Acanthopanax Senticosus Saponins Prevent Cognitive Decline in Rats with Alzheimer’s Disease
Abstract
1. Introduction
2. Results
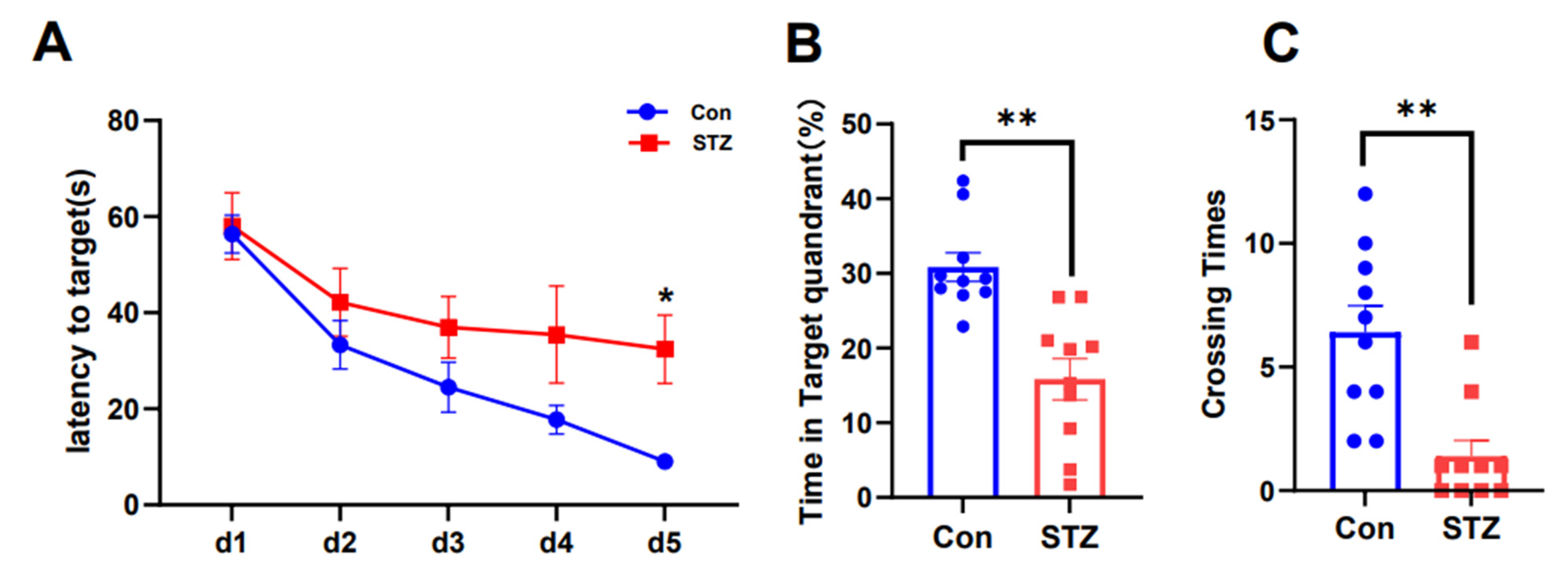
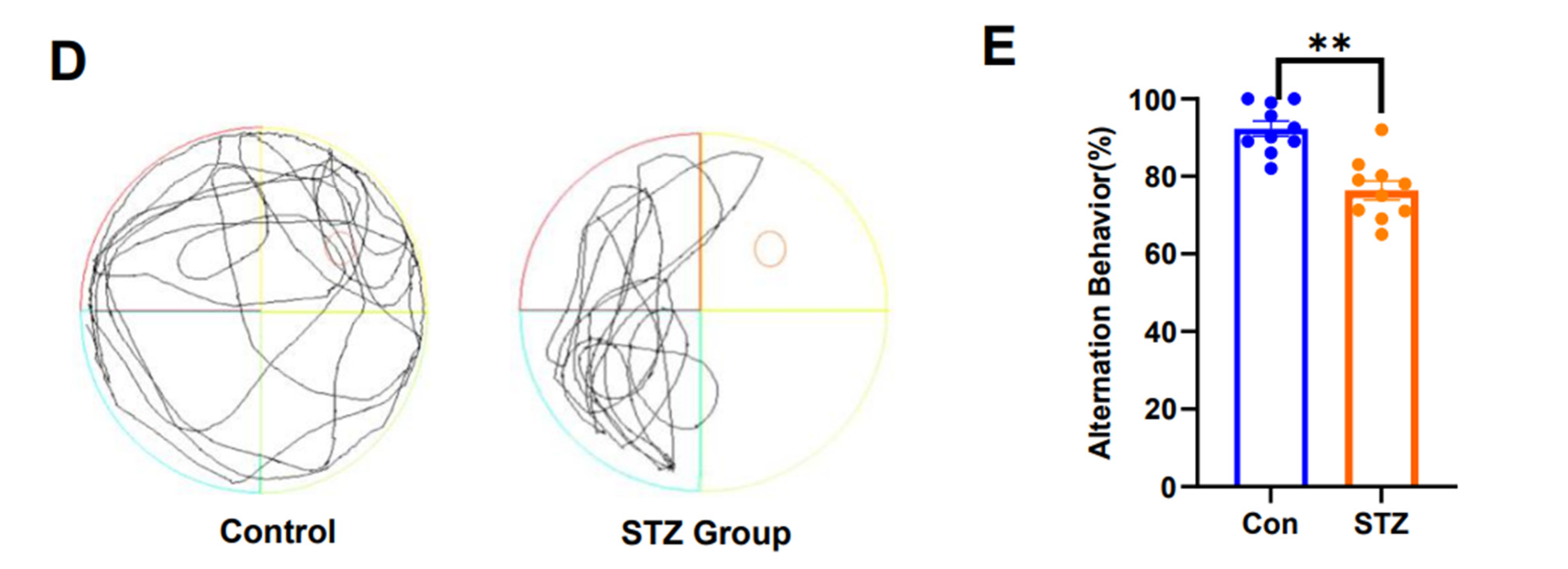
2.1. AD Model Rats Induced by STZ Show Cognitive Dysfunction
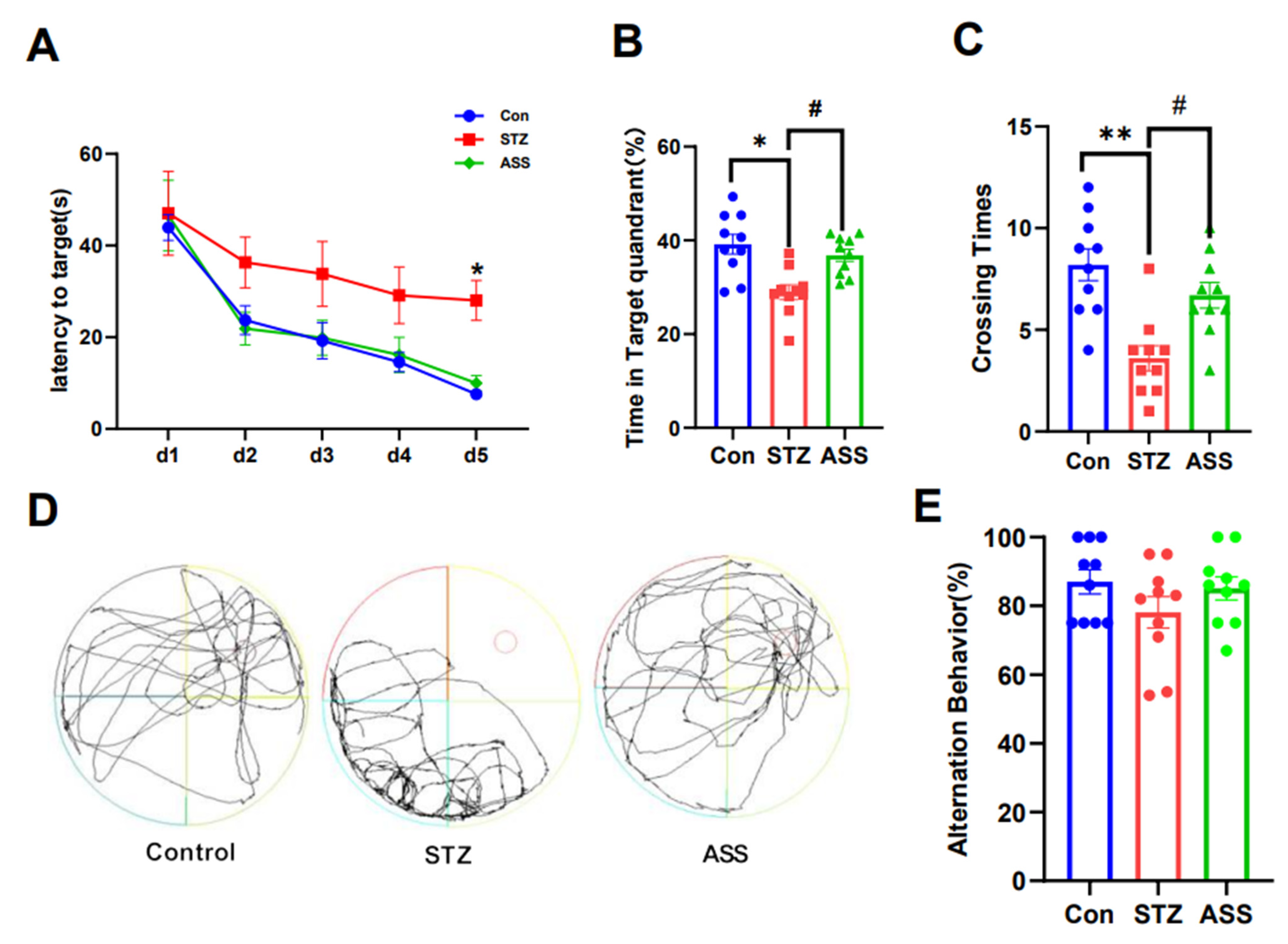
2.2. ASS Can Improve Cognitive Dysfunction in STZ-Treated Rats
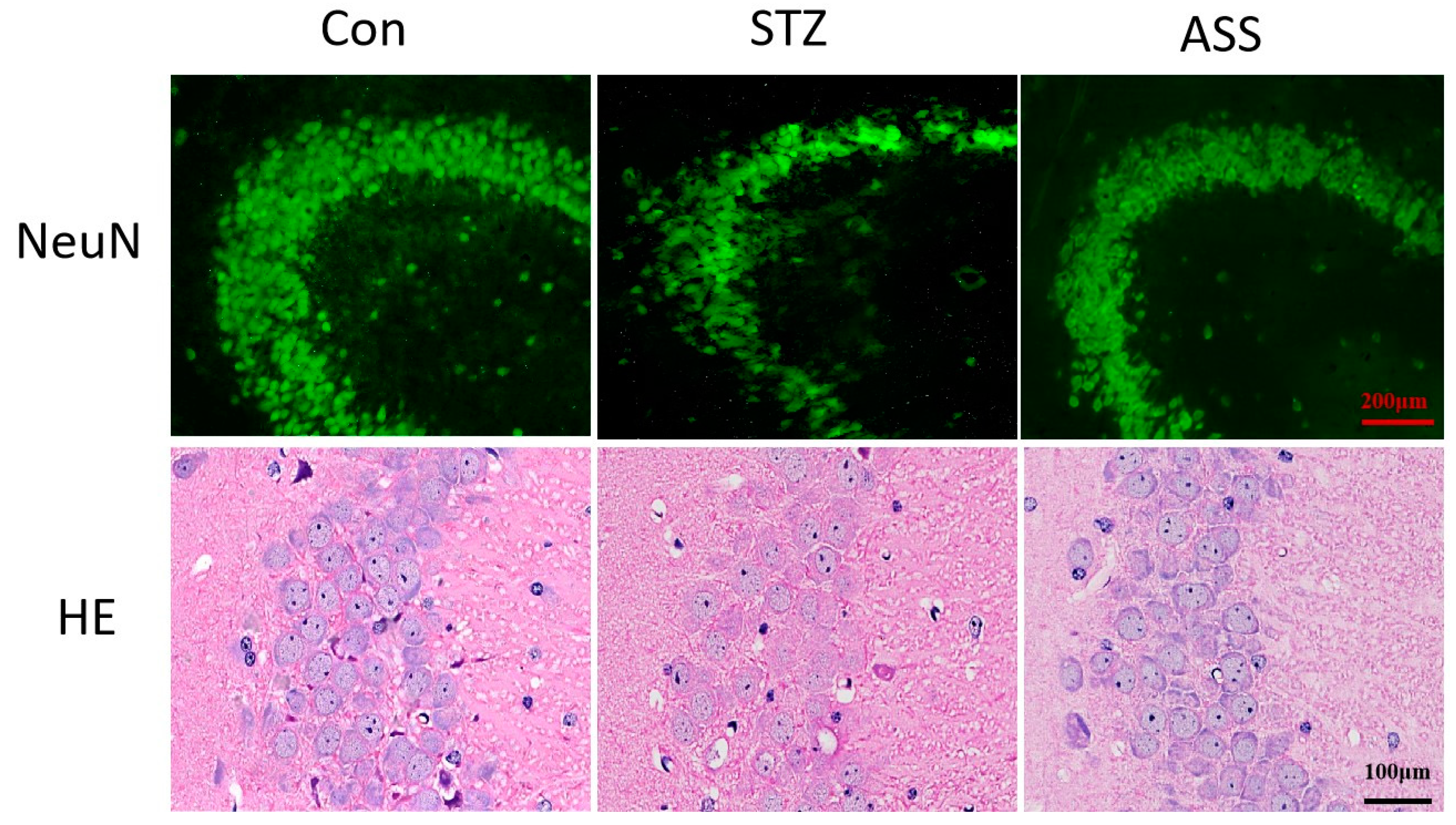
2.3. ASS Causes Neuronal Changes in the Hippocampal Region
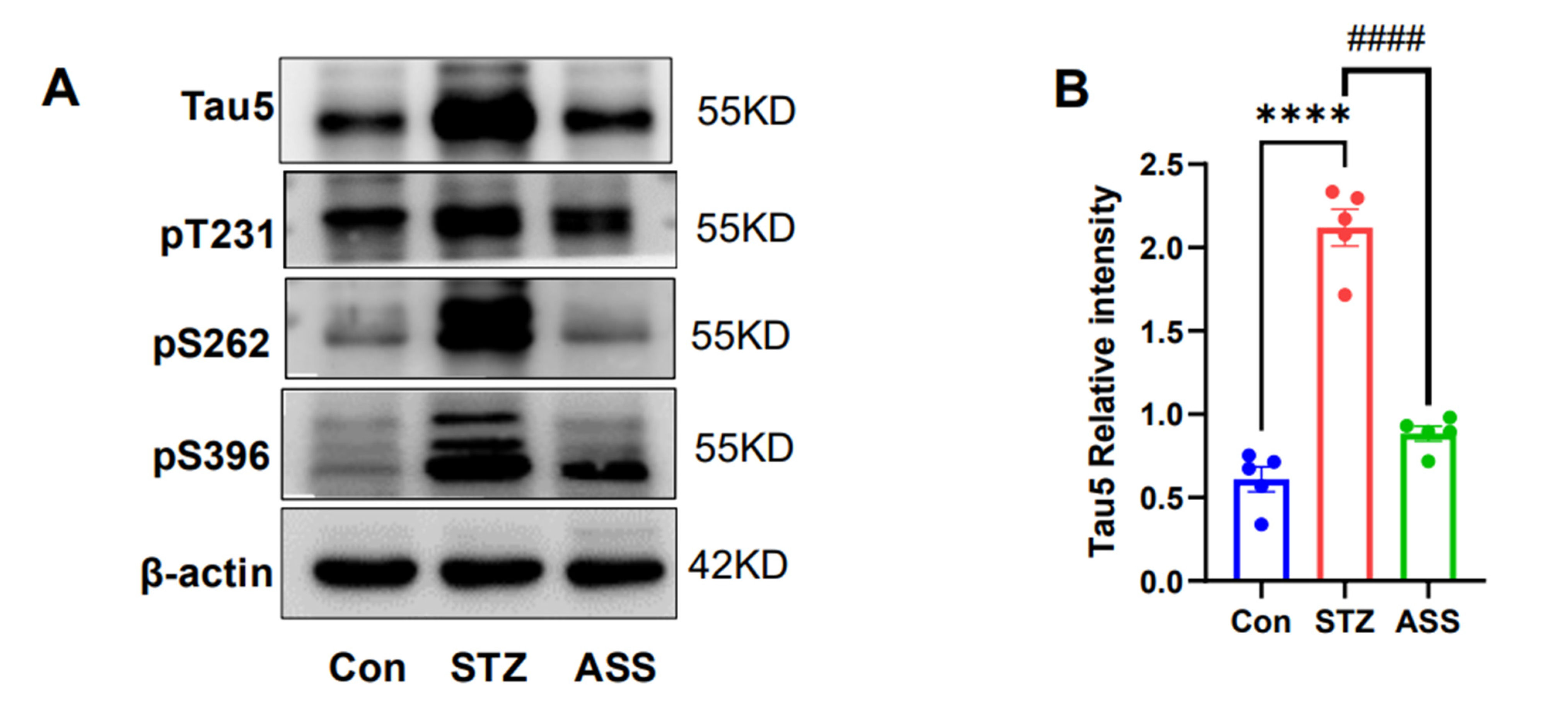
2.4. Effects of ASS on the Hyperphosphorylation of Tau Protein in the Hippocampus of STZ-Treated Rats
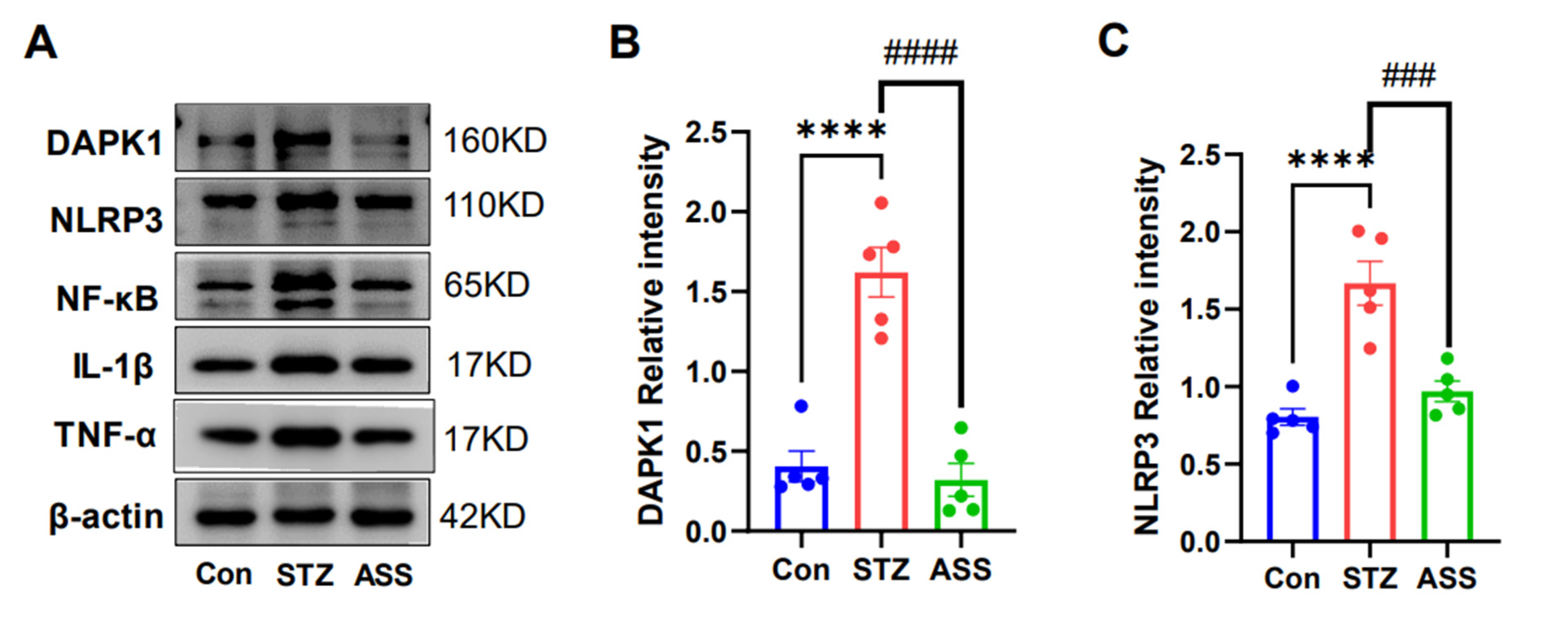
2.5. Effects of ASS on the STZ-Induced Inflammatory Cytokines in STZ-Treated Rats
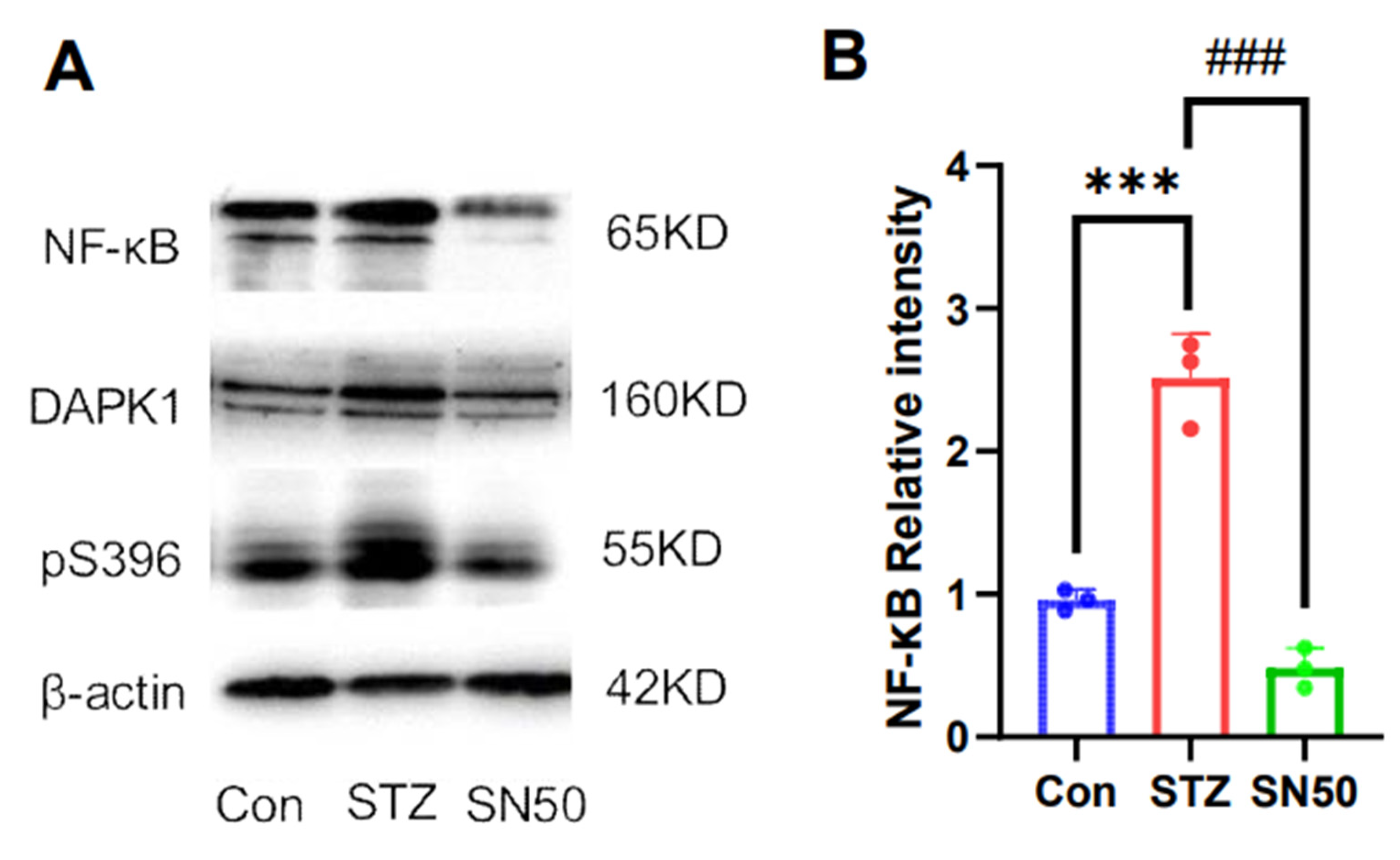
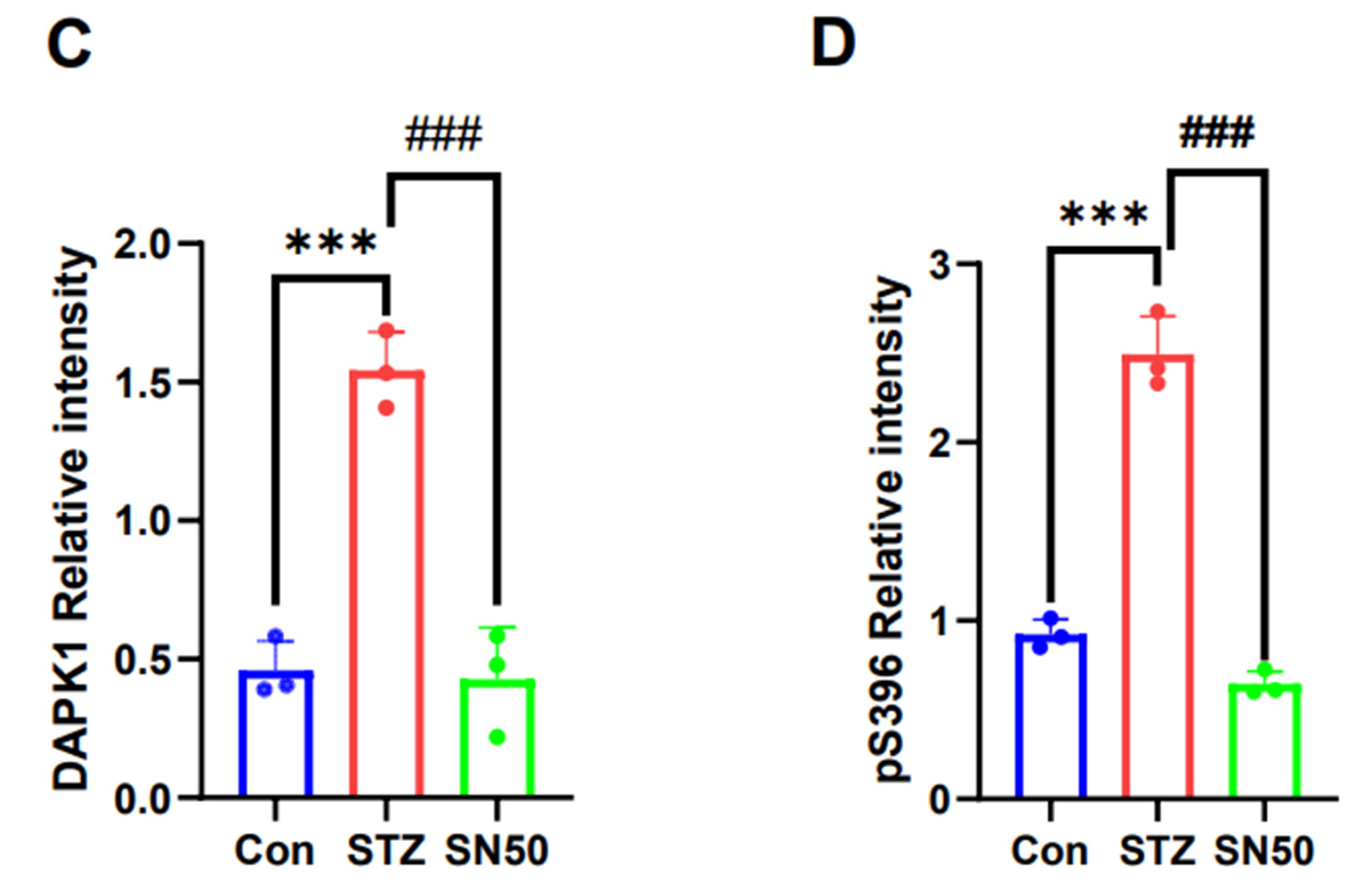
2.6. DAPK1 Protein Expression in the Hippocampal Region of STZ-Treated Rats Is Associated with NF-κB
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Model Development and Treatment
4.3. Behavioural Tests
4.3.1. Morris Water Maze Test (MWMT)
4.3.2. Y-Maze
4.4. Sample Preparation and Molecular Biology Experiments
4.4.1. Haematoxylin and Eosin (HE) Staining
4.4.2. Immunofluorescent Staining
4.4.3. Western Blot
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bondi, M.W.; Edmonds, E.C.; Salmon, D.P. Alzheimer’s Disease: Past, Present, and Future. J. Int. Neuropsychol. Soc. 2017, 23, 818–831. [Google Scholar] [CrossRef]
