The Potential of Siraitia grosvenorii to Promote Bone Regeneration via Modulating Macrophage Polarization: A Network Pharmacology and Experimental Study
Abstract
1. Introduction
2. Results
2.1. Identification of Bioactive Compounds in SG
2.2. Identification of Gene Targets for SG
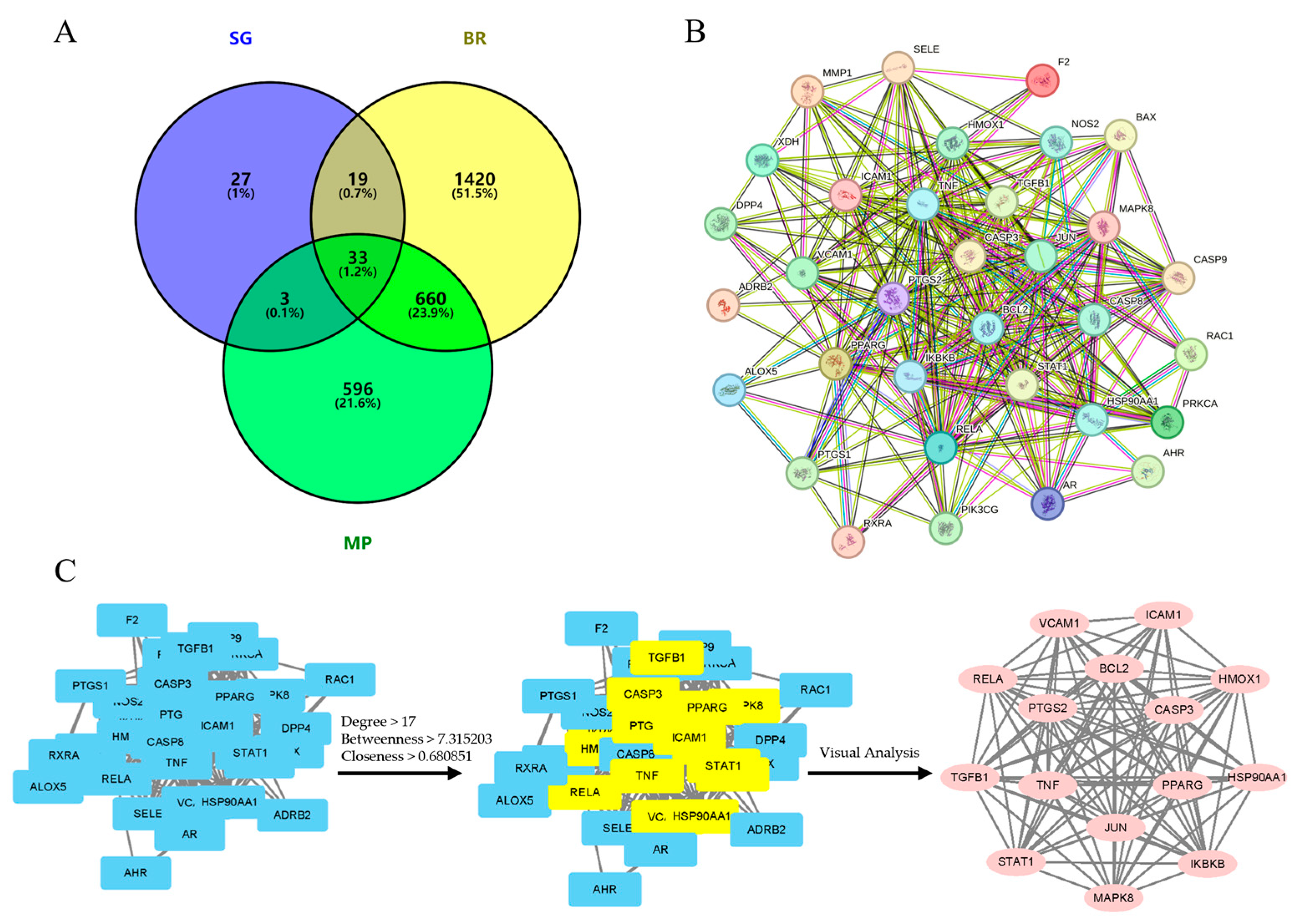
2.3. Identification of Therapeutic Targets for SG in Bone Regeneration and Macrophage Polarization
2.4. PPI Analysis
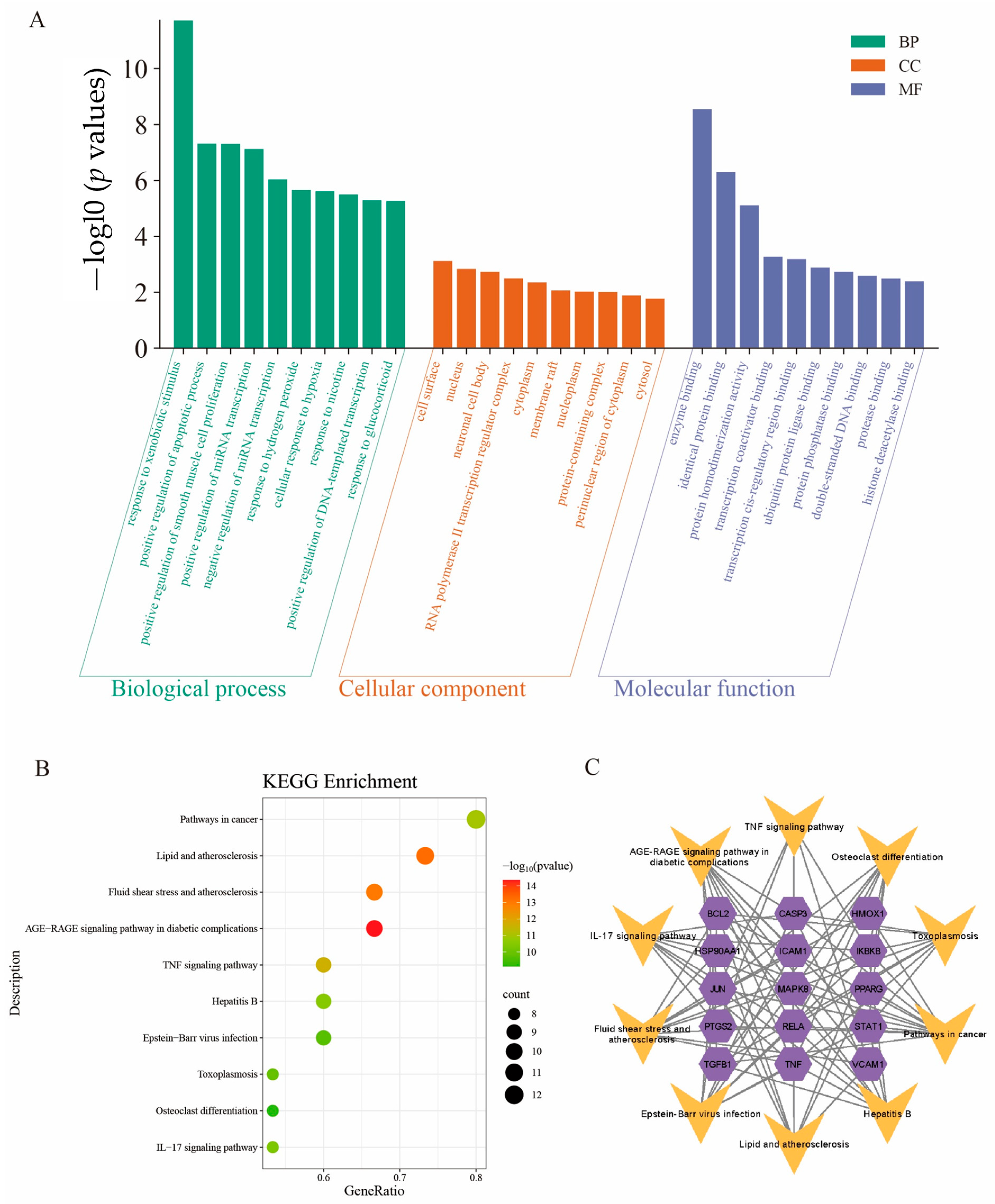
2.5. Gene Ontology (GO) Enrichment Analysis
2.6. Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Enrichment Analysis
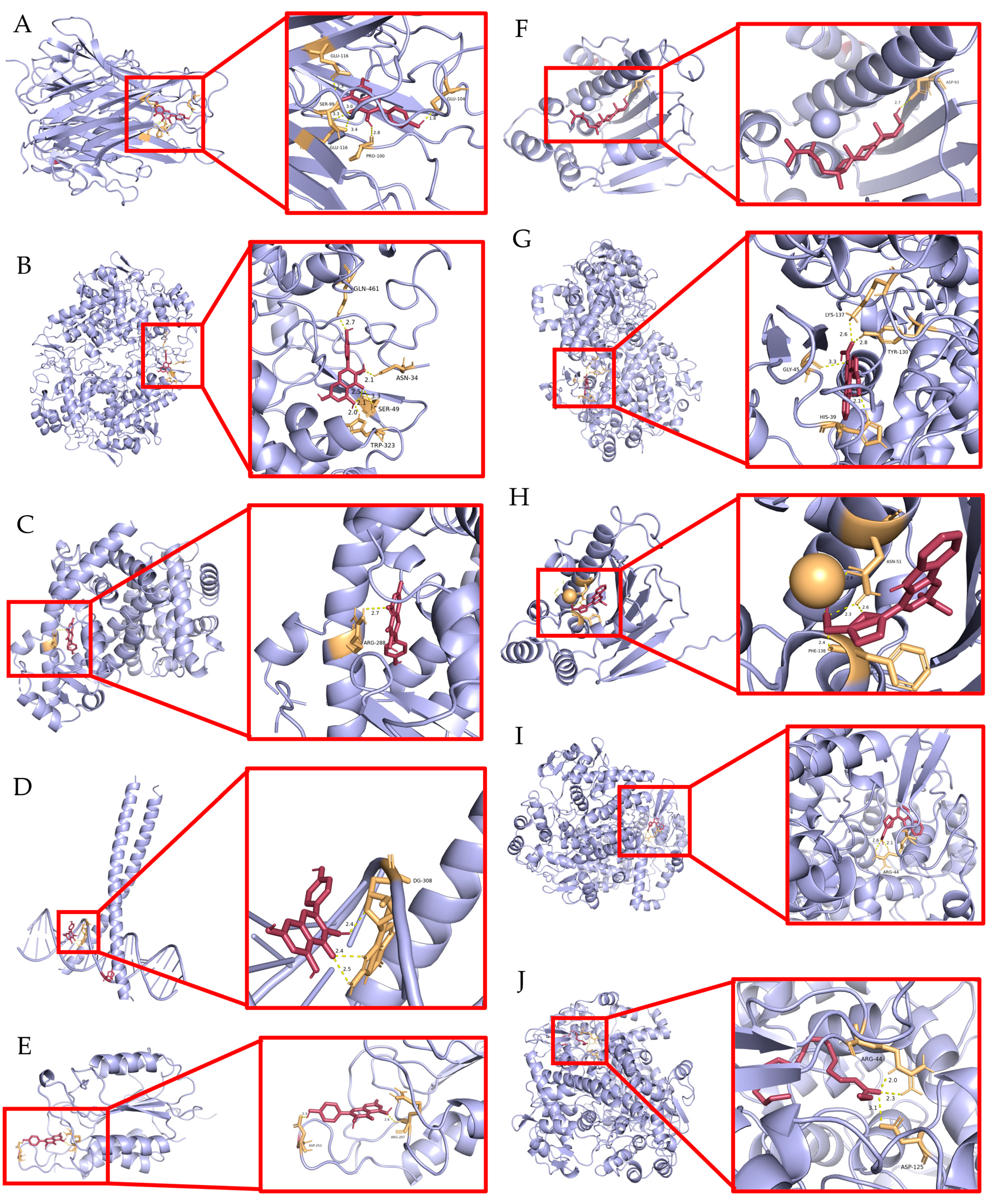
2.7. Validation of Molecular Docking
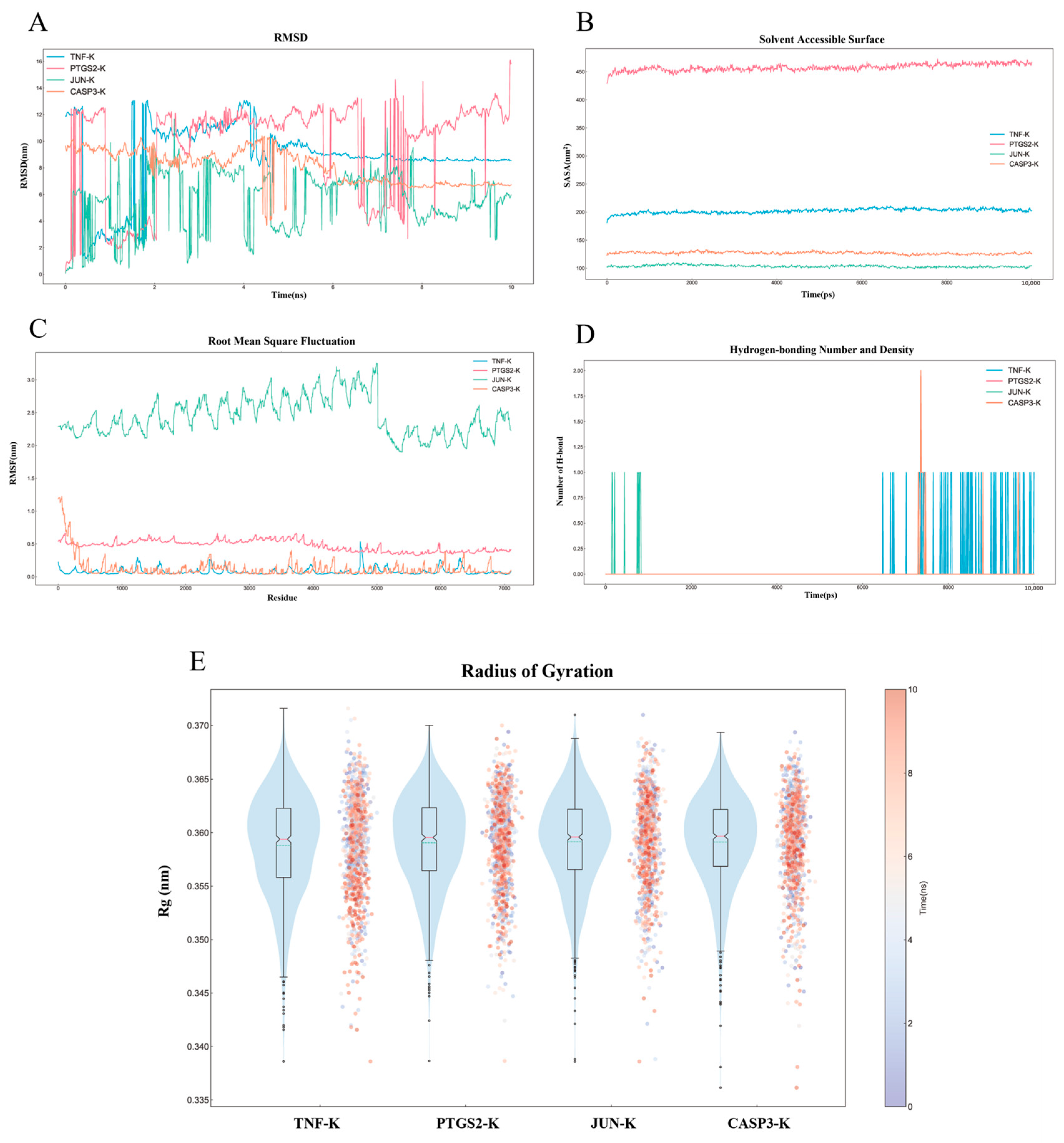
2.8. MD Simulation Analysis
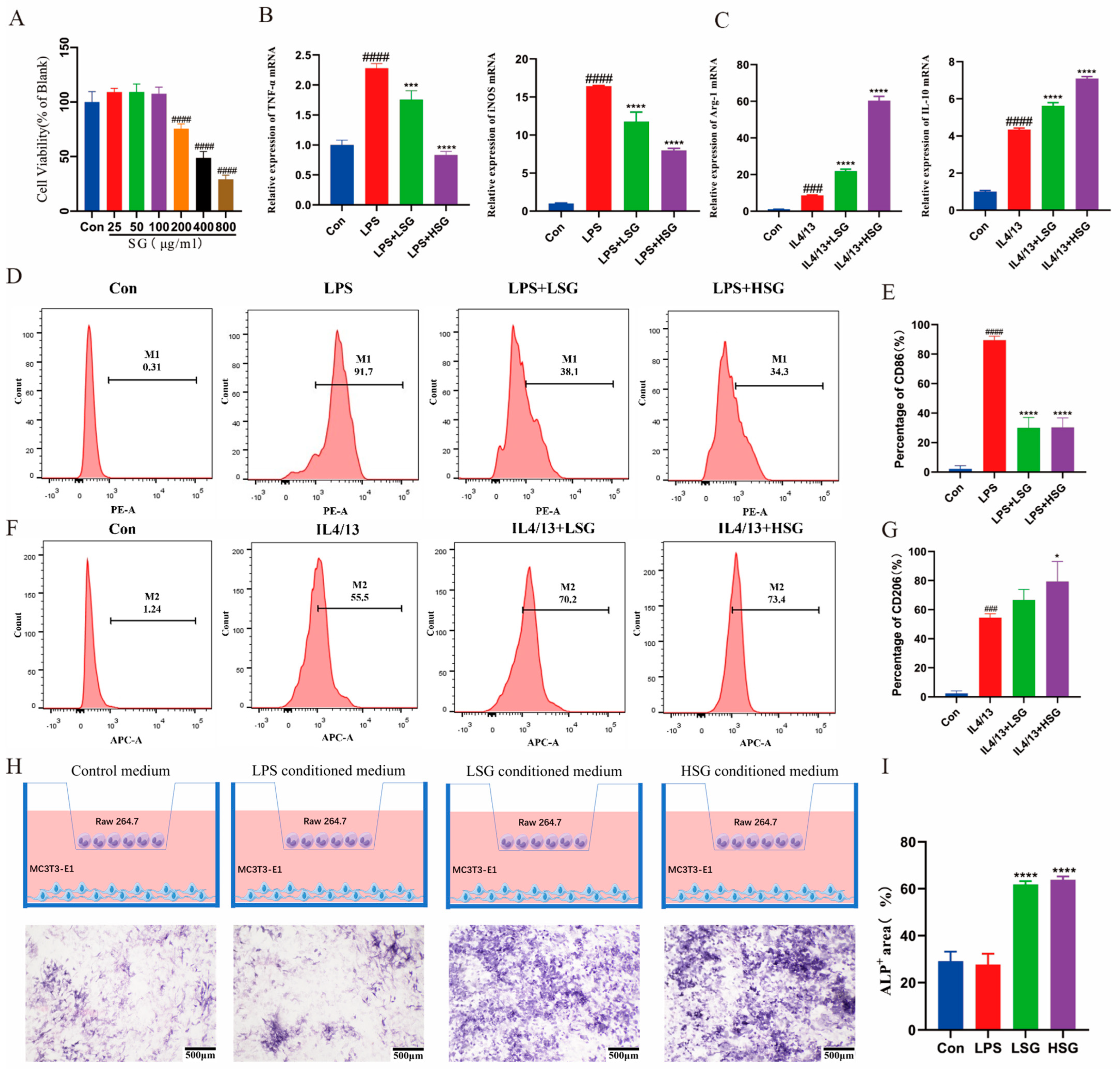
2.9. Cytotoxicity of SG
2.10. Effect of SG on the Inflammatory Factors in Macrophages
2.11. SG-Induced Macrophage M2-Type Differentiation In Vitro
2.12. Osteogenic Differentiation of MC3T3-E1 Cells in a Co-Culture System
2.13. Histological Analyses of Immunomodulatory and Osteogenesis Capability
3. Discussion
4. Materials and Methods
4.1. Identification of Bioactive Components and Target Genes in SG
4.2. Identification of Target Genes in SG
4.3. Construction of Protein–Protein Interaction (PPI) Network and Identification of Core Targets
4.4. GO and KEGG Pathway Enrichment Analyses
4.5. Molecular Docking
4.6. MD Simulation
4.7. Cell Culture
4.8. Preparation of SG-Conditioned Medium
4.9. Cell Viability and Proliferation
4.10. Flow Cytometry Analysis
4.11. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.12. Osteogenic Differentiation Assay
4.13. Preparation of SG/PLGA/Gelatin Composite Scaffolds
4.14. Calvarial Defect Model
4.15. Histological Evaluation
4.16. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SG | Siraitia grosvenorii |
| TCM | Traditional Chinese Medicine |
| TNF | tumor necrosis factor |
| IL | interleukin |
| iNOS | Inducible Nitric Oxide Synthase |
| ARG | arginase |
| MAPK | mitogen-activated protein kinase |
| NF-κB | nuclear factor-kappa B |
| PPI | protein–protein interaction |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| MD | molecular dynamics |
| ROS | Reactive Oxygen Species |
| OB | oral bioavailability |
| DL | drug-likeness |
| RMSD | root mean square deviation |
| FCM | flow cytometry |
| ALP | alkaline phosphatase |
