Plasma Levels of MicroRNA Let-7c-5p May Predict Risk of Acute Chest Syndrome in Patients with Sickle Cell Disease
Abstract
1. Introduction
2. Results
2.1. Characteristics of Subjects Used for RNA Sequencing
2.2. MicroRNA Contents of Small EVs in Subjects with SCD
2.3. Characteristics of Subjects Used for RT-qPCR of Let-7c-5p (Replication Cohort)
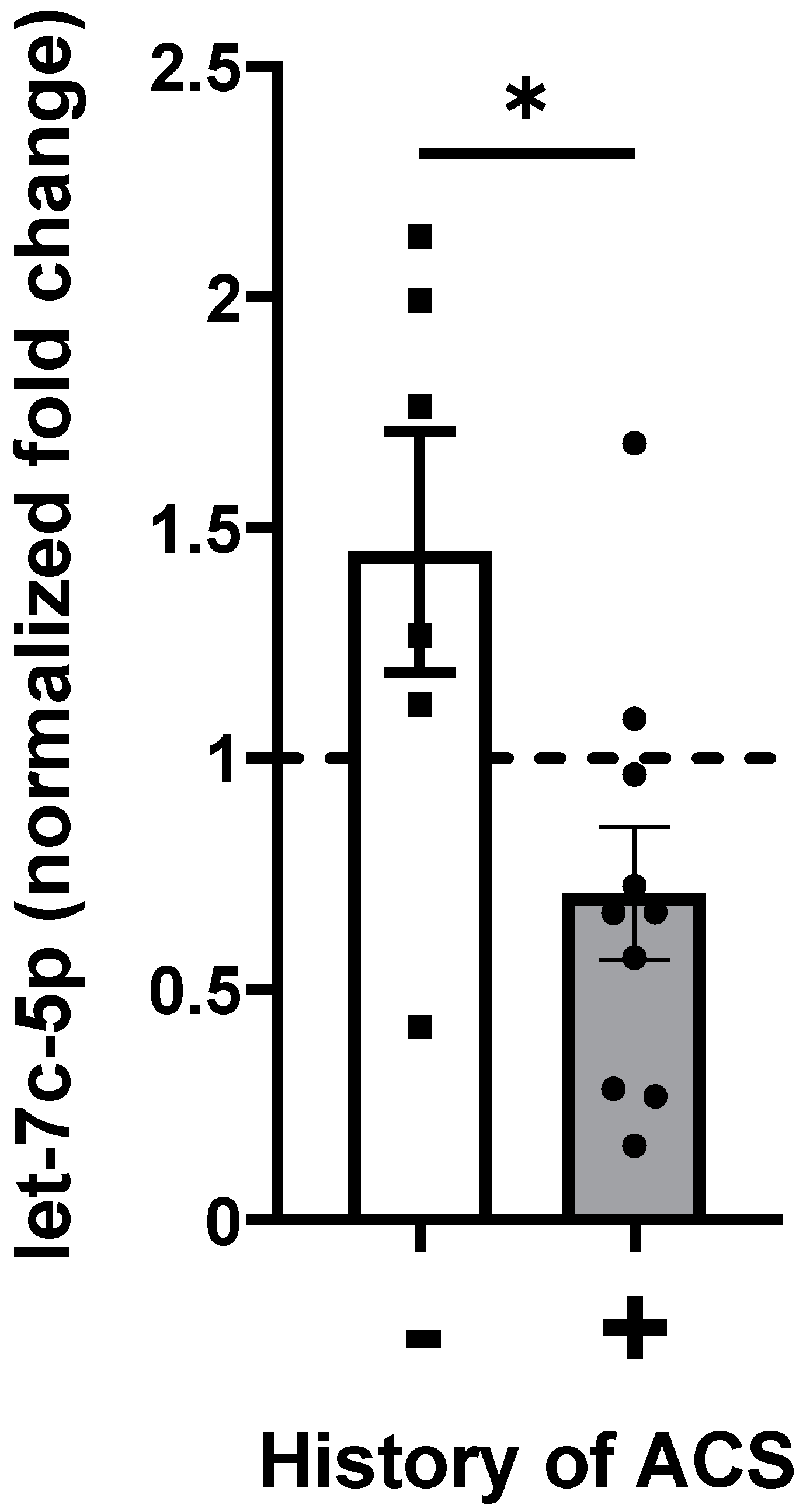
2.4. Let-7c-5p Levels in Subjects with SCD
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Isolation of Platelet-Free Plasma
4.3. Small RNA Isolation and Its Sequencing in Discovery Cohort
4.4. Sequencing Data Analysis
4.5. MicroRNA Extraction and RT-qPCR in Replication Cohort
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SCD | Sickle cell disease |
| ACS | Acute chest syndrome |
| RT-qPCR | Quantitative reverse transcription polymerase chain reaction |
| EV | Extracellular vesicle |
| NS | Not Significant |
| sPLA2 | Secretory phospholipase A2 |
References
- Hoppe, C.C. Inflammatory mediators of endothelial injury in sickle cell disease. Hematol. Oncol. Clin. N. Am. 2014, 28, 265–286. [Google Scholar] [CrossRef] [PubMed]
- Sundd, P.; Gladwin, M.T.; Novelli, E.M. Pathophysiology of sickle cell disease. Annu. Rev. Pathol. 2019, 14, 263–292. [Google Scholar] [CrossRef]
- Gladwin, M.T.; Vichinsky, E. Pulmonary complications of sickle cell disease. N. Engl. J. Med. 2008, 359, 2254–2265. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.T. How I treat acute chest syndrome in children with sickle cell disease. Blood 2011, 117, 5297–5305. [Google Scholar] [CrossRef]
- Howard, J.; Hart, N.; Roberts-Harewood, M.; Cummins, M.; Awogbade, M.; Davis, B. BCSH Committee guideline on the management of acute chest syndrome in sickle cell disease. Br. J. Haematol. 2015, 169, 492–505. [Google Scholar] [CrossRef]
- DeBaun, M.R.; Rodeghier, M.; Cohen, R.; Kirkham, F.J.; Rosen, C.L.; Roberts, I.; Cooper, B.; Stocks, J.; Wilkey, O.; Inusa, B.; et al. Factors predicting future ACS episodes in children with sickle cell anemia. Am. J. Hematol. 2014, 89, E212–E217. [Google Scholar] [CrossRef] [PubMed]
- Hebbel, R.P.; Key, N.S. Microparticles in sickle cell anaemia: Promise and pitfalls. Br. J. Haematol. 2016, 174, 16–29. [Google Scholar] [CrossRef]
- Ridger, V.C.; Boulanger, C.M.; Angelillo-Scherrer, A.; Badimon, L.; Blanc-Brude, O.; Bochaton-Piallat, M.-L.; Boilard, E.; Buzas, E.I.; Caporali, A.; Dignat-George, F.; et al. Microvesicles in vascular homeostasis and diseases. Position Paper of the European Society of Cardiology (ESC) Working Group on Atherosclerosis and Vascular Biology. Thromb. Haemost. 2017, 117, 1296–1316. [Google Scholar] [CrossRef]
- Oggero, S.; Austin-Williams, S.; Norling, L.V. The contrasting role of extracellular vesicles in vascular inflammation and tissue repair. Front. Pharmacol. 2019, 10, 1479. [Google Scholar] [CrossRef]