- Bredesen, D.E.; Amos, E.C.; Canick, J.; Ackerley, M.; Raji, C.; Fiala, M.; Ahdidan, J. Reversal of cognitive decline in Alzheimer’s disease. Aging 2016, 8, 1250–1258. [Google Scholar] [CrossRef]
- Nakamura, A.; Kaneko, N.; Villemagne, V.L.; Kato, T.; Doecke, J.; Doré, V.; Fowler, C.; Li, Q.X.; Martins, R.; Rowe, C.; et al. High performance plasma amyloid-beta biomarkers for Alzheimer’s disease. Nature 2018, 554, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Naseri, N.N.; Wang, H.; Guo, J.; Sharma, M.; Luo, W. The complexity of tau in Alzheimer’s disease. Neurosci. Lett. 2019, 705, 183–194. [Google Scholar] [PubMed]
- Drummond, E.; Pires, G.; MacMurray, C.; Askenazi, M.; Nayak, S.; Bourdon, M.; Safar, J.; Ueberheide, B.; Wisniewski, T. Phosphorylated tau interactome in the human Alzheimer’s disease brain. Brain 2020, 143, 2803–2817. [Google Scholar] [CrossRef]
- Yuca, H.; Aydin, B.; Karakaya, S.; Goger, G.; Bingöl, Z.; Civas, A.; Koca, M.; Demirci, B.; Sytar, O.; Gulcin, I.; et al. Exploring Astrodaucus orientalis (L.) Drude: Phytochemical Analysis and its Biological Potential Against Alzheimer’s and Diabetes. Chem. Biodivers. 2024, 21, e202301753. [Google Scholar] [CrossRef] [PubMed]
- Kurkinen, M. Donanemab: Not two without a third. Adv. Clin. Exp. Med. 2023, 32, 1085–1087. [Google Scholar] [CrossRef]
- Thakral, S.; Yadav, A.; Singh, V.; Kumar, M.; Kumar, P.; Narang, R.; Sudhakar, K.; Verma, A.; Khalilullah, H.; Jaremko, M.; et al. Alzheimer’s disease: Molecular aspects and treatment opportunities using herbal drugs. Ageing Res. Rev. 2023, 88, 101960. [Google Scholar]