References
- Perier-Metz, C.; Duda, G.N.; Checa, S. A mechanobiological computer optimization framework to design scaffolds to enhance bone regeneration. Front. Bioeng. Biotechnol. 2022, 10, 980727. [Google Scholar] [CrossRef] [PubMed]
- Sparks, D.S.; Medeiros Savi, F.; Saifzadeh, S.; Wille, M.L.; Wagels, M.; Hutmacher, D.W. Bone Regeneration Exploiting Corticoperiosteal Tissue Transfer for Scaffold-Guided Bone Regeneration. Tissue Eng. Part C Methods 2022, 28, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari-Jafari, F.; Amoabediny, G.; Dehghan, M.M. Role of biomechanics in vascularization of tissue-engineered bones. J. Biomech. 2020, 110, 109920. [Google Scholar] [CrossRef]
- Shen, C.; Wang, M.M.; Witek, L.; Tovar, N.; Cronstein, B.N.; Torroni, A.; Flores, R.L.; Coelho, P.G. Transforming the Degradation Rate of β-tricalcium Phosphate Bone Replacement Using 3-Dimensional Printing. Ann. Plast. Surg. 2021, 87, e153–e162. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, W.; Li, L.; Zhou, Y.; Yao, G.; Chen, G.; Xu, L.; Yang, N.; Yan, Z.; Zhu, C.; et al. Concrete-Inspired Bionic Bone Glue Repairs Osteoporotic Bone Defects by Gluing and Remodeling Aging Macrophages. Adv. Sci. 2024, 11, e2408044. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, B.; Tang, M.; Jin, D.; Lai, P. Tuberous sclerosis complex 1 targeted depletion in macrophages promotes osteogenesis by modulating secretion of Oncostatin M in the inflammatory stage of bone healing. Int. Immunopharmacol. 2023, 124, 110895. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, M.; Lu, S.; Dai, S.; Liu, J. Role of macrophage polarization in heart failure and traditional Chinese medicine treatment. Front. Pharmacol. 2024, 15, 1434654. [Google Scholar] [CrossRef]
- Chen, S.; Zeng, J.; Li, R.; Zhang, Y.; Tao, Y.; Hou, Y.; Yang, L.; Zhang, Y.; Wu, J.; Meng, X. Traditional Chinese medicine in regulating macrophage polarization in immune response of inflammatory diseases. J. Ethnopharmacol. 2024, 325, 117838. [Google Scholar] [CrossRef]
- Yuan, L.; He, Q.; Zhang, Y.; Luo, H.; Xiang, W.; Deng, C.; Li, C.; Li, X.; Yao, L.; Ke, D.; et al. 6-Gingerol microneedle promotes diabetic wound healing by regulating macrophage polarization. Int. Immunopharmacol. 2025, 151, 114288. [Google Scholar] [CrossRef]
