Promising Gastric Cancer Biomarkers—Focus on Tryptophan Metabolism via the Kynurenine Pathway
Abstract
1. Introduction
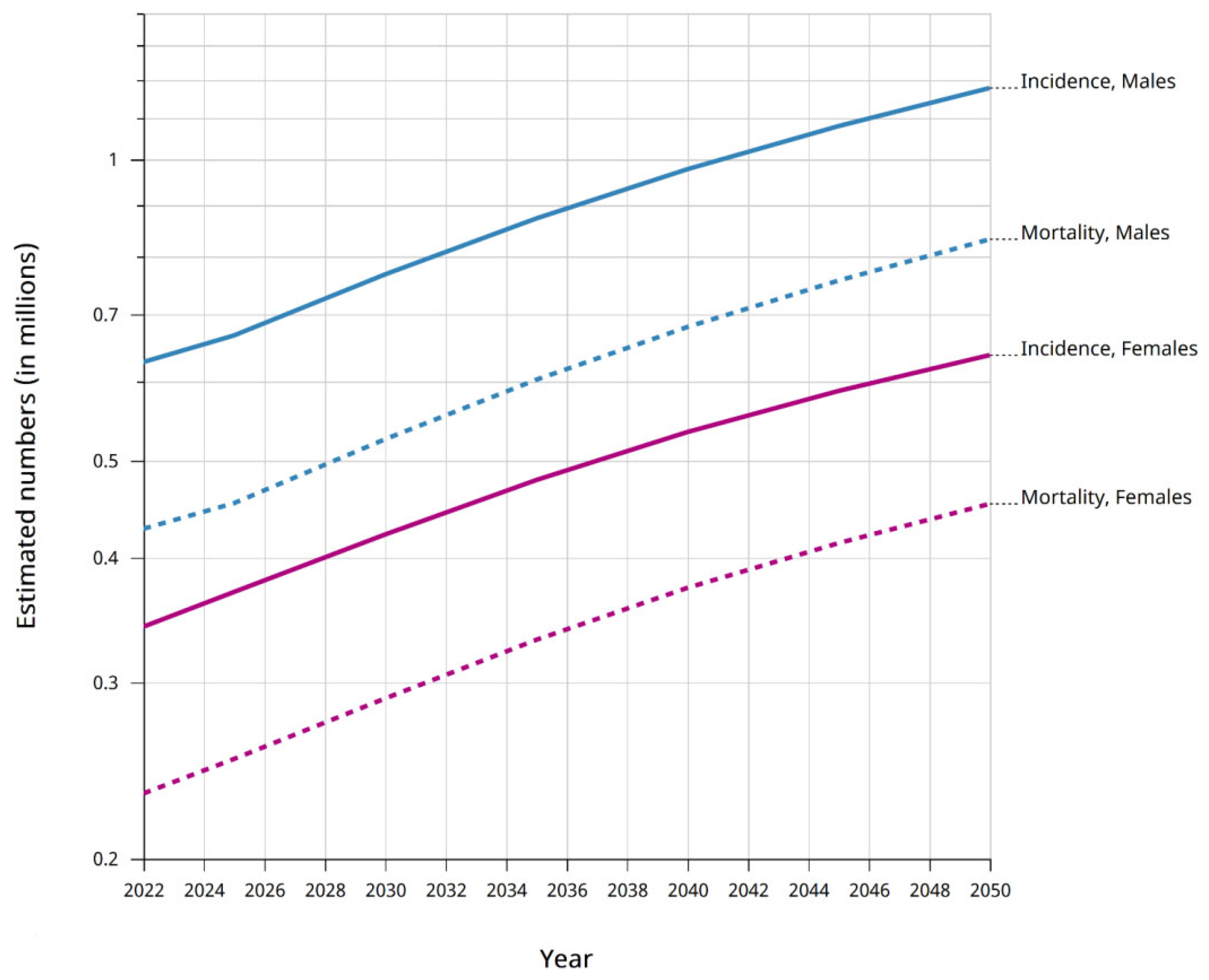
2. Gastric Cancer Etiology and Diagnosis
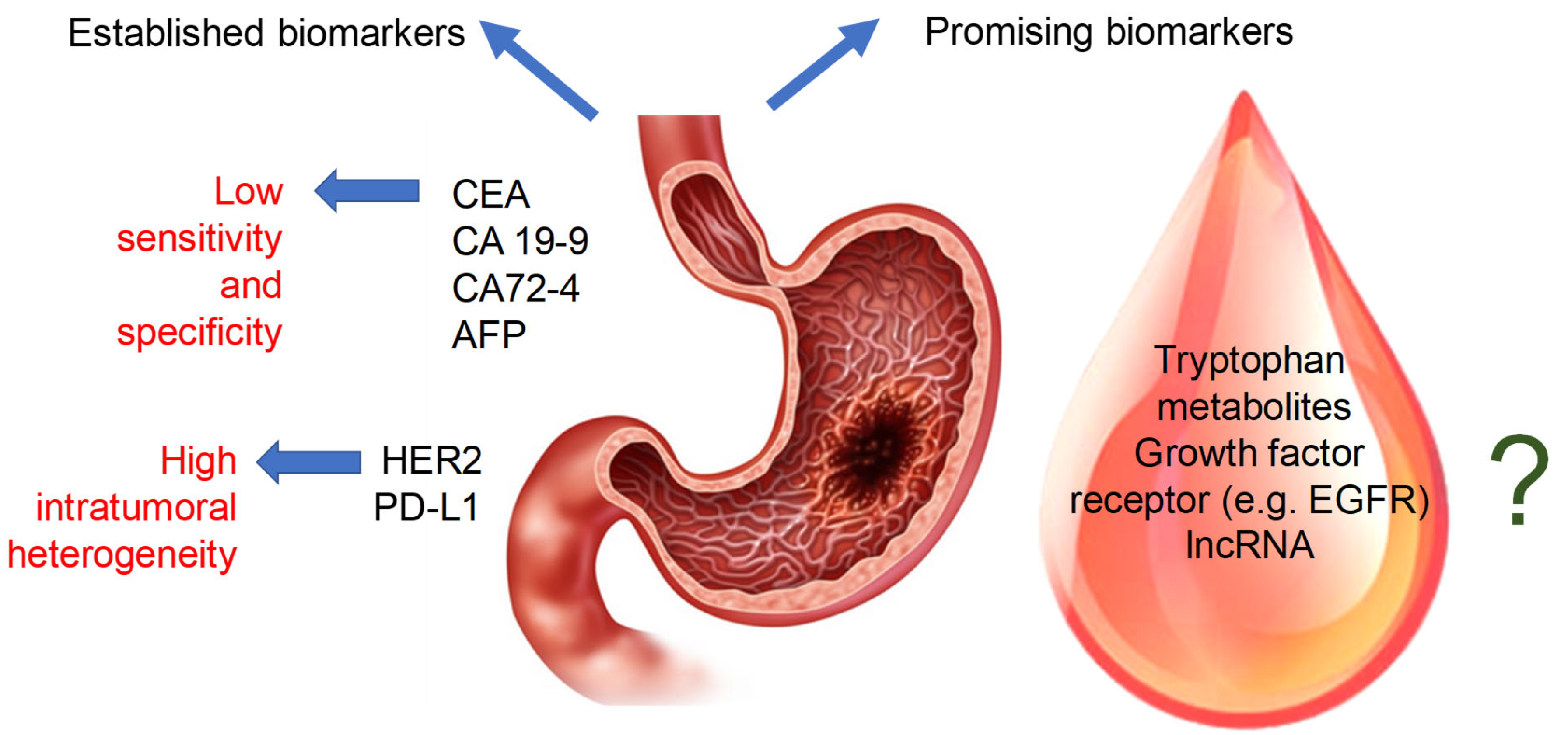
3. Established Biomarkers of Gastric Cancer
4. Putative Biomarkers of Gastric Cancer
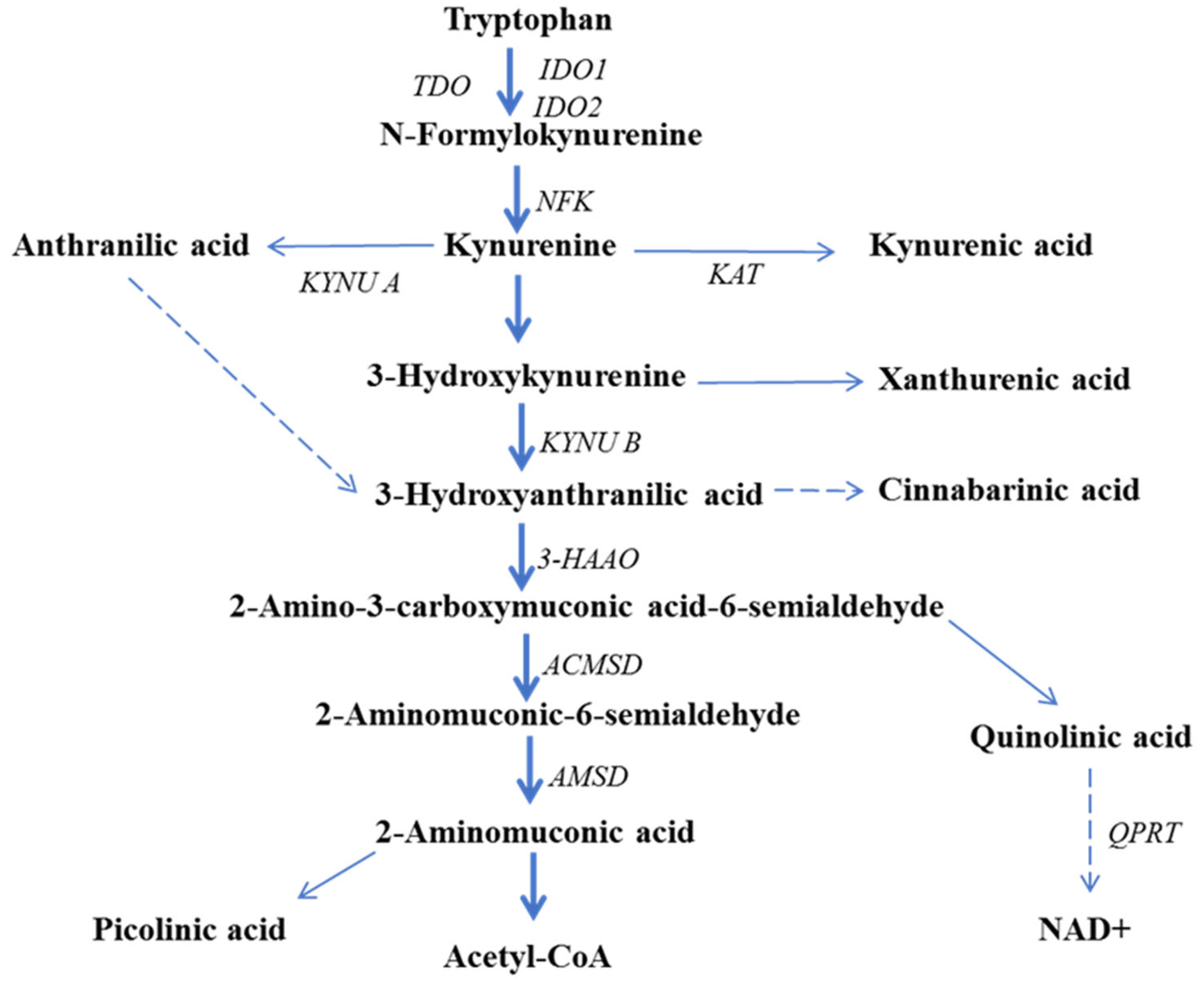
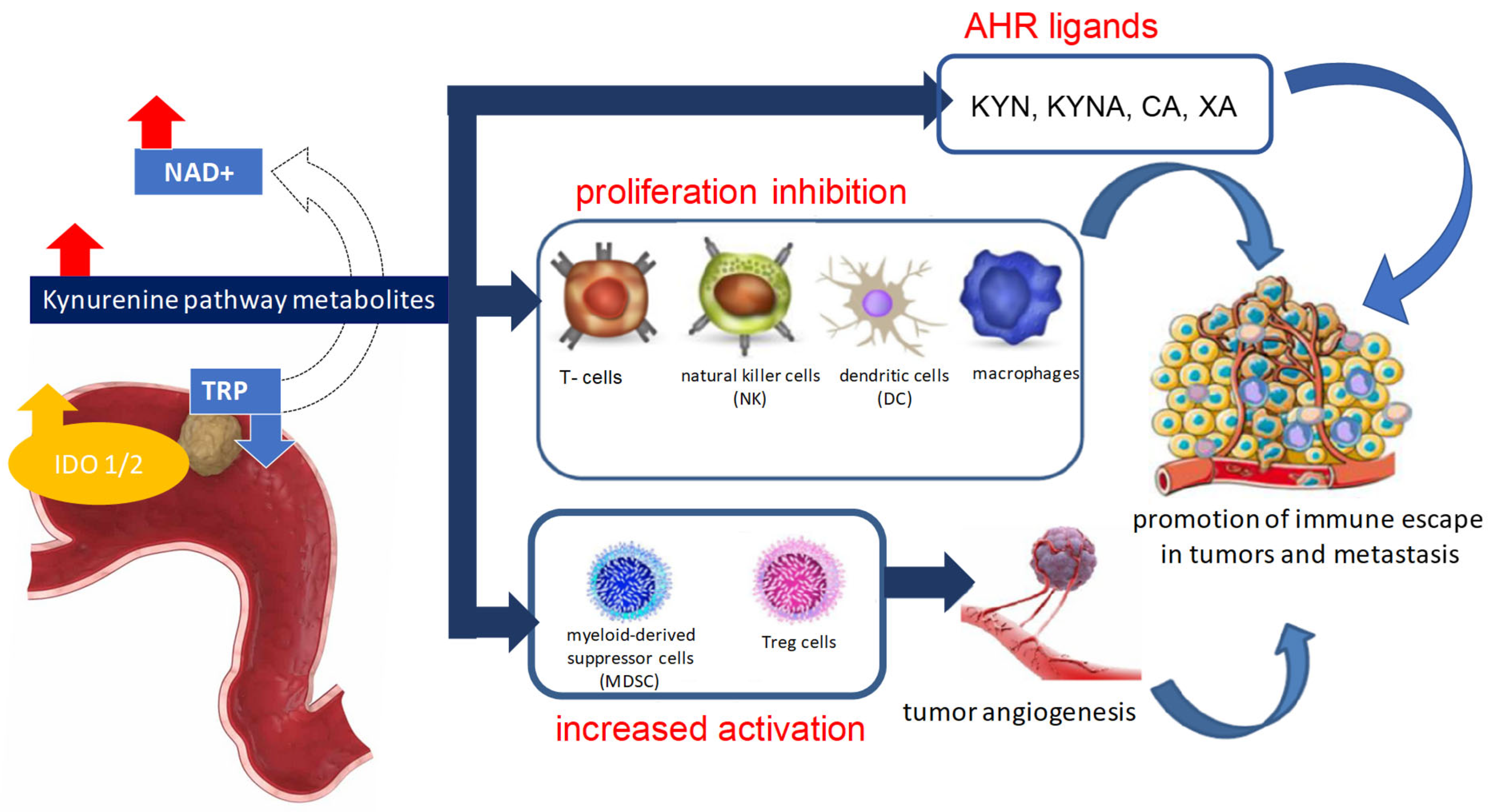
5. The Role of Kynurenine Pathway Enzymes in Tumorigenesis
6. Kynurenine Pathway Metabolites in Cancer Pathogenesis
6.1. Kynurenine
6.2. Kynurenic Acid
6.3. 3-Hydroxyanthranilic Acid
6.4. Quinolinic Acid
6.5. Picolinic Acid
7. Kynurenine Pathway Metabolites and Enzymes in Gastric Cancer
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Feng, A.; Zheng, S.; Chen, C.; Lyu, J. Recent Estimates and Predictions of 5-Year Survival in Patients with Gastric Cancer: A Model-Based Period Analysis. Cancer Control 2022, 29, 10732748221099227. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.N.; Teng, Q.X.; Tian, Q.; Chen, W.; Xie, Y.; Wu, K.; Zeng, Q.; Zeng, L.; Pan, Y.; Chen, Z.; et al. Signaling pathways and therapeutic interventions in gastric cancer. Signal Transduct. Target. Ther. 2022, 7, 358. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; Yeoh, K.G. Genetics and Molecular Pathogenesis of Gastric Adenocarcinoma. Gastroenterology 2015, 149, 1153–1162. [Google Scholar] [CrossRef]
- Cancer Tomorrow. Available online: https://gco.iarc.fr/tomorrow/en/dataviz/trends?multiple_populations=1&cancers=7 (accessed on 12 February 2024).
- Cheng, X.J.; Lin, J.C.; Tu, S.P. Etiology and Prevention of Gastric Cancer. Gastrointest. Tumors 2016, 3, 25–36. [Google Scholar] [CrossRef]
- Aronson, J.K.; Ferner, R.E. Biomarkers—A General Review. Curr. Protoc. Pharmacol. 2017, 76, 9.23.1–9.23.17. [Google Scholar] [CrossRef]
- Matsuoka, T.; Yashiro, M. Biomarkers of Gastric Cancer: Current Topics and Future Perspective. World J. Gastroenterol. 2018, 24, 2818–2832. [Google Scholar] [CrossRef]
- Elimova, E.; Wadhwa, R.; Shiozaki, H.; Sudo, K.; Estrella, J.S.; Badgwell, B.D.; Da, P.; Matamoros, A., Jr.; Song, S.; Ajani, J.A. Molecular Biomarkers in Gastric Cancer. J. Natl. Compr. Cancer Netw. 2015, 13, 19–29. [Google Scholar] [CrossRef]
- Smith, C.; Chang, M.Y.; Parker, K.H.; Beury, D.W.; DuHadaway, J.B.; Flick, H.E.; Boulden, J.; Sutanto-Ward, E.; Soler, A.P.; Laury-Kleintop, L.D.; et al. IDO is a Nodal Pathogenic Driver of Lung Cancer and Metastasis Development. Cancer Discov. 2012, 2, 722–735. [Google Scholar] [CrossRef]
- Munn, D.H.; Zhou, M.; Attwood, J.T.; Bondarev, I.; Conway, S.J.; Marshall, B.; Brown, C.; Mellor, A.L. Prevention of Allogeneic Fetal Rejection by Tryptophan Catabolism. Science 1998, 281, 1191–1193. [Google Scholar] [CrossRef]
- Bender, D.A. Biochemistry of Tryptophan in Health and Disease. Mol. Aspects Med. 1983, 6, 101–197. [Google Scholar] [CrossRef] [PubMed]
- Platten, M.; Nollen, E.A.A.; Röhrig, U.F.; Fallarino, F.; Opitz, C.A. Tryptophan Metabolism as a Common Therapeutic Target in Cancer, Neurodegeneration and Beyond. Nat. Rev. Drug Discov. 2019, 18, 379–401. [Google Scholar] [CrossRef]
- Badawy, A.A.B.; Guillemin, G. The Plasma [Kynurenine]/[Tryptophan] Ratio and Indoleamine 2,3-Dioxygenase: Time for Appraisal. Int. J. Tryptophan Res. 2019, 12, 1178646919868978. [Google Scholar] [CrossRef]
- Opitz, C.A.; Litzenburger, U.M.; Sahm, F.; Ott, M.; Tritschler, I.; Trump, S.; Schumacher, T.; Jestaedt, L.; Schrenk, D.; Weller, M.; et al. An Endogenous Tumour-promoting Ligand of the Human Aryl Hydrocarbon Receptor. Nature 2011, 478, 197–203. [Google Scholar] [CrossRef]
- Badawy, A.A.B. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Int. J. Tryptophan Res. 2017, 10, 1178646917691938. [Google Scholar] [CrossRef]
- Schwarch, R.; Bruno, J.P.; Muchowski, P.J.; Wu, H.Q. Kynurenines in the Mammalian Brain: When Physiology Meets Pathology. Nat. Rev. Neurosci. 2012, 13, 465–477. [Google Scholar] [CrossRef]
- He, Z.; Davis, J.K.; Spain, J.C. Purification, Characterization, and Sequence analysis of 2-aminomuconic 6-semialdehyde dehydrogenase from Pseudomonas Pseudoalcaligenes JS45. J. Bacteriol. 1998, 180, 4591–4595. [Google Scholar] [CrossRef]
- Pilotte, L.; Larrieu, P.; Stroobant, V.; Colau, D.; Dolušić, E.; Frédérick, R.; De Plaen, E.; Uyttenhove, C.; Wouters, J.; Masereel, B.; et al. Reversal of Tumoral Immune Resistance by Inhibition of Tryptophan 2,3-dioxygenase. Proc. Natl. Acad. Sci. USA 2012, 109, 2497–2502. [Google Scholar] [CrossRef]
- Gouasmi, R.; Ferraro-Peyret, C.; Nancey, S.; Coste, I.; Renno, T.; Chaveroux, C.; Aznar, N.; Ansieau, S. The Kynurenine Pathway and Cancer: Why Keep It Simple When You Can Make It Complicated. Cancers 2022, 14, 2793. [Google Scholar] [CrossRef]
- Choi, J.M.; Park, W.S.; Song, K.Y.; Lee, H.J.; Jung, B.H. Development of Simultaneous Analysis of Tryptophan Metabolites in Serum and Gastric Juice—An Investigation Towards Establishing a Biomarker Test for Gastric Cancer Diagnosis. Biomed. Chromatogr. 2016, 30, 1963–1974. [Google Scholar] [CrossRef]
- Gęca, K.; Rawicz-Pruszyński, K.; Mlak, R.; Sadok, I.; Polkowski, W.P.; Staniszewska, M. Kynurenine and Anthranilic Acid in the Peritoneum Correlate with the Stage of Gastric Cancer Disease. Int. J. Tryptophan Res. 2022, 15, 11786469211065620. [Google Scholar] [CrossRef] [PubMed]
- Sadok, I.; Jędruchniewicz, K.; Rawicz-Pruszyński, K.; Staniszewska, M. UHPLC-ESI-MS/MS Quantification of Relevant Substrates and Metabolites of the Kynurenine Pathway Present in Serum and Peritoneal Fluid from Gastric Cancer Patients—Method Development and Validation. Int. J. Mol. Sci. 2022, 22, 6972. [Google Scholar] [CrossRef]
- Engin, A.B.; Karahalil, B.; Karakaya, A.E.; Engin, A. Exposure to Helicobacter pylori and Serum Kynurenine to Tryptophan Ratio in Patients with Gastric Cancer. Pteridines 2010, 21, 110–120. [Google Scholar] [CrossRef]
- Helicobacter and Cancer Collaborative Group. Gastric Cancer and Helicobacter Pylori: A Combined Analysis of 12 Case Control Studies Nested Within Prospective Cohorts. Gut 2001, 49, 347–353. [Google Scholar] [CrossRef]
