Identifying Genes Associated with the Anticancer Activity of a Fluorinated Chalcone in Triple-Negative Breast Cancer Cells Using Bioinformatics Tools
Abstract
1. Introduction
2. Results
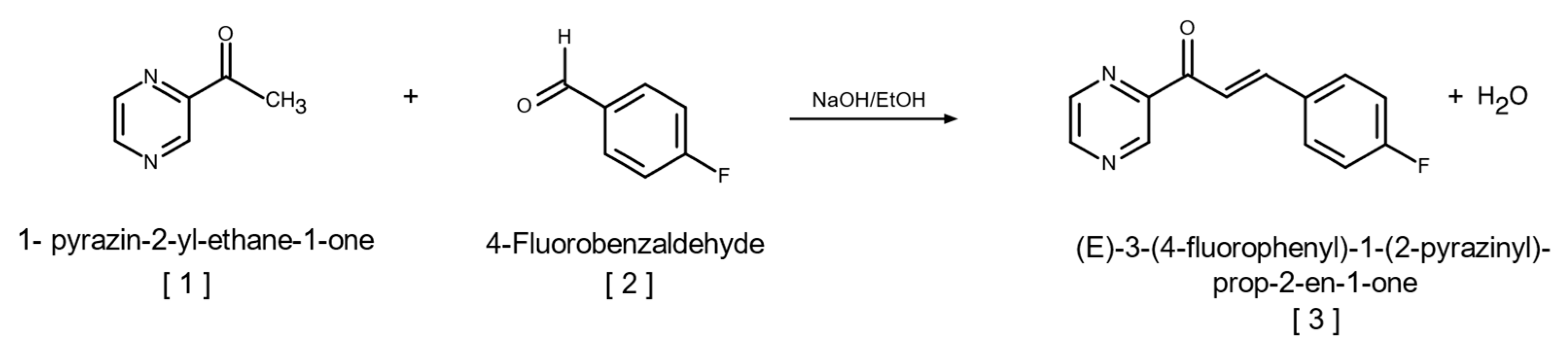
2.1. Synthesis of (E)-3-(4-Fluorophenyl)-1-(2-pyrazinyl)-prop-2-en-1-one
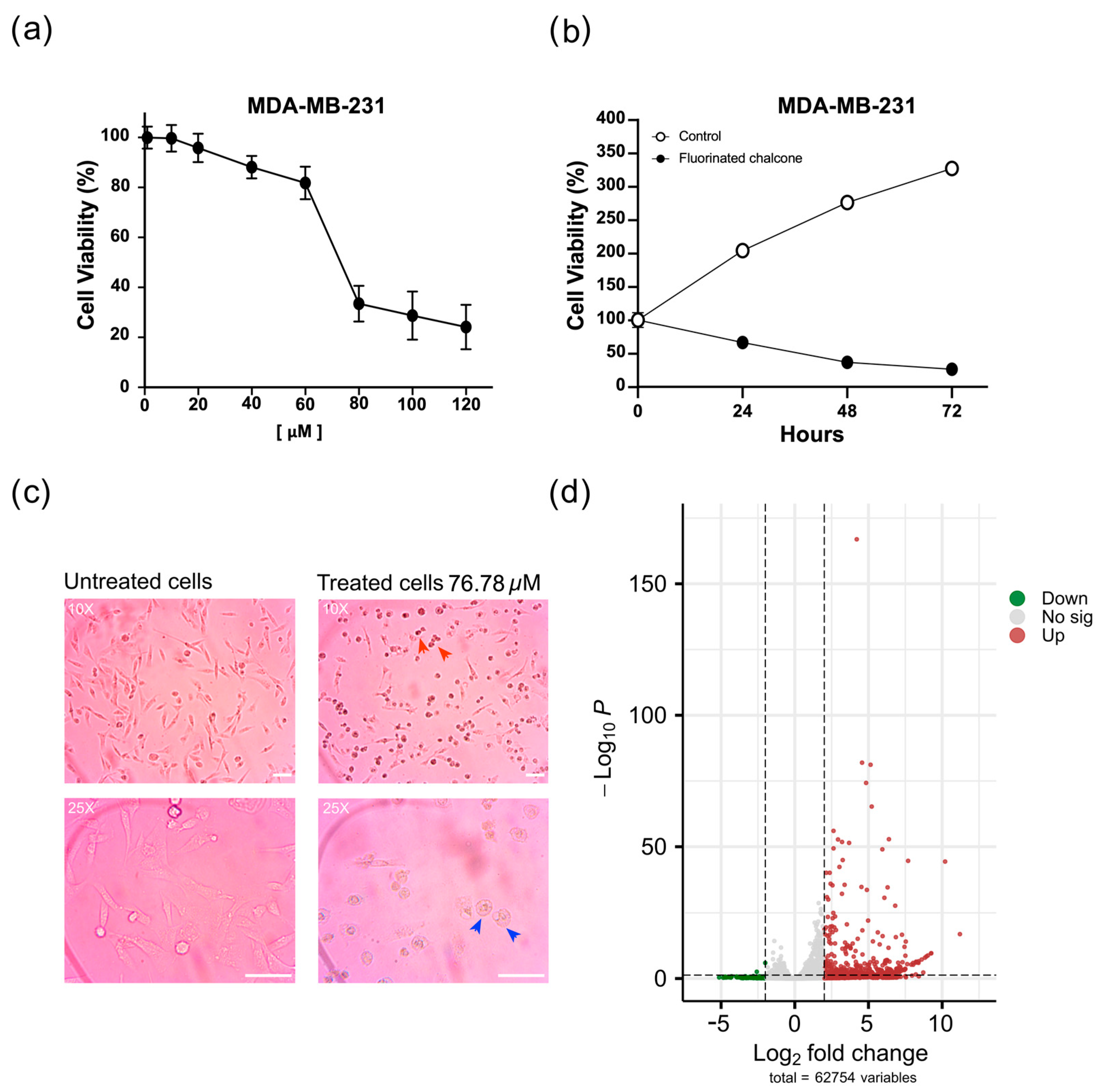
2.2. Fluorinated Chalcone Induces Morphological Changes Suggesting Apoptosis
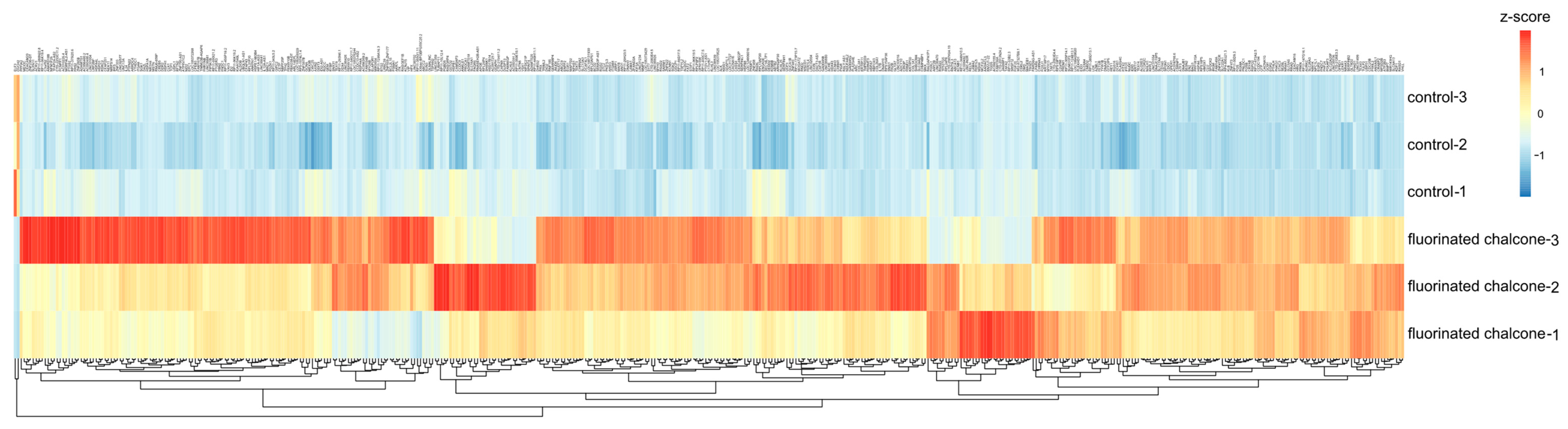
2.3. Identifying DE Genes Associated with the Anticancer Activity of Fluorinated Chalcone
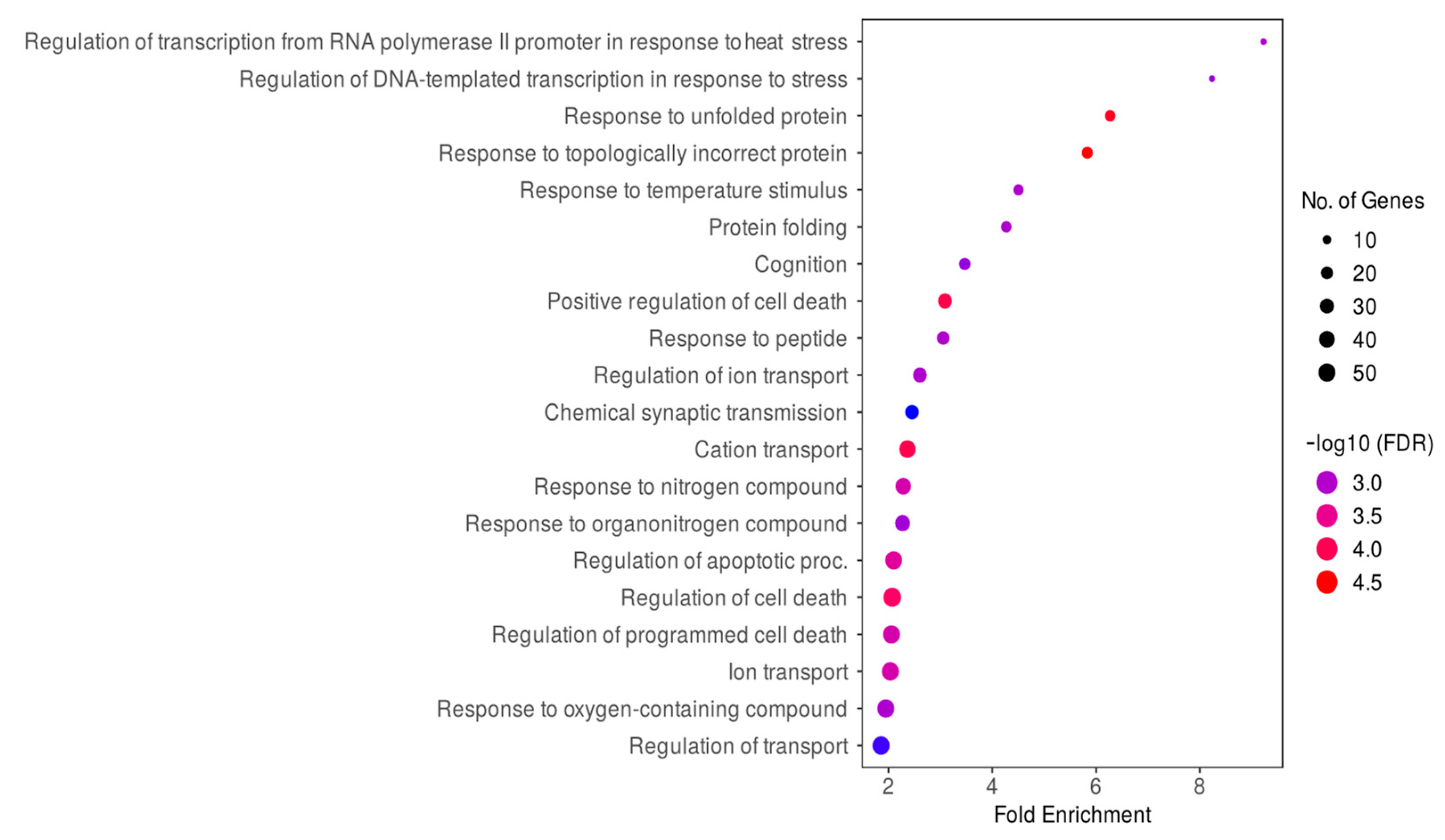
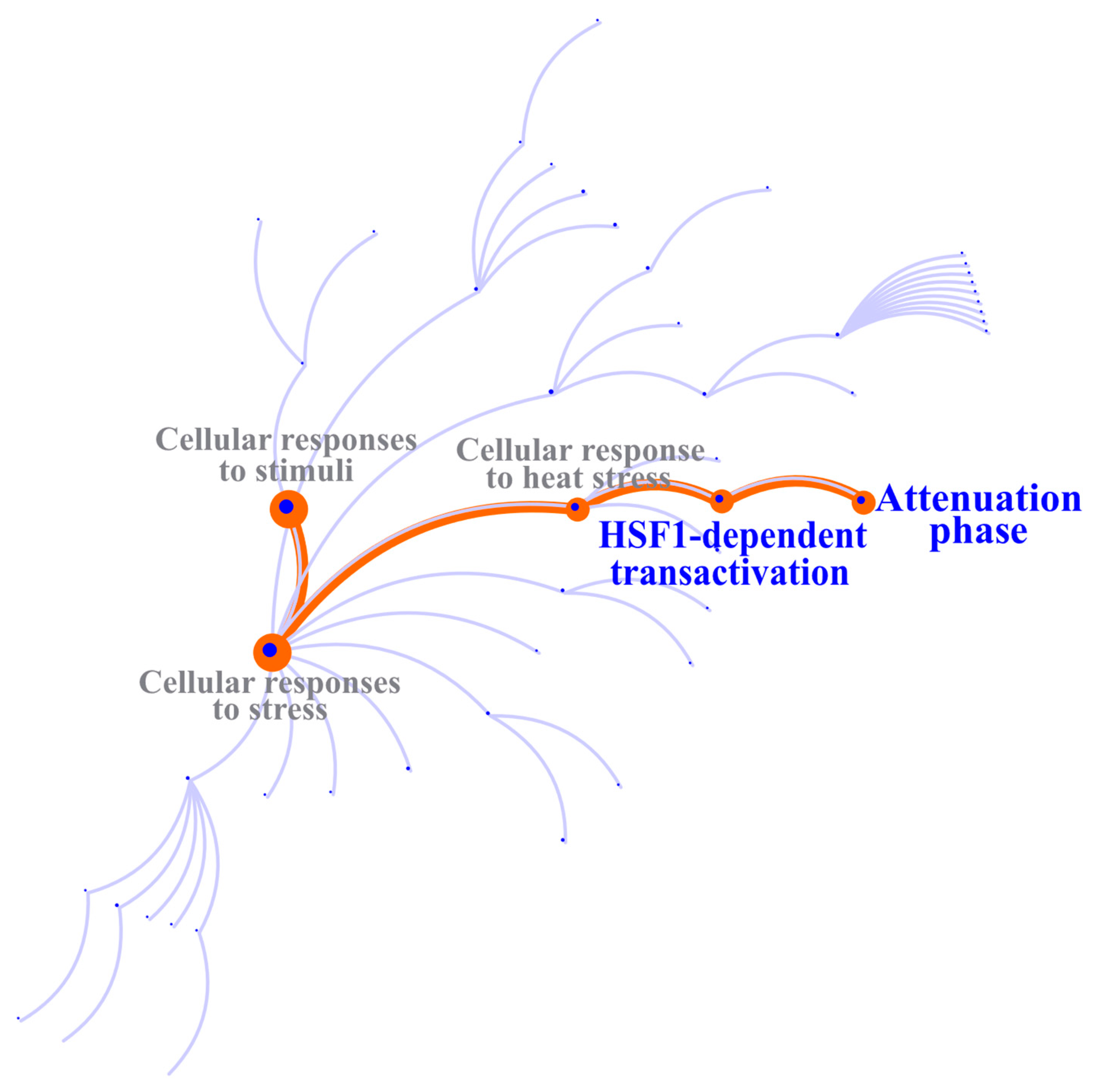
2.4. Identifying the Biological Processes and Molecular Pathways of DE Genes
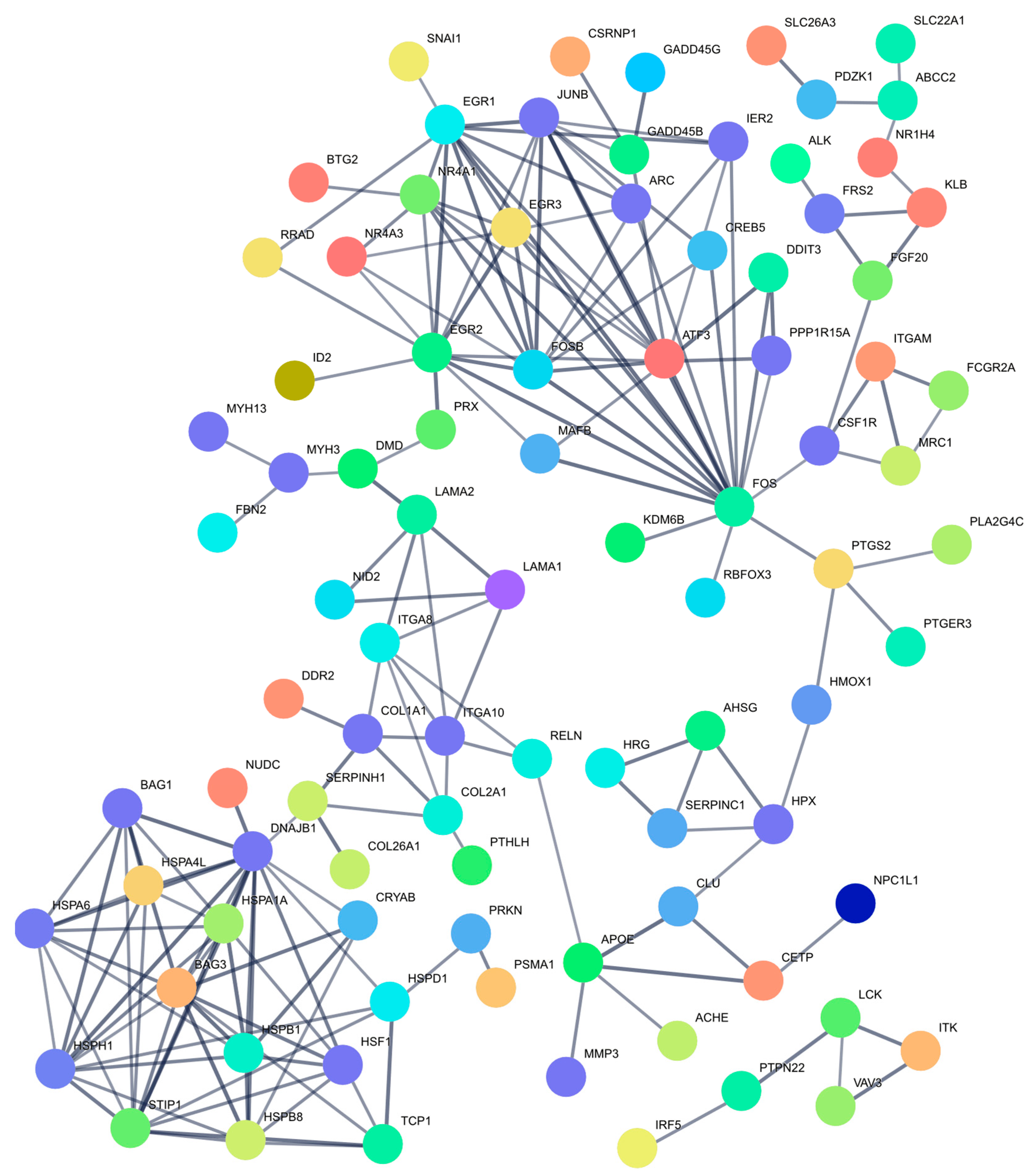
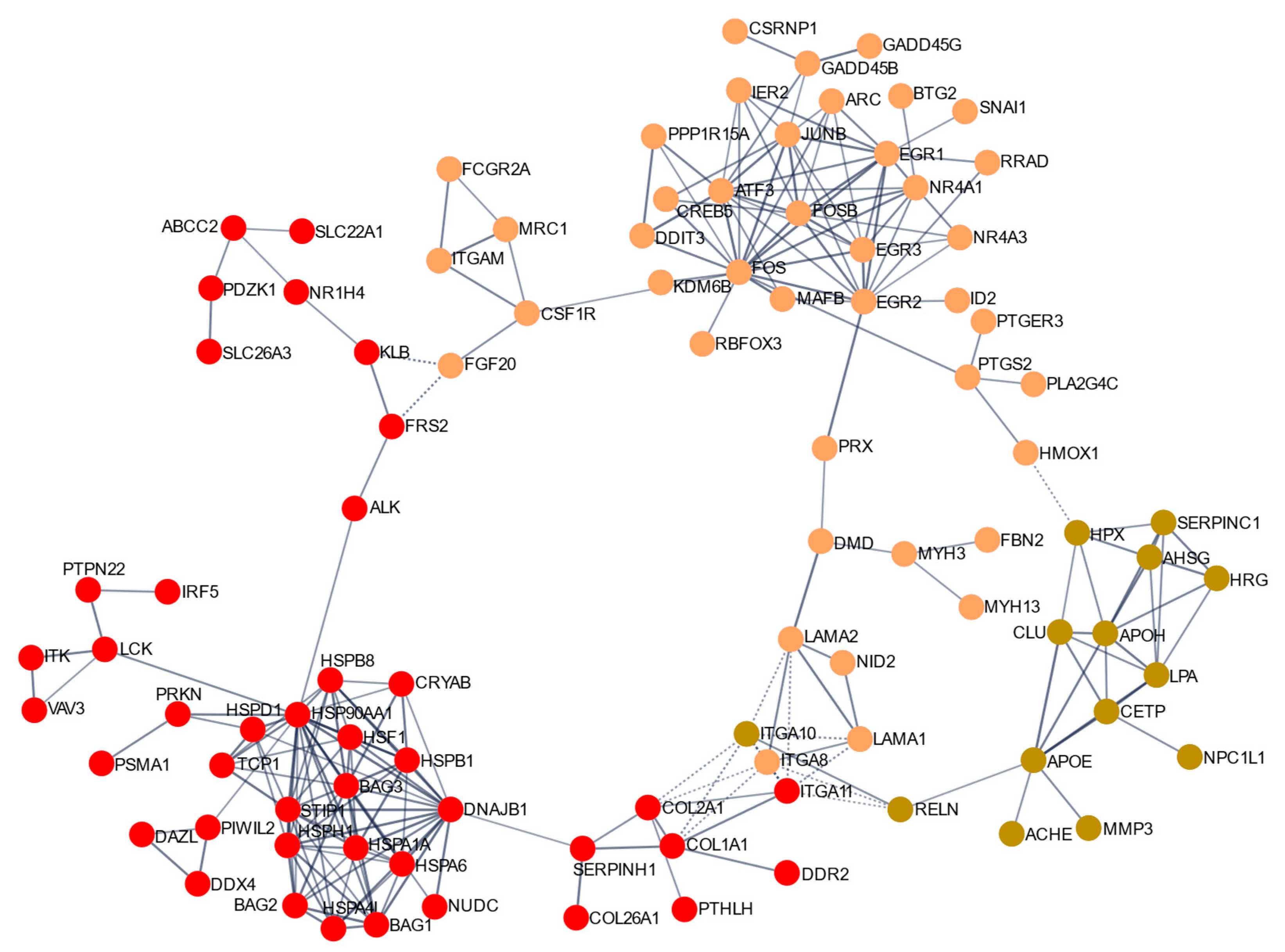
2.5. Clusters of Genes Interact Synergistically to Inhibit Cell Proliferation of MDA-MB-231 Cells
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.1.1. Equipment and Experimental Conditions
4.1.2. Synthesis of (E)-3-(4-Fluorophenyl)-1-(2-pyrazinyl)-prop-2-en-1-one (Fluorinated Chalcone)
4.2. Cell Culture and Cell Viability Assay
Treatment of MDA-MB-231 Cells with the IC50
4.3. Library Preparation and RNA-Seq
4.4. Bioinformatics Analysis
4.4.1. Quality Control, Transcriptome Assembly, and Mapping
4.4.2. Identification of Differentially Expressed (DE) Genes
4.4.3. Functional Analysis and Construction of a Protein–Protein Interaction (PPI) Network
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATF3 | Activation of Transcription Factor 3 |
| DBD | DNA binding domain |
| DISC | death-inducing signaling complex |
| DMEM | dulbecco’s Modified Eagle Medium |
| DMSO | dimethyl sulfoxide |
| DNA | deoxyribonucleic acid |
| EDTA | ethylene diamine tetraacetic acid |
| ER | estrogen receptor |
| FBS | fetal bovine serum |
| FDR | false discovery rate |
| GO | gene ontology |
| HER-2 | human epidermal growth factor receptor 2 |
| HSEs | heat shock elements |
| HSF1 | Heat Shock Factor 1 |
| HSP | heat shock protein |
| HSR | heat shock responsive |
| IC50 | half maximal inhibitory concentration |
| VGKC | voltage-gated potassium channel |
| KLHL gene | Kelch-like gene |
| LZ | leucine zipper |
| PARP | poly ADP-ribose polymerase |
| PBS | phosphate-buffered saline |
| PD-1 | programmed death-1 |
| PD-L1 | programmed death-ligand 1 |
| PPI | protein–protein interaction |
| PR | progesterone receptor |
| RD | regulatory domain |
| RIN | RNA integrity number |
| RNA | ribonucleic acid |
| RNA-seq | RNA sequencing |
| RT-PCR | reverse transcription polymerase chain reaction |
| SLC | solute carrier |
| TAD | transactivation domain |
| TNBC | triple-negative breast cancer |
| VGCC | voltage-gated calcium channel |
References
- Howard, F.M.; Olopade, O.I. Epidemiology of Triple-Negative Breast Cancer: A Review. Cancer J. 2021, 27, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Duan, J.J.; Bian, X.W.; Yu, S.C. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. BCR 2020, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- Vagia, E.; Mahalingam, D.; Cristofanilli, M. The Landscape of Targeted Therapies in TNBC. Cancers 2020, 12, 916. [Google Scholar] [CrossRef]
- Obidiro, O.; Battogtokh, G.; Akala, E.O. Triple Negative Breast Cancer Treatment Options and Limitations: Future Outlook. Pharmaceutics 2023, 15, 1796. [Google Scholar] [CrossRef] [PubMed]
- Tavares, D.F.; Chaves Ribeiro, V.; Andrade, M.A.V.; Moreira Cardoso-Júnior, L.; Rhangel Gomes Teixeira, T.; Ramos Varrone, G.; Lopes Britto, R. Immunotherapy using PD-1/PDL-1 inhibitors in metastatic triple-negative breast cancer: A systematic review. Oncol. Rev. 2021, 15, 497. [Google Scholar] [CrossRef]
- Elmakaty, I.; Abdo, R.; Elsabagh, A.; Elsayed, A.; Malki, M.I. Comparative efficacy and safety of PD-1/PD-L1 inhibitors in triple negative breast cancer: A systematic review and network meta-analysis of randomized controlled trials. Cancer Cell Int. 2023, 23, 90. [Google Scholar] [CrossRef]
- El Gazzar, W.B.; Albakri, K.A.; Hasan, H.; Badr, A.M.; Farag, A.A.; Saleh, O.M. Poly(ADP-ribose) polymerase inhibitors in the treatment landscape of triple-negative breast cancer (TNBC). J. Oncol. Pharm. Pract. 2023, 29, 1467–1479. [Google Scholar] [CrossRef]
- Soung, Y.H.; Ju, J.; Chung, J. The Sensitization of Triple-Negative Breast Cancers to Poly ADP Ribose Polymerase Inhibition Independent of BRCA1/2 Mutation Status by Chemically Modified microRNA-489. Cells 2023, 13, 49. [Google Scholar] [CrossRef]
- Lee, J. Current Treatment Landscape for Early Triple-Negative Breast Cancer (TNBC). J. Clin. Med. 2023, 12, 1524. [Google Scholar] [CrossRef]
- Bukhari, S.N.; Jantan, I.; Jasamai, M. Anti-inflammatory trends of 1, 3-diphenyl-2-propen-1-one derivatives. Mini Rev. Med. Chem. 2013, 13, 87–94. [Google Scholar] [CrossRef]
- Jang, S.; Jung, J.C.; Oh, S. Synthesis of 1,3-diphenyl-2-propen-1-one derivatives and evaluation of their biological activities. Bioorg. Med. Chem. 2007, 15, 4098–4105. [Google Scholar] [CrossRef]
- Rodríguez, I.; Saavedra, E.; Del Rosario, H.; Perdomo, J.; Quintana, J.; Prencipe, F.; Oliva, P.; Romagnoli, R.; Estévez, F. Apoptosis Pathways Triggered by a Potent Antiproliferative Hybrid Chalcone on Human Melanoma Cells. Int. J. Mol. Sci. 2021, 22, 13462. [Google Scholar] [CrossRef] [PubMed]
- Michalkova, R.; Kello, M.; Kudlickova, Z.; Gazdova, M.; Mirossay, L.; Mojzisova, G.; Mojzis, J. Programmed Cell Death Alterations Mediated by Synthetic Indole Chalcone Resulted in Cell Cycle Arrest, DNA Damage, Apoptosis and Signaling Pathway Modulations in Breast Cancer Model. Pharmaceutics 2022, 14, 503. [Google Scholar] [CrossRef] [PubMed]
- Kuruc, T.; Kello, M.; Petrova, K.; Kudlickova, Z.; Kubatka, P.; Mojzis, J. The Newly Synthetized Chalcone L1 Is Involved in the Cell Growth Inhibition, Induction of Apoptosis and Suppression of Epithelial-to-Mesenchymal Transition of HeLa Cells. Molecules 2021, 26, 1356. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Kang, C.Y.; Niu, Z.X.; Zhou, H.C.; Yang, H.M. A chalcone inhibits the growth and metastasis of KYSE-4 esophageal cancer cells. J. Int. Med. Res. 2020, 48, 300060520928831. [Google Scholar] [CrossRef]
