Gut Microbiota and Colorectal Cancer: A Balance Between Risk and Protection
Abstract
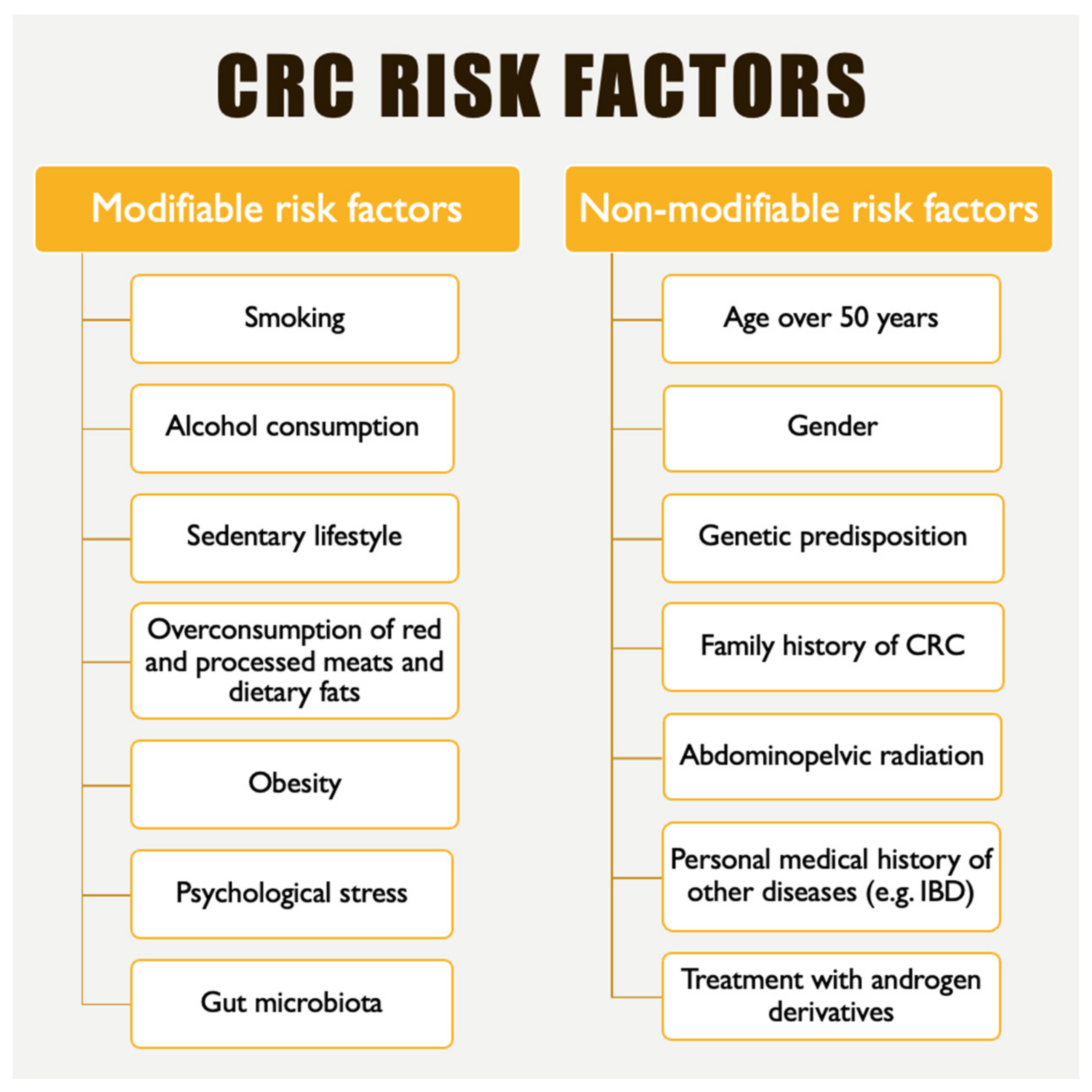
1. Introduction
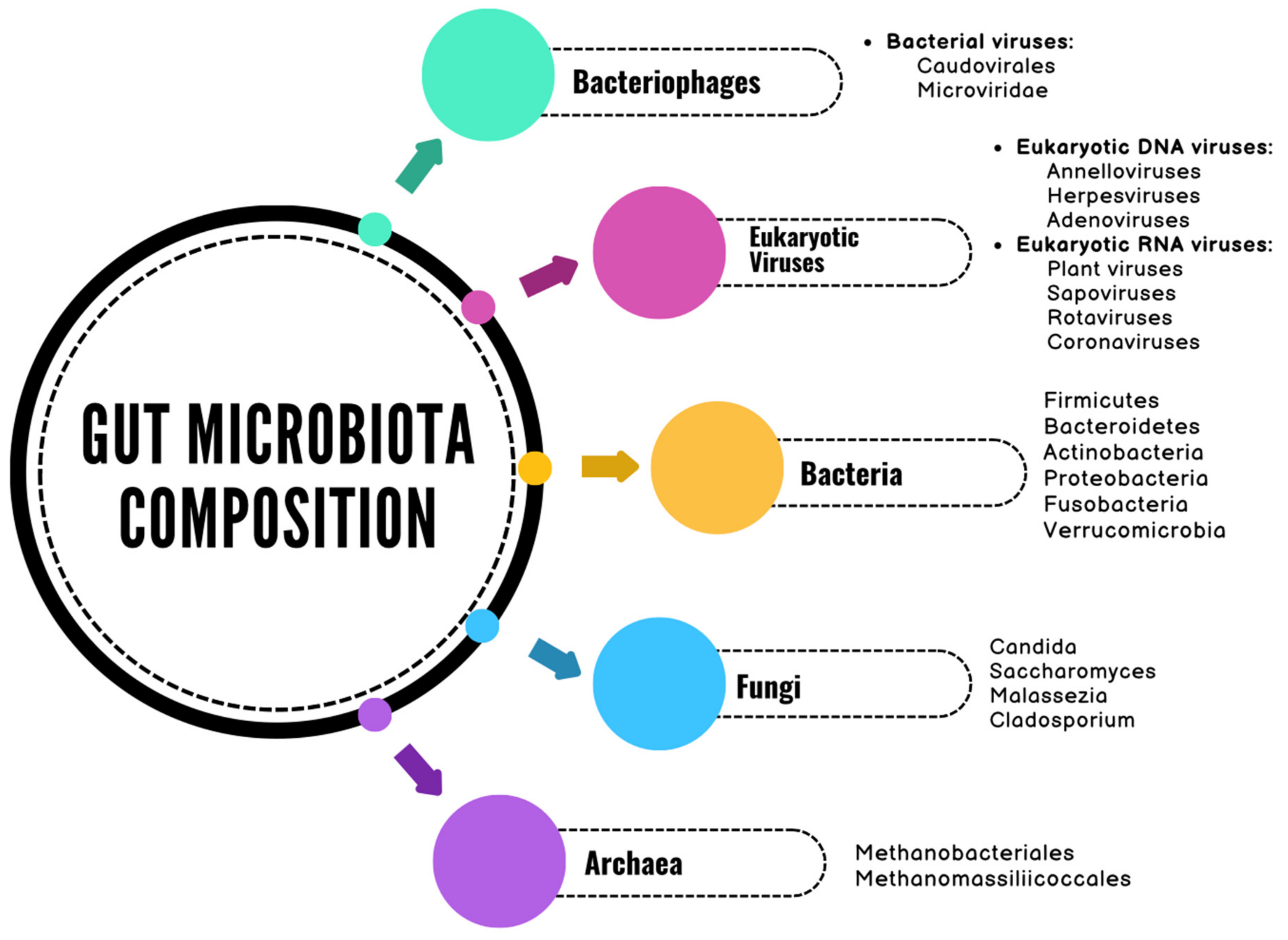
2. The Gut Microbiome
3. Mechanisms Linking Microbiota to Colorectal Cancer
3.1. Microbiota-Mediated Inflammation and Immune Modulation
- The MyD88-dependent pathway, which involves the myeloid differentiation factor 88 (MyD88) adapter protein;
- The TRIF-dependent pathway, which relies on the TIR-domain-containing adapter-inducing interferon-β (TRIF) [77].
