The Clinical Relevance of Epithelial-to-Mesenchymal Transition Hallmarks: A Cut-Off-Based Approach in Healthy and Cancerous Cell Lines
Abstract
1. Introduction
2. Results
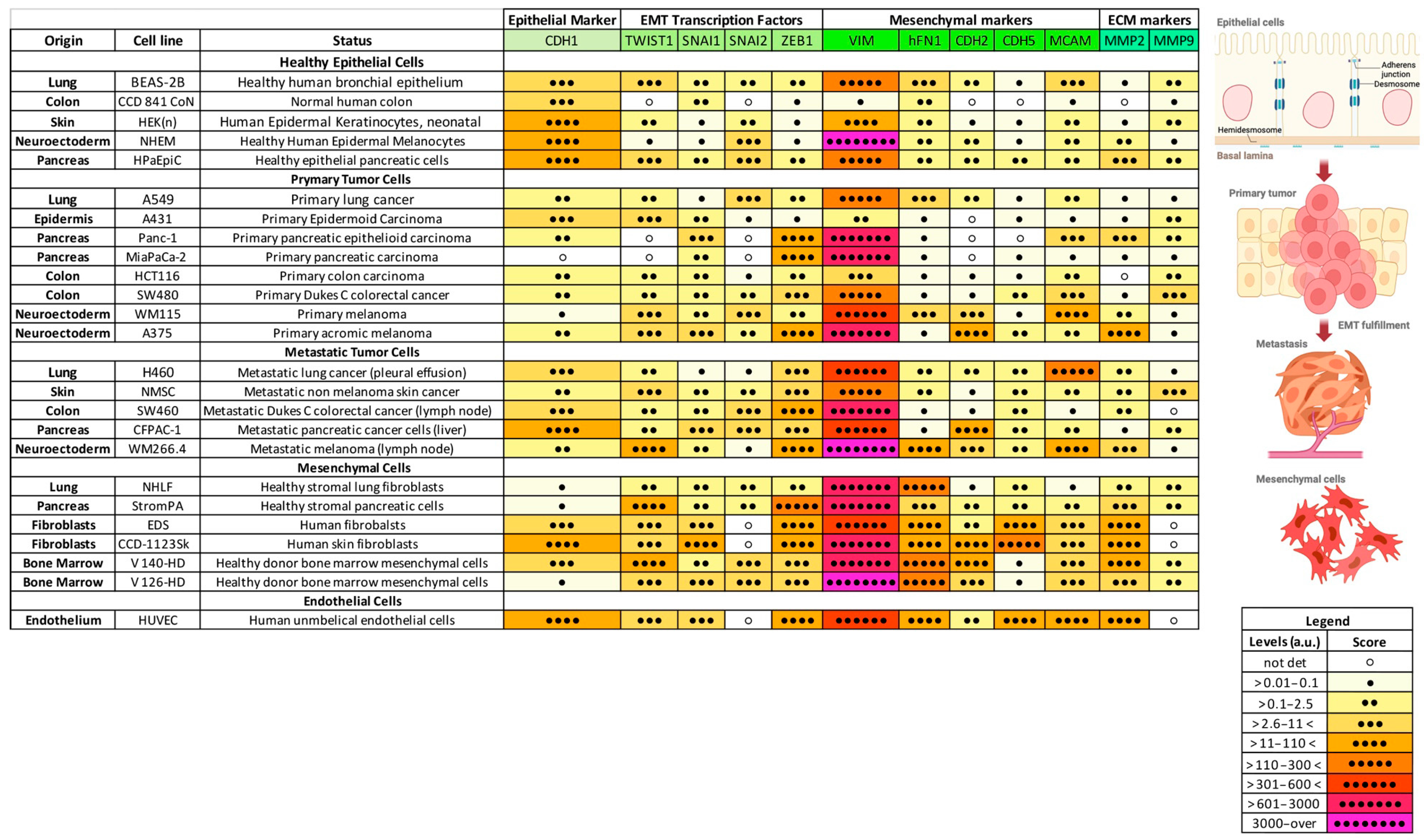
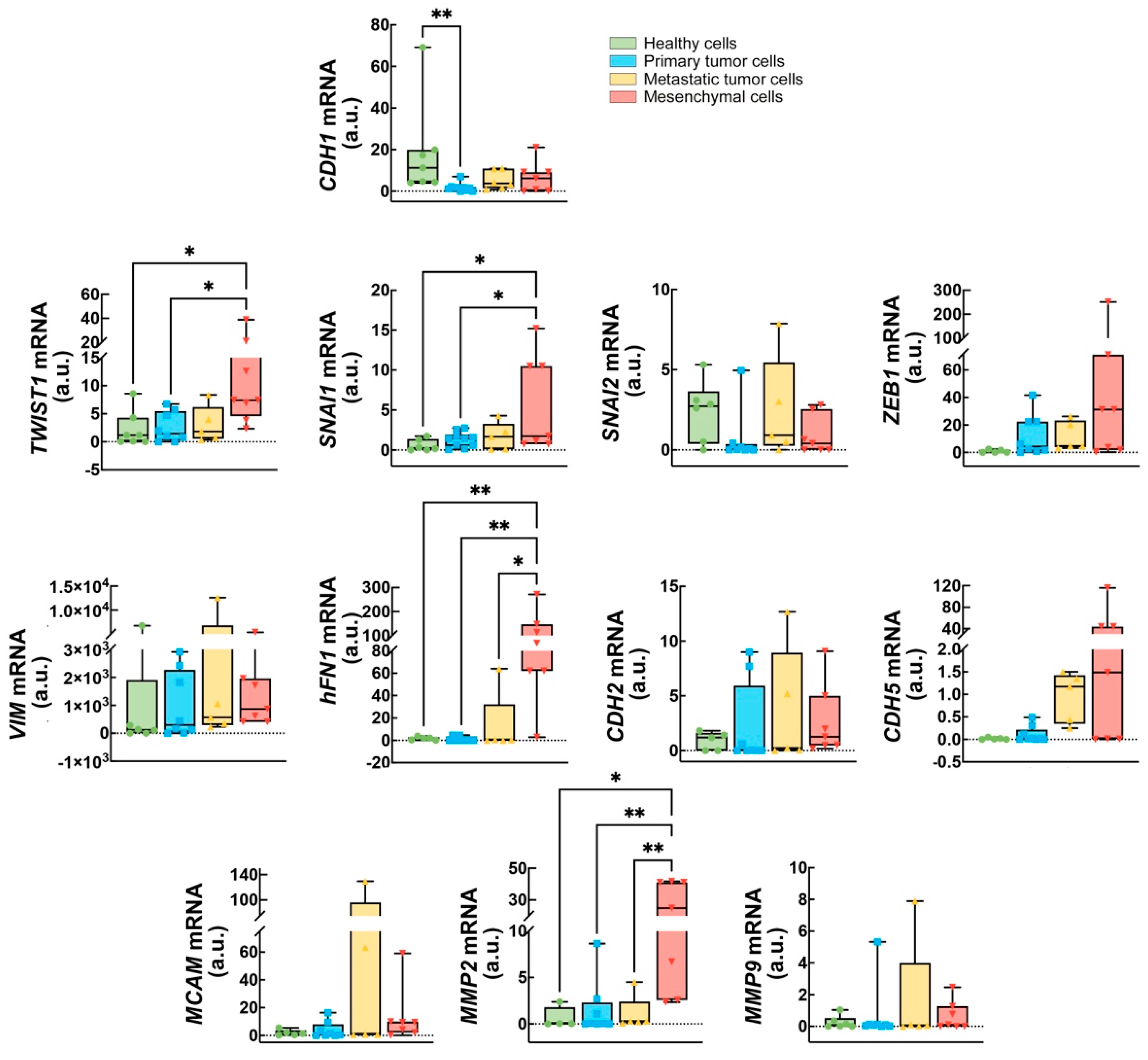
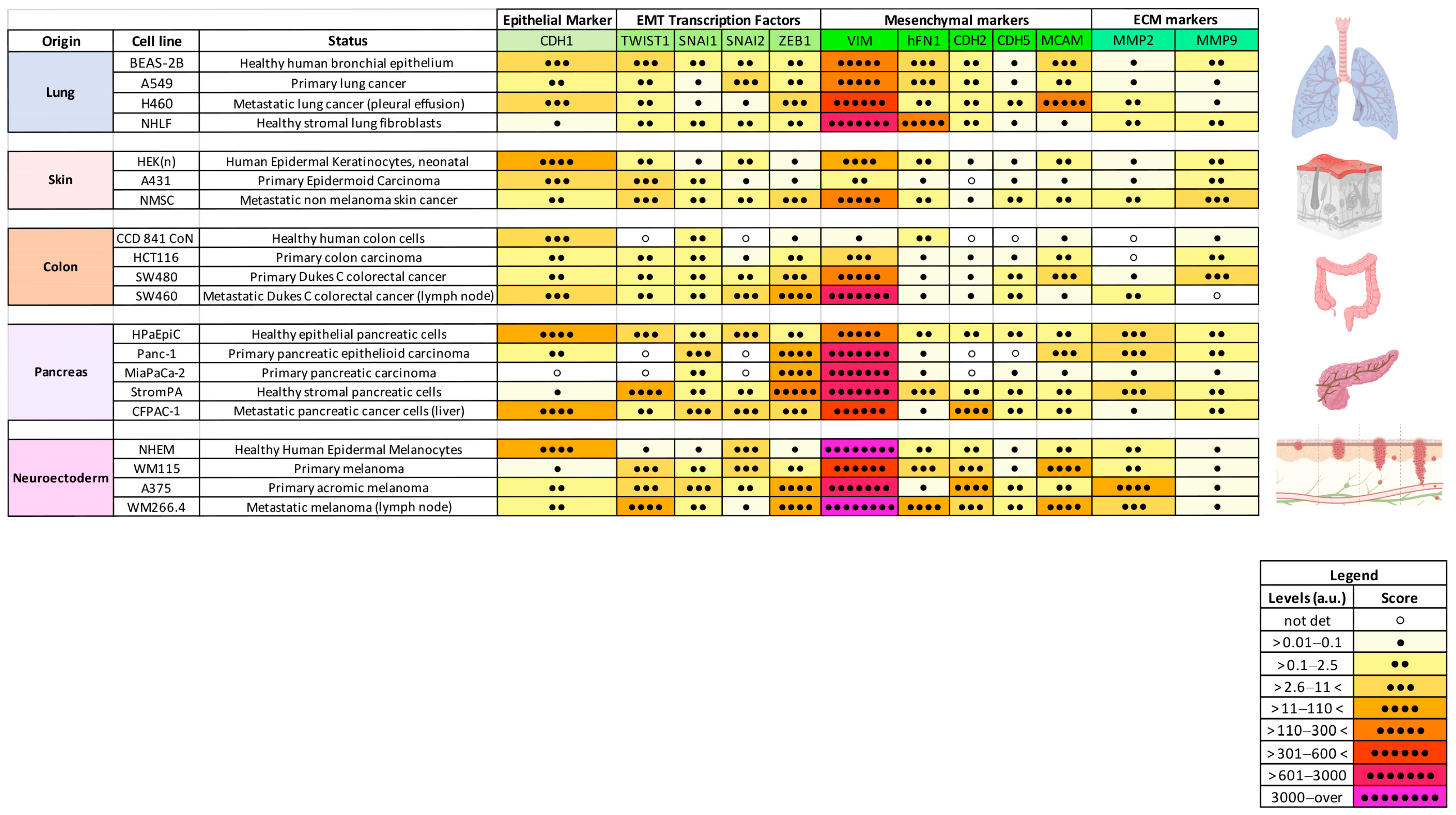
2.1. Expression of a Subset of EMT Markers Defines Cancer Progression
2.2. CDH1, ZEB1, and CDH5 Levels Could Discriminate Between Healthy and Cancer-Derived Cells
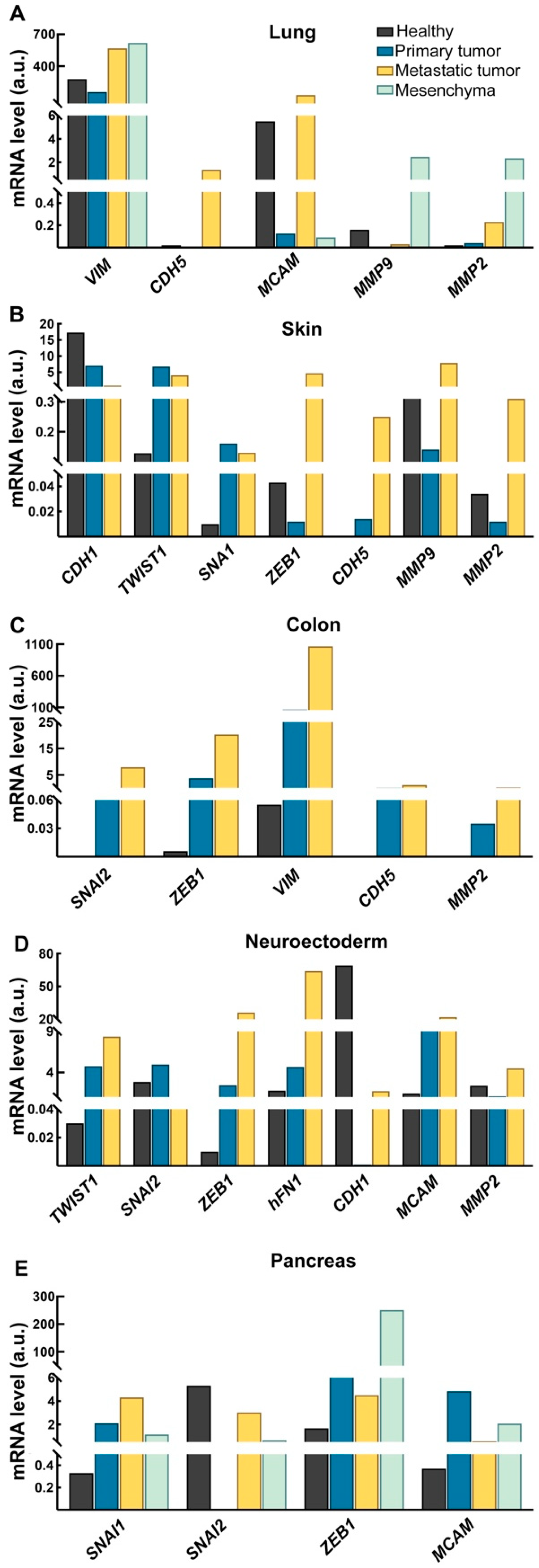
2.3. EMT Profile During Cancer Progression Is Tissue-Specific
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Selection of Reference Genes Panel
4.3. Quantitative Real-Time PCR Assay for Molecular EMT Profiling
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Focaccio, A.; Rossi, L.; De Luca, A. A Spotlight on the Role of Copper in the Epithelial to Mesenchymal Transition. Life Sci. 2024, 354, 122972. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Tamma, R.; Annese, T. Epithelial-Mesenchymal Transition in Cancer: A Historical Overview. Transl. Oncol. 2020, 13, 100773. [Google Scholar] [CrossRef] [PubMed]
- Polyak, K.; Weinberg, R.A. Transitions between Epithelial and Mesenchymal States: Acquisition of Malignant and Stem Cell Traits. Nat. Rev. Cancer 2009, 9, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Yeung, K.T.; Yang, J. Epithelial–Mesenchymal Transition in Tumor Metastasis. Mol. Oncol. 2017, 11, 28–39. [Google Scholar] [CrossRef]
- Thiery, J.P. Epithelial–Mesenchymal Transitions in Tumour Progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef]
- Scheel, C.; Weinberg, R.A. Cancer Stem Cells and Epithelial–Mesenchymal Transition: Concepts and Molecular Links. Semin. Cancer Biol. 2012, 22, 396–403. [Google Scholar] [CrossRef]
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and Definitions for Research on Epithelial–Mesenchymal Transition. Nat. Rev. Mol. Cell Biol. 2020, 21, 341–352. [Google Scholar] [CrossRef]
- Lu, W.; Kang, Y. Epithelial-Mesenchymal Plasticity in Cancer Progression and Metastasis. Dev. Cell 2019, 49, 361–374. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, X.; Peng, Y.; Wu, M.; Zhang, P.; Xie, R.; Wu, Y.; Yan, Q.; Liu, S.; Wang, J. HIF-1α Promotes Epithelial-Mesenchymal Transition and Metastasis through Direct Regulation of ZEB1 in Colorectal Cancer. PLoS ONE 2015, 10, e0129603. [Google Scholar] [CrossRef]
- Dongre, A.; Weinberg, R.A. New Insights into the Mechanisms of Epithelial–Mesenchymal Transition and Implications for Cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef]
- Li, X.; Pei, D.; Zheng, H. Transitions between Epithelial and Mesenchymal States during Cell Fate Conversions. Protein Cell 2014, 5, 580–591. [Google Scholar] [CrossRef]
- Wang, C.; Mu, Z.; Chervoneva, I.; Austin, L.; Ye, Z.; Rossi, G.; Palazzo, J.P.; Sun, C.; Abu-Khalaf, M.; Myers, R.E.; et al. Longitudinally Collected CTCs and CTC-Clusters and Clinical Outcomes of Metastatic Breast Cancer. Breast Cancer Res. Treat. 2017, 161, 83–94. [Google Scholar] [CrossRef]
- Singh, D.; Deshmukh, R.K.; Das, A. SNAI1-Mediated Transcriptional Regulation of Epithelial-to-Mesenchymal Transition Genes in Breast Cancer Stem Cells. Cell. Signal. 2021, 87, 110151. [Google Scholar] [CrossRef]
- Saitoh, M. Transcriptional Regulation of EMT Transcription Factors in Cancer. Semin. Cancer Biol. 2023, 97, 21–29. [Google Scholar] [CrossRef]