- Joe, E.; Ringman, J.M. Cognitive symptoms of Alzheimer’s disease: Clinical management and prevention. BMJ 2019, 367, l6217. [Google Scholar] [CrossRef]
- Mummery, C.J.; Börjesson-Hanson, A.; Blackburn, D.J.; Vijverberg, E.G.B.; De Deyn, P.P.; Ducharme, S.; Jonsson, M.; Schneider, A.; Rinne, J.O.; Ludolph, A.C.; et al. Tau-targeting antisense oligonucleotide MAPT(Rx) in mild Alzheimer’s disease: A phase 1b, randomized, placebo-controlled trial. Nat. Med. 2023, 29, 1437–1447. [Google Scholar] [CrossRef]
- Dujardin, S.; Commins, C.; Lathuiliere, A.; Beerepoot, P.; Fernandes, A.R.; Kamath, T.V.; De Los Santos, M.B.; Klickstein, N.; Corjuc, D.L.; Corjuc, B.T. Tau molecular diversity contributes to clinical heterogeneity in Alzheimer’s disease. Nat. Med. 2020, 26, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Sytar, O.; Hajihashemi, S. Specific Secondary Metabolites of Medicinal Plants and Their Role in Stress Adaptation. In Plant Secondary Metabolites and Abiotic Stress; Wiley: Hoboken, NJ, USA, 2024. [Google Scholar]
- Wang, Y.; Hu, H.; Liu, X.; Guo, X. Hypoglycemic medicines in the treatment of Alzheimer’s disease: Pathophysiological links between AD and glucose metabolism. Front. Pharmacol. 2023, 14, 1138499. [Google Scholar] [CrossRef]