3.2. Oxidative Stress
3.3. Evading the Antitumor Immune Response
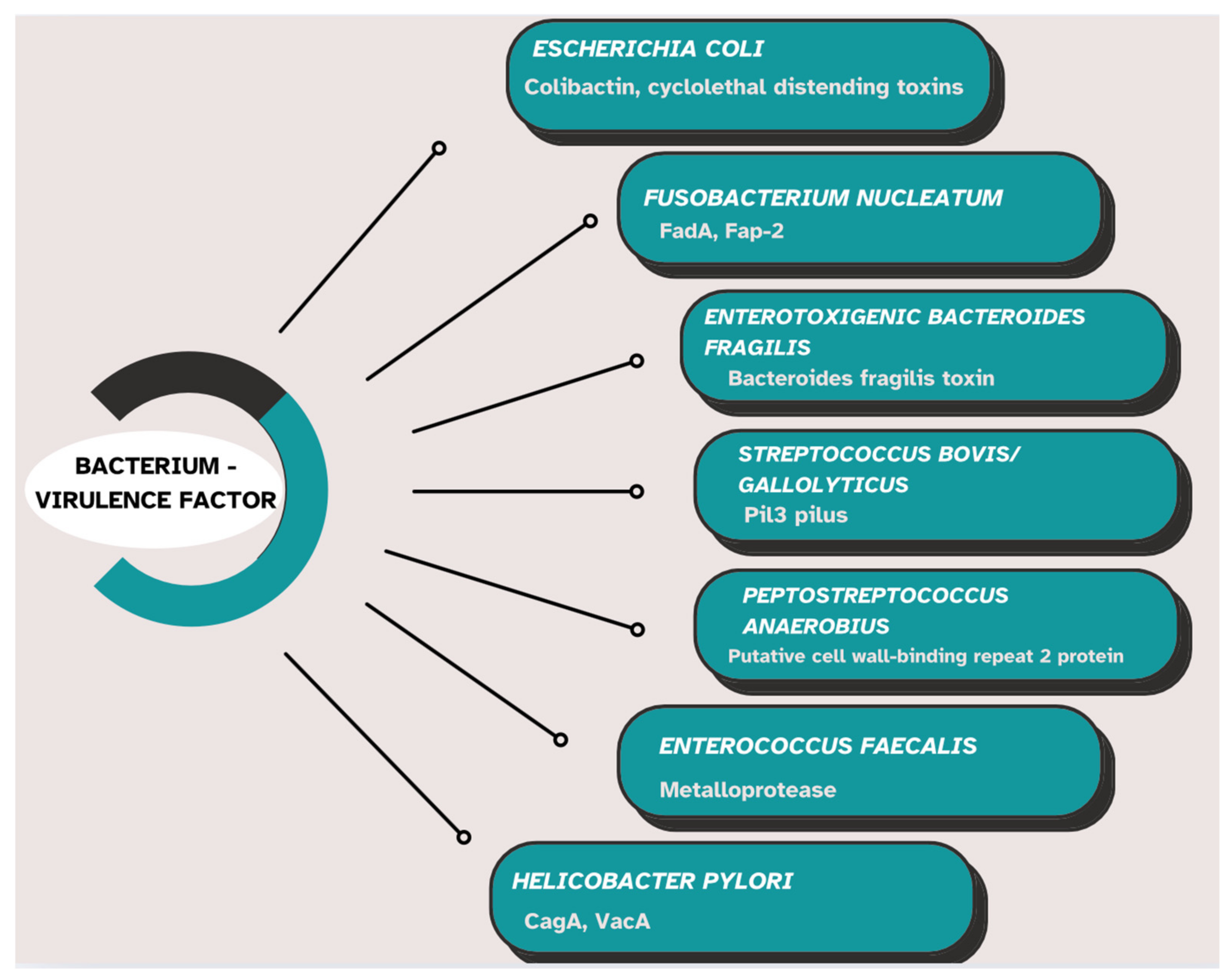
3.4. Production of Genotoxins, Virulence Factors, and Intestinal Microbial Metabolites
4. Pathogenic Microbiota Associated with Colorectal Cancer
5. Protective Microbiota in Colorectal Cancer
6. Concluding Remarks and Future Direction
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Agency for Research on Cancer. Available online: https://gco.iarc.fr/today/en/dataviz/maps-heatmap?mode=population (accessed on 20 January 2025).
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Roshandel, G.; Ghasemi-Kebria, F.; Malekzadeh, R. Colorectal Cancer: Epidemiology, Risk Factors, and Prevention. Cancers 2024, 16, 1530. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, V.A.; Gheorghe, G.; Bacalbasa, N.; Chiotoroiu, A.L.; Diaconu, C. Colorectal Cancer: From Risk Factors to Oncogenesis. Medicina 2023, 59, 1646. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Przegląd Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef]
- Saraiva, M.R.; Rosa, I.; Claro, I. Early-onset colorectal cancer: A review of current knowledge. World J. Gastroenterol. 2023, 29, 1289–1303. [Google Scholar] [CrossRef]
- Waddell, O.; Pearson, J.; McCombie, A.; Marshall, H.; Purcell, R.; Keenan, J.; Glyn, T.; Frizelle, F. The incidence of early onset colorectal cancer in Aotearoa New Zealand: 2000–2020. BMC Cancer 2024, 24, 456. [Google Scholar] [CrossRef]
- Ionescu, V.A.; Gheorghe, G.; Baban, I.A.; Barbu, A.; Georgescu, T.F.; Tiuca, L.C.; Antonie, N.I.; Diaconu, C.C. Prognostic differences between early-onset and late-onset colorectal cancer. Medicina 2025, 61, 390. [Google Scholar] [CrossRef]
- Yeo, H.; Betel, D.; Abelson, J.S.; Zheng, X.E.; Yantiss, R.; Shah, M.A. Early-onset Colorectal Cancer is Distinct from Traditional Colorectal Cancer. Clin. Color. Cancer 2017, 16, 293–299.e296. [Google Scholar] [CrossRef]
- Wong, C.C.; Yu, J. Gut microbiota in colorectal cancer development and therapy. Nat. Rev. Clin. Oncol. 2023, 20, 429–452. [Google Scholar] [CrossRef]
- Garvey, M. Intestinal Dysbiosis: Microbial Imbalance Impacts on Colorectal Cancer Initiation, Progression and Disease Mitigation. Biomedicines 2024, 12, 740. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.K. Potential Role of the Gut Microbiome in Colorectal Cancer Progression. Front. Immunol. 2022, 12, 807648. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wu, J.; Wang, Y.; Zhang, L.; Ren, J.; Zhang, Z.; Chen, B.; Zhang, K.; Zhu, B.; Liu, W.; et al. Lifestyle patterns influence the composition of the gut microbiome in a healthy Chinese population. Sci. Rep. 2023, 13, 14425. [Google Scholar] [CrossRef] [PubMed]
- Dekaboruah, E.; Suryavanshi, M.V.; Chettri, D.; Verma, A.K. Human microbiome: An academic update on human body site specific surveillance and its possible role. Arch. Microbiol. 2020, 202, 2147–2167. [Google Scholar] [CrossRef] [PubMed]
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M.; Rusu, A.V.; et al. Human gut microbiota in health and disease: Unveiling the relationship. Front. Microbiol. 2022, 13, 999001. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Verges, M.C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar]
- Fouhy, F.; Watkins, C.; Hill, C.J.; O’Shea, C.A.; Nagle, B.; Dempsey, E.M.; O’Toole, P.W.; Ross, R.P.; Ryan, C.A.; Stanton, C. Perinatal factors affect the gut microbiota up to four years after birth. Nat. Commun. 2019, 10, 1517. [Google Scholar] [CrossRef]
- Hollister, E.B.; Riehle, K.; Luna, R.A.; Weidler, E.M.; Rubio-Gonzale, M.; Mistretta, T.A.; Raza, S.; Doddapaneni, H.V.; Metcalf, G.A.; Muzny, D.M.; et al. Structure and function of the healthy pre-adolescent pediatric gut microbiome. Microbiome 2015, 3, 36. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Cao, Z.; Sugimura, N.; Burgermeister, E.; Ebert, M.P.; Zuo, T.; Lan, P. The gut virome: A new microbiome component in health and disease. EBioMedicine 2022, 81, 104113. [Google Scholar] [CrossRef]
- Mafra, D.; Ribeiro, M.; Fonseca, L.; Regis, B.; Cardozo, L.F.M.F.; dos Santos, H.F.; de Jesus, H.E.; Schultz, J.; Shiels, P.G.; Stenvinkel, P.; et al. Archaea from the gut microbiota of humans: Could be linked to chronic diseases? Anaerobe 2022, 77, 102629. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Abele, G.; Miggiano, D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Shkoporov, A.N.; Hill, C. Bacteriophages of the Human Gut: The “known unknown” of the microbiome. Cell Host Microbe 2019, 25, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Bushman, F.D. The human virome: Assembly, composition and host interactions. Nat. Rev. Microbiol. 2021, 19, 514–527. [Google Scholar] [CrossRef]
- Patel, M.M.; Hall, A.J.; Vinjé, J.; Parashar, U.D. Noroviruses: A comprehensive review. J. Clin. Virol. 2009, 44, 1–8. [Google Scholar] [CrossRef]
- Lopman, B.A.; Steele, D.; Kirkwood, C.D.; Parashar, U.D. The vast and varied global burden of norovirus: Prospects for prevention and control. PLoS Med. 2016, 13, e1001999. [Google Scholar] [CrossRef]
- Sadiq, A.; Khan, J. Rotavirus in developing countries: Molecular diversity, epidemiological insights, and strategies for effective vaccination. Front. Microbiol. 2024, 14, 1297269. [Google Scholar] [CrossRef]
- Omatola, C.A.; Mshelbwala, P.P.; Okolo, M.L.O.; Onoja, A.B.; Abraham, J.O.; Adaji, D.M.; Samson, S.O.; Okeme, T.O.; Aminu, R.F.; Akor, M.E.; et al. Noroviruses: Evolutionary Dynamics, Epidemiology, Pathogenesis, and Vaccine Advances—A Comprehensive Review. Vaccines 2024, 12, 590. [Google Scholar] [CrossRef]
- Bosch, A.; Pintó, R.M.; Guix, S. Human astroviruses. Clin. Microbiol. Rev. 2014, 27, 1048–1074. [Google Scholar] [CrossRef]
- Cortes-Penfield, N.W.; Ramani, S.; Estes, M.K.; Atmar, R.L. Astrovirus infection in humans: Molecular epidemiology, immune response, and clinical disease. Infect. Immun. 2017, 85, e00207-17. [Google Scholar]
- Rubinstein, R.J.; Gutiérrez, L.; Toval-Ruíz, C.; Hammond, K.; Bode, L.; Vinjé, J.; Vilchez, S.; Becker-Dreps, S.; Bucardo, F.; Vielot, N.A.; et al. Epidemiology of Pediatric Astrovirus Gastroenteritis in a Nicaraguan Birth Cohort. Open Forum Infect. Dis. 2024, 11, ofae465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, R. The human gut phageome: Composition, development, and alterations in disease. Front. Microbiol. 2023, 14, 1213625. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hou, X.; Chu, H. The Novel Role of Phage Particles in Chronic Liver Diseases. Microorganisms 2023, 11, 1181. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhou, C. Bacteriophages: A double-edged sword in the gastrointestinal tract. Front. Microbiomes 2024, 3, 1450523. [Google Scholar] [CrossRef]
- Bernardes, E.T.; Pettersen, V.K.; Gutierrez, M.W.; Laforest-Lapointe, I.; Jendzjowsky, N.G.; Cavn, J.B.; Vicentini, F.A.; Keenan, C.M.; Ramay, H.R.; Samara, J.; et al. Intestinal fungi are causally implicated in microbiome assembly and immune development in mice. Nat. Commun. 2020, 11, 2577. [Google Scholar] [CrossRef]
- Perez, J.C. Fungi of the human gut microbiota: Roles and significance. Int. J. Med. Microbiol. 2021, 311, 151490. [Google Scholar] [CrossRef]