- Ilic, M.; Ilic, I. Epidemiology of Stomach Cancer. World J. Gastroenterol. 2022, 28, 1187–1203. [Google Scholar] [CrossRef]
- Karimi, P.; Islami, F.; Anandasabapathy, S.; Freedman, N.D.; Kamangar, F. Gastric Cancer: Descriptive Epidemiology, Risk Factors, Screening, and Prevention. Cancer Epidemiol. Biomarkers Prev. 2014, 23, 700–713. [Google Scholar] [CrossRef]
- Lynch, H.T.; Grady, W.; Suriano, G.; Huntsman, D. Gastric Cancer: New Genetic Developments. J. Surg. Oncol. 2005, 90, 114–133. [Google Scholar] [CrossRef]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric Cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef]
- Röcken, C. Predictive Biomarkers in Gastric Cancer. J. Cancer Res. Clin. Oncol. 2023, 149, 467–481. [Google Scholar] [CrossRef]
- Rutkowski, P.; Jeziorski, A.; Wysocki, W.M. Chirurgia Onkologiczna; PZWL: Warsow, Poland, 2022; pp. 150–200. Volume 3. [Google Scholar]
- Richter, P.; Wallner, G.; Zegarski, W.; Sierżęga, M.; Kołodziejczyk, P.; Nasierowska-Guttmejer, A.; Kielan, W.; Murawa, D.; Wyrwicz, L.; Konopka, K.; et al. Polski Konsensus Diagnostyki i Leczenia Raka Żołądka—Aktualizacja 2022. Biul. Pol. Tow. Onkol. Nowotw. 2022, 7, 381–408. [Google Scholar]
- Johnston, F.M.; Beckman, M. Updates on Management of Gastric Cancer. Curr. Oncol. Rep. 2019, 21, 67. [Google Scholar] [CrossRef] [PubMed]
- Dohi, O.; Seya, M.; Iwai, N.; Ochiai, T.; Yumoto, J.; Mukai, H.; Yamauchi, K.; Kobayashi, R.; Hirose, R.; Inoue, K.; et al. Endoscopic Detection and Diagnosis of Gastric Cancer Using Image-Enhanced Endoscopy: A Systematic Review and Meta-analysis. DEN Open 2025, 5, e418. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, O.; Nishizawa, T.; Koike, K. Endoscopic Kyoto Classification of Helicobacter Pylori Infection and Gastric Cancer Risk Diagnosis. World J. Gastroenterol. 2020, 26, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Shao, Y.; Yu, X.; Chen, C.; Guo, J.; Ye, G. Global Progress and Future Prospects of Early Gastric Cancer Screening. J. Cancer 2024, 15, 3045–3064. [Google Scholar] [CrossRef]
- Li, X.; Yang, D.H.; Ranganathan, P. Editorial: Chemo-Resistance in Gastrointestinal Cancers. Front. Oncol. 2022, 12, 821212. [Google Scholar] [CrossRef]
- Feng, F.; Tian, Y.; Xu, G.; Liu, Z.; Liu, S.; Zheng, G.; Guo, M.; Lian, X.; Fan, D.; Zhang, H. Diagnostic and Prognostic Value of CEA, CA19–9, AFP and CA125 for Early Gastric Cancer. BMC Cancer 2017, 17, 737. [Google Scholar] [CrossRef]
- Kankanala, V.; Zubair, M.; Mukkamalla, S.K. Carcinoembryonic Antigen; StatPearls: Treasure Island, FL, USA, 2024; pp. 1–4. [Google Scholar]
- Marrelli, D.; Pinto, E.; De Stefano, A.; Farnetani, M.; Garosi, L.; Roviello, F. Clinical Utility of CEA, CA 19-9, and CA 72-4 in the Follow-up of Patients with Resectable Gastric Cancer. Am. J. Surg. 2001, 181, 16–19. [Google Scholar] [CrossRef]
- Hu, P.J.; Chen, M.Y.; Wu, M.S.; Lin, Y.C.; Shih, P.H.; Lai, C.H.; Lin, H. Clinical Evaluation of CA72-4 for Screening Gastric Cancer in a Healthy Population: A Multicenter Retrospective Study. Cancers 2019, 11, 733. [Google Scholar] [CrossRef]
- Fong, C.; Chau, I. HER2 Inhibition in Gastric Cancer—Novel Therapeutic Approaches for an Established Target. Cancers 2022, 14, 3824. [Google Scholar] [CrossRef]
- Ye, M.; Huang, D.; Zhang, Q.; Weng, W.; Tan, C.; Qin, G.; Wang, L. Heterogeneous Programmed Death-Ligand 1 Expression in Gastric Cancer: Comparison of Tissue Microarrays and Whole Sections. Cancer Cell Int. 2020, 20, 186. [Google Scholar] [CrossRef]
- Yamashita, K.; Iwatsuki, M.; Harada, K.; Koga, Y.; Kiyozumi, Y.; Eto, K.; Baba, H. Can PD-L1 Expression Evaluated by Biopsy Sample Accurately Reflect its Expression In the Whole Tumour In Gastric Cancer? Br. J. Cancer 2019, 121, 278–280. [Google Scholar] [CrossRef]
- Kanda, M.; Kodera, Y. Recent Advances in the Molecular Diagnostics of Gastric Cancer. World J. Gastroenterol. 2015, 21, 9838–9852. [Google Scholar] [CrossRef]
- Choi, S.; Park, S.; Kim, H.; Kang, S.Y.; Ahn, S.; Kim, K.M. Gastric Cancer: Mechanisms, Biomarkers, and Therapeutic Approaches. Biomedicines 2022, 10, 543. [Google Scholar] [CrossRef]
- Gamboa-Dominguez, A.; Dominguez-Fonseca, C.; Chavarri-Guerra, Y.; Vargas, R.; Reyes-Gutierrez, E.; Green, D.; Quintanilla-Martinez, L.; Luber, B.; Busch, R.; Becker, K.; et al. E-Cadherin Expression in Sporadic Gastric Cancer from Mexico: Exon 8 and 9 Deletions are Infrequent Events Associated with Poor Survival. Hum. Pathol. 2005, 36, 29–35. [Google Scholar] [CrossRef]
- Carlomagno, N.; Incollingo, P.; Tammaro, V.; Peluso, G.; Rupealta, N.; Chiacchio, G.; Sotelo, M.L.S.; Minieri, G.; Pisani, A.; Riccio, E.; et al. Diagnostic, Predictive, Prognostic, and Therapeutic Molecular Biomarkers in Third Millennium: A Breakthrough in Gastric Cancer. BioMed Res. Int. 2017, 2017, 7869802. [Google Scholar] [CrossRef]
- Park, C.K.; Park, J.S.; Kim, H.S.; Rha, S.Y.; Hyung, W.J.; Cheong, J.H.; Noh, S.H.; Lee, S.K.; Lee, Y.C.; Huh, Y.; et al. Receptor Tyrosine Kinase Amplified Gastric Cancer: Clinicopathologic Characteristics and Proposed Screening Algorithm. Oncotarget 2016, 7, 72099–72112. [Google Scholar] [CrossRef]
- Schrumpf, T.; Behrens, H.M.; Haag, J.; Krüger, S.; Röcken, C. FGFR2 Overexpression and Compromised Survival in Diffuse-Type Gastric Cancer in a Large Central European Cohort. PLoS ONE 2022, 17, e0264011. [Google Scholar] [CrossRef]
- Dong, S.C.; Sha, H.H.; Xu, X.Y.; Hu, T.M.; Lou, R.; Li, H.; Wu, J.; Dan, C.; Feng, J. Glutathione S-transferase π: A Potential Role in Antitumor Therapy. Drug Des. Dev. Ther. 2018, 12, 3535–3547. [Google Scholar] [CrossRef]
- Yu, P.; Du, Y.; Cheng, X.; Yu, Q.; Huang, L.; Dong, R. Expression of Multidrug Resistance-Associated Proteins and Their Relation to Postoperative Individualized Chemotherapy in Gastric Cancer. World J. Surg. Oncol. 2014, 12, 307. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, F.; Jin, J.; Xu, W.; Qian, H. Exosomal LncRNAs in Gastrointestinal Cancer: Biological Functions and Emerging Clinical Applications. Cancers 2023, 15, 959. [Google Scholar] [CrossRef]