- Kim, M.G.; Ooi, S.L.; Kim, G.W.; Pak, S.C.; Koo, B.S. Effectiveness and Safety of Pattern Identification-Based Herbal Medicine for Alzheimer’s Disease: A Systematic Review and Meta-Analysis. J. Integr. Complement. Med. 2023, 29, 605–620. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhao, H.; Huang, B.; Zheng, C.; Peng, W.; Qin, L. Acanthopanax senticosus: Review of botany, chemistry and pharmacology. Pharmazie 2011, 66, 83–97. [Google Scholar]
- Li, X.; Tang, S.Q.; Huang, H.; Luo, J.; Zhang, X.D.; Yook, C.S.; Whang, W.K.; Kim, Y.C.; Liu, X.Q. Acanthopanax henryi: Review of Botany, Phytochemistry and Pharmacology. Molecules 2021, 26, 2215. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, A.; Sun, H.; Zhang, Y.; Meng, X.; Yan, G.; Liu, L.; Wang, X. High-throughput ultra high performance liquid chromatography combined with mass spectrometry approach for the rapid analysis and characterization of multiple constituents of the fruit of Acanthopanax senticosus (Rupr. et Maxim.) Harms. J. Sep. Sci. 2017, 40, 2178–2187. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Li, Z.; Wu, H.; Zou, B.; Xu, Y. Exploring Natural Products as Radioprotective Agents for Cancer Therapy: Mechanisms, Challenges, and Opportunities. Cancers 2023, 15, 3585. [Google Scholar] [CrossRef]
- Liang, Q.; Yu, X.; Qu, S.; Xu, H.; Sui, D. Acanthopanax senticosides B ameliorates oxidative damage induced by hydrogen peroxide in cultured neonatal rat cardiomyocytes. Eur. J. Pharmacol. 2010, 627, 209–215. [Google Scholar] [CrossRef]