- Hill, J.H.; Round, J.L. Intestinal fungal-host interactions in promoting and maintaining health. Cell Host Microbe 2024, 32, 1668–1680. [Google Scholar] [CrossRef]
- Hallen-Adams, H.E.; Suhr, M.J. Fungi in the healthy human gastrointestinal tract. Virulence 2017, 8, 352–358. [Google Scholar] [CrossRef]
- Gaci, N.; Borrel, G.; Tottey, W.; O’Toole, P.W.; Brugere, J.F. Archaea and the human gut: New beginning of an old story. World J. Gastroenterol. 2014, 20, 16062–26078. [Google Scholar] [CrossRef]
- Kim, J.Y.; Whon, T.W.; Lim, M.Y.; Kim, Y.N.; Kim, N.; Kwon, M.S.; Kim, J.; Lee, S.H.; Choi, H.J.; Nam, I.H.; et al. The human gut archaeome: Identification of diverse haloarchaea in Korean subjects. Microbiome 2020, 8, 114. [Google Scholar] [CrossRef]
- Chibani, C.M.; Mahnert, A.; Borrel, G.; Almeida, A.; Werner, A.; Brugere, J.F.; Gribaldo, S.; Finn, R.D.; Schmitz, R.A.; Miossl-Eichinger, C. A catalogue of 1,167 genomes from the human gut archaeome. Nat. Microbiol. 2022, 7, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Hul, M.V.; Cani, P.D.; Petitfils, C.; De Vos, W.M.; Tilg, H.; El-Omar, E.M. What defines a healthy gut microbiome? Gut 2024, 73, 1893–1908. [Google Scholar] [PubMed]
- Maciel-Fiuza, M.F.; Muller, G.C.; Campos, D.M.S. Role of gut microbiota in infectious and inflammatory disease. Front. Microbiol. 2023, 14, 1098386. [Google Scholar] [CrossRef] [PubMed]
- Takiishi, T.; Fenero, C.I.M.; Camara, N.O.S. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers 2017, 5, e1373208. [Google Scholar] [CrossRef]
- Sommer, F.; Bäckhed, F. The gut microbiota-masters of host development and physiology. Nat Rev. Microbiol. 2013, 11, 227–238. [Google Scholar] [CrossRef]
- Jang, Y.J.; Kim, W.K.; Han, D.H.; Lee, K.; Ko, G. Lactobacillus fermentum species ameliorate dextran sulfate sodium-induced colitis by regulating the immune response and altering gut microbiota. Gut Microbes 2019, 10, 696–711. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.R.; Kim, N.R.; Jeong, B.J.; Lee, J.S.; Jang, S.; Chung, D.K. Oral administration of lactobacillus plantarum lysates attenuates the development of atopic dermatitis lesions in mouse models. J. Microbiol. 2015, 53, 47–52. [Google Scholar] [CrossRef]
- Huang, R.; Wu, F.; Zhou, Q.; Wei, W.; Yue, J.; Xiao, B.; Luo, Z. Lactobacillus and intestinal diseases: Mechanisms of action and clinical applications. Microbiol. Res. 2022, 260, 127019. [Google Scholar] [CrossRef]
- Round, J.L.; Lee, S.M.; Li, J.; Tran, G.; Jabri, B.; Chatila, T.A.; Mazmanian, S.K. The toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 2011, 332, 974–977. [Google Scholar] [CrossRef]
- Bachmann, R.; van Hul, M.; Baldin, P.; Léonard, D.; Delzenne, N.M.; Belzer, C.; Ouwerkerk, J.P.; Repsilber, D.; Rangel, I.; Kartheuser, A.; et al. Akkermansia muciniphila reduces peritonitis and improves intestinal tissue wound healing after a colonic Transmural defect by a MyD88-dependent mechanism. Cells 2022, 11, 2666. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, K.; El Abbar, F.; Dobranowski, P.; Manoogian, J.; Butcher, J.; Figeys, D.; Mack, D.; Stintzi, A. Butyrate’s role in human health and the current progress towards its clinical application to treat gastrointestinal disease. Clin. Nutr. 2023, 42, 61–75. [Google Scholar] [CrossRef]
- Soldan, M.; Argalasova, L.; Hadvinova, L.; Galilelo, B.; Babjakova, J. The Effect of Dietary Types on Gut Microbiota Composition and Development of Non-Communicable Diseases: A Narrative Review. Nutrients 2024, 16, 3134. [Google Scholar] [CrossRef] [PubMed]
- Parizadeh, M.; Arrieta, M.C. The global human gut microbiome: Genes, lifestyles, and diet. Trends Mol. Med. 2023, 29, 789–801. [Google Scholar] [CrossRef]
- Hall, A.B.; Tolonen, A.C.; Xavier, R.J. Human genetic variation and the gut microbiome in disease. Nat. Rev. Genet. 2017, 18, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Menafra, D.; Progano, M.; Tecce, N.; Pivonello, R.; Colao, A. Diet and gut microbiome: Impact of each factor and mutual interactions on prevention and treatment of type 1, type 2, and gestational diabetes mellitus. Hum. Nutr. Metab. 2024, 38, 200286. [Google Scholar] [CrossRef]
- Kendong, S.M.A.; Ali, R.A.R.; Nawawi, K.N.M.; Ahmad, H.F.; Mokhtar, N.M. Gut Dysbiosis and Intestinal Barrier Dysfunction: Potential Explanation for Early-Onset Colorectal Cancer. Front. Cell. Infect. Microbiol. 2021, 11, 744606. [Google Scholar] [CrossRef]
- Mo, Z.; Huang, P.; Yang, C.; Xiao, S.; Zhang, G.; Ling, F.; Li, L. Meta-Analysis of 16S rRNA Microbial Data Identified Distinctive and Predictive Microbiota Dysbiosis in Colorectal Carcinoma Adjacent Tissue. mSystems 2020, 5, e00138-20. [Google Scholar] [CrossRef]
- Mira-Pascual, L.; Cabrera-Rubio, R.; Ocon, S.; Costales, P.; Parra, A.; Suarez, A.; Moris, F.; Rodrigo, L.; Mira, A.; Collado, M.C. Microbial Mucosal Colonic Shifts Associated with the Development of Colorectal Cancer Reveal the Presence of Different Bacterial and Archaeal Biomarkers. J. Gastroenterol. 2015, 50, 167–179. [Google Scholar] [CrossRef]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef]
- Petersen, C.; Round, J.L. Defining dysbiosis and its influence on host immunity and disease. Cell. Microbiol. 2014, 16, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.M.; Bercik, P. The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology 2009, 136, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Wei, H.; Liu, W.; Coker, O.O.; Gou, H.; Liu, C.; Zhao, L.; Li, C.; Zhou, Y.; Wang, G.; et al. Cigarette smoke promotes colorectal cancer through modulation of gut microbiota and related metabolites. Gut 2022, 71, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Claesson, M.J.; Cusack, S.; O’Sullivan, O.; Greene-Diniz, R.; de Weerd, H.; Flannery, E.; Marchesi, J.R.; Falush, D.; Dinan, T.; Fitzgerald, G.; et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc. Natl. Acad. Sci. USA 2011, 108, 4586–4591. [Google Scholar] [CrossRef]
- DeJong, E.N.; Surette, M.G.; Bowdish, D.M.E. The gut microbiota and unhealthy aging: Disentangling cause from consequence. Cell Host Microbe 2020, 28, 180–189. [Google Scholar] [CrossRef]
- Raskov, H.; Pommergaard, H.C.; Burcharth, J.; Rosenberg, J. Colorectal carcinogenesis—Update and perspectives. World J. Gastroenterol. 2014, 20, 18151–18164. [Google Scholar] [CrossRef]
- Hernandez-Luna, M.A.; Lopez-Briones, S.; Luria-Perez, R. The Four Horsemen in Colon Cancer. J. Oncol. 2019, 2019, 5636272. [Google Scholar] [CrossRef]
- Pandey, H.; Tang, D.W.T.; Wong, S.H.; Lal, D. Gut Microbiota in Colorectal Cancer: Biological Role and Therapeutic Opportunities. Cancers 2023, 15, 866. [Google Scholar] [CrossRef]
- Ionescu, V.A.; Gheorghe, G.; Georgescu, T.F.; Buica, V.; Catanescu, M.S.; Cercel, I.A.; Budeanu, B.; Budan, M.; Bacalbasa, N.; Diaconu, C. Exploring the Role of the Gut Microbiota in Colorectal Cancer Development. Gastrointest. Disord. 2024, 6, 526–537. [Google Scholar] [CrossRef]
- Miron, A.; Giurcaneanu, C.; Mihai, M.M.; Popescu, M.N.; Soare, E.; Popa, L.G. Antimicrobial Biomaterials for Chronic Wound Care. Pharmaceutics 2023, 28, 1606. [Google Scholar] [CrossRef]