- Vesuna, F.; van Diest, P.; Chen, J.H.; Raman, V. Twist Is a Transcriptional Repressor of E-Cadherin Gene Expression in Breast Cancer. Biochem. Biophys. Res. Commun. 2008, 367, 235–241. [Google Scholar] [CrossRef]
- Brlek, P.; Bukovac, A.; Kafka, A.; Pećina-Šlaus, N. TWIST1 Upregulation Affects E-Cadherin Expression in Brain Metastases. Clin. Transl. Oncol. Off. Publ. Fed. Spanish Oncol. Soc. Natl. Cancer Inst. Mex. 2021, 23, 1085–1095. [Google Scholar] [CrossRef]
- Rick, J.W.; Chandra, A.; Dalle Ore, C.; Nguyen, A.T.; Yagnik, G.; Aghi, M.K. Fibronectin in Malignancy: Cancer-Specific Alterations, Protumoral Effects, and Therapeutic Implications. Semin. Oncol. 2019, 46, 284–290. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, R.; Feng, H. Fibronectin Promotes Tumor Cells Growth and Drugs Resistance through a CDC42-YAP-Dependent Signaling Pathway in Colorectal Cancer. Cell Biol. Int. 2020, 44, 1840–1849. [Google Scholar] [CrossRef]
- Li, B.; Shen, W.; Peng, H.; Li, Y.; Chen, F.; Zheng, L.; Xu, J.; Jia, L. Fibronectin 1 Promotes Melanoma Proliferation and Metastasis by Inhibiting Apoptosis and Regulating EMT. OncoTargets Ther. 2019, 12, 3207–3221. [Google Scholar] [CrossRef]
- Gaggioli, C.; Robert, G.; Bertolotto, C.; Bailet, O.; Abbe, P.; Spadafora, A.; Bahadoran, P.; Ortonne, J.-P.; Baron, V.; Ballotti, R.; et al. Tumor-Derived Fibronectin Is Involved in Melanoma Cell Invasion and Regulated by V600E B-Raf Signaling Pathway. J. Investig. Dermatol. 2007, 127, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, Y.-Z.; Xu, L.; Han, T.; Luan, J.; Li, X.; Liu, Y.; Wang, Z.; Liu, Q.; Kong, X.; et al. Exploring New Frontiers: Cell Surface Vimentin as an Emerging Marker for Circulating Tumor Cells and a Promising Therapeutic Target in Advanced Gastric Cancer. J. Exp. Clin. Cancer Res. 2024, 43, 129. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, W.S.; Apostolatos, C.A.; Apostolatos, A.H.; Schutte, R.J.; Huynh, M.A.; Ostrov, D.A.; Acevedo-Duncan, M. Oncogenic PKC-ι Activates Vimentin during Epithelial-Mesenchymal Transition in Melanoma; a Study Based on PKC-ι and PKC-ζ Specific Inhibitors. Cell Adhes. Migr. 2018, 12, 447–463. [Google Scholar] [CrossRef]
- Hashemi, M.; Arani, H.Z.; Orouei, S.; Fallah, S.; Ghorbani, A.; Khaledabadi, M.; Kakavand, A.; Tavakolpournegari, A.; Saebfar, H.; Heidari, H.; et al. EMT Mechanism in Breast Cancer Metastasis and Drug Resistance: Revisiting Molecular Interactions and Biological Functions. Biomed. Pharmacother. 2022, 155, 113774. [Google Scholar] [CrossRef]
- Wang, L.; Mohanasundaram, P.; Lindström, M.; Asghar, M.N.; Sultana, G.; Misiorek, J.O.; Jiu, Y.; Chen, H.; Chen, Z.; Toivola, D.M.; et al. Vimentin Suppresses Inflammation and Tumorigenesis in the Mouse Intestine. Front. Cell Dev. Biol. 2022, 10, 862237. [Google Scholar] [CrossRef]
- Zhang, Y.; Weinberg, R.A. Epithelial-to-Mesenchymal Transition in Cancer: Complexity and Opportunities. Front. Med. 2018, 12, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Banyard, J.; Bielenberg, D.R. The Role of EMT and MET in Cancer Dissemination. Connect. Tissue Res. 2015, 56, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Bardia, A.; Wittner, B.S.; Stott, S.L.; Smas, M.E.; Ting, D.T.; Isakoff, S.J.; Ciciliano, J.C.; Wells, M.N.; Shah, A.M.; et al. Circulating Breast Tumor Cells Exhibit Dynamic Changes in Epithelial and Mesenchymal Composition. Science 2013, 339, 580–584. [Google Scholar] [CrossRef]
