Virus-Free Micro-Corm Induction and the Mechanism of Corm Development in Taro
Abstract
1. Introduction
2. Results
2.1. Establishing a Rapid Propagation System for Tissue Culture and Obtaining Virus-Free Taro Plantlets
2.2. Synergistic Effects of ABA and Sucrose on Taro Corm Expansion
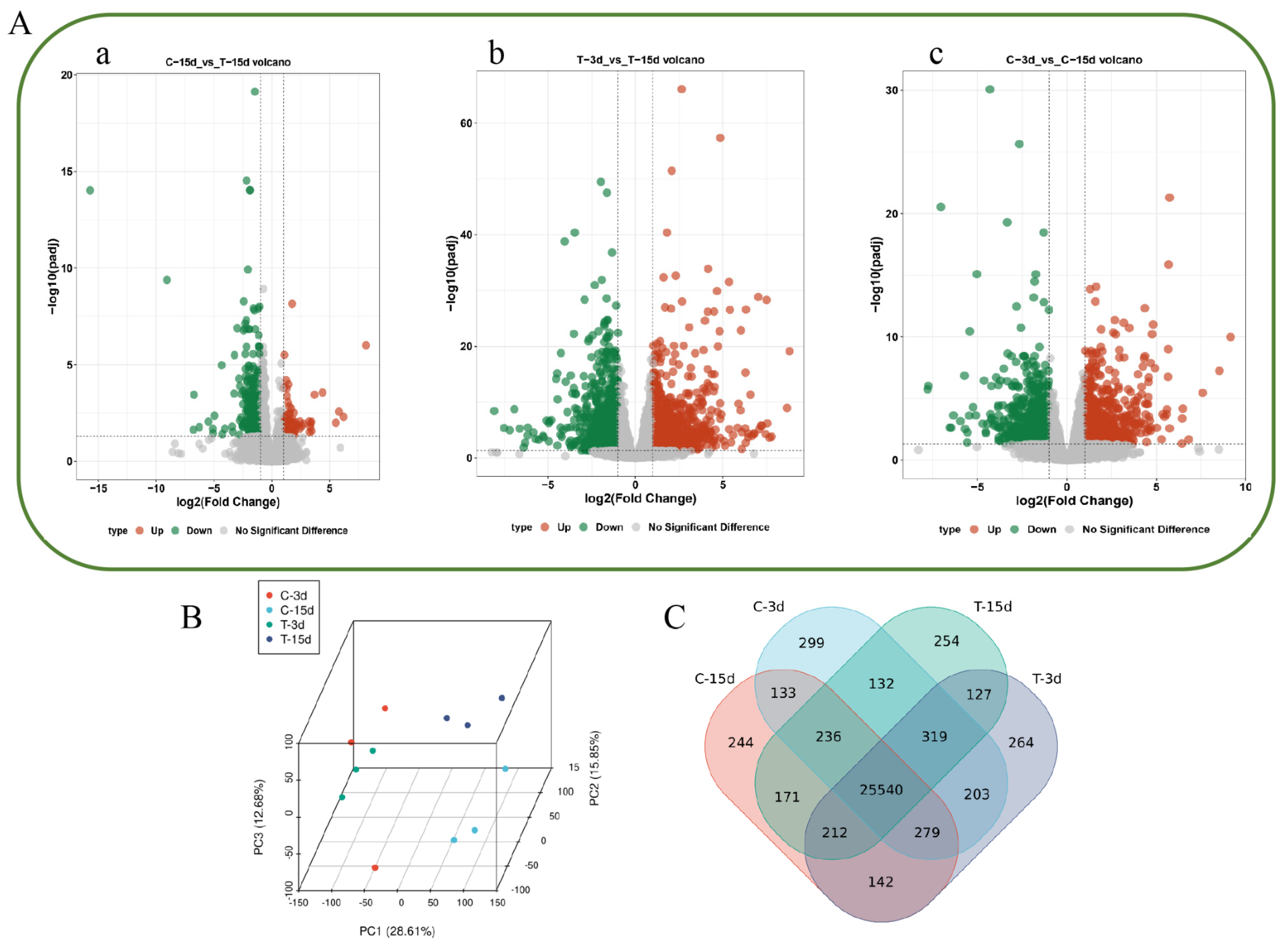
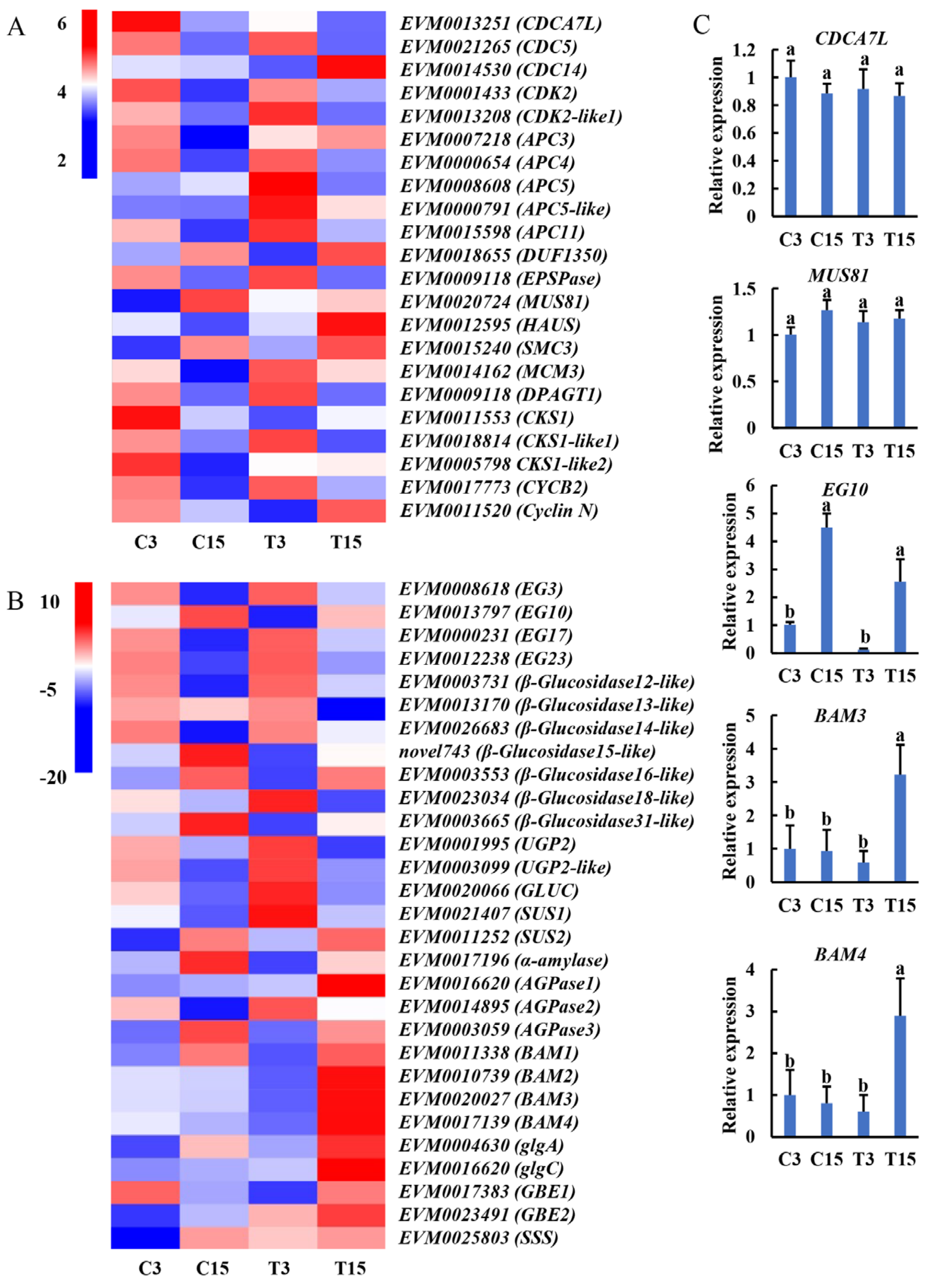
2.3. Analysis of Transcriptome Sequencing Data
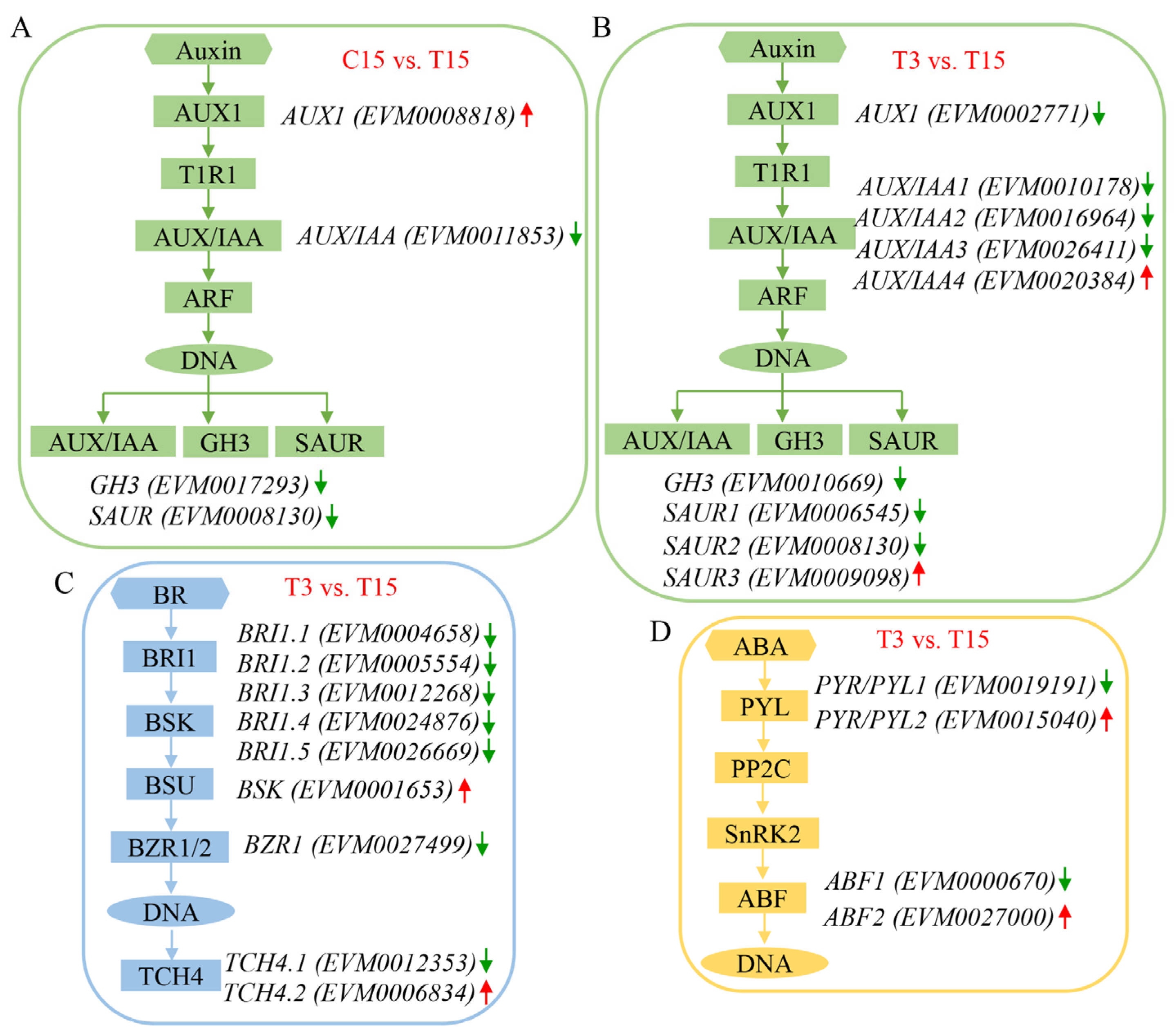
2.4. ABA Treatment Affects the Expression of Genes Involved in Hormone Signaling Pathways
2.5. Expression Patterns of Key Genes Associated with Taro Expansion
3. Discussion
3.1. The Shoot Apical Meristem Tissue Culture Is an Effective Method for Obtaining Virus-Free Taro
3.2. High Concentrations of Sucrose and Exogenous ABA Are the Key Factors That Induce Taro Corm Expansion
3.3. Working Model for Obtaining Virus-Free Taro Plantlets and Test-Tube Taro Corms via Shoot Apical Meristems
4. Materials and Methods
4.1. Obtainment of Shoot Apical Meristems
4.2. Preparation of the Medium
4.3. The Shoot Apical Meristem Culture
4.4. RNA Extraction, RT-PCR Assays, and Taro Virus Detection
4.5. Proliferation of Adventitious Shoots
4.6. Induction of Corm Expansion in Taro
4.7. RNA-Seq and Bioinformatics Analysis
4.8. Validation of the Transcriptome Data via qRT-PCR Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ram, R.; Joshi, A.; Verma, N.; Kulshrestha, S.; Raikhy, G.; Hallan, V.; Zaidi, A.A. First report of Dasheen mosaic virus infecting four ornamental aroids in India. Plant Pathol. 2003, 52, 411. [Google Scholar] [CrossRef]
- Park, H.R.; Lee, H.S.; Cho, S.Y.; Kim, Y.S.; Shin, K.S. Anti-metastatic effect of polysaccharide isolated from Colocasia esculenta is exerted through immunostimulation. Int. J. Mol. Med. 2013, 31, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dong, Z.; Liu, X.; Chen, H.; Lai, F.; Zhang, M. Structure characterization of two novel polysaccharides from Colocasia esculenta (taro) and a comparative study of their immunomodulatory activities. J. Funct. Foods 2018, 42, 47–57. [Google Scholar] [CrossRef]
