Construction of Simple Sequence Repeat-Based Genetic Linkage Map and Identification of QTLs for Accumulation of Floral Volatiles in Lavender (Lavandula angustifolia Mill.)
Abstract
1. Introduction
2. Results and Discussion
2.1. Development of an F2 Segregating Population by Self-Pollination of L. angustifolia var. Hemus
2.2. SSR Marker Development
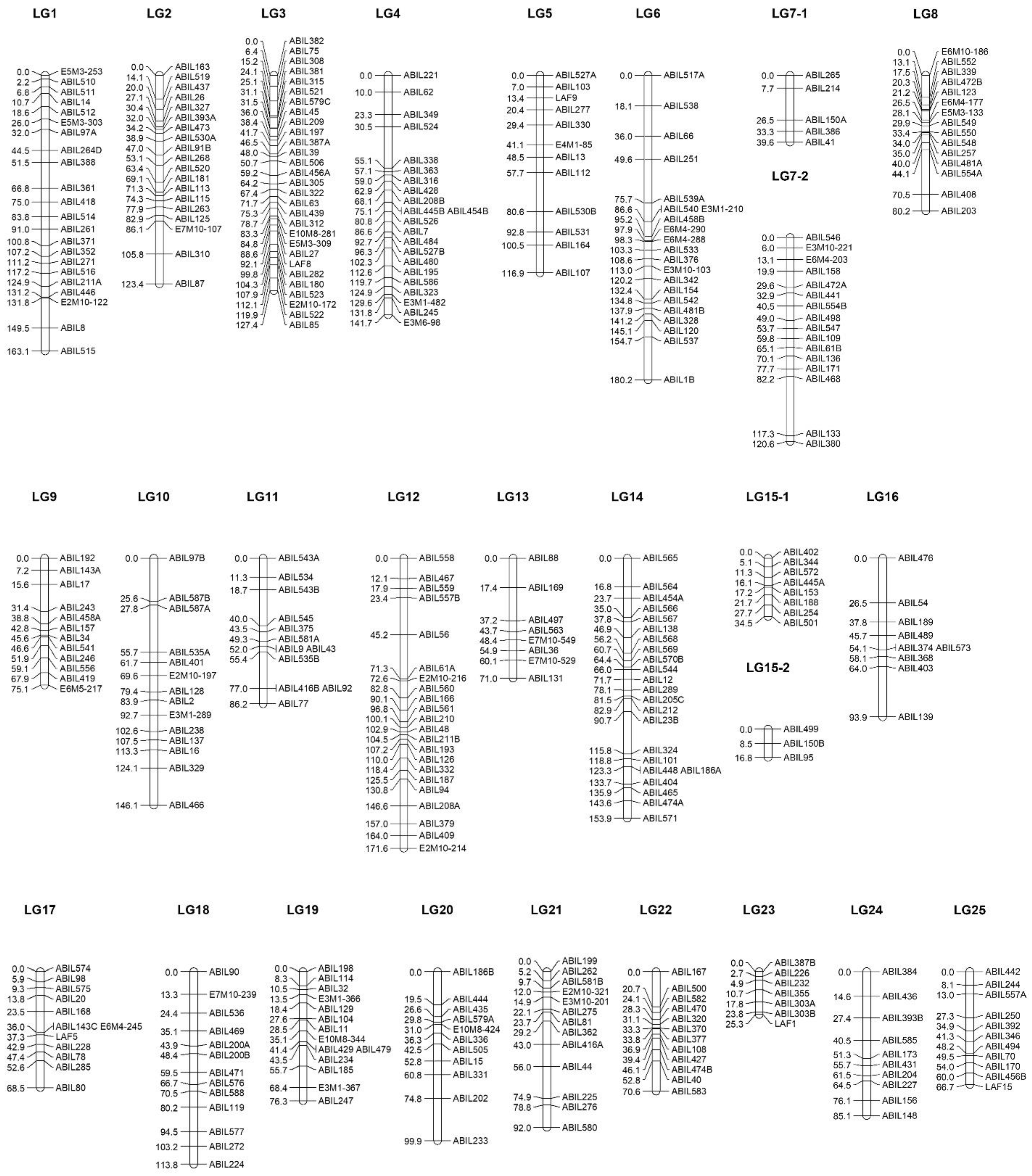
2.3. Linkage Map Construction
2.4. SSR Marker Transferability
2.5. Variations of Volatile Contents in the Flowers of Plants from the Segregating Population
2.6. Mapping of QTLs Related to the Accumulation of Flower Volatiles in L. angustifolia var. Hemus
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Genomic DNA Isolation
4.3. Next Generation Sequencing, SSR Identification, and Primer Design
4.4. SSR Identification After Search of Reference Genome Sequence and Primer Design
4.5. PCR Amplification of SSR Regions
4.6. PCR Amplification of SSR Regions with Tailed Primers
4.7. PCR Amplification of SRAP Fragments
4.8. SSR and SRAP Fragment Analysis
4.9. Linkage Analysis and Genetic Map Construction
4.10. Sample Preparation and GC/MS Analysis of Floral Volatiles
4.11. QTL Mapping
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SSR | simple sequence repeat |
| SRAP | sequence-related amplified polymorphism |
| QTL | quantitative trait locus |
| GC/MS | gas chromatography/mass spectrometry |
References
- Cáceres-Cevallos, G.J.; Quílez, M.; Ortiz de Elguea-Culebras, G.; Melero-Bravo, E.; Sánchez-Vioque, R.; Jordán, M.J. Agronomic Evaluation and Chemical Characterization of Lavandula latifolia Medik. under the Semiarid Conditions of the Spanish Southeast. Plants 2023, 12, 1986. [Google Scholar] [CrossRef] [PubMed]
- Gallotte, P.; Fremondière, G.; Gallois, P.; Bernier, J.-P.B.; Buchwalder, A.; Walton, A.; Piasentin, J.; Fopa-Fomeju, B. Lavandula angustifolia Mill. and Lavandula × Intermedia Emeric Ex Loisel: Lavender and Lavandin. In Medicinal, Aromatic and Stimulant Plants; Springer: Berlin/Heidelberg, Germany, 2020; pp. 303–311. [Google Scholar]
- Giray, F.H. An analysis of world lavender oil markets and lessons for Turkey. J. Essent. Oil Bear. Plants 2018, 21, 1612–1623. [Google Scholar] [CrossRef]
- Lis-Balchin, M. Lavender: The Genus Lavandula; CRC Press: London, UK, 2002. [Google Scholar]
- Pokajewicz, K.; Czarniecka-Wiera, M.; Krajewska, A.; Maciejczyk, E.; Wieczorek, P.P. Lavandula × intermedia—A Bastard lavender or a plant of many values? Part I. Biology and chemical composition of lavandin. Molecules 2023, 28, 2943. [Google Scholar] [CrossRef]
- Crișan, I.; Ona, A.; Vârban, D.; Muntean, L.; Vârban, R.; Stoie, A.; Mihăiescu, T.; Morea, A. Current trends for lavender (Lavandula angustifolia Mill.) crops and products with emphasis on essential oil quality. Plants 2023, 12, 357. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Gille, E.; Trifan, A.; Luca, V.S.; Miron, A. Essential oils of Lavandula genus: A systematic review of their chemistry. Phytochem. Rev. 2017, 16, 761–799. [Google Scholar] [CrossRef]
- Diass, K.; Merzouki, M.; Elfazazi, K.; Azzouzi, H.; Challioui, A.; Azzaoui, K.; Hammouti, B.; Touzani, R.; Depeint, F.; Ayerdi Gotor, A. Essential Oil of Lavandula officinalis: Chemical Composition and Antibacterial Activities. Plants 2023, 12, 1571. [Google Scholar] [CrossRef]
- Malloggi, E.; Menicucci, D.; Cesari, V.; Frumento, S.; Gemignani, A.; Bertoli, A. Lavender aromatherapy: A systematic review from essential oil quality and administration methods to cognitive enhancing effects. Appl. Psychol. Health Well-Being 2022, 14, 663–690. [Google Scholar] [CrossRef]
- Wells, R.; Truong, F.; Adal, A.M.; Sarker, L.S.; Mahmoud, S.S. Lavandula essential oils: A current review of applications in medicinal, food, and cosmetic industries of lavender. Nat. Prod. Commun. 2018, 13, 1934578X1801301038. [Google Scholar] [CrossRef]
- Stanev, S.; Zagorcheva, T.; Atanassov, I. Lavender cultivation in Bulgaria-21 st century developments, breeding challenges and opportunities. Bulg. J. Agric. Sci. 2016, 22, 584–590. [Google Scholar]
- Détár, E.; Németh, É.Z.; Gosztola, B.; Demján, I.; Pluhár, Z. Effects of variety and growth year on the essential oil properties of lavender (Lavandula angustifolia Mill.) and lavandin (Lavandula × intermedia Emeric ex Loisel.). Biochem. Syst. Ecol. 2020, 90, 104020. [Google Scholar] [CrossRef]
- Kiprovski, B.; Zeremski, T.; Varga, A.; Čabarkapa, I.; Filipović, J.; Lončar, B.; Aćimović, M. Essential oil quality of lavender grown outside its native distribution range: A study from Serbia. Horticulturae 2023, 9, 816. [Google Scholar] [CrossRef]
- Vijulie, I.; Lequeux-Dincă, A.-I.; Preda, M.; Mareci, A.; Matei, E. Could Lavender Farming Go from a Niche Crop to a Suitable Solution for Romanian Small Farms? Land 2022, 11, 662. [Google Scholar] [CrossRef]