- Hao, N.B.; He, Y.F.; Li, X.Q.; Wang, K.; Wang, R.L. The Role of miRNA and lncRNA In Gastric Cancer. Oncotarget 2017, 8, 81572–81582. [Google Scholar] [CrossRef]
- Metzger, M.; Behrens, H.; Böger, C.; Haag, J.; Krüger, S.; Röcken, C. MET In Gastric Cancer—Discarding a 10% Cutoff Rule. Histopathology 2016, 68, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Geng, Q.; Tian, Y.; Cai, M.; Fang, X.; Zhan, Y.; Zhou, Z.; Li, W.; Chen, Y.; Sun, X.; et al. Activated Mammalian Target of Rapamycin Is a Potential Therapeutic Target in Gastric Cancer. BMC Cancer 2010, 10, 536. [Google Scholar] [CrossRef]
- Kim, Y.I.; Pecha, R.L.; Keihanian, T.; Mercado, M.; Pena-Munoz, S.V.; Lang, K.; Van Buren, G.; Dhingra, S.; Othman, M. MUC1 Expressions and Its Prognostic Values in US Gastric Cancer Patients. Cancers 2023, 15, 998. [Google Scholar] [CrossRef]
- Lee, H.S.; Lee, H.K.; Kim, H.S.; Yang, H.K.; Kim, Y.I.; Kim, W.H. MUC1, MUC2, MUC5AC, and MUC6 Expressions in Gastric Carcinomas. Cancer 2001, 92, 1427–1434. [Google Scholar] [CrossRef]
- Carrasco-Garcia, E.; Álvarez-Satta, M.; García-Puga, M.; Ribeiro, M.L.; Arevalo, S.; Arauzo-Bravo, M.; Matheu, A. Therapeutic Relevance of SOX9 Stem Cell Factor in Gastric Cancer. Expert. Opin. Ther. Targets 2019, 23, 143–152. [Google Scholar] [CrossRef]
- Busuttil, R.A.; Zapparoli, G.V.; Haupt, S.; Fennell, C.; Wong, S.Q.; Pang, J.M.B.; Takeno, E.; Mitcheli, C.; Di Costanzo, N.; Fox, S.; et al. Role of p53 In the Progression of Gastric Cancer. Oncotarget 2014, 5, 12016–12026. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Badawy, A.A.B. Tryptophan Metabolism, Disposition and Utilization in Pregnancy. Biosci. Rep. 2015, 35, e00261. [Google Scholar] [CrossRef]
- Perez-Castro, L.; Garcia, R.; Venkateswaran, N.; Barnes, S.; Conacci-Sorrell, M. Tryptophan and Its Metabolites in Normal Physiology and Cancer Etiology. FEBS J. 2023, 290, 7–27. [Google Scholar] [CrossRef]
- Théate, I.; van Baren, N.; Pilotte, L.; Moulin, P.; Larrieu, P.; Renauld, J.C.; Hervé, C.; Gutierrez Roelens, I.; Marbaix, E.; Sempoux, C.; et al. Extensive Profiling of the Expression of the Indoleamine 2,3-Dioxygenase 1 Protein in Normal and Tumoral Human Tissues. Cancer Immunol. Res. 2015, 3, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, M.; Yamamoto, Y.; Kawasoe, M.; Arioka, Y.; Murakami, Y.; Hoshi, M.; Saito, K. Studies On Tissue and Cellular Distribution of Indoleamine 2,3-dioxygenase 2: The Absence of IDO1 Upregulates IDO2 Expression in the Epididymis. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2012, 60, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Karanikas, V.; Zamanakou, M.; Kerenidi, T.; Dahabreh, J.; Hevas, A.; Nakou, M.; Gourgoulianis, K.; Germenis, A. Indoleamine 2,3-dioxygenase (IDO) Expression in Lung Cancer. Cancer Biol. Ther. 2007, 6, 1258–1262. [Google Scholar] [CrossRef]
- Hornyák, L.; Dobos, N.; Koncz, G.; Karányi, Z.; Páll, D.; Szabó, Z.; Székvölgyi, L. The Role of Indoleamine-2, 3-dioxygenase in Cancer Development, Diagnostics, and Therapy. Front. Immunol. 2018, 9, 151. [Google Scholar] [CrossRef]
- Oxenkrug, G.F. Tryptophan–Kynurenine Metabolism as a Common Mediator of Genetic and Environmental Impacts in Major Depressive Disorder: The Serotonin Hypothesis Revisited 40 Years Later. Isr. J. Psychiatry Relat. Sci. 2010, 47, 56–63. [Google Scholar]
- Prendergast, G.C.; Smith, C.; Thomas, S.; Mandik-Nayak, L.; Laury-Kleintop, L.; Metz, R.; Muller, A.J. IDO in Inflammatory Programming and Immune Suppression in Cancer. In Tumor-Induced Immune Suppression: Mechanisms and Therapeutic Reversal; Gabrilovich, D.I., Hurwitz, A.A., Eds.; Springer: New York, NY, USA, 2014; pp. 311–346. [Google Scholar]
- Li, P.; Xu, W.; Liu, F.; Zhu, H.; Zhang, L.; Ding, Z.; Liang, H. The Emerging Roles of IDO2 in Cancer and Its Potential as a Therapeutic Target. Biomed. Pharmacother. 2021, 137, 111295. [Google Scholar] [CrossRef]
- Bilir, C.; Sarisozen, C. Indoleamine 2,3-dioxygenase (IDO): Only an Enzyme or a Checkpoint Controller? J. Oncol. Sci. 2017, 3, 52–56. [Google Scholar] [CrossRef]
- Gibney, S.M.; Fagan, E.M.; Waldron, A.M.; O’Byrne, J.; Connor, T.J.; Harkin, A. Inhibition of Stress-induced Hepatic Tryptophan 2,3-dioxygenase Exhibits Antidepressant Activity in an Animal Model of Sepressive Behaviour. Int. J. Neuropsychopharmacol. 2014, 17, 917–928. [Google Scholar] [CrossRef]
- Badawy, A.A.B. Kynurenine Pathway and Human Systems. Exp. Gerontol. 2020, 129, 110770. [Google Scholar] [CrossRef]
- Ye, Z.; Yue, L.; Shi, J.; Shao, M.; Wu, T. Role of IDO and TDO in Cancers and Related Diseases and the Therapeutic Implications. J. Cancer 2019, 10, 2771–2782. [Google Scholar] [CrossRef]
- González Esquivel, D.; Ramírez-Ortega, D.; Pineda, B.; Castro, N.; Ríos, C.; Pérez de la Cruz, V. Kynurenine Pathway Metabolites and Enzymes Involved in Redox Reactions. Neuropharmacology 2017, 112, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Venkateswaran, N.; Lafita-Navarro, M.C.; Hao, Y.H.; Kilgore, J.A.; Perez-Castro, L.; Braverman, J.; Borenstein-Auerbach, N.; Kim, M.; Lesner, N.; Mishra, P.; et al. MYC Promotes Tryptophan Uptake and Metabolism by the Kynurenine Pathway in Colon Cancer. Genes. Dev. 2019, 33, 1236–1251. [Google Scholar] [CrossRef] [PubMed]
- Skorobogatov, K.; De Picker, L.; Verkerk, R.; Coppens, V.; Leboyer, M.; Müller, N.; Morrens, M. Brain Versus Blood: A Systematic Review on the Concordance Between Peripheral and Central Kynurenine Pathway Measures in Psychiatric Disorders. Front. Immunol. 2021, 12, 716980. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.R.; Jamie, J.F.; Guillemin, G.J. Kynurenine-3-monooxygenase: A Review of Structure, Mechanism, and Inhibitors. Drug Discov. Today. 2016, 21, 315–324. [Google Scholar] [CrossRef]
