Restoration of pp60Src Re-Establishes Electron Transport Chain Complex I Activity in Pulmonary Hypertensive Endothelial Cells
Abstract
1. Introduction
2. Results
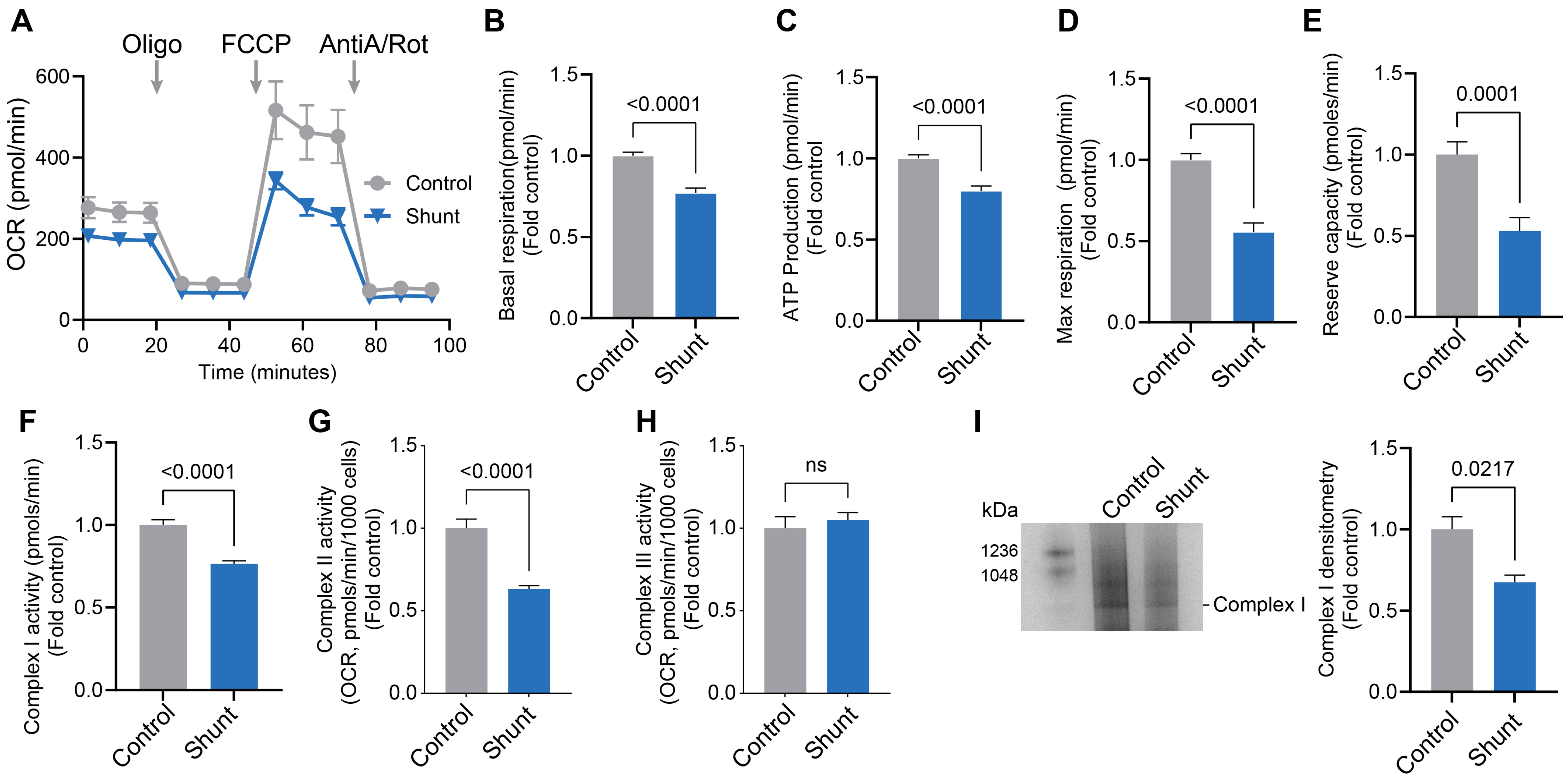
2.1. Pulmonary Arterial Endothelial Cells Isolated from a Lamb Model of PH (Shunt) Have Reduced Mitochondrial Bioenergetics and Decreased ETC Complex I Activity
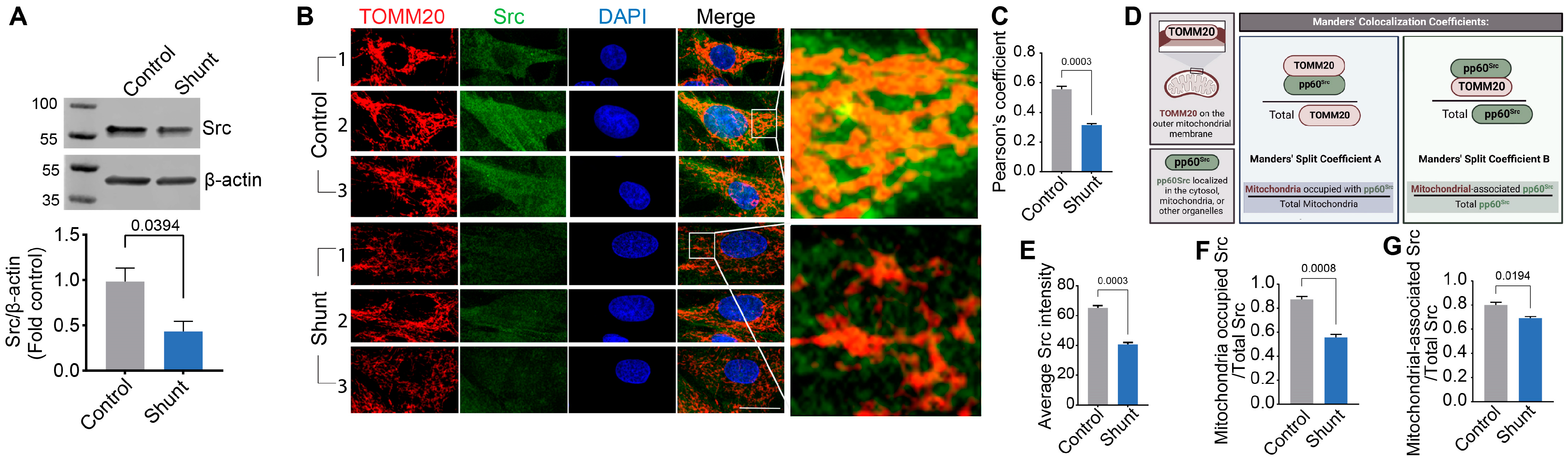
2.2. Shunt PAECs Have Reduced Mitochondrial pp60Src Accumulation
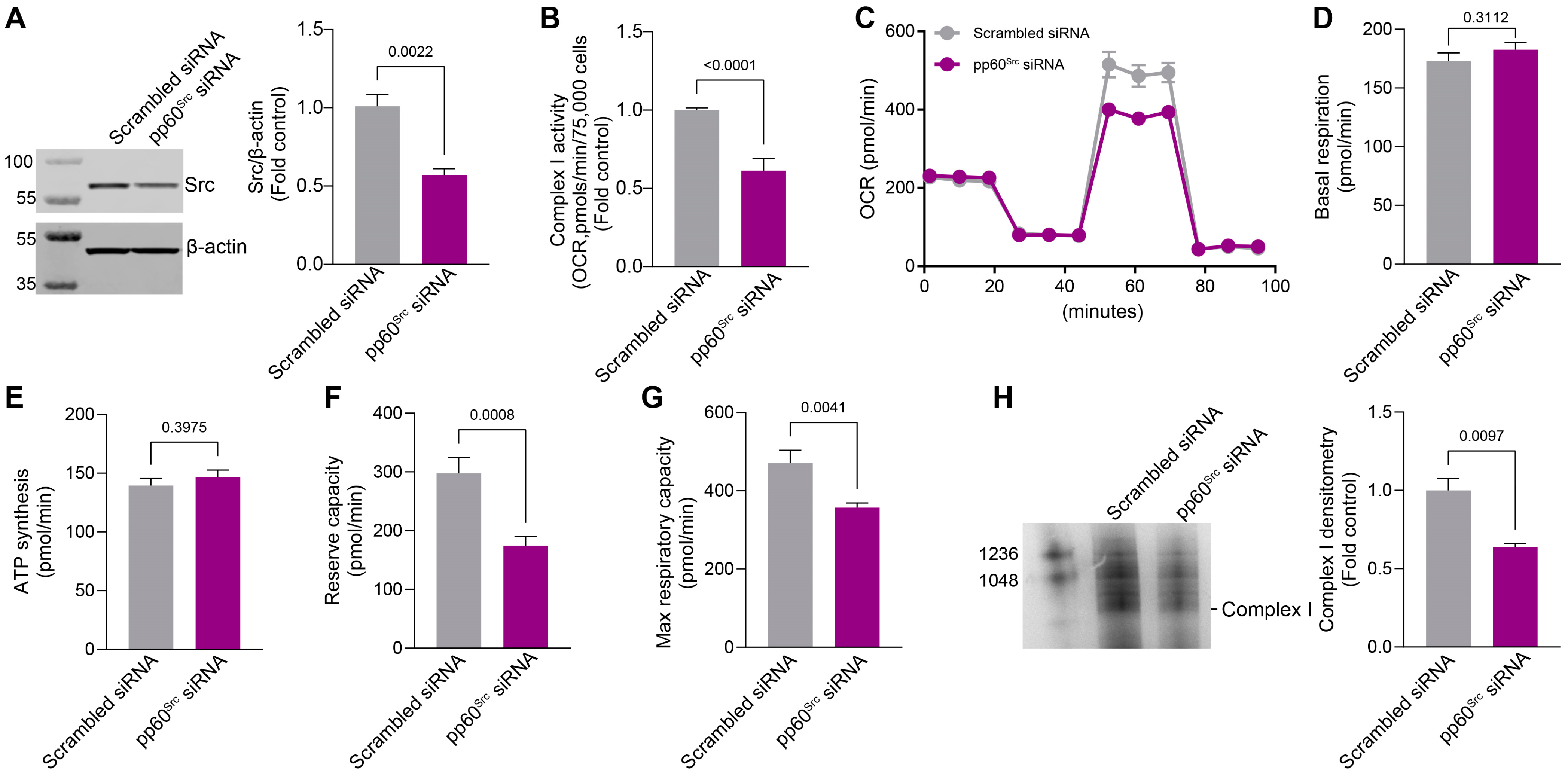
2.3. siRNA-Mediated Knockdown of pp60Src Alters Complex I Activity, Complex I Assembly, and Mitochondrial Respiration
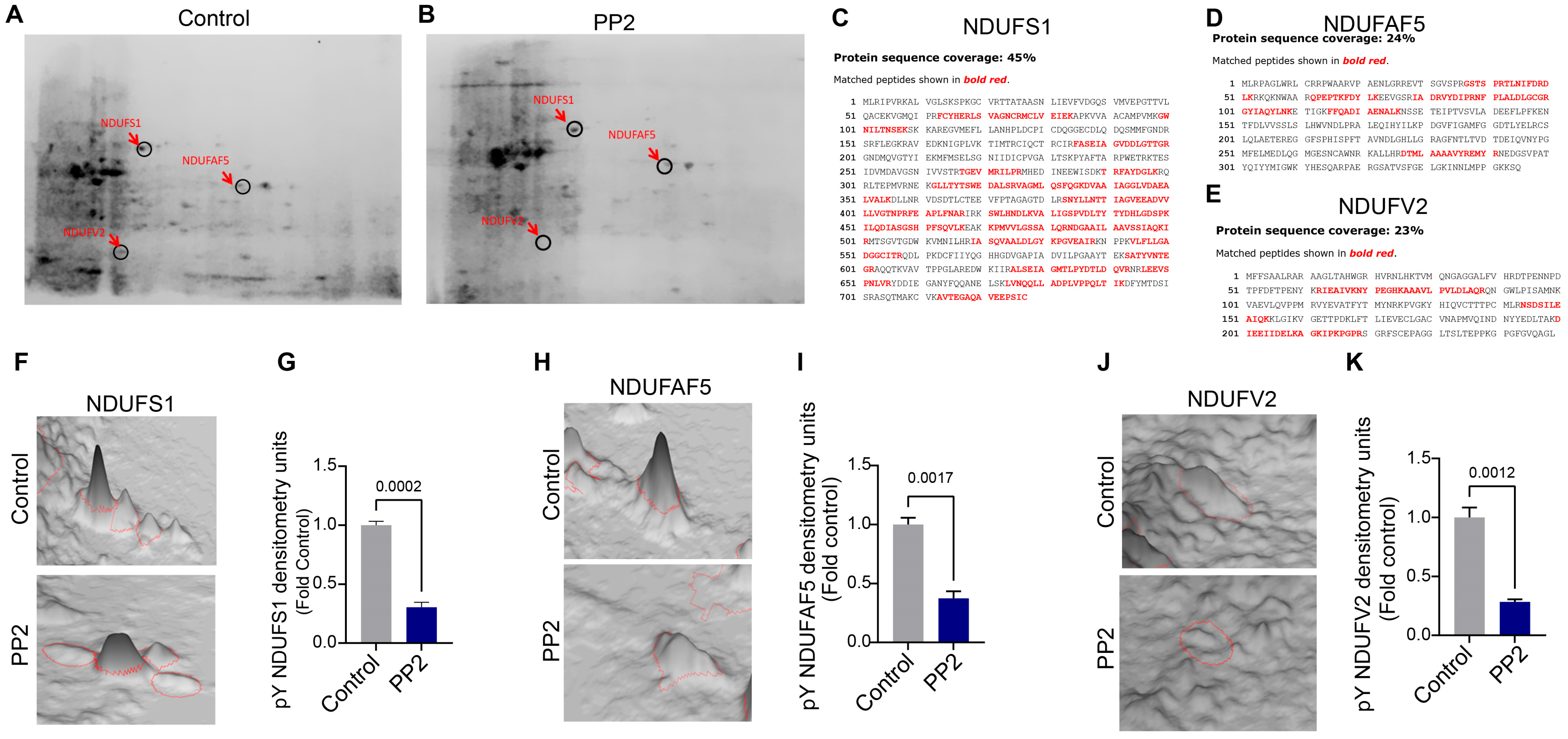
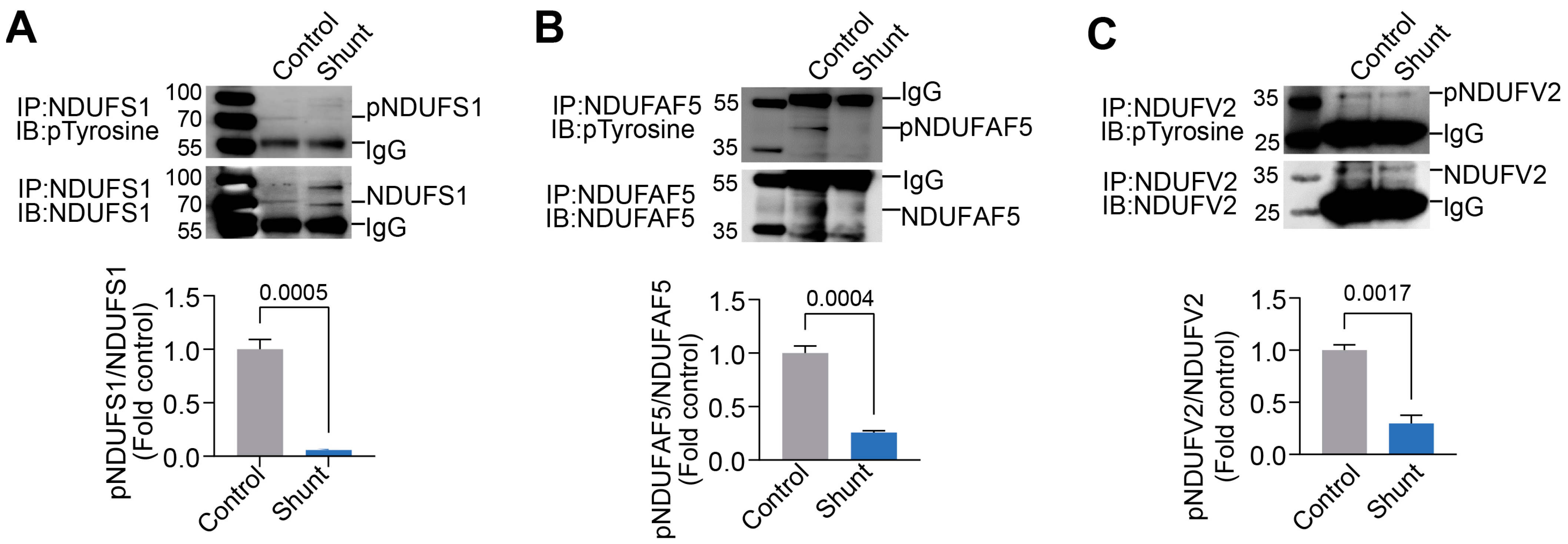
2.4. Identification of pp60Src Protein Substrates in ETC Complex I
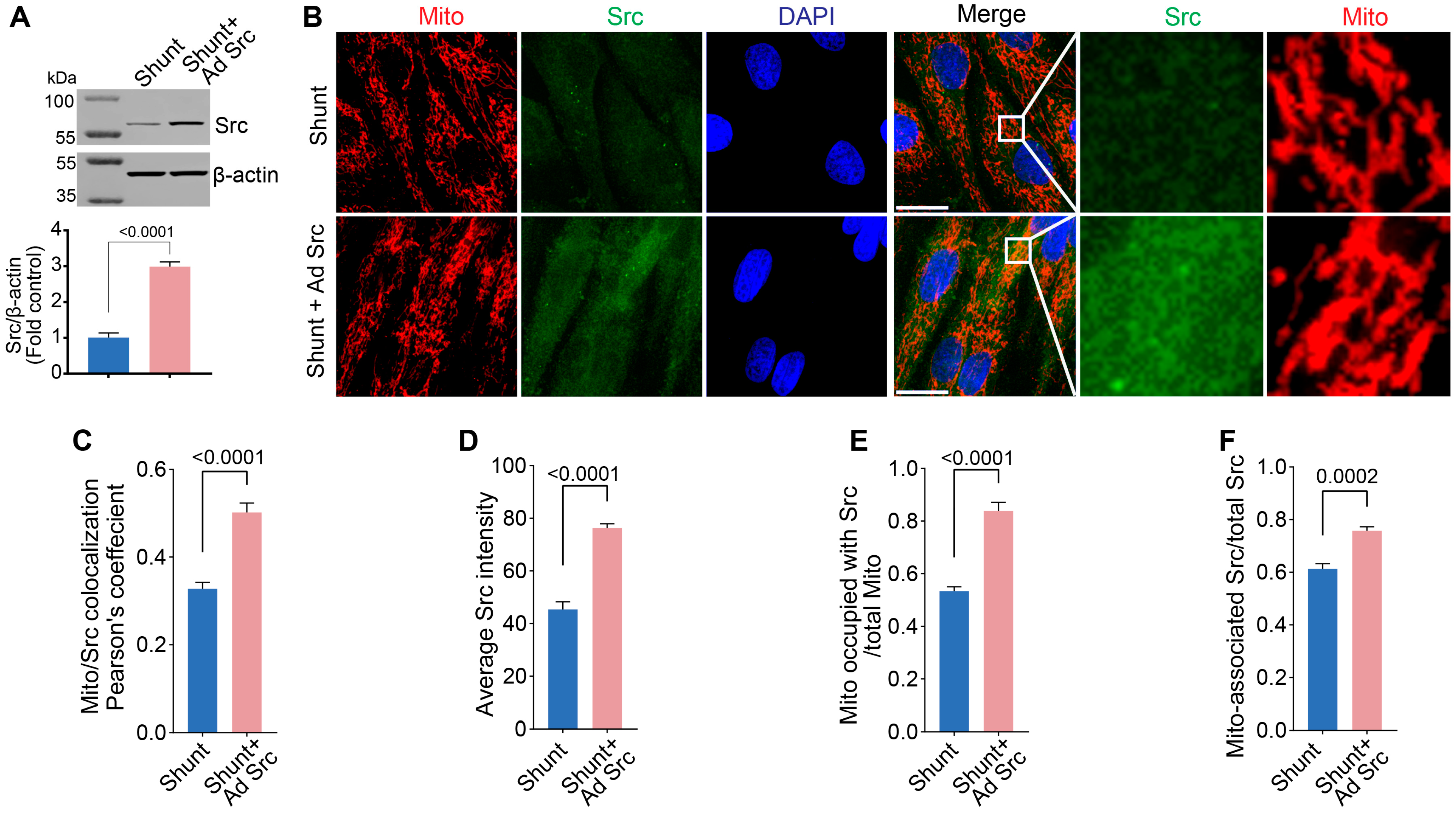
2.5. Restoration of pp60Src Protein Levels Improves ETC Complex I Activity and Bioenergetics in Shunt PAECs
3. Discussion
4. Materials and Methods
4.1. Antibodies, Reagents, and Chemicals
4.2. Cell Culture and Adenoviral Transduction of Pulmonary Arterial Endothelial Cells (PAECs)
4.3. Small Interfering RNA (siRNA) Treatment
4.4. Mitochondria Isolation and Blue Native Polyacrylamide Gel Electrophoresis (BN-PAGE)
4.5. Immunoprecipitation and Western Blot Analysis
4.6. Phosphoprotein Enrichment and 2-Dimentional Electrophoresis
4.7. DE Gel Analysis for Protein Expression Profiling
4.8. In-Gel Protein Digestion
4.9. Mass Spectrometry
4.10. Fluorescent Microscopy
4.11. Measurement of Oxygen Consumption Rate
4.12. Functional Analysis of Measurement of Electron Transport Chain (ETC) Complex I
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vakifahmetoglu-Norberg, H.; Ouchida, A.T.; Norberg, E. The role of mitochondria in metabolism and cell death. Biochem. Biophys. Res. Commun. 2017, 482, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Federico, M.; De la Fuente, S.; Palomeque, J.; Sheu, S.-S. The role of mitochondria in metabolic disease: A special emphasis on heart dysfunction. J. Physiol. 2021, 599, 3477–3493. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.H.; Park, K.S.; Lee, K.-U.; Lee, H.K. Mitochondrial metabolism and diabetes. J. Diabetes Investig. 2010, 1, 161–169. [Google Scholar] [CrossRef]
- Grasso, D.; Zampieri, L.X.; Capelôa, T.; Van de Velde, J.A.; Sonveaux, P. Mitochondria in cancer. Cell Stress 2020, 4, 114–146. [Google Scholar] [CrossRef] [PubMed]
- Missiroli, S.; Perrone, M.; Genovese, I.; Pinton, P.; Giorgi, C. Cancer metabolism and mitochondria: Finding novel mechanisms to fight tumours. eBioMedicine 2020, 59, 102943. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Ivanova, E.A.; Sobenin, I.A.; Yet, S.-F.; Orekhov, A.N. The Role of Mitochondria in Cardiovascular Diseases. Biology 2020, 9, 137. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Shkurat, T.P.; Melnichenko, A.A.; Grechko, A.V.; Orekhov, A.N. The role of mitochondrial dysfunction in cardiovascular disease: A brief review. Ann. Med. 2018, 50, 121–127. [Google Scholar] [CrossRef]
- Liang, S.; Yegambaram, M.; Wang, T.; Wang, J.; Black, S.M.; Tang, H. Mitochondrial Metabolism, Redox, and Calcium Homeostasis in Pulmonary Arterial Hypertension. Biomedicines 2022, 10, 341. [Google Scholar] [CrossRef]
- Hroudová, J.; Singh, N.; Fišar, Z. Mitochondrial Dysfunctions in Neurodegenerative Diseases: Relevance to Alzheimer’s Disease. BioMed Res. Int. 2014, 2014, 175062. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, F.; Ma, X.; Perry, G.; Zhu, X. Mitochondria dysfunction in the pathogenesis of Alzheimer’s disease: Recent advances. Mol. Neurodegener. 2020, 15, 30. [Google Scholar] [CrossRef]