- Despinasse, Y.; Moja, S.; Soler, C.; Jullien, F.; Pasquier, B.; Bessière, J.-M.; Baudino, S.; Nicolè, F. Structure of the chemical and genetic diversity of the true lavender over its natural range. Plants 2020, 9, 1640. [Google Scholar] [CrossRef] [PubMed]
- Dobreva, A.; Petkova, N.; Todorova, M.; Gerdzhikova, M.; Zherkova, Z.; Grozeva, N. Organic vs. Conventional Farming of Lavender: Effect on Yield, Phytochemicals and Essential Oil Composition. Agronomy 2023, 14, 32. [Google Scholar] [CrossRef]
- Jug-Dujaković, M.; Ninčević Runjić, T.; Grdiša, M.; Liber, Z.; Šatović, Z. Intra-and Inter-Cultivar Variability of Lavandin (Lavandula × intermedia Emeric ex Loisel.) Landraces from the Island of Hvar, Croatia. Agronomy 2022, 12, 1864. [Google Scholar] [CrossRef]
- Perović, A.B.; Karabegović, I.T.; Krstić, M.S.; Veličković, A.V.; Avramović, J.M.; Danilović, B.R.; Veljković, V.B. Novel hydrodistillation and steam distillation methods of essential oil recovery from lavender: A comprehensive review. Ind. Crops Prod. 2024, 211, 118244. [Google Scholar] [CrossRef]
- Ashraf, A.; Sultan, P.; Qazi, P.; Rasool, S. Approaches for the genetic improvement of Lavender: A short review. J. Pharmacogn. Phytochem. 2019, 8, 736–740. [Google Scholar]
- Sharan, H.; Pandey, P.; Singh, S. Genetic Resources and Breeding Strategies for Lavender (Lavandula angustifolia Mill.). In Ethnopharmacology and OMICS Advances in Medicinal Plants Volume 2: Revealing the Secrets of Medicinal Plants; Nandave, M., Joshi, R., Upadhyay, J., Eds.; Springer Nature: Singapore, 2024; pp. 33–54. [Google Scholar]
- Adal, A.M.; Mahmoud, S.S. Short-chain isoprenyl diphosphate synthases of lavender (Lavandula). Plant Mol. Biol. 2020, 102, 517–535. [Google Scholar] [CrossRef]
- Adal, A.M.; Najafianashrafi, E.; Sarker, L.S.; Mahmoud, S.S. Cloning, functional characterization and evaluating potential in metabolic engineering for lavender (+)-bornyl diphosphate synthase. Plant Mol. Biol. 2023, 111, 117–130. [Google Scholar] [CrossRef]
- Adal, A.M.; Sarker, L.S.; Malli, R.P.; Liang, P.; Mahmoud, S.S. RNA-Seq in the discovery of a sparsely expressed scent-determining monoterpene synthase in lavender (Lavandula). Planta 2019, 249, 271–290. [Google Scholar] [CrossRef]
- Demissie, Z.A.; Sarker, L.S.; Mahmoud, S.S. Cloning and functional characterization of β-phellandrene synthase from Lavandula angustifolia. Planta 2011, 233, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Despinasse, Y.; Fiorucci, S.; Antonczak, S.; Moja, S.; Bony, A.; Nicolè, F.; Baudino, S.; Magnard, J.-L.; Jullien, F. Bornyl-diphosphate synthase from Lavandula angustifolia: A major monoterpene synthase involved in essential oil quality. Phytochemistry 2017, 137, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Guitton, Y.; Nicolè, F.; Moja, S.; Valot, N.; Legrand, S.; Jullien, F.; Legendre, L. Differential accumulation of volatile terpene and terpene synthase mRNAs during lavender (Lavandula angustifolia and L. × intermedia) inflorescence development. Physiol. Plant. 2010, 138, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhang, W.; Li, J.; Wang, D.; Bai, H.; Li, H.; Shi, L. The transcription factor LaMYC4 from lavender regulates volatile Terpenoid biosynthesis. BMC Plant Biol. 2022, 22, 289. [Google Scholar] [CrossRef]
- Sarker, L.S.; Adal, A.M.; Mahmoud, S.S. Diverse transcription factors control monoterpene synthase expression in lavender (Lavandula). Planta 2020, 251, 1–5. [Google Scholar] [CrossRef]
- Cheng, S.; Zhang, X.; Mu, H.; Wang, J.; Jiang, Y.; Li, X.; Wen, Y.; Ma, Q.; Guo, S. Transcriptome analysis unravels differential genes involved in essential oil content in callus and tissue culture seedlings of Lavandula angustifolia. Biotechnol. Biotechnol. Equip. 2024, 38, 2367097. [Google Scholar] [CrossRef]
- Guo, D.; Kang, K.; Wang, P.; Li, M.; Huang, X. Transcriptome profiling of spike provides expression features of genes related to terpene biosynthesis in lavender. Sci. Rep. 2020, 10, 6933. [Google Scholar] [CrossRef]
- Lane, A.; Boecklemann, A.; Woronuk, G.N.; Sarker, L.; Mahmoud, S.S. A genomics resource for investigating regulation of essential oil production in Lavandula angustifolia. Planta 2010, 231, 835–845. [Google Scholar] [CrossRef]
- Kelimujiang, K.; Zhang, W.; Zhang, X.; Waili, A.; Tang, X.; Chen, Y.; Chen, L. Genome-wide investigation of WRKY gene family in Lavandula angustifolia and potential role of LaWRKY57 and LaWRKY75 in the regulation of terpenoid biosynthesis. Front. Plant Sci. 2024, 15, 1449299. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Dong, Y.; Hao, H.; Ling, Z.; Bai, H.; Wang, H.; Cui, H.; Shi, L. Time-series transcriptome provides insights into the gene regulation network involved in the volatile terpenoid metabolism during the flower development of lavender. BMC Plant Biol. 2019, 19, 313. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.; Dong, Y.; Huang, Y.; Qi, Y.; Bai, H.; Li, H.; Shi, L. Genome-wide identification and expression of BAHD acyltransferase gene family shed novel insights into the regulation of linalyl acetate and lavandulyl acetate in lavender. J. Plant Physiol. 2024, 292, 154143. [Google Scholar] [CrossRef] [PubMed]