- Burmaoglu, S.; Gobek, A.; Anil, D.A.; Alagoz, M.A.; Guner, A.; Güler, C.; Hepokur, C.; Karabay Yavasoglu, N.U.; Algul, O.J.P.A.C. Assessing the Antiangiogenic Effects of Chalcones and Their Derivatives. Polycycl. Aromat. Compd. 2024, 44, 51–66. [Google Scholar] [CrossRef]
- Sun, M.; Yuan, M.; Kang, Y.; Qin, J.; Zhang, Y.; Duan, Y.; Wang, L.; Yao, Y. Identification of novel non-toxic and anti-angiogenic α-fluorinated chalcones as potent colchicine binding site inhibitors. J. Enzym. Inhib. Med. Chem. 2022, 37, 339–354. [Google Scholar] [CrossRef]
- Padhye, S.; Ahmad, A.; Oswal, N.; Dandawate, P.; Rub, R.A.; Deshpande, J.; Swamy, K.V.; Sarkar, F.H. Fluorinated 2′-hydroxychalcones as garcinol analogs with enhanced antioxidant and anticancer activities. Bioorg. Med. Chem. Lett. 2010, 20, 5818–5821. [Google Scholar] [CrossRef]
- Olotu, F.A.; Joy, M.; Abdelgawad, M.A.; Narayanan, S.E.; Soliman, M.E.; Mathew, B. Revealing the role of fluorine pharmacophore in chalcone scaffold for shifting the MAO-B selectivity: Investigation of a detailed molecular dynamics and quantum chemical study. J. Biomol. Struct. Dyn. 2021, 39, 6126–6139. [Google Scholar] [CrossRef]
- Meanwell, N.A. Fluorine and Fluorinated Motifs in the Design and Application of Bioisosteres for Drug Design. J. Med. Chem. 2018, 61, 5822–5880. [Google Scholar] [CrossRef]
- Glyn, R.J.; Pattison, G. Effects of Replacing Oxygenated Functionality with Fluorine on Lipophilicity. J. Med. Chem. 2021, 64, 10246–10259. [Google Scholar] [CrossRef] [PubMed]
- Zafrani, Y.; Parvari, G.; Amir, D.; Ghindes-Azaria, L.; Elias, S.; Pevzner, A.; Fridkin, G.; Berliner, A.; Gershonov, E.; Eichen, Y.; et al. Modulation of the H-Bond Basicity of Functional Groups by α-Fluorine-Containing Functions and its Implications for Lipophilicity and Bioisosterism. J. Med. Chem. 2021, 64, 4516–4531. [Google Scholar] [CrossRef]
- Swallow, S. Fluorine in medicinal chemistry. Prog. Med. Chem. 2015, 54, 65–133. [Google Scholar] [PubMed]
- Khairul, W.M.; Hashim, F.; Mohammed, M.; Shah, N.; Johari, S.; Rahamathullah, R.; Daud, A.I.; Ma, N.L. Synthesis, Molecular Docking and Biological Activity Evaluation of Alkoxy Substituted Chalcone Derivatives: Potential Apoptosis Inducing Agent on MCF-7 Cells. Anti-Cancer Agents Med. Chem. 2021, 21, 1738–1750. [Google Scholar] [CrossRef]
- Lim, Y.H.; Oo, C.W.; Koh, R.Y.; Voon, G.L.; Yew, M.Y.; Yam, M.F.; Loh, Y.C. Synthesis, characterization, and anti-cancer activity of new chalcone derivatives containing naphthalene and fluorine moieties. Drug Dev. Res. 2020, 81, 994–1003. [Google Scholar] [CrossRef]
- Li, K.; Zhao, S.; Long, J.; Su, J.; Wu, L.; Tao, J.; Zhou, J.; Zhang, J.; Chen, X.; Peng, C. A novel chalcone derivative has antitumor activity in melanoma by inducing DNA damage through the upregulation of ROS products. Cancer Cell Int. 2020, 20, 36. [Google Scholar] [CrossRef] [PubMed]
- Hou, G.; Yuan, X.; Li, Y.; Hou, G.; Liu, X. Cardamonin, a natural chalcone, reduces 5-fluorouracil resistance of gastric cancer cells through targeting Wnt/β-catenin signal pathway. Investig. New Drugs 2020, 38, 329–339. [Google Scholar] [CrossRef]
- Shimada, T.; Naito, R.; Toriumi, R.; Nakagawa, R.; Aoyama, S.; Kamijima, T.; Kano, H.; Kadomoto, S.; Iwamoto, H.; Yaegashi, H.; et al. Novel α-Trifluoromethyl Chalcone Exerts Antitumor Effects Against Prostate Cancer Cells. Anticancer Res. 2023, 43, 2433–2444. [Google Scholar] [CrossRef]
- Huang, Z.; Yu, P.; Tang, J. Characterization of Triple-Negative Breast Cancer MDA-MB-231 Cell Spheroid Model. OncoTargets Ther. 2020, 13, 5395–5405. [Google Scholar] [CrossRef]
- Hero, T.; Bühler, H.; Kouam, P.N.; Priesch-Grzeszowiak, B.; Lateit, T.; Adamietz, I.A. The Triple-negative Breast Cancer Cell Line MDA-MB 231 Is Specifically Inhibited by the Ionophore Salinomycin. Anticancer Res. 2019, 39, 2821–2827. [Google Scholar] [CrossRef]
- Shubin, A.V.; Demidyuk, I.V.; Komissarov, A.A.; Rafieva, L.M.; Kostrov, S.V. Cytoplasmic vacuolization in cell death and survival. Oncotarget 2016, 7, 55863–55889. [Google Scholar] [CrossRef]
- Wickman, G.; Julian, L.; Olson, M.F. How apoptotic cells aid in the removal of their own cold dead bodies. Cell Death Differ. 2012, 19, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Brevnova, E.E.; Platoshyn, O.; Zhang, S.; Yuan, J.X. Overexpression of human KCNA5 increases IK V and enhances apoptosis. American journal of physiology. Cell Physiol. 2004, 287, C715–C722. [Google Scholar] [CrossRef] [PubMed]
- Andersson, B.; Janson, V.; Behnam-Motlagh, P.; Henriksson, R.; Grankvist, K. Induction of apoptosis by intracellular potassium ion depletion: Using the fluorescent dye PBFI in a 96-well plate method in cultured lung cancer cells. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2006, 20, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Remillard, C.V.; Yuan, J.X. Activation of K+ channels: An essential pathway in programmed cell death. American journal of physiology. Lung Cell. Mol. Physiol. 2004, 286, L49–L67. [Google Scholar] [CrossRef]
- Lansu, K.; Gentile, S. Potassium channel activation inhibits proliferation of breast cancer cells by activating a senescence program. Cell Death Dis. 2013, 4, e652. [Google Scholar] [CrossRef]
- Perez-Neut, M.; Rao, V.R.; Gentile, S. hERG1/Kv11.1 activation stimulates transcription of p21waf/cip in breast cancer cells via a calcineurin-dependent mechanism. Oncotarget 2016, 7, 58893–58902. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Ni, B.; Zhang, J.; Li, S.; Huang, Y.; Cai, Y.; Mei, H.; Li, Z. Loss of KCNJ15 expression promotes malignant phenotypes and correlates with poor prognosis in renal carcinoma. Cancer Manag. Res. 2019, 11, 1211–1220. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Y.; Chen, Y.; Xin, S.; Jin, L.; Liu, X.; Zhou, Z.; Zhang, J.; Mei, W.; Zhang, B.; et al. In Silico Establishment and Validation of Novel Lipid Metabolism-Related Gene Signature in Bladder Cancer. Oxidative Med. Cell. Longev. 2022, 2022, 3170950. [Google Scholar] [CrossRef]
