Assessment of Blood Endothelial Cell Biomarkers in Women and Men with Abnormal Body Mass and Paroxysmal Atrial Fibrillation Based on CHA2DS2-VASC Score: A Retrospective Study
Abstract
1. Introduction
2. Results
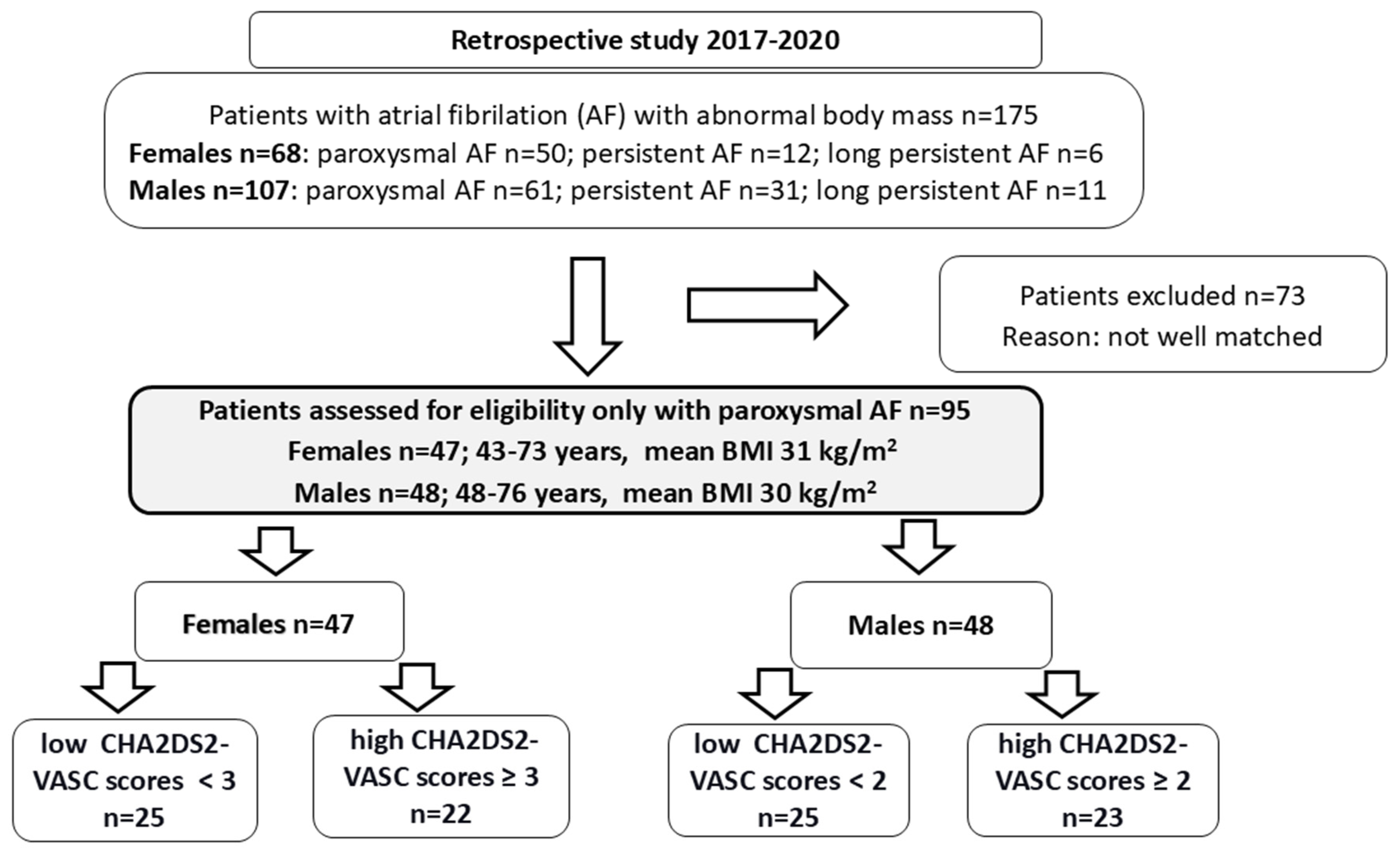
2.1. Females and Males with Paroxysmal AF (Table 1)
| Parameters | Females Paroxysmal AF n = 47 | Males Paroxysmal AF n = 48 | p |
|---|---|---|---|
| Age, years | 64 (42–73) | 62 (48–76) | 0.0563 |
| BMI, kg/m2 | 31.9 (23.3–42.5) | 29.9 (22.2–37.4) | 0.0757 |
| BMI > 25 ≤ 45, n (%) | 45 (96) | 43 (95) | 0.2505 |
| Il-6, pg/mL | 3.2 (2.0–11.4) | 3.2 (1.9–11.5) | 0.6044 |
| EHRA 1 | 0 | 0 | ------ |
| EHRA 2 | 24 (37) | 25 (40) | 0.9208 |
| EHRA 3 | 20 (31) | 20 (32) | 0.9303 |
| EHRA 4 | 3 (5) | 4 (6) | 0.9787 |
| Mean CHA2DS2-VASC | 2 (1–6) | 1 (0–3) | <0.0001 |
| Mean HAS-BLED | 1 (0–3) | 1 (0–3) | 0.1042 |
| % EF | 59 (50–61) | 60 (40–63) | 0.8014 |
| TnT, µg/L | 0.007 (0.003–0.87) | 0.008 (0.004–0.010) | 0.1262 |
| CK-MB, U/L | 15 (10–37) | 14 (7–46) | 0.3511 |
| SBP | 130 (100–160) | 125 (110–140) | 0.0859 |
| DBP | 80 (52–95) | 80 (55–110) | 0.1848 |
| D-Dimers mg/dL | 0.18 (0.03–1.4) | 0.14 (0.02–0.95) | 0.0303 |
| ST2 | 0.85 (0.50–5.0) | 1.35 (0.46–4.0) | 0.0118 |
| TSH, µU/mL | 1.60 (0.40–7.7) | 1.25 (0.22–12.3) | 0.0398 |
| GFR, mL/min | 66 (40–114) | 79.5 (37–135) | <0.0001 |
| sCD40L, pg/mL | 558 (179–985) | 498 (190–1020) | 0.5895 |
| Cholesterol, mg/dL | 193 (132–290) | 152 (116–269) | 0.1184 |
| Glucose, mg/dL | 98 (87–127) | 104 (80–138) | 0.2254 |
| Smoking, n (%) | 3 (6) | 2 (4) | 0.6286 |
| Endothelial cell markers | |||
| t-PA, ng/mL | 1.47 (0.67–3.1) | 1.31 (0.71–2.1) | 0.2713 |
| PAI-1, ng/mL | 18.0 (10.4–37.5) | 17.4 (9.5–49.4) | 0.8313 |
| sVCAM-1, ng/mL | 252.1 (70.1–601.3) | 223.1 (74.3–650.9) | 0.1155 |
| sICAM-1, ng/mL | 34.3 (16.7–295.9) | 25.5 (9.3–320.2) | 0.0076 |
| vWF, ng/mL | 1.9 (0.17–4.7) | 2.1 (0.46–5.43) | 0.2096 |
| sTM, ng/mL | 3.6 (2.3–5.7) | 3.2 (0.94–7.9) | 0.0263 |
| Comorbidities and medications | |||
| Dyslipidemia, n (%) | 23 (49) | 15(31) | 0.0785 |
| Hypertension, n (%) | 33 (72) | 31 (64) | 0.5585 |
| Heart Failure, n (%) | 1 (2) | 1 (2) | 0.988 |
| Thyroid Diseases, n (%) | 17 (36) | 10 (21) | 0.0975 |
| Statins, n(%) | 22 (47) | 24 (50) | 0.7556 |
| ACE inhibitor, n (%) | 10 (21) | 9 (19) | 0.7582 |
| VKA, n (%) | 17 (36) | 9(19) | 0.0569 |
| NOAC, n (%) | 30 (64) | 39 (81) | 0.0569 |
| Beta-blockers, n (%) | 43 (91) | 37 (77) | 0.0542 |
| ARB, n (%) | 19 (40) | 17 (35) | 0.6149 |
| CCB, n (%) | 14 (30) | 9 (19) | 0.2093 |
| Diuretics, n (%) | 11 (23) | 7(15) | 0.2727 |
| Antiarrhythmic, n (%) | 26 (55) | 12 (25) | 0.0026 |
2.2. Criteria for Division Based on the CHA2DS2-VASc Score for Females and Males
2.3. Comparison of Females and Males with Low CHA2DS2-VASC Score
2.4. Comparison of Females and Males with High CHA2DS2-VASC Score
2.5. Correlation Between the CHA2DS2-VASc Score and Endothelial Cell Biomarkers in Females and Males with Paroxysmal AF (Table 4)
