlich, K.; Wan, W.L.; et al. The EDS1-PAD4-ADR1 node mediates Arabidopsis pattern-triggered immunity. Nature 2021, 598, 495–499. [Google Scholar] [CrossRef]
- Heidrich, K.; Wirthmueller, L.; Tasset, C.; Pouzet, C.; Deslandes, L.; Parker, J.E. Arabidopsis EDS1 Connects Pathogen Effector Recognition to Cell Compartment-Specific Immune Responses. Science 2011, 334, 1401–1404. [Google Scholar] [CrossRef]
- Halane, M.K.; Kim, S.H.; Spears, B.J.; Garner, C.M.; Rogan, C.J.; Okafor, E.C.; Su, J.; Bhattacharjee, S.; Gassmann, W. The bacterial type III-secreted protein AvrRps4 is a bipartite effector. PLoS Pathog. 2018, 14, e1006984. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.; Chaturvedi, R.; Chowdhury, Z.; Venables, B.; Petros, R.A. Signaling by small metabolites in systemic acquired resistance. Plant J. 2014, 79, 645–658. [Google Scholar] [CrossRef]
- Cecchini, N.M.; Jung, H.W.; Engle, N.L.; Tschaplinski, T.J.; Greenberg, J.T. ALD1 Regulates Basal Immune Components and Early Inducible Defense Responses in Arabidopsis. Mol. Plant Microbe Interact. 2015, 28, 455–466. [Google Scholar] [CrossRef]
- Cui, H.; Gobbato, E.; Kracher, B.; Qiu, J.; Bautor, J.; Parker, J.E. A core function of EDS1 with PAD4 is to protect the salicylic acid defense sector in Arabidopsis immunity. New Phytol. 2017, 213, 1802–1817. [Google Scholar] [CrossRef]
- Huang, M.; Yuan, M.; Sun, C.; Li, M.; Wu, P.; Jiang, H.; Wu, G.; Chen, Y. Roles of AGD2a in Plant Development and Microbial Interactions of Lotus japonicus. Int. J. Mol. Sci. 2022, 23, 6863. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, X.; Tian, L.; Wu, P.; Li, M.; Jiang, H.; Chen, Y.; Wu, G. Knockdown of LjALD1, AGD2-like defense response protein 1, influences plant growth and nodulation in Lotus japonicus. J. Integr. Plant Biol. 2014, 56, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Feys, B.J.; Moisan, L.J.; Newman, M.A.; Parker, J.E. Direct interaction between the Arabidopsis disease resistance signaling proteins, EDS1 and PAD4. EMBO J. 2001, 20, 5400–5411. [Google Scholar] [CrossRef]
- Wagner, S.; Stuttmann, J.; Rietz, S.; Guerois, R.; Brunstein, E.; Bautor, J.; Niefind, K.; Parker, J.E. Structural basis for signaling by exclusive EDS1 heteromeric complexes with SAG101 or PAD4 in plant innate immunity. Cell Host Microbe 2013, 14, 619–630. [Google Scholar] [CrossRef]
- Lapin, D.; Bhandari, D.D.; Parker, J.E. Origins and Immunity Networking Functions of EDS1 Family Proteins. Annu. Rev. Phytopathol. 2020, 58, 253–276. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, M.; Chen, Y.; Wu, P.; Wu, G.; Jiang, H. Knockdown of OsPAO and OsRCCR1 cause different plant death phenotypes in rice. J. Plant Physiol. 2011, 168, 1952–1959. [Google Scholar] [CrossRef]
- Wang, J.; Shine, M.B.; Gao, Q.M.; Navarre, D.; Jiang, W.; Liu, C.; Chen, Q.; Hu, G.; Kachroo, A. Enhanced Disease Susceptibility1 Mediates Pathogen Resistance and Virulence Function of a Bacterial Effector in Soybean. Plant Physiol. 2014, 165, 1269–1284. [Google Scholar] [CrossRef]
- Lapin, D.; Kovacova, V.; Sun, X.; Dongus, J.A.; Bhandari, D.; Von Born, P.; Bautor, J.; Guarneri, N.; Rzemieniewski, J.; Stuttmann, J.; et al. A Coevolved EDS1-SAG101-NRG1 Module Mediates Cell Death Signaling by TIR-Domain Immune Receptors. Plant Cell 2019, 31, 2430–2455. [Google Scholar] [CrossRef]
- Bhandari, D.D.; Lapin, D.; Kracher, B.; Von Born, P.; Bautor, J.; Niefind, K.; Parker, J.E. An EDS1 heterodimer signalling surface enforces timely reprogramming of immunity genes in Arabidopsis. Nat. Commun. 2019, 10, 772. [Google Scholar]
- Gantner, J.; Ordon, J.; Kretschmer, C.; Guerois, R.; Stuttmann, J. An EDS1-SAG101 Complex Is Essential for TNL-Mediated Immunity in Nicotiana benthamiana. Plant Cell 2019, 31, 2456–2474. [Google Scholar] [CrossRef]