- Kasimir-Bauer, S.; Hoffmann, O.; Wallwiener, D.; Kimmig, R.; Fehm, T. Expression of Stem Cell and Epithelial-Mesenchymal Transition Markers in Primary Breast Cancer Patients with Circulating Tumor Cells. Breast Cancer Res. 2012, 14, R15. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Gao, H.; Anfossi, S.; Cohen, E.; Mego, M.; Lee, B.-N.; Tin, S.; De Laurentiis, M.; Parker, C.A.; Alvarez, R.H.; et al. Epithelial–Mesenchymal Transition and Stem Cell Markers in Patients with HER2-Positive Metastatic Breast Cancer. Mol. Cancer Ther. 2012, 11, 2526–2534. [Google Scholar] [CrossRef]
- Mani, S.A.; Guo, W.; Liao, M.-J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The Epithelial-Mesenchymal Transition Generates Cells with Properties of Stem Cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef]
- Li, H.; Qiu, Z.; Li, F.; Wang, C. The Relationship between MMP-2 and MMP-9 Expression Levels with Breast Cancer Incidence and Prognosis. Oncol. Lett. 2017, 14, 5865–5870. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, H. Prognostic Values of Tumoral MMP2 and MMP9 Overexpression in Breast Cancer: A Systematic Review and Meta-Analysis. BMC Cancer 2021, 21, 149. [Google Scholar] [CrossRef]
- Langers, A.M.J.; Verspaget, H.W.; Hawinkels, L.J.A.C.; Kubben, F.J.G.M.; van Duijn, W.; van der Reijden, J.J.; Hardwick, J.C.H.; Hommes, D.W.; Sier, C.F.M. MMP-2 and MMP-9 in Normal Mucosa Are Independently Associated with Outcome of Colorectal Cancer Patients. Br. J. Cancer 2012, 106, 1495–1498. [Google Scholar] [CrossRef] [PubMed]
- Luan, H.; Jian, L.; Huang, Y.; Guo, Y.; Zhou, L. Identification of Novel Therapeutic Target and Prognostic Biomarker in Matrix Metalloproteinase Gene Family in Pancreatic Cancer. Sci. Rep. 2023, 13, 17211. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Gao, M.; Lin, D.; Du, G.; Cai, Y. Prognostic and Immunological Roles of MMP-9 in Pan-Cancer. Biomed Res. Int. 2022, 2022, 2592962. [Google Scholar] [CrossRef]
- Dejana, E.; Bazzoni, G.; Lampugnani, M.G. Vascular Endothelial (VE)-Cadherin: Only an Intercellular Glue? Exp. Cell Res. 1999, 252, 13–19. [Google Scholar] [CrossRef]
- Hendrix, M.J.C.; Seftor, E.A.; Meltzer, P.S.; Gardner, L.M.G.; Hess, A.R.; Kirschmann, D.A.; Schatteman, G.C.; Seftor, R.E.B. Expression and Functional Significance of VE-Cadherin in Aggressive Human Melanoma Cells: Role in Vasculogenic Mimicry. Proc. Natl. Acad. Sci. USA 2001, 98, 8018–8023. [Google Scholar] [CrossRef]
- Delgado-Bellido, D.; Zamudio-Martínez, E.; Fernández-Cortés, M.; Herrera-Campos, A.B.; Olmedo-Pelayo, J.; Perez, C.J.; Expósito, J.; de Álava, E.; Amaral, A.T.; Valle, F.O.; et al. VE-Cadherin Modulates β-Catenin/TCF-4 to Enhance Vasculogenic Mimicry. Cell Death Dis. 2023, 14, 135. [Google Scholar] [CrossRef]
- Liu, Z.; Qi, L.; Li, Y.; Zhao, X.; Sun, B. VEGFR2 Regulates Endothelial Differentiation of Colon Cancer Cells. BMC Cancer 2017, 17, 593. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, D.-M.; Zhou, X.-G.; Yin, N.; Zhang, Y.; Zhang, Z.-X.; Li, D.-C.; Zhou, J. HIF-2α Promotes the Formation of Vasculogenic Mimicry in Pancreatic Cancer by Regulating the Binding of Twist1 to the VE-Cadherin Promoter. Oncotarget 2017, 8, 47801–47815. [Google Scholar] [CrossRef]
- Jolly, M.K.; Boareto, M.; Huang, B.; Jia, D.; Lu, M.; Ben-Jacob, E.; Onuchic, J.N.; Levine, H. Implications of the Hybrid Epithelial/Mesenchymal Phenotype in Metastasis. Front. Oncol. 2015, 5, 155. [Google Scholar] [CrossRef] [PubMed]