- Tlaskalová-Hogenová, H.; Stepánková, R.; Hudcovic, T.; Tucková, L.; Cukrowska, B.; Lodinová-Zádníková, R.; Kozáková, H.; Rossmann, P.; Bártová, J.; Sokol, D.; et al. Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunol. Lett. 2004, 93, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Mowat, A.M. To respond or not to respond–A personal perspective of intestinal tolerance. Nat. Rev. Immunol. 2018, 18, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.P.; Amstad, P.; Raja, K.; Ambs, S.; Nagashima, M.; Bennett, W.P.; Shields, P.G.; Ham, A.J.; Swenberg, J.A.; Marrogi, A.J.; et al. Increased p53 mutation load in noncancerous colon tissue from ulcerative colitis: A cancer-prone chronic inflammatory disease. Cancer Res. 2000, 60, 3333–3337. [Google Scholar] [PubMed]
- Lavelle, E.C.; Murphy, C.; O’Neill, L.A.; Creagh, E.M. The role of TLRs, NLRs, and RLRs in mucosal innate immunity and homeostasis. Mucosal Immunol. 2010, 3, 17–28. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal permeability—A new target for disease prevention and therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef]
- O’Neill, L.A.J.; Golenbock, D.; Bowie, A.G. The history of Toll-like receptors—Redefining innate immunity. Nat. Rev. Immunol. 2013, 13, 453–460. [Google Scholar] [CrossRef]
- Salcedo, R.; Worschech, A.; Cardone, M.; Jones, Y.; Gyulai, Z.; Dai, R.M.; Wang, E.; Ma, W.; Haines, D.; O’hUigin, C.; et al. MyD88-mediated signaling prevents development of adenocarcinomas of the colon: Role of interleukin 18. J. Exp. Med. 2010, 207, 1625–1636. [Google Scholar] [CrossRef]
- Yesudhas, D.; Gosu, V.; Anwar, M.A.; Choi, S. Multiple roles of toll-like receptor 4 in colorectal cancer. Front. Immunol. 2014, 5, 334. [Google Scholar] [CrossRef]
- Lowe, E.L.; Crother, T.R.; Rabizadeh, S.; Hu, B.; Wang, H.; Chen, S.; Shimada, K.; Wong, M.H.; Michelsen, K.S.; Arditi, M. Toll-like receptor 2 signaling protects mice from tumor development in a mouse model of colitis-induced cancer. PLoS ONE 2010, 5, e13027. [Google Scholar] [CrossRef]
- Couturier-Maillard, A.; Secher, T.; Rehman, A.; Normand, S.; De Arcangelis, A.; Haesler, R.; Huot, L.; Grandjean, T.; Bressenot, A.; Delanoye-Crespin, A.; et al. NOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancer. J. Clin. Investig. 2013, 123, 700–711. [Google Scholar] [CrossRef]
- Mager, L.F.; Wasmer, M.H.; Rau, T.T.; Krebs, P. Cytokine-induced modulation of colorectal cancer. Front. Oncol. 2016, 6, 96. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Baba, Y.; Yamauchi, M.; Kuchiba, A.; Nosho, K.; Shima, K.; Tanaka, N.; Huttenhower, C.; Frank, D.A.; Fuchs, C.S.; et al. STAT3 expression, molecular features, inflammation patterns, and prognosis in a database of 724 colorectal cancers. Clin. Cancer Res. 2011, 17, 1452–1462. [Google Scholar] [CrossRef] [PubMed]
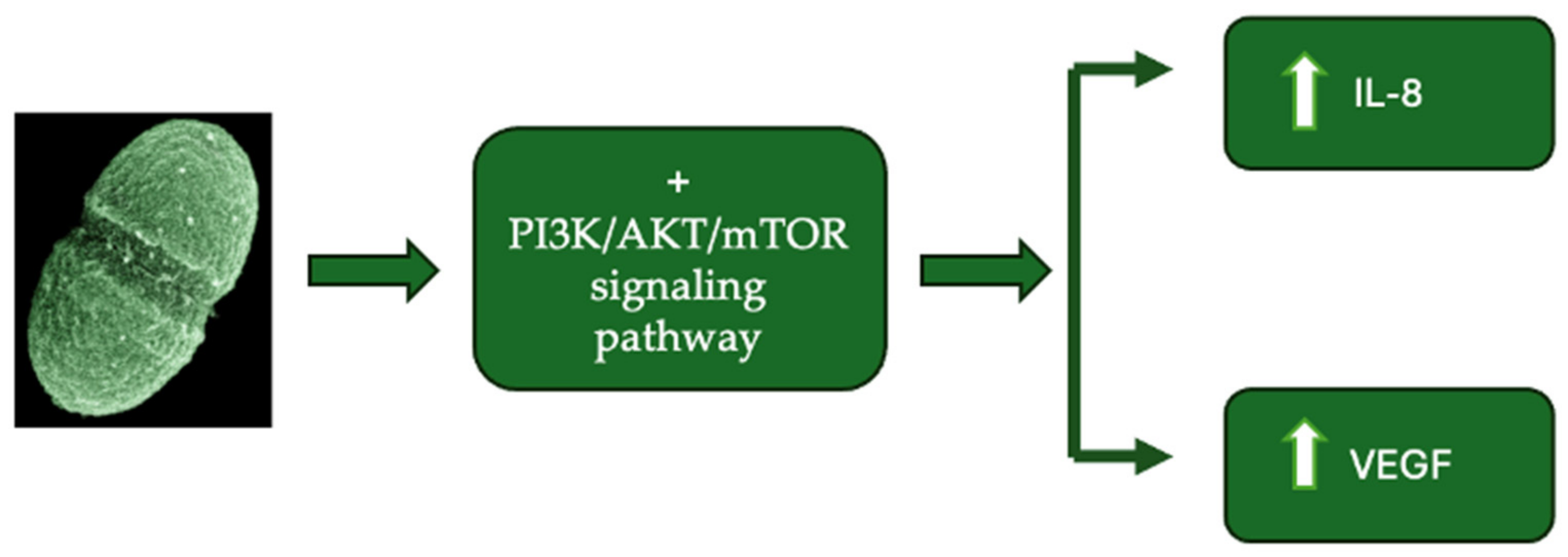
- Gur, C.; Ibrahim, Y.; Isaacson, B.; Yamin, R.; Abed, J.; Gamliel, M.; Enk, J.; Bar-On, Y.; Stanietsky-Kaynan, N.; Coppenhagen-Glazer, S.; et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 2015, 42, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Mima, K.; Sukawa, Y.; Nishihara, R.; Qian, Z.R.; Yamauchi, M.; Inamura, K.; Kim, S.A.; Masuda, A.; Nowak, J.A.; Nosho, K.; et al. Fusobacterium nucleatum and T cells in colorectal carcinoma. JAMA Oncol. 2015, 1, 653–661. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.; Li, L.; Zhong, C.; Zhang, Y.; Yang, X.; Li, M.; Yang, C. The role of gut microbiota in intestinal disease: From an oxidative stress perspective. Front. Microbiol. 2024, 15, 1328324. [Google Scholar] [CrossRef]
- Jackson, D.N.; Theiss, A.L. Gut bacteria signaling to mitochondria in intestinal inflammation and cancer. Gut Microbes 2020, 11, 285–304. [Google Scholar] [CrossRef]
- Singh, T.P.; Kadyan, S.; Devi, H.; Park, G.; Nagpal, R. Gut microbiome as a therapeutic target for liver diseases. Life Sci. 2023, 322, 121685. [Google Scholar] [CrossRef]
- Ma, C.; Han, M.; Heinrich, B.; Fu, Q.; Zhang, Q.; Sandhu, M.; Agdashian, D.; Terabe, M.; Berzofsky, J.A.; Fako, V.; et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science 2018, 360, eaan593. [Google Scholar] [CrossRef]
- Klampfer, L. Cytokines, inflammation and colon cancer. Curr. Cancer Drug Targets 2011, 11, 451–464. [Google Scholar] [CrossRef]
- Bardelcikova, A.; Soltys, J.; Mojzis, J. Oxidative Stress, Inflammation and Colorectal Cancer: An Overview. Antioxidants 2023, 12, 901. [Google Scholar] [CrossRef]
- Sorolla, M.A.; Hidalgo, I.; Sorolla, A.; Montal, R.; Pallise, O.; Salud, A.; Parisi, E. Microenvironmental Reactive Oxygen Species in Colorectal Cancer: Involved Processes and Therapeutic Opportunities. Cancers 2021, 13, 5037. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.P.; Zhou, H.L.; Chen, H.B.; Zheng, M.Y.; Liang, Y.W.; Gu, Y.T.; Li, W.T.; Qiu, W.L.; Zhou, H.G. Gut microbiota interactions with antitumor immunity in colorectal cancer: From understanding to application. Biomed. Pharmacother. 2023, 165, 115040. [Google Scholar] [CrossRef] [PubMed]
- Gagniere, J.; Raisch, J.; Veziant, J.; Barnich, N.; Bonnet, R.; Buc, E.; Bringer, M.A.; Pezet, D.; Bonnet, M. Gut microbiota imbalance and colorectal cancer. World J. Gastroenterol. 2016, 22, 501–518. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, D.; Wu, D.; Gao, X.; Shao, F.; Zhao, M.; Wang, J.; Ma, J.; Wang, W.; Qin, X.; et al. Tissue-resident Lachnospiraceae family bacteria protect against colorectal carcinogenesis by promoting tumor immune surveillance. Cell Host Microbe 2023, 31, 418–432. [Google Scholar] [CrossRef]
- Yu, A.I.; Zhao, K.A.; Eaton, K.A.; Ho, S.; Chen, J.; Poe, S.; Becker, J.; Gonzalez, A.; McKinstry, D.; Hasso, M.; et al. Gut microbiota modulate CD8 T cell responses to influence colitis-associated tumorigenesis. Cell Rep. 2020, 31, 107471. [Google Scholar] [CrossRef]
- Chen, H.; Tong, T.; Lu, S.Y.; Ji, L.; Xuan, B.; Zhao, G.; Yan, Y.; Song, L.; Zhao, L.; Xie, Y.; et al. Urea cycle activation triggered by host-microbiota maladaptation driving colorectal tumorigenesis. Cell Metab. 2023, 35, 651–666.e7. [Google Scholar] [CrossRef]
- Shakhpazyan, N.K.; Mikhaleva, L.M.; Bedzhanyan, A.L.; Gioeva, Z.V.; Mikhalev, A.I.; Midiber, K.Y.; Pechnikova, V.V.; Biryukov, A.E. Exploring the Role of the Gut Microbiota in Modulating Colorectal Cancer Immunity. Cells 2024, 13, 1437. [Google Scholar] [CrossRef]
- He, R.; Huang, S.; Lu, J.; Su, L.; Gao, X.; Chi, H. Unveiling the immune symphony: Decoding colorectal cancer metastasis through immune interactions. Front. Immunol. 2024, 15, 1362709. [Google Scholar] [CrossRef]
- Ratajczak, K.; Grel, H.; Olejnik, P.; Jakiela, S.; Stobiecka, M. Current progress, strategy, and prospects of PD-1/PDL-1 immune checkpoint biosensing platforms for cancer diagnostics, therapy monitoring, and drug screening. Biosens. Bioelectron. 2023, 240, 115644. [Google Scholar] [CrossRef]
- Schwartz, J.C.; Zhang, X.; Fedorov, A.A.; Nathenson, S.G.; Almo, S.C. Structural basis for co-stimulation by the human CTLA-4/B7-2 complex. Nature 2001, 410, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhou, R.; Wang, H.; Li, W.; Pan, M.; Yao, X.; Zhan, W.; Yang, S.; Xu, L.; Ding, Y.; et al. Gut microbiota-stimulated cathepsin K secretion mediates TLR4-dependent M2 macrophage polarization and promotes tumor metastasis in colorectal cancer. Cell Death Differ. 2019, 26, 2447–2463. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Fan, L.; Lin, Y.; Shen, W.; Qi, Y.; Zhang, Y.; Chen, Z.; Wang, L.; Long, Y.; Hou, T.; et al. Fusobacterium nucleatum promotes colorectal cancer metastasis through miR-1322/CCL20 axis and M2 polarization. Gut Microbes 2021, 13, 1980347. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Wong, C.C.; Tong, L.; Chu, E.S.H.; Szeto, C.H.; Go, M.Y.Y.; Coker, O.O.; Chan, A.W.H.; Chan, F.K.L.; Sung, J.J.Y.; et al. Peptostreptococcus anaerobius promotes colorectal carcinogenesis and modulates tumour immunity. Nat. Microbiol. 2019, 4, 2319–2330. [Google Scholar] [CrossRef]