- Malli, R.P.; Adal, A.M.; Sarker, L.S.; Liang, P.; Mahmoud, S.S. De novo sequencing of the Lavandula angustifolia genome reveals highly duplicated and optimized features for essential oil production. Planta 2019, 249, 251–256. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Dong, Y.; Zhang, W.; Wang, D.; Bai, H.; Li, K.; Li, H.; Shi, L. The chromosome-based lavender genome provides new insights into Lamiaceae evolution and terpenoid biosynthesis. Hortic. Res. 2021, 8, 90. [Google Scholar] [CrossRef]
- Hamilton, J.P.; Vaillancourt, B.; Wood, J.C.; Wang, H.; Jiang, J.; Soltis, D.E.; Buell, C.R.; Soltis, P.S. Chromosome-scale genome assembly of the ‘Munstead’ cultivar of Lavandula angustifolia. BMC Genom. Data 2023, 24, 75. [Google Scholar] [CrossRef]
- Fontez, M.; Bony, A.; Nicole, F.; Moja, S.; Jullien, F. Lavandula angustifolia Mill. a model of aromatic and medicinal plant to study volatile organic compounds synthesis, evolution and ecological functions. Bot. Lett. 2023, 170, 65–76. [Google Scholar] [CrossRef]
- Guitton, Y.; Nicolè, F.; Moja, S.; Benabdelkader, T.; Valot, N.; Legrand, S.; Jullien, F.; Legendre, L. Lavender inflorescence: A model to study regulation of terpenes synthesis. Plant Signal. Behav. 2010, 5, 749–751. [Google Scholar] [CrossRef]
- Habán, M.; Korczyk-Szabó, J.; Čerteková, S.; Ražná, K. Lavandula Species, Their Bioactive Phytochemicals, and Their Biosynthetic Regulation. Int. J. Mol. Sci. 2023, 24, 8831. [Google Scholar] [CrossRef]
- Adal, A.M.; Demissie, Z.A.; Mahmoud, S.S. Identification, validation and cross-species transferability of novel Lavandula EST-SSRs. Planta 2015, 241, 987–1004. [Google Scholar] [CrossRef]
- Zagorcheva, T.; Stanev, S.; Rusanov, K.; Atanassov, I. SRAP markers for genetic diversity assessment of lavender (Lavandula angustifolia mill.) varieties and breeding lines. Biotechnol. Biotechnol. Equip. 2020, 34, 303–308. [Google Scholar] [CrossRef]
- Ražná, K.; Čerteková, S.; Štefúnová, V.; Habán, M.; Korczyk-Szabó, J.; Ernstová, M. Lavandula spp. diversity assessment by molecular markers as a tool for growers. Agrobiodivers. Improv.Nutr. Health Life Qual. 2023, 7, 27–39. [Google Scholar] [CrossRef]
- Chahota, R.K.; Katoch, M.; Sharma, P.K.; Thakur, S.R. QTLs and Gene Tagging in Crop Plants. In Agricultural Biotechnology: Latest Research and Trends; Kumar Srivastava, D., Kumar Thakur, A., Kumar, P., Eds.; Springer Nature: Singapore, 2021; pp. 537–552. [Google Scholar]
- Chaturvedi, T.; Gupta, A.K.; Lal, R.K.; Tiwari, G. March of molecular breeding techniques in the genetic enhancement of herbal medicinal plants: Present and future prospects. Nucleus 2022, 65, 413–436. [Google Scholar] [CrossRef]
- Stanev, S. Evaluation of the stability and adaptability of the Bulgarian lavender (Lavandula angustifolia Mill.) sorts yield. Agric. Sci. Technol. 2010, 2, 121–123. [Google Scholar]
- Valchev, H.; Kolev, Z.; Stoykova, B.; Kozuharova, E. Pollinators of Lavandula angustifolia Mill. an important factor for optimal production of lavender essential oil. BioRisk 2022, 17, 297–307. [Google Scholar] [CrossRef]
- Alekseeva, M.E.; Rusanova, M.G.; Georgieva, L.N.; Rusanov, K.E.; Atanassov, I.I. High cross-pollination rate of Greek oregano (O. vulgare ssp. hirtum) with Common oregano (O. vulgare ssp. vulgare) under open field conditions as revealed by microsatellite marker analysis. Biotechnol. Biotechnol. Equip. 2023, 37, 2279636. [Google Scholar] [CrossRef]
- Valchev, H.; Kozuharova, E. In situ and ex situ investigations on breeding systems and pollination of Sideritis scardica Griseb.(Lamiaceae) in Bulgaria. Proc. Bulg. Acad. Sci. 2022, 75, 527–535. [Google Scholar] [CrossRef]
- Alekseeva, M.; Rusanova, M.; Rusanov, K.; Atanassov, I. A set of highly polymorphic microsatellite markers for genetic diversity studies in the genus Origanum. Plants 2023, 12, 824. [Google Scholar] [CrossRef]
- Manco, R.; Chiaiese, P.; Basile, B.; Corrado, G. Comparative analysis of genomic-and EST-SSRs in European plum (Prunus domestica L.): Implications for the diversity analysis of polyploids. 3 Biotech 2020, 10, 543. [Google Scholar] [CrossRef]
- Garavello, M.; Cuenca, J.; Dreissig, S.; Fuchs, J.; Navarro, L.; Houben, A.; Aleza, P. Analysis of crossover events and allele segregation distortion in interspecific citrus hybrids by single pollen genotyping. Front. Plant Sci. 2020, 11, 615. [Google Scholar] [CrossRef]
- Ma, Z.; Gao, W.; Liu, L.; Liu, M.; Zhao, N.; Han, M.; Wang, Z.; Jiao, W.; Gao, Z.; Hu, Y. Identification of QTL for resistance to root rot in sweetpotato (Ipomoea batatas (L.) Lam) with SSR linkage maps. BMC Genom. 2020, 21, 366. [Google Scholar] [CrossRef]