- Brabletz, S.; Schuhwerk, H.; Brabletz, T.; Stemmler, M.P. Dynamic EMT: A Multi-Tool for Tumor Progression. EMBO J. 2021, 40, e108647. [Google Scholar] [CrossRef] [PubMed]
- Mego, M.; Mani, S.A.; Lee, B.-N.; Li, C.; Evans, K.W.; Cohen, E.N.; Gao, H.; Jackson, S.A.; Giordano, A.; Hortobagyi, G.N.; et al. Expression of Epithelial–Mesenchymal Transition-Inducing Transcription Factors in Primary Breast Cancer: The Effect of Neoadjuvant Therapy. Int. J. Cancer 2012, 130, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Mego, M.; Karaba, M.; Minarik, G.; Benca, J.; Silvia, J.; Sedlackova, T.; Manasova, D.; Kalavska, K.; Pindak, D.; Cristofanilli, M.; et al. Circulating Tumor Cells with Epithelial-to-Mesenchymal Transition Phenotypes Associated with Inferior Outcomes in Primary Breast Cancer. Anticancer Res. 2019, 39, 1829–1837. [Google Scholar] [CrossRef]
- Matikas, A.; Kotsakis, A.; Apostolaki, S.; Politaki, H.; Perraki, M.; Kalbakis, K.; Nikolaou, M.; Economopoulou, P.; Hatzidaki, D.; Georgoulias, V. Detection of Circulating Tumour Cells before and Following Adjuvant Chemotherapy and Long-Term Prognosis of Early Breast Cancer. Br. J. Cancer 2022, 126, 1563–1569. [Google Scholar] [CrossRef]
- Rapanotti, M.C.; Campione, E.; Suarez Viguria, T.M.; Spallone, G.; Costanza, G.; Rossi, P.; Orlandi, A.; Valenti, P.; Bernardini, S.; Bianchi, L. Stem-Mesenchymal Signature Cell Genes Detected in Heterogeneous Circulating Melanoma Cells Correlate With Disease Stage in Melanoma Patients. Front. Mol. Biosci. 2020, 7, 92. [Google Scholar] [CrossRef]
- Rapanotti, M.C.; Cugini, E.; Campione, E.; Di Raimondo, C.; Costanza, G.; Rossi, P.; Ferlosio, A.; Bernardini, S.; Orlandi, A.; De Luca, A.; et al. Epithelial-to-Mesenchymal Transition Gene Signature in Circulating Melanoma Cells: Biological and Clinical Relevance. Int. J. Mol. Sci. 2023, 24, 11792. [Google Scholar] [CrossRef]
- Vasaikar, S.V.; Deshmukh, A.P.; den Hollander, P.; Addanki, S.; Kuburich, N.A.; Kudaravalli, S.; Joseph, R.; Chang, J.T.; Soundararajan, R.; Mani, S.A. EMTome: A Resource for Pan-Cancer Analysis of Epithelial-Mesenchymal Transition Genes and Signatures. Br. J. Cancer 2021, 124, 259–269. [Google Scholar] [CrossRef]
- Blume, J.D. Bounding Sample Size Projections for the Area under a ROC Curve. J. Stat. Plan. Inference 2009, 139, 711–721. [Google Scholar] [CrossRef]
- Suhail, Y.; Cain, M.P.; Vanaja, K.; Kurywchak, P.A.; Levchenko, A.; Kalluri, R.; Kshitiz. Systems Biology of Cancer Metastasis. Cell Syst. 2019, 9, 109–127. [Google Scholar] [CrossRef]
- Fidler, I.J. The Pathogenesis of Cancer Metastasis: The “seed and Soil” Hypothesis Revisited. Nat. Rev. Cancer 2003, 3, 453–458. [Google Scholar] [CrossRef]
- Książkiewicz, M.; Markiewicz, A.; Zaczek, A.J. Epithelial-Mesenchymal Transition: A Hallmark in Metastasis Formation Linking Circulating Tumor Cells and Cancer Stem Cells. Pathobiology 2012, 79, 195–208. [Google Scholar] [CrossRef]
- Raja, R.; Pandey, A.; Kumar, P. Epithelial to Mesenchymal Plasticity: Role in Cancer Progression. Front. Biosci. Landmark Ed. 2020, 25, 838–873. [Google Scholar] [CrossRef] [PubMed]
- Demirkan, B. The Roles of Epithelial-to-Mesenchymal Transition (EMT) and Mesenchymal-to-Epithelial Transition (MET) in Breast Cancer Bone Metastasis: Potential Targets for Prevention and Treatment. J. Clin. Med. 2013, 2, 264–282. [Google Scholar] [CrossRef] [PubMed]