- Lin, Y.; Fan, L.; Qi, Y.; Xu, C.; Jia, D.; Jiang, Y.; Chen, S.; Wang, L. Bifidobacterium adolescentis induces decorin(+) macrophages via TLR2 to suppress colorectal carcinogenesis. J. Exp. Clin. Cancer Res. 2023, 42, 172. [Google Scholar] [CrossRef]
- Davrolis, N.; Gazouli, M.; Rigal, F.; Whittaker, R.J.; Matthews, T.J.; Georgiou, K.; Thedoropoulos, G.; Triantis, K.A. Rower-law scaling in intratumoral microbiota of colorectal cancer. Gut Pathog. 2024, 16, 34. [Google Scholar] [CrossRef]
- Khan, M.A.W.; Ologun, G.; Arora, R.; McQuade, J.; Wargo, J.A. The gut microbiome modulates response to cancer immunotherapy. Dig. Dis. Sci. 2021, 65, 885–896. [Google Scholar] [CrossRef]
- Lu, Y.; Yuan, X.; Wang, M.; He, Z.; Li, H.; Wang, J.; Li, Q. Gut microbiota influence immunotherapy responses: Mechanisms and therapeutic strategies. J. Hematol. Oncol. 2022, 15, 47. [Google Scholar] [CrossRef]
- Kang, X.; Lau, H.C.H.; Yu, J. Modulating gut microbiome in cancer immunotherapy: Harnessing microbes to enhance treatment efficacy. Cell Rep. Med. 2024, 5, 101478. [Google Scholar] [CrossRef]
- Routy, B.; Gopalakrishnan, V.; Daillere, R.; Zitvogel, L.; Wargo, J.A.; Kroemer, G. The gut microbiota influences anticancer immunosurveillance and general health. Nat. Rev. Clin. Oncol. 2018, 15, 382–396. [Google Scholar] [CrossRef]
- Peng, Z.; Cheng, S.; Kou, Y.; Wang, Z.; Jin, R.; Hu, H.; Zhang, X.; Gong, J.F.; Li, J.; Lu, M.; et al. The Gut Microbiome Is Associated with Clinical Response to Anti-PD-1/PD-L1 Immunotherapy in Gastrointestinal Cancer. Cancer Immunol. Res. 2020, 8, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Tanoue, T.; Morita, S.; Plichta, D.R.; Skelly, A.N.; Suda, W.; Sugiura, Y.; Narushima, S.; Vlamakis, H.; Motoo, I.; Sugita, K.; et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature 2019, 565, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Leake, I. Genotoxins from gut bacteria. Nat. Biotechnol. 2022, 40, 1765. [Google Scholar] [CrossRef] [PubMed]
- Fardini, Y.; Wang, X.; Témoin, S.; Nithianantham, S.; Lee, D.; Shoham, M.; Han, Y.W. Fusobacterium nucleatum adhesin FadA binds vascular endothelial cadherin and alters endothelial integrity. Mol. Microbiol. 2011, 82, 1468–1480. [Google Scholar] [CrossRef]
- Abed, J.; Emgard, J.E.M.; Zamir, G.; Faroja, M.; Almogy, G.; Grenov, A.; Sol, A.; Naor, R.; Pikarsky, E.; Atlan, K.A.; et al. Fap2 Mediates Fusobacterium nucleatum Colorectal Adenocarcinoma Enrichment by Binding to Tumor-Expressed Gal-GalNAc. Cell Host Microbe 2016, 20, 215–225. [Google Scholar] [CrossRef]
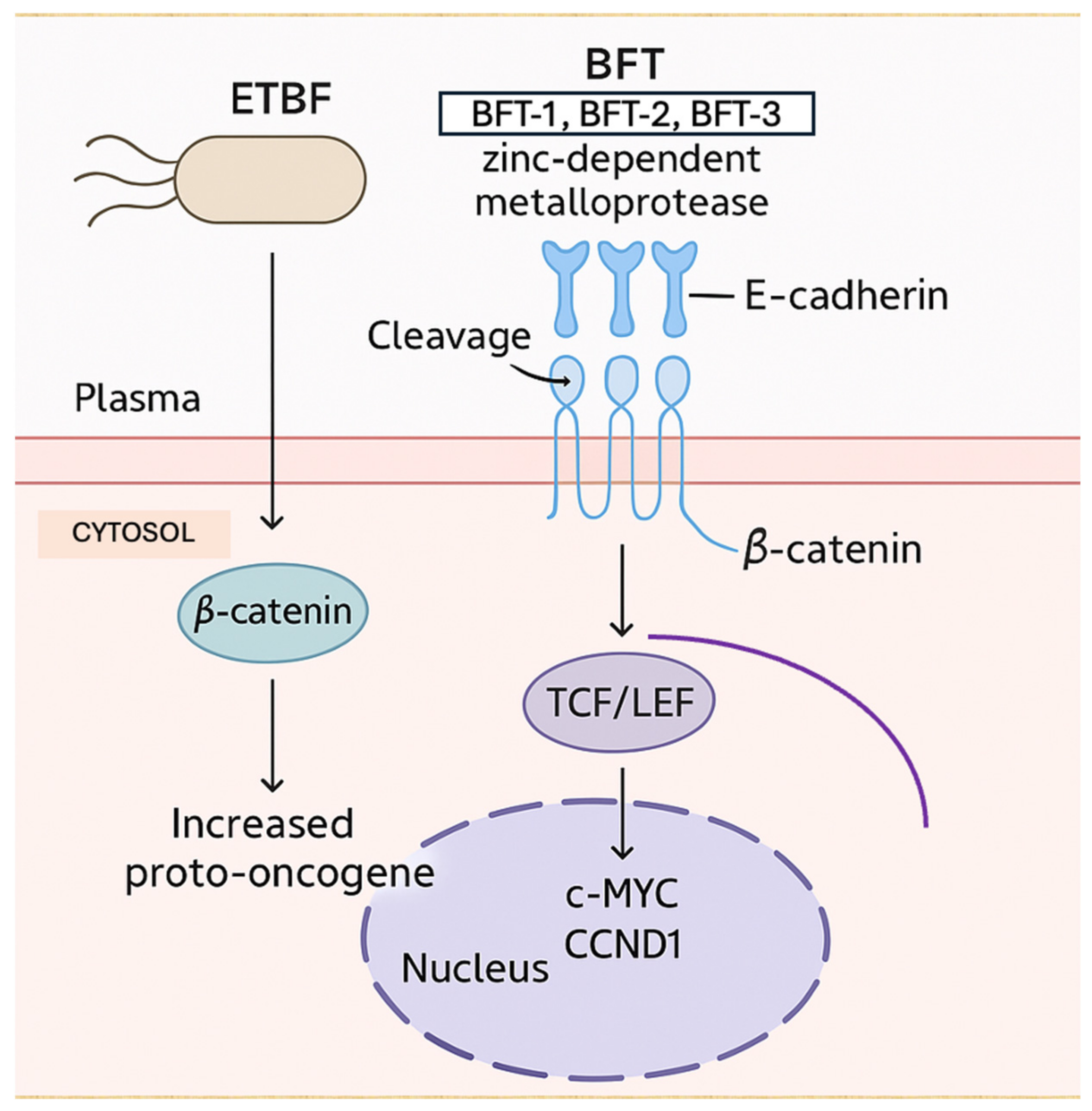
- Boleij, A.; Hechenbleikner, E.M.; Goodwin, A.C.; Badani, R.; Stein, E.M.; Lazarev, M.G.; Ellis, B.; Carroll, K.C.; Albesiano, E.; Wick, E.C.; et al. The Bacteroides fragilis toxin gene is prevalent in the colon mucosa of colorectal cancer patients. Clin. Infect. Dis. 2015, 60, 208–215. [Google Scholar] [CrossRef]
- Toprak, N.U.; Yagci, A.; Gulluoglu, B.M.; Akin, M.L.; Demirkalem, P.; Celenk, T.; Soyletir, G. A possible role of Bacteroides fragilis enterotoxin in the aetiology of colorectal cancer. Clin. Microbiol. Infect. 2006, 12, 782–786. [Google Scholar] [CrossRef]
- Martins, M.; du Merle, L.; Trieu-Cuot, P.; Dramsi, S. Heterogeneous expression of Pil3 pilus is critical for Streptococcus gallolyticus translocation across polarized colonic epithelial monolayers. Microbes Infect. 2020, 22, 55–59. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Y.; Moore, D.R.; Nimmo, S.L.; Lightfoot, S.A.; Huycke, M.M. 4-hydroxy-2-nonenal mediates genotoxicity and bystander effects caused by Enterococcus faecalis-infected macrophages. Gastroenterology 2012, 142, 543–547. [Google Scholar] [CrossRef]
- Steck, N.; Hoffmann, M.; Sava, I.G.; Kim, S.C.; Hahne, H.; Tonkonogy, S.L.; Mair, K.; Krueger, D.; Pruteanu, M.; Shanahan, F.; et al. Enterococcus faecalis metalloprotease compromises epithelial barrier and contributes to intestinal inflammation. Gastroenterology 2011, 141, 959–971. [Google Scholar] [CrossRef]
- Jones, M.; Helliwell, P.; Pritchard, C.; Tharakan, J.; Mathew, J. Helicobacter pylori in colorectal neoplasms: Is there an aetiological relationship? World J. Surg. Oncol. 2007, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Higashi, H.; Tsutsumi, R.; Fujita, A.; Yamazaki, S.; Asaka, M.; Azuma, T.; Hatakeyama, M. Biological activity of the Helicobacter pylori virulence factor CagA is determined by variation in the tyrosine phosphorylation sites. Proc. Natl. Acad. Sci. USA 2002, 99, 14428–14433. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.P.; Dumas, M.E.; Wang, Y.; Legido-Quigley, C.; Yap, I.K.; Tang, H. A top-down systems biology view of microbiome-mammalian metabolic interactions in a mouse model. Mol. Syst. Biol. 2007, 3, 112. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Huang, J.; Chen, Z.; Jiang, Z.; Li, X.; Chen, Z. Metabolomics in gut microbiota: Applications and challenges. Sci. Bull. 2016, 61, 1151–1153. [Google Scholar] [CrossRef]
- Ionescu, V.A.; Gheorghe, G.; Georgescu, T.F.; Bacalbasa, N.; Gheorghe, F.; Diaconu, C.C. The latest data concernung the etiology and pathogenesis of irritable bowel syndrome. J. Clin. Med. 2024, 13, 5124. [Google Scholar] [CrossRef]
- Peng, Y.; Nie, Y.; Yu, J.; Wong, C.C. Microbial Metabolites in Colorectal Cancer: Basic and Clinical Implications. Metabolites 2021, 11, 159. [Google Scholar] [CrossRef]
- O’Keefe, S.J.D. Diet, microorganisms and their metabolites, and colon cancer. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 691–706. [Google Scholar] [CrossRef]
- Wilson, A.J.; Chueh, A.C.; Togel, L.; Corner, G.A.; Ahmed, N.; Goel, S. Apoptotic sensitivity of colon cancer cells to histone deacetylase inhibitors is mediated by an Sp1/Sp3-activated transcriptional program involving immediate-early gene induction. Cancer Res. 2010, 70, 609–620. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 2006, 47, 241–259. [Google Scholar] [CrossRef]
- Ocvirk, S.; Wilson, A.S.; Posma, J.M.; Li, J.V.; Koller, K.R.; Day, G.M.; Flanagan, A.C.; Otto, J.E.; Sacco, E.P.; Sacco, F.D.; et al. A prospective cohort analysis of gut microbial co-metabolism in Alaska Native and rural African people at high and low risk of colorectal cancer. Am. J. Clin. Nutr. 2020, 111, 406–419. [Google Scholar] [CrossRef]