- Martin, G.; Baurens, F.C.; Hervouet, C.; Salmon, F.; Delos, J.M.; Labadie, K.; Perdereau, A.; Mournet, P.; Blois, L.; Dupouy, M. Chromosome reciprocal translocations have accompanied subspecies evolution in bananas. Plant J. 2020, 104, 1698–1711. [Google Scholar] [CrossRef]
- Mittelsten Scheid, O. Mendelian and non-Mendelian genetics in model plants. Plant Cell 2022, 34, 2455–2461. [Google Scholar] [CrossRef] [PubMed]
- Yoosefzadeh Najafabadi, M.; Hesami, M.; Rajcan, I. Unveiling the mysteries of non-mendelian heredity in plant breeding. Plants 2023, 12, 1956. [Google Scholar] [CrossRef] [PubMed]
- Canaguier, A.; Grimplet, J.; Di Gaspero, G.; Scalabrin, S.; Duchêne, E.; Choisne, N.; Mohellibi, N.; Guichard, C.; Rombauts, S.; Le Clainche, I. A new version of the grapevine reference genome assembly (12X. v2) and of its annotation (VCost. v3). Genom. Data 2017, 14, 56. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Chow, W.; Collins, J.; Pelan, S.; Pointon, D.-L.; Sims, Y.; Torrance, J.; Tracey, A.; Wood, J. Significantly improving the quality of genome assemblies through curation. GigaScience 2021, 10, giaa153. [Google Scholar] [CrossRef]
- Li, S.; Yang, G.; Yang, S.; Just, J.; Yan, H.; Zhou, N.; Jian, H.; Wang, Q.; Chen, M.; Qiu, X.; et al. The development of a high-density genetic map significantly improves the quality of reference genome assemblies for rose. Sci. Rep. 2019, 9, 5985. [Google Scholar] [CrossRef]
- Anilkumar, C.; Sunitha, N.; Harikrishna; Devate, N.B.; Ramesh, S. Advances in integrated genomic selection for rapid genetic gain in crop improvement: A review. Planta 2022, 256, 87. [Google Scholar] [CrossRef]
- Liu, Z.; Bernard, A.; Wang, Y.; Dirlewanger, E.; Wang, X. Genomes and integrative genomic insights into the genetic architecture of main agronomic traits in the edible cherries. Hortic. Res. 2025, 12, uhae269. [Google Scholar] [CrossRef]
- Vervalle, J.A.; Costantini, L.; Lorenzi, S.; Pindo, M.; Mora, R.; Bolognesi, G.; Marini, M.; Lashbrooke, J.G.; Tobutt, K.R.; Vivier, M.A. A high-density integrated map for grapevine based on three mapping populations genotyped by the Vitis 18K SNP chip. Theor. Appl. Genet. 2022, 135, 4371–4390. [Google Scholar] [CrossRef]
- Seira, E.; Poulaki, S.; Hassiotis, C.; Poulios, S.; Vlachonasios, K.E. Gene Expression of Monoterpene Synthases Is Affected Rhythmically during the Day in Lavandula angustifolia Flowers. Physiologia 2023, 3, 433–441. [Google Scholar] [CrossRef]
- Yildirim, M.U.; Sarihan, E.O.; Kul, H.; Khawar, K.M. Diurnal and nocturnal variability of essential oil content and components of Lavandula angustifolia Mill.(Lavender). Mustafa Kemal Univ. J. Agric. Sci./Mustafa Kemal Üniversitesi Tarım Bilim. Derg. 2019, 24, 268–278. [Google Scholar]
- Zagorcheva, T.; Rusanov, K.; Stanev, S.; Atanassov, I. A simple procedure for comparative GC-MS analysis of lavender (Lavandula angustifolia Mill.) flower volatile composition. IOSR J. Pharm. Biol. Sci. 2016, 11, 9–14. [Google Scholar] [CrossRef]
- Rusanov, K.; Vassileva, P.; Rusanova, M.; Atanassov, I. Identification of QTL controlling the ratio of linalool to linalyl acetate in the flowers of Lavandula angustifolia Mill var. Hemus. Biotechnol. Biotechnol. Equip. 2023, 37, 2288929. [Google Scholar] [CrossRef]
- Li, J.; Li, H.; Wang, Y.; Zhang, W.; Wang, D.; Dong, Y.; Ling, Z.; Bai, H.; Jin, X.; Hu, X. Decoupling subgenomes within hybrid lavandin provide new insights into speciation and monoterpenoid diversification of Lavandula. Plant Biotechnol. J. 2023, 21, 2084. [Google Scholar] [CrossRef] [PubMed]
- Landmann, C.; Fink, B.; Festner, M.; Dregus, M.; Engel, K.-H.; Schwab, W. Cloning and functional characterization of three terpene synthases from lavender (Lavandula angustifolia). Arch. Biochem. Biophys. 2007, 465, 417–429. [Google Scholar] [CrossRef]
- Jullien, F.; Moja, S.; Bony, A.; Legrand, S.; Petit, C.; Benabdelkader, T.; Poirot, K.; Fiorucci, S.; Guitton, Y.; Nicolè, F. Isolation and functional characterization of a τ-cadinol synthase, a new sesquiterpene synthase from Lavandula angustifolia. Plant Mol. Biol. 2014, 84, 227–241. [Google Scholar] [CrossRef]
- Sarker, L.S.; Mahmoud, S.S. Cloning and functional characterization of two monoterpene acetyltransferases from glandular trichomes of L. × intermedia. Planta 2015, 242, 709–719. [Google Scholar] [CrossRef]
- Adal, A.M.; Sarker, L.S.; Lemke, A.D.; Mahmoud, S.S. Isolation and functional characterization of a methyl jasmonate-responsive 3-carene synthase from Lavandula x intermedia. Plant Mol. Biol. 2017, 93, 641–657. [Google Scholar] [CrossRef]
- Murray, M.; Thompson, W. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4326. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 23 August 2023).