- Budzinska, M.; Zimna, A.; Kurpisz, M. The role of mitochondria in Duchenne muscular dystrophy. J. Physiol. Pharmacol. 2021, 72, 157–166. [Google Scholar] [CrossRef]
- Pokharel, M.D.; Marciano, D.P.; Fu, P.; Franco, M.C.; Unwalla, H.; Tieu, K.; Fineman, J.R.; Wang, T.; Black, S.M. Metabolic reprogramming, oxidative stress, and pulmonary hypertension. Redox Biol. 2023, 64, 102797. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, B.; Wang, Y.; Zhang, H.; He, L.; Wang, P.; Dong, M. Mitochondrial dysfunction in pulmonary arterial hypertension. Front. Physiol. 2022, 13, 1079989. [Google Scholar] [CrossRef] [PubMed]
- Suliman, H.B.; Nozik-Grayck, E. Mitochondrial Dysfunction: Metabolic Drivers of Pulmonary Hypertension. Antioxid. Redox Signal. 2019, 31, 843–857. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.D.; Bazan, I.; Zhang, Y.; Fares, W.H.; Lee, P.J. Mitochondrial dysfunction and pulmonary hypertension: Cause, effect, or both. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 314, L782–L796. [Google Scholar] [CrossRef]
- Rafikov, R.; Sun, X.; Rafikova, O.; Louise Meadows, M.; Desai, A.A.; Khalpey, Z.; Yuan, J.X.; Fineman, J.R.; Black, S.M. Complex I dysfunction underlies the glycolytic switch in pulmonary hypertensive smooth muscle cells. Redox Biol. 2015, 6, 278–286. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, P.; Vang, A.; Zimmer, A.; Huck, S.; Nicely, P.; Wang, E.; Mancini, T.J.; Owusu-Sarfo, J.; Cavarsan, C.F.; et al. Reduction in activity and abundance of mitochondrial electron transport chain supercomplexes in pulmonary hypertension-induced right ventricular dysfunction. bioRxiv 2024. [Google Scholar] [CrossRef]
- Mooers, E.A.; Johnson, H.M.; Michalkiewicz, T.; Rana, U.; Joshi, C.; Afolayan, A.J.; Teng, R.J.; Konduri, G.G. Aberrant PGC-1alpha signaling in a lamb model of persistent pulmonary hypertension of the newborn. Pediatr. Res. 2024, 96, 1636–1644. [Google Scholar] [CrossRef]
- Redout, E.M.; Wagner, M.J.; Zuidwijk, M.J.; Boer, C.; Musters, R.J.; van Hardeveld, C.; Paulus, W.J.; Simonides, W.S. Right-ventricular failure is associated with increased mitochondrial complex II activity and production of reactive oxygen species. Cardiovasc. Res. 2007, 75, 770–781. [Google Scholar] [CrossRef]
- Yang, Z.; Zhuan, B.; Yan, Y.; Jiang, S.; Wang, T. Roles of different mitochondrial electron transport chain complexes in hypoxia-induced pulmonary vasoconstriction. Cell Biol. Int. 2016, 40, 188–195. [Google Scholar] [CrossRef]
- Sun, X.; Lu, Q.; Yegambaram, M.; Kumar, S.; Qu, N.; Srivastava, A.; Wang, T.; Fineman, J.R.; Black, S.M. TGF-beta1 attenuates mitochondrial bioenergetics in pulmonary arterial endothelial cells via the disruption of carnitine homeostasis. Redox Biol. 2020, 36, 101593. [Google Scholar] [CrossRef] [PubMed]
- Ardito, F.; Giuliani, M.; Perrone, D.; Troiano, G.; Lo Muzio, L. The crucial role of protein phosphorylation in cell signaling and its use as targeted therapy (Review). Int. J. Mol. Med. 2017, 40, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef]
- Niemi, N.M.; MacKeigan, J.P. Mitochondrial phosphorylation in apoptosis: Flipping the death switch. Antioxid. Redox Signal. 2013, 19, 572–582. [Google Scholar] [CrossRef]