- Lapping-Carr, G.; Gemel, J.; Mao, Y.; Beyer, E.C. Circulating extracellular vesicles and endothelial damage in sickle cell disease. Front. Physiol. 2020, 11, 1063. [Google Scholar] [CrossRef]
- Khalyfa, A.; Khalyfa, A.A.; Akbarpour, M.; Connes, P.; Romana, M.; Lapping-Carr, G.; Zhang, C.; Andrade, J.; Gozal, D. Extracellular microvesicle microRNAs in children with sickle cell anaemia with divergent clinical phenotypes. Br. J. Haematol. 2016, 174, 786–798. [Google Scholar] [CrossRef]
- Lapping-Carr, G.; Khalyfa, A.; Rangel, S.; Darlington, W.; Beyer, E.C.; Peddinti, R.; Cunningham, J.M.; Gozal, D. Exosomes contribute to endothelial integrity and acute chest syndrome risk: Preliminary findings. Pediatr. Pulmonol. 2017, 52, 1478–1485. [Google Scholar] [CrossRef]
- Lapping-Carr, G.; Gemel, J.; Mao, Y.; Sparks, G.; Harrington, M.; Peddinti, R.; Beyer, E.C. Circulating extracellular vesicles from patients with acute chest syndrome disrupt adherens junctions between endothelial cells. Pediatr. Res. 2021, 89, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Gemel, J.; Mao, Y.; Lapping-Carr, G.; Beyer, E.C. Gap junctions between endothelial cells are disrupted by circulating extracellular vesicles from sickle cell patients with acute chest syndrome. Int. J. Mol. Sci. 2020, 21, 884. [Google Scholar] [CrossRef] [PubMed]
- Gemel, J.; Zhang, J.; Mao, Y.; Lapping-Carr, G.; Beyer, E.C. Circulating small extracellular vesicles may contribute to vaso-occlusive crises in sickle cell disease. J. Clin. Med. 2022, 11, 816. [Google Scholar] [CrossRef] [PubMed]
- Roush, S.; Slack, F.J. The let-7 family of microRNAs. Trends Cell Biol. 2008, 18, 505–516. [Google Scholar] [CrossRef]
- Reinhart, B.J.; Slack, F.J.; Basson, M.; Pasquinelli, A.E.; Bettinger, J.C.; Rougvie, A.E.; Horvitz, H.R.; Ruvkun, G. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature 2000, 403, 901–906. [Google Scholar] [CrossRef]
- Thornton, J.E.; Gregory, R.I. How does Lin28 let-7 control development and disease? Trends Cell Biol. 2012, 22, 474–482. [Google Scholar] [CrossRef]
- Rybak, A.; Fuchs, H.; Smirnova, L.; Brandt, C.; Pohl, E.E.; Nitsch, R.; Wulczyn, F.G. A feedback loop comprising lin-28 and let-7 controls pre-let-7 maturation during neural stem-cell commitment. Nat. Cell Biol. 2008, 10, 987–993. [Google Scholar] [CrossRef]
- Viswanathan, S.R.; Daley, G.Q.; Gregory, R.I. Selective blockade of microRNA processing by Lin28. Science 2008, 320, 97–100. [Google Scholar] [CrossRef]
- Jiang, S. Recent findings regarding let-7 in immunity. Cancer Lett. 2018, 434, 130–131. [Google Scholar] [CrossRef] [PubMed]