- Yusop, M.S.M.; Saad, M.F.M.; Talip, N.; Baharum, S.N.; Bunawan, H. A Review on Viruses Infecting Taro (Colocasia esculenta (L.) Schott). Pathogens 2019, 8, 56. [Google Scholar] [CrossRef]
- Hartman, R.D. Dasheen Mosaic Virus and Other Phytopathogens Elimination from Caladium, Taro, and Cocoyam by Culture of Shoot Tips. Phytopathology 1973, 64, 237–240. [Google Scholar] [CrossRef]
- Wagih, M.E. Eradication of taro viruses from seedlings via seed rescue culture coupled with thermotheraphy. Afr. Crop Sci. J. 1997, 5, 419–424. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, X.; Jishuang, C. Establishment of Virus-Free Taro (Colocasia esulenta cv. Fenghuayunaitou) by Meristem-Tip Culture Combined with Thermotherapy. Plant Pathol. J. 2002, 1, 40–43. [Google Scholar] [CrossRef]
- Domingo, R.S. Applying Meristem Tip Culture and Thermo Therapy to Eliminate Viruses from Pineapple (Ananas comosus) and Taro (Colocasia esculenta); University of Hawai’i at Hilo: Hilo, HI, USA, 2020. [Google Scholar]
- Hong, S.; Li, Y.; Yu, X.; Ye, S.; Ning, B. RT-PCR Detection of Detoxification of Red Bud Taro Shoot Tip by Cryotherapy. Mol. Plant Breed. 2018, 16, 4678–4684. [Google Scholar] [CrossRef]
- Liu, L.; Chen, X.; Yan, L.; Jin, Y.; Sun, L.; Yang, Y.; Wang, Y.; Zhao, Z. Different eradication effects of latent viruses by combining thermotherapy with shoot tip culture or cryotherapy in four apple cultivars. Sci. Hortic. 2021, 288, 110356. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Mathew, L.; Pathirana, R.; Wiedow, C.; Hunter, D.A.; McLachlan, A.; Khan, S.; Tang, J.; Nadarajan, J. Eradication of Potato Virus S, Potato Virus A, and Potato Virus M From Infected in vitro-Grown Potato Shoots Using in vitro Therapies. Front. Plant Sci. 2022, 13, 878733. [Google Scholar] [CrossRef]
- Zierer, W.; Rüscher, D.; Sonnewald, U.; Sonnewald, S. Tuber and Tuberous Root Development. Annu. Rev. Plant Biol. 2021, 72, 551–580. [Google Scholar] [CrossRef] [PubMed]
- Wambui Njuguna, J.; Karuma, A.N.; Gicheru, P.; Onwonga, R. Effects of Watering Regimes and Planting Density on Taro (Colocasia esculenta) Growth, Yield, and Yield Components in Embu, Kenya. Int. J. Agron. 2023, 2023, 1–9. [Google Scholar] [CrossRef]
