Increased Myocardial MARK4 Expression in Patients with Heart Failure and Sleep-Disordered Breathing
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics
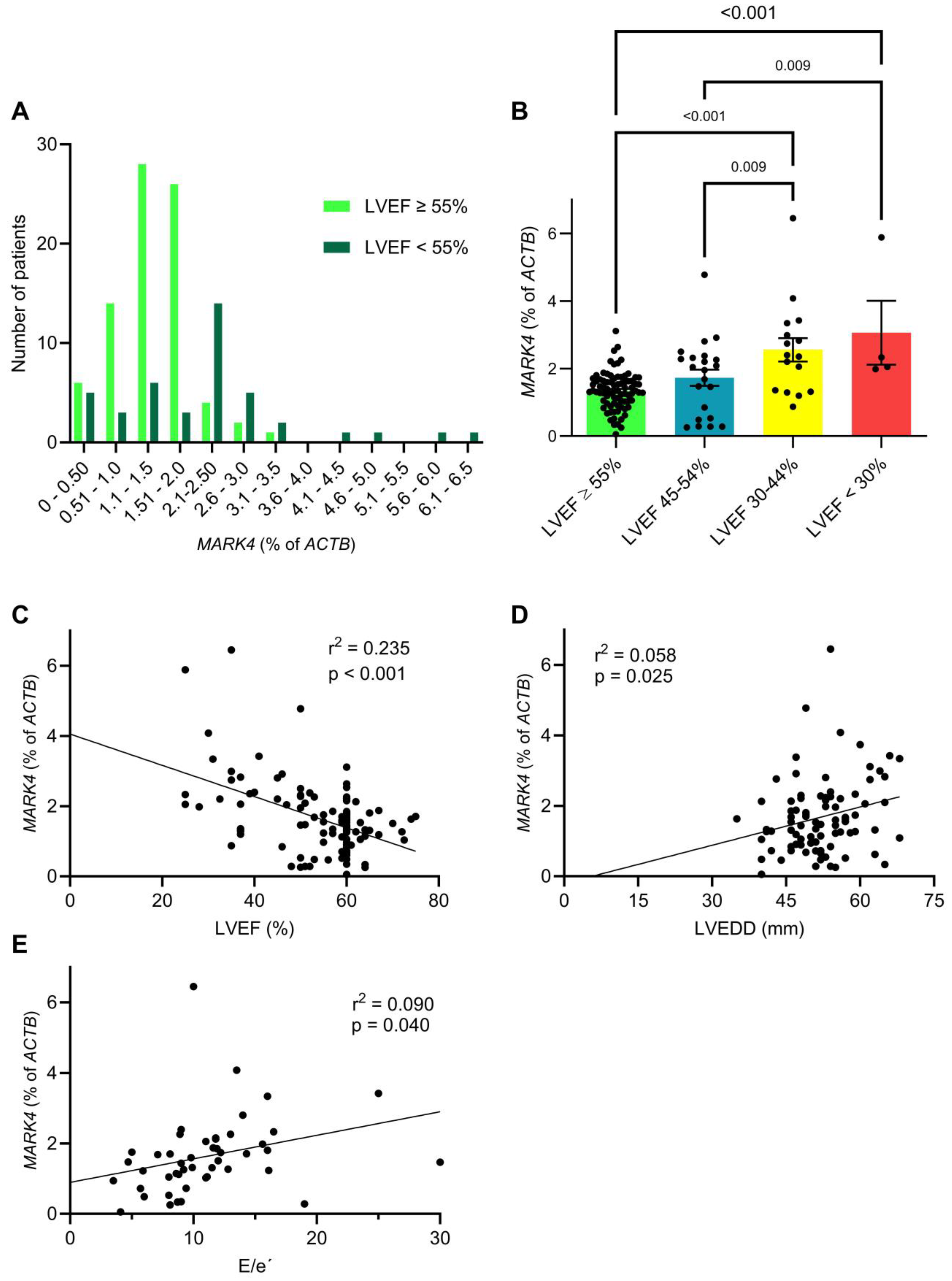
2.2. Myocardial MARK4 Expression Is Increased in Patients with Heart Failure
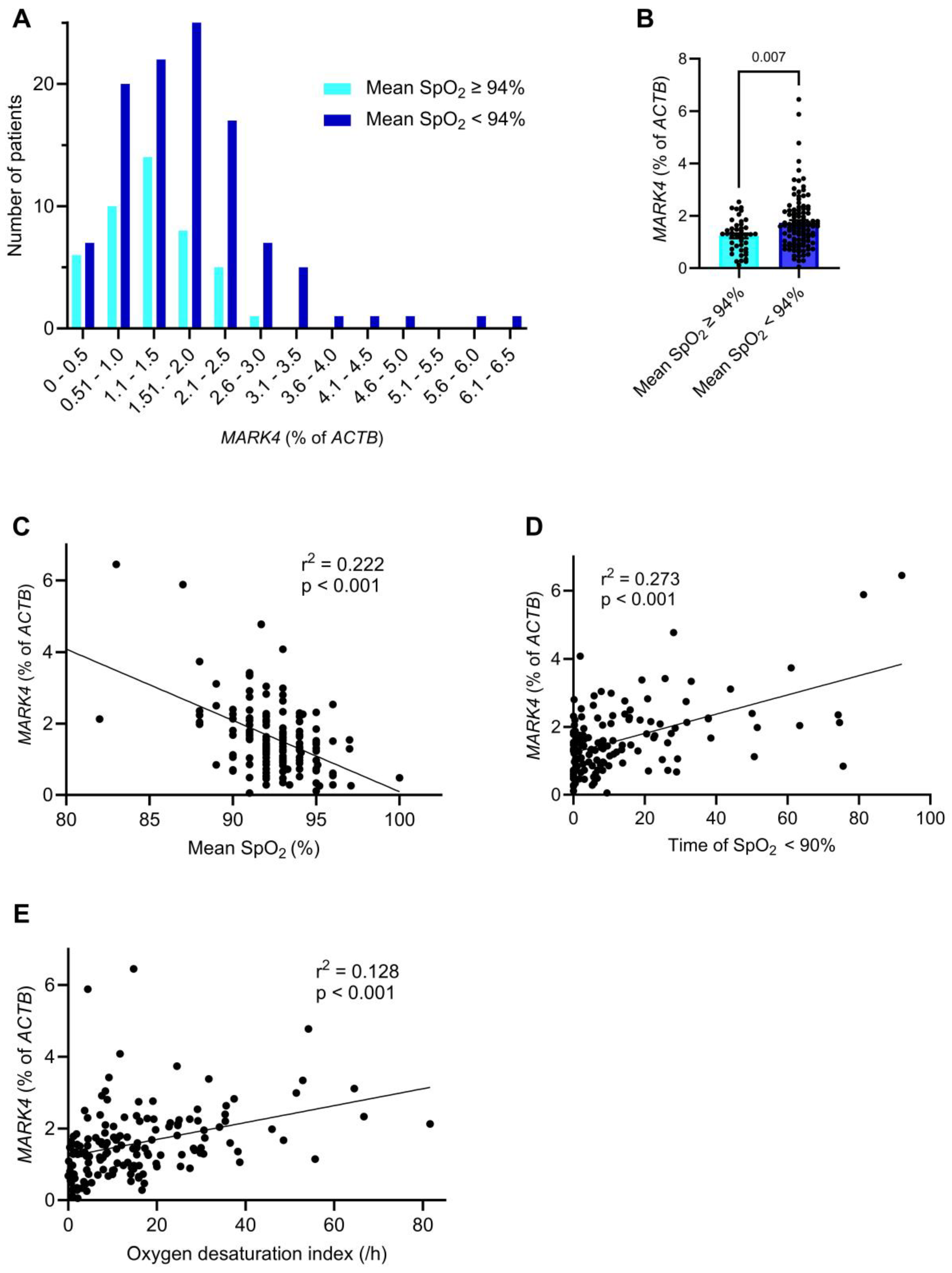
2.3. Myocardial MARK4 Expression Is Increased in Patients with SDB
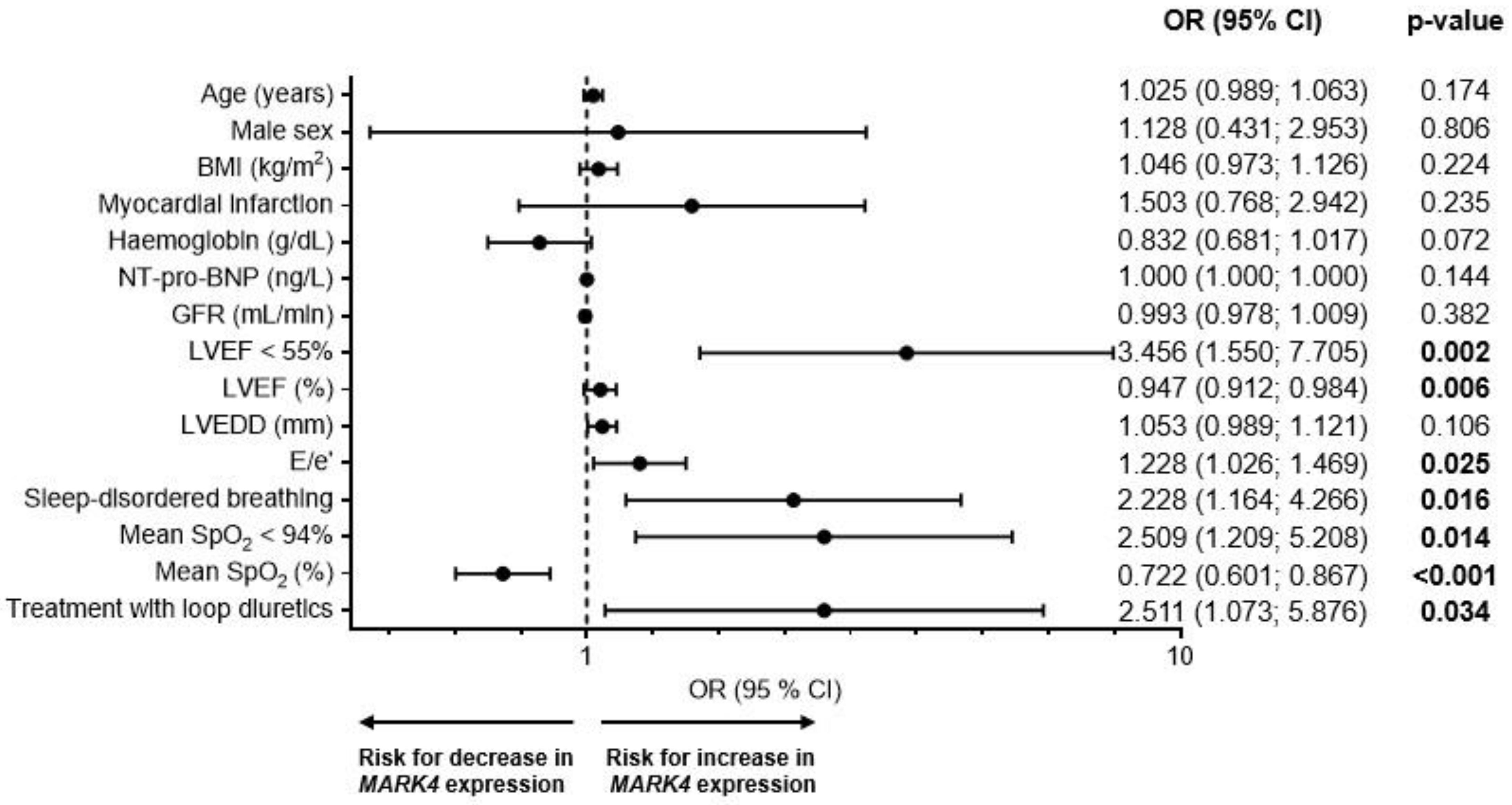
2.4. Risk Factors for an Increased Myocardial MARK4 Expression
3. Discussion
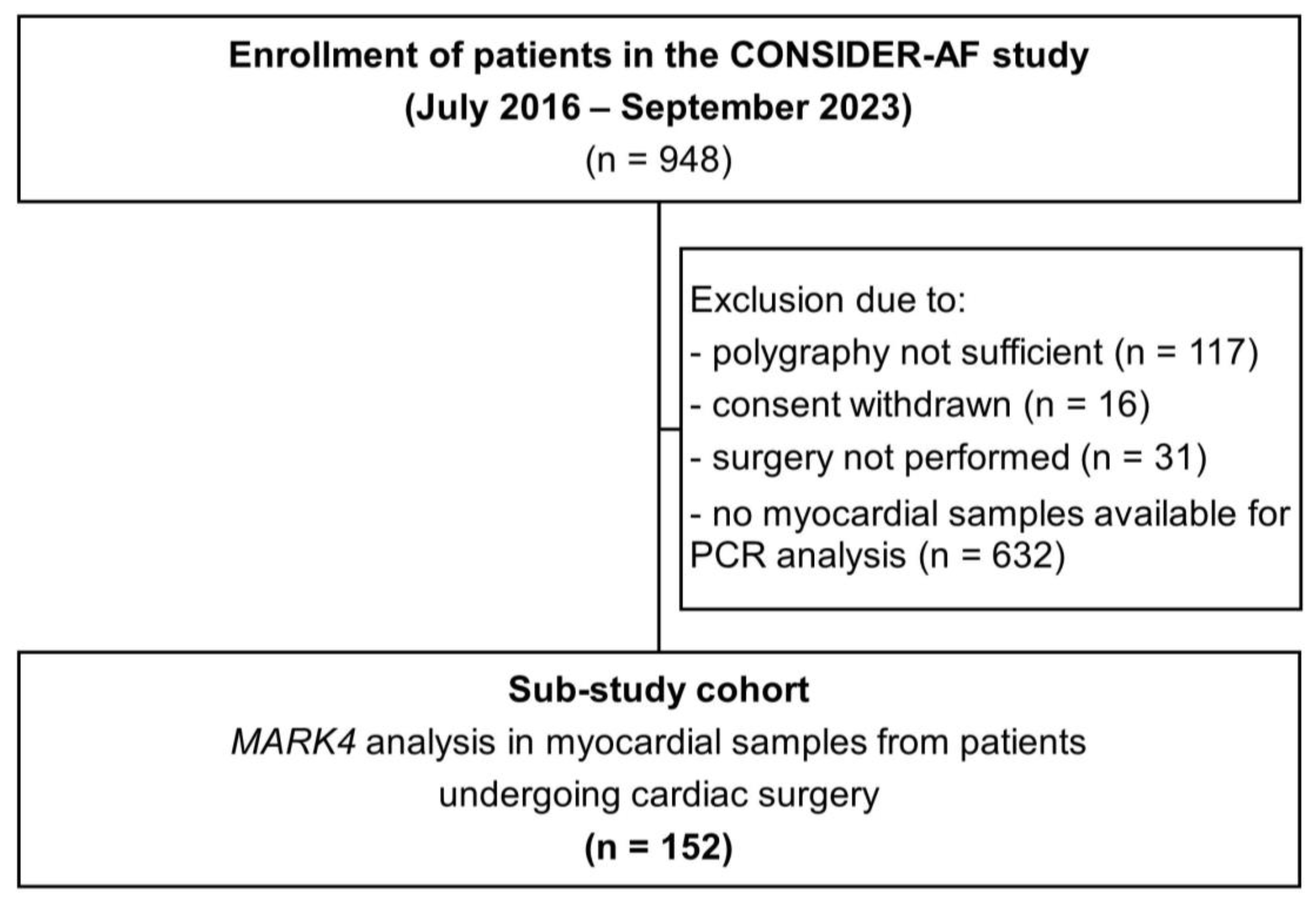
4. Materials and Methods
4.1. Study Design
4.2. Assessment of SDB
4.3. RNA Isolation, Transcription into cDNA, and Quantification of MARK4
4.4. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACTB | Actin beta |
| AHI | Apnea-hypopnea index |
| ASV | Adaptive servoventilation |
| BMI | Body mass index |
| CSA | Central sleep apnea |
| CPAP | Continues positive airway pressure |
| CRP | C-reactive protein |
| LAVI | Left atrial volume index |
| LVEDD | Left ventricular end-diastolic diameter |
| LVEF | Left ventricular ejection fraction |
| MAP | Microtubule-associated protein |
| MARK | MAP/microtubule affinity-regulating kinase |
| NT-pro-BNP | N-terminal pro b-type natriuretic peptide |
| NYHA | New York Heart Association |
| OSA | Obstructive sleep apnea |
| SDB | Sleep-disordered breathing |
| sPAP | Systolic pulmonary artery pressure |
| SpO2 | Peripheral oxygen saturation |
References
- Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [CrossRef] [PubMed]
- Wei, S.; Miranda, J.J.; Mamas, M.A.; Zühlke, L.J.; Kontopantelis, E.; Thabane, L.; Van Spall, H.G. Sex differences in the etiology and burden of heart failure across country income level: Analysis of 204 countries and territories 1990–2019. Eur. Heart J. Qual. Care Clin. Outcomes 2023, 9, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Barasa, A.; Schaufelberger, M.; Lappas, G.; Swedberg, K.; Dellborg, M.; Rosengren, A. Heart failure in young adults: 20-year trends in hospitalization, aetiology, and case fatality in Sweden. Eur. Heart J. 2014, 35, 25–32. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
- Jones, N.R.; Roalfe, A.K.; Adoki, I.; Hobbs, F.D.R.; Taylor, C.J. Survival of patients with chronic heart failure in the community: A systematic review and meta-analysis. Eur. J. Heart Fail. 2019, 21, 1306–1325. [Google Scholar] [CrossRef] [PubMed]
- Kasai, T.; Floras, J.S.; Bradley, T.D. Sleep apnea and cardiovascular disease: A bidirectional relationship. Circulation 2012, 126, 1495–1510. [Google Scholar] [CrossRef]
- Wester, M.; Arzt, M.; Sinha, F.; Maier, L.S.; Lebek, S. Insights into the Interaction of Heart Failure with Preserved Ejection Fraction and Sleep-Disordered Breathing. Biomedicines 2023, 11, 3038. [Google Scholar] [CrossRef]