- Yachida, S.; Mizutani, S.; Shiroma, H.; Shiba, S.; Nakajima, T.; Sakamoto, T.; Watanabe, H.; Masuda, K.; Nishimoto, Y.; Kubo, M.; et al. Metagenomic and metabolomic analyses reveal distinct stage-specific phenotypes of the gut microbiota in colorectal cancer. Nat. Med. 2019, 25, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Dong, W.; Wang, S.; Zhang, Y.; Liu, T.; Xie, R.; Wang, B.; Cao, H. Deoxycholic acid disrupts the intestinal mucosal barrier and promotes intestinal tumorigenesis. Food Funct. 2018, 9, 5588–5597. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Coulter, S.; Yoshihara, E.; Oh, T.G.; Fang, S.; Cayabyab, F.; Zhu, Q.; Zhang, T.; Leblanc, M.; Liu, S.; et al. FXR Regulates Intestinal Cancer Stem Cell Proliferation. Cell 2019, 176, 1098–1112. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Cho, J.H.; Kim, E.; Kim, Y.J. Ursodeoxycholic acid inhibits the proliferation of colon cancer cells by regulating oxidative stress and cancer stem-like cell growth. PLoS ONE 2017, 12, e0181183. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, J.H.; Kim, B.G.; Lee, K.L.; Kim, J.W.; Koh, S.J. Tauroursodeoxycholic acid attenuates colitis-associated colon cancer by inhibiting nuclear factor kappaB signaling. J. Gastroenterol. Hepatol. 2019, 34, 544–551. [Google Scholar] [CrossRef]
- Casero, R.A., Jr.; Stewart, T.M.; Pegg, A.E. Polyamine metabolism and cancer: Treatments, challenges and opportunities. Nat. Rev. Cancer. 2018, 18, 681–695. [Google Scholar] [CrossRef]
- Pegg, A.E. Functions of Polyamines in Mammals. J. Biol. Chem. 2016, 291, 14904–14912. [Google Scholar] [CrossRef]
- Goodwin, A.C.; Shields, C.E.D.; Wu, S.; Huso, D.L.; Wu, X.; Murray-Stewart, T.R.; Hacker-Prietz, A.; Rabizadeh, S.; Woster, P.M.; Sears, C.L.; et al. Polyamine catabolism contributes to enterotoxigenic Bacteroides fragilis-induced colon tumorigenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 15354–15359. [Google Scholar] [CrossRef]
- Wang, C.; Ruan, P.; Zhao, Y.; Li, X.; Wang, J.; Wu, X. Spermidine/spermine N1-acetyltransferase regulates cell growth and metastasis via AKT/beta-catenin signaling pathways in hepatocellular and colorectal carcinoma cells. Oncotarget 2017, 8, 1092–1109. [Google Scholar] [CrossRef]
- Hayes, C.S.; Shicora, A.C.; Keough, M.P.; Snook, A.E.; Burns, M.R.; Gilmour, S.K. Polyamine-blocking therapy reverses immunosup-pression in the tumor microenvironment. Cancer Immunol. Res. 2014, 2, 274–285. [Google Scholar] [CrossRef]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2018, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Mahmood, N.; Raza, M.A.; Mushtaq, Z.; Saeed, F.; Afzaal, M.; Hussain, M.; Amjad, H.W.; Al-Awadi, H.M. Gut microbiota and their derivatives in the progression of colorectal cancer: Mechanisms of action, genome and epigenome contributions. Heliyon 2024, 10, e29495. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.X.; Law, J.H.; Png, C.W.; Alberts, R.; Zhang, Y.; Chu, J.H.; Tan, K.K. Alterations in colorectal cancer virome and its persistence after surgery. Sci. Rep. 2024, 14, 2819. [Google Scholar] [CrossRef] [PubMed]
- Nakatsu, G.; Zhou, H.; Wu, W.K.K.; Wong, S.H.; Coker, O.O.; Dai, Z.; Li, X.; Szeto, C.H.; Sugimura, N.; Lam, T.Y.T.; et al. Alterations in enteric virome are associated with colorectal cancer and survival outcomes. Gastroenterology 2018, 155, 529–541.e5. [Google Scholar] [CrossRef]
- Hannigan, G.D.; Duhaime, M.B.; Ruffin, M.T.; Koumpouras, C.C.; Schloss, P.D. Diagnostic potential and interactive dynamics of the colorectal cancer virome. MBio 2018, 9, e02248-18. [Google Scholar] [CrossRef]
- Zepeda-Rivera, M.; Minot, S.S.; Bouzek, H.; Wu, H.; Blanco-Miguez, A.; Manghi, P.; Jones, D.S.; LaCourse, K.D.; Wu, Y.; McMahon, E.F.; et al. A distinct Fusobacterium nucleatum clade dominates the colorectal cancer niche. Nature 2024, 628, 424–432. [Google Scholar] [CrossRef]
- Zhu, H.; Li, M.; Bi, D.; Yang, H.; Gao, Y.; Song, F.; Zheng, J.; Xie, R.; Zhagn, Y.; Liu, H.; et al. Fusobacterium nucleatum promotes tumor progression in KRAS p.G12D-mutant colorectal cancer by binding to DHX15. Nat. Commun. 2024, 15, 1688. [Google Scholar] [CrossRef]
- Mima, K.; Nishihara, R.; Qian, Z.R.; Cao, Y.; Sukawa, Y.; Nowak, J.A.; Yang, J.; Dou, R.; Masugi, Y.; Song, M.; et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut 2016, 65, 1973–1980. [Google Scholar] [CrossRef]
- Ye, C.; Liu, X.; Liu, Z.; Pan, C.; Zhang, X.; Zhao, Z.; Sun, H. Fusobacterium nucleatum in tumors: From tumorigenesis to tumor metastasis and tumor resistance. Cancer Biol. Ther. 2024, 25, 2306676. [Google Scholar] [CrossRef]
- Dadgar-Zankbar, L.; Elahi, Z.; Shariati, A.; Khaledi, A.; Razavi, S.; Khoshbayan, A. Exploring the role of Fusobacterium nucleatum in colorectal cancer: Implications for tumor proliferation and chemoresistance. Cell Commun. Signal. 2024, 22, 547. [Google Scholar] [CrossRef]
- Dadgar-Zankbar, L.; Shariati, A.; Bostanghadiri, N.; Elahi, Z.; Mirkalantari, S.; Razavi, S.; Kamali, F.; Darban-Sarokhalil, D. Evaluation of enterotoxigenic Bacteroides fragiliscorrelation with the expression of cellular signaling pathway genes in Iranian patients with colorectal cancer. Infect. Agents Cancer 2023, 18, 48. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.C.; Kumar, R.; Xu, J.; Xu, Y. A pathogenicity locus of Streptococcus gallolyticus subspecies gallolyticus. Sci. Rep. 2023, 13, 6291. [Google Scholar] [CrossRef] [PubMed]
- Abdulamir, A.S.; Hafidh, R.R.; Bakar, F.A. The association of Streptococcus bovis/gallolyticus with colorectal tumors: The nature and the underlying mechanisms of its etiological role. J. Exp. Clin. Cancer Res 2011, 30, 11. [Google Scholar] [CrossRef] [PubMed]
- Corredoira, J.; Garcia-Pais, M.J.; Coira, A.; Rabunal, R.; Garcia-Garrote, F.; Pita, J.; Rodriguez-Marcias, A.; Blanco, M.; Lopez-Roses, L.; Lopez-Alvarez, J.; et al. Differences between endocarditis caused by Streptococcus bovis and Enterococcus spp. and their association with colorectal cancer. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1657–1665. [Google Scholar] [CrossRef]
- Kumar, R.; Herold, J.L.; Schady, D.; Davis, J.; Kopetz, S.; Martinez-Moczygemba, M.; Murray, B.E.; Han, F.; Li, Y.; Callaway, E.; et al. Streptococcus gallolyticus subsp. gallolyticus promotes colorectal tumor development. PLoS Pathog. 2017, 13, e1006440. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Deng, M.; Chen, X.; Jiang, L.; Zhang, J.; Tao, l.; Yu, W.; Qiu, Y. Enterococcus faecalis promotes the progression of colorectal cancer via its metabolite: Biliverdin. J. Transl. Med. 2023, 21, 72. [Google Scholar] [CrossRef]
- Huycke, M.M.; Abrams, V.; Moore, D.R. Enterococcus faecalis produces extracellular superoxide and hydrogen peroxide that damages colonic epithelial cell DNA. Carcinogenesis 2002, 23, 529–536. [Google Scholar] [CrossRef]
- Ruiz, P.A.; Shkoda, A.; Kim, S.C.; Sartor, R.B.; Haller, D. Il-10 gene-deficient mice lack Tgf-Beta/Smad signaling and fail to inhibit proinflammatory gene expression in intestinal epithelial cells after the colonization with colitogenic Enterococcus Faecalis. J. Immunol. 2005, 174, 2990–2999. [Google Scholar] [CrossRef]
- Wassenaar, T.M. E. coli and colorectal cancer: A complex relationship that deserves a critical mindset. Clin. Rev. Microbiol. 2017, 44, 619–632. [Google Scholar] [CrossRef]
- Barbosa de Souza, J.; de Almeida Campos, L.A.; Palacio, S.B.; Brelaz-deCastro, M.C.A.; Cavalcanti, I.M.F. Prevalence and implications of pKs-positive Escherichia coli in colorectal cancer. Life Sci. 2024, 341, 122462. [Google Scholar] [CrossRef]