| Parameters | Females with Paroxysmal AF | Males with Paroxysmal AF | ||
|---|---|---|---|---|
| r | p | r | p | |
| t-PA | −0.002 | 0.9852 | 0.273 | 0.0604 |
| PAI-1 | −0.071 | 0.6312 | 0.029 | 0.8410 |
| sVCAM-1 | −0.346 | 0.0568 | 0.247 | 0.0902 |
| sICAM-1 | 0.150 | 0.3134 | −0.202 | 0.1665 |
| vWF | 0.208 | 0.1601 | 0.265 | 0.0686 |
| TM | 0.189 | 0.2019 | 0.292 | 0.0440 |
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Study Population
4.3. Biochemical Analyses
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Linz, D.; Gawalko, M.; Betz, K.; Hendriks, J.M.; Lip, G.Y.H.; Vinter, N.; Guo, Y.; Johnsen, S. Atrial fibrillation: Epidemiology, screening and digital health. Lancet Reg. Health Eur. 2024, 37, 100786. [Google Scholar] [CrossRef]
- Heeringa, J.; van der Kuip, D.A.; Hofman, A.; Kors, J.A.; van Herpen, G.; Stricker, B.H.; Stijnen, T.; Lip, G.Y.; Witteman, J.C. Prevalence, incidence and lifetime risk of atrial fibrillation: The Rotterdam study. Eur. Heart J. 2006, 27, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Khaing, E.; Aroudaky, A.; Dircks, D.; Almerstani, M.; Alziadin, N.; Frankel, S.; Hollenberg, B.; Limsiri, P.; Schleifer, W.; Easley, A.; et al. Representation of Women in Atrial Fibrillation Ablation Randomized Controlled Trials: Systematic Review. J. Am. Heart Assoc. 2025, 14, e035181. [Google Scholar] [CrossRef] [PubMed]
- Cheung, J.W.; Cheng, E.P.; Wu, X.; Yeo, I.; Christos, P.J.; Kamel, H.; Markowitz, S.M.; Liu, C.F.; Thomas, G.; Ip, J.E.; et al. Sex-based differences in outcomes, 30-day readmissions, and costs following catheter ablation of atrial fibrillation: The United States Nationwide Readmissions Database 2010–2014. Eur. Heart J. 2019, 40, 3035–3043. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Mohanty, P.; Di, B.L.; Sanchez, J.E.; Shaheen, M.H.; Burkhardt, J.D.; Bassouni, M.; Cummings, J.; Wang, Y.; Lewis, W.R.; et al. Outcomes and complications of catheter ablation for atrial fibrillation in females. Heart Rhythm. 2010, 7, 167–172. [Google Scholar] [CrossRef]
- Lang, C.; Seyfang, L.; Ferrari, J.; Gattringer, T.; Greisenegger, S.; Willeit, K.; Toell, T.; Krebs, S.; Brainin, M.; Kiechl, S.; et al. Do Women with Atrial Fibrillation Experience More Severe Strokes? Results From the Austrian Stroke Unit Registry. Stroke 2017, 48, 778–780. [Google Scholar] [CrossRef]
- Westerman, S.; Wenger, N. Gender Differences in Atrial Fibrillation: A Review of Epidemiology, Management, and Outcomes. Curr. Cardiol. Rev. 2019, 15, 136–144. [Google Scholar] [CrossRef]
- Moreau, K.L. Intersection between gonadal function and vascular aging in women. J. Appl. Physiol. 2018, 125, 1881–1887. [Google Scholar] [CrossRef]
- Stanhewicz, A.E.; Wenner, M.M.; Stachenfeld, N.S. Sex differences in endothelial function important to vascular health and overall cardiovascular disease risk across the lifespan. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1569–H1588. [Google Scholar] [CrossRef]
- Gohar, E.Y.; Pollock, D.M. Sex-Specific Contributions of Endothelin to Hypertension. Curr. Hypertens. Rep. 2018, 20, 58. [Google Scholar] [CrossRef]