- Letafati, A.; Najafi, S.; Mottahedi, M.; Karimzadeh, M.; Shahini, A.; Garousi, S.; Abbasi-Kolli, M.; Sadri Nahand, J.; Tamehri Zadeh, S.S.; Hamblin, M.R.; et al. MicroRNA let-7 and viral infections: Focus on mechanisms of action. Cell. Mol. Biol. Lett. 2022, 27, 14. [Google Scholar] [CrossRef]
- Han, J.; Cui, X.; Yuan, T.; Yang, Z.; Liu, Y.; Ren, Y.; Wu, C.; Bian, Y. Plasma-derived exosomal let-7c-5p, miR-335-3p, and miR-652-3p as potential diagnostic biomarkers for stable coronary artery disease. Front. Physiol. 2023, 14, 1161612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, S.; Zhu, W.; Niu, T.; Wang, J.; Yang, M.; Liu, E.; Wang, J.; Li, S.; Zhang, H. Exosomal miR-let-7c-5p is involved in the cognitive function of type 2 diabetes mellitus patients by interleukin 10: A cross-sectional study. J. Diabetes 2023, 15, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Law, Y.-Y.; Lee, W.-F.; Hsu, C.-J.; Lin, Y.-Y.; Tsai, C.-H.; Huang, C.-C.; Wu, M.-H.; Tang, C.-H.; Liu, J.-F. miR-let-7c-5p and miR-149-5p inhibit proinflammatory cytokine production in osteoarthritis and rheumatoid arthritis synovial fibroblasts. Aging 2021, 13, 17227–17236. [Google Scholar] [CrossRef]
- Ni, J.; Wang, X.; Chen, S.; Liu, H.; Wang, Y.; Xu, X.; Cheng, J.; Jia, J.; Zhen, X. MicroRNA let-7c-5p protects against cerebral ischemia injury via mechanisms involving the inhibition of microglia activation. Brain Behav. Immun. 2015, 49, 75–85. [Google Scholar] [CrossRef]
- Kostiniuk, D.; Marttila, S.; Raitoharju, E. Circulatory miRNAs in essential hypertension. Atherosclerosis 2024, 401, 119069. [Google Scholar] [CrossRef]
- Naprawa, J.T.; Bonsu, B.K.; Goodman, D.G.; Ranalli, M.A. Serum biomarkers for identifying acute chest syndrome among patients who have sickle cell disease and present to the emergency department. Pediatrics 2005, 116, e420–e425. [Google Scholar] [CrossRef]
- Styles, L.A.; Schalkwijk, C.G.; Aarsman, A.J.; Vichinsky, E.P.; Lubin, B.H.; Kuypers, F.A. Phospholipase A2 levels in acute chest syndrome of sickle cell disease. Blood 1996, 87, 2573–2578. [Google Scholar] [CrossRef]
- Alsabri, M.; Elsnhory, A.B.; Alattar, O.; Ahmed, N.; Ashraf, S.; Alassri, R. Secretory Phospholipase A2 as a promising biomarker for predicting acute chest syndrome in children with sickle cell disease: A Systematic Review and Meta-Analysis. Cureus 2024, 16, e69053. [Google Scholar] [CrossRef]
- Hammerman, S.I.; Kourembanas, S.; Conca, T.J.; Tucci, M.; Brauer, M.; Farber, H.W. Endothelin-1 production during the acute chest syndrome in sickle cell disease. Am. J. Respir. Crit. Care Med. 1997, 156, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Graido-Gonzalez, E.; Doherty, J.C.; Bergreen, E.W.; Organ, G.; Telfer, M.; McMillen, M.A. Plasma endothelin-1, cytokine, and prostaglandin E2 levels in sickle cell disease and acute vaso-occlusive sickle crisis. Blood 1998, 92, 2551–2555. [Google Scholar] [CrossRef] [PubMed]