- Shah, S.C.; Camargo, M.C.; Lamm, M.; Bustamante, R.; Roumie, C.L.; Wilson, O.; Halvorson, A.E.; Greevy, R.; Liu, L.; Gupta, S.; et al. Impact of Helicobacter pylori Infection and Treatment on Colorectal Cancer in a Large, Nationwide Cohort. J. Clin. Oncol. 2024, 42, 1881–1889. [Google Scholar] [CrossRef] [PubMed]
- Ralser, A.; Dietl, A.; Jarosch, S.; Engelsberger, V.; Wanisch, A.; Janssen, K.P.; Middelhoff, M.; Vieth, M.; Quante, M.; Haller, D.; et al. Helicobacter pylori promotes colorectal carcinogenesis by deregulating intestinal immunity and inducing a mucus-degrading microbiota signature. Gut 2023, 72, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Dahmus, J.D.; Kotler, D.L.; Kastenberg, D.M.; Kistler, C.A. The gut microbiome and colorectal cancer: A review of bacterial pathogenesis. J. Gastrointest. Oncol. 2018, 9, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, C.L.; Krejany, E.O.; Young, L.F.; O’Connor, J.R.; Awad, M.M.; Boyd, R.L.; Emmins, J.J.; Lyras, D.; Rood, J.I. The α-toxin of Clostridium septicum is essential for virulence. Mol. Microbiol. 2005, 57, 1357–1366. [Google Scholar] [CrossRef]
- Wang, M.; Liu, K.; Bao, W.; Hang, B.; Chen, X.; Zhu, X.; Li, G.; Liu, L.; Xiang, H.; Hu, H.; et al. Gut microbiota protect against colorectal tumorigenesis through lncRNA Snhg9. Deev. Cell 2025, 60, 1008–1017.e7. [Google Scholar] [CrossRef]
- Pires, L.; Gonzalez-Paramas, A.M.; Heleno, S.A.; Calhelha, R.C. Gut Microbiota as an Endocrine Organ: Unveiling Its Role in Human Physiology and Health. Appl. Sci. 2024, 14, 9383. [Google Scholar] [CrossRef]
- Chen, G.; Ren, Q.; Zhong, Z.; Li, Q.; Huang, Z.; Zhang, C.; Yuan, H.; Feng, Z.; Chen, B.; Wang, N.; et al. Exploring the gut microbiome’s role in colorectal cancer: Diagnostic and prognostic implications. Front. Immunol. 2024, 15, 1431747. [Google Scholar] [CrossRef]
- Wong, S.H.; Yu, J. Gut microbiota in colorectal cancer: Mechanisms of action and clinical applications. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 690–704. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Mei, J.X.; Yu, G.; Lei, L.; Zhang, W.H.; Liu, K.; Chen, X.L.; Kolat, D.; Yang, K.; Hu, J.K. Role of the gut microbiota in anticancer therapy: From molecular mechanisms to clinical applications. Signal Transduct. Target. Ther. 2023, 8, 201. [Google Scholar] [CrossRef]
- Dikeocha, I.J.; Al-Kabsi, A.M.; Chiu, H.T.; Alshawsh, M.A. Faecalibacterium prausnitzii Ameliorates Colorectal Tumorigenesis and Suppresses Proliferation of HCT116 Colorectal Cancer Cells. Biomedicines 2022, 10, 1128. [Google Scholar] [CrossRef]
- Dong, J.; Wang, B.; Xiao, Y.; Liu, J.; Wang, Q.; Xiao, H.; Jin, Y.; Liu, Z.; Chen, Z.; Li, Y.; et al. Roseburia intestinalis sensitizes colorectal cancer to radiotherapy through the butyrate/OR51E1/RALB axis. Cell Rep. 2024, 43, 113846. [Google Scholar] [CrossRef] [PubMed]
- Gubernatorova, E.O.; Gorshkova, E.A.; Bondareva, M.A.; Podosokorskaya, O.A.; Sheynova, A.D.; Yakoleva, A.S.; Bonch-Osmolovskaya, E.A.; Nedospasov, S.A.; Kruglov, A.A.; Drutskaya, M.S. Akkermansia muciniphila–Friend or foe in colorectal cancer? Front Immunol. 2023, 14, 1303795. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.T.; Seo, Y.; Ahn, J.S.; Oh, S.J.; Park, H.J.; Yu, J.H.; Kim, S.H.; Lee, Y.; Yang, J.W.; Cho, J.; et al. De novo interleukin-10 production primed by Lactobacillus sakei CVL-001 amplifies the immunomodulatory abilities of mesenchymal stem cells to alleviate colitis. Biomed. Pharmacother. 2025, 182, 117745. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, E.; Corr, S.C. Lactobacillus spp. for Gastrointestinal Health: Current and Future Perspectives. Front. Immunol. 2022, 13, 840245. [Google Scholar] [CrossRef]
- Alakomi, H.L.; Skytta, E.; Saarela, M.; Mattila-Sandholm, T.; Latva-Kala, K.; Helander, I.M. Lactic Acid Permeabilizes Gram-Negative Bacteria by Disrupting the Outer Membrane. Appl. Environ. Microbiol. 2000, 66, 2001–2005. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, Y.; Wang, P.; Huang, Y.; Wang, F. Short-Chain Fatty Acids Manifest Stimulative and Protective Effects on Intestinal Barrier Function Through the Inhibition of NLRP3 Inflammasome and Autophagy. Cell. Physiol. Biochem. 2018, 49, 190–205. [Google Scholar] [CrossRef]
- Todorov, S.D. Bacteriocins From Lactobacillus Plantarum–Production, Genetic Organization and Mode of Action: Produção, Organização Genética E Modo De Ação. Braz. J. Microbiol. 2009, 40, 209–221. [Google Scholar] [CrossRef]
- Rachwal, K.; Gustaw, K. Lactic Acid Bacteria in Sustainable Food Production. Sustainability 2024, 16, 3362. [Google Scholar] [CrossRef]
- O’Flaherty, S.; Briner Crawley, A.; Theriot, C.M.; Barrangou, R. The Lactobacillus Bile Salt Hydrolase Repertoire Reveals Niche-Specific Adaptation. mSphere 2018, 3, e00140-18. [Google Scholar] [CrossRef]
- Riviere, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef]
- Shang, F.; Jiang, X.; Wang, H.; Guo, S.; Kang, S.; Xu, B.; Wang, X.; Chen, S.; Li, N.; Liu, B.; et al. Bifidobacterium longum suppresses colorectal cancer through the modulation of intestinal microbes and immune function. Front. Microbiol. 2024, 15, 1327464. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Song, M.H.; Ji, Y.S.; Patk, J.W.; Shin, Y.K.; Kim, S.C.; Kim, G.; Cho, B.; Park, H.; Ku, J.L.; et al. Cell free supernatants of Bifidobacterium adolescentis and Bifidobacterium longumsuppress the tumor growth in colorectal cancer organoid model. Sci. Rep. 2025, 15, 935. [Google Scholar]
- Lopez-Siles, M.; Duncan, S.H.; Garcia-Gil, L.J.; Martinez-Medina, M. Faecalibacterium prausnitzii: From microbiology to diagnostics and prognostics. ISME J. 2017, 11, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.J.; Yang, J.Y.; Yan, Z.H.; Hu, S.; Li, J.Q.; Xu, Z.X.; Jian, Y.P. Recent findings in Akkermansia muciniphila-regulated metabolism and its role in intestinal diseases. Clin. Nutr. 2022, 41, 2333–2344. [Google Scholar] [CrossRef]
- Derosa, L.; Routy, B.; Thomas, A.M.; Iebba, V.; Zalcman, G.; Friard, S.; Mazieres, J.; Audigier-Valette, C.; Moro-Sibilot, D.; Goldwasser, F.; et al. Intestinal Akkermansia muciniphila predicts clinical response to PD-1 blockade in advanced non-small cell lung cancer patients. Nat. Med. 2022, 28, 315–324. [Google Scholar] [CrossRef]
- Kang, X.; Liu, C.; Ding, Y.; Ni, Y.; Ji, F.; Lau, H.C.H.; Jiang, L.; Sung, J.J.; Wong, S.H.; Yu, J. Roseburia intestinalis generated butyrate boosts anti-PD-1 efficacy in colorectal cancer by activating cytotoxic CD8+ T cells. Gut 2023, 72, 2112–2122. [Google Scholar] [CrossRef]
- Zagato, E.; Pozzi, C.; Bertocchi, A.; Schioppa, T.; Saccheri, F.; Guglietta, S.; Fosso, B.; Melocchi, L.; Nizzoli, G.; Troisi, J.; et al. Endogenous murine microbiota member Faecalibaculum rodentium and its human homologue protect from intestinal tumour growth. Nat. Microbiol. 2020, 5, 511–524. [Google Scholar] [CrossRef]
- Li, Q.; Hu, W.; Liu, W.X.; Zhao, L.Y.; Huang, D.; Liu, X.D.; Chan, H.; Zhang, Y.; Zeng, J.D.; Coker, O.O.; et al. Streptococcus thermophilus inhibits colorectal tumorigenesis through secreting beta-galactosidase. Gastroenterology 2021, 160, 1179–1193.e1114. [Google Scholar] [CrossRef]
- Singh, R.; Chandrashekharappa, S.; Bodduluri, S.R.; Baby, B.V.; Hegde, B.; Kotla, N.G.; Hiwale, A.A.; Saiyed, T.; Patel, P.; Vijay-Kumar, M.; et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat. Commun. 2019, 10, 89. [Google Scholar] [CrossRef]