- Mayer, G.; Arzt, M.; Braumann, B.; Ficker, J.H.; Fietze, I.; Frohnhofen, H.; Galetke, W.; Maurer, J.T.; Orth, M.; Penzel, T.; et al. German S3 Guideline Nonrestorative Sleep/Sleep Disorders, chapter “Sleep-Related Breathing Disorders in Adults,” short version: German Sleep Society (Deutsche Gesellschaft für Schlafforschung und Schlafmedizin, DGSM). Somnologie 2017, 21, 290–301. [Google Scholar] [CrossRef]
- Kaneko, Y.; Floras, J.S.; Usui, K.; Plante, J.; Tkacova, R.; Kubo, T.; Ando, S.; Bradley, T.D. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N. Engl. J. Med. 2003, 348, 1233–1241. [Google Scholar] [CrossRef]
- Bradley, T.D.; Logan, A.G.; Kimoff, R.J.; Sériès, F.; Morrison, D.; Ferguson, K.; Belenkie, I.; Pfeifer, M.; Fleetham, J.; Hanly, P.; et al. Continuous positive airway pressure for central sleep apnea and heart failure. N. Engl. J. Med. 2005, 353, 2025–2033. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, R.D.; Antic, N.A.; Heeley, E.; Luo, Y.; Ou, Q.; Zhang, X.; Mediano, O.; Chen, R.; Drager, L.F.; Liu, Z.; et al. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. N. Engl. J. Med. 2016, 375, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Kemphues, K.J. par-1, a gene required for establishing polarity in C. elegans embryos, encodes a putative Ser/Thr kinase that is asymmetrically distributed. Cell 1995, 81, 611–620. [Google Scholar] [CrossRef]
- Schneider, A.; Laage, R.; von Ahsen, O.; Fischer, A.; Rossner, M.; Scheek, S.; Grünewald, S.; Kuner, R.; Weber, D.; Krüger, C.; et al. Identification of regulated genes during permanent focal cerebral ischaemia: Characterization of the protein kinase 9b5/MARKL1/MARK4. J. Neurochem. 2004, 88, 1114–1126. [Google Scholar] [CrossRef]
- Drewes, G.; Ebneth, A.; Preuss, U.; Mandelkow, E.M.; Mandelkow, E. MARK, a novel family of protein kinases that phos-phorylate microtubule-associated proteins and trigger microtubule disruption. Cell 1997, 89, 297–308. [Google Scholar] [CrossRef]
- Drewes, G.; Trinczek, B.; Illenberger, S.; Biernat, J.; Schmitt-Ulms, G.; Meyer, H.E.; Mandelkow, E.M.; Mandelkow, E. Micro-tubule-associated protein/microtubule affinity-regulating kinase (p110mark). A novel protein kinase that regulates tau-microtubule interactions and dynamic instability by phosphorylation at the Alzheimer-specific site serine 262. J. Biol. Chem. 1995, 270, 7679–7688. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Xu, L.; Jeddo, S.F.; Li, K.; Li, X.; Li, J. MARK2 enhances cisplatin resistance via PI3K/AKT/NF-κB signaling pathway in osteosarcoma cells. Am. J. Transl. Res. 2020, 12, 1807–1823. [Google Scholar]
- Hubaux, R.; Thu, K.L.; Vucic, E.A.; Pikor, L.A.; Kung, S.H.Y.; Martinez, V.D.; Mosslemi, M.; Becker-Santos, D.D.; Gazdar, A.F.; Lam, S.; et al. Microtubule affinity-regulating kinase 2 is associated with DNA damage response and cisplatin resistance in non-small cell lung cancer. Int. J. Cancer 2015, 137, 2072–2082. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, J.; Wang, W.; Wu, D.; Kang, Y.; Fu, L. MARK4 aggravates cardiac dysfunction in mice with STZ-induced diabetic cardiomyopathy by regulating ACSL4-mediated myocardial lipid metabolism. Sci. Rep. 2024, 14, 12978. [Google Scholar] [CrossRef]