- Sharan, K.; Surrey, S.; Ballas, S.; Borowski, M.; Devoto, M.; Wang, K.F.; Sandler, E.; Keller, M. Association of T-786C eNOS gene polymorphism with increased susceptibility to acute chest syndrome in females with sickle cell disease. Br. J. Haematol. 2004, 124, 240–243. [Google Scholar] [CrossRef]
- Chaar, V.; Tarer, V.; Etienne-Julan, M.; Diara, J.P.; Elion, J.; Romana, M. ET-1 and ecNOS gene polymorphisms and susceptibility to acute chest syndrome and painful vaso-occlusive crises in children with sickle cell anemia. Haematologica 2006, 91, 1277–1278. [Google Scholar] [PubMed]
- Schaefer, E.; Anderson-Crannage, M.; Hochberg, J.; Kanarfogel, T.; Ktena, Y.P.; Herrick, J.; Thatcher, E.; Shi, Q.; Hochberg, B.; Chu, Y.; et al. A Phase II Study to Evaluate the Safety and Efficacy of Defibrotide and Changes in Plasma Biomarkers in Sickle Cell Disease-Related Acute Chest Syndrome (IND 127812). Blood 2023, 142, 2520. [Google Scholar] [CrossRef]
- Babraham Bioinformatics-FastQC a Quality Control Tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 1 March 2025).
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Picard Tools-By Broad Institute. Available online: https://broadinstitute.github.io/picard/ (accessed on 1 March 2025).
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Bioconductor-DESeq2. Available online: https://bioconductor.org/packages/3.8/bioc/html/DESeq2.html (accessed on 1 March 2025).
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Roberts, T.C.; Coenen-Stass, A.M.L.; Wood, M.J.A. Assessment of RT-qPCR normalization strategies for accurate quantification of extracellular microRNAs in murine serum. PLoS ONE 2014, 9, e89237. [Google Scholar] [CrossRef]


| No History of ACS (n = 12) | History of ACS (n = 17) | p-Value | |
|---|---|---|---|
| Demographics | |||
| Age (years), median (range) | 14 (3–18) | 14 (5–20) | NS |
| Sex, n (%) | NS | ||
| Male | 7 (58) | 11 (65) | |
| Female | 5 (42) | 6 (35) | |
| Hematologic Values | Median (25%, 75%) | Median (25%, 75%) | |
| White blood cell count (×103/µL) | 9.2 (8.4, 14.3) | 11.8 (10.7, 14.6) | NS |
| Hemoglobin (g/dL) | 8.4 (8.1, 9.1) | 9.3 (7.8, 10.7) | NS |
| MCV (fL) | 84.2 (77.3, 93.8) | 95.2 (88.3, 101) | <0.05 |
| Reticulocyte (×103/µL) | 266 (212, 384) | 249(161, 303) | NS |
| Platelet (×103/µL) | 404 (291, 462) | 414 (400, 458) | NS |
| Bilirubin (mg/dL) | 3.0 (2.0, 4.6) | 2.9 (1.8, 6.3) | NS |
| Hemoglobin Genotype, n (%) | |||
| SS | 11 (92) | 15 (88) | |
| Sβ0Thal | 0 | 1 (6) | |
| SC | 1 (8) | 1 (6) | |
| Clinical Characteristics | |||
| Rate of ACS (n/year) | 0 | 0.17 (0.08, 0.23) | <0.05 |
| Absolute ACS, n | 0 | 1 (1, 3.5) | <0.005 |