- Horbinski, C.; Chu, C.T. Kinase signaling cascades in the mitochondrion: A matter of life or death. Free Radic. Biol. Med. 2005, 38, 2–11. [Google Scholar] [CrossRef]
- Pagliarini, D.J.; Dixon, J.E. Mitochondrial modulation: Reversible phosphorylation takes center stage? Trends Biochem. Sci. 2006, 31, 26–34. [Google Scholar] [CrossRef]
- McBride, H.M.; Neuspiel, M.; Wasiak, S. Mitochondria: More than just a powerhouse. Curr. Biol. 2006, 16, R551–R560. [Google Scholar] [CrossRef]
- Koc, E.C.; Koc, H. Regulation of mammalian mitochondrial translation by post-translational modifications. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2012, 1819, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Hofer, A.; Wenz, T. Post-translational modification of mitochondria as a novel mode of regulation. Exp. Gerontol. 2014, 56, 202–220. [Google Scholar] [CrossRef]
- Tibaldi, E.; Brunati, A.M.; Massimino, M.L.; Stringaro, A.; Colone, M.; Agostinelli, E.; Arancia, G.; Toninello, A. Src-Tyrosine kinases are major agents in mitochondrial tyrosine phosphorylation. J. Cell. Biochem. 2008, 104, 840–849. [Google Scholar] [CrossRef]
- Hebert-Chatelain, E. Src kinases are important regulators of mitochondrial functions. Int. J. Biochem. Cell Biol. 2013, 45, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Yeatman, T.J. A renaissance for SRC. Nat. Rev. Cancer 2004, 4, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Ishizawar, R.; Parsons, S.J. c-Src and cooperating partners in human cancer. Cancer Cell 2004, 6, 209–214. [Google Scholar] [CrossRef]
- Demory, M.L.; Boerner, J.L.; Davidson, R.; Faust, W.; Miyake, T.; Lee, I.; Hüttemann, M.; Douglas, R.; Haddad, G.; Parsons, S.J. Epidermal growth factor receptor translocation to the mitochondria: Regulation and effect. J. Biol. Chem. 2009, 284, 36592–36604. [Google Scholar] [CrossRef]
- Bard, F.; Patel, U.; Levy, J.B.; Jurdic, P.; Horne, W.C.; Baron, R. Molecular complexes that contain both c-Cbl and c-Src associate with Golgi membranes. Eur. J. Cell Biol. 2002, 81, 26–35. [Google Scholar] [CrossRef]
- Kaplan, K.B.; Swedlow, J.R.; Varmus, H.E.; Morgan, D.O. Association of p60c-src with endosomal membranes in mammalian fibroblasts. J. Cell Biol. 1992, 118, 321–333. [Google Scholar] [CrossRef]
- Ge, H.; Zhao, M.; Lee, S.; Xu, Z. Mitochondrial Src tyrosine kinase plays a role in the cardioprotective effect of ischemic preconditioning by modulating complex I activity and mitochondrial ROS generation. Free Radic. Res. 2015, 49, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Johnson Kameny, R.; Datar, S.A.; Boehme, J.B.; Morris, C.; Zhu, T.; Goudy, B.D.; Johnson, E.G.; Galambos, C.; Raff, G.W.; Sun, X.; et al. Ovine Models of Congenital Heart Disease and the Consequences of Hemodynamic Alterations for Pulmonary Artery Remodeling. Am. J. Respir. Cell Mol. Biol. 2019, 60, 503–514. [Google Scholar] [CrossRef]
- Xu, W.; Erzurum, S.C. Endothelial cell energy metabolism, proliferation, and apoptosis in pulmonary hypertension. Compr. Physiol. 2011, 1, 357–372. [Google Scholar] [CrossRef]