- Yu, X.; Chen, X.; Amrute-Nayak, M.; Allgeyer, E.; Zhao, A.; Chenoweth, H.; Clement, M.; Harrison, J.; Doreth, C.; Sirinakis, G.; et al. MARK4 controls ischaemic heart failure through microtubule detyrosination. Nature 2021, 594, 560–565. [Google Scholar] [CrossRef]
- Lebek, S.; Tafelmeier, M.; Messmann, R.; Provaznik, Z.; Schmid, C.; Maier, L.S.; Birner, C.; Arzt, M.; Wagner, S. Angioten-sin-converting enzyme inhibitor/angiotensin II receptor blocker treatment and haemodynamic factors are associated with increased cardiac mRNA expression of angiotensin-converting enzyme 2 in patients with cardiovascular disease. Eur. J. Heart Fail. 2020, 22, 2248–2257. [Google Scholar] [CrossRef] [PubMed]
- Hegner, P.; Wester, M.; Tafelmeier, M.; Provaznik, Z.; Klatt, S.; Schmid, C.; Maier, L.S.; Arzt, M.; Wagner, S.; Lebek, S. Systemic inflammation predicts diastolic dysfunction in patients with sleep disordered breathing. Eur. Respir. J. 2024, 63, 2400579. [Google Scholar] [CrossRef] [PubMed]
- Manilall, A.; Mokotedi, L.; Gunter, S.; Le Roux, R.; Fourie, S.; Flanagan, C.A.; Millen, A.M.E. Tumor Necrosis Factor-α Mediates Inflammation-induced Early-Stage Left Ventricular Systolic Dysfunction. J. Cardiovasc. Pharmacol. 2023, 81, 411–422. [Google Scholar] [CrossRef]
- Yang, J.; Niu, H.; Pang, S.; Liu, M.; Chen, F.; Li, Z.; He, L.; Mo, J.; Yi, H.; Xiao, J.; et al. MARK3 kinase: Regulation and physiologic roles. Cell. Signal. 2023, 103, 110578. [Google Scholar] [CrossRef]
- Anwar, S.; Shahwan, M.; Hasan, G.M.; Islam, A.; Hassan, M.I. Microtubule-affinity regulating kinase 4: A potential drug target for cancer therapy. Cell. Signal. 2022, 99, 110434. [Google Scholar] [CrossRef]
- Peng, C.Y.; Graves, P.R.; Ogg, S.; Thoma, R.S.; Byrnes, M.J., III; Wu, Z.; Stephenson, M.T.; Piwnica-Worms, H. C-TAK1 protein kinase phosphorylates human Cdc25C on serine 216 and promotes 14-3-3 protein binding. Cell Growth Differ. 1998, 9, 197–208. [Google Scholar]
- Müller, J.; Ory, S.; Copeland, T.; Piwnica-Worms, H.; Morrison, D.K. C-TAK1 regulates Ras signaling by phosphorylating the MAPK scaffold, KSR1. Mol. Cell 2001, 8, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Jansson, D.; Ng, A.C.-H.; Fu, A.; Depatie, C.; Al Azzabi, M.; Screaton, R.A. Glucose controls CREB activity in islet cells via regulated phosphorylation of TORC2. Proc. Natl. Acad. Sci. USA 2008, 105, 10161–10166. [Google Scholar] [CrossRef]
- Lennerz, J.K.; Hurov, J.B.; White, L.S.; Lewandowski, K.T.; Prior, J.L.; Planer, G.J.; Gereau, R.W., 4th; Piwnica-Worms, D.; Schmidt, R.E.; Piwnica-Worms, H. Loss of Par-1a/MARK3/C-TAK1 kinase leads to reduced adiposity, resistance to hepatic steatosis, and defective gluconeogenesis. Mol. Cell. Biol. 2010, 30, 5043–5056. [Google Scholar] [CrossRef]
- Moroni, R.F.; de Biasi, S.; Colapietro, P.; Larizza, L.; Beghini, A. Distinct expression pattern of microtubule-associated pro-tein/microtubule affinity-regulating kinase 4 in differentiated neurons. Neuroscience 2006, 143, 83–94. [Google Scholar] [CrossRef]