- Huang, T.T.; Tseng, L.M.; Chen, J.L.; Chu, P.Y.; Lee, C.H.; Huang, C.T.; Wang, W.; Lau, K.; Tseng, M.; Chang, Y.; et al. Kynurenine 3-monooxygenase Upregulates Pluripotent Genes Through β-catenin and Promotes Triple-negative Breast Cancer Progression. eBioMedicine 2020, 54, 102717. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, Y.; You, H.; Tao, X.; Wang, C.; Jin, G.; Wang, N.; Ruan, H.; Gu, D.; Huo, X.; et al. Prognostic Significance of Kynurenine 3-monooxygenase and Effects on Proliferation, Migration, and Invasion of Human Hepatocellular Carcinoma. Sci. Rep. 2015, 5, srep10466. [Google Scholar] [CrossRef]
- Adams, S.; Braidy, N.; Bessesde, A.; Brew, B.J.; Grant, R.; Teo, C.; Guillemin, G.J. The Kynurenine Pathway in Brain Tumor Pathogenesis. Cancer Res. 2012, 72, 5649–5657. [Google Scholar] [CrossRef]
- Thirtamara-Rajamani, K.; Li, P.; Galvis, M.L.E.; Labrie, V.; Brundin, P.; Brundin, L. Is the Enzyme ACMSD a Novel Therapeutic Target in Parkinson’s Disease? J. Park. Dis. 2017, 7, 577–587. [Google Scholar] [CrossRef]
- Shen, H.; Xu, X.; Bai, Y.; Wang, X.; Wu, Y.; Zhong, J.; Wu, Q.; Luo, Y.; Shang, T.; Shen, R.; et al. Therapeutic Potential of Targeting Kynurenine Pathway in Neurodegenerative Diseases. Eur. J. Med. Chem. 2023, 251, 115258. [Google Scholar] [CrossRef]
- Liu, C.L.; Cheng, S.P.; Chen, M.J.; Lin, C.H.; Chen, S.N.; Kuo, Y.H.; Chang, Y. Quinolinate Phosphoribosyltransferase Promotes Invasiveness of Breast Cancer Through Myosin Light Chain Phosphorylation. Front. Endocrinol. 2021, 11, 621944. [Google Scholar] [CrossRef]
- Zhou, L.; Mu, L.; Jiang, W.; Yang, Q. QPRT Acts as an Independent Prognostic Factor in Invasive Breast Cancer. J. Oncol. 2022, 2022, 6548644. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Xu, H.; Zeng, Y.; Lan, Q.; Liu, L.; Lai, W.; Chu, Z. Identification of a 3-gene Signature for Predicting the Prognosis of Stage II Colon Cancer Based on Microsatellite Status. J. Gastrointest. Oncol. 2021, 12, 2749–2762. [Google Scholar] [CrossRef] [PubMed]
- Pantouris, G.; Mowat, C.G. Antitumour Agents as Inhibitors of Tryptophan 2,3-dioxygenase. Biochem. Biophys. Res. Commun. 2014, 443, 28–31. [Google Scholar] [CrossRef]
- Dolušić, E.; Larrieu, P.; Moineaux, L.; Stroobant, V.; Pilotte, L.; Colau, D.; Pochet, L.; Van den Eynde, B.; Masereel, B.; Wouters, J.; et al. Tryptophan 2,3-Dioxygenase (TDO) Inhibitors. 3-(2-(Pyridyl)ethenyl)indoles as Potential Anticancer Immunomodulators. J. Med. Chem. 2011, 54, 5320–5334. [Google Scholar] [CrossRef]
- Prendergast, G.C.; Malachowski, W.P.; DuHadaway, J.B.; Muller, A.J. Discovery of IDO1 Inhibitors: From Bench to Bedside. Cancer Res. 2017, 77, 6795–6811. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, H.; Li, F.; Li, H.; Yu, J.; Ren, X. The Correlation Between the Subsets of Tumor Infiltrating Memory T Cells and the Expression of Indoleamine 2,3-Dioxygenase in Gastric Cancer. Dig. Dis. Sci. 2013, 58, 3494–3502. [Google Scholar] [CrossRef]
- Yoshii, M.; Tanaka, H.; Ohira, M.; Muguruma, K.; Iwauchi, T.; Lee, T.; Sakurai, K.; Kubo, N.; Yashiro, M.; Sawada, T.; et al. Expression of Forkhead Box P3 in Tumour Cells Causes Immunoregulatory Function of Signet Ring Cell Carcinoma of the Ctomach. Br. J. Cancer 2012, 106, 1668–1674. [Google Scholar] [CrossRef]
- Uyttenhove, C.; Pilotte, L.; Théate, I.; Stroobant, V.; Colau, D.; Parmentier, N.; Boon, T.; Van den Eynde, B. Evidence for a Tumoral Immune Resistance Mechanism Based on Tryptophan Degradation by Indoleamine 2,3-dioxygenase. Nat. Med. 2003, 9, 1269–1274. [Google Scholar] [CrossRef]
- Liu, M.; Wang, X.; Wang, L.; Ma, X.; Gong, Z.; Zhang, S.; Li, Y. Targeting the IDO1 Pathway in Cancer: From Bench to Bedside. J. Hematol. Oncol. 2018, 11, 100. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Zheng, X.; Zhang, X.; Wang, H.; Li, Q.; Yuan, K.; Zhou, N.; Yu, Y.; Song, N.; et al. Gene Silencing of Indoleamine 2,3-dioxygenase 2 in Melanoma Cells Induces Apoptosis Through the Suppression of NAD+ and Inhibits In Vivo Tumor Growth. Oncotarget 2016, 7, 32329–32340. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, P.; Liu, H.; Fang, C.; Guo, H.; Chen, X.; Tan, M.; Zhang, Y.; Min, W. Silencing IDO2 in Dendritic Cells: A Novel Strategy to Strengthen Cancer Immunotherapy in a Murine Lung Cancer Model. Int. J. Oncol. 2020, 57, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Yamasuge, W.; Yamamoto, Y.; Fujigaki, H.; Hoshi, M.; Nakamoto, K.; Kunisawa, K.; Mouri, A.; Nabeshima, T.; Saito, K. Indoleamine 2,3-dioxygenase 2 Depletion Suppresses Tumor Growth in a Mouse model of Lewis Lung Carcinoma. Cancer Sci. 2019, 110, 3061–3070. [Google Scholar] [CrossRef] [PubMed]
- Ci, C.; Wu, C.; Lyu, D.; Chang, X.; He, C.; Liu, W.; Chen, L.; Ding, W. Downregulation of Kynureninase Restrains Cutaneous Squamous Cell Carcinoma Proliferation and Represses the PI3K/AKT Pathway. Clin. Exp. Dermatol. 2020, 45, 194–201. [Google Scholar] [CrossRef]
- Al-Mansoob, M.; Gupta, I.; Rusyniak, R.S.; Ouhtit, A. KYNU, a Novel Potential Target that Underpins CD44-promoted Breast Tumour Cell Invasion. J. Cell. Mol. Med. 2021, 25, 2309–2314. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, X.; Lai, J.; Yi, W.; Yang, J.; Du, T.; Du, T.; Long, X.; Zhang, Y.; Xiao, Y. A Novel Role of Kynureninase in the Growth Control of Breast Cancer Cells and Its Relationships with Breast Cancer. J. Cell. Mol. Med. 2019, 23, 6700–6707. [Google Scholar] [CrossRef]
- Guillemin, G.J.; Cullen, K.M.; Lim, C.K.; Smythe, G.A.; Garner, B.; Kapoor, V.; Takikawa, O.; Brew, B.J. Characterization of the Kynurenine Pathway in Human Neurons. J. Neurosci. 2007, 27, 12884–12992. [Google Scholar] [CrossRef]
- Walczak, K.; Wnorowski, A.; Turski, W.A.; Plech, T. Kynurenic Acid and Cancer: Facts and Controversies. Cell. Mol. Life Sci. 2020, 77, 1531–1550. [Google Scholar] [CrossRef]
- Cervantes, G.I.V.; Cossio, J.Á.N.; de la Cruz, G.P.; Salazar, A.; de la Cruz, V.P.; Pineda, B. Bioinformatic Analysis of Kynurenine Pathway Enzymes and Their Relationship with Glioma Hallmarks. Metabolites 2022, 12, 1054. [Google Scholar] [CrossRef]