- Tian, Z.; Gu, R.; Xie, W.; Su, X.; Yuan, Z.; Wan, Z.; Wang, H.; Liu, Y.; Feng, Y.; Liu, X.; et al. Hydrogen bonding-mediated phase-transition gelatin-based bioadhesives to regulate immune microenvironment for diabetic wound healing. Bioact. Mater. 2025, 46, 434–447. [Google Scholar] [CrossRef]
- Song, J.L.; Qian, B.; Pan, C.; Lv, F.; Wang, H.; Gao, Y.; Zhou, Y. Protective activity of mogroside V against ovalbumin-induced experimental allergic asthma in Kunming mice. J. Food Biochem. 2019, 43, e12973. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, D.S.; Yuk, H.J.; Kim, S.H.; Yang, W.K.; Park, G.D.; Kim, K.S.; Ham, W.J.; Sung, Y.Y. Siraitia grosvenorii Extract Attenuates Airway Inflammation in a Murine Model of Chronic Obstructive Pulmonary Disease Induced by Cigarette Smoke and Lipopolysaccharide. Nutrients 2023, 15, 468. [Google Scholar] [CrossRef]
- Li, J.; Bi, D.; Zhang, X.; Cao, Y.; Lv, K.; Jiang, L. Network Pharmacology and Inflammatory Microenvironment Strategy Approach to Finding the Potential Target of Siraitia grosvenorii (Luo Han Guo) for Glioblastoma. Front. Genet. 2021, 12, 799799. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Guo, M.; Zhao, L.; Zhang, J.; He, J.; Xu, A.; Yu, Z.; Ma, X.; Yong, Y.; Li, Y.; et al. Siraitia grosvenorii Extract Protects Lipopolysaccharide-Induced Intestinal Inflammation in Mice via Promoting M2 Macrophage Polarization. Pharmaceuticals 2024, 17, 1023. [Google Scholar] [CrossRef]
- Duan, J.; Zhu, D.; Zheng, X.; Ju, Y.; Wang, F.; Sun, Y.; Fan, B. Siraitia grosvenorii (Swingle) C. Jeffrey: Research Progress of Its Active Components, Pharmacological Effects, and Extraction Methods. Foods 2023, 12, 1373. [Google Scholar] [CrossRef]
- Lee, Y.M.; Kim, M.; Yuk, H.J.; Kim, S.H.; Kim, D.S. Siraitia grosvenorii Residual Extract Inhibits Inflammation in RAW264.7 Macrophages and Attenuates Osteoarthritis Progression in a Rat Model. Nutrients 2023, 15, 1417. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Tang, Q.; Tian, Y.; Fan, Q.; Huang, Y.; Tan, X. Network pharmacology-based prediction of the active ingredients and potential targets of Mahuang Fuzi Xixin decoction for application to allergic rhinitis. J. Ethnopharmacol. 2015, 176, 402–412. [Google Scholar] [CrossRef]
- Shou, X.; Wang, Y.; Zhang, X.; Zhang, Y.; Yang, Y.; Duan, C.; Yang, Y.; Jia, Q.; Yuan, G.; Shi, J.; et al. Network Pharmacology and Molecular Docking Analysis on Molecular Mechanism of Qingzi Zhitong Decoction in the Treatment of Ulcerative Colitis. Front. Pharmacol. 2022, 13, 727608. [Google Scholar] [CrossRef]