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Benson, G. Tandem repeats finder: A program to analyze DNA sequences. Nucleic Acids Res. 1999, 27, 573–580. [Google Scholar] [CrossRef]
- Koressaar, T.; Remm, M. Enhancements and modifications of primer design program Primer3. Bioinformatics 2007, 23, 1289–1291. [Google Scholar] [CrossRef] [PubMed]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, N.A.; Cox, E.; Holmes, J.B.; Anderson, W.R.; Falk, R.; Hem, V.; Tsuchiya, M.T.; Schuler, G.D.; Zhang, X.; Torcivia, J. Exploring and retrieving sequence and metadata for species across the tree of life with NCBI Datasets. Sci. Data 2024, 11, 732. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Zhang, C.; Liu, Q.; Zhang, X.; Yue, B. Krait: An ultrafast tool for genome-wide survey of microsatellites and primer design. Bioinformatics 2018, 34, 681–683. [Google Scholar] [CrossRef]
- Blacket, M.; Robin, C.; Good, R.; Lee, S.; Miller, A. Universal primers for fluorescent labelling of PCR fragments—An efficient and cost-effective approach to genotyping by fluorescence. Mol. Ecol. Resour. 2012, 12, 456–463. [Google Scholar] [CrossRef]
- Schuelke, M. An economic method for the fluorescent labeling of PCR fragments. Nat. Biotechnol. 2000, 18, 233–234. [Google Scholar] [CrossRef]
- Li, G.; Quiros, C.F. Sequence-related amplified polymorphism (SRAP), a new marker system based on a simple PCR reaction: Its application to mapping and gene tagging in Brassica. Theor. Appl. Genet. 2001, 103, 455–461. [Google Scholar] [CrossRef]
- Ooijen, V. JoinMap® 5, Software for the Calculation of Genetic Linkage Maps in Experimental Populations of Diploid Species; Kyazma BV: Wageningen, The Netherlands, 2018. [Google Scholar]
- Voorrips, R.E. MapChart: Software for the Graphical Presentation of Linkage Maps and QTLs. J. Hered. 2002, 93, 77–78. [Google Scholar] [CrossRef]
| Type of the Tested Markers | Total Number of Tested Markers | Markers Showing Positive Amplification and Distinct Pattern (% from the Total Number of Tested Markers) | Number of Polymorphic Markers (% from the Total Number of Tested Markers) [% from the Markers with Positive Amplification] | Total Number of Loci Identified (Average Number of Detected Loci per Marker) |
|---|---|---|---|---|
| NGS-SSR | 471 | 442 (93.8%) | 255 (54.1%) [57.7%] | 282 (1.11) |
| GEN-SSR | 170 | 154 (90.6%) | 79 (46.5%) [51.3%] | 90 (1.14) |
| EST-SSR | 22 | 17 (77.3%) | 5 (22.7%) [29.4%] | 5 (1.0) |
| SRAP | 11 | 11 (100%) | 11 (100%) [100%] | 33 (3.0) |
| Total for the SSR markers | 663 | 613 | 339 | 377 |
| Linkage Group | Number of Loci ** | Map Length (cM) | Map Density (Loci/cM) | Largest Gap (cM) | Chromosome Sequence (Mbp) | Foremost Position of the Loci (Mbp) | Rearmost Position of the Loci (Mbp) | Coverage of the Chromosome (%) |
|---|---|---|---|---|---|---|---|---|
| LG1 | 22 | 154.23 | 7.01 | 17.69 | 43.13 | 1.64 | 40.38 | 89.8% |
| LG2 | 19 | 123.38 | 6.49 | 19.68 | 40.25 | 1.08 | 39.51 | 95.5% |
| LG3 | 29 | 127.30 | 4.39 | 8.93 | 39.13 | 1.54 | 39.09 | 96.0% |
| LG4 | 22 | 141.74 | 6.44 | 24.50 | 37.48 | 0.30 | 34.65 | 91.6% |
| LG5 | 12 | 116.89 | 9.74 | 22.92 | 35.70 | 1.86 | 34.68 | 91.9% |
| LG6 | 21 | 180.24 | 8.58 | 26.06 | 35.32 | 0.08 | 35.18 | 99.4% |
| LG7 * | 21 | 160.20 | 7.63 | 18.83 | 34.57 | 0.05 | 34.21 | 98.8% |
| LG8 | 15 | 80.21 | 5.35 | 26.38 | 33.58 | 1.31 | 33.47 | 95.8% |
| LG9 | 12 | 75.12 | 6.26 | 15.83 | 33.03 | 10.06 | 30.87 | 63.0% |
| LG10 | 14 | 146.12 | 10.44 | 27.91 | 32.61 | 0.14 | 32.21 | 98.3% |
| LG11 | 12 | 86.21 | 7.18 | 21.59 | 31.76 | 0.78 | 31.00 | 95.2% |
| LG12 | 22 | 171.62 | 7.80 | 26.10 | 31.37 | 0.02 | 26.38 | 84.0% |
| LG13 | 8 | 71.03 | 8.88 | 19.81 | 30.09 | 7.42 | 26.93 | 64.8% |
| LG14 | 23 | 153.85 | 6.69 | 11.26 | 29.44 | 0.25 | 28.75 | 96.8% |
| LG15 * | 11 | 51.39 | 4.67 | 8.46 | 28.69 | 1.16 | 27.98 | 93.5% |
| LG16 | 9 | 93.88 | 10.43 | 29.89 | 28.47 | 1.04 | 15.06 | 49.2% |
| LG17 | 12 | 68.54 | 5.71 | 15.70 | 27.94 | 2.54 | 22.52 | 71.5% |