- Farah, A.; Kabbage, M.; Atafi, S.; Gabteni, A.J.; Barbirou, M.; Madhioub, M.; Hamzaoui, L.; Mohamed, M.A.; Touinsi, H.; Kchaou, A.O.; et al. Selective expression of KCNA5 and KCNB1 genes in gastric and colorectal carcinoma. BMC Cancer 2020, 20, 1179. [Google Scholar] [CrossRef]
- Dubois, C.; Vanden Abeele, F.; Prevarskaya, N. Targeting apoptosis by the remodelling of calcium-transporting proteins in cancerogenesis. FEBS J. 2013, 280, 5500–5510. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.O. Calcium’s Role in Orchestrating Cancer Apoptosis: Mitochondrial-Centric Perspective. Int. J. Mol. Sci. 2023, 24, 8982. [Google Scholar] [CrossRef] [PubMed]
- Toyota, M.; Ho, C.; Ohe-Toyota, M.; Baylin, S.B.; Issa, J.P. Inactivation of CACNA1G, a T-type calcium channel gene, by aberrant methylation of its 5′ CpG island in human tumors. Cancer Res. 1999, 59, 4535–4541. [Google Scholar]
- Sekar, S.; Subbamanda, Y.; Pullaguri, N.; Sharma, A.; Sahu, C.; Kumar, R.; Bhargava, A.J.C.R.i.B. Isoform-specific expression of T-type voltage-gated calcium channels and estrogen receptors in breast cancer reveals specific isoforms that may be potential targets. Curr. Res. Biotechnol. 2022, 4, 459–467. [Google Scholar] [CrossRef]
- Phan, N.N.; Wang, C.Y.; Chen, C.F.; Sun, Z.; Lai, M.D.; Lin, Y.C. Voltage-gated calcium channels: Novel targets for cancer therapy. Oncol. Lett. 2017, 14, 2059–2074. [Google Scholar] [CrossRef] [PubMed]
- Liu, X. SLC Family Transporters. Adv. Exp. Med. Biol. 2019, 1141, 101–202. [Google Scholar]
- Pizzagalli, M.D.; Bensimon, A.; Superti-Furga, G. A guide to plasma membrane solute carrier proteins. FEBS J. 2021, 288, 2784–2835. [Google Scholar] [CrossRef]
- Ma, Y.; Bai, X.Y.; Du, X.; Fu, B.; Chen, X. NaDC3 Induces Premature Cellular Senescence by Promoting Transport of Krebs Cycle Intermediates, Increasing NADH, and Exacerbating Oxidative Damage. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016, 71, 1–12. [Google Scholar] [CrossRef]
- Lee, J.H.; Nam, J.H.; Park, J.; Kang, D.W.; Kim, J.Y.; Lee, M.G.; Yoon, J.S. Regulation of SLC26A3 activity by NHERF4 PDZ-mediated interaction. Cell. Signal. 2012, 24, 1821–1830. [Google Scholar] [CrossRef]
- Schweinfest, C.W.; Henderson, K.W.; Suster, S.; Kondoh, N.; Papas, T.S. Identification of a colon mucosa gene that is down-regulated in colon adenomas and adenocarcinomas. Proc. Natl. Acad. Sci. USA 1993, 90, 4166–4170. [Google Scholar] [CrossRef]
- Heise, M.; Lautem, A.; Knapstein, J.; Schattenberg, J.M.; Hoppe-Lotichius, M.; Foltys, D.; Weiler, N.; Zimmermann, A.; Schad, A.; Gründemann, D.; et al. Downregulation of organic cation transporters OCT1 (SLC22A1) and OCT3 (SLC22A3) in human hepatocellular carcinoma and their prognostic significance. BMC Cancer 2012, 12, 109. [Google Scholar] [CrossRef]
- Schaeffeler, E.; Hellerbrand, C.; Nies, A.T.; Winter, S.; Kruck, S.; Hofmann, U.; van der Kuip, H.; Zanger, U.M.; Koepsell, H.; Schwab, M. DNA methylation is associated with downregulation of the organic cation transporter OCT1 (SLC22A1) in human hepatocellular carcinoma. Genome Med. 2011, 3, 82. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, R.; Meeson, A.; Lowes, S. Solute transporters and malignancy: Establishing the role of uptake transporters in breast cancer and breast cancer metastasis. Cancer Metastasis Rev. 2020, 39, 919–932. [Google Scholar] [CrossRef]
- Mohelnikova-Duchonova, B.; Brynychova, V.; Hlavac, V.; Kocik, M.; Oliverius, M.; Hlavsa, J.; Honsova, E.; Mazanec, J.; Kala, Z.; Melichar, B.; et al. The association between the expression of solute carrier transporters and the prognosis of pancreatic cancer. Cancer Chemother. Pharmacol. 2013, 72, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Lautem, A.; Heise, M.; Gräsel, A.; Hoppe-Lotichius, M.; Weiler, N.; Foltys, D.; Knapstein, J.; Schattenberg, J.M.; Schad, A.; Zimmermann, A.; et al. Downregulation of organic cation transporter 1 (SLC22A1) is associated with tumor progression and reduced patient survival in human cholangiocellular carcinoma. Int. J. Oncol. 2013, 42, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, W.; Pu, Q.; Yang, Y.; Ye, S.; Ma, Q.; Ren, J.; Cao, Z.; Zhong, G.; Zhang, X.; et al. The effects and mechanisms of SLC34A2 in tumorigenesis and progression of human non-small cell lung cancer. J. Biomed. Sci. 2015, 22, 52. [Google Scholar] [CrossRef]
- Di Marco, G.S.; Hausberg, M.; Hillebrand, U.; Rustemeyer, P.; Wittkowski, W.; Lang, D.; Pavenstädt, H. Increased inorganic phosphate induces human endothelial cell apoptosis in vitro. Am. J. Physiol. Ren. Physiol. 2008, 294, F1381–F1387. [Google Scholar] [CrossRef]
- Park, J.W.; Yook, J.M.; Ryu, H.M.; Choi, S.Y.; Morishita, M.; Do, J.Y.; Park, S.H.; Kim, C.D.; Choi, J.Y.; Chung, H.Y.; et al. Phosphate-induced apoptosis in human peritoneal mesothelial cells in vitro. Am. J. Nephrol. 2011, 34, 77–86. [Google Scholar] [CrossRef]
- Hlaváč, V.; Brynychová, V.; Václavíková, R.; Ehrlichová, M.; Vrána, D.; Pecha, V.; Koževnikovová, R.; Trnková, M.; Gatěk, J.; Kopperová, D.; et al. The expression profile of ATP-binding cassette transporter genes in breast carcinoma. Pharmacogenomics 2013, 14, 515–529. [Google Scholar] [CrossRef]
- Hlavata, I.; Mohelnikova-Duchonova, B.; Vaclavikova, R.; Liska, V.; Pitule, P.; Novak, P.; Bruha, J.; Vycital, O.; Holubec, L.; Treska, V.; et al. The role of ABC transporters in progression and clinical outcome of colorectal cancer. Mutagenesis 2012, 27, 187–196. [Google Scholar] [CrossRef]
- Nelson, P.T.; Jicha, G.A.; Wang, W.X.; Ighodaro, E.; Artiushin, S.; Nichols, C.G.; Fardo, D.W. ABCC9/SUR2 in the brain: Implications for hippocampal sclerosis of aging and a potential therapeutic target. Ageing Res. Rev. 2015, 24 Pt B, 111–125. [Google Scholar] [CrossRef]
- Hendig, D.; Langmann, T.; Zarbock, R.; Schmitz, G.; Kleesiek, K.; Götting, C. Characterization of the ATP-binding cassette transporter gene expression profile in Y79: A retinoblastoma cell line. Mol. Cell. Biochem. 2009, 328, 85–92. [Google Scholar] [CrossRef] [PubMed]