- Liu, L.; Jiao, Y.; Yang, M.; Wu, L.; Long, G.; Hu, W. Network Pharmacology, Molecular Docking and Molecular Dynamics to Explore the Potential Immunomodulatory Mechanisms of Deer Antler. Int. J. Mol. Sci. 2023, 24, 10370. [Google Scholar] [CrossRef]
- Gaillard, T. Evaluation of AutoDock and AutoDock Vina on the CASF-2013 Benchmark. J. Chem. Inf. Model. 2018, 58, 1697–1706. [Google Scholar] [CrossRef]
- Brüschweiler, R. Efficient RMSD measures for the comparison of two molecular ensembles. Root-mean-square deviation. Proteins 2003, 50, 26–34. [Google Scholar] [CrossRef]
- Baskin, L.S. Electric conductance and pH measurements of isoionic salt-free bovine mercaptalbumin solutions. An evaluation of root-mean-square proton fluctuations. J. Phys. Chem. 1968, 72, 2958–2962. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, M.; Chen, F.; Wang, J.; Li, X.; Liang, J.; Fan, Y.; Xiao, Y.; Zhang, X. Correlations between macrophage polarization and osteoinduction of porous calcium phosphate ceramics. Acta Biomater. 2020, 103, 318–332. [Google Scholar] [CrossRef]
- Guo, Q.; Shi, M.; Sarengaowa; Xiao, Z.; Xiao, Y.; Feng, K. Recent Advances in the Distribution, Chemical Composition, Health Benefits, and Application of the Fruit of Siraitia grosvenorii. Foods 2024, 13, 2278. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Yu, X.H.; Maskey, A.R.; Musa, I.; Wang, Z.Z.; Garcia, V.; Guo, A.; Yang, N.; Srivastava, K.; Dunkin, D.; et al. Cytochrome P450 3A4 suppression by epimedium and active compound kaempferol leads to synergistic anti-inflammatory effect with corticosteroid. Front. Pharmacol. 2022, 13, 1042756. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Chen, S.; Deng, W.; Gong, Z.; Guo, Y.; Zeng, S.; Xu, Q. Kaempferol Attenuates Gouty Arthritis by Regulating the Balance of Th17/Treg Cells and Secretion of IL-17. Inflammation 2023, 46, 1901–1916. [Google Scholar] [CrossRef]
- Wang, T.; Liu, B.; Zhang, C.; Fang, T.; Ding, Y.; Song, G.; Zhang, S.; Shi, L.; Deng, X.; Wang, J. Kaempferol-Driven Inhibition of Listeriolysin O Pore Formation and Inflammation Suppresses Listeria monocytogenes Infection. Microbiol. Spectr. 2022, 10, e0181022. [Google Scholar] [CrossRef]