- Sun, X.; Sharma, S.; Fratz, S.; Kumar, S.; Rafikov, R.; Aggarwal, S.; Rafikova, O.; Lu, Q.; Burns, T.; Dasarathy, S.; et al. Disruption of endothelial cell mitochondrial bioenergetics in lambs with increased pulmonary blood flow. Antioxid. Redox Signal. 2013, 18, 1739–1752. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, J.; Zhang, R.; Zhang, M.; Cui, H.; Wang, L.; Cui, Y.; Wang, W.; Sun, Y.; Wang, C. Targeting Endothelial ENO1 (Alpha-Enolase) -PI3K-Akt-mTOR Axis Alleviates Hypoxic Pulmonary Hypertension. Hypertension 2023, 80, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Koeck, T.; Lara, A.R.; Neumann, D.; DiFilippo, F.P.; Koo, M.; Janocha, A.J.; Masri, F.A.; Arroliga, A.C.; Jennings, C.; et al. Alterations of cellular bioenergetics in pulmonary artery endothelial cells. Proc. Natl. Acad. Sci. USA 2007, 104, 1342–1347. [Google Scholar] [CrossRef]
- Rafikova, O.; Rafikov, R.; Kangath, A.; Qu, N.; Aggarwal, S.; Sharma, S.; Desai, J.; Fields, T.; Ludewig, B.; Yuan, J.X.Y.; et al. Redox regulation of epidermal growth factor receptor signaling during the development of pulmonary hypertension. Free. Radic. Biol. Med. 2016, 95, 96–111. [Google Scholar] [CrossRef] [PubMed]
- Paulin, R.; Meloche, J.; Courboulin, A.; Lambert, C.; Haromy, A.; Courchesne, A.; Bonnet, P.; Provencher, S.; Michelakis, E.D.; Bonnet, S. Targeting cell motility in pulmonary arterial hypertension. Eur. Respir. J. 2014, 43, 531–544. [Google Scholar] [CrossRef]
- Dai, X.; Wang, L.J.; Wu, J.; Shi, Y.X.; Li, G.P.; Yang, X.Q. Src kinase inhibitor PP2 regulates the biological characteristics of A549 cells via the PI3K/Akt signaling pathway. Oncol. Lett. 2018, 16, 5059–5065. [Google Scholar] [CrossRef] [PubMed]
- Oda, Y.; Renaux, B.; Bjorge, J.; Saifeddine, M.; Fujita, D.J.; Hollenberg, M.D. cSrc is a major cytosolic tyrosine kinase in vascular tissue. Can. J. Physiol. Pharmacol. 1999, 77, 606–617. [Google Scholar] [CrossRef]
- MacKay, C.E.; Knock, G.A. Control of vascular smooth muscle function by Src-family kinases and reactive oxygen species in health and disease. J. Physiol. 2015, 593, 3815–3828. [Google Scholar] [CrossRef]
- Chou, M.T.; Wang, J.; Fujita, D.J. Src Kinase becomes preferentially associated with the VEGFR, KDR/Flk-1, following VEGF stimulation of vascular endothelial cells. BMC Biochem. 2002, 3, 32. [Google Scholar] [CrossRef]
- Wallez, Y.; Cand, F.; Cruzalegui, F.; Wernstedt, C.; Souchelnytskyi, S.; Vilgrain, I.; Huber, P. Src kinase phosphorylates vascular endothelial-cadherin in response to vascular endothelial growth factor: Identification of tyrosine 685 as the unique target site. Oncogene 2007, 26, 1067–1077. [Google Scholar] [CrossRef]
- Duval, M.; Bœuf, F.L.; Huot, J.; Gratton, J.-P. Src-mediated Phosphorylation of Hsp90 in Response to Vascular Endothelial Growth Factor (VEGF) Is Required for VEGF Receptor-2 Signaling to Endothelial NO Synthase. Mol. Biol. Cell 2007, 18, 4659–4668. [Google Scholar] [CrossRef]
- Pullamsetti, S.S.; Berghausen, E.M.; Dabral, S.; Tretyn, A.; Butrous, E.; Savai, R.; Butrous, G.; Dahal, B.K.; Brandes, R.P.; Ghofrani, H.A.; et al. Role of Src tyrosine kinases in experimental pulmonary hypertension. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1354–1365. [Google Scholar] [CrossRef]
- Hu, G.; Place, A.T.; Minshall, R.D. Regulation of endothelial permeability by Src kinase signaling: Vascular leakage versus transcellular transport of drugs and macromolecules. Chem. Biol. Interact. 2008, 171, 177–189. [Google Scholar] [CrossRef]