- Rovina, D.; Fontana, L.; Monti, L.; Novielli, C.; Panini, N.; Sirchia, S.M.; Erba, E.; Magnani, I.; Larizza, L. Microtubule-associated protein/microtubule affinity-regulating kinase 4 (MARK4) plays a role in cell cycle progression and cytoskeletal dynamics. Eur. J. Cell Biol. 2014, 93, 355–365. [Google Scholar] [CrossRef]
- Gu, G.J.; Lund, H.; Wu, D.; Blokzijl, A.; Classon, C.; von Euler, G.; Landegren, U.; Sunnemark, D.; Kamali-Moghaddam, M. Role of individual MARK isoforms in phosphorylation of tau at Ser262 in Alzheimer’s disease. Neuromol. Med. 2013, 15, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Beghini, A.; Magnani, I.; Roversi, G.; Piepoli, T.; Di Terlizzi, S.; Moroni, R.F.; Pollo, B.; Fuhrman Conti, A.M.; Cowell, J.K.; Finocchiaro, G.; et al. The neural progenitor-restricted isoform of the MARK4 gene in 19q13.2 is upregulated in human gliomas and overexpressed in a subset of glioblastoma cell lines. Oncogene 2003, 22, 2581–2591. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Satoh, S.; Okabe, H.; Kitahara, O.; Ono, K.; Kihara, C.; Tanaka, T.; Tsunoda, T.; Yamaoka, Y.; Nakamura, Y.; et al. Isolation of a novel human gene, MARKL1, homologous to MARK3 and its involvement in hepatocellular carcinogenesis. Neoplasia 2001, 3, 4–9. [Google Scholar] [CrossRef]
- Liu, Z.; Gan, L.; Chen, Y.; Luo, D.; Zhang, Z.; Cao, W.; Zhou, Z.; Lin, X.; Sun, C. Mark4 promotes oxidative stress and in-flammation via binding to PPARγ and activating NF-κB pathway in mice adipocytes. Sci. Rep. 2016, 6, 21382. [Google Scholar] [CrossRef]
- Feng, M.; Tian, L.; Gan, L.; Liu, Z.; Sun, C. Mark4 promotes adipogenesis and triggers apoptosis in 3T3-L1 adipocytes by activating JNK1 and inhibiting p38MAPK pathways. Biol. Cell 2014, 106, 294–307. [Google Scholar] [CrossRef]
- Sun, C.; Tian, L.; Nie, J.; Zhang, H.; Han, X.; Shi, Y. Inactivation of MARK4, an AMP-activated protein kinase (AMPK)-related kinase, leads to insulin hypersensitivity and resistance to diet-induced obesity. J. Biol. Chem. 2012, 287, 38305–38315. [Google Scholar] [CrossRef]
- Alam, M.; Ahmed, S.; Abid, M.; Hasan, G.M.; Islam, A.; Hassan, M.I. Therapeutic targeting of microtubule affinity-regulating kinase 4 in cancer and neurodegenerative diseases. J. Cell. Biochem. 2023, 124, 1223–1240. [Google Scholar] [CrossRef]
- Robison, P.; Caporizzo, M.A.; Ahmadzadeh, H.; Bogush, A.I.; Chen, C.Y.; Margulies, K.B.; Shenoy, V.B.; Prosser, B.L. Dety-rosinated microtubules buckle and bear load in contracting cardiomyocytes. Science 2016, 352, aaf0659. [Google Scholar] [CrossRef]
- Gundersen, G.G.; Kalnoski, M.H.; Bulinski, J.C. Distinct populations of microtubules: Tyrosinated and nontyrosinated alpha tubulin are distributed differently in vivo. Cell 1984, 38, 779–789. [Google Scholar] [CrossRef]