- Xu, Y.H.; Deng, J.L.; Wang, L.P.; Zhang, H.B.; Tang, L.; Huang, Y.; Tang, J.; Wang, S.; Wang, G. Identification of Candidate Genes Associated with Breast Cancer Prognosis. DNA Cell Biol. 2020, 39, 1205–1227. [Google Scholar] [CrossRef]
- Ullmark, T.; Montano, G.; Järvstråt, L.; Nilsson, H.J.; Håkansson, E.; Drott, K.; Nilsson, B.; Vidovic, K.; Gullberg, U. Anti-apoptotic Quinolinate Phosphoribosyltransferase (QPRT) Is a Target Gene of Wilms’ Tumor Gene 1 (WT1) Protein in Leukemic Cells. Biochem. Biophys. Res. Commun. 2017, 482, 802–807. [Google Scholar] [CrossRef]
- Sahm, F.; Oezen, I.; Opitz, C.A.; Radlwimmer, B.; von Deimling, A.; Ahrendt, T.; Adams, S.; Bode, H.B.; Guillemin, G.J.; Wick, W.; et al. The Endogenous Tryptophan Metabolite and NAD+ Precursor Quinolinic Acid Confers Resistance of Gliomas to Oxidative Stress. Cancer Res. 2013, 73, 3225–3334. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.T.; Yeh, K.C.; Lee, C.H.; Chen, Y.Y.; Ho, P.J.; Chang, K.Y.; Chen, C.; Lai, Y.; Chen, C. Molecular Profiling of Afatinib-resistant Non-small Cell Lung Cancer Cells In Vivo Derived from Mice. Pharmacol. Res. 2020, 161, 105183. [Google Scholar] [CrossRef] [PubMed]
- Jensen, N.F.; Stenvang, J.; Beck, M.K.; Hanáková, B.; Belling, K.C.; Do, K.N.; Viuff, B.; Nygård, S.B.; Gupta, R.; Rasmussen, M.F.; et al. Establishment and Characterization of Models of Chemotherapy Resistance in Colorectal Cancer: Towards a Predictive Signature of Chemoresistance. Mol. Oncol. 2015, 9, 1169–1185. [Google Scholar] [CrossRef]
- Rothhammer, V.; Quintana, F.J. The Aryl Hydrocarbon Receptor: An Environmental Sensor Integrating Immune Responses in Health and Disease. Nat. Rev. Immunol. 2019, 19, 184–197. [Google Scholar] [CrossRef]
- Cheong, J.E.; Sun, L. Targeting the IDO1/TDO2–KYN–AhR Pathway for Cancer Immunotherapy—Challenges and Opportunities. Trends Pharmacol. Sci. 2018, 39, 307–325. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Fu, Y.; Yin, Y.; Xu, K. Modulating AHR Function Offers Exciting Therapeutic Potential in Gut Immunity and Inflammation. Cell Biosci. 2023, 13, 85. [Google Scholar] [CrossRef]
- Chen, L.B.; Zhu, S.P.; Liu, T.P.; Zhao, H.; Chen, P.F.; Duan, Y.J.; Hu, R. Cancer Associated Fibroblasts Promote Renal Cancer Progression Through a TDO/Kyn/AhR Dependent Signaling Pathway. Front. Oncol. 2021, 11, 628821. [Google Scholar] [CrossRef]
- Fallarini, S.; Magliulo, L.; Paoletti, T.; de Lalla, C.; Lombardi, G. Expression of Functional GPR35 in Human iNKT Cells. Biochem. Biophys. Res. Commun. 2010, 398, 420–425. [Google Scholar] [CrossRef]
- Elizei, S.S.; Poormasjedi-Meibod, M.S.; Wang, X.; Kheirandish, M.; Ghahary, A. Kynurenic Acid Downregulates IL-17/1L-23 Axis In Vitro. Mol. Cell. Biochem. 2017, 431, 55–65. [Google Scholar] [CrossRef]
- Kaszaki, J.; Palásthy, Z.; Érczes, D.; Rácz, A.; Torday, C.; Varga, G.; Vécsei, L.; Boros, M. Kynurenic Acid Inhibits Intestinal Hypermotility and Xanthine Oxidase Activity During Experimental Colon Obstruction in Dogs. Neurogastroenterol. Motil. 2008, 20, 53–62. [Google Scholar] [CrossRef]
- Wang, J.; Simonavicius, N.; Wu, X.; Swaminath, G.; Reagan, J.; Tian, H.; Ling, L. Kynurenic Acid as a Ligand for Orphan G Protein-coupled Receptor GPR35. J. Biol. Chem. 2006, 281, 22021–22028. [Google Scholar] [CrossRef] [PubMed]
- Tankiewicz, A.; Dziemiańczyk, D.; Buczko, P.; Szarmach, I.J.; Grabowska, S.Z.; Pawlak, D. Tryptophan and Its Metabolites in Patients with Oral Squamous Cell Carcinoma: Preliminary Study. Adv. Med. Sci. 2006, 51, 221–224. [Google Scholar]
- Zdzisińska, B.; Wejksza, K.; Walter-Croneck, A.; Turski, W.A.; Kandefer-Szerszeń, M. Kynurenic Acid in Blood and Bone Marrow Plasma of Monoclonal Gammopathy of Undetermined Significance (MGUS) and Multiple Myeloma (MM) Patients. Leuk. Res. 2010, 34, 38–45. [Google Scholar] [CrossRef]
- Walczak, K.; Żurawska, M.; Kiś, J.; Starownik, R.; Zgrajka, W.; Bar, K.; Turski, W.; Rzeski, W. Kynurenic Acid in Human Renal Cell Carcinoma: Its Antiproliferative and Antimigrative Action on Caki-2 Cells. Amino Acids 2012, 43, 1663–1670. [Google Scholar] [CrossRef]
- Berthon, C.; Fontenay, M.; Corm, S.; Briche, I.; Allorge, D.; Hennart, B.; Lhermitte, M.; Quesnel, B. Metabolites of Tryptophan Catabolism are Elevated in Sera of Patients with Myelodysplastic Syndromes and Inhibit Hematopoietic Progenitor Amplification. Leuk. Res. 2013, 37, 573–579. [Google Scholar] [CrossRef]
- Fotopoulou, C.; Sehouli, J.; Pschowski, R.; Haehling, S.V.; Domanska, G.; Braicu, E.I.; Fusch, G.; Reinke, P.; Schefold, J.C. Systemic Changes of Tryptophan Catabolites via the Indoleamine-2,3-dioxygenase Pathway in Primary Cervical Cancer. Anticancer Res. 2011, 31, 2629–2635. [Google Scholar]
- Adams, S.; Teo, C.; McDonald, K.L.; Zinger, A.; Bustamante, S.; Lim, C.K.; Sundaram, G.; Braidy, N.; Brew, B.J.; Guillemin, G.J. Involvement of the Kynurenine Pathway in Human Glioma Pathophysiology. PLoS ONE 2014, 9, e112945. [Google Scholar] [CrossRef]
- Sagan, D.; Kocki, T.; Patel, S.; Kocki, J. Utility of Kynurenic Acid for Non-Invasive Detection of Metastatic Spread to Lymph Nodes in Non-Small Cell Lung Cancer. Int. J. Med. Sci. 2015, 12, 146–153. [Google Scholar] [CrossRef]
- Serio, C.D.; Cozzi, A.; Angeli, I.; Doria, L.; Micucci, I.; Pellerito, S.; Mirone, P.; Masotti, G.; Moroni, F.; Tarantini, F. Kynurenic Acid Inhibits the Release of the Neurotrophic Fibroblast Growth Factor (FGF)-1 and Enhances Proliferation of Glia Cells, in vitro. Cell Mol. Neurobiol. 2005, 25, 981–993. [Google Scholar] [CrossRef]
- Walczak, K.; Deneka-Hannemann, S.; Jarosz, B.; Zgrajka, W.; Stoma, F.; Trojanowski, T.; Turski, W.; Rzeski, W. Kynurenic Acid Inhibits Proliferation and Migration of Human Glioblastoma T98G cells. Pharmacol. Rep. 2014, 66, 130–136. [Google Scholar] [CrossRef]
- Dorta, E.; Aspée, A.; Pino, E.; González, L.; Lissi, E.; López-Alarcón, C. Controversial Alkoxyl and Peroxyl Radical Scavenging Activity of the Tryptophan Metabolite 3-hydroxyanthranilic acid. Biomed. Pharmacother. 2017, 90, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Gu, X.; Zheng, Q.; Shi, Q.; Yuan, X.; Chu, Q.; Jia, J.; Su, Y.; Bao, Z.; Lu, J.; et al. Effects of 3-HAA on HCC by Regulating the Heterogeneous Macrophages—A scRNA-Seq Analysis. Adv. Sci. 2023, 10, 2207074. [Google Scholar] [CrossRef] [PubMed]