- Somani, Y.B.; Pawelczyk, J.A.; De Souza, M.J.; Kris-Etherton, P.M.; Proctor, D.N. Aging women and their endothelium: Probing the relative role of estrogen on vasodilator function. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H395–H404. [Google Scholar] [CrossRef]
- Dungan, G.D.; Kantarcioglu, B.; Odeh, A.; Hoppensteadt, D.; Siddiqui, F.; Rohde, L.; Fareed, J.; Syed, M.A. Vascular Endothelial Dysfunction and Immunothrombosis in the Pathogenesis of Atrial Fibrillation. Clin. Appl. Thromb. Hemost. 2024, 30, 10760296241296138. [Google Scholar] [CrossRef]
- Cines, D.B.; Pollak, E.S.; Buck, C.A.; Loscalzo, J.; Zimmerman, G.A.; McEver, R.P.; Pober, J.S.; Wick, T.M.; Konkle, B.A.; Schwartz, B.S.; et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood 1998, 91, 3527–3561. [Google Scholar] [PubMed]
- Gupta, V.; Sachdeva, S.; Khan, A.S.; Haque, S.F. Endothelial dysfunction and inflammation in different stages of essential hypertension. Saudi J. Kidney Dis. Transpl. 2011, 22, 97–103. [Google Scholar] [PubMed]
- Kajikawa, M.; Higashi, Y. Obesity and Endothelial Function. Biomedicines 2022, 10, 1745. [Google Scholar] [CrossRef]
- Matz, R.L.; Schott, C.; Stoclet, J.C.; Andriantsitohaina, R. Age-related endothelial dysfunction with respect to nitric oxide, endothelium-derived hyperpolarizing factor and cyclooxygenase products. Physiol. Res. 2000, 49, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Mussbacher, M.; Schossleitner, K.; Kral-Pointner, J.B.; Salzmann, M.; Schrammel, A.; Schmid, J.A. More than Just a Monolayer: The Multifaceted Role of Endothelial Cells in the Pathophysiology of Atherosclerosis. Curr. Atheroscler. Rep. 2022, 24, 483–492. [Google Scholar] [CrossRef]
- Nerpin, E.; Ingelsson, E.; Riserus, U.; Helmersson-Karlqvist, J.; Sundstrom, J.; Jobs, E.; Larsson, A.; Lind, L.; Arnlov, J. Association between glomerular filtration rate and endothelial function in an elderly community cohort. Atherosclerosis 2012, 224, 242–246. [Google Scholar] [CrossRef]
- Black, N.; Mohammad, F.; Saraf, K.; Morris, G. Endothelial function and atrial fibrillation: A missing piece of the puzzle? J. Cardiovasc. Electrophysiol. 2022, 33, 109–116. [Google Scholar] [CrossRef]
- Corban, M.T.; Godo, S.; Burczak, D.R.; Noseworthy, P.A.; Toya, T.; Lewis, B.R.; Lerman, L.O.; Gulati, R.; Lerman, A. Coronary Endothelial Dysfunction Is Associated with Increased Risk of Incident Atrial Fibrillation. J. Am. Heart Assoc. 2020, 9, e014850. [Google Scholar] [CrossRef]
- Okawa, K.; Sogo, M.; Morimoto, T.; Tsushima, R.; Sudo, Y.; Saito, E.; Ozaki, M.; Takahashi, M. Relationship Between Endothelial Dysfunction and the Outcomes After Atrial Fibrillation Ablation. J. Am. Heart Assoc. 2023, 12, e028482. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, T.; Kunugita, F.; Ozawa, M.; Satoh, Y.; Yoshizawa, R.; Owada, S.; Sawa, Y.; Morino, Y.; Nakamura, M. Relationship between Impairment of the Vascular Endothelial Function and the CHA(2)DS(2)-VASc Score in Patients with Sinus Rhythm and Non-valvular Atrial Fibrillation. Intern. Med. 2018, 57, 2131–2139. [Google Scholar] [CrossRef] [PubMed]