- Carrera, E.; Bou, J.; García Martínez, J.L.; Prat, S. Changes in GA 20-oxidase gene expression strongly affect stem length, tuber induction and tuber yield of potato plants. Plant J. 2000, 22, 247–256. [Google Scholar] [CrossRef]
- Li, J.; Seng, S.; Li, D.; Zhang, F.; Liu, Y.; Yao, T.; Liang, J.; Yi, M.; Wu, J. Antagonism between abscisic acid and gibberellin regulates starch synthesis and corm development in Gladiolus hybridus. Hortic. Res. 2021, 8, 155. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, B.; Liu, X.; Shan, N.; Sun, J.; Zhang, H.; Huang, Y.; Xiao, Y.; Zhou, Q. Uncovering the mechanism preliminarily of formation and development of taro corm in vitro by morphological physiology and transcriptomic analysis. Sci. Hortic. 2022, 291, 110575. [Google Scholar] [CrossRef]
- Roumeliotis, E.; Kloosterman, B.; Oortwijn, M.; Kohlen, W.; Bouwmeester, H.J.; Visser, R.G.; Bachem, C.W. The effects of auxin and strigolactones on tuber initiation and stolon architecture in potato. J. Exp. Bot. 2012, 63, 4539–4547. [Google Scholar] [CrossRef]
- Romanov, A.G.; Aksenova, N.P.; Konstantinova, T.N.; Golyanovskaya, S.A.; Kossmann, J.; Willmitzer, L. Effect of indole-3-acetic acid and kinetin on tuberisation parameters of different cultivars and transgenic lines of potato in vitro. Plant Growth Regul. 2000, 32, 245–251. [Google Scholar] [CrossRef]
- Zhou, T.; Song, B.; Liu, T.; Shen, Y.; Dong, L.; Jing, S.; Xie, C.; Liu, J. Phytochrome F plays critical roles in potato photoperiodic tuberization. Plant J. 2019, 98, 42–54. [Google Scholar] [CrossRef]
- Tiessen, A.; Hendriks, J.H.M.; Stitt, M.; Branscheid, A.; Gibon, Y.; Farré, E.M.; Geigenberger, P. Starch Synthesis in Potato Tubers Is Regulated by Post-Translational Redox Modification of ADP-Glucose Pyrophosphorylase. Plant Cell 2002, 14, 2191–2213. [Google Scholar] [CrossRef]
- Geigenberger, P. Regulation of sucrose to starch conversion in growing potato tubers. J. Exp. Bot. 2003, 54, 457–465. [Google Scholar] [CrossRef]