- Pan, J.; Li, X.; Guo, F.; Yang, Z.; Zhang, L.; Yang, C. Ginkgetin attenuates cerebral ischemia-reperfusion induced autophagy and cell death via modulation of the NF-kappaB/p53 signaling pathway. Biosci. Rep. 2019, 39, BSR20191452. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. 2021, 1, e78. [Google Scholar] [CrossRef]
- Qureshi, S.; Ali, G.; Muhammad, T.; Idrees, M.; Ullah, S.; Khan, S.A.; Ullah, R.; Khan, R.; Ul-Haq, Z.; Mohsin, A.H.; et al. Corrigendum to “Thiadiazine-thione derivatives ameliorate STZ-induced diabetic neuropathy by regulating insulin and neuroinflammatory signaling” [Int. Immunopharmacol. 113 (2022) 109421]. Int. Immunopharmacol. 2024, 138, 112649. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Cui, L.; Liu, B.; Liu, W.; Hayashi, T.; Mizuno, K.; Hattori, S.; Ushiki-Kaku, Y.; Onodera, S.; Ikejima, T.; et al. Silibinin ameliorates STZ-induced impairment of memory and learning by up- regulating insulin signaling pathway and attenuating apoptosis. Physiol. Behav. 2020, 213, 112689. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Liu, Y.; Xu, Q.Q.; Xian, Y.F.; Lin, Z.X. Sulforaphene Ameliorates Neuroinflammation and Hyperphosphorylated Tau Protein via Regulating the PI3K/Akt/GSK-3beta Pathway in Experimental Models of Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2020, 2020, 4754195. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Hou, X.J.; Che, X.H.; Zhou, T.S.; Liu, X.Q.; Wu, C.F.; Yang, J.Y. Pseudoginsenoside-F11 attenuates cognitive dysfunction and tau phosphorylation in sporadic Alzheimer’s disease rat model. Acta Pharmacol. Sin. 2021, 42, 1401–1408. [Google Scholar] [CrossRef]