- Krittayaphong, R.; Pumprueg, S.; Sairat, P. Soluble ST2 in the prediction of heart failure and death in patients with atrial fibrillation. Clin. Cardiol. 2022, 45, 447–456. [Google Scholar] [CrossRef]
- Califano, F.; Giovanniello, T.; Pantone, P.; Campana, E.; Parlapiano, C.; Alegiani, F.; Vincentelli, G.M.; Turchetti, P. Clinical importance of thrombomodulin serum levels. Eur. Rev. Med. Pharmacol. Sci. 2000, 4, 9–66. [Google Scholar]
- Takano, S.; Kimura, S.; Ohdama, S.; Aoki, N. Plasma thrombomodulin in health and diseases. Blood 1990, 76, 2024–2029. [Google Scholar] [CrossRef]
- Champsi, A.; Mobley, A.R.; Subramanian, A.; Nirantharakumar, K.; Wang, X.; Shukla, D.; Bunting, K.V.; Molgaard, I.; Dwight, J.; Arroyo, R.C.; et al. Gender and contemporary risk of adverse events in atrial fibrillation. Eur. Heart J. 2024, 45, 3707–3717. [Google Scholar] [CrossRef]
- Teppo, K.; Lip, G.Y.H.; Airaksinen, K.E.J.; Halminen, O.; Haukka, J.; Putaala, J.; Mustonen, P.; Linna, M.; Hartikainen, J.; Lehto, M. Comparing CHA2DS2-VA and CHA2DS2-VASc scores for stroke risk stratification in patients with atrial fibrillation: A temporal trends analysis from the retrospective Finnish AntiCoagulation in Atrial Fibrillation (FinACAF) cohort. Lancet Reg. Health Eur. 2024, 43, 100967. [Google Scholar] [CrossRef]
- Freestone, B.; Chong, A.Y.; Nuttall, S.; Blann, A.D.; Lip, G.Y. Soluble E-selectin, von Willebrand factor, soluble thrombomodulin, and total body nitrate/nitrite product as indices of endothelial damage/dysfunction in paroxysmal, persistent, and permanent atrial fibrillation. Chest 2007, 132, 1253–1258. [Google Scholar] [CrossRef]
- Schnabel, R.B.; Larson, M.G.; Yamamoto, J.F.; Kathiresan, S.; Rong, J.; Levy, D.; Keaney, J.F., Jr.; Wang, T.J.; Vasan, R.S.; Benjamin, E.J. Relation of multiple inflammatory biomarkers to incident atrial fibrillation. Am. J. Cardiol. 2009, 104, 92–96. [Google Scholar] [CrossRef]
- Willeit, K.; Pechlaner, R.; Willeit, P.; Skroblin, P.; Paulweber, B.; Schernthaner, C.; Toell, T.; Egger, G.; Weger, S.; Oberhollenzer, M.; et al. Association Between Vascular Cell Adhesion Molecule 1 and Atrial Fibrillation. JAMA Cardiol. 2017, 2, 516–523. [Google Scholar] [CrossRef]
- Llaurado, G.; Ceperuelo-Mallafre, V.; Vilardell, C.; Simo, R.; Albert, L.; Berlanga, E.; Vendrell, J.; Gonzalez-Clemente, J.M. Impaired endothelial function is not associated with arterial stiffness in adults with type 1 diabetes. Diabetes Metab. 2013, 39, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Remmelzwaal, S.; Beulens, J.W.J.; Elders, P.J.M.; Stehouwer, C.D.A.; Zhang, Z.; Handoko, M.L.; Appelman, Y.; van Empel, V.; Heymans, S.R.B.; Thijs, L.; et al. Sex differences in the longitudinal relationship of low-grade inflammation and echocardiographic measures in the Hoorn and FLEMENGHO Study. PLoS ONE 2021, 16, e0251148. [Google Scholar] [CrossRef] [PubMed]