- Ferreira, S.J.; Sonnewald, U. The mode of sucrose degradation in potato tubers determines the fate of assimilate utilization. Front. Plant Sci. 2012, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; van Lammeren, A.A.; Vermeer, E.; Vreugdenhil, D. The role of gibberellin, abscisic acid, and sucrose in the regulation of potato tuber formation in vitro. Plant Physiol. 1998, 117, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Begum, S.; Jing, S.; Yu, L.; Sun, X.; Wang, E.; Abu Kawochar, M.; Qin, J.; Liu, J.; Song, B. Modulation of JA signalling reveals the influence of StJAZ1-like on tuber initiation and tuber bulking in potato. Plant J. 2022, 109, 952–964. [Google Scholar] [CrossRef]
- Liu, H.; Si, C.; Shi, C.; Wang, S.; Sun, Z.; Shi, Y. Switch from Apoplasmic to Symplasmic Phloem Unloading during Storage Roots Formation and Bulking of Sweetpotato. Crop Sci. 2019, 59, 675–683. [Google Scholar] [CrossRef]
- Hajirezaei, M.R.; Bornke, F.; Peisker, M.; Takahata, Y.; Lerchl, J.; Kirakosyan, A.; Sonnewald, U. Decreased sucrose content triggers starch breakdown and respiration in stored potato tubers (Solanum tuberosum). J. Exp. Bot. 2003, 54, 477–488. [Google Scholar] [CrossRef]
- Kühn, C.; Hajirezaei, M.; Fernie, A.R.; Roessner-Tunali, U.; Czechowski, T.; Hirner, B.; Frommer, W.B. The Sucrose Transporter StSUT1 Localizes to Sieve Elements in Potato Tuber Phloem and Influences Tuber Physiology and Development. Plant Physiol. 2003, 131, 102–113. [Google Scholar] [CrossRef]
- Yoon, J.; Cho, L.; Tun, W.; Jeon, J.; An, G. Sucrose signaling in higher plants. Plant Sci. 2021, 302, 110703. [Google Scholar] [CrossRef]
- Chen, J.; Chen, J.; Chen, J.; Adams, M.J. Molecular characterisation of an isolate of Dasheen mosaic virus from Zantedeschia aethiopica in China and comparisons in the genus Potyvirus. Arch. Virol. 2001, 146, 1821–1829. [Google Scholar] [CrossRef]
- Yang, I.C.; Hafner, G.J.; Dale, J.L.; Harding, R.M. Genomic characterisation of taro bacilliform virus. Arch. Virol. 2003, 148, 937–949. [Google Scholar] [CrossRef]
- Zettler, F.W. Dasheen Mosaic Virus as a Pathogen of Cultivated Aroids and Control of the Virus by Tissue Culture. Plant Dis. 1987, 71, 958. [Google Scholar] [CrossRef]
- Bradamante, G.; Mittelsten, S.O.; Incarbone, M. Under siege: Virus control in plant meristems and progeny. Plant Cell 2021, 33, 2523–2537. [Google Scholar] [CrossRef]