- Murtishaw, A.S.; Heaney, C.F.; Bolton, M.M.; Sabbagh, J.J.; Langhardt, M.A.; Kinney, J.W. Effect of acute lipopolysaccharide-induced inflammation in intracerebroventricular-streptozotocin injected rats. Neuropharmacology 2016, 101, 110–122. [Google Scholar] [CrossRef]
- Babaei, P.; Eyvani, K.; Kouhestani, S. Sex-Independent Cognition Improvement in Response to Kaempferol in the Model of Sporadic Alzheimer’s Disease. Neurochem. Res. 2021, 46, 1480–1486. [Google Scholar] [CrossRef]
- Li, X.H.; Zhu, H.C.; Cui, X.M.; Wang, W.; Yang, L.; Wang, L.B.; Hu, N.W.; Duan, D.X. Death-associated protein kinase 1 is associated with cognitive dysfunction in major depressive disorder. Neural Regen. Res. 2023, 18, 1795–1801. [Google Scholar] [CrossRef]
- Duan, D.; Chai, G.S.; Ni, Z.F.; Hu, Y.; Luo, Y.; Cheng, X.S.; Chen, N.N.; Wang, J.Z.; Liu, G.P. Phosphorylation of tau by death-associated protein kinase 1 antagonizes the kinase-induced cell apoptosis. J. Alzheimers Dis. 2013, 37, 795–808. [Google Scholar] [CrossRef]
- Long, L.N.; Zhang, H.H.; Wang, F.; Yin, Y.X.; Yang, L.Y.; Chen, J.S. Research Note: Effects of polysaccharide-enriched Acanthopanax senticosus extract on growth performance, immune function, antioxidation, and ileal microbial populations in broiler chickens. Poult. Sci. 2021, 100, 101028. [Google Scholar] [CrossRef]