- Zeng, H.Y.; Liu, Y.; Chen, D.K.; Bao, H.N.; Huang, L.Q.; Yin, J.; Chen, Y.L.; Xiao, S.; Yao, N. The immune components ENHANCED DISEASE SUSCEPTIBILITY 1 and PHYTOALEXIN DEFICIENT 4 are required for cell death caused by overaccumulation of ceramides in Arabidopsis. Plant J. 2021, 107, 1447–1465. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Kang, Y.; Wu, M.; Liu, H.; Hui, S.; Zhang, Q.; Li, X.; Xiao, J.; Wang, S. Jasmonic Acid-Involved OsEDS1 Signaling in Rice-Bacteria Interactions. Rice 2019, 12, 25. [Google Scholar] [CrossRef]
- Naseem, M.; Srivastava, M.; Dandekar, T. Stem-cell-triggered immunity safeguards cytokinin enriched plant shoot apexes from pathogen infection. Front. Plant Sci. 2014, 5, 588. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Jeong, R.D.; Venugopal, S.C.; Lapchyk, L.; Navarre, D.; Kachroo, A.; Kachroo, P. SAG101 forms a ternary complex with EDS1 and PAD4 and is required for resistance signaling against turnip crinkle virus. PLoS Pathog. 2011, 7, e1002318. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.V.; Blanvillain-Baufume, S.; Huibers, R.P.; Wiermer, M.; Li, G.; Gobbato, E.; Rietz, S.; Parker, J.E. Balanced nuclear and cytoplasmic activities of EDS1 are required for a complete plant innate immune response. PLoS Pathog. 2010, 6, e1000970. [Google Scholar] [CrossRef]
- Khan, M.S.S.; Islam, F.; Chen, H.; Chang, M.; Wang, D.; Liu, F.; Fu, Z.Q.; Chen, J. Transcriptional Coactivators: Driving Force of Plant Immunity. Front. Plant Sci. 2022, 13, 823937. [Google Scholar] [CrossRef]
- Yan, Z.; Xingfen, W.; Wei, R.; Jun, Y.; Zhiying, M. Island Cotton Enhanced Disease Susceptibility 1 Gene Encoding a Lipase-Like Protein Plays a Crucial Role in Response to Verticillium dahliae by Regulating the SA Level and H(2)O(2) Accumulation. Front. Plant Sci. 2016, 7, 1830. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Hu, Y.; Liu, H.; He, M.; Yang, Z.; Kong, F.; Liu, X.; Hou, X. DELLA and EDS1 Form a Feedback Regulatory Module to Fine-Tune Plant Growth-Defense Tradeoff in Arabidopsis. Mol. Plant. 2019, 12, 1485–1498. [Google Scholar] [CrossRef]
- Stuttmann, J.; Peine, N.; Garcia, A.V.; Wagner, C.; Choudhury, S.R.; Wang, Y.; James, G.V.; Griebel, T.; Alcazar, R.; Tsuda, K.; et al. Arabidopsis thaliana DM2h (R8) within the Landsberg RPP1-like Resistance Locus Underlies Three Different Cases of EDS1-Conditioned Autoimmunity. PLoS Genet. 2016, 12, e1005990. [Google Scholar] [CrossRef]
- Poncini, L.; Wyrsch, I.; Denervaud Tendon, V.; Vorley, T.; Boller, T.; Geldner, N.; Metraux, J.P.; Lehmann, S. In roots of Arabidopsis thaliana, the damage-associated molecular pattern AtPep1 is a stronger elicitor of immune signalling than flg22 or the chitin heptamer. PLoS ONE 2017, 12, e0185808. [Google Scholar] [CrossRef]
- Jing, Y.P.; Zheng, X.J.; Zhang, D.L.; Shen, N.; Wang, Y.; Yang, L.; Fu, A.G.; Shi, J.S.; Zhao, F.G.; Lan, W.Z.; et al. Danger-Associated Peptides Interact with PIN-Dependent Local Auxin Distribution to Inhibit Root Growth in Arabidopsis. Plant Cell. 2019, 31, 1767–1787. [Google Scholar] [CrossRef]
- Lukan, T.; Coll, A. Intertwined Roles of Reactive Oxygen Species and Salicylic Acid Signaling Are Crucial for the Plant Response to Biotic Stress. Int. J. Mol. Sci. 2022, 23, 5568. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.F.; Xu, L.; Tan, W.J.; Chen, L.; Qi, H.; Xie, L.J.; Chen, M.X.; Liu, B.Y.; Yu, L.J.; Yao, N.; et al. Disruption of the Arabidopsis Defense Regulator Genes SAG101, EDS1, and PAD4 Confers Enhanced Freezing Tolerance. Mol. Plant 2015, 8, 1536–1549. [Google Scholar]