- Schreiber, J.M.; Limpens, E.; de Keijzer, J. Distributing Plant Developmental Regulatory Proteins via Plasmodesmata. Plants 2024, 13, 684. [Google Scholar] [CrossRef]
- Syller, J.; Grupa, A. Antagonistic within-host interactions between plant viruses: Molecular basis and impact on viral and host fitness. Mol. Plant Pathol. 2016, 17, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Alam, N.C.N.; Kadir, A.M.A. In vitro micropropagation of two local taro cultivars for large-scale cultivation. J. Plant Biotechnol. 2022, 49, 124–130. [Google Scholar] [CrossRef]
- Tsafack, T.; Charles, G.P.; Hourmant, A.; Omokolo, N.D.; Branchard, M.J.P.C.; Culture, O. Effect of photoperiod and thermoperiod on microtuberization and carbohydrate levels in Cocoyam (Xanthosoma sagittifolium L. Schott). Plant Cell Tissue Organ Cult. 2009, 96, 151–159. [Google Scholar] [CrossRef]
- Motallebi-Azar, A.; Kazemiani, S.; Yarmohamadi, F.J.R.A. Effect of sugar/osmotica levels on in vitro microtuberization of potato (Solanum tuberosum L.). Plant Grow. 2013, 39, 112–116. [Google Scholar] [CrossRef]
- Li, M.; Li, J.; Liu, W.; Liu, L.; Lu, J.; Niu, J.; Liu, X.; Yang, Q. A protocol for in vitro production of microtubers in Chinese yam (Dioscorea opposita). Biosci. Biotechnol. Biochem. 2014, 78, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Zhu, M.; Yu, J.; Han, R.; Tang, C.; Xu, T.; Liu, J.; Li, Z. RNA-Seq and iTRAQ reveal multiple pathways involved in storage root formation and development in sweet potato (Ipomoea batatas L.). BMC Plant Biol. 2019, 19, 136. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, Q.; Li, H.; Sun, J.; Li, B.; Zhang, H.; Huang, Y.; Fang, J.; Zhou, Q. Induction and Plant Regeneration of Virus-free Microtuber in Red Bud Taro. Acta Hortic. Sin. 2020, 47, 2427–2438. [Google Scholar] [CrossRef]
- Yu, X.; Sheng, J.J.; Zheng, X.W.; Diao, Y.; Zheng, X.F.; Xie, K.Q.; Zhou, M.Q.; Hu, Z.L. First Report of Dasheen mosaic virus Infecting Lotus (Nelumbo nucifera) in China. Plant Dis. 2015, 99, 1449. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Feng, Z.; Wang, X.; Wang, X.; Zhang, X. DEGseq: An R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 2010, 26, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