- Jung, H.; Park, H.J.; Kim, R.G.; Shin, K.M.; Ha, J.; Choi, J.W.; Kim, H.J.; Lee, Y.S.; Lee, K.T. In vivo anti-inflammatory and antinociceptive effects of liriodendrin isolated from the stem bark of Acanthopanax senticosus. Planta Med. 2003, 69, 610–616. [Google Scholar]
- Su, J.; Fu, X.; Liu, R.; Zhang, X.; Xue, J.; Li, Y.; Zhang, R.; Li, X.; Wang, X.; Wang, X.; et al. Antioxidant and Antiapoptotic Properties of n-Butanol Fraction the Acanthopanax senticosus Extracts in H(2)O(2 Liver Injury in Mice. Evid. Based Complement. Altern. Med. 2023, 2023, 9190198. [Google Scholar] [CrossRef] [PubMed]
- Kuang, F.; Xiang, T. Molecular mechanism of Acanthopanax senticosus in the treatment of Alzheimer’s disease based on network pharmacology and molecular docking. Mol. Divers. 2023, 27, 2849–2865. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Mohammed, S.A.D.; Wang, T.; Fu, J.; Wang, Y.; Nan, Y.; Lu, F.; Liu, S. Efficacy and mechanism study of Baichanting compound, a combination of Acanthopanax senticosus (Rupr. and Maxim.) Harms, Paeonia lactiflora Pall and Uncaria rhynchophylla (Miq.) Miq. ex Havil, on Parkinson’s disease based on metagenomics and metabolomics. J. Ethnopharmacol. 2024, 319 Pt 1, 117182. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Yan, W.; Gu, Z.; Li, Y.; Chen, L.; He, B. Anti-Neuroinflammatory Potential of Natural Products in the Treatment of Alzheimer’s Disease. Molecules 2023, 28, 1486. [Google Scholar] [CrossRef]