- Jiang, L.; Wan, Y.; Pan, J.; Mao, X.; Sun, X.; Zan, L.; Wang, H. Transcriptomic analysis reveals the inhibitory effect of beta-sitosterol on proliferation of bovine preadipocytes. Anim. Biotechnol. 2024, 35, 2339406. [Google Scholar] [CrossRef]
- Liao, P.C.; Lai, M.H.; Hsu, K.P.; Kuo, Y.H.; Chen, J.; Tsai, M.C.; Li, C.X.; Yin, X.J.; Jeyashoke, N.; Chao, L.K. Identification of β-Sitosterol as in Vitro Anti-Inflammatory Constituent in Moringa oleifera. J. Agric. Food Chem. 2018, 66, 10748–10759. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, N.; Xue, M.; Zhang, M.; Liu, W.; Xu, C.; Fan, Y.; Meng, Y.; Zhang, Q.; Zhou, Y. Anti-Inflammatory and Antioxidant Properties of β-Sitosterol in Copper Sulfate-Induced Inflammation in Zebrafish (Danio rerio). Antioxidants 2023, 12, 391. [Google Scholar] [CrossRef]
- van Loo, G.; Bertrand, M.J.M. Death by TNF: A road to inflammation. Nat. Rev. Immunol. 2023, 23, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Zhang, T.; Zhang, C.; Ji, H.; Tong, X.; Xia, R.; Wang, W.; Ma, Z.; Shi, X. Exosomal miR-30d-5p of neutrophils induces M1 macrophage polarization and primes macrophage pyroptosis in sepsis-related acute lung injury. Crit. Care 2021, 25, 356. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, X.; Gong, P.; Long, H.; Wang, J.; Yang, W.; Yao, W. Siraitia grosvenorii As a Homologue of Food and Medicine: A Review of Biological Activity, Mechanisms of Action, Synthetic Biology, and Applications in Future Food. J. Agric. Food Chem. 2024, 72, 6850–6870. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, R.; Jiang, W.; Zhou, L. Research progress of pharmacological effects of Siraitia grosvenorii extract. J. Pharm. Pharmacol. 2022, 74, 953–960. [Google Scholar] [CrossRef]
- Walke, P.B.; Bansode, S.B.; More, N.P.; Chaurasiya, A.H.; Joshi, R.S.; Kulkarni, M.J. Molecular investigation of glycated insulin-induced insulin resistance via insulin signaling and AGE-RAGE axis. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166029. [Google Scholar] [CrossRef]
- Liu, R.; Chen, Y.; Fu, W.; Wang, S.; Cui, Y.; Zhao, X.; Lei, Z.N.; Hettinghouse, A.; Liu, J.; Wang, C.; et al. Fexofenadine inhibits TNF signaling through targeting to cytosolic phospholipase A2 and is therapeutic against inflammatory arthritis. Ann. Rheum. Dis. 2019, 78, 1524–1535. [Google Scholar] [CrossRef]