- Morita, T.; Saito, K.; Takemura, M.; Maekawa, N.; Fujigaki, S.; Fujii, H.; Wada, H.; Takeuchi, S.; Noma, A.; Seishima, M. 3-Hydroxyanthranilic Acid, an L-tryptophan Metabolite, Induces Apoptosis in Monocyte-derived Cells Stimulated by Interferon-γ. Ann. Clin. Biochem. Int. J. Lab. Med. 2001, 38, 242–251. [Google Scholar] [CrossRef]
- Fallarino, F.; Grohmann, U.; Vacca, C.; Bianchi, R.; Orabona, C.; Spreca, A.; Fioretti, M.C.; Puccetti, P. T Cell Apoptosis by Tryptophan Catabolism. Cell Death Differ. 2002, 9, 1069–1077. [Google Scholar] [CrossRef]
- Hayashi, T.; Mo, J.H.; Gong, X.; Rossetto, C.; Jang, A.; Beck, L.; Elliott, G.I.; Kufareva, I.; Abagyan, R.; Broide, D.H.; et al. 3-Hydroxyanthranilic acid Inhibits PDK1 Activation and Suppresses Experimental Asthma by Inducing T cell Apoptosis. Proc. Natl. Acad. Sci. USA 2007, 104, 18619–18624. [Google Scholar] [CrossRef]
- Zaher, S.S.; Germain, C.; Fu, H.; Larkin, D.F.P.; George, A.J.T. 3-Hydroxykynurenine Suppresses CD4+ T-Cell Proliferation, Induces T-Regulatory-Cell Development, and Prolongs Corneal Allograft Survival. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2640–2648. [Google Scholar] [CrossRef]
- Thaker, A.I.; Rao, M.S.; Bishnupuri, K.S.; Kerr, T.A.; Foster, L.; Marinshaw, J.M.; Newberry, R.D.; Stenson, W.F.; Ciorba, M.A. IDO1 Metabolites Activate β-catenin Signaling to Promote Cancer Cell Proliferation and Colon Tumorigenesis in Mice. Gastroenterology 2013, 145, 416–425. [Google Scholar] [CrossRef]
- Frumento, G.; Rotondo, R.; Tonetti, M.; Damonte, G.; Benatti, U.; Ferrara, G.B. Tryptophan-derived Catabolites Are Responsible for Inhibition of T and Natural Killer Cell Proliferation Induced by Indoleamine 2,3-Dioxygenase. J. Exp. Med. 2002, 196, 459–468. [Google Scholar] [CrossRef]
- Abbas, A.H.; Mahmood, A.A.R.; Tahtamouni, L.H.; Al-Mazaydeh, Z.A.; Rammaha, M.S.; Alsoubani, F.; Al-bayati, R.I. A Novel Derivative of Picolinic Acid Induces Endoplasmic Reticulum Stress-mediated Apoptosis in Human Non-small Cell Lung Cancer Cells: Synthesis, Docking Study, and Anticancer Activity. Pharmacia 2021, 68, 679–692. [Google Scholar] [CrossRef]
- Grant, R.S.; Coggan, S.E.; Smythe, G.A. The Physiological Action of Picolinic Acid in the Human Brain. Int. J. Tryptophan Res. 2009, 2, 71–79. [Google Scholar] [CrossRef]
- Larussa, T.; Leone, I.; Suraci, E.; Nazionale, I.; Procopio, T.; Conforti, F.; Abenavoli, L.; Hribal, M.L.; Imeneo, M.; Luzza, F. Enhanced Expression of Indoleamine 2,3-Dioxygenase in Helicobacter pylori-Infected Human Gastric Mucosa Modulates Th1/Th2 Pathway and Interleukin 17 Production. Helicobacter 2015, 20, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Chen, B.; Yang, L.; Gao, W. KYNU as a Biomarker of Tumor-Associated Macrophages and Correlates with Immunosuppressive Microenvironment and Poor Prognosis in Gastric Cancer. Int. J. Genom. 2023, 2023, 4662480. [Google Scholar] [CrossRef] [PubMed]
- Mansorunov, D.; Apanovich, N.; Kipkeeva, F.; Nikulin, M.; Malikhova, O.; Stilidi, I.; Karpukhin, A. The Correlation of Ten Immune Checkpoint Gene Expressions and Their Association with Gastric Cancer Development. Int. J. Mol. Sci. 2022, 23, 13846. [Google Scholar] [CrossRef]
- Santhanam, S.; Alvarado, D.M.; Ciorba, M.A. Therapeutic Targeting of Inflammation and Tryptophan Metabolism in Colon and Gastrointestinal Cancer. Transl. Res. J. Lab. Clin. Med. 2016, 167, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Li, J.; Song, S.; Wang, J.; Cai, W.; Hu, W.; Ji, J.; Zhu, Z.; Zang, L.; Yan, R.; et al. A Positive Feedback Between IDO1 Metabolite and COL12A1 via MAPK Pathway to Promote Gastric Cancer Metastasis. J. Exp. Clin. Cancer Res. 2019, 38, 314. [Google Scholar] [CrossRef]
- Yan, J.; Chen, D.; Ye, Z.; Zhu, X.; Li, X.; Jiao, H.; Zhang, C.; Cheng, J.; Xu, L.; Li, H.; et al. Molecular Mechanisms and Therapeutic Significance of Tryptophan Metabolism and Signaling in Cancer. Mol. Cancer. 2024, 23, 241. [Google Scholar] [CrossRef]
- Liu, H.; Shen, Z.; Wang, Z.; Wang, X.; Zhang, H.; Qin, J.; Qin, X.; Xu, J.; Sun, Y. Increased Expression of IDO Associates with Poor Postoperative Clinical Outcome of Patients with Gastric Adenocarcinoma. Sci. Rep. 2016, 6, 21319. [Google Scholar] [CrossRef]
- Lu, S.; Wang, L.J.; Lombardo, K.; Kwak, Y.; Kim, W.H.; Resnick, M.B. Expression of Indoleamine 2, 3-dioxygenase 1 (IDO1) and Tryptophanyl-tRNA Synthetase (WARS) in Gastric Cancer Molecular Subtypes. Appl. Immunohistochem. Mol. Morphol. 2020, 28, 360–368. [Google Scholar] [CrossRef]
- Nishi, M.; Yoshikawa, K.; Higashijima, J.; Tokunaga, T.; Kashihara, H.; Takasu, C.; Ishikawa, D.; Wada, Y.; Shimada, M. The Impact of Indoleamine 2,3-dioxygenase (IDO) Expression on Stage III Gastric Cancer. Anticancer Res. 2018, 38, 3387–3392. [Google Scholar] [CrossRef]
- Li, F.; Sun, Y.; Huang, J.; Xu, W.; Liu, J.; Yuan, Z. CD4/CD8 + T cells, DC Subsets, Foxp3, and IDO Expression are Predictive Indictors of Gastric Cancer Prognosis. Cancer Med. 2019, 8, 7330–7344. [Google Scholar] [CrossRef]
- El-Zaatari, M.; Bass, A.J.; Bowlby, R.; Zhang, M.; Syu, L.J.; Yang, Y.; Grasberger, H.; Shreiner, A.; Tan, B.; Bishu, S.; et al. Indoleamine 2,3-Dioxygenase 1, Increased in Human Gastric Pre-Neoplasia, Promotes Inflammation and Metaplasia in Mice and Is Associated with Type II Hypersensitivity/Autoimmunity. Gastroenterology 2018, 154, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Zhai, X.Y. Role of Tryptophan Metabolism in Cancers and Therapeutic Implications. Biochimie 2021, 182, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, C.; Cui, X.; Liu, C.; Xiao, P.; Yang, W. Kynureninase Induce Cuproptosis Resistance in Gastric Cancer Progression Through Downregulating Lipotic Acid Synthetase Mediated Non-canonical Mechanism. Cell. Signal. 2025, 127, 111565. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Hu, C.; Wang, Y.; Shi, Y.; Yuan, L.; Xu, J.; Zhang, Y.; Chen, J.; Wei, Q.; Qin, J.; et al. Glutathione Peroxidase 2 Knockdown Suppresses Gastric Cancer Progression and Metastasis via Regulation of Kynurenine Metabolism. Oncogene 2023, 42, 1994–2006. [Google Scholar] [CrossRef]