- Novoa, C.; Salazar, P.; Cisternas, P.; Gherardelli, C.; Vera-Salazar, R.; Zolezzi, J.M.; Inestrosa, N.C. Inflammation context in Alzheimer’s disease, a relationship intricate to define. Biol. Res. 2022, 55, 39. [Google Scholar] [CrossRef]
- Sun, E.; Motolani, A.; Campos, L.; Lu, T. The Pivotal Role of NF-kB in the Pathogenesis and Therapeutics of Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 8972. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, X.-M.; Wang, W.; Yang, L.; Nie, B.-W.; Liu, Q.; Li, X.-H.; Duan, D.-X. Acanthopanax Senticosus Saponins Prevent Cognitive Decline in Rats with Alzheimer’s Disease. Int. J. Mol. Sci. 2025, 26, 3715. https://doi.org/10.3390/ijms26083715
Cui X-M, Wang W, Yang L, Nie B-W, Liu Q, Li X-H, Duan D-X. Acanthopanax Senticosus Saponins Prevent Cognitive Decline in Rats with Alzheimer’s Disease. International Journal of Molecular Sciences. 2025; 26(8):3715. https://doi.org/10.3390/ijms26083715
Chicago/Turabian StyleCui, Xue-Min, Wang Wang, Lin Yang, Bao-Wen Nie, Qian Liu, Xiao-Hui Li, and Dong-Xiao Duan. 2025. "Acanthopanax Senticosus Saponins Prevent Cognitive Decline in Rats with Alzheimer’s Disease" International Journal of Molecular Sciences 26, no. 8: 3715. https://doi.org/10.3390/ijms26083715
APA StyleCui, X.-M., Wang, W., Yang, L., Nie, B.-W., Liu, Q., Li, X.-H., & Duan, D.-X. (2025). Acanthopanax Senticosus Saponins Prevent Cognitive Decline in Rats with Alzheimer’s Disease. International Journal of Molecular Sciences, 26(8), 3715. https://doi.org/10.3390/ijms26083715