- de Grouw, E.P.; Raaijmakers, M.H.; Boezeman, J.B.; van der Reijden, B.A.; van de Locht, L.T.; de Witte, T.J.; Jansen, J.H.; Raymakers, R.A. Preferential expression of a high number of ATP binding cassette transporters in both normal and leukemic CD34+CD38- cells. Leukemia 2006, 20, 750–754. [Google Scholar] [CrossRef]
- Demidenko, R.; Razanauskas, D.; Daniunaite, K.; Lazutka, J.R.; Jankevicius, F.; Jarmalaite, S. Frequent down-regulation of ABC transporter genes in prostate cancer. BMC Cancer 2015, 15, 683. [Google Scholar] [CrossRef]
- Gillet, J.P.; Schneider, J.; Bertholet, V.; De Longueville, F.; Remacle, J.; Efferth, T. Microarray Expression Profiling of ABC Transporters in Human Breast Cancer. Cancer Genom. Proteom. 2006, 3, 97–106. [Google Scholar]
- Lanneau, D.; Brunet, M.; Frisan, E.; Solary, E.; Fontenay, M.; Garrido, C. Heat shock proteins: Essential proteins for apoptosis regulation. J. Cell. Mol. Med. 2008, 12, 743–761. [Google Scholar] [CrossRef]
- Kennedy, D.; Jäger, R.; Mosser, D.D.; Samali, A. Regulation of apoptosis by heat shock proteins. IUBMB Life 2014, 66, 327–338. [Google Scholar] [CrossRef]
- Kenny, M.K.; Mendez, F.; Sandigursky, M.; Kureekattil, R.P.; Goldman, J.D.; Franklin, W.A.; Bases, R. Heat shock protein 70 binds to human apurinic/apyrimidinic endonuclease and stimulates endonuclease activity at abasic sites. J. Biol. Chem. 2001, 276, 9532–9536. [Google Scholar] [CrossRef] [PubMed]
- Mendez, F.; Sandigursky, M.; Franklin, W.A.; Kenny, M.K.; Kureekattil, R.; Bases, R. Heat-shock proteins associated with base excision repair enzymes in HeLa cells. Radiat. Res. 2000, 153, 186–195. [Google Scholar] [CrossRef]
- Mendez, F.; Kozin, E.; Bases, R. Heat shock protein 70 stimulation of the deoxyribonucleic acid base excision repair enzyme polymerase beta. Cell Stress Chaperones 2003, 8, 153–161. [Google Scholar] [CrossRef]
- Hai, T.; Wolfgang, C.D.; Marsee, D.K.; Allen, A.E.; Sivaprasad, U. ATF3 and stress responses. Gene Expr. 1999, 7, 321–335. [Google Scholar] [PubMed]
- Lu, D.; Wolfgang, C.D.; Hai, T. Activating transcription factor 3, a stress-inducible gene, suppresses Ras-stimulated tumorigenesis. J. Biol. Chem. 2006, 281, 10473–10481. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.J.; Seo, Y.H. A novel chalcone-based molecule, BDP inhibits MDA-MB-231 triple-negative breast cancer cell growth by suppressing Hsp90 function. Oncol. Rep. 2017, 38, 2343–2350. [Google Scholar] [CrossRef] [PubMed]
- Mai, C.W.; Yaeghoobi, M.; Abd-Rahman, N.; Kang, Y.B.; Pichika, M.R. Chalcones with electron-withdrawing and electron-donating substituents: Anticancer activity against TRAIL resistant cancer cells, structure-activity relationship analysis and regulation of apoptotic proteins. Eur. J. Med. Chem. 2014, 77, 378–387. [Google Scholar] [CrossRef]
- Silva, G.; Marins, M.; Chaichanasak, N.; Yoon, Y.; Fachin, A.L.; Pinhanelli, V.C.; Regasini, L.O.; Dos Santos, M.B.; Ayusso, G.M.; Marques, B.C.; et al. Trans-chalcone increases p53 activity via DNAJB1/HSP40 induction and CRM1 inhibition. PLoS ONE 2018, 13, e0202263. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, J.; Huang, G.; Zhao, Y.; Yue, X.; Wu, H.; Li, J.; Zhu, J.; Shen, Z.; Haffty, B.G.; et al. Cullin3-KLHL25 ubiquitin ligase targets ACLY for degradation to inhibit lipid synthesis and tumor progression. Genes Dev. 2016, 30, 1956–1970. [Google Scholar] [CrossRef]
- Dhanoa, B.S.; Cogliati, T.; Satish, A.G.; Bruford, E.A.; Friedman, J.S. Update on the Kelch-like (KLHL) gene family. Hum. Genom. 2013, 7, 13. [Google Scholar] [CrossRef]
- Ye, G.; Wang, J.; Yang, W.; Li, J.; Ye, M.; Jin, X. The roles of KLHL family members in human cancers. Am. J. Cancer Res. 2022, 12, 5105–5139. [Google Scholar]
- Choi, J.; Zhou, N.; Busino, L. KLHL6 is a tumor suppressor gene in diffuse large B-cell lymphoma. Cell Cycle 2019, 18, 249–256. [Google Scholar] [CrossRef]
- Kroll, J.; Shi, X.; Caprioli, A.; Liu, H.H.; Waskow, C.; Lin, K.M.; Miyazaki, T.; Rodewald, H.R.; Sato, T.N. The BTB-kelch protein KLHL6 is involved in B-lymphocyte antigen receptor signaling and germinal center formation. Mol. Cell. Biol. 2005, 25, 8531–8540. [Google Scholar] [CrossRef]
- Taniguchi, K.; Wada, M.; Kohno, K.; Nakamura, T.; Kawabe, T.; Kawakami, M.; Kagotani, K.; Okumura, K.; Akiyama, S.; Kuwano, M. A human canalicular multispecific organic anion transporter (cMOAT) gene is overexpressed in cisplatin-resistant human cancer cell lines with decreased drug accumulation. Cancer Res. 1996, 56, 4124–4129. [Google Scholar] [PubMed]
- Tada, Y.; Wada, M.; Migita, T.; Nagayama, J.; Hinoshita, E.; Mochida, Y.; Maehara, Y.; Tsuneyoshi, M.; Kuwano, M.; Naito, S. Increased expression of multidrug resistance-associated proteins in bladder cancer during clinical course and drug resistance to doxorubicin. Int. J. Cancer 2002, 98, 630–635. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; He, X.; Yao, R.; Fan, L.; Zhao, L.; Lu, B.; Pang, Z. Genome instability-related LINC02577, LINC01133 and AC107464.2 are lncRNA prognostic markers correlated with immune microenvironment in pancreatic adenocarcinoma. BMC Cancer 2023, 23, 430. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wu, Y.; Li, C.; Qu, Z.; Lou, G.; Guo, X.; Ji, J.; Li, N.; Guo, M.; Zhang, M.; et al. Comprehensive analysis of the SLC16A gene family in pancreatic cancer via integrated bioinformatics. Sci. Rep. 2020, 10, 7315. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Luo, Y.; Zheng, Y.; Zheng, L.; Lin, W.; Chen, Z.; Wu, S.; Chen, J.; Xie, Y. Long non-coding RNA LINC00426 contributes to doxorubicin resistance by sponging miR-4319 in osteosarcoma. Biol. Direct 2020, 15, 11. [Google Scholar] [CrossRef]
- Sun, J.; Nagel, R.; Zaal, E.A.; Ugalde, A.P.; Han, R.; Proost, N.; Song, J.Y.; Pataskar, A.; Burylo, A.; Fu, H.; et al. SLC1A3 contributes to L-asparaginase resistance in solid tumors. EMBO J. 2019, 38, e102147. [Google Scholar] [CrossRef]