| Rate of pain (n/year) | 0 (0, 0.23) | 0.18 (0.08, 0.65) | NS |
| Absolute pain, n | 0 (0, 3.5) | 2 (1, 9) | NS |
| Hydroxyurea, n (%) | 3 (25) | 12 (71) | <0.05 |
| Asthma, n (%) | 0 | 5 (29) | <0.05 |
| Obstructive sleep apnea (%) | 6 (50) | 4 (24) | NS |
| Splenectomy, n (%) | 3 (25) | 4 (24) | NS |
| Cholecystectomy, n (%) | 1 (8) | 4 (24) | NS |
| No History of ACS (n = 6) | History of ACS (n = 10) | p-Value | |
|---|---|---|---|
| Demographics | |||
| Age (years), median (range) | 4 (4–8) | 8 (4–9) | NS |
| Sex, n (%) | NS | ||
| Male | 3 (50) | 3 (30) | |
| Female | 3 (50) | 7 (70) | |
| Hematologic Values | Median (25%, 75%) | Median (25%, 75%) | |
| White blood cell count (×103/µL) | 12.3 (10.6, 13.2) | 10.9 (9.9, 12.4) | NS |
| Hemoglobin (g/dL) | 8.3 (8.2, 8.7) | 9.2 (8.0, 9.7) | NS |
| MCV (fL) | 88.6 (84.1, 92.4) | 93.5 (91.4, 98.0) | NS |
| Reticulocyte (×103/µL) | 258 (217, 298) | 231 (203, 259) | NS |
| Platelet (×103/µL) | 402 (282, 509) | 429 (383, 459) | NS |
| Bilirubin (mg/dL) | 1.9 (1.9, 2.6) | 3.1 (2.1, 4.7) | NS |
| Hemoglobin Genotype, n (%) | |||
| SS | 5 (83) | 10 (100) | |
| Sβ0Thal | 1 (14) | 0 (0) | |
| Clinical Characteristics | |||
| Rate of ACS (n/year) | 0 | 0.25 (0.20, 0.25) | <0.005 |
| Absolute ACS, n | 0 | 1.00 (1.00, 2.75) | <0.005 |
| Rate of pain (n/year) | 0.6 (0, 0.2) | 0.5 (0.1, 0.8) | NS |
| Absolute pain, n | 0.5 (0, 1.0) | 2.0 (1.0, 7.0) | NS |
| Hydroxyurea, n (%) | 1 (12) | 6 (60) | <0.05 |
| Asthma, n (%) | 1 (12) | 3 (30) | NS |
| Obstructive sleep apnea (%) | 0 | 4 (40) | <0.05 |
| Splenectomy, n (%) | 1 (12) | 2 (20) | NS |
| Cholecystectomy, n (%) | 0 | 1 (10) | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, J.; Gemel, J.; Beyer, E.C.; Lapping-Carr, G. Plasma Levels of MicroRNA Let-7c-5p May Predict Risk of Acute Chest Syndrome in Patients with Sickle Cell Disease. Int. J. Mol. Sci. 2025, 26, 3831. https://doi.org/10.3390/ijms26083831
Fan J, Gemel J, Beyer EC, Lapping-Carr G. Plasma Levels of MicroRNA Let-7c-5p May Predict Risk of Acute Chest Syndrome in Patients with Sickle Cell Disease. International Journal of Molecular Sciences. 2025; 26(8):3831. https://doi.org/10.3390/ijms26083831
Chicago/Turabian StyleFan, James, Joanna Gemel, Eric C. Beyer, and Gabrielle Lapping-Carr. 2025. "Plasma Levels of MicroRNA Let-7c-5p May Predict Risk of Acute Chest Syndrome in Patients with Sickle Cell Disease" International Journal of Molecular Sciences 26, no. 8: 3831. https://doi.org/10.3390/ijms26083831
APA StyleFan, J., Gemel, J., Beyer, E. C., & Lapping-Carr, G. (2025). Plasma Levels of MicroRNA Let-7c-5p May Predict Risk of Acute Chest Syndrome in Patients with Sickle Cell Disease. International Journal of Molecular Sciences, 26(8), 3831. https://doi.org/10.3390/ijms26083831