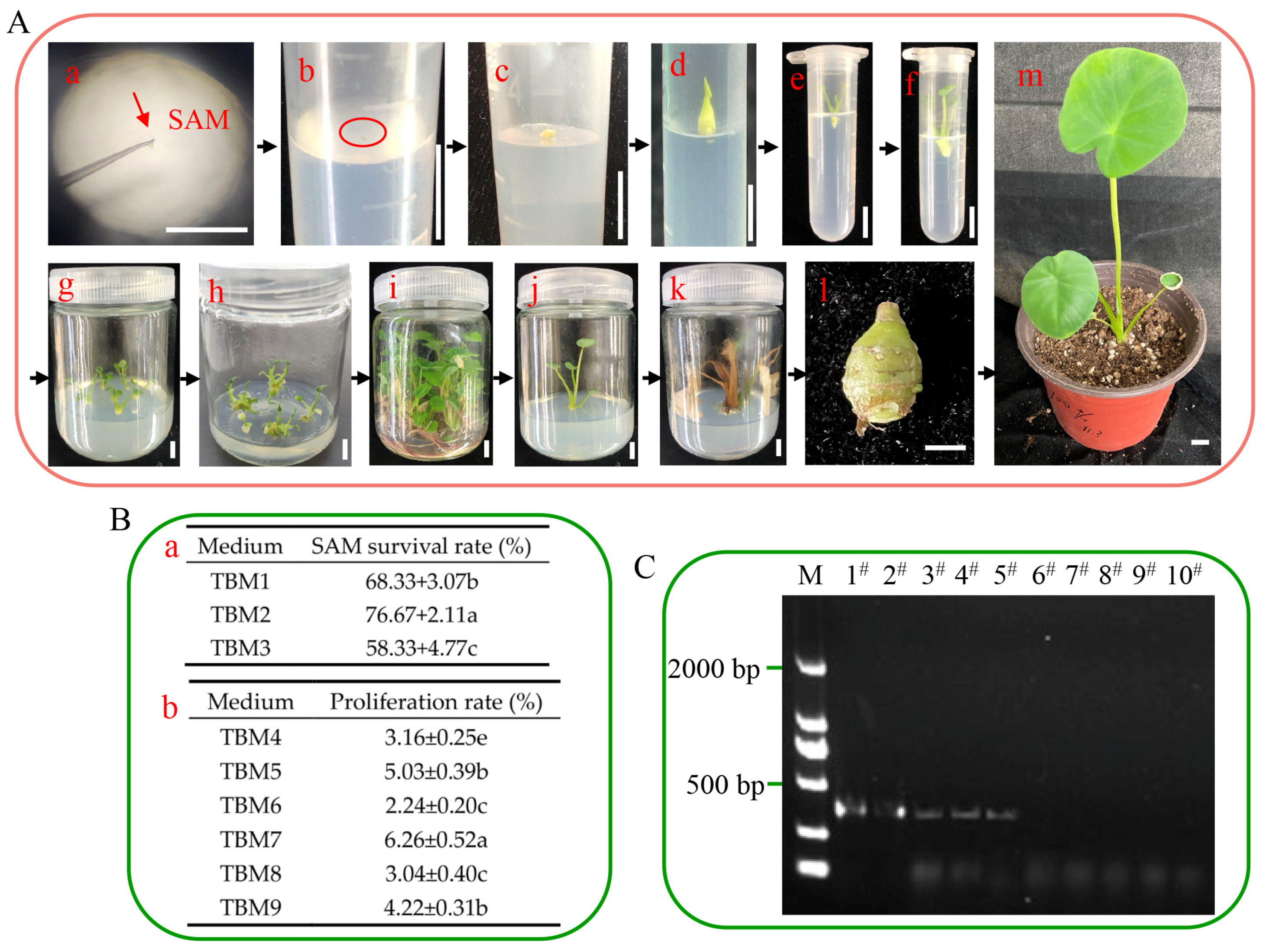
 represents the taro virus, and the area above the dotted line was used to induce adventitious shoots. (C) The shoot apical meristems for inducing adventitious shoots. (D) The shoot apical meristem was cultured in vitro. (E) The shoot apical meristem was cultured into adventitious shoots, and an RT-PCR assay was used to verify the elimination effect. (F) Adventitious shoots only grow roots in 3% sucrose conditions, and the solid circles represent sucrose. (G) Adventitious shoots induced taro corms in 8% sucrose or 3% sucrose + 5 μM ABA conditions; the solid circles represent sucrose, and the dotted circles represent ABA. (H) The virus-free taro corm, which is easy to transport and store.
represents the taro virus, and the area above the dotted line was used to induce adventitious shoots. (C) The shoot apical meristems for inducing adventitious shoots. (D) The shoot apical meristem was cultured in vitro. (E) The shoot apical meristem was cultured into adventitious shoots, and an RT-PCR assay was used to verify the elimination effect. (F) Adventitious shoots only grow roots in 3% sucrose conditions, and the solid circles represent sucrose. (G) Adventitious shoots induced taro corms in 8% sucrose or 3% sucrose + 5 μM ABA conditions; the solid circles represent sucrose, and the dotted circles represent ABA. (H) The virus-free taro corm, which is easy to transport and store.
 represents the taro virus, and the area above the dotted line was used to induce adventitious shoots. (C) The shoot apical meristems for inducing adventitious shoots. (D) The shoot apical meristem was cultured in vitro. (E) The shoot apical meristem was cultured into adventitious shoots, and an RT-PCR assay was used to verify the elimination effect. (F) Adventitious shoots only grow roots in 3% sucrose conditions, and the solid circles represent sucrose. (G) Adventitious shoots induced taro corms in 8% sucrose or 3% sucrose + 5 μM ABA conditions; the solid circles represent sucrose, and the dotted circles represent ABA. (H) The virus-free taro corm, which is easy to transport and store.