- Chen, C.Y.; Caporizzo, M.A.; Bedi, K.; Vite, A.; Bogush, A.I.; Robison, P.; Heffler, J.G.; Salomon, A.K.; Kelly, N.A.; Babu, A.; et al. Suppression of detyrosinated microtubules improves cardiomyocyte function in human heart failure. Nat. Med. 2018, 24, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Cowie, M.R.; Woehrle, H.; Wegscheider, K.; Angermann, C.; d’Ortho, M.-P.; Erdmann, E.; Levy, P.; Simonds, A.K.; Somers, V.K.; Zannad, F.; et al. Adaptive Servo-Ventilation for Central Sleep Apnea in Systolic Heart Failure. N. Engl. J. Med. 2015, 373, 1095–1105. [Google Scholar] [CrossRef]
- Bradley, T.D.; Logan, A.G.; Lorenzi Filho, G.; Kimoff, R.J.; Durán Cantolla, J.; Arzt, M.; Redolfi, S.; Parati, G.; Kasai, T.; Dunlap, M.E.; et al. Adaptive servo-ventilation for sleep-disordered breathing in patients with heart failure with reduced ejection fraction (ADVENT-HF): A multicentre, multinational, parallel-group, open-label, phase 3 randomised controlled trial. Lancet Respir. Med. 2024, 12, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Traaen, G.M.; Aakerøy, L.; Hunt, T.-E.; Øverland, B.; Bendz, C.; Sande, L.Ø.; Aakhus, S.; Fagerland, M.W.; Steinshamn, S.; Anfinsen, O.-G.; et al. Effect of Continuous Positive Airway Pressure on Arrhythmia in Atrial Fibrillation and Sleep Apnea: A Randomized Controlled Trial. Am. J. Respir. Crit. Care Med. 2021, 204, 573–582. [Google Scholar] [CrossRef]
- Peker, Y.; Glantz, H.; Eulenburg, C.; Wegscheider, K.; Herlitz, J.; Thunström, E. Effect of Positive Airway Pressure on Car-diovascular Outcomes in Coronary Artery Disease Patients with Nonsleepy Obstructive Sleep Apnea. The RICCADSA Randomized Controlled Trial. Am. J. Respir. Crit. Care Med. 2016, 194, 613–620. [Google Scholar] [CrossRef]
- Zinchuk, A.V.; Chu, J.-H.; Liang, J.; Celik, Y.; Op de Beeck, S.; Redeker, N.S.; Wellman, A.; Yaggi, H.K.; Peker, Y.; Sands, S.A. Physiological Traits and Adherence to Sleep Apnea Therapy in Individuals with Coronary Artery Disease. Am. J. Respir. Crit. Care Med. 2021, 204, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Hegner, P.; Ofner, F.; Schaner, B.; Gugg, M.; Trum, M.; Lauerer, A.-M.; Maier, L.S.; Arzt, M.; Lebek, S.; Wagner, S. CaMKIIδ-dependent dysregulation of atrial Na(+) homeostasis promotes pro-arrhythmic activity in an obstructive sleep apnea mouse model. Front. Pharmacol. 2024, 15, 1411822. [Google Scholar] [CrossRef]
- Hegner, P.; Lebek, S.; Schaner, B.; Ofner, F.; Gugg, M.; Maier, L.S.; Arzt, M.; Wagner, S. CaMKII-Dependent Contractile Dys-function and Pro-Arrhythmic Activity in a Mouse Model of Obstructive Sleep Apnea. Antioxidants 2023, 12, 315. [Google Scholar] [CrossRef]
- Lebek, S.; Pichler, K.; Reuthner, K.; Trum, M.; Tafelmeier, M.; Mustroph, J.; Camboni, D.; Rupprecht, L.; Schmid, C.; Maier, L.S.; et al. Enhanced CaMKII-Dependent Late I(Na) Induces Atrial Proarrhythmic Activity in Patients With Sleep-Disordered Breathing. Circ. Res. 2020, 126, 603–615. [Google Scholar] [CrossRef]