- Qiang, Y.W.; Ye, S.; Huang, Y.; Chen, Y.; Van Rhee, F.; Epstein, J.; Walker, B.A.; Morgan, G.J.; Davies, F.E. MAFb protein confers intrinsic resistance to proteasome inhibitors in multiple myeloma. BMC Cancer 2018, 18, 724. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, C.; Liu, W.; Du, X.; Liu, X.; Xing, B. Long noncoding RNA LINC01234 promotes hepatocellular carcinoma progression through orchestrating aspartate metabolic reprogramming. Mol. Ther. J. Am. Soc. Gene Ther. 2022, 30, 2354–2369. [Google Scholar] [CrossRef]
- Lei, Z.N.; Tian, Q.; Teng, Q.X.; Wurpel, J.N.D.; Zeng, L.; Pan, Y.; Chen, Z.S. Understanding and targeting resistance mechanisms in cancer. MedComm 2023, 4, e265. [Google Scholar] [CrossRef]
- Nishino, K.; Yamasaki, S.; Nakashima, R.; Zwama, M.; Hayashi-Nishino, M. Function and Inhibitory Mechanisms of Multidrug Efflux Pumps. Front. Microbiol. 2021, 12, 737288. [Google Scholar] [CrossRef]
- Gumilar, K.E.; Chin, Y.; Ibrahim, I.H.; Tjokroprawiro, B.A.; Yang, J.Y.; Zhou, M.; Gassman, N.R.; Tan, M. Heat Shock Factor 1 Inhibition: A Novel Anti-Cancer Strategy with Promise for Precision Oncology. Cancers 2023, 15, 5167. [Google Scholar] [CrossRef] [PubMed]
- Dastidar, S.G.; De Kumar, B.; Lauckner, B.; Parrello, D.; Perley, D.; Vlasenok, M. Transcriptional responses of cancer cells to heat shock-inducing stimuli involve amplification of robust HSF1 binding. Nat. Commun. 2023, 14, 7420. [Google Scholar] [CrossRef] [PubMed]
- Trinklein, N.D.; Murray, J.I.; Hartman, S.J.; Botstein, D.; Myers, R.M. The role of heat shock transcription factor 1 in the genome-wide regulation of the mammalian heat shock response. Mol. Biol. Cell 2004, 15, 1254–1261. [Google Scholar] [CrossRef]
- Neudegger, T.; Verghese, J.; Hayer-Hartl, M.; Hartl, F.U.; Bracher, A. Structure of human heat-shock transcription factor 1 in complex with DNA. Nat. Struct. Mol. Biol. 2016, 23, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Cyran, A.M.; Zhitkovich, A. Heat Shock Proteins and HSF1 in Cancer. Front. Oncol. 2022, 12, 860320. [Google Scholar] [CrossRef]
- Abravaya, K.; Phillips, B.; Morimoto, R.I. Attenuation of the heat shock response in HeLa cells is mediated by the release of bound heat shock transcription factor and is modulated by changes in growth and in heat shock temperatures. Genes Dev. 1991, 5, 2117–2127. [Google Scholar] [CrossRef]
- Wilson, A.J.; Chueh, A.C.; Tögel, L.; Corner, G.A.; Ahmed, N.; Goel, S.; Byun, D.S.; Nasser, S.; Houston, M.A.; Jhawer, M.; et al. Apoptotic sensitivity of colon cancer cells to histone deacetylase inhibitors is mediated by an Sp1/Sp3-activated transcriptional program involving immediate-early gene induction. Cancer Res. 2010, 70, 609–620. [Google Scholar] [CrossRef]
- Chüeh, A.C.; Tse, J.W.T.; Dickinson, M.; Ioannidis, P.; Jenkins, L.; Togel, L.; Tan, B.; Luk, I.; Davalos-Salas, M.; Nightingale, R.; et al. ATF3 Repression of BCL-X(L) Determines Apoptotic Sensitivity to HDAC Inhibitors across Tumor Types. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 5573–5584. [Google Scholar] [CrossRef]
- Yoo, K.H.; Kim, D.H.; Oh, S.; Park, M.S.; Kim, H.; Ha, H.H.; Cho, S.H.; Chung, I.J.; Bae, W.K. Transcriptome analysis upon potassium usnate exposure reveals ATF3-induced apoptosis in human gastric and colon cancer cells. Phytomedicine Int. J. Phytother. Phytopharm. 2021, 91, 153655. [Google Scholar] [CrossRef]
- Tanaka, Y.; Nakamura, A.; Morioka, M.S.; Inoue, S.; Tamamori-Adachi, M.; Yamada, K.; Taketani, K.; Kawauchi, J.; Tanaka-Okamoto, M.; Miyoshi, J.; et al. Systems analysis of ATF3 in stress response and cancer reveals opposing effects on pro-apoptotic genes in p53 pathway. PLoS ONE 2011, 6, e26848. [Google Scholar] [CrossRef]
- Liu, G.; Su, L.; Hao, X.; Zhong, N.; Zhong, D.; Singhal, S.; Liu, X. Salermide up-regulates death receptor 5 expression through the ATF4-ATF3-CHOP axis and leads to apoptosis in human cancer cells. J. Cell. Mol. Med. 2012, 16, 1618–1628. [Google Scholar] [CrossRef] [PubMed]
- Kucerova-Chlupacova, M.; Vyskovska-Tyllova, V.; Richterova-Finkova, L.; Kunes, J.; Buchta, V.; Vejsova, M.; Paterova, P.; Semelkova, L.; Jandourek, O.; Opletalova, V. Novel Halogenated Pyrazine-Based Chalcones as Potential Antimicrobial Drugs. Molecules 2016, 21, 1421. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. Feature Counts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Love, M.; Anders, S.; Huber, W.J.G.B. Differential analysis of count data–the DESeq2 package. Genome Biol. 2014, 15, 10–1186. [Google Scholar]
- Ge, S.X.; Jung, D.; Yao, R. ShinyGO: A graphical gene-set enrichment tool for animals and plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef]
- Jassal, B.; Matthews, L.; Viteri, G.; Gong, C.; Lorente, P.; Fabregat, A.; Sidiropoulos, K.; Cook, J.; Gillespie, M.; Haw, R.; et al. The reactome pathway knowledgebase. Nucleic Acids Res. 2020, 48, D498–D503. [Google Scholar] [CrossRef]
- Franceschini, A.; Szklarczyk, D.; Frankild, S.; Kuhn, M.; Simonovic, M.; Roth, A.; Lin, J.; Minguez, P.; Bork, P.; von Mering, C.; et al. STRING v9.1: Protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2013, 41, D808–D815. [Google Scholar] [CrossRef]
- Fabregat, A.; Sidiropoulos, K.; Garapati, P.; Gillespie, M.; Hausmann, K.; Haw, R.; Jassal, B.; Jupe, S.; Korninger, F.; McKay, S.; et al. The Reactome pathway Knowledgebase. Nucleic Acids Res. 2015, 44, D481–D487. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, A.; Sidiropoulos, K.; Viteri, G.; Forner, O.; Marin-Garcia, P.; Arnau, V.; D’eustachio, P.; Stein, L.; Hermjakob, H. Reactome pathway analysis: A high-performance in-memory approach. BMC Bioinform. 2017, 18, 142. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Rungger, D.; Voellmy, R. Multiple Layers of Regulation of Human Heat Shock Transcription Factor 1. Mol. Cell. Biol. 1995, 15, 4319–4330. [Google Scholar] [CrossRef] [PubMed]
- Baler, R.; Dahl, G.; Voellmy, R. Activation of Human Heat Shock Genes Is Accompanied by Oligomerization, Modification, and Rapid Translocation of Heat Shock Transcription Factor HSF1. Mol. Cell. Biol. 1993, 13, 2486–2496. [Google Scholar] [CrossRef]
- Sarge, K.D.; Murphy, S.P.; Morimoto, R.I. Activation of Heat Shock Gene Transcription by Heat Shock Factor 1 Involves Oligomerization, Acquisition of DNA-Binding Activity, and Nuclear Localization and Can Occur in the Absence of Stress. Mol. Cell. Biol. 1993, 13, 1392–1407. [Google Scholar] [CrossRef]