- Hartman, R.J.G.; Owsiany, K.; Ma, L.; Koplev, S.; Hao, K.; Slenders, L.; Civelek, M.; Mokry, M.; Kovacic, J.; Pasterkamp, G.; et al. Sex-Stratified Gene Regulatory Networks Reveal Female Key Driver Genes of Atherosclerosis Involved in Smooth Muscle Cell Phenotype Switching. Circulation 2021, 143, 713–726. [Google Scholar] [CrossRef]
- Dion-Albert, L.; Cadoret, A.; Doney, E.; Kaufmann, F.N.; Dudek, K.A.; Daigle, B.; Parise, L.F.; Cathomas, F.; Samba, N.; Hudson, N.; et al. Vascular and blood-brain barrier-related changes underlie stress responses and resilience in female mice and depression in human tissue. Nat. Commun. 2022, 13, 164. [Google Scholar] [CrossRef]
- Piercy, K.T.; Donnell, R.L.; Kirkpatrick, S.S.; Timaran, C.H.; Stevens, S.L.; Freeman, M.B.; Goldman, M.H. Effects of estrogen, progesterone, and combination exposure on interleukin-1 beta-induced expression of VCAM-1, ICAM-1, PECAM, and E-selectin by human female iliac artery endothelial cells. J. Surg. Res. 2002, 105, 215–219. [Google Scholar] [CrossRef]
- Nathan, L.; Pervin, S.; Singh, R.; Rosenfeld, M.; Chaudhuri, G. Estradiol inhibits leukocyte adhesion and transendothelial migration in rabbits in vivo: Possible mechanisms for gender differences in atherosclerosis. Circ. Res. 1999, 85, 377–385. [Google Scholar] [CrossRef]
- Trenti, A.; Tedesco, S.; Boscaro, C.; Trevisi, L.; Bolego, C.; Cignarella, A. Estrogen, Angiogenesis, Immunity and Cell Metabolism: Solving the Puzzle. Int. J. Mol. Sci. 2018, 19, 859. [Google Scholar] [CrossRef]
- Howard, B.V.; Rossouw, J.E. Estrogens and cardiovascular disease risk revisited: The Women’s Health Initiative. Curr. Opin. Lipidol. 2013, 24, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Medenwald, D.; Dietz, S.; Tiller, D.; Kluttig, A.; Greiser, K.; Loppnow, H.; Thiery, J.; Nuding, S.; Russ, M.; Fahrig, A.; et al. Inflammation and echocardiographic parameters of ventricular hypertrophy in a cohort with preserved cardiac function. Open Heart 2014, 1, e000004. [Google Scholar] [CrossRef]
- Shin, J.; Hong, J.; Edwards-Glenn, J.; Krukovets, I.; Tkachenko, S.; Adelus, M.L.; Romanoski, C.E.; Rajagopalan, S.; Podrez, E.; Byzova, T.V.; et al. Unraveling the Role of Sex in Endothelial Cell Dysfunction: Evidence from Lineage Tracing Mice and Cultured Cells. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 238–253. [Google Scholar] [CrossRef]
- Korybalska, K.; Kawka, E.; Breborowicz, A.; Witowski, J. Atorvastatin does not impair endothelial cell wound healing in an in vitro model of vascular injury. J. Physiol. Pharmacol. 2012, 63, 389–395. [Google Scholar] [PubMed]
- Li, K.; Zemmrich, C.; Bramlage, P.; Persson, A.B.; Sacirovic, M.; Ritter, O.; Buschmann, E.; Buschmann, I.; Hillmeister, P. Effect of ACEI and ARB treatment on nitric oxide-dependent endothelial function. Vasa 2021, 50, 413–422. [Google Scholar] [CrossRef]
- Peller, M.; Ozieranski, K.; Balsam, P.; Grabowski, M.; Filipiak, K.J.; Opolski, G. Influence of beta-blockers on endothelial function: A meta-analysis of randomized controlled trials. Cardiol. J. 2015, 22, 708–716. [Google Scholar] [CrossRef]