| LG18 | 13 | 113.77 | 8.75 | 14.26 | 27.54 | 2.61 | 27.51 | 90.4% |
| LG19 | 14 | 76.30 | 5.45 | 12.75 | 27.40 | 0.11 | 19.59 | 71.1% |
| LG20 | 11 | 99.86 | 9.08 | 25.05 | 27.08 | 4.13 | 26.87 | 84.0% |
| LG21 | 13 | 92.03 | 7.08 | 18.86 | 27.05 | 5.20 | 26.54 | 78.9% |
| LG22 | 12 | 70.64 | 5.89 | 20.45 | 26.81 | 0.59 | 25.15 | 91.6% |
| LG23 | 7 | 25.23 | 3.60 | 7.07 | 26.63 | 14.18 | 24.09 | 37.2% |
| LG24 | 10 | 85.09 | 8.51 | 14.64 | 23.28 | 1.45 | 17.98 | 71.0% |
| LG25 | 11 | 66.70 | 6.06 | 14.25 | 22.94 | 1.37 | 16.55 | 66.2% |
| Minimal value for LG | 7 | 25.23 | 3.60 | 37.2% | ||||
| Maximal value for LG | 29 | 180.24 | 10.44 | 99.4% | ||||
| Average value per LG | 15 +/−5.67 | 105.26 +/−40.73 | 82.6% +/−16.7% | |||||
| Total value for the map | 375 | 2631.57 | 7.01 |
| PCR Amplification (*) | Results of SSR Amplification from DNA of the Tested Plants ** | |||
|---|---|---|---|---|
| L. angustifolia var. Hidcote Blue | L. latifolia Ll_abi2 | L. latifolia Bastin Nursery | L. × heterophylla var. Big Boy James | |
| (+) | 291 | 243 | 172 | 251 |
| 93.9% | 78.4% | 78.5% | 81.0% | |
| (w) | 10 | 15 | 7 | 10 |
| 3.2% | 4.8% | 3.2% | 3.2% | |
| (−) | 9 | 52 | 40 | 49 |
| 2.9% | 16.8% | 18.3% | 15.8% | |
| Total number of tested SSRs | 310 | 310 | 219 | 310 |
| No | Compound | RT(s) | RI | Average * +/− Stdev (%) | Minimal * Content (%) | Maximal * Content (%) | Min/ Average ** (%) | Max/ Average ** (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | Toluene | 527.8 | 1039.0 | 0.329 +/− 0.117 | 0.007 | 0.778 | 2.0 | 236.8 |
| 2 | Camphene | 563.2 | 1055.2 | 0.131 +/− 0.079 | 0.027 | 0.416 | 20.8 | 317.2 |
| 3 | β-Pinene | 657.8 | 1098.8 | 0.045 +/− 0.031 | 0.007 | 0.186 | 16.0 | 411.5 |
| 4 | Sabinene | 688.3 | 1112.9 | 0.068 +/− 0.025 | 0.025 | 0.134 | 37.5 | 198.4 |
| 5 | 3-Carene | 756.1 | 1144.2 | 0.082 +/− 0.072 | 0.010 | 0.371 | 11.9 | 453.1 |
| 6 | β-Myrcene | 795.3 | 1162.3 | 0.188 +/− 0.086 | 0.079 | 0.614 | 42.2 | 326.4 |
| 7 | D-Limonene | 893.6 | 1204.9 | 0.453 +/− 0.178 | 0.181 | 1.050 | 40.0 | 231.9 |
| 8 | Eucalyptol | 916.4 | 1211.8 | 0.403 +/− 0.446 | 0.000 | 1.442 | 0.0 | 357.8 |
| 9 | β-Phellandrene | 921.7 | 1213.4 | 0.627 +/− 0.889 | 0.000 | 3.678 | 0.0 | 586.7 |
| 10 | trans-β-Ocimene | 1003.9 | 1238.2 | 2.040 +/− 1.082 | 0.477 | 5.561 | 23.4 | 272.6 |
| 11 | β-Ocimene | 1057.7 | 1254.4 | 1.572 +/− 1.464 | 0.048 | 6.102 | 3.1 | 388.2 |
| 12 | 3-Octanone | 1065.7 | 1256.9 | 1.108 +/− 0.723 | 0.000 | 2.878 | 0.0 | 259.7 |
| 13 | Hexyl acetate | 1124.2 | 1274.5 | 0.285 +/− 0.117 | 0.093 | 0.566 | 32.5 | 198.7 |
| 14 | 3-Octanol, acetate | 1337.2 | 1338.8 | 0.070 +/− 0.075 | 0.000 | 0.395 | 0.0 | 566.4 |
| 15 | 1-Octen-3-yl-acetate | 1489.7 | 1384.8 | 0.828 +/− 0.468 | 0.069 | 2.016 | 8.3 | 243.4 |
| 16 | 1-Octen-3-ol | 1742.7 | 1457.0 | 0.190 +/− 0.132 | 0.035 | 0.618 | 18.4 | 324.8 |
| 17 | Camphor | 1965.3 | 1519.6 | 0.088 +/− 0.039 | 0.037 | 0.232 | 42.4 | 263.3 |
| 18 | Linalool | 2096.0 | 1556.4 | 24.788 +/− 8.364 | 7.457 | 45.286 | 30.1 | 182.7 |
| 19 | Linalyl acetate | 2130.8 | 1566.2 | 44.435 +/− 9.455 | 19.247 | 61.662 | 43.3 | 138.8 |
| 20 | α-Santalene | 2168.2 | 1576.7 | 0.387 +/− 0.345 | 0.000 | 1.511 | 0.0 | 390.1 |
| 21 | Borneol acetate | 2198.9 | 1585.4 | 0.176 +/− 0.119 | 0.000 | 0.534 | 0.0 | 302.7 |
| 22 | α-Bergamotene | 2209.6 | 1588.4 | 0.080 +/− 0.073 | 0.000 | 0.312 | 0.0 | 388.2 |
| 23 | β-Caryophyllene | 2248.0 | 1599.2 | 4.739 +/− 2.819 | 1.457 | 16.888 | 30.7 | 356.3 |
| 24 | Lavandulyl acetate | 2299.1 | 1614.4 | 2.739 +/− 2.158 | 0.170 | 11.249 | 6.2 | 410.7 |
| 25 | α-Humulene | 2496.7 | 1673.3 | 0.991 +/− 1.581 | 0.000 | 6.266 | 0.0 | 632.6 |
| 26 | Crypton | 2503.6 | 1675.4 | 0.433 +/− 0.384 | 0.000 | 1.129 | 0.0 | 260.6 |
| 27 | Lavandulol | 2540.6 | 1686.4 | 0.148 +/− 0.153 | 0.000 | 0.987 | 0.0 | 668.9 |
| 28 | Borneol | 2614.7 | 1708.5 | 0.512 +/− 0.266 | 0.016 | 1.443 | 3.2 | 281.6 |
| 29 | γ-Cadinene | 2793.5 | 1761.9 | 0.226 +/− 0.188 | 0.004 | 0.801 | 1.9 | 355.1 |