- Cotto, J.J.; Kline, M.; Morimoto, R.I. Activation of Heat Shock Factor 1 DNA Binding Precedes Stress-induced Serine Phosphorylation. J. Biol. Chem. 1996, 271, 3355–3358. [Google Scholar] [CrossRef]
- Vasudevan, D.; Takeuchi, H.; Johar, S.S.; Majerus, E.; Haltiwanger, R.S. Peters Plus Syndrome Mutations Disrupt a Noncanonical ER Quality-Control Mechanism. Curr. Biol. 2015, 25, 286–295. [Google Scholar] [CrossRef]
- Maki, M.; Heinonen, T.Y. Peters’-plus syndrome is a congenital disorder of glycosylation caused by a defect in the beta1,3-glucosyltransferase that modifies thrombospondin type 1 repeats. Ann. Med. 2009, 41, 2–10. [Google Scholar]
- Bamforth, S.D.; Bragança, J.; Farthing, C.R.; Schneider, J.E.; Broadbent, C.; Michell, A.C.; Clarke, K.; Neubauer, S.; Norris, D.; Brown, N.A.; et al. Cited2 controls left-right patterning and heart development through a Nodal-Pitx2c pathway. Nat. Genet. 2004, 36, 1189–1196. [Google Scholar] [CrossRef]
- Li, Q.; Pan, H.; Guan, L.; Su, D.; Ma, X. CITED2 mutation links congenital heart defects to dysregulation of the cardiac gene VEGF and PITX2C expression. Biochem. Biophys. Res. Commun. 2012, 423, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, L.M.; Dlugosz, M.; Luther, K.B.; Haltiwanger, R.S.; Majerus, E.M. O-Fucosylation Is Required for ADAMTS13 Secretion. J. Biol. Chem. 2007, 282, 17014–17023. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.C.; Tucker, R.P. The thrombospondin type 1 repeat (TSR) superfamily: Diverse proteins with related roles in neuronal development. Dev. Dyn. 2000, 218, 280–299. [Google Scholar] [CrossRef]
- Luo, Y.; Nita-Lazar, A.; Haltiwanger, R.S. Two Distinct Pathways for O-Fucosylation of Epidermal Growth Factor-like or Thrombospondin Type 1 Repeats. J. Biol. Chem. 2006, 281, 9385–9392. [Google Scholar] [CrossRef]
- Hofsteenge, J.; Huwiler, K.G.; Macek, B.; Hess, D.; Lawler, J.; Mosher, D.F.; Peter-Katalinic, J. C-Mannosylation and O-Fucosylation of the Thrombospondin Type 1 Module. J. Biol. Chem. 2001, 276, 6485–6498. [Google Scholar] [CrossRef]
- Gonzalez de Peredo, A.; Klein, D.; Macek, B.; Hess, D.; Peter-Katalinic, J.; Hofsteenge, J. C-Mannosylation and O-Fucosylation of Thrombospondin Type 1 Repeats. Mol. Cell. Proteom. 2002, 1, 11–18. [Google Scholar] [CrossRef]
- Della Corte, C.M.; Viscardi, G.; Di Liello, R.; Fasano, M.; Martinelli, E.; Troiani, T.; Ciardiello, F.; Morgillo, F. Role and targeting of anaplastic lymphoma kinase in cancer. Mol. Cancer 2018, 17, 30. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R., Jr. Anaplastic lymphoma kinase (ALK): Structure, oncogenic activation, and pharmacological inhibition. Pharmacol. Res. 2013, 68, 68–94. [Google Scholar] [CrossRef]
- Lin, J.J.; Riely, G.J.; Shaw, A.T. Targeting ALK: Precision Medicine Takes on Drug Resistance. Cancer Discov. 2017, 7, 137–155. [Google Scholar] [CrossRef]
- Heuckmann, J.M.; Hölzel, M.; Sos, M.L.; Heynck, S.; Balke-Want, H.; Koker, M.; Peifer, M.; Weiss, J.; Lovly, C.M.; Grütter, C.; et al. ALK mutations conferring differential resistance to structurally diverse ALK inhibitors. Clin. Cancer Res. 2011, 17, 7394–7401. [Google Scholar] [CrossRef]
- George, R.E.; Sanda, T.; Hanna, M.; Fröhling, S.; Luther, W., II; Zhang, J.; Ahn, Y.; Zhou, W.; London, W.B.; McGrady, P.; et al. Activating mutations in ALK provide a therapeutic target in neuroblastoma. Nature 2008, 455, 975–978. [Google Scholar] [CrossRef] [PubMed]
- Ceccon, M.; Mologni, L.; Scapozza, L.; Bisson, W.; Gambacorti-Passerini, C. Crizotinib-resistant NPM-ALK mutants confer differential sensitivity to unrelated Alk inhibitors. Mol. Cancer Res. 2013, 11, 122–132. [Google Scholar] [CrossRef]
- Sasaki, T.; Koivunen, J.; Ogino, A.; Yanagita, M.; Nikiforow, S.; Zheng, W.; Lathan, C.; Marcoux, J.P.; Du, J.; Okuda, K.; et al. A novel ALK secondary mutation and EGFR signaling cause resistance to ALK kinase inhibitors. Cancer Res. 2011, 71, 6051–6060. [Google Scholar] [CrossRef]
- Lovly, C.M.; Pao, W. Escaping ALK inhibition: Mechanisms of and strategies to overcome resistance. Sci. Transl. Med. 2012, 4, 120ps2. [Google Scholar] [CrossRef] [PubMed]
- Facchinetti, F.; Tiseo, M.; Di Maio, M.; Graziano, P.; Bria, E.; Rossi, G.; Novello, S. Tackling ALK in non-small cell lung cancer: The role of novel inhibitors. Transl. Lung Cancer Res. 2016, 5, 301–321. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Seto, Y.; Okada, K.; Uematsu, S.; Uchibori, K.; Tsukahara, M.; Oh-Hara, T.; Fujita, N.; Yanagitani, N.; Nishio, M.; et al. Overcoming resistance by ALK compound mutation (I1171S + G1269A) after sequential treatment of multiple ALK inhibitors in non-small cell lung cancer. Thorac. Cancer 2020, 11, 581–587. [Google Scholar] [CrossRef]
- Yoda, S.; Lin, J.J.; Lawrence, M.S.; Burke, B.J.; Friboulet, L.; Langenbucher, A.; Dardaei, L.; Prutisto-Chang, K.; Dagogo-Jack, I.; Timofeevski, S.; et al. Sequential ALK Inhibitors Can Select for Lorlatinib-Resistant Compound ALK Mutations in ALK-Positive Lung Cancer. Cancer Discov. 2018, 8, 714–729. [Google Scholar] [CrossRef]
- Katayama, R.; Friboulet, L.; Koike, S.; Lockerman, E.L.; Khan, T.M.; Gainor, J.F.; Iafrate, A.J.; Takeuchi, K.; Taiji, M.; Okuno, Y.; et al. Two novel ALK mutations mediate acquired resistance to the next-generation ALK inhibitor alectinib. Clin. Cancer Res. 2014, 20, 5686–5696. [Google Scholar] [CrossRef]
- Amin, A.D.; Li, L.; Rajan, S.S.; Gokhale, V.; Groysman, M.J.; Pongtornpipat, P.; Tapia, E.O.; Wang, M.; Schatz, J.H. TKI sensitivity patterns of novel kinase-domain mutations suggest therapeutic opportunities for patients with resistant ALK+ tumors. Oncotarget 2016, 7, 23715–23729. [Google Scholar] [CrossRef][Green Version]
- Hallberg, B.; Palmer, R.H. Mechanistic insight into ALK receptor tyrosine kinase in human cancer biology. Nat. Rev. Cancer 2013, 13, 685–700. [Google Scholar] [CrossRef]