- Lebek, S.; Caravia, X.M.; Chemello, F.; Tan, W.; McAnally, J.R.; Chen, K.; Xu, L.; Liu, N.; Bassel-Duby, R.; Olson, E.N. Elimi-nation of CaMKIIδ Autophosphorylation by CRISPR-Cas9 Base Editing Improves Survival and Cardiac Function in Heart Failure in Mice. Circulation 2023, 148, 1490–1504. [Google Scholar] [CrossRef]
- Duran, J.; Nickel, L.; Estrada, M.; Backs, J.; van den Hoogenhof, M.M. CaMKIIδ Splice Variants in the Healthy and Diseased Heart. Front. Cell Dev. Biol. 2021, 9, 644630. [Google Scholar] [CrossRef] [PubMed]
- Beckendorf, J.; van den Hoogenhof, M.M.; Backs, J. Physiological and unappreciated roles of CaMKII in the heart. Basic Res. Cardiol. 2018, 113, 29. [Google Scholar] [CrossRef] [PubMed]
- Mandelkow, E.-M.; Thies, E.; Trinczek, B.; Biernat, J.; Mandelkow, E. MARK/PAR1 kinase is a regulator of microtu-bule-dependent transport in axons. J. Cell Biol. 2004, 167, 99–110. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, S.; Liang, Y.; He, Y.; Li, Q.; Zhan, J.; Hou, H.; Qiu, X. Single dose of intravenous miR199a-5p delivery targeting ischemic heart for long-term repair of myocardial infarction. Nat. Commun. 2024, 15, 5565. [Google Scholar] [CrossRef] [PubMed]
- Marcum, Z.A.; Gellad, W.F. Medication adherence to multidrug regimens. Clin. Geriatr. Med. 2012, 28, 287–300. [Google Scholar] [CrossRef]
- González-Bueno, J.; Sevilla-Sánchez, D.; Puigoriol-Juvanteny, E.; Molist-Brunet, N.; Codina-Jané, C.; Espaulella-Panicot, J. Factors Associated with Medication Non-Adherence among Patients with Multimorbidity and Polypharmacy Admitted to an Intermediate Care Center. Int. J. Environ. Res. Public Health 2021, 18, 9606. [Google Scholar] [CrossRef]
- Cutler, R.L.; Fernandez-Llimos, F.; Frommer, M.; Benrimoj, C.; Garcia-Cardenas, V. Economic impact of medication non-adherence by disease groups: A systematic review. BMJ Open 2018, 8, e016982. [Google Scholar] [CrossRef]
- Lauerer, A.-M.; Caravia, X.M.; Maier, L.S.; Chemello, F.; Lebek, S. Gene editing in common cardiovascular diseases. Pharmacol. Ther. 2024, 263, 108720. [Google Scholar] [CrossRef]
- Tafelmeier, M.; Knapp, M.; Lebek, S.; Floerchinger, B.; Camboni, D.; Creutzenberg, M.; Wittmann, S.; Zeman, F.; Schmid, C.; Maier, L.S.; et al. Predictors of delirium after cardiac surgery in patients with sleep disordered breathing. Eur. Respir. J. 2019, 54, 1900354. [Google Scholar] [CrossRef]
- Tafelmeier, M.; Knapp, M.; Lebek, S.; Floerchinger, B.; Camboni, D.; Wittmann, S.; Creutzenberg, M.; Zeman, F.; Schmid, C.; Maier, L.S.; et al. Rationale and design of the CONSIDER AF study. Somnologie 2019, 23, 17–28. [Google Scholar] [CrossRef]
- Lebek, S.; Hegner, P.; Hultsch, R.; Rohde, J.; Rupprecht, L.; Schmid, C.; Sossalla, S.; Maier, L.S.; Arzt, M.; Wagner, S. Volt-age-Gated Sodium Channel Na(V)1.8 Dysregulates Na and Ca, Leading to Arrhythmias in Patients with Sleep-Disordered Breathing. Am. J. Respir. Crit. Care Med. 2022, 206, 1428–1431. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
| Total Cohort (n = 152) | <Median of MARK4 (n = 76) | >Median of MARK4 (n = 76) | p-Value | |