| 30 | p-Isopropylbenzaldehyde | 2880.7 | 1787.9 | 0.200 +/− 0.051 | 0.099 | 0.313 | 49.6 | 156.0 |
| 31 | Caryophyllene oxide | 3293.1 | 1994.5 | 0.408 +/− 0.205 | 0.127 | 1.274 | 31.2 | 312.6 |
| 32 | p-Cymen-7-ol | 3406.2 | 2123.2 | 0.299 +/− 0.134 | 0.054 | 1.232 | 18.2 | 412.4 |
| 33 | tau.-Cadinol | 3465.8 | 2194.7 | 0.953 +/− 0.634 | 0.153 | 2.910 | 16.0 | 305.2 |
| 34 | Coumarin | 3791.1 | 2510.7 | 0.590 +/− 0.219 | 0.186 | 1.308 | 31.6 | 221.8 |
| No | Trait | QTL Name | LOD * | LG | Peak (cM) | Left Marker | Right Marker | PVE (%) ** | Additive (%) *** |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Toluene | q01-5.1 | 5.27 | 5 | 2.00 | ABIL527A | ABIL103 | 25.9 | −0.083 |
| 2 | q01-5.2 | 5.26 | 5 | 95.82 | ABIL531 | ABIL164 | 25.6 | −0.075 | |
| 3 | Camphene | q02-11.1 | 4.80 | 11 | 81.94 | ABIL416B | ABIL77 | 23.9 | −0.064 |
| 4 | q02-20.1 | 4.58 | 20 | 48.52 | ABIL505 | ABIL15 | 22.6 | −0.025 | |
| 5 | β-Pinene | q03-11.1 | 5.35 | 11 | 86.12 | ABIL416B | ABIL77 | 26.2 | −0.024 |
| 6 | Sabinene | q04-3.1 | 4.04 | 3 | 54.74 | ABIL506 | ABIL456A | 20.1 | −0.019 |
| 7 | q04-3.2 | 4.79 | 3 | 61.19 | ABIL322 | ABIL63 | 23.8 | −0.022 | |
| 8 | q04-19.1 | 4.17 | 19 | 38.07 | E10M8-344 | ABIL429 | 21.1 | 0.015 | |
| 9 | 3-Carene | q05-5.1 | 15.61 | 5 | 38.45 | ABIL330 | E4M1-85 | 58.8 | 0.083 |
| 10 | D-Limonene | q06-19.1 | 5.43 | 19 | 32.53 | ABIL11 | E10M8-344 | 26.6 | 0.121 |
| 11 | β-Phellandrene | q07-2.1 | 4.67 | 2 | 38.20 | ABIL473 | ABIL530A | 23.3 | −0.563 |
| 12 | q07-19.1 | 4.81 | 19 | 31.53 | ABIL11 | E10M8-344 | 23.9 | 0.605 | |
| 13 | trans-β-Ocimene | q08-2.1 | 4.45 | 2 | 14.08 | ABIL163 | ABIL519 | 22.3 | −0.644 |
| 14 | q08-2.2 | 4.03 | 2 | 49.02 | ABIL91B | ABIL268 | 20.5 | −0.660 | |
| 15 | q08-6.1 | 6.96 | 6 | 13.00 | ABIL517A | ABIL538 | 32.7 | 0.811 | |
| 16 | q08-24.1 | 4.09 | 24 | 33.42 | ABIL393B | ABIL585 | 20.7 | −1.042 | |
| 17 | 3-Octanone | q09-1.1 | 12.20 | 1 | 6.96 | ABIL510 | ABIL14 | 50.0 | 0.681 |
| 18 | q09-1.2 | 6.24 | 1 | 52.47 | ABIL388 | ABIL361 | 29.8 | 0.565 | |
| 19 | q09-1.3 | 4.49 | 1 | 106.83 | ABIL371 | ABIL352 | 22.8 | 0.471 | |
| 20 | 3-Octanol acetate | q10-1.1 | 6.20 | 1 | 17.72 | ABIL14 | ABIL512 | 29.7 | 0.057 |
| 21 | 1-Octen-3-yl-acetate | q11-4.1 | 4.10 | 4 | 128.91 | ABIL323 | ABIL245 | 20.8 | 0.317 |
| 22 | q11-8.1 | 6.03 | 8 | 33.40 | ABIL550 | ABIL548 | 29.0 | 0.206 | |
| 23 | 1-Octen-3-ol | q12-8.1 | 4.39 | 8 | 59.13 | ABIL554A | ABIL408 | 22.1 | 0.105 |
| 24 | Linalool | q13-6.1 | 4.33 | 6 | 21.09 | ABIL538 | ABIL66 | 21.8 | −5.217 |
| 25 | q13-8.1 | 12.17 | 8 | 34.01 | ABIL550 | ABIL548 | 50.2 | 9.134 | |
| 26 | Linalyl acetate | q14-8.1 | 11.95 | 8 | 33.40 | ABIL550 | ABIL548 | 49.3 | −10.576 |
| 27 | α-Santalene | q15-12.1 | 4.04 | 12 | 165.01 | ABIL409 | E2M10-214 | 20.5 | 0.384 |
| 28 | Borneol acetate | q16-11.1 | 4.86 | 11 | 82.96 | ABIL416B | ABIL77 | 24.2 | −0.092 |
| 29 | α-Bergamotene | q17-12.1 | 4.44 | 12 | 165.01 | ABIL409 | E2M10-214 | 22.8 | 0.048 |
| 30 | β-Caryophyllene | q18-6.1 | 5.46 | 6 | 61.64 | ABIL251 | ABIL539A | 27.4 | 2.180 |
| 31 | q18-12.1 | 6.39 | 12 | 101.13 | ABIL210 | ABIL48 | 30.5 | 1.878 | |
| 32 | q18-12.2 | 5.41 | 12 | 168.01 | ABIL409 | E2M10-214 | 26.5 | 1.859 | |
| 33 | Lavandulyl acetate | q19-3.1 | 17.35 | 3 | 84.29 | ABIL312 | ABIL27 | 62.7 | −2.187 |
| 34 | α-Humulene | q20-6.1 | 5.30 | 6 | 19.09 | ABIL538 | ABIL66 | 26.0 | 1.053 |
| 35 | q20-12.1 | 7.26 | 12 | 82.97 | ABIL61A | ABIL560 | 33.8 | −1.262 | |
| 36 | q20-12.2 | 8.15 | 12 | 125.52 | ABIL332 | ABIL187 | 37.1 | −1.252 | |
| 37 | Lavandulol | q21-3.1 | 8.47 | 3 | 84.29 | ABIL312 | ABIL27 | 38.2 | −0.135 |
| 38 | q21-8.1 | 6.16 | 8 | 16.05 | ABIL552 | ABIL339 | 29.5 | 0.122 | |
| 39 | Borneol | q22-11.1 | 3.50 * | 11 | 82.96 | ABIL416B | ABIL77 | 18.1 | −0.179 |
| 40 | q22-20.1 | 4.50 | 20 | 44.52 | ABIL505 | ABIL15 | 22.6 | −0.034 | |
| 41 | γ-Cadinene | q23-5.1 | 11.58 | 5 | 58.69 | ABIL112 | ABIL503B | 48.2 | −0.175 |