represents the taro virus, and the area above the dotted line was used to induce adventitious shoots. (C) The shoot apical meristems for inducing adventitious shoots. (D) The shoot apical meristem was cultured in vitro. (E) The shoot apical meristem was cultured into adventitious shoots, and an RT-PCR assay was used to verify the elimination effect. (F) Adventitious shoots only grow roots in 3% sucrose conditions, and the solid circles represent sucrose. (G) Adventitious shoots induced taro corms in 8% sucrose or 3% sucrose + 5 μM ABA conditions; the solid circles represent sucrose, and the dotted circles represent ABA. (H) The virus-free taro corm, which is easy to transport and store.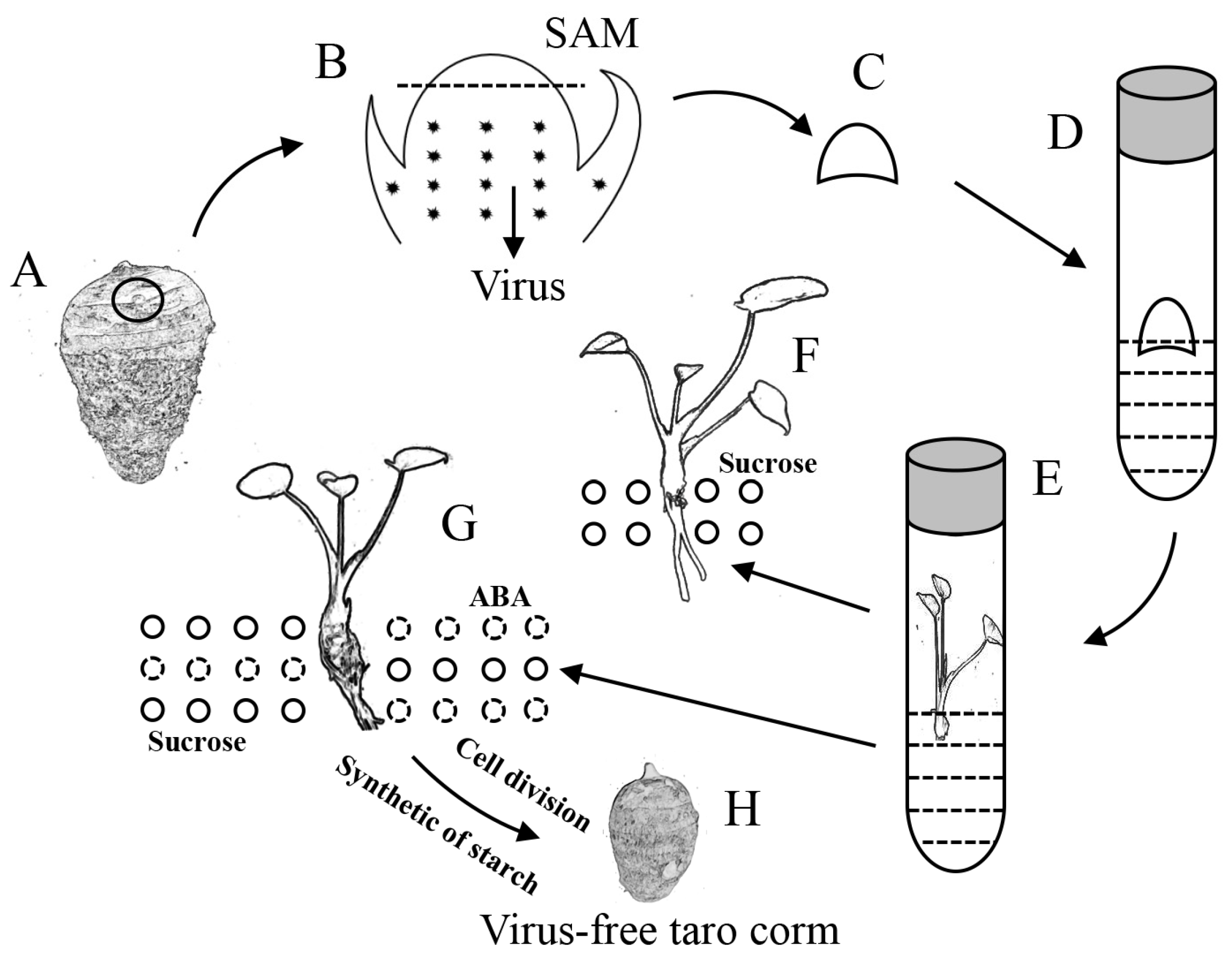
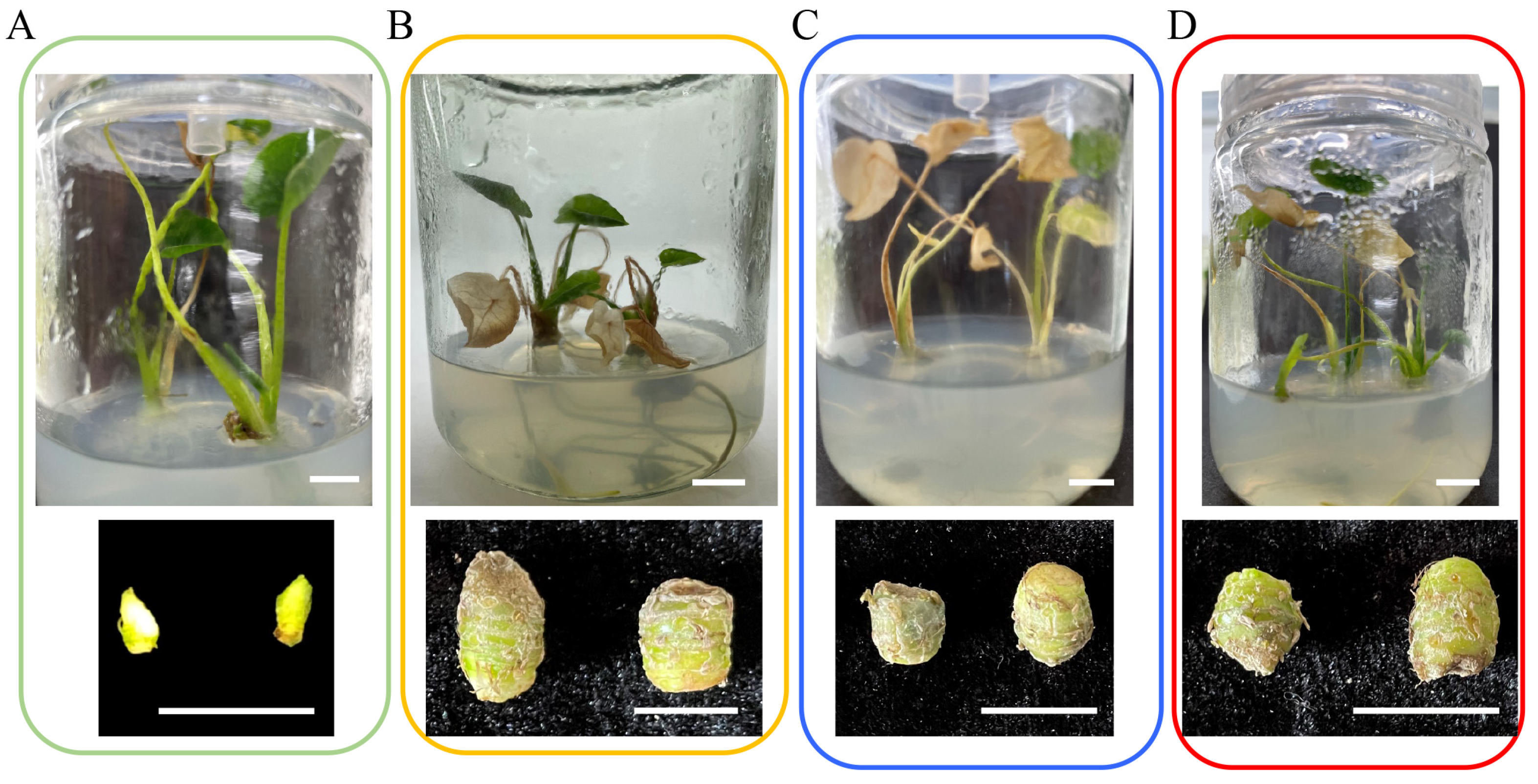
| Medium | Corm Weight (g) | Corm Transverse Diameter (mm) | Corm Longitudinal Diameter (mm) |
|---|---|---|---|
| TBM10 | - | - | - |
| TBM11 | 0.40 ± 0.04 c | 8.41 ± 0.26 b | 9.64 ± 0.78 ab |
| TBM12 | 0.28 ± 0.03 d | 7.23 ± 0.43 c | 9.26 ± 0.99 b |
| TBM13 | 0.50 ± 0.01 b | 8.79 ± 0.30 ab | 11.85 ± 0.93 a |
| TBM14 | 0.62 ± 0.05 a | 9.72 ± 0.54 a | 11.98 ± 1.00 a |
| TBM15 | 0.26 ± 0.02 d | 6.79 ± 0.40 c | 8.80 ± 0.33 b |