- Pham, Q.T.; Taniyama, D.; Akabane, S.; Takashima, T.; Maruyama, R.; Sekino, Y.; Sentani, K.; Yasui, W.; Oue, N. Essential Roles of TDO2 in Gastric Cancer: TDO2 Is Associated with Cancer Progression, Patient Survival, PD-L1 Expression, and Cancer Stem Cells. Pathobiology 2023, 90, 44–55. [Google Scholar] [CrossRef]
- Kuligowski, J.; Sanjuan-Herráez, D.; Vázquez-Sánchez, M.A.; Brunet-Vega, A.; Pericay, C.; Ramírez-Lázaro, M.J.; Lario, A.; Gombau, L.; Junguera, F.; Calvet, X.; et al. Metabolomic Analysis of Gastric Cancer Progression within the Correa’s Cascade Using Ultraperformance Liquid Chromatography-Mass Spectrometry. J. Proteome Res. 2016, 15, 2729–2738. [Google Scholar] [CrossRef]
- Wu, D.; Wang, Z. Gastric Cancer Cell-Derived Kynurenines Hyperactive Regulatory T Cells to Promote Chemoresistance via the IL-10/STAT3/BCL2 Signaling Pathway. DNA Cell Biol. 2022, 41, 447–455. [Google Scholar] [CrossRef]
- Cui, J.X.; Xu, X.H.; He, T.; Liu, J.J.; Xie, T.Y.; Tian, W.; Liu, J. L-kynurenine Induces NK Cell Loss in Gastric Cancer Microenvironment via Promoting Ferroptosis. J. Exp. Clin. Cancer Res. 2023, 42, 52. [Google Scholar] [CrossRef]
- Liang, X.; Du, W.; Huang, L.; Xiang, L.; Pan, W.; Yang, F.; Zheng, F.; Xie, U.; Geng, L.; Gong, S.; et al. Helicobacter Pylori Promotes Gastric Intestinal Metaplasia Through Activation of IRF3-mediated Kynurenine Pathway. Cell Commun. Signal. 2023, 21, 141. [Google Scholar] [CrossRef]
| Biomarker | Type | Role in Carcinogenesis | Potential Clinical Value | Material for Analysis | Detection Method | Ref. |
|---|---|---|---|---|---|---|
| B7–H3 | Transmembrane protein | It is involved in cancer progression and metastasis and may inhibit CD8+ T cells | Predictive biomarker | Gastric tissue | IHC | [30] |
| CLDN 18.2 | Claudin family protein | It is involved in cancer cell proliferation, invasion, and metastasis | Prognostic and therapeutic biomarker | Gastric tissue | IHC | [46] |
| E-Cadherin | Transmembrane glycoprotein | It is involved in cancer cell proliferation, invasion, and metastasis. Reduces response to conventional and targeted therapy | Prognostic and predictive biomarker | Gastric tissue | IHC | [47,48] |
| EGFR | Growth factor receptor | Regulates cell proliferation, migration, survival, and tumor angiogenesis. Amplified or overexpressed in intestinal-type GC | Prognostic biomarker | Gastric tissue, serum exosomes | IHC | [9,30,49] |
| FGFR2 | Growth factor receptor | Its overexpression is associated with greater depth of tumor invasion, higher rates of lymph node metastasis, more advanced disease stage, and worse outcome. It is amplified or overexpressed in diffuse-type GC | Predictive biomarker | Tissue from distant metastases, primary tumors | IHC, NGS, ISH | [9,30,46,50] |
| GST-π | Enzyme | It is associated with tumor invasion and recurrence | Predictive biomarker | Gastric tissue | IHC | [51,52] |
| lncRNA | RNA molecule | Its expression regulates GC cell proliferation, cell cycle, apoptosis, invasion, migration, metastasis, and tumorigenicity | Diagnostic, predictive, and therapeutic biomarker. Promising biomarker for early diagnosis | Serum exosomes, plasma | qRT-PCR | [53,54] |
| MET | Hepatocyte growth factor receptor | Induces proliferation, survival, motility, cell scattering, angiogenesis, and tubulogenesis. Drives epithelial–mesenchymal transition and tumor invasion. Amplified or overexpressed in diffuse-type GC | Predictive and therapeutic biomarker | Gastric tissue | IHC, FISH, NGS | [9,30,46,55] |
| MRP2 | Protein | Involved in mechanisms mediating multidrug resistance | Predictive biomarker | Gastric tissue | IHC | [52] |
| mTOR | Kinase | Involved in cell growth, differentiation, and survival. Amplified or overexpressed in intestinal-type and diffuse-type GC | Therapeutic target | Gastric tissue | IHC | [56] |
| MUC1 | High molecular weight glycoprotein | Oncoprotein involved in tumor proliferation, metabolism, invasion, and metastasis | Prognostic biomarker | Gastric tissue | IHC | [57,58] |
| SOX9 | Transcription factor | Critical for GC cell survival and promotes cancer cell proliferation and chemoresistance. Its expression is related to tumor progression and associated with advanced stages, lymph node metastasis, and extra-capsular growth in lymph node metastasis | Prognostic biomarker | Gastric tissue | IHC | [9,59] |
| T53 | Nuclear protein | Supports cancer progression. Tumor suppressor | Prognostic and predictive biomarker | Gastric tissue | IHC | [48,60] |
| VEFGR | Growth factor receptor | Involved in angiogenesis and lymphangiogenesis | Prognostic, therapeutic, and predictive biomarker | Gastric tissue, serum | IHC | [46] |
| VISTA | Membrane protein | Suppresses T cell activation. Supports immune escape | Predictive biomarker | Gastric tissue | IHC | [30] |
| Enzyme | Localization | Activators | Inhibitors | Up-Regulation or Down-Regulation in Disorders | Ref. |
|---|---|---|---|---|---|
| IDO1 | Placenta, colon, epididymis, dendritic cells, macrophages, reticular cells, cancer cells, innate immune cells, stromal cells, brain, kidney, lung, spleen | IFN-γ, IFN-α, TNF-α, IL-1, IL-12, IL-18, IL-6, IL-10 | Nitric oxide, TRP excess (>50 µM) | Cervical carcinomas, endometrial carcinomas, bladder carcinomas, kidney carcinomas, non-small cell lung carcinomas, ovarian carcinomas, melanomas, stomach carcinomas, colorectal carcinomas, head and neck carcinomas, esophageal carcinomas, prostate carcinomas, breast carcinomas, pancreatic carcinomas, glioblastomas | [18,64,65,66,67,68,69] |