- Miyazaki, T.; Neff, L.; Tanaka, S.; Horne, W.C.; Baron, R. Regulation of cytochrome c oxidase activity by c-Src in osteoclasts. J. Cell Biol. 2003, 160, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Hebert-Chatelain, E.; Jose, C.; Gutierrez Cortes, N.; Dupuy, J.W.; Rocher, C.; Dachary-Prigent, J.; Letellier, T. Preservation of NADH ubiquinone-oxidoreductase activity by Src kinase-mediated phosphorylation of NDUFB10. Biochim. Biophys. Acta (BBA) Bioenerg. 2012, 1817, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Oishi, P.E.; Sharma, S.; Datar, S.A.; Kumar, S.; Aggarwal, S.; Lu, Q.; Raff, G.; Azakie, A.; Hsu, J.H.; Sajti, E.; et al. Rosiglitazone preserves pulmonary vascular function in lambs with increased pulmonary blood flow. Pediatr. Res. 2013, 73, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Oishi, P.E.; Wiseman, D.A.; Sharma, S.; Kumar, S.; Hou, Y.; Datar, S.A.; Azakie, A.; Johengen, M.J.; Harmon, C.; Fratz, S.; et al. Progressive dysfunction of nitric oxide synthase in a lamb model of chronically increased pulmonary blood flow: A role for oxidative stress. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 295, L756–L766. [Google Scholar] [CrossRef]
- Black, S.M.; Fineman, J.R.; Johengen, M.; Bristow, J.; Soifer, S.J. Increased Pulmonary Blood Flow Alters the Molecular Regulation of Vascular Reactivity in the Lamb. • 122. Pediatr. Res. 1996, 39, 23. [Google Scholar] [CrossRef][Green Version]
- Seltana, A.; Guezguez, A.; Lepage, M.; Basora, N.; Beaulieu, J.-F. Src family kinase inhibitor PP2 accelerates differentiation in human intestinal epithelial cells. Biochem. Biophys. Res. Commun. 2013, 430, 1195–1200. [Google Scholar] [CrossRef]
- Alcalá, S.; Mayoral-Varo, V.; Ruiz-Cañas, L.; López-Gil, J.C.; Heeschen, C.; Martín-Pérez, J.; Sainz, B., Jr. Targeting SRC Kinase Signaling in Pancreatic Cancer Stem Cells. Int. J. Mol. Sci. 2020, 21, 7437. [Google Scholar] [CrossRef]
- Bain, J.; Plater, L.; Elliott, M.; Shpiro, N.; Hastie, C.J.; Mclauchlan, H.; Klevernic, I.; Arthur, J.S.C.; Alessi, D.R.; Cohen, P. The selectivity of protein kinase inhibitors: A further update. Biochem. J. 2007, 408, 297–315. [Google Scholar] [CrossRef]
- Brandvold, K.R.; Steffey, M.E.; Fox, C.C.; Soellner, M.B. Development of a highly selective c-Src kinase inhibitor. ACS Chem. Biol. 2012, 7, 1393–1398. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, M.; Ryan, M.T. Assembly factors of human mitochondrial complex I and their defects in disease. IUBMB Life 2010, 62, 497–502. [Google Scholar] [CrossRef]
- Mimaki, M.; Wang, X.; McKenzie, M.; Thorburn, D.R.; Ryan, M.T. Understanding mitochondrial complex I assembly in health and disease. Biochim. Biophys. Acta (BBA) Bioenerg. 2012, 1817, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.K.; Lu, J.; Bai, Y. Mitochondrial respiratory complex I: Structure, function and implication in human diseases. Curr. Med. Chem. 2009, 16, 1266–1277. [Google Scholar] [CrossRef]
- Cassina, A.M.; Hodara, R.; Souza, J.M.; Thomson, L.; Castro, L.; Ischiropoulos, H.; Freeman, B.A.; Radi, R. Cytochrome c nitration by peroxynitrite. J. Biol. Chem. 2000, 275, 21409–21415. [Google Scholar] [CrossRef]
- Wang, T.; Yegambaram, M.; Gross, C.; Sun, X.; Lu, Q.; Wang, H.; Wu, X.; Kangath, A.; Tang, H.; Aggarwal, S.; et al. RAC1 nitration at Y32 IS involved in the endothelial barrier disruption associated with lipopolysaccharide-mediated acute lung injury. Redox Biol. 2021, 38, 101794. [Google Scholar] [CrossRef] [PubMed]
- Beer, S.M.; Taylor, E.R.; Brown, S.E.; Dahm, C.C.; Costa, N.J.; Runswick, M.J.; Murphy, M.P. Glutaredoxin 2 catalyzes the reversible oxidation and glutathionylation of mitochondrial membrane thiol proteins: Implications for mitochondrial redox regulation and antioxidant DEFENSE. J. Biol. Chem. 2004, 279, 47939–47951. [Google Scholar] [CrossRef] [PubMed]