|---|---|---|---|---|
| Age (years), mean ± SD | 65.7 ± 9.0 | 64.7 ± 8.8 | 66.7 ± 9.2 | 0.119 MWU |
| Male sex, n (%) | 133 (87.5) | 66 (86.8) | 67 (88.2) | 0.806 Chi |
| BMI (kg/m2) mean ± SD | 28.6 ± 4.5 | 28.1 ± 4.1 | 29.0 ± 4.8 | 0.224 T |
| NYHA functional class, n (%) | ||||
| I | 44 (28.9) | 32 (42.1) | 12 (15.8) | <0.001 Chi |
| II | 67 (44.1) | 27 (35.5) | 40 (52.6) | 0.034 Chi |
| III | 39 (26) | 17 (22) | 22 (29) | 0.353 Chi |
| IV | 2 (1) | 0 (0) | 2 (3) | 0.492 F |
| Cardiovascular risk factors | ||||
| Arterial hypertension, n (%) | 127 (83.6) | 65 (85.5) | 62 (81.6) | 0.512 Chi |
| Systolic blood pressure (mmHg), mean ± SD | 132.5 ± 17.4 | 134.5 ± 17.9 | 130.6 ± 16.2 | 0.144 MWU |
| Diastolic blood pressure (mmHg), mean ± SD | 77.2 ± 8.7 | 77.6 ± 8.1 | 76.9 ± 9.2 | 0.552 MWU |
| Smoker, n (%) | 98 (64.5) | 43 (56.5) | 55 (72.4) | 0.042 Chi |
| Previous smoker | 79 (59.4) | 33 (50.0) | 46 (68.7) | 0.028 Chi |
| Current smoker | 18 (25.0) | 10 (23.3) | 8 (27.6) | 0.677 Chi |
| Diabetes mellitus, n (%) | 55 (36.2) | 26 (34.2) | 29 (38.2) | 0.613 Chi |
| HbA1c (%), mean ± SD | 6.4 ± 1.3 | 6.4 ± 1.4 | 6.4 ± 1.2 | 0.505 MWU |
| Hyperlipidaemia, n (%) | 105 (69.5) | 51 (67.1) | 54 (72.0) | 0.513 Chi |
| History of TIA or Stroke, n (%) | 25 (16.4) | 15 (19.7) | 10 (13.2) | 0.274 Chi |
| Atrial fibrillation, n (%) | 20 (13.6) | 9 (12.3) | 11 (14.9) | 0.654 Chi |
| Postoperative atrial fibrillation, n (%) | 33 (22.3) | 12 (16.4) | 21 (28.0) | 0.091 Chi |
| Haemoglobin (g/dL), mean ± SD | 14.1 ± 1.6 | 14.3 ± 1.7 | 13.9 ± 1.6 | 0.070 T |
| NT-pro-BNP (ng/L), mean ± SD | 1165.7 ± 3233.9 | 726.4 ± 1307.7 | 1604.9 ± 4356.2 | 0.070 MWU |
| GFR (mL/min), mean ± SD | 75.0 ± 20.7 | 76.5 ± 20.1 | 73.5 ± 21.4 | 0.331 MWU |
| CRP (mg/L), mean ± SD | 6.9 ± 15.9 | 6.0 ± 13.0 | 7.8 ± 18.3 | 0.006 MWU |
| Medical treatment, n (%) | ||||
| Angiotensin-converting enzyme inhibitors | 70 (51.5) | 38 (55.1) | 32 (47.8) | 0.394 Chi |
| Angiotensin receptor blockers | 35 (25.7) | 16 (23.2) | 19 (28.4) | 0.491 Chi |
| Calcium Channel Blocker | 40 (29.4) | 19 (27.5) | 21 (31.3) | 0.626 Chi |
| Beta-blockers | 90 (66.2) | 43 (62.3) | 47 (70.1) | 0.957 Chi |
| Mineralocorticoid receptor antagonists | 12 (8.8) | 6 (8.7) | 6 (8.8) | 0.335 Chi |
| Loop diuretics | 30 (22.1) | 10 (14.5) | 20 (29.9) | 0.031 Chi |
| Thiazide diuretics | 26 (19.1) | 10 (14.5) | 16 (23.9) | 0.164 Chi |
| Total Cohort (n = 123) | <Median of MARK4 (n = 59) | >Median of MARK4 (n = 64) | p-Value | |
|---|---|---|---|---|
| LVEF (%), mean ± SD | 54.5 ± 10.7 | 57.4 ± 7.9 | 51.9 ± 12.3 | 0.004 MWU |
| LVEF < 55%, n (%) | 42 (34.1) | 12 (20.3) | 30 (46.9) | 0.002 Chi |
| LVEDD (mm), mean ± SD | 51.2 ± 9.2 | 50.8 ± 6.7 | 53.3 ± 7.2 | 0.103 T |
| LAVI (mL/m2) mean ± SD | 33.5 ± 12.9 | 31.5 ±11.2 | 35.7 ± 14.6 | 0.320 T |
| E/e’ ratio, mean ± SD | 11.1 ± 5.0 | 9.3 ± 3.6 | 12.8 ± 5.5 | 0.005 MWU |
| sPAP (mmHg), mean ± SD | 26.8 ± 11.8 | 23.8 ± 12.4 | 29.6 ± 10.6 | 0.035 MWU |