- de Wit, S.; van Dalum, G.; Lenferink, A.T.M.; Tibbe, A.G.J.; Hiltermann, T.J.N.; Groen, H.J.M.; van Rijn, C.J.M.; Terstappen, L.W.M.M. The Detection of EpCAM+ and EpCAM– Circulating Tumor Cells. Sci. Rep. 2015, 5, 12270. [Google Scholar] [CrossRef]
- Eslami-S, Z.; Cortés-Hernández, L.E.; Alix-Panabières, C. Epithelial Cell Adhesion Molecule: An Anchor to Isolate Clinically Relevant Circulating Tumor Cells. Cells 2020, 9, 1836. [CrossRef]
- Rapanotti, M.C.; Cugini, E.; Nuccetelli, M.; Terrinoni, A.; Di Raimondo, C.; Lombardo, P.; Costanza, G.; Cosio, T.; Rossi, P.; Orlandi, A.; et al. Molecular Sciences MCAM/MUC18/CD146 as a Multifaceted Warning Marker of Melanoma Progression in Liquid Biopsy. Int. J. Mol. Sci. 2021, 22, 12416. [Google Scholar] [CrossRef]
- Novikova, I.A.; Kit, O.I.; Zlatnik, E.Y.; Ulianova, E.P.; Sagakyants, A.B.; Sitkovskaya, A.O.; Shulgina, O.G.; Gevorkyan, Y.A.; Soldatkina, N.V.; Kolesnikov, V.E.; et al. Relationship between Circulating Tumor Cells and ZEB1 Expression in Tumor Cells in Colorectal Cancer. J. Clin. Oncol. 2021, 39, e15509. [Google Scholar] [CrossRef]
- Gao, Y.; Cheng, X.; Han, M. ZEB1-Activated Notch1 Promotes Circulating Tumor Cell Migration and Invasion in Lung Squamous Cell Carcinoma. Clin. Transl. Oncol. 2023, 25, 817–829. [Google Scholar] [CrossRef]
- Kröger, C.; Afeyan, A.; Mraz, J.; Eaton, E.N.; Reinhardt, F.; Khodor, Y.L.; Thiru, P.; Bierie, B.; Ye, X.; Burge, C.B.; et al. Acquisition of a Hybrid E/M State Is Essential for Tumorigenicity of Basal Breast Cancer Cells. Proc. Natl. Acad. Sci. USA 2019, 116, 7353–7362. [Google Scholar] [CrossRef]
- Sahoo, S.; Ramu, S.; Nair, M.G.; Pillai, M.; San Juan, B.P.; Milioli, H.Z.; Mandal, S.; Naidu, C.M.; Mavatkar, A.D.; Subramaniam, H.; et al. Increased Prevalence of Hybrid Epithelial/Mesenchymal State and Enhanced Phenotypic Heterogeneity in Basal Breast Cancer. iScience 2024, 27, 110116. [Google Scholar] [CrossRef] [PubMed]
- Youssef, K.K.; Narwade, N.; Arcas, A.; Marquez-Galera, A.; Jiménez-Castaño, R.; Lopez-Blau, C.; Fazilaty, H.; García-Gutierrez, D.; Cano, A.; Galcerán, J.; et al. Two Distinct Epithelial-to-Mesenchymal Transition Programs Control Invasion and Inflammation in Segregated Tumor Cell Populations. Nat. Cancer 2024, 5, 1660–1680. [Google Scholar] [CrossRef] [PubMed]
- Druzhkova, I.; Shirmanova, M.; Ignatova, N.; Dudenkova, V.; Lukina, M.; Zagaynova, E.; Safina, D.; Kostrov, S.; Didych, D.; Kuzmich, A.; et al. Expression of EMT-Related Genes in Hybrid E/M Colorectal Cancer Cells Determines Fibroblast Activation and Collagen Remodeling. Int. J. Mol. Sci. 2020, 21, 8119. [Google Scholar] [CrossRef]
- Parodi, M.; Centonze, G.; Murianni, F.; Orecchia, P.; Andriani, F.; Roato, I.; Gardelli, C.; Balsamo, M.; Moro, M.; Taiè, G.; et al. Hybrid Epithelial-Mesenchymal Status of Lung Cancer Dictates Metastatic Success through Differential Interaction with NK Cells. J. Immunother. Cancer 2024, 12, e007895. [Google Scholar] [CrossRef]
- Lobb, R.J.; Visan, K.S.; Wu, L.-Y.; Norris, E.L.; Hastie, M.L.; Everitt, S.; Yang, I.A.; Bowman, R.V.; Siva, S.; Larsen, J.E.; et al. An Epithelial-to-Mesenchymal Transition Induced Extracellular Vesicle Prognostic Signature in Non-Small Cell Lung Cancer. Commun. Biol. 2023, 6, 68. [Google Scholar] [CrossRef]
- Guarino, M. Epithelial-Mesenchymal Transition and Tumour Invasion. Int. J. Biochem. Cell Biol. 2007, 39, 2153–2160. [Google Scholar] [CrossRef]