- Guan, K.L.; Xiong, Y. Regulation of intermediary metabolism by protein acetylation. Trends Biochem. Sci. 2011, 36, 108–116. [Google Scholar] [CrossRef]
- Cesaro, L.; Salvi, M. Mitochondrial tyrosine phosphoproteome: New insights from an up-to-date analysis. Biofactors 2010, 36, 437–450. [Google Scholar] [CrossRef]
- Falfushynska, H.I.; Sokolov, E.; Piontkivska, H.; Sokolova, I.M. The Role of Reversible Protein Phosphorylation in Regulation of the Mitochondrial Electron Transport System During Hypoxia and Reoxygenation Stress in Marine Bivalves. Front. Mar. Sci. 2020, 7, 467. [Google Scholar] [CrossRef]
- Lucero, M.; Suarez, A.E.; Chambers, J.W. Phosphoregulation on mitochondria: Integration of cell and organelle responses. CNS Neurosci. Ther. 2019, 25, 837–858. [Google Scholar] [CrossRef] [PubMed]
- Mathers, K.E.; Staples, J.F. Differential posttranslational modification of mitochondrial enzymes corresponds with metabolic suppression during hibernation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 317, R262–R269. [Google Scholar] [CrossRef]
- Salvi, M.; Brunati, A.M.; Bordin, L.; La Rocca, N.; Clari, G.; Toninello, A. Characterization and location of Src-dependent tyrosine phosphorylation in rat brain mitochondria. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2002, 1589, 181–195. [Google Scholar] [CrossRef]
- Salvi, M.; Stringaro, A.; Brunati, A.M.; Agostinelli, E.; Arancia, G.; Clari, G.; Toninello, A. Tyrosine phosphatase activity in mitochondria: Presence of Shp-2 phosphatase in mitochondria. Cell. Mol. Life Sci. 2004, 61, 2393–2404. [Google Scholar] [CrossRef]
- Salvi, M.; Brunati, A.M.; Toninello, A. Tyrosine phosphorylation in mitochondria: A new frontier in mitochondrial signaling. Free Radic. Biol. Med. 2005, 38, 1267–1277. [Google Scholar] [CrossRef]
- Pagliarini, D.J.; Wiley, S.E.; Kimple, M.E.; Dixon, J.R.; Kelly, P.; Worby, C.A.; Casey, P.J.; Dixon, J.E. Involvement of a mitochondrial phosphatase in the regulation of ATP production and insulin secretion in pancreatic beta cells. Mol. Cell 2005, 19, 197–207. [Google Scholar] [CrossRef]
- Brink, J.; Hovmöller, S.; Ragan, C.I.; Cleeter, M.W.J.; Boekema, E.J.; van Bruggen, E.F.J. The structure of NADH:ubiquinone oxidoreductase from beef-heart mitochondria. Eur. J. Biochem. 1987, 166, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Hagras, M.A.; Konstantopoulou, V.; Mayr, J.A.; Stuchebrukhov, A.A.; Meierhofer, D. Mutations in NDUFS1 Cause Metabolic Reprogramming and Disruption of the Electron Transfer. Cells 2019, 8, 1149. [Google Scholar] [CrossRef]
- Hébert Chatelain, E.; Dupuy, J.W.; Letellier, T.; Dachary-Prigent, J. Functional impact of PTP1B-mediated Src regulation on oxidative phosphorylation in rat brain mitochondria. Cell. Mol. Life Sci. 2011, 68, 2603–2613. [Google Scholar] [CrossRef]
- Rhein, V.F.; Carroll, J.; Ding, S.; Fearnley, I.M.; Walker, J.E. NDUFAF5 Hydroxylates NDUFS7 at an Early Stage in the Assembly of Human Complex, I. J. Biol. Chem. 2016, 291, 14851–14860. [Google Scholar] [CrossRef]