- Tolan, D.R. Molecular basis of hereditary fructose intolerance: Mutations and polymorphisms in the human aldolase B gene. Hum. Mutat. 1995, 6, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Cross, N.C.; Tolan, D.R.; Cox, T.M. Catalytic deficiency of human aldolase B in hereditary fructose intolerance caused by a common missense mutation. Cell 1988, 53, 881–885. [Google Scholar] [CrossRef]
- Domogatskaya, A.; Rodin, S.; Tryggvason, K. Functional diversity of laminins. Annu. Rev. Cell Dev. Biol. 2012, 28, 523–553. [Google Scholar] [CrossRef] [PubMed]
- Alper, S.L.; Sharma, A.K. The SLC26 gene family of anion transporters and channels. Mol. Asp. Med. 2013, 34, 494–515. [Google Scholar] [CrossRef] [PubMed]
- Wedenoja, S.; Pekansaari, E.; Höglund, P.; Mäkelä, S.; Holmberg, C.; Kere, J. Update on SLC26A3 mutations in congenital chloride diarrhea. Hum. Mutat. 2011, 32, 715–722. [Google Scholar] [CrossRef]
- Akil, O.; Seal, R.P.; Burke, K.; Wang, C.; Alemi, A.; During, M.; Edwards, R.H.; Lustig, L.R. Restoration of Hearing in the VGLUT3 Knockout Mouse Using Virally Mediated Gene Therapy. Neuron 2012, 75, 283–293. [Google Scholar] [CrossRef]
- Ruel, J.; Emery, S.; Nouvian, R.; Bersot, T.; Amilhon, B.; Van Rybroek, J.M.; Rebillard, G.; Lenoir, M.; Eybalin, M.; Delprat, B.; et al. Impairment of SLC17A8 Encoding Vesicular Glutamate Transporter-3, VGLUT3, Underlies Nonsyndromic Deafness DFNA25 and Inner Hair Cell Dysfunction in Null Mice. Am. J. Hum. Genet. 2008, 83, 278–292. [Google Scholar] [CrossRef]
- Harakalova, M.; Van Harssel, J.J.; Terhal, P.A.; Van Lieshout, S.; Duran, K.; Renkens, I.; Amor, D.J.; Wilson, L.C.; Kirk, E.P.; Turner, C.L.; et al. Dominant missense mutations in ABCC9 cause Cantú syndrome. Nat. Genet. 2012, 44, 793–796. [Google Scholar] [CrossRef]
- van Bon, B.W.; Gilissen, C.; Grange, D.K.; Hennekam, R.C.; Kayserili, H.; Engels, H.; Reutter, H.; Ostergaard, J.R.; Morava, E.; Tsiakas, K.; et al. Cantú Syndrome Is Caused by Mutations in ABCC9. Am. J. Hum. Genet. 2012, 90, 1094–1101. [Google Scholar] [CrossRef]
- Ashcroft, F.M.; Tammaro, P. A mutation in the ATP-binding site of the Kir6.2 subunit of the KATP channel alters coupling with the SUR2A subunit. J. Physiol. 2007, 584, 743–753. [Google Scholar]
- Olson, T.M.; Alekseev, A.E.; Moreau, C.; Liu, X.K.; Zingman, L.V.; Miki, T.; Seino, S.; Asirvatham, S.J.; Jahangir, A.; Terzic, A. KATP channel mutation confers risk for vein of Marshall adrenergic atrial fibrillation. Nat. Clin. Pract. Cardiovasc. Med. 2007, 4, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Bryan, J.; Gonzalez, G.; Aguilar-Bryan, L.; Babenko, A.P. Reconstituted human cardiac KATP channels: Functional identity with the native channels from the sarcolemma of human ventricular cells. Circ. Res. 1998, 83, 1132–1143. [Google Scholar]
- Corut, A.; Senyigit, A.; Ugur, S.A.; Altin, S.; Ozcelik, U.; Calisir, H.; Yildirim, Z.; Gocmen, A.; Tolun, A. Mutations in SLC34A2 Cause Pulmonary Alveolar Microlithiasis and Are Possibly Associated with Testicular Microlithiasis. Am. J. Hum. Genet. 2006, 79, 650–656. [Google Scholar] [CrossRef]
- Biber, J.; Forster, I.C.; Hernando, N.; Murer, H. Phosphate transporters of the SLC20 and SLC34 families. Mol. Asp. Med. 2013, 34, 386–395. [Google Scholar]
- Shida, Y.; Rydz, N.; Stegner, D.; Brown, C.; Mewburn, J.; Sponagle, K.; Danisment, O.; Crawford, B.; Vidal, B.; Hegadorn, C.A.; et al. Analysis of the role of von Willebrand factor, platelet glycoprotein VI-, and α2β1-mediated collagen binding in thrombus formation. Blood 2014, 124, 1799–1807. [Google Scholar] [CrossRef] [PubMed]
- Riddell, A.F.; Gomez, K.; Millar, C.M.; Mellars, G.; Gill, S.; Brown, S.A.; Sutherland, M.; Laffan, M.A.; McKinnon, T.A.J. Characterization of W1745C and S1783A: 2 novel mutations causing defective collagen binding in the A3 domain of von Willebrand factor. Blood 2009, 114, 3489–3496. [Google Scholar] [CrossRef]
- Flood, V.H.; Lederman, C.A.; Wren, J.S.; Christopherson, P.A.; Friedman, K.D.; Hoffmann, R.G.; Montgomery, R.R. Absent collagen binding in a VWF A3 domain mutant: Utility of the VWF:CB in diagnosis of VWD. J. Thromb. Haemost. 2010, 8, 1431–1433. [Google Scholar] [CrossRef]
- Maas, D.P.; Atiq, F.; Blijlevens, N.M.; Brons, P.P.; Krouwel, S.; Gorkom, B.A.L.; Leebeek, F.W.; Nieuwenhuizen, L.; Schoormans, S.C.; Simons, A.; et al. Von Willebrand disease type 2M: Correlation between genotype and phenotype. J. Thromb. Haemost. 2021, 20, 316–327. [Google Scholar] [CrossRef]
- Yin, X.; Wang, H.; Wu, D.; Zhao, G.; Shao, J.; Dai, Y. SLC34A2 Gene mutation of pulmonary alveolar microlithiasis: Report of four cases and review of literatures. Respir. Med. 2012, 107, 217–222. [Google Scholar] [CrossRef][Green Version]
- Whitsett, J.A.; Wert, S.E.; Weaver, T.E. Diseases of Pulmonary Surfactant Homeostasis. Annu. Rev. Pathol. Mech. Dis. 2015, 10, 371–393. [Google Scholar] [CrossRef]
- Marchiori, E.; Pereira e Silva, J.L.; Ferreira Francisco, F.A.; Hochhegger, B.; Zanetti, G. Pulmonary alveolar microlithiasis. State-of-the-art review. Respir. Med. 2013, 107, 1–9. [Google Scholar]

| Symbol | Gene Name | log2FC | p-Value | p-Adjusted |
|---|---|---|---|---|
| Members of the VGKC family | ||||
| KCNB1 | Potassium voltage-gated channel subfamily B member 1 | 5.0077 | 0.0034 | 0.0456 |
| KCNH3 | Potassium voltage-gated channel subfamily H member 3 | 5.7809 | 2.19 × 10−5 | 0.0008 |
| KCNJ15 | Potassium inwardly rectifying channel subfamily J member 15 | 3.6421 | 0.0023 | 0.0337 |
| KCNN1 | Potassium calcium-activated channel subfamily N member 1 | 5.8723 | 1.74 × 10−5 | 0.0006 |
| Members of the VGCC family | ||||
| CACNA1E | Calcium voltage-gated channel subunit alpha1 E | 7.0921 | 7.82 × 10−5 | 0.0024 |
| CACNA1G | Calcium voltage-gated channel subunit alpha1 G | 6.3592 | 1.31 × 10−14 | 2.07 × 10−12 |
| Members of the SLC family | ||||
| SLC13A3 | Solute carrier family 13 member 3 | 5.5741 | 5.71 × 10−6 | 0.0002 |
| SLC16A1 | Solute carrier family 16 member 1 | 5.8161 | 0.0001 | 0.0045 |
| SLC17A8 | Solute carrier family 17 member 8 | 6.6355 | 0.0012 | 0.0220 |
| SLC22A1 | Solute carrier family 22 member 1 | 4.0406 | 0.0031 | 0.0430 |