- Goel, H.L.; Mercurio, A.M. VEGF Targets the Tumour Cell. Nat. Rev. Cancer 2013, 13, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Mazeda, I.; Martins, S.F.; Garcia, E.A.; Rodrigues, M.; Longatto, A. VEGF Expression in Colorectal Cancer Metastatic Lymph Nodes: Clinicopathological Correlation and Prognostic Significance. Gastrointest. Disord. 2020, 2, 267–280. [Google Scholar] [CrossRef]
- Fujimoto, J.; Sakaguchi, H.; Aoki, I.; Khatun, S.; Tamaya, T. Clinical Implications of Expression of Vascular Endothelial Growth Factor in Metastatic Lesions of Ovarian Cancers. Br. J. Cancer 2001, 85, 313–316. [Google Scholar] [CrossRef]
- Vand-Rajabpour, F.; Sadeghipour, N.; Saee-Rad, S.; Fathi, H.; Noormohammadpour, P.; Yaseri, M.; Hesari, K.K.; Bagherpour, Z.; Tabrizi, M. Differential BMI1, TWIST1, SNAI2 MRNA Expression Pattern Correlation with Malignancy Type in a Spectrum of Common Cutaneous Malignancies: Basal Cell Carcinoma, Squamous Cell Carcinoma, and Melanoma. Clin. Transl. Oncol. 2017, 19, 489–497. [Google Scholar] [CrossRef]
- Bachmann, I.M.; Puntervoll, H.E.; Otte, A.P.; Akslen, L.A. Loss of BMI-1 Expression Is Associated with Clinical Progress of Malignant Melanoma. Mod. Pathol. 2008, 21, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Shirley, S.H.; Greene, V.R.; Duncan, L.M.; Torres Cabala, C.A.; Grimm, E.A.; Kusewitt, D.F. Slug Expression during Melanoma Progression. Am. J. Pathol. 2012, 180, 2479–2489. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, J.; Rho, O.; Youssef, R.M.; DiGiovanni, J. Twist1 Regulates Keratinocyte Proliferation and Skin Tumor Promotion. Mol. Carcinog. 2016, 55, 941–952. [Google Scholar] [CrossRef]
- Beck, B.; Lapouge, G.; Rorive, S.; Drogat, B.; Desaedelaere, K.; Delafaille, S.; Dubois, C.; Salmon, I.; Willekens, K.; Marine, J.-C.; et al. Different Levels of Twist1 Regulate Skin Tumor Initiation, Stemness, and Progression. Cell Stem Cell 2015, 16, 67–79. [Google Scholar] [CrossRef]
- Melnikova, V.O.; Bar-Eli, M. Transcriptional Control of the Melanoma Malignant Phenotype. Cancer Biol. Ther. 2008, 7, 997–1003. [Google Scholar] [CrossRef]
- Chen, Y.; Sumardika, I.W.; Tomonobu, N.; Winarsa Ruma, I.M.; Kinoshita, R.; Kondo, E.; Inoue, Y.; Sato, H.; Yamauchi, A.; Murata, H.; et al. Melanoma Cell Adhesion Molecule Is the Driving Force behind the Dissemination of Melanoma upon S100A8/A9 Binding in the Original Skin Lesion. Cancer Lett. 2019, 452, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Rapanotti, M.C.; Campione, E.; Spallone, G.; Orlandi, A.; Bernardini, S.; Bianchi, L. Minimal Residual Disease in Melanoma: Circulating Melanoma Cells and Predictive Role of MCAM/MUC18/MelCAM/CD146. Cell Death Discov. 2017, 3, 17005. [Google Scholar] [CrossRef]
- Rapanotti, M.C.; Viguria, T.M.S.; Spallone, G.; Terrinoni, A.; Rossi, P.; Costanza, G.; Campione, E.; Lombardo, P.; Pathirannehalage, C.D.; Orlandi, A.; et al. Minimal Residual Disease in Melanoma:Molecular Characterization of in Transit Cutaneous Metastases and Circulating Melanoma Cells Recognizes an Expression Panel Potentially Related to Disease Progression. Cancer Treat. Res. Commun. 2020, 25, 100262. [Google Scholar] [CrossRef]
- Rapanotti, M.C.; Suarez Viguria, T.M.; Costanza, G.; Ricozzi, I.; Pierantozzi, A.; Di Stefani, A.; Campione, E.; Bernardini, S.; Chimenti, S.; Orlandi, A.; et al. Sequential Molecular Analysis of Circulating MCAM/MUC18 Expression: A Promising Disease Biomarker Related to Clinical Outcome in Melanoma. Arch. Dermatol. Res. 2014, 306, 527–537. [Google Scholar] [CrossRef]