- Sugiana, C.; Pagliarini, D.J.; McKenzie, M.; Kirby, D.M.; Salemi, R.; Abu-Amero, K.K.; Dahl, H.H.; Hutchison, W.M.; Vascotto, K.A.; Smith, S.M.; et al. Mutation of C20orf7 disrupts complex I assembly and causes lethal neonatal mitochondrial disease. Am. J. Hum. Genet. 2008, 83, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Gerards, M.; Sluiter, W.; van den Bosch, B.J.; de Wit, L.E.; Calis, C.M.; Frentzen, M.; Akbari, H.; Schoonderwoerd, K.; Scholte, H.R.; Jongbloed, R.J.; et al. Defective complex I assembly due to C20orf7 mutations as a new cause of Leigh syndrome. J. Med. Genet. 2010, 47, 507–512. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Liao, P.-C.; Chuang, K.-T.; Kao, M.-C. Mitochondrial targeting of human NADH dehydrogenase (ubiquinone) flavoprotein 2 (NDUFV2) and its association with early-onset hypertrophic cardiomyopathy and encephalopathy. J. Biomed. Sci. 2011, 18, 29. [Google Scholar] [CrossRef] [PubMed]
- Ogura, M.; Yamaki, J.; Homma, M.K.; Homma, Y. Mitochondrial c-Src regulates cell survival through phosphorylation of respiratory chain components. Biochem. J. 2012, 447, 281–289. [Google Scholar] [CrossRef]
- Cohen, P. The role of protein phosphorylation in human health and disease. The Sir Hans Krebs Medal Lecture. Eur. J. Biochem. 2001, 268, 5001–5010. [Google Scholar] [CrossRef]
- Weatherald, J.; Chaumais, M.C.; Savale, L.; Jaïs, X.; Seferian, A.; Canuet, M.; Bouvaist, H.; Magro, P.; Bergeron, A.; Guignabert, C.; et al. Long-term outcomes of dasatinib-induced pulmonary arterial hypertension: A population-based study. Eur. Respir. J. 2017, 50, 1700217. [Google Scholar] [CrossRef]
- Sharma, S.; Sud, N.; Wiseman, D.A.; Carter, A.L.; Kumar, S.; Hou, Y.; Rau, T.; Wilham, J.; Harmon, C.; Oishi, P.; et al. Altered carnitine homeostasis is associated with decreased mitochondrial function and altered nitric oxide signaling in lambs with pulmonary hypertension. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2008, 294, L46–L56. [Google Scholar] [CrossRef]
- Sharma, S.; Sun, X.; Kumar, S.; Rafikov, R.; Aramburo, A.; Kalkan, G.; Tian, J.; Rehmani, I.; Kallarackal, S.; Fineman, J.R.; et al. Preserving mitochondrial function prevents the proteasomal degradation of GTP cyclohydrolase I. Free Radic. Biol. Med. 2012, 53, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Black, S.M.; Field-Ridley, A.; Sharma, S.; Kumar, S.; Keller, R.L.; Kameny, R.; Maltepe, E.; Datar, S.A.; Fineman, J.R. Altered Carnitine Homeostasis in Children With Increased Pulmonary Blood Flow Due to Ventricular Septal Defects. Pediatr. Crit. Care Med. 2017, 18, 931–934. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Zhang, W. Metabolic reprogramming: A novel metabolic model for pulmonary hypertension. Front. Cardiovasc. Med. 2022, 9, 957524. [Google Scholar] [CrossRef]
- Kelly, L.K.; Wedgwood, S.; Steinhorn, R.H.; Black, S.M. Nitric oxide decreases endothelin-1 secretion through the activation of soluble guanylate cyclase. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 286, L984–L991. [Google Scholar] [CrossRef] [PubMed]
- Wedgwood, S.; Mitchell, C.J.; Fineman, J.R.; Black, S.M. Developmental differences in the shear stress-induced expression of endothelial NO synthase: Changing role of AP-1. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003, 284, L650–L662. [Google Scholar] [CrossRef] [PubMed]
- Barabutis, N.; Handa, V.; Dimitropoulou, C.; Rafikov, R.; Snead, C.; Kumar, S.; Joshi, A.; Thangjam, G.; Fulton, D.; Black, S.M.; et al. LPS induces pp60c-src-mediated tyrosine phosphorylation of Hsp90 in lung vascular endothelial cells and mouse lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 304, L883–L893. [Google Scholar] [CrossRef] [PubMed]