- Reriani, M.K.; Dunlay, S.M.; Gupta, B.; West, C.P.; Rihal, C.S.; Lerman, L.O.; Lerman, A. Effects of statins on coronary and peripheral endothelial function in humans: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Cardiovasc. Prev. Rehabil. 2011, 18, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, B.A.; Piccini, J.P. Anticoagulation in atrial fibrillation. BMJ 2014, 348, g2116. [Google Scholar] [CrossRef] [PubMed]
- Kaplanski, G.; Marin, V.; Fabrigoule, M.; Boulay, V.; Benoliel, A.M.; Bongrand, P.; Kaplanski, S.; Farnarier, C. Thrombin-activated human endothelial cells support monocyte adhesion in vitro following expression of intercellular adhesion molecule-1 (ICAM-1; CD54) and vascular cell adhesion molecule-1 (VCAM-1; CD106). Blood 1998, 92, 1259–1267. [Google Scholar] [CrossRef]
- Minami, T.; Sugiyama, A.; Wu, S.Q.; Abid, R.; Kodama, T.; Aird, W.C. Thrombin and phenotypic modulation of the endothelium. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 41–53. [Google Scholar] [CrossRef]
- Ossei-Gerning, N.; Wilson, I.J.; Grant, P.J. Sex differences in coagulation and fibrinolysis in subjects with coronary artery disease. Thromb. Haemost. 1998, 79, 736–740. [Google Scholar] [CrossRef]
- Schwertz, D.W.; Penckofer, S. Sex differences and the effects of sex hormones on hemostasis and vascular reactivity. Heart Lung 2001, 30, 401–426. [Google Scholar] [CrossRef]
- Lip, G.Y.; Blann, A.D.; Jones, A.F.; Beevers, D.G. Effects of hormone-replacement therapy on hemostatic factors, lipid factors, and endothelial function in women undergoing surgical menopause: Implications for prevention of atherosclerosis. Am. Heart J. 1997, 134, 764–771. [Google Scholar] [CrossRef]
- Joundi, R.A.; Cipriano, L.E.; Sposato, L.A.; Saposnik, G. Ischemic Stroke Risk in Patients with Atrial Fibrillation and CHA2DS2-VASc Score of 1: Systematic Review and Meta-Analysis. Stroke 2016, 47, 1364–1367. [Google Scholar] [CrossRef] [PubMed]
- Flammer, A.J.; Anderson, T.; Celermajer, D.S.; Creager, M.A.; Deanfield, J.; Ganz, P.; Hamburg, N.M.; Luscher, T.F.; Shechter, M.; Taddei, S.; et al. The assessment of endothelial function: From research into clinical practice. Circulation 2012, 126, 753–767. [Google Scholar] [CrossRef] [PubMed]
- Camm, A.J.; Lip, G.Y.; De, C.R.; Savelieva, I.; Atar, D.; Hohnloser, S.H.; Hindricks, G.; Kirchhof, P. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: An update of the 2010 ESC Guidelines for the management of atrial fibrillation—Developed with the special contribution of the European Heart Rhythm Association. Europace 2012, 14, 1385–1413. [Google Scholar] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., III; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van, L.F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Andrassy, K.M. Comments on ‘KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease’. Kidney Int. 2013, 84, 622–623. [Google Scholar] [CrossRef]
| Parameters | Female CHA2DS2-VASC < 3 n = 25 | Males CHA2DS2-VASC < 2 n = 25 | p |