| SLC22A20P | Solute carrier family 22 member 20, pseudogene | 3.5557 | 0.0025 | 0.0368 |
| SLC26A3 | Solute carrier family 26 member 3 | 5.3321 | 0.0011 | 0.0198 |
| SLC34A2 | Solute carrier family 34 member 2 | 5.8169 | 0.0016 | 0.0268 |
| SLC5A5 | Solute carrier family 5 member 5 | 6.1385 | 2.52 × 10−8 | 1.92 × 10−6 |
| SLC6A13 | Solute carrier family 6 member 13 | 6.2167 | 0.0014 | 0.0238 |
| SLC6A4 | Solute carrier family 6 member 4 | 5.0679 | 0.0037 | 0.0487 |
| Members of the ABC family | ||||
| ABCA13 | ATP-binding cassette subfamily A member 13 | 5.6202 | 0.0033 | 0.0447 |
| ABCC2 | ATP-binding cassette subfamily C member 2 | 2.1551 | 1.39 × 10−7 | 9.08 × 10−6 |
| ABCC9 | ATP-binding cassette subfamily C member 9 | 5.0750 | 3.47 × 10−6 | 0.0001 |
| Members of the HSP family | ||||
| HSPA1A | Heat shock protein family A (Hsp70) member 1A | 8.0281 | 1.44 × 10−8 | 1.16 × 10−6 |
| HSPA4L | Heat shock protein family A (Hsp70) member 4 like | 2.1221 | 9.57 × 10−23 | 3.57 × 10−20 |
| HSPA6 | Heat shock protein family A (Hsp70) member 6 | 10.1994 | 3.68 × 10−48 | 4.39 × 10−45 |
| HSPA7 | Heat shock protein family A (Hsp70) member 7 (pseudogene) | 9.2484 | 2.1 × 10−12 | 2.77 × 10−10 |
| HSPB1 | Heat shock protein family B (small) member 1 | 2.5661 | 9.23 × 10−4 | 0.0170 |
| HSPB8 | Heat shock protein family B (small) member 8 | 3.3302 | 1.11 × 10−6 | 5.99 × 10−5 |
| HSPD1 | Heat shock protein family D (Hsp60) member 1 | 2.1816 | 1.61 × 10−34 | 1.11 × 10−31 |
| Members of the KLHL family | ||||
| KLHL25 | Kelch-like family member 25 | 2.0169 | 2.92 × 10−6 | 0.0001 |
| KLHL3 | Kelch-like family member 3 | 3.0216 | 4.1 × 10−5 | 0.0014 |
| KLHL6 | Kelch-like family member 6 | 6.1169 | 0.0021 | 0.0328 |
| Identifier | Pathway * | Genes Found | Ratio | p-Value * | FDR * |
|---|---|---|---|---|---|
| R-HSA-3371571 | HSF1-dependent transactivation | 11/59 | 0.003 | 3.63 × 10−7 | 2.96 × 10−4 |
| R-HSA-3371568 | Attenuation phase | 9/47 | 0.002 | 3.37 × 10−6 | 0.001 |
| R-HSA-3371556 | Cellular response to heat stress | 16/305 | 0.013 | 0.004 | 0.977 |
| R-HSA-3371453 | Regulation of HSF1-mediated heat shock response | 14/260 | 0.011 | 0.006 | 0.977 |
| R-HSA-3371511 | HSF1 activation | 7/99 | 0.004 | 0.012 | 0.977 |
| R-HSA-5083635 | Defective B3GALTL causes PPs | 4/39 | 0.002 | 0.016 | 0.977 |
| R-HSA-8866906 | TFAP2 (AP-2) family regulates transcription of other transcription factors | 2/8 | 3.49 × 10−4 | 0.017 | 0.977 |
| R-HSA-5173214 | O-glycosylation of TSR domain-containing proteins | 4/41 | 0.002 | 0.019 | 0.977 |
| R-HSA-383280 | Nuclear Receptor transcription pathway | 7/113 | 0.005 | 0.023 | 0.977 |
| R-HSA-9700649 | Drug resistance of ALK mutants | 1/1 | 4.37 × 10−5 | 0.024 | 0.977 |
| R-HSA-9717301 | NVP-TAE684-resistant ALK mutants | 1/1 | 4.37 × 10−5 | 0.024 | 0.977 |
| R-HSA-9717323 | Ceritinib-resistant ALK mutants | 1/1 | 4.37 × 10−5 | 0.024 | 0.977 |
| R-HSA-9717329 | Lorlatinib-resistant ALK mutants | 1/1 | 4.37 × 10−5 | 0.024 | 0.977 |
| R-HSA-9717264 | ASP-3026-resistant ALK mutants | 1/1 | 4.37 × 10−5 | 0.024 | 0.977 |
| R-HSA-9717316 | Alectinib-resistant ALK mutants | 1/1 | 4.37 × 10−5 | 0.024 | 0.977 |
| R-HSA-9717319 | Brigatinib-resistant ALK mutants | 1/1 | 4.37 × 10−5 | 0.024 | 0.977 |
| R-HSA-9717326 | Crizotinib-resistant ALK mutants | 1/1 | 4.37 × 10−5 | 0.024 | 0.977 |
| R-HSA-5657560 | Hereditary fructose intolerance | 1/2 | 8.73 × 10−5 | 0.048 | 0.977 |
| R-HSA-3000157 | Laminin interactions | 3/34 | 0.001 | 0.052 | 0.977 |
| R-HSA-5619085 | Defective SLC26A3 causes DIAR1 | 1/3 | 1.31 × 10−4 | 0.071 | 0.977 |
| R-HSA-5619076 | Defective SLC17A8 causes DFNA25 | 1/3 | 1.31 × 10−4 | 0.071 | 0.977 |
| R-HSA-5678420 | Defective ABCC9 causes CMD10, ATFB12 and Cantu syndrome | 1/3 | 1.31 × 10−4 | 0.071 | 0.977 |
| R-HSA-5619045 | Defective SLC34A2 causes PALM | 1/3 | 1.31 × 10−4 | 0.071 | 0.977 |
| R-HSA-9845622 | Defective VWF binding to collagen type I | 1/3 | 1.31 × 10−4 | 0.071 | 0.977 |
| R-HSA-5687583 | Defective SLC34A2 causes PALM | 1/3 | 1.31 × 10−4 | 0.071 | 0.977 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De la Cruz-Cano, E.; González-Díaz, J.Á.; Olivares-Corichi, I.M.; Ayala-Sumuano, J.T.; Díaz-Gandarilla, J.A.; Torres-Sauret, Q.; Larios-Serrato, V.; Vilchis-Reyes, M.Á.; López-Victorio, C.J.; González-Garrido, J.A.; et al. Identifying Genes Associated with the Anticancer Activity of a Fluorinated Chalcone in Triple-Negative Breast Cancer Cells Using Bioinformatics Tools. Int. J. Mol. Sci. 2025, 26, 3662. https://doi.org/10.3390/ijms26083662
De la Cruz-Cano E, González-Díaz JÁ, Olivares-Corichi IM, Ayala-Sumuano JT, Díaz-Gandarilla JA, Torres-Sauret Q, Larios-Serrato V, Vilchis-Reyes MÁ, López-Victorio CJ, González-Garrido JA, et al. Identifying Genes Associated with the Anticancer Activity of a Fluorinated Chalcone in Triple-Negative Breast Cancer Cells Using Bioinformatics Tools. International Journal of Molecular Sciences. 2025; 26(8):3662. https://doi.org/10.3390/ijms26083662
Chicago/Turabian StyleDe la Cruz-Cano, Eduardo, José Ángel González-Díaz, Ivonne María Olivares-Corichi, Jorge Tonatiuh Ayala-Sumuano, José Alfredo Díaz-Gandarilla, Quirino Torres-Sauret, Violeta Larios-Serrato, Miguel Ángel Vilchis-Reyes, Carlos Javier López-Victorio, José Arnold González-Garrido, and et al. 2025. "Identifying Genes Associated with the Anticancer Activity of a Fluorinated Chalcone in Triple-Negative Breast Cancer Cells Using Bioinformatics Tools" International Journal of Molecular Sciences 26, no. 8: 3662. https://doi.org/10.3390/ijms26083662
APA StyleDe la Cruz-Cano, E., González-Díaz, J. Á., Olivares-Corichi, I. M., Ayala-Sumuano, J. T., Díaz-Gandarilla, J. A., Torres-Sauret, Q., Larios-Serrato, V., Vilchis-Reyes, M. Á., López-Victorio, C. J., González-Garrido, J. A., & García-Sánchez, J. R. (2025). Identifying Genes Associated with the Anticancer Activity of a Fluorinated Chalcone in Triple-Negative Breast Cancer Cells Using Bioinformatics Tools. International Journal of Molecular Sciences, 26(8), 3662. https://doi.org/10.3390/ijms26083662