| IDO2 | Brain, liver, kidney, epididymis, dendritic cells, B-cells, placenta, epididymis | IFN-γ, IL-10, PGE2, lipopolysaccharide | 1-methyl-DL-tryptophan | Non-small cell lung cancer, pancreatic cancer, colon cancer, gastric cancer, renal tumors | [64,65,70,71,72] |
| TDO | Liver, brain kidney, skin, placenta, pregnant uterus, epididymis, testis | Glucocorticoids, TRP substrates, estrogens, heme cofactor | NAD(P)H-mediated feedback mechanism, progesterone, estrogens, IFN-γ | Bladder carcinoma, brain tumor, breast carcinoma, cervix carcinoma, colorectal carcinoma, Ewing sarcoma, head and neck carcinoma, hepatocarcinoma, leukemia, lung, carcinoma, melanoma, mesothelioma, B-cell lymphoma, neuroblastoma, ovarian carcinoma, renal cell carcinoma, sarcoma | [14,19,51,62,73,74] |
| NFK | Liver, kidney, brain | o-aminophenol, o-aminotoluidine | Organophosphate, insecticides, metal cations | Colon cancer | [16,73,75,76] |
| KMO | The outer membrane of mitochondria, liver, kidney, macrophages and monocytes, central nervous system, placenta | Inflammatory cytokines, IFN-γ, ROS | 4-aryl-4-oxobutanoic acids, sulfonamides, 6-phenylpyrimidines, phenyloxadiazoles, riboflavin, excess of TRP, AA, XA | Schizophrenia, infectious diseases, renal clear cell carcinoma, lower-grade brain glioma, acute myeloid leukemia, TNBC, hepatocellular carcinoma | [16,63,73,75,77,78,79,80] |
| KYNU | Liver, kidney | IFN-γ | Vitamin B6 deficiency, leucine-rich diets | Chronic inflammatory skin diseases, many autoimmune and autoinflammatory diseases, renal papillary cell carcinoma, ovarian serous cyst adenocarcinoma, pancreatic adenocarcinoma, lung adenocarcinoma, lower-grade brain glioma, renal papillary cell carcinoma, acute myeloid leukemia | [14,16,63,73] |
| KAT | Kidney, placenta, heart, macrophages, liver, brain | KYN, 2-oxoglutarate, pyruvate, oxaloacetic acid | Vitamin B6 deficiency, IFN-γ | Schizophrenia | [16,73,74,77] |
| 3HAAO | Liver, kidney, brain | IFN-γ, Fe2+ | Iron chelators | Neurological disorders, esophageal carcinoma | [16,63,72] |
| ACMSD | Kidney, liver | Leucine, Fe2+, Co2+ | QA, PIC, KYNA, glycolytic intermediates: glyceraldehyde-3-phosphate, 3-phosphoglycerate, phosphoenolpyruvate, 2-phosphoglycerate, some metal ions: Zn2+, Fe3+, Cr3+, Cd2+ | Brain tumors, neuroinflammatory diseases | [16,75,81,82] |
| QPRT | Liver, kidney, brain | - | Phthalic acid, O2, pyridine analogs of QA, lysine, histidine, arginine, some cations (Cu2+, Fe2+, Fe3+), various carboxylic acids, IFN-γ | Inflammatory diseases, glioma, breast cancer, stomach adenocarcinoma, cutaneous melanoma, glioblastoma, colon cancer | [16,73,75,83,84,85,86] |
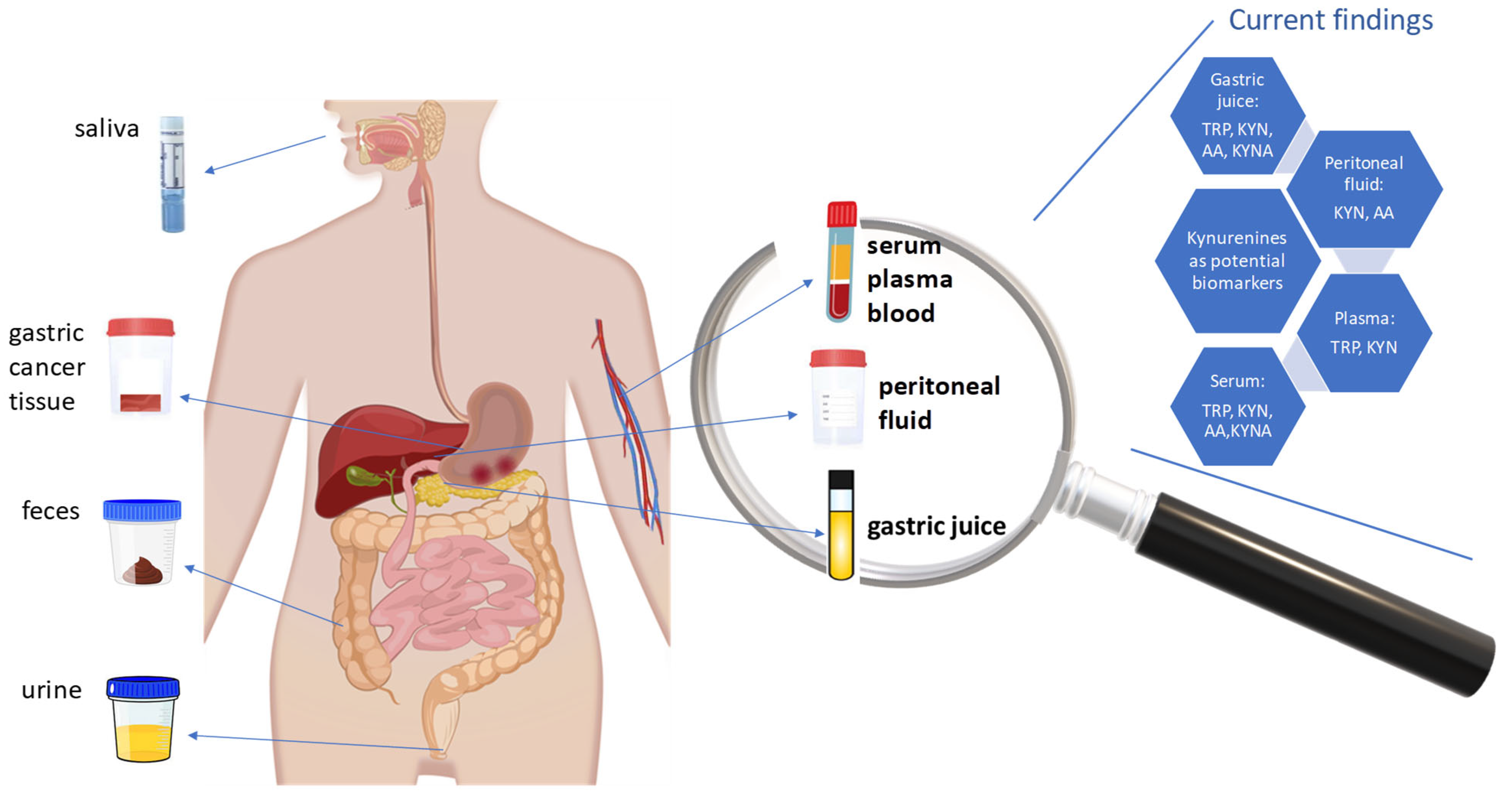
| Metabolite | Sample Type | Major Findings | Ref. |
|---|---|---|---|
| TRP, KYN | Plasma | Statistically significant lower relative level of TRP in samples from patients with GC compared to H. pylori–negative patients with NAG-, H. pylori–positive patients with CAG+, and patients having precursor lesions of GC (atrophy and/or intestinal metaplasia, PLGC). Slightly higher relative concentration of KYN in plasma from patients with GC than in NAG-, CAG+, and PLGC samples (not statistically significant differences). | [150] |
| TRP, KYN | Serum | Significant decrease in TRP level and not statistically significant increase in KYN level in serum from patients with GC than in controls without malignant disease. Significant increase in [KYN]/[TRP] ratio in serum from GC patients. In the H. pylori–seronegative subgroup, there were no significant differences in serum KYN and [KYN]/[TRP] ratio, and a significant decrease in serum TRP levels, between cancer-free individuals and patients with GC. In the H. pylori–seropositive subgroup, there was a significant increase in serum [KYN]/[TRP] ratio and a decrease in TRP serum levels in patients with GC compared to controls. Significant correlation between serum neopterin levels and [KYN]/[TRP] ratio in H. pylori–seronegative and –seropositive controls and GC individuals. | [24] |
| TRP, KYN, AA, KYNA | Serum, gastric juice | Statistically significant increase in AA and KYNA levels, and a significant decrease in KYN levels, in serum from the GC group compared with the controls. Comparable serum TRP levels between the studied groups. Statistically significant increase in TRP, AA, and KYNA levels in gastric juice from patients with GC compared with the control group. The KYN level also increases, but with no statistical significance. | [21] |
| KYN, AA | Serum, peritoneal fluid, lavage washings | Significantly higher AA levels in peritoneal lavage washings in patients with pN1-3 compared to those with pN0. Positive correlations between AA level in peritoneal fluid with pN stage and between AA level in peritoneal lavage washings with cT stage. Significantly higher KYN levels in peritoneal lavage washings in cM1 patients than in cM0 patients. Positive correlation between KYN level with cM (peritoneum) stage. Positive correlation of KYN level with cT and negative correlation of 3HKyn and XA levels with cM in patients with GC. | [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ożga, K.; Stepuch, P.; Maciejewski, R.; Sadok, I. Promising Gastric Cancer Biomarkers—Focus on Tryptophan Metabolism via the Kynurenine Pathway. Int. J. Mol. Sci. 2025, 26, 3706. https://doi.org/10.3390/ijms26083706
Ożga K, Stepuch P, Maciejewski R, Sadok I. Promising Gastric Cancer Biomarkers—Focus on Tryptophan Metabolism via the Kynurenine Pathway. International Journal of Molecular Sciences. 2025; 26(8):3706. https://doi.org/10.3390/ijms26083706
Chicago/Turabian StyleOżga, Kinga, Paweł Stepuch, Ryszard Maciejewski, and Ilona Sadok. 2025. "Promising Gastric Cancer Biomarkers—Focus on Tryptophan Metabolism via the Kynurenine Pathway" International Journal of Molecular Sciences 26, no. 8: 3706. https://doi.org/10.3390/ijms26083706
APA StyleOżga, K., Stepuch, P., Maciejewski, R., & Sadok, I. (2025). Promising Gastric Cancer Biomarkers—Focus on Tryptophan Metabolism via the Kynurenine Pathway. International Journal of Molecular Sciences, 26(8), 3706. https://doi.org/10.3390/ijms26083706