- Papadaki, M.A.; Stoupis, G.; Theodoropoulos, P.A.; Mavroudis, D.; Georgoulias, V.; Agelaki, S. Circulating Tumor Cells with Stemness and Epithelial-to-Mesenchymal Transition Features Are Chemoresistant and Predictive of Poor Outcome in Metastatic Breast Cancer. Mol. Cancer Ther. 2019, 18, 437–447. [Google Scholar] [CrossRef]
- Nakayama, F.; Miyoshi, M.; Kimoto, A.; Kawano, A.; Miyashita, K.; Kamoshida, S.; Shimizu, K.; Hori, Y. Pancreatic Cancer Cell-Derived Exosomes Induce Epithelial-Mesenchymal Transition in Human Pancreatic Cancer Cells Themselves Partially via Transforming Growth Factor Β1. Med. Mol. Morphol. 2022, 55, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Pastushenko, I.; Brisebarre, A.; Sifrim, A.; Fioramonti, M.; Revenco, T.; Boumahdi, S.; Van Keymeulen, A.; Brown, D.; Moers, V.; Lemaire, S.; et al. Identification of the Tumour Transition States Occurring during EMT. Nature 2018, 556, 463–468. [Google Scholar] [CrossRef]
- Falconi, G.; Fabiani, E.; Fianchi, L.; Criscuolo, M.; Raffaelli, C.S.; Bellesi, S.; Hohaus, S.; Voso, M.T.; D’Alò, F.; Leone, G. Impairment of PI3K/AKT and WNT/β-Catenin Pathways in Bone Marrow Mesenchymal Stem Cells Isolated from Patients with Myelodysplastic Syndromes. Exp. Hematol. 2016, 44, 75–83.e4. [Google Scholar] [CrossRef]
- Loh, C.Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef] [PubMed]
- HAZAN, R.B.; QIAO, R.U.I.; KEREN, R.; BADANO, I.; SUYAMA, K. Cadherin Switch in Tumor Progression. Ann. N. Y. Acad. Sci. 2004, 1014, 155–163. [Google Scholar] [CrossRef]
- Stemmler, M.P.; Eccles, R.L.; Brabletz, S.; Brabletz, T. Non-Redundant Functions of EMT Transcription Factors. Nat. Cell Biol. 2019, 21, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Luca, M.; Huang, S.; Gutman, M.; Reich, R.; Johnson, J.P.; Bar-Eli, M. Expression of MCAM/MUC18 by Human Melanoma Cells Leads to Increased Tumor Growth and Metastasis. Cancer Res. 1997, 57, 2295–2303. [Google Scholar]
- Chomczynski, P.; Sacchi, N. Single-Step Method of RNA Isolation by Acid Guanidinium Thiocyanate-Phenol-Chloroform Extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]

| Gene | Healthy vs. Primary Tumor | ||||
| mRNA levels (a.u.) | ROC AUC | Sensitivity (%) | Specificity (%) | p value | |
| CDH1 | >3.085 | 0.96 | 100 | 90 | 0.002 |
| CDH1/ZEB1 | <4.552 | 0.90 | 87.5 | 100 | 0.019 |
| CDH5/CDH1 | >0.001807 | 0.87 | 87.5 | 85.7 | 0.018 |
| Gene | Healthy vs. Metastatic Tumor | ||||
| mRNA levels (a.u.) | ROC AUC | Sensitivity (%) | Specificity (%) | p value | |
| ZEB1 | >0.2765 | 1 | 100 | 100 | 0.009 |
| CDH5 | >0.135 | 0.92 | 100 | 80 | 0.028 |
| CDH5/CDH1 | >0.06031 | 1 | 100 | 100 | 0.005 |
| CDH1/ZEB1 | >4.186 | 1 | 100 | 100 | 0.009 |
| Gene | Primary vs. Metastatic Tumor | ||||
| mRNA levels (a.u.) | ROC AUC | Sensitivity (%) | Specificity (%) | p value | |
| CDH5 | >0.1865 | 0.93 | 100 | 77.8 | 0.009 |
| CDH5/ZEB1 | <0.05273 | 0.88 | 87.5 | 100 | 0.028 |
| Origin | Cell Line | Status |
|---|---|---|
| HUMAN LUNG | Beas B2 | Healthy Bronchial Epithelium |
| A-549 | Primary Lung Carcinoma | |
| H460 | Metastatic Lung Carcinoma | |
| HUMAN COLON | CCD 841 CoN | Healthy Colon Epithelium |
| HCT 116 | Primary Colon Carcinoma | |
| SW 480 | Primary Dukes C Colon–Rectal cancer | |
| SW 460 | Metastatic Dukes C Colon–Rectal cancer | |
| HUMAN PANCREAS | HPaEpiC | Healthy Pancreas Epithelium |
| Panc1 | Primary Epithelioid Pancreatic carcinoma | |
| MiaPaCa-2 | Primary Pancreatic Carcinoma | |