|---|---|---|---|
| Age, years | 62 (43–73) | 60 (48–69) | 0.5242 |
| BMI, kg/m2 | 30.9 (23.3–42.5) | 30 (22–37) | 0.2691 |
| BMI > 25 ≤ 45, n (%) | 23 (92) | 22 (88) | 0.6374 |
| Il-6, pg/mL | 3.2 (2.2–11.4) | 3.2 (1.9–6.7) | 0.3085 |
| EHRA 1 | 0 | 0 | ------ |
| EHRA 2 | 15 (60) | 15 (60) | 1.000 |
| EHRA 3 | 10 (40) | 9 (36) | 0.7708 |
| EHRA 4 | 0 | 1 (4) | 0.3124 |
| Mean HAS-BLED | 1 (0–2) mean 0.76 | 1 (0–1) mean 0.68 | 0.7756 |
| % EF | 59 (50–60) | 59 (50–63) | 0.7806 |
| TnT, µg/L | 0.007 (0.003–0.084) | 0.007 (0.004–0.024) | 0.5071 |
| CK-MB, U/L | 15 (10–37) | 14 (7–31) | 0.2092 |
| SBP | 125 (100–160) | 125 (110–140) | 0.7147 |
| DBP | 75 (52–91) | 80 (55–95) | 0.2562 |
| D-Dimers mg/dL | 0.19 (0.03–1.36) | 0.13 (0.03–0.66) | 0.1235 |
| ST2 | 1.2 (0.50–5.0) | 1.4 (0.46–4.0) | 0.1201 |
| TSH, µU/mL | 2.5 (0.66–7.7) | 1.2 (0.22–12.3) | 0.0063 |
| GFR, mL/min | 67 (44–114) | 73 (50–135) | 0.0320 |
| sCD40L, pg/mL | 559 (261–985) | 512 (274–1020) | 0.2293 |
| Cholesterol, mg/dL | 198 (160–267) | 196 (116–269) | 0.8972 |
| Glucose, mg/dL | 98 (88–127) | 105 (80–138) | 0.6754 |
| Smoking, n (%) | 2 | 3 | 0.6374 |
| Endothelial cell markers | |||
| t-PA, ng/mL | 1.5 (0.67–3.1) | 1.2 (0.71–2.1) | 0.1453 |
| PAI-1, ng/mL | 18.0 (12.1–32.5) | 17.5 (10.2–38.1) | 0.8588 |
| sVCAM-1, ng/mL | 300.4 (111.4–601.3) | 178.1 (74.3–510.4) | 0.0055 |
| sICAM-1, ng/mL | 36.1 (16.7–261.9) | 36.9 (9.3–108.1) | 0.4212 |
| vWF, ng/mL | 1.8 (1.2–3.2) | 1.8 (0.46–5.4) | 0.9693 |
| sTM, ng/mL | 3.6 (2.4–5.1) | 3.0 (1.1–4.9) | 0.0150 |
| Comorbidities and medications | |||
| Dyslipidemia, n (%) | 15 (60) | 10 (40) | 0.1573 |
| Hypertension, n (%) | 14 (56) | 13 (52) | 0.7766 |
| Heart Failure, n (%) | 1 (4) | 0 (0) | 0.3124 |
| Thyroid Diseases, n (%) | 11 (44) | 5 (20) | 0.0689 |
| Statins, n (%) | 10 (40) | 7 (28) | 0.3705 |
| ACE inhibitor, n (%) | 5 (20) | 4 (16) | 0.7128 |
| VKA, n (%) | 7 (28) | 2 (9) | 0.0657 |
| NOAC, n (%) | 18 (72) | 23 (92) | 0.0657 |
| Beta-blockers, n (%) | 23 (92) | 17 (68) | 0.0339 |
| ARB, n (%) | 9 (36) | 7 (30) | 0.5443 |
| CCB, n (%) | 6 (24) | 3 (12) | 0.2695 |
| Diuretics, n (%) | 5 (20) | 4 (16) | 0.7128 |
| Antiarrhythmic, n (%) | 15 (60) | 6 (24) | 0.0099 |
| Parameters | Females CHA2DS2-VASC ≥ 3 n = 22 | Males CHA2DS2-VASC ≥ 2 n = 23 | p |
|---|---|---|---|
| Age, years | 68 (60–73) | 66 (54–76) | 0.0242 |
| BMI, kg/m2 | 32.3 (25.3–36.7) | 30 (22–36) | 0.1022 |
| BMI > 25 ≤ 45, n (%) | 22 (100) | 21(91) | 0.1571 |
| Il-6, pg/mL | 3.4 (2.0–9.2) | 3.2 (2.2–11.5) | 0.8439 |
| EHRA 1 | 0 | 0 | ------ |
| EHRA 2 | 9 (41) | 10 (43) | 0.8615 |
| EHRA 3 | 10 (45) | 11 (48) | 0.8734 |
| EHRA 4 | 3 (14) | 2 (9) | 0.5981 |
| Mean HAS-BLED | 2 (1–3) mean 2.0 | 2 (0–3) mean 1.5 | 0.013 |
| %EF | 59 (50–61) | 60 (40–60) | 0.9982 |
| TnT, µg/L | 0.007 (0.003–0.87) | 0.009 (0.005–0.022) | 0.1286 |
| CK-MB, U/L | 15 (11–27) | 17 (10–46) | 0.6720 |
| SBP | 130 (110–160) | 125 (110–140) | 0.0210 |
| DBP | 80 (60–95) | 80 (60–110) | 0.5029 |