| TBM16 | 0.30 ± 0.03 d | 6.65 ± 0.30 c | 8.01 ± 0.95 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Xiao, Y.; Li, Z.; Liu, T.; Cui, J.; Li, B.; Zhu, Q.; Luo, S.; Shan, N.; Sun, J.; et al. Virus-Free Micro-Corm Induction and the Mechanism of Corm Development in Taro. Int. J. Mol. Sci. 2025, 26, 3740. https://doi.org/10.3390/ijms26083740
Wang S, Xiao Y, Li Z, Liu T, Cui J, Li B, Zhu Q, Luo S, Shan N, Sun J, et al. Virus-Free Micro-Corm Induction and the Mechanism of Corm Development in Taro. International Journal of Molecular Sciences. 2025; 26(8):3740. https://doi.org/10.3390/ijms26083740
Chicago/Turabian StyleWang, Shenglin, Yao Xiao, Zihao Li, Tao Liu, Jiarui Cui, Bicong Li, Qianglong Zhu, Sha Luo, Nan Shan, Jingyu Sun, and et al. 2025. "Virus-Free Micro-Corm Induction and the Mechanism of Corm Development in Taro" International Journal of Molecular Sciences 26, no. 8: 3740. https://doi.org/10.3390/ijms26083740
APA StyleWang, S., Xiao, Y., Li, Z., Liu, T., Cui, J., Li, B., Zhu, Q., Luo, S., Shan, N., Sun, J., Huang, Y., & Zhou, Q. (2025). Virus-Free Micro-Corm Induction and the Mechanism of Corm Development in Taro. International Journal of Molecular Sciences, 26(8), 3740. https://doi.org/10.3390/ijms26083740