- Chen, H.; Li, M.; Qi, G.; Zhao, M.; Liu, L.; Zhang, J.; Chen, G.; Wang, D.; Liu, F.; Fu, Z.Q. Two interacting transcriptional coactivators cooperatively control plant immune responses. Sci. Adv. 2021, 7, eabl7173. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xin, J.; Liu, L.; Song, A.; Guan, Z.; Fang, W.; Chen, F. A temporal gene expression map of Chrysanthemum leaves infected with Alternaria alternata reveals different stages of defense mechanisms. Hortic. Res. 2020, 7, 23. [Google Scholar] [CrossRef]
- Li, S.; Zhao, J.; Zhai, Y.; Yuan, Q.; Zhang, H.; Wu, X.; Lu, Y.; Peng, J.; Sun, Z.; Lin, L.; et al. The hypersensitive induced reaction 3 (HIR3) gene contributes to plant basal resistance via an EDS1 and salicylic acid-dependent pathway. Plant J. 2019, 98, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Tarkowska, D.; Sedaghatmehr, M.; Welsch, M.; Gupta, S.; Mueller-Roeber, B.; Balazadeh, S. The HB40-JUB1 transcriptional regulatory network controls gibberellin homeostasis in Arabidopsis. Mol. Plant 2022, 15, 322–339. [Google Scholar] [CrossRef]
- Bigeard, J.; Colcombet, J.; Hirt, H. Signaling mechanisms in pattern-triggered immunity (PTI). Mol. Plant 2015, 8, 521–539. [Google Scholar]
- Zhang, J.; Shao, F.; Li, Y.; Cui, H.; Chen, L.; Li, H.; Zou, Y.; Long, C.; Lan, L.; Chai, J.; et al. A Pseudomonas syringae effector inactivates MAPKs to suppress PAMP-induced immunity in plants. Cell Host Microbe. 2007, 1, 175–185. [Google Scholar] [CrossRef]
- Tsuda, K.; Mine, A.; Bethke, G.; Igarashi, D.; Botanga, C.J.; Tsuda, Y.; Glazebrook, J.; Sato, M.; Katagiri, F. Dual regulation of gene expression mediated by extended MAPK activation and salicylic acid contributes to robust innate immunity in Arabidopsis thaliana. PLoS Genet. 2013, 9, e1004015. [Google Scholar]
- Yang, L.; Li, B.; Zheng, X.-Y.; Li, J.; Yang, M.; Dong, X.; He, G.; An, C.; Deng, X.W. Salicylic acid biosynthesis is enhanced and contributes to increased biotrophic pathogen resistance in Arabidopsis hybrids. Nat. Commun. 2015, 6, 7309. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; William, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; Baren, M.J.; Salzberg, L.S.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Stiller, J.; Martirani, L.; Tuppale, S.; Chian, R.J.; Chiurazzi, M.; Gresshoff, P.M. High frequency transformation and regeneration of transgenic plants in the model legume Lotus japonicus. J. Exp. Bot. 1997, 48, 1357–1365. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.D.; Cho, Y.H.; Sheen, J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2007, 2, 1565–1572. [Google Scholar]
- Son, G.H.; Moon, J.; Shelake, R.M.; Vuong, U.T.; Ingle, R.A.; Gassmann, W.; Kim, J.Y.; Kim, S.H. Conserved Opposite Functions in Plant Resistance to Biotrophic and Necrotrophic Pathogens of the Immune Regulator SRFR1. Int. J. Mol. Sci. 2021, 22, 6427. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, M.; Li, Q.; Huang, M.; Huang, H.; Sun, C.; Jiang, H.; Wu, G.; Chen, Y. Enhanced Disease Susceptibility1 Regulates Immune Response in Lotus japonicus. Int. J. Mol. Sci. 2025, 26, 3848. https://doi.org/10.3390/ijms26083848
Yuan M, Li Q, Huang M, Huang H, Sun C, Jiang H, Wu G, Chen Y. Enhanced Disease Susceptibility1 Regulates Immune Response in Lotus japonicus. International Journal of Molecular Sciences. 2025; 26(8):3848. https://doi.org/10.3390/ijms26083848
Chicago/Turabian StyleYuan, Mengru, Qiong Li, Mingchao Huang, Hongdou Huang, Chunyu Sun, Huawu Jiang, Guojiang Wu, and Yaping Chen. 2025. "Enhanced Disease Susceptibility1 Regulates Immune Response in Lotus japonicus" International Journal of Molecular Sciences 26, no. 8: 3848. https://doi.org/10.3390/ijms26083848
APA StyleYuan, M., Li, Q., Huang, M., Huang, H., Sun, C., Jiang, H., Wu, G., & Chen, Y. (2025). Enhanced Disease Susceptibility1 Regulates Immune Response in Lotus japonicus. International Journal of Molecular Sciences, 26(8), 3848. https://doi.org/10.3390/ijms26083848