| 42 | p-Isopropylbenzaldehyde | q24-6.1 | 4.65 | 6 | 25.09 | ABIL538 | ABIL66 | 21.3 | 0.035 |
| 43 | tau.-Cadinol | q25-5.1 | 11.67 | 5 | 58.69 | ABIL112 | ABIL503B | 48.5 | −0.594 |
| Chromosome | Trait (QTL Name) | QTL Markers and Candidate Gene(s) | Chromosome Locations, kBp | Comment References |
|---|---|---|---|---|
| CH11 | Camphene (q02-11.1) β-Pinene (q03-11.1) Borneol acetate (q16-11.1) Borneol (q22-11.1) | Left QTL marker ABIL416B | 30,426 | LaBPPS (L. angustifolia bornyl diphosphate synthase) LaBPPS catalyzes the production of various by-products, including borneol, camphene, and β-pinene [25]. BLAST search with sequence acc. number KM015221. |
| LaBPPS-like | 30,794 30,802 30,819 30,831 30,834 30,841 30,855 30,872 30,880 30,922 | |||
| Right QTL marker ABIL77 | 31,003 | |||
| CH12 | α-Santalene (q15-12.1) α-Bergamotene (q17-12.1) β-Caryophyllene (q18-12.2) | Left QTL marker ABIL409 | 26,385 | LaBERS (L. angustifolia trans-alpha-bergamotene synthase) LaBERS catalyzes the production of various by-products including α-santalene, α-bergamotene, and β-caryophyllene [68]. BLAST search with sequence acc. number DQ263742 |
| LaBERS-like | 28,836 28,848 30,868 30,899 | |||
| Right QTL marker E2M10-214 | SRAP marker | |||
| CH5 | γ-Cadinene (q23-5.1) tau.-Cadinol (q25-5.1) | Left QTL marker ABIL112 | 29,285 | LaCADS (L. angustifolia tau-cadinol synthase) LaCADS catalyzes the production of various by-products, including γ-cadinene and tau.-cadinol [69]. BLAST search with sequence acc. number JX401282 |
| LaCADS-like | 30,139 30,143 30,154 30,158 | |||
| Right QTL marker ABIL503B | 32,864 | |||
| CH8 | Linalool (q13-8.1) Linalyl acetate (q14-8.1) | Left QTL marker ABIL550 | 20,261 | LiATT4 (Lavandula × intermedia alcohol acetyltransferase) [70]. LiATT4 catalyzes the production of linalyl acetate from linalool as substrate [67]. BLAST search with sequence acc. number KM275344. |
| LiAAT4 | 20,389 | |||
| Right QTL marker ABIL548 | 20,883 | |||
| CH6 | Linalool (q13-6.1) | Left QTL marker ABIL538 | 27 | LaLINS (L. angustifolia linalool synthase) LaLINS catalyzes the production of linalool from geranyl diphosphate [68]. BLAST search with sequence acc. number DQ263741 |
| LaLINS | 362 | |||
| Right QTL marker ABIL66 | 548 | |||
| CH5 | 3-Carene (q05-5.1) | Left QTL marker ABIL330 | 15,474 | Li3CARS (L. × intermedia 3-carene synthase) Li3CARS catalyzes the production of 3-carene [71]. BLAST search with sequence acc. number KX024762 |
| Li3CARS | 26,547 26,570 26,605 | |||
| Right QTL marker E4M1-85/ABIL13 | 28,143 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgieva, P.; Rusanov, K.; Rusanova, M.; Kitanova, M.; Atanassov, I. Construction of Simple Sequence Repeat-Based Genetic Linkage Map and Identification of QTLs for Accumulation of Floral Volatiles in Lavender (Lavandula angustifolia Mill.). Int. J. Mol. Sci. 2025, 26, 3705. https://doi.org/10.3390/ijms26083705
Georgieva P, Rusanov K, Rusanova M, Kitanova M, Atanassov I. Construction of Simple Sequence Repeat-Based Genetic Linkage Map and Identification of QTLs for Accumulation of Floral Volatiles in Lavender (Lavandula angustifolia Mill.). International Journal of Molecular Sciences. 2025; 26(8):3705. https://doi.org/10.3390/ijms26083705
Chicago/Turabian StyleGeorgieva, Pavlina, Krasimir Rusanov, Mila Rusanova, Meglena Kitanova, and Ivan Atanassov. 2025. "Construction of Simple Sequence Repeat-Based Genetic Linkage Map and Identification of QTLs for Accumulation of Floral Volatiles in Lavender (Lavandula angustifolia Mill.)" International Journal of Molecular Sciences 26, no. 8: 3705. https://doi.org/10.3390/ijms26083705
APA StyleGeorgieva, P., Rusanov, K., Rusanova, M., Kitanova, M., & Atanassov, I. (2025). Construction of Simple Sequence Repeat-Based Genetic Linkage Map and Identification of QTLs for Accumulation of Floral Volatiles in Lavender (Lavandula angustifolia Mill.). International Journal of Molecular Sciences, 26(8), 3705. https://doi.org/10.3390/ijms26083705