| CFPAC-1 | Metastatic Pancreatic Carcinoma | |
| HUMAN SKIN | HEK (n) | Healthy Neonatal Epidermal Keratinocytes |
| A431 | Primary Epidermoid Carcinoma | |
| NMSC | Non-Melanoma Skin Cancer | |
| HUMANNEURO-ECTODERMA | NHEM | Normal Human Epidermal Melanocytes |
| WM 115 | Primary Melanoma | |
| A375 | Achromic Primary Melanoma | |
| WM 266.4 | Metastatic Melanoma | |
| HUMANENDOTHELIUM | HUVEC | Human Umbilical Endothelial cells |
| HUMANMESENCHYMA | NHLF | Stromal Lung Fibroblasts |
| StromPA | Stromal Pancreatic cells | |
| EDS | Human Skin Fibroblasts | |
| CCD-1123SK | Human Skin Fibroblasts | |
| V140-HD | Healthy Bone Marrow Donor-Derived Mesenchymal Cells [83] | |
| V126-HD | Healthy Bone Marrow Donor-Derived Mesenchymal cells [83] |
| Gene | Primer Sequences |
|---|---|
| CD146/MCAM * | F: 5′-AGCTCCGCGTCTACAAAGC-3′ |
| R: 5′-CTACACAGGTAGCGACCTCC-3′ | |
| CDH1 § | F: 5′AAAGGCCCATTTCCTAAAAACCT-3′ |
| R: 5′TGCGTTCTCTATCCAGAGGCT-3′ | |
| CDH2 § | F: 5′CTCCTATGA GTGGAA CAG GAA CG-3′ |
| R: 5′-TTG GAT CAA TGT CAT AAT CAA GTG CTGTA-3′ | |
| CDH5 * | F. 5′-CACTGGAACCCCCACAGGAAAAGA-3′ |
| R. 5′-GGACAGCGTTCTCACACACTTTGG-3′ | |
| HFN1 § | F: 5′-AGCCGAGGTTTTAACTGCGA-3′ |
| R: 5′-CCC ACT CGGTAAGTGTTCCC-3′ | |
| VIM § | R: 5′-GACGCCATCAACACCGAGTT-3′ |
| F: 5′-CTTTGTCGTTGGTTAGCTGGT-3′ | |
| SNAI1 § | F: 5′-CCCAGTGCCTCGACCACTAT-3′ |
| R: 5′-GCTGGAAGGTAAACTCTGGATTAGA-3′ | |
| SNAI2/SLUG § | R: 5′-CCAAGCTTTCAGACCCCCAT-3′ |
| F: 5′-GAAAAAGGCTTCTCCCCCGT-3′ | |
| TWIST1 § | R: 5′- GCTTGAGGGTCTGAATCTTGCT-3′ |
| F: 5′-GTCCGCAGTCTTACGAGGAG-3′ | |
| ZEB1 § | R: 5′-CAGCTTGATACCTGTGAATGGG-3′ |
| F: 5′-TATCTGTGGTCGTGTGGGACT-3′ | |
| MMP2 * | F: 5′-CCTGCCCCTCCCTTCAACCA-3′ |
| R: 5′-GTTTCCGCTTCTGGCTGGGTC-3′ | |
| MMP9 * | F: 5′-CGGAGTGGCAGGGGGAAGATG |
| R. 5′-CGGAGTGGCAGGGGGAAGATG | |
| ACTB * | R: 5′-GAGACCTTCAACACCCCAGCC-3 |
| R. 5′-AATGTCACGCACGATTTCCC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rapanotti, M.C.; Cugini, E.; Scioli, M.G.; Cenci, T.; Anzillotti, S.; Puzzuoli, M.; Terrinoni, A.; Ferlosio, A.; De Luca, A.; Orlandi, A. The Clinical Relevance of Epithelial-to-Mesenchymal Transition Hallmarks: A Cut-Off-Based Approach in Healthy and Cancerous Cell Lines. Int. J. Mol. Sci. 2025, 26, 3617. https://doi.org/10.3390/ijms26083617
Rapanotti MC, Cugini E, Scioli MG, Cenci T, Anzillotti S, Puzzuoli M, Terrinoni A, Ferlosio A, De Luca A, Orlandi A. The Clinical Relevance of Epithelial-to-Mesenchymal Transition Hallmarks: A Cut-Off-Based Approach in Healthy and Cancerous Cell Lines. International Journal of Molecular Sciences. 2025; 26(8):3617. https://doi.org/10.3390/ijms26083617
Chicago/Turabian StyleRapanotti, Maria Cristina, Elisa Cugini, Maria Giovanna Scioli, Tonia Cenci, Silvia Anzillotti, Martina Puzzuoli, Alessandro Terrinoni, Amedeo Ferlosio, Anastasia De Luca, and Augusto Orlandi. 2025. "The Clinical Relevance of Epithelial-to-Mesenchymal Transition Hallmarks: A Cut-Off-Based Approach in Healthy and Cancerous Cell Lines" International Journal of Molecular Sciences 26, no. 8: 3617. https://doi.org/10.3390/ijms26083617
APA StyleRapanotti, M. C., Cugini, E., Scioli, M. G., Cenci, T., Anzillotti, S., Puzzuoli, M., Terrinoni, A., Ferlosio, A., De Luca, A., & Orlandi, A. (2025). The Clinical Relevance of Epithelial-to-Mesenchymal Transition Hallmarks: A Cut-Off-Based Approach in Healthy and Cancerous Cell Lines. International Journal of Molecular Sciences, 26(8), 3617. https://doi.org/10.3390/ijms26083617