- Yu, H.; Li, X.X.; Han, X.; Chen, B.X.; Zhang, X.H.; Gao, S.; Xu, D.Q.; Wang, Y.; Gao, Z.K.; Yu, L.; et al. Fecal microbiota transplantation inhibits colorectal cancer progression: Reversing intestinal microbial dysbiosis to enhance anti-cancer immune responses. Front. Microbiol. 2023, 14, 1126808. [Google Scholar] [CrossRef]
- Yang, Y.; An, Y.; Dong, Y.; Chu, Q.; Wei, J.; Wang, B.; Cao, H. Fecal microbiota transplantation: No longer cinderella in tumour immunotherapy. EBioMedicine 2024, 100, 104967. [Google Scholar] [CrossRef] [PubMed]
- Dikeocha, I.J.; Al-Kabsi, A.M.; Eid, E.E.M.; Hussin, S.; Alshawsh, M.A. Probiotics supplementation in patients with colorectal cancer: A systematic review of randomized controlled trials. Nutr. Rev. 2021, 80, 22–49. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Lei, J.; Ke, S.; Chen, Y.; Xiao, J.; Tang, Z.; Wang, L.; Ren, Y.; Alnaggar, M.; Qiu, H.; et al. Fecal microbiota transplantation plus tislelizumab and fruquintinib in refractory microsatellite stable metastatic colorectal cancer: An open-label, single-arm, phase II trial (RENMIN-215). EClinicalMedicine 2023, 66, 102315. [Google Scholar] [CrossRef] [PubMed]
- Zaharuddin, L.; Mokhtar, N.M.; Nawawi, K.N.M.; Ali, R.A.R. A randomized double-blind placebo-controlled trial of probiotics in post-surgical colorectal cancer. BMC Gastroenterol. 2019, 19, 131. [Google Scholar] [CrossRef]
- Yang, Y.; Xia, Y.; Chen, H.; Hong, L.; Feng, J.; Yang, J.; Yang, Z.; Shi, C.; Wu, W.; Gao, R.; et al. The effect of perioperative probiotics treatment for colorectal cancer: Short-term outcomes of a randomized controlled trial. Oncotarget 2016, 7, 8432–8440. [Google Scholar] [CrossRef]
- Pilot Trial of Fecal Microbiota Transplantation and Re-Introduction of Anti-PD-1 Therapy in dMMR Solid Tumor Anti-PD-1 Non-Responders. Available online: https://www.mdanderson.org/patients-family/diagnosis-treatment/clinical-trials/clinical-trials-index/clinical-trials-detail.ID2020-0186.html (accessed on 10 April 2025).
- Karimi, M.; Shirsalimi, N.; Hashempour, Z.; Omran, H.S.; Sedighi, E.; Beigi, F.; Mortezazadeh, M. Safety and efficacy of fecal microbiota transplantation (FMT) as a modern adjuvant therapy in various diseases and disorders: A comprehensive literature review. Front. Immunol. 2024, 15, 1439176. [Google Scholar] [CrossRef]
- Merrick, B.; Allen, L.; Zain, N.M.M.; Forbes, B.; Shaecross, D.L.; Goldenberg, S.D. Regulations, risk and safety of Faecal Microbiota Transplant. Infect. Prev. Pract. 2020, 2, 100069. [Google Scholar] [CrossRef]
- Rezasoltani, S.; Looha, M.A.; Aghdaei, H.A.; Jasemi, S.; Sechi, L.A.; Gazouli, M.; Sadeghi, A.; Torkashvand, S.; Baniali, R.; Schluter, H.; et al. 16S rRNA sequencing analysis of the oral and fecal microbiota in colorectal cancer positives versus colorectal cancer negatives in Iranian population. Gut Pathog. 2024, 16, 9. [Google Scholar] [CrossRef]
- Rezasoltani, S.; Aghdaei, H.A.; Jasemi, S.; Gazouli, M.; Dovrolis, N.; Sadechi, A.; Schluter, H.; Zali, M.R.; Sechi, L.; Feizabadi, M.M. Oral Microbiota as Novel Biomarkers for Colorectal Cancer Screening. Cancers 2022, 15, 192. [Google Scholar] [CrossRef]
| Microorganism | Effects on Gene Expression | Effects on Colorectal Oncogenesis |
|---|---|---|
| Fusobacterium nucleatum | Increased abundance of Fusobacterium nucleatum—Downregulation of Fc Receptor-Like A (FCRLA), Peptidase Inhibitor 16 (PI16), Lymphocyte Specific Protein 1 (LSP1), and Tumor Necrosis Factor Superfamily Member 9 (TNFSF9). | Facilitates immune evasion of tumor cells and promotes CRC progression. |
| Prevotella spp. | Decreased abundance of Prevotella spp.—Downregulation of Metallothionein 1M (MT1M). | May disrupt cellular defense mechanisms against CRC progression. |
| Halomonadaceae | Increased abundance of Halomonadaceae—Downregulation of Reelin (RELN). | May contribute to tumor invasion and poor prognosis in CRC patients. |
| Paeniclostridium spp. | Increased abundance of Paeniclostridium spp.—Upregulation of Phospholipase C Beta 1 (PLCB1). | PLCB1 is involved in cellular processes such as proliferation and apoptosis. The effect of increased PLCB1 expression in CRC patients remains unclear. |
| Enterococcus spp. | Decreased abundance of Enterococcus spp.—Downregulation of Immunoglobulin Superfamily Member 9 (IGSF9). | Facilitates immune evasion of tumor cells and promotes CRC progression. |
| Bacterial Species | Protective Mechanism Against CRC |
|---|---|
| Lactobacillus spp. |
|
| Bifidobacterium spp. |
|
| Faecalibacterium prausnitzii | |
| Akkermansia muciniphila |
|
| Roseburia spp. |
|
| Faecalibaculum rodentium |
|
| Streptococcus thermophilus |
|
| Study | Study Design | Intervention | Combined Therapy | Conclusions |
|---|---|---|---|---|
| Dikeocha et al., 2021 [193]. | Systematic Review of 23 RCTs | Probiotics (Lactobacillus, Bifidobacterium) | Not specified | Probiotic supplementation improved quality of life, enhanced gut microbiota diversity, reduced postoperative infections, and inhibited pro-inflammatory cytokine production in CRC patients. |
| Zhao et al., 2023 [194]. | Open-label, Single-arm, Phase II Trial (RENMIN-215) | FMT + Tislelizumab + Fruquintinib | Anti-PD-1 and anti-angiogenic therapy | Combination therapy showed improved survival with manageable safety in refractory MSS mCRC patients. |
| Zaharuddin et al., 2019 [195]. | Open-label, Single-arm, Phase II Trial | Probiotics | Not specified | Probiotic supplementation reduced postoperative infections and improved gut microbiota composition in post-surgical CRC patients. |
| Yang et al., 2016 [196]. | Randomized Controlled Trial | Perioperative Probiotics Treatment | Not specified | Probiotic supplementation after CRC surgery may exert anti-inflammatory effects by modulating the intestinal microenvironment. |
| MD Anderson Pilot Trial [197]. | Open-label, Single-arm, Phase II Trial | FMT + Reintroduction of Anti-PD-1 Therapy (Pembrolizumab or Nivolumab) | Anti-PD-1 therapy | Study aims to assess whether FMT can enhance response to anti–PD-1 therapy in dMMR solid tumor patients; results pending. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ionescu, V.A.; Diaconu, C.C.; Gheorghe, G.; Mihai, M.-M.; Diaconu, C.C.; Bostan, M.; Bleotu, C. Gut Microbiota and Colorectal Cancer: A Balance Between Risk and Protection. Int. J. Mol. Sci. 2025, 26, 3733. https://doi.org/10.3390/ijms26083733
Ionescu VA, Diaconu CC, Gheorghe G, Mihai M-M, Diaconu CC, Bostan M, Bleotu C. Gut Microbiota and Colorectal Cancer: A Balance Between Risk and Protection. International Journal of Molecular Sciences. 2025; 26(8):3733. https://doi.org/10.3390/ijms26083733
Chicago/Turabian StyleIonescu, Vlad Alexandru, Camelia Cristina Diaconu, Gina Gheorghe, Mara-Madalina Mihai, Carmen Cristina Diaconu, Marinela Bostan, and Coralia Bleotu. 2025. "Gut Microbiota and Colorectal Cancer: A Balance Between Risk and Protection" International Journal of Molecular Sciences 26, no. 8: 3733. https://doi.org/10.3390/ijms26083733
APA StyleIonescu, V. A., Diaconu, C. C., Gheorghe, G., Mihai, M.-M., Diaconu, C. C., Bostan, M., & Bleotu, C. (2025). Gut Microbiota and Colorectal Cancer: A Balance Between Risk and Protection. International Journal of Molecular Sciences, 26(8), 3733. https://doi.org/10.3390/ijms26083733