| D-Dimers mg/dL | 0.16 (0.07–0.54) | 0.14 (0.02–0.95) | 0.1839 |
| ST2 | 0.78 (0.51–3.6) | 1.3 (0.49–3.7) | 0.0314 |
| TSH, µU/mL | 1.2 (0.40–6.3) | 1.4 (0.58–3.2) | 0.9684 |
| GFR, mL/min | 64 (40–91) | 82 (37–113) | 0.0006 |
| sCD40L, pg/mL | 512 (179–845) | 494 (189–986) | 0.6626 |
| Cholesterol, mg/dL | 177 (132–290) | 138 (116–211) | 0.0392 |
| Glucose, mg/dL | 100 (87–112) | 98 (85–135) | 0.8765 |
| Smoking, n (%) | 1 | 0 | 0.3011 |
| Endothelial cell markers | |||
| t-PA, ng/mL | 1.5 (0.69–1.9) | 1.4 (0.77–2.0) | 0.9811 |
| PAI-1, ng/mL | 17.8 (10.4–37.5) | 16.6 (9.5–49.4) | 0.9958 |
| VCAM, ng/mL | 204.6 (70.1–590.9) | 237.6 (102.1–690.9) | 0.5471 |
| ICAM, ng/mL | 34.3 (19.3–295.9) | 23.3 (9.6–320.2) | 0.0016 |
| vWF, ng/mL | 1.9 (1.6–4.7) | 2.7 (0.85–5.3) | 0.0844 |
| TM, ng/mL | 3.6 (2.3–5.7) | 3.3 (0.94–7.9) | 0.7899 |
| Comorbidities and medications | |||
| Dyslipidemia, n (%) | 8 (36) | 5 (22) | 0.2793 |
| Hypertension, n (%) | 19 (84) | 18 (78) | 0.4773 |
| Heart Failure, n (%) | 0 (0) | 1 (4) | 0.3226 |
| Thyroid Diseases, n (%) | 6 (27) | 5 (22) | 0.6659 |
| Statins, n (%) | 12 (55) | 17 (74) | 0.1749 |
| ACE inhibitor, n (%) | 5 (23) | 7 (30) | 0.5589 |
| VKA, n (%) | 10 (45) | 7(30) | 0.2989 |
| NOAC, n (%) | 12 (54) | 16 (69) | 0.2989 |
| Beta-blockers, n (%) | 19 (86) | 20 (87) | 0.9534 |
| ARB, n (%) | 10(45) | 10(43) | 0.8939 |
| CCB, n (%) | 3 (14) | 6 (26) | 0.2966 |
| Diuretics, n (%) | 5 (23) | 3 (11) | 0.3957 |
| Antiarrhythmic, n (%) | 11 (50) | 7 (30) | 0.1805 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikora, W.; Kanikowska, D.; Budzianowski, J.; Kawka, E.; Rutkowski, R.; Korybalska, K. Assessment of Blood Endothelial Cell Biomarkers in Women and Men with Abnormal Body Mass and Paroxysmal Atrial Fibrillation Based on CHA2DS2-VASC Score: A Retrospective Study. Int. J. Mol. Sci. 2025, 26, 3627. https://doi.org/10.3390/ijms26083627
Sikora W, Kanikowska D, Budzianowski J, Kawka E, Rutkowski R, Korybalska K. Assessment of Blood Endothelial Cell Biomarkers in Women and Men with Abnormal Body Mass and Paroxysmal Atrial Fibrillation Based on CHA2DS2-VASC Score: A Retrospective Study. International Journal of Molecular Sciences. 2025; 26(8):3627. https://doi.org/10.3390/ijms26083627
Chicago/Turabian StyleSikora, Wiesław, Dominika Kanikowska, Jan Budzianowski, Edyta Kawka, Rafał Rutkowski, and Katarzyna Korybalska. 2025. "Assessment of Blood Endothelial Cell Biomarkers in Women and Men with Abnormal Body Mass and Paroxysmal Atrial Fibrillation Based on CHA2DS2-VASC Score: A Retrospective Study" International Journal of Molecular Sciences 26, no. 8: 3627. https://doi.org/10.3390/ijms26083627
APA StyleSikora, W., Kanikowska, D., Budzianowski, J., Kawka, E., Rutkowski, R., & Korybalska, K. (2025). Assessment of Blood Endothelial Cell Biomarkers in Women and Men with Abnormal Body Mass and Paroxysmal Atrial Fibrillation Based on CHA2DS2-VASC Score: A Retrospective Study. International Journal of Molecular Sciences, 26(8), 3627. https://doi.org/10.3390/ijms26083627