- Zhang, X.M.; Min, X.R.; Xie, H.X.; Jiang, Y.N.; Rui, Y.X.; Li, B.; Zeng, N.; Liu, R. Piperazine ferulate inhibits diabetic nephropathy by suppressing AGE/RAGE-mediated inflammatory signaling in rats and podocytes. Front. Pharmacol. 2024, 15, 1394369. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Y.; Wang, L.; Chen, Y.; Han, X.; Sun, L.; Chen, H.; Chen, Q. Effect of Bifidobacterium on osteoclasts: TNF-α/NF-κB inflammatory signal pathway-mediated mechanism. Front. Endocrinol. 2023, 14, 1109296. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Li, Z.; Wu, Z.; Lin, X.; Li, N.; Shen, R.; Wei, G.; Yu, N.; Gong, F.; et al. Mogrol Attenuates Osteoclast Formation and Bone Resorption by Inhibiting the TRAF6/MAPK/NF-κB Signaling Pathway In vitro and Protects Against Osteoporosis in Postmenopausal Mice. Front. Pharmacol. 2022, 13, 803880. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, H.; Wu, P.; Chen, Y.; Deng, Z.; Cai, L.; Wu, M. Multifunctional injectable hydrogel system as a mild photothermal-assisted therapeutic platform for programmed regulation of inflammation and osteo-microenvironment for enhanced healing of diabetic bone defects in situ. Theranostics 2024, 14, 7140–7198. [Google Scholar] [CrossRef]
- Wang, S.; Xu, C.; Yu, Y.; Li, J.; Chen, T.; Wang, J.; Liu, C. Immunoregulative coating for scarless healing in anterior cruciate ligament reconstruction. Bioact. Mater. 2025, 45, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Zhang, X.; Shen, W.; Wang, P.; Yin, X.; Liu, J.; Xu, H.; Liu, B.; Man, Z.; Li, W. Multifunctional Scaffold Comprising Metal-Organic Framework, Hydrogel, and Demineralized Bone Matrix for the Treatment of Steroid-Induced Femoral Head Necrosis. Small 2024, 21, e2407758. [Google Scholar] [CrossRef] [PubMed]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2019 update: Improved access to chemical data. Nucleic Acids Res. 2019, 47, D1102–D1109. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- UniProt: The Universal Protein Knowledgebase in 2023. Nucleic Acids Res. 2023, 51, D523–D531. [CrossRef]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinform. 2016, 54, 1.30.31–31.30.33. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Kohl, M.; Wiese, S.; Warscheid, B. Cytoscape: Software for visualization and analysis of biological networks. Methods Mol. Biol. 2011, 696, 291–303. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Burley, S.K.; Bhikadiya, C.; Bi, C.; Bittrich, S.; Chen, L.; Crichlow, G.V.; Christie, C.H.; Dalenberg, K.; Di Costanzo, L.; Duarte, J.M.; et al. RCSB Protein Data Bank: Powerful new tools for exploring 3D structures of biological macromolecules for basic and applied research and education in fundamental biology, biomedicine, biotechnology, bioengineering and energy sciences. Nucleic Acids Res. 2021, 49, D437–D451. [Google Scholar] [CrossRef] [PubMed]
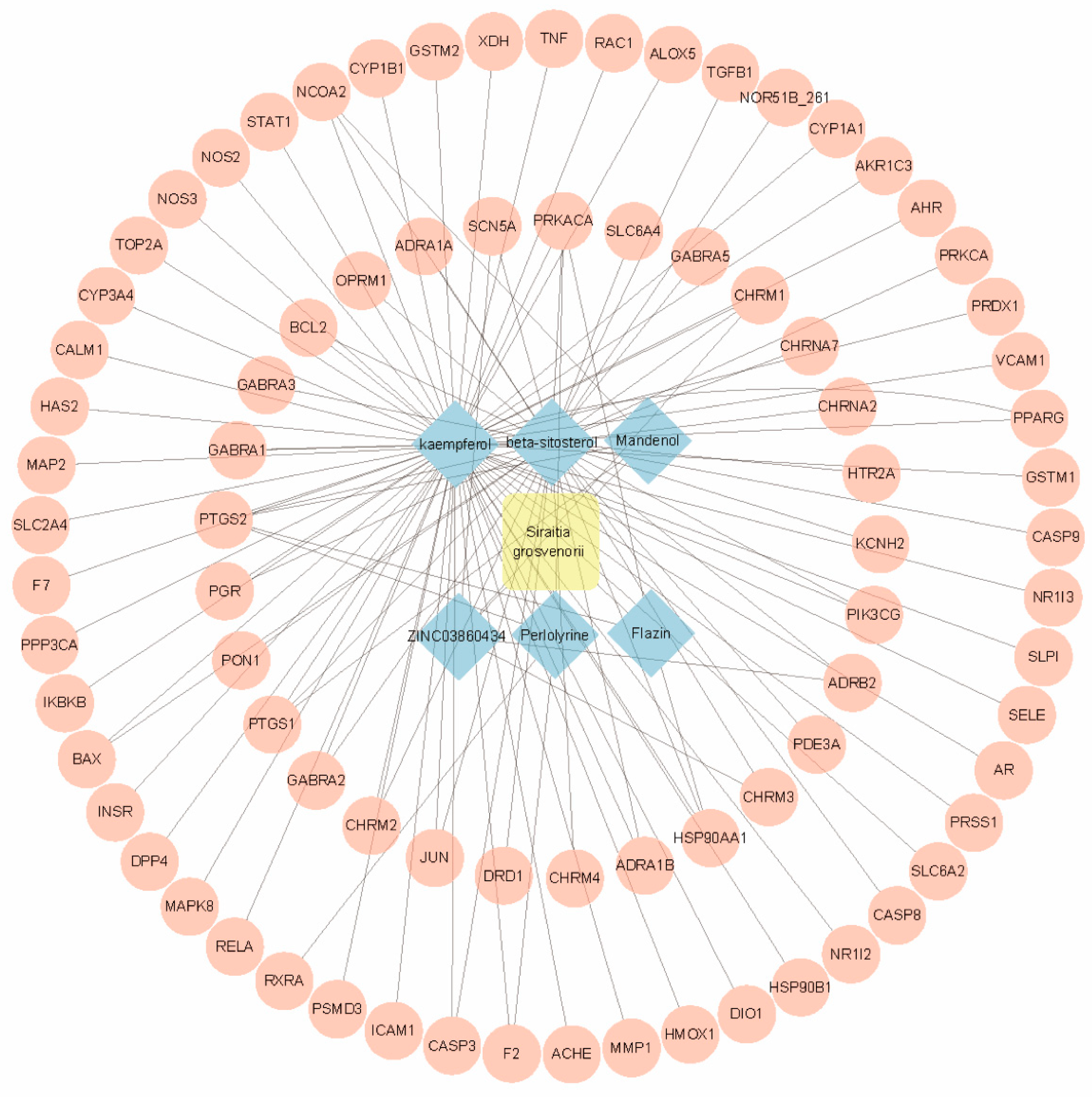

| Mol ID | Molecule Name | OB(%) | DL |
|---|---|---|---|
| MOL009295 | flazin | 94.27 | 0.38 |
| MOL002140 | perlolyrine | 65.94 | 0.27 |
| MOL010105 | (S)-2-methylbutyl-4-(4-decyloxybenzylideneamino) | 45.01 | 0.7 |
| MOL001749 | ZINC03860434 | 43.59 | 0.34 |
| MOL001494 | mandenol | 41.99 | 0.19 |
| MOL000422 | kaempferol | 41.88 | 0.24 |
| MOL010131 | mogroester | 41.68 | 0.31 |
| MOL010070 | 11-oxomogroside IIA1 | 37.62 | 0.22 |
| MOL000358 | beta-sitosterol | 36.91 | 0.75 |
| MOL001506 | supraene | 33.54 | 0.42 |
| MOL010072 | 11-oxomogroside IIE | 32.77 | 0.21 |
| Gene Names | Uniprot ID | Protein Names | Degree | Betweenness | Closeness |
|---|---|---|---|---|---|
| TNF | P01375 | Tumor necrosis factor | 30 | 104.0552585 | 0.941176 |
| PTGS2 | P35354 | Prostaglandin G/H synthase 2 | 28 | 64.36478232 | 0.888889 |
| PPARG | P37231 | Peroxisome proliferator-activated receptor gamma | 27 | 58.5240787 | 0.864865 |
| CASP3 | P42574 | Caspase-3 | 25 | 25.35190353 | 0.820513 |
| JUN | P05412 | Transcription factor Jun | 25 | 30.21752402 | 0.820513 |
| HSP90AA1 | P07900 | Heat shock protein HSP 90-alpha | 19 | 28.56351398 | 0.711111 |
| BCL2 | P10415 | Apoptosis regulator Bcl-2 | 25 | 23.21023686 | 0.820513 |
| RELA | Q04206 | Transcription factor p65 | 24 | 57.11209028 | 0.800000 |
| TGFB1 | P01137 | Transforming growth factor beta-1 proprotein | 24 | 19.89555432 | 0.800000 |
| ICAM1 | P05362 | Intercellular adhesion molecule 1 | 22 | 30.17604244 | 0.761905 |
| STAT1 | P42224 | Signal transducer and activator of transcription 1-alpha/beta | 21 | 15.2762432 | 0.744186 |
| HMOX1 | P09601 | Heme oxygenase 1 | 21 | 9.067893137 | 0.744186 |
| VCAM1 | P19320 | Vascular cell adhesion protein 1 | 19 | 16.00899837 | 0.711111 |
| IKBKB | O14920 | Inhibitor of nuclear factor kappa-B kinase subunit beta | 19 | 7.896653347 | 0.711111 |
| MAPK8 | P45983 | Mitogen-activated protein kinase 8 | 18 | 8.979000629 | 0.695652 |