| Vena cava inferior (mm), mean ± SD | 15.3 ± 4.2 | 14.9 ± 4.2 | 15.9 ± 4.2 | 0.325 T |
| Total Cohort (n = 152) | <Median of MARK4 (n = 76) | >Median of MARK4 (n = 76) | p-Value | |
|---|---|---|---|---|
| Total recording time (min), mean ± SD | 481.0 ± 51.8 | 481.4 ± 63.2 | 480.5 ± 37.5 | 0.137 MWU |
| Sleep-disordered breathing, n (%) | 73 (48.0) | 29 (38.2) | 44 (57.9) | 0.015 Chi |
| AHI (/h), mean ± SD | 18.2 ± 15.8 | 14.0 ± 12.2 | 22.4 ± 17.8 | 0.002 MWU |
| Obstructive apnea index (/h), mean ± SD | 5.1 ± 8.7 | 3.9 ± 5.0 | 6.3 ± 11.2 | 0.123 MWU |
| Central apnea index (/h), mean ± SD | 6.0 ± 8.6 | 4.8 ± 7.1 | 7.2 ± 9.7 | 0.283 MWU |
| Mean SpO2 (%), mean ± SD | 92.5 ± 2.3 | 93.2 ± 1.8 | 91.8 ± 2.5 | <0.001 MWU |
| Minimum SpO2 (%), mean ± SD | 81.2 ± 7.1 | 82.7 ± 6.7 | 79.8 ± 7.2 | 0.009 MWU |
| Time of SpO2 < 90% (min), mean ± SD | 12.3 ± 18.1 | 6.9 ± 11.8 | 17.8 ± 21.4 | <0.001 MWU |
| Oxygen desaturation index (/h), mean ± SD | 15.6 ± 14.9 | 11.4 ± 10.8 | 19.8 ± 17.1 | <0.001 MWU |
| Mean heart rates (/min), mean ± SD | 71.2 ± 14.3 | 69.3 ± 11.1 | 73.2 ± 16.8 | 0.139 MWU |
| Multivariable Model for an Increased MARK4 Expression r² = 0.188, n = 121 | OR (95% CI) | p-Value |
|---|---|---|
| Age (years) | 1.014 (0.970; 1.061) | 0.538 |
| Male sex | 0.543 (0.149; 1.978) | 0.355 |
| BMI (kg/m2) | 1.008 (0.915; 1.111) | 0.867 |
| LVEF (%) | 0.962 (0.927; 0.998) | 0.039 |
| E/e’ | 0.999 (0.992; 1.007) | 0.868 |
| Existing SDB | 1.546 (0.661; 3.613) | 0.315 |
| Mean SpO2 (%) | 0.798 (0.652; 0.976) | 0.028 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seydel, B.; Hegner, P.; Lauerer, A.-M.; Schildt, S.; Bayram, F.; Tafelmeier, M.; Wermers, D.; Rupprecht, L.; Schmid, C.; Wagner, S.; et al. Increased Myocardial MARK4 Expression in Patients with Heart Failure and Sleep-Disordered Breathing. Int. J. Mol. Sci. 2025, 26, 3614. https://doi.org/10.3390/ijms26083614
Seydel B, Hegner P, Lauerer A-M, Schildt S, Bayram F, Tafelmeier M, Wermers D, Rupprecht L, Schmid C, Wagner S, et al. Increased Myocardial MARK4 Expression in Patients with Heart Failure and Sleep-Disordered Breathing. International Journal of Molecular Sciences. 2025; 26(8):3614. https://doi.org/10.3390/ijms26083614
Chicago/Turabian StyleSeydel, Bettina, Philipp Hegner, Anna-Maria Lauerer, Sönke Schildt, Fatma Bayram, Maria Tafelmeier, Dominik Wermers, Leopold Rupprecht, Christof Schmid, Stefan Wagner, and et al. 2025. "Increased Myocardial MARK4 Expression in Patients with Heart Failure and Sleep-Disordered Breathing" International Journal of Molecular Sciences 26, no. 8: 3614. https://doi.org/10.3390/ijms26083614
APA StyleSeydel, B., Hegner, P., Lauerer, A.-M., Schildt, S., Bayram, F., Tafelmeier, M., Wermers, D., Rupprecht, L., Schmid, C., Wagner, S., Maier, L. S., Arzt, M., & Lebek, S. (2025). Increased Myocardial MARK4 Expression in Patients with Heart Failure and Sleep-Disordered Breathing. International Journal of Molecular Sciences, 26(8), 3614. https://doi.org/10.3390/ijms26083614