- Van Coster, R.; Smet, J.; George, E.; De Meirleir, L.; Seneca, S.; Van Hove, J.; Sebire, G.; Verhelst, H.; De Bleecker, J.; Van Vlem, B.; et al. Blue Native Polyacrylamide Gel Electrophoresis: A Powerful Tool in Diagnosis of Oxidative Phosphorylation Defects. Pediatr. Res. 2001, 50, 658–665. [Google Scholar] [CrossRef]
- Sud, N.; Sharma, S.; Wiseman, D.A.; Harmon, C.; Kumar, S.; Venema, R.C.; Fineman, J.R.; Black, S.M. Nitric oxide and superoxide generation from endothelial NOS: Modulation by HSP90. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 293, L1444–L1453. [Google Scholar] [CrossRef]
- Yegambaram, M.; Sun, X.; Flores, A.G.; Lu, Q.; Soto, J.; Richards, J.; Aggarwal, S.; Wang, T.; Gu, H.; Fineman, J.R.; et al. Novel Relationship between Mitofusin 2-Mediated Mitochondrial Hyperfusion, Metabolic Remodeling, and Glycolysis in Pulmonary Arterial Endothelial Cells. Int. J. Mol. Sci. 2023, 24, 17533. [Google Scholar] [CrossRef]
- Manivannan, B.; Rawson, P.; Jordan, T.W.; Secor, W.E.; La Flamme, A.C. Differential patterns of liver proteins in experimental murine hepatosplenic schistosomiasis. Infect. Immun. 2010, 78, 618–628. [Google Scholar] [CrossRef]
- Appel, R.D.; Palagi, P.M.; Walther, D.; Vargas, J.R.; Sanchez, J.-C.; Ravier, F.; Pasquali, C.; Hochstrasser, D.F. Melanie II—A third-generation software package for analysis of two-dimensional electrophoresis images: I. Features and user interface. Electrophoresis 1997, 18, 2724–2734. [Google Scholar] [CrossRef]
- Appel, R.D.; Vargas, J.R.; Palagi, P.M.; Walther, D.; Hochstrasser, D.F. Melanie II—A third-generation software package for analysis of two-dimensional electrophoresis images: II. Algorithms. Electrophoresis 1997, 18, 2735–2748. [Google Scholar] [CrossRef]
- Yegambaram, M.; Kumar, S.; Wu, X.; Lu, Q.; Sun, X.; Garcia Flores, A.; Meadows, M.L.; Barman, S.; Fulton, D.; Wang, T.; et al. Endothelin-1 acutely increases nitric oxide production via the calcineurin mediated dephosphorylation of Caveolin-1. Nitric Oxide 2023, 140, 50–57. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yegambaram, M.; Pokharel, M.D.; Sun, X.; Lu, Q.; Soto, J.; Aggarwal, S.; Maltepe, E.; Fineman, J.R.; Wang, T.; Black, S.M. Restoration of pp60Src Re-Establishes Electron Transport Chain Complex I Activity in Pulmonary Hypertensive Endothelial Cells. Int. J. Mol. Sci. 2025, 26, 3815. https://doi.org/10.3390/ijms26083815
Yegambaram M, Pokharel MD, Sun X, Lu Q, Soto J, Aggarwal S, Maltepe E, Fineman JR, Wang T, Black SM. Restoration of pp60Src Re-Establishes Electron Transport Chain Complex I Activity in Pulmonary Hypertensive Endothelial Cells. International Journal of Molecular Sciences. 2025; 26(8):3815. https://doi.org/10.3390/ijms26083815
Chicago/Turabian StyleYegambaram, Manivannan, Marissa D. Pokharel, Xutong Sun, Qing Lu, Jamie Soto, Saurabh Aggarwal, Emin Maltepe, Jeffery R. Fineman, Ting Wang, and Stephen M. Black. 2025. "Restoration of pp60Src Re-Establishes Electron Transport Chain Complex I Activity in Pulmonary Hypertensive Endothelial Cells" International Journal of Molecular Sciences 26, no. 8: 3815. https://doi.org/10.3390/ijms26083815
APA StyleYegambaram, M., Pokharel, M. D., Sun, X., Lu, Q., Soto, J., Aggarwal, S., Maltepe, E., Fineman, J. R., Wang, T., & Black, S. M. (2025). Restoration of pp60Src Re-Establishes Electron Transport Chain Complex I Activity in Pulmonary Hypertensive Endothelial Cells. International Journal of Molecular Sciences, 26(8), 3815. https://doi.org/10.3390/ijms26083815