| Component | Targets | Vina Score | Component | Targets | Vina Score |
|---|---|---|---|---|---|
| kaempferol | TNF | −8.60 | beta-sitosterol | JUN | −7.50 |
| kaempferol | PTGS2 | −9.20 | beta-sitosterol | HSP90AA1 | −6.60 |
| kaempferol | PPARG | −8.40 | beta-sitosterol | CASP3 | −7.30 |
| kaempferol | JUN | −7.40 | flazin | PTGS2 | −9.60 |
| kaempferol | HSP90AA1 | −7.20 | flazin | HSP90AA1 | −8.20 |
| kaempferol | CASP3 | −7.30 | perlolyrine | PTGS2 | −9.20 |
| beta-sitosterol | PTGS2 | −9.20 | mandenol | PTGS2 | −6.00 |
| Gene | Primer Sequence |
|---|---|
| iNOS | 5′-ATGGCTCGGGATGTGGCTAC-3′ (Forward) |
| 3′-ACTTCTATAGAAGCCACGTCAGAAA-5′ (Reverse) | |
| TNF-α | 5′-GCCAGGAGGGAGAACAGAAACTC-3′ (Forward) |
| 3′-ACAGGGAAAGTGAGTGACCGG-5′ (Reverse) | |
| Arg-1 | 5′-AGCTCTGGGAATCTGCATGG-3′ (Forward) |
| 3′-ATAGACGGTTTCTGTAGCACATGTA-5′ (Reverse) | |
| IL-10 | 5′-TAGAGCTGCGGACTGCCTTC-3′ (Forward) |
| 3′-CTTCGTACCGGGTCTTTAGT-5′ (Reverse) | |
| GAPDH | 5′-TGTGTCCGTCGTGGATCTGA-3′ (Forward) |
| 3′-GAGGACGCTGAAGTTGTCGTT-5′ (Reverse) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mai, Y.; Huang, L.; Qiao, Y.; Qin, Y.; Wang, L.; Liao, H. The Potential of Siraitia grosvenorii to Promote Bone Regeneration via Modulating Macrophage Polarization: A Network Pharmacology and Experimental Study. Int. J. Mol. Sci. 2025, 26, 3609. https://doi.org/10.3390/ijms26083609
Mai Y, Huang L, Qiao Y, Qin Y, Wang L, Liao H. The Potential of Siraitia grosvenorii to Promote Bone Regeneration via Modulating Macrophage Polarization: A Network Pharmacology and Experimental Study. International Journal of Molecular Sciences. 2025; 26(8):3609. https://doi.org/10.3390/ijms26083609
Chicago/Turabian StyleMai, Yuying, Linhui Huang, Yang Qiao, Yuan Qin, Lufei Wang, and Hongbing Liao. 2025. "The Potential of Siraitia grosvenorii to Promote Bone Regeneration via Modulating Macrophage Polarization: A Network Pharmacology and Experimental Study" International Journal of Molecular Sciences 26, no. 8: 3609. https://doi.org/10.3390/ijms26083609
APA StyleMai, Y., Huang, L., Qiao, Y., Qin, Y., Wang, L., & Liao, H. (2025). The Potential of Siraitia grosvenorii to Promote Bone Regeneration via Modulating Macrophage Polarization: A Network Pharmacology and Experimental Study. International Journal of Molecular Sciences, 26(8), 3609. https://doi.org/10.3390/ijms26083609